ABSTRACT
We previously reported that high temperature impacts ganoderic acid (GA) biosynthesis in Ganoderma lucidum via Ca2+. Therefore, to further understand the signal-regulating network of the organism's response to heat stress (HS), we examined the role of nitric oxide (NO) under HS. After HS treatment, the NO level was significantly increased by 120% compared to that under the control conditions. The application of a NO scavenger resulted in a 25% increase in GA compared with that found in the sample treated only with HS. Additionally, the application of a NO donor to increase NO resulted in a 30% lower GA content than that in the sample treated only with HS. These results show that the increase in NO levels alleviates HS-induced GA accumulation. Subsequently, we aimed to detect the effects of the interaction between NO and Ca2+ on GA biosynthesis under HS in G. lucidum. Our pharmacological approaches revealed that the NO and Ca2+ signals promoted each other in response to HS. We further constructed the silenced strain of nitrate reductase (NR) and calmodulin (CaM), and the results are in good agreement with the silenced strain and pharmacological experiment. The cross-promotion between NO and Ca2+ signals is involved in the regulation of HS-induced GA biosynthesis in G. lucidum, and this finding is supported by studies with NR-silenced (NRi) and CaM-silenced (CaMi) strains. However, Ca2+ may have a more direct and significant effect on the HS-induced GA increase than NO. These data indicate that NO functions in signaling and has a close relationship with Ca2+ in HS-induced GA biosynthesis.
IMPORTANCE HS is an important environmental stress affecting the growth and development of organisms. We previously reported that HS modulates GA biosynthesis in G. lucidum via Ca2+. However, the signal-regulating network of the organism's response to HS has not yet been elucidated. In this study, we found that NO relieved HS-induced GA accumulation, and NO and Ca2+ could exert promoting effects on each other in response to HS. Further research on the effect of NO and Ca2+ on the production of GAs in response to HS indicated that Ca2+ has a notably more direct and significant effect on the HS-induced GA increase than NO. Our results improve our understanding of the mechanism of HS signal transduction in fungi. A greater understanding of the regulation of secondary metabolism in response to environmental stimuli will provide clues regarding the role of these products in fungal biology.
KEYWORDS: Ganoderma lucidum, heat stress, nitric oxide, calcium-calmodulin, ganoderic acid
INTRODUCTION
For all living organisms, temperatures only moderately above the respective optimal growth temperature represent a challenging problem for survival (1). Heat stress (HS) reduces the fungal growth of Metarhizium robertsii (2) and leads to cell death in Saccharomyces cerevisiae (3). A series of studies have focused on the damage phenomenon caused by HS, but few studies have evaluated the mechanism of HS signal transduction (4, 5). In addition, the fungus is sessile and cannot flee unfavorable temperature conditions; therefore, dissecting the HS response of the fungus and identifying key components of HS sensing and the signal transduction pathway are high priorities for fungi.
In fungi, a 6-h treatment with heat increases the nitric oxide (NO) concentration in Trichoderma harzianum mycelia, and the exogenous NO donor sodium nitroprusside (SNP) can avoid the thermal damage caused by high temperature (6). NO can effectively alleviate oxidative damage induced by HS in the mycelia of Pleurotus eryngii var. tuoliensis (7). However, the function of NO has been poorly studied in the fungal kingdom, and the participation of NO in the HS response remains unclear. Thus, studying the relationship between NO and HS becomes particularly important.
Ganoderma lucidum is one of the best-known medicinal basidiomycetes in the world and has been commonly used throughout China and Southeast Asia for many centuries as a home remedy for the treatment of minor disorders and the promotion of vitality and longevity (8). Current studies on G. lucidum primarily address the separation and purification of the effective components (9, 10), but fewer studies have evaluated their biosynthesis. In recent years, complete genome sequencing, genetic transformation, and transcriptional gene silencing have been gradually developed (11–13), and these offer powerful tools for further basic biological studies. Therefore, as a higher basidiomycete with bioactive secondary metabolites, G. lucidum is becoming a potential model system for studies examining how environmental factors regulate the development and secondary metabolism of basidiomycetes. Previous studies have shown that HS increases ganoderic acid (GA) biosynthesis in G. lucidum (14). Further analyses have revealed that cytosolic Ca2+ plays a vital role in HS signal transduction in G. lucidum (14). However, the other signals involved in the regulatory process, as well as the types of regulatory interactions between these signals, have yet be elucidated. Whether GA biosynthesis is related to the linkages between these signals in G. lucidum remains unclear.
In the present study, we studied the effect of NO on HS-induced GA biosynthesis in G. lucidum and assessed the effect of the relationship between NO and Ca2+ on the production of GAs in response to HS. These results improve our understanding of the mechanism of HS signal transduction in fungi and provide evidence for the mechanism of environmental regulation of secondary metabolism in fungi.
RESULTS
The NO concentration is increased in heat-stressed strains.
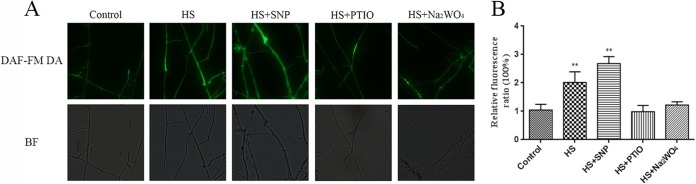
To examine NO accumulation in G. lucidum under HS treatment, a fluorescent probe, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA), was used for the detection of the intracellular NO formation. The fluorescence analysis revealed that under HS growth conditions (42°C), the NO level increased by 120% (P < 0.01) compared with that under control growth conditions (28°C) (Fig. 1A and B). To verify that the observed fluorescence corresponded to NO detection, the wild type (WT) was treated with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO) and the nitrate reductase (NR) inhibitor Na2WO4. cPTIO and Na2WO4 efficiently impaired the fluorescence increase triggered by HS, and each of these treatments significantly reduced (P < 0.01) the percentages of fluorescence to 100% and 75% compared with heat treatment alone, respectively. The exogenous application of SNP, an NO donor, led to a further increase (P < 0.01) in the NO level by 35% compared with heat treatment alone (Fig. 1). These results showed that HS treatment could induce NO accumulation in G. lucidum.
FIG 1.
NO levels were increased in heat-stressed strains. (A) The WT strains were cultured on PDA plates for 5 days and then treated with 500 μM SNP, 500 μM cPTIO, or 500 μM Na2WO4 for 30 min. The strains were exposed to 42°C for 20 min. Change in the level of NO detected by DAF-FM DA staining. BF, bright-field microscopy. (B) Changes in the NO fluorescence ratios in the strains subjected to different treatments. The values are the means ± SD of the results from three independent experiments. Asterisks indicate significant differences compared with the untreated strains (Student's t test; **, P < 0.01).
NR plays a key role in HS-induced NO accumulation.
NR is a major enzymatic source of nitric oxide (NO) production in plants and fungi. To clarify whether the production of NO stimulation by HS was mediated by the NR gene, we cloned the NR gene in G. lucidum and constructed an NR gene-silenced strain to detect its role in the development and metabolism of G. lucidum. First, we used the sequences of the Coprinopsis cinerea NR gene to determine whether G. lucidum contains genes encoding NR. The corresponding NR gene was found in the G. lucidum genome database (G. lucidum strain 22281-R1, GenBank accession no. MG601724). The NR gene sequence is 3,729 bp and contains an open reading frame (ORF) of 2,409 bp encoding a protein of 802 amino acids. The presence of introns was confirmed by real-time reverse transcriptase PCR (qRT-PCR) analysis (data not shown) using the primers shown in Table S1 in the supplemental material. The G. lucidum NR gene coding regions contain a putative molybdopterin-binding domain, a cytochrome b5-like heme/steroid binding domain, an oxidoreductase flavin adenine dinucleotide (FAD)-binding domain, a Moco-binding site, an FAD-binding motif, and an NAD binding pocket similar to that of plant and animal NRs (Fig. S1). The NR-silencing vectors carry the hygromycin B (Hyg) resistance gene as the selectable marker. The vector pAN7-ura3-dual was used as an empty vector control. qRT-PCR analyses and the effect on NO levels were used to confirm silencing in the transformants, and the NR gene-silenced (NRi) strains NRi-6 and NRi-8 were selected for further analyses (Fig. S2).
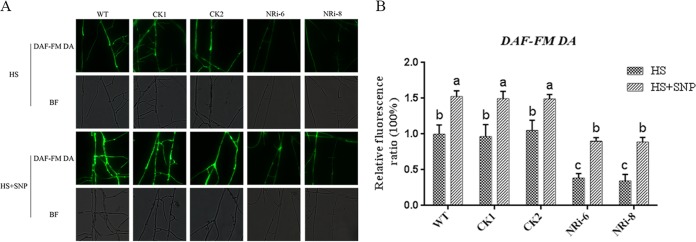
To further demonstrate that the role of NO production was mediated by NR under HS conditions, we compared the NO levels in the WT and the NR gene-silenced strains under HS treatment. The results of the DAF-FM DA fluorescence analysis showed that the NO content of NRi-6 and NRi-8 decreased (P < 0.05) by approximately 70% compared with the WT sample treated with only HS. However, these effects were recovered by the addition of SNP to the NR-silenced strains (Fig. 2). Both the NR inhibitor experiment (Fig. 1) and the NR-silenced strains showed lower NO levels than the WT strain. The results indicated that NO production from NR was involved in the HS treatment conditions.
FIG 2.
Effect of different treatments on the NO content in the WT and silenced strains. (A) The WT and NRi strains were cultured on PDA plates for 5 days, treated with 500 μM SNP for 30 min, and then exposed to 42°C for 20 min. The change in the NO amount was detected by fluorescence microscopy after staining with DAF-FM DA. (B) Changes in the NO fluorescence ratios in the WT and NR-silenced strains that were subjected to different treatments. The values are the means ± SD of the results from three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test).
HS-induced NO participates in the regulation of GA biosynthesis.
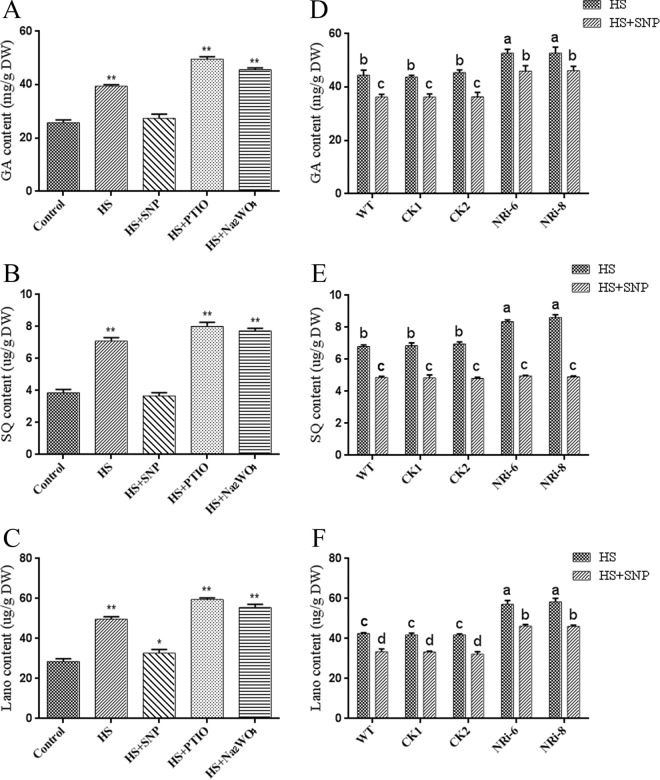
Previous studies have found that HS increases GA biosynthesis, and we thus examined whether the NO level was responsible for GA biosynthesis in the HS sample. We pretreated the WT sample with SNP, cPTIO, and Na2WO4 before HS treatment to detect the effect of the NO content on HS-induced GA biosynthesis. Exogenous application of cPTIO and Na2WO4 resulted in 25% and 15% increases (P < 0.01) in GA levels compared with heat treatment alone. The GA content was 30% lower after exogenous application of SNP (P < 0.01) than after heat treatment (Fig. 3A). To reflect the changes in GA accumulation from various aspects, two important intermediate products in the GA biosynthesis pathway, squalene (SQ) and lanosterol (Lano), were detected. The results showed similar changing trends in GAs, and two important intermediate products were increased in the cPTIO- and Na2WO4-treated strains compared with the sample treated with only HS. Exogenous application of SNP was used to alleviate the accumulation of the two intermediate products induced by HS (Fig. 3B and C), and changes in these intermediate products also indirectly indicated changes in the GA level. The results showed that different concentrations of NO differentially affected the GA accumulation induced by HS treatment. The data suggest that NO alleviates HS-induced GA biosynthesis.
FIG 3.
Effect of different concentrations of NO on GA biosynthesis in heat-stressed strains. The WT strains were cultured in PDA liquid cultures with shaking for 5 days, pretreated with or without 500 μM SNP, 500 μM cPTIO, and 500 μM Na2WO4, and then exposed to 42°C for 12 h. (A) Measurement of the amount of total GA in G. lucidum subjected to different treatments. (B) Measurement of the amount of SQ in G. lucidum subjected to different treatments. (C) Measurement of the amount of Lano in G. lucidum subjected to different treatments. The WT and NRi strains were cultured in PDA liquid cultures with shaking for 5 days, pretreated with or without 500 μM SNP, and then exposed to 42°C for 12 h. (D to F) Measurement of the amount of total GA (D), SQ (E), and Lano (F) in the WT and NRi strains with HS treatment. The values are the means ± SD of the results from three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test). DW, dry weight.
To further explore the role of NO in the HS regulation of GA accumulation with genetic evidence, GA accumulation in the WT and NRi strains in response to HS was detected. After HS treatment at 42°C, the GA level in the NRi-6 and NRi-8 strains was 20% higher (P < 0.05) than that in the WT. To examine whether the suppression of the NO level in the NRi strains was responsible for GA accumulation under HS, we pretreated the WT and NRi strains with SNP. Upon exogenous application of SNP, the GA level in the NRi-6 and NRi-8 strains was almost fully rescued to the level of the WT with heat treatment alone (Fig. 3D). The intermediate products SQ and Lano were detected in the WT and NRi strains. The NRi strains exhibited a 22% increase (P < 0.05) in SQ content compared with that in the WT strains under HS treatment. The level of Lano was increased by 34% (P < 0.05) in the NRi strains compared with the WT. Pretreatment with SNP alleviated the increase in SQ and Lano induced by HS (Fig. 3E and F), and the changing trends in SQ and Lano were similar to those of GAs. Our data revealed that in response to HS treatment, the WT plants maintained a higher level of NO and a lower GA content than the NRi strains under HS. These results from physiology and genetics studies (Fig. 1 to 3) demonstrate that the NO level is involved in GA biosynthesis following heat treatment, and NO relieves HS-induced GA accumulation in G. lucidum.
Effect of NO on the Ca2+ signaling pathway under HS treatment conditions.
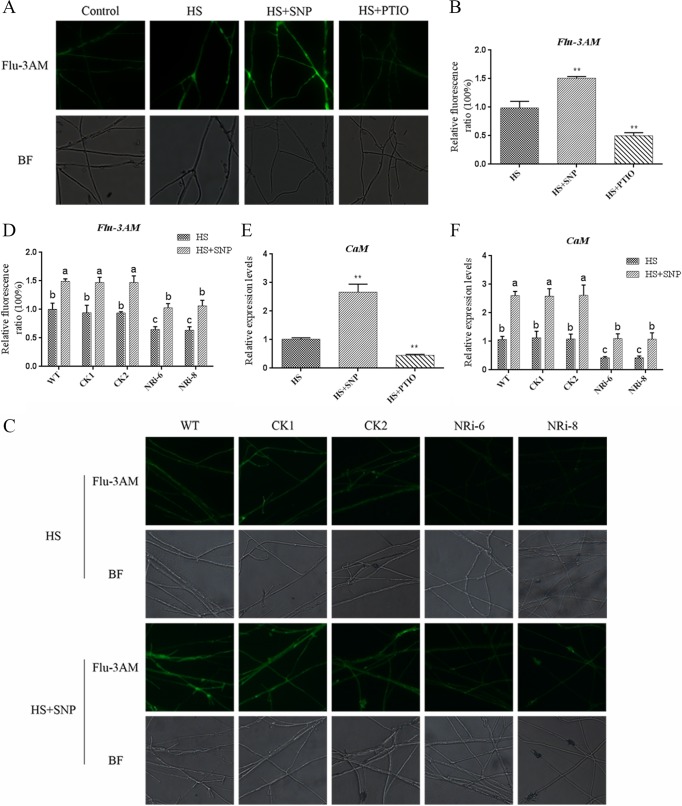
We previously reported that an increase in cytosolic Ca2+ was induced by heat and involved HS signal transduction in G. lucidum. To gain insight into NO signal transduction, the effect of NO on the Ca2+ signaling pathway was examined under heat treatment. The experimental results showed that pretreatment with SNP resulted in 45% further increases (P < 0.01) in the Ca2+ content compared with treatment with only HS, as detected by Fluo-3AM, a Ca2+-sensitive fluorescent probe (Fig. 4A and B). However, pretreatment with a specific NO scavenger, cPTIO, arrested (P < 0.01) 50% of the HS-induced accumulation of Ca2+ compared with the treatment with only HS. These data demonstrate that NO can induce an increase in the concentration of Ca2+ under HS. Calmodulin (CaM) is the predominant Ca2+ sensor and plays a crucial role in the response to external factors. To further examine the effect of NO on the Ca2+ signaling pathway under HS conditions, we examined the effect of NO on CaM gene expression by qRT-PCR. CaM gene expression in the SNP-and-HS-cotreated sample was 200% higher (P < 0.01) than that in the WT with HS alone. The CaM gene expression level in the cPTIO-and-HS-cotreated sample was 60% lower (P < 0.01) than that in the WT treated with only HS (Fig. 4E). The results show that NO is involved in HS-induced Ca2+ production and CaM gene expression.
FIG 4.
Effect of NO on the Ca2+ signaling pathway under HS treatment conditions. (A) WT strains were cultured on PDA plates for 5 days, treated with 500 μM SNP or 500 μM cPTIO for 30 min, and then exposed to 42°C for 20 min. The changes in the Ca2+ levels were detected by fluorescence microscopy after staining with Fluo-3AM. (B) Changes in the Ca2+ fluorescence ratios in the strains subjected to different treatments. (C) The WT and NRi strains were cultured on PDA plates for 5 days, treated with or without 500 μM SNP for 30 min, and then exposed to 42°C for 20 min. The changes in Ca2+ levels were detected by fluorescence microscopy after staining with Fluo-3AM. (D) Changes in the Ca2+ fluorescence ratios in the WT and NRi strains subjected to different treatments. (E) Effect of the NO donor and NO scavengers on the expression of the CaM gene in G. lucidum under HS treatment. (F) Changes in the expression of the CaM gene in the WT and NRi strains under HS treatment with or without SNP. The values are the means ± SD of the results from three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test).
To further clarify this point, Ca2+ accumulation under HS was compared between the WT and NRi strains. The result showed that the Ca2+ level was 40% higher (P < 0.05) in the WT than in the NRi strains and that exogenous application of SNP restored the level of Ca2+ in the NRi strains (Fig. 4C and D). The expression of the CaM gene was 55% lower (P < 0.05) in the NRi strains than in the WT (Fig. 4F). The application of SNP restored the level of CaM gene expression in NRi strains (Fig. 4F). These genetics-based data suggest that NO is involved in HS-induced Ca2+ production and CaM gene expression. NO relieved HS-induced GA accumulation, and Ca2+ positively regulated GA biosynthesis; however, NO could help increase the Ca2+ content. This contradiction requires further study.
Effect of Ca2+ on the NO signaling pathway under HS treatment conditions.
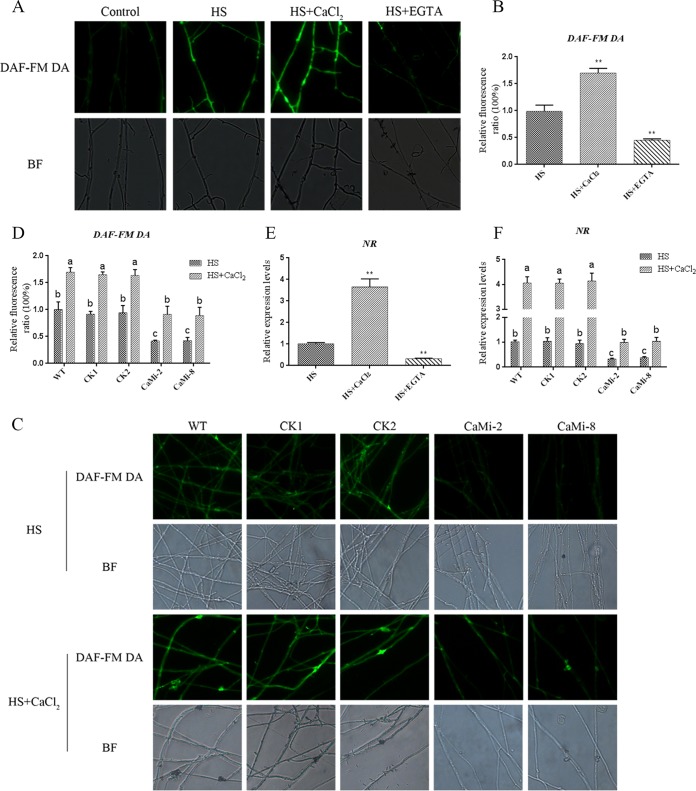
To investigate the complex relationships between NO and Ca2+, CaCl2 and EGTA were used for NO detection. Exogenous pretreatment with CaCl2 greatly increased (P < 0.01) the NO levels by 70% under HS conditions, and pretreatment with EGTA significantly reduced (P < 0.01) the production of NO induced by HS treatment by 60% compared with treatment with HS alone (Fig. 5A and B). To determine the influence of Ca2+ on NO levels under HS, we examined the effect of Ca2+ on NR gene expression by qRT-PCR. The expression of NR was increased (P < 0.01) by 350% in the CaCl2-and-HS-cotreated sample and decreased (P < 0.01) by 70% in the EGTA-and-HS-cotreated sample compared with the WT treated with HS alone (Fig. 5E). These results suggest that Ca2+ is required for HS-induced NO production and NR gene expression in G. lucidum. It is therefore reasonable to propose a link between the functions of NO and Ca2+ under HS conditions.
FIG 5.
Effect of Ca2+ on the NO signaling pathway under HS treatment conditions. (A) The WT strains were cultured on PDA plates for 5 days, treated with 2 mM CaCl2 or 500 μM EGTA for 30 min, and then exposed to 42°C for 20 min. The changes in NO levels were detected by fluorescence microscopy after staining with DAF-FM DA. (B) Changes in the NO fluorescence ratios in the strains subjected to different treatments. (C) The WT and CaMi strains were cultured on PDA plates for 5 days, treated with or without 2 mM CaCl2 for 30 min, and then exposed to 42°C for 20 min. The changes in NO levels were detected by fluorescence microscopy after staining with DAF-FM DA. (D) Changes in the NO fluorescence ratios in WT and CaMi strains subjected to different treatments. (E) Effect of CaCl2 and EGTA on the expression of the NR gene in G. lucidum under HS treatment. (F) Changes in the expression of the NR gene in the WT and CaMi strains under HS treatment with or without CaCl2. The values are the means ± SD of the results from three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test).
In addition, we constructed CaM gene-silencing strains by RNA interference (RNAi) to examine the effect of NO signaling on the Ca2+ level in HS signaling. The RNAi silencing vector pAN7-dual was used to express the sense-antisense structure of the CaM gene. We constructed a CaM-silencing vector named pAN7-dual-CaMi using the same construction method used for the NR-silenced strains. qRT-PCR analyses were used to determine the silencing efficiency of the transformants, and CaM-silenced (CaMi-2 and CaMi-8) strains were selected for further analyses (Fig. S3A and B). Under HS conditions, the NO level in the WT was 55% higher (P < 0.01) than that in the CaMi strains (Fig. 5C and D), and the level of NR gene expression in CaMi strains was 65% lower (P < 0.01) than that in the WT sample (Fig. 5F). The application of CaCl2 restored the level of NO and the NR gene expression in the CaMi strains (Fig. 5F). These data, combined with the results shown in Fig. 3 and 4, demonstrate the presence of cross talk between NO and calcium in HS signaling.
Analysis of the effects of Ca2+ on GA biosynthesis in WT and NRi strains.
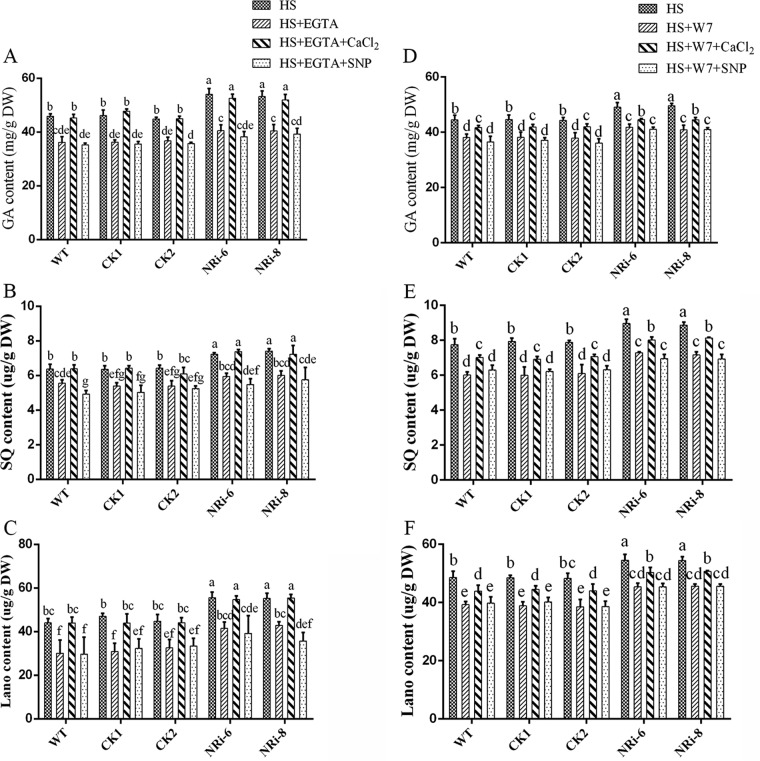
To examine the relationship between NO and Ca2+ in GA biosynthesis under HS treatment conditions, NRi strains were exogenously pretreated with EGTA and the CaM antagonist N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide hydrochloride (W7) to prevent both Ca2+ and NO. Exogenous CaCl2 and SNP were added in the presence of EGTA or W7 to the NRi strains, respectively. The same treatment was performed for the WT as a control. Under HS treatment conditions, the GA content was reduced (P < 0.05) by 20% and 15% in the presence of EGTA and W7 in the WT and empty vector control (CK) samples, respectively. The exogenous application of CaCl2 after pretreatment with EGTA could fully restore the GA content in the WT and CK samples, and the exogenous application of CaCl2 after pretreatment with W7 showed only a 9% recovery (P < 0.05) of the GA content in the WT and CK samples. However, the readdition of SNP after pretreatment with EGTA and W7 had no significant (P > 0.05) effect on the GA levels in the WT and CK samples under HS treatment (Fig. 6A and D). The SQ and Lano contents were similar to the total GA content in the WT (Fig. 6B, C, E, and F). The GA level was notably decreased (P < 0.05) by approximately 23% and 18% by pretreatment with EGTA and W7, respectively, in the NRi-6 and NRi-8 strains compared with the strains treated with only HS, and they approached the WT level. After exogenous application of CaCl2, the GA contents of the NRi-6 and NRi-8 strains could be restored to the levels in the samples treated with only HS, despite the presence of EGTA. Under HS, the GA content recovered (P < 0.05) by only 10% in the CaCl2-and-W7-cotreated sample compared with the sample treated with only W7. However, supplementation with SNP after pretreatment with EGTA and W7 had no significant (P > 0.05) effect on the GA content in NRi-6 and NRi-8 strains (Fig. 6A and D). To reflect the changes in GA accumulation from various aspects, the SQ and Lano levels were detected, which showed the same changing patterns as the GAs under HS conditions in the NRi-6 and NRi-8 strains (Fig. 6B, C, E, and F). These observations suggest that Ca2+ participates in the NO pathway of HS signal transduction and that Ca2+ is involved in GA biosynthesis in the NRi strains.
FIG 6.
Effects of Ca2+ on GA biosynthesis in the WT and NRi strains. The WT strains were cultured in PDA liquid cultures with shaking for 5 days with the addition of exogenous 2 mM CaCl2 and 500 μM SNP in the presence of 500 μM EGTA and then exposed to 42°C for 12 h. (A to C) Measurements of the total GA (A), squalene (B), and lanosterol (C) contents in heat-stressed strains are shown. The WT and NRi strains were cultured in PDA liquid cultures with shaking for 5 days, and exogenous 2 mM CaCl2 and 500 μM SNP were added in the presence of 50 μM W7. (D to F) Measurements of the total GA (D), squalene (E), and lanosterol (F) contents in the heat-stressed strains are shown. The values are the means ± SD of the results from three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test).
Analysis of the effects of NO on GA biosynthesis in the WT and CaMi strains.
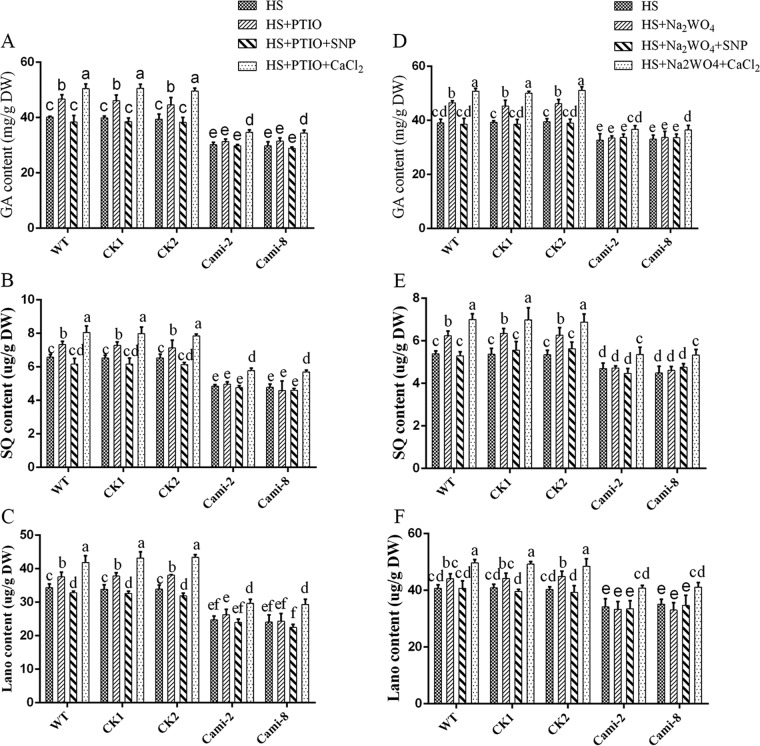
To further examine the interaction between NO and Ca2+ in GA biosynthesis under HS conditions, we examined the effect of NO on the CaMi strains. Exogenous pretreatment of the CaMi strains with cPTIO and Na2WO4 prevented both NO and Ca2+. Then, exogenous SNP and CaCl2 were added in the presence of cPTIO and Na2WO4 to the CaMi strains, respectively. The same treatment of the WT served as a control. Analysis of the level of GA revealed that pretreatment with cPTIO and Na2WO4 led to no significant (P > 0.05) difference in the CaMi strains, although the effect clearly enhanced the GA levels in the WT (Fig. 7A and D). Under HS, exogenous application of SNP could effectively alleviate cPTIO- and Na2WO4-induced GA accumulation in the WT. However, supplementation with SNP after pretreatment with cPTIO and Na2WO4 had no significant (P > 0.05) effect on the GA content in the CaMi-2 and CaMi-8 strains. Under HS, supplementation with CaCl2 after pretreatment with cPTIO and Na2WO4 led to 15% and 13% increases (P < 0.05) in GA accumulation relative to that observed in the WT samples treated with only cPTIO or Na2WO4. However, partial recovery rates of only 10% and 7% (P < 0.05) in the CaMi strains were found after supplementation with CaCl2 after pretreatment with cPTIO and Na2WO4, respectively (Fig. 7A and D). To monitor the metabolic synthesis of GAs, the intermediate products SQ and Lano were also analyzed. The SQ and Lano levels suggested the same trend in GA content (Fig. 7B, C, E, and F), whereas exogenous application of cPTIO showed adverse effects on the WT and CaMi strains. These results suggest that Ca2+ may have a more direct and significant effect than NO on the HS-induced GA increase.
FIG 7.
Effects of NO on GA biosynthesis in the WT and CaMi strains. The WT and CaMi strains were cultured in PDA liquid cultures with shaking for 5 days, and exogenous 2 mM CaCl2 and 500 μM SNP were added in the presence of 500 μM cPTIO, respectively. The strains were then exposed to 42°C for 12 h. (A to C) Measurements of the total GA (A), squalene (B), and lanosterol (C) contents in the heat-stressed strains are shown. The WT and CaMi strains were cultured in PDA liquid cultures with shaking for 5 days, and exogenous 2 mM CaCl2 and 500 μM SNP were added in the presence of 500 μM Na2WO4. The strains were then exposed to 42°C for 12 h. (D to F) Measurements of the total GA (D), squalene (E), and lanosterol (F) contents in the heat-stressed strains are shown. The values are the means ± SD of three independent experiments. Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple-range test).
DISCUSSION
Secondary metabolites are a diverse set of compounds that are believed to have numerous functions in plant-environment interactions and adaptation to the changing environment. HS is an important environmental stress that influences secondary metabolites. Ectoine and 5-hydroxyectoine biosynthesis is triggered in response to a high growth temperature in Streptomyces coelicolor. Increased levels of phenylpropanoid metabolites have been observed in citrus plants in response to HS. Research on the regulatory mechanism of the environment in the synthesis of secondary metabolite products has aroused general concern. Our recent research has also shown a significant increase in GA content after HS in G. lucidum (14). However, little is known regarding the mechanism through which HS induces secondary metabolism in fungi. G. lucidum is widely used in Asia, notably in East Asia. As a nonmodel organism, it is difficult to extensively research the molecular interaction from a genetic perspective. However, from the physiological perspective, further research will be necessary to determine which signaling molecules are involved in controlling this process. This research will also aid the explanation of the mutual influence between these signals and provide a detailed understanding of the signal transduction mechanisms of HS. In this work, we obtained evidence for the involvement of NO in HS-induced GA biosynthesis. These results provide insights into the mechanism underlying the environmental control of secondary metabolism via signal transduction pathways.
NO is an important signal molecule in stress responses. However, stress-induced NO occurs via multiple pathways. In mammals, NO production is mainly mediated by NO synthase (NOS), which oxidizes arginine to generate citrulline and NO (15). However, the molecular identity of plant NOS is unknown. In the absence of functional NOS proteins, one of the most important sources of NO in land plants is thought to be the reduction of nitrite (NO2−) by NR. However, little is known about the role and biosynthesis of NO in fungi. In Aspergillus nidulans, NO synthesis by NR is regulated during development (16). Our study demonstrated that HS treatment increased the amount of NO produced from NR to alleviate GA accumulation induced by HS in G. lucidum. The involvement of NR-mediated NO production has been reported under both biotic and abiotic stresses in plants. In Triticum aestivum, the NO burst mediated through NR relieved Al-induced root inhibition and oxidative damage, and antioxidant enzyme activities were significantly enhanced by an NO donor but suppressed by an NO scavenger or NR inhibitor under Al stress (17). In Arabidopsis, cold acclimation induced an increase in the endogenous NO level by stimulating NR activity, and the use of an NR inhibitor, an NO scavenger, and an NO donor revealed that the NR-dependent NO level was positively correlated with freezing tolerance (18). In summary, both in plants and in fungi, the NO product from NR plays a key role in normal growth and in alleviating the stress response.
The function of Ca2+, which acts as a signaling molecule in organisms, is now well established (19). The possibility that NO works together with the universal second messenger Ca2+ in animals and in plant biology has emerged. However, in microorganisms, fewer correlative research studies on the relationship between signals have been reported, particularly in fungi. Studies of NO and Ca2+ in plants and animals have shown significant overlap in their individual pathways, and growing evidence suggests that these two messengers may interact in subtle ways; however, it remains controversial regarding which pathway is upstream of the other. Our current data indicate that NO is required for HS-induced GA biosynthesis both upstream and downstream of Ca2+ in G. lucidum. Similar results have been reported in research on plants and animals (Table S2). Studies examining animal cells highlight the ability of NO to modulate the gating of Ca2+ channels and to impact the activity of Ca2+ pumps (20). Moreover, Ca2+ sensors, such as Ca2+/CaM-dependent protein kinases, which are regulated through NO-dependent mechanisms, are being discovered (21). NO is not only a step in the signaling cascades initiated by Ca2+ but also one of the key messengers governing the overall control of Ca2+ homeostasis (22). The occurrence of such cross talk is well documented in plants. Ca2+ induces NO production in guard cells of the Arabidopsis epidermis, and NO generation in guard cells further triggers transient Ca2+ and finally stomatal closure. The results describing the Ca2+ signal transduction pathway indicate that it cross talks with NO, leading to stomatal closure (23). In Ulva compressa, copper-induced NO synthesis and increases in NOS activity are dependent on Ca2+ release, and copper-induced calcium release is activated by NO. These results suggest that Ca2+ functions both upstream and downstream of NO production under exposure to copper stress (24). The present research is of great importance for determining the molecular mechanisms underlying the cross talk between Ca2+ and NO under exposure to biotic stress.
Numerous studies indicate that Ca2+, a key messenger in regulating many growth and developmental processes, plays a crucial role in stress signaling. In various ways, signal-induced cellular Ca2+ levels can regulate response genes either directly or indirectly through Ca2+ sensors. Elevated Ca2+ levels influence the binding of Ca2+ transcription factors, which directly bind to specific DNA sequences and modulate gene expression. In addition, Ca2+ can activate calcium sensors (Ca2+/CaM or Ca2+/calmodulin-like [CML] sensors) that interact with DNA-binding proteins and modulate their activity. Finally, Ca2+ activates a protein kinase (calcium-dependent protein kinase [CDPK], CaM-binding protein kinase [CBK], and/or CaM kinase [CaMK]) directly or a protein phosphatase, which in turn phosphorylates or dephosphorylates a transcription factor (TF), resulting in the activation or repression of transcription (25, 26). Although there are various downstream receptors (Ca2+ receptor protein), these studies provide evidence that CaM might be one of the key players in the response to biotic and abiotic stresses (27). Our current results have shown that CaM plays an important role in HS-induced GA biosynthesis. The addition of CaCl2 could partially restore the accumulation of GA in W7-treated and in CaM-silenced strains, potentially because the other Ca2+ receptor plays a role in this process.
Due to the importance of high-temperature stress, a large amount of research has been undertaken to understand how to integrate these signals via a signaling network, which involves second-messenger molecules as well as signal-sensing proteins. A variety of signaling pathways are thought to be triggered by reactive oxygen species (ROS) and redox imbalance under HS conditions and play an important role in plants. H2O2 is quickly produced in response to heat treatment in Arabidopsis species (28). Heat-induced overaccumulation of ROS occurs in Arabidopsis species (29). Considerable evidence has accumulated supporting the roles of proline, membrane fluidity, and protein kinases in HS signal transduction (30, 31). However, little is known regarding the relationships between these signaling molecules in the biosynthesis of secondary metabolism under HS. Thus, the relationship between these signaling molecules in HS-induced secondary metabolism in G. lucidum merits further investigation.
In a recent study, our data suggested that HS-induced cytosolic Ca2+ participates in regulating GA biosynthesis in G. lucidum (14). In the present study, our data suggested that HS treatment could induce NO accumulation in G. lucidum and that NO alleviates GA accumulation induced by HS treatment. NO and Ca2+ could promote each other in response to HS. However, the roles of NO and Ca2+ in response to HS were reversed. This phenomenon has also been reported in muscle cells. Cytosolic Ca2+ increases NO release, and the generation of NO induces the release of Ca2+ (Table S2). Ca2+ activates muscle growth and regeneration; however, NO represses muscle growth and regeneration (32). This finding illustrates the complexity of the signal-regulating network. On the one hand, the signal exercises its functions, and on the other hand, the signal promotes the reverse function. Collectively, our data confirm the existence of a novel signaling pathway in which NO production is stimulated by HS to regulate GA accumulation (Fig. 8). This finding allows us to further study the biological processes in HS at the physical level, and combined with genetic modification, these studies will allow us to shed light on the mechanisms of HS signal transduction at a more advanced level. A greater understanding of the regulation of secondary metabolism in response to environmental stimuli will provide clues regarding the roles of these products in fungal biology.
FIG 8.
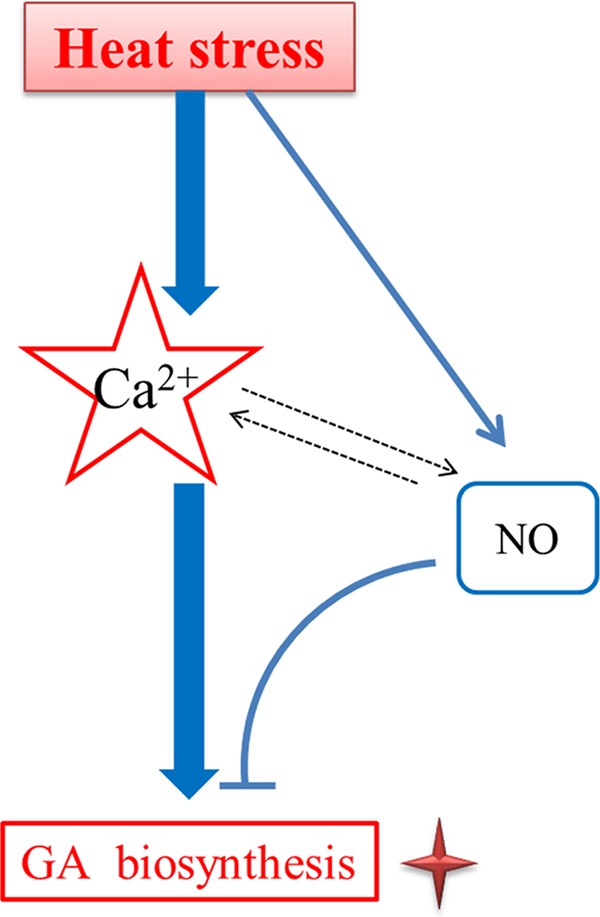
Schematic representation of a hypothetical model of the HS signaling pathway in GA biosynthesis in G. lucidum. HS treatment could induce NO accumulation, and NO production from NR is involved in alleviating HS-induced GA biosynthesis. There is cross talk between the NO and calcium signals in response to HS-induced GA biosynthesis. Ca2+ may have a more direct and significant effect than NO on the HS-induced GA increase. The solid arrows indicate data supported by our own experiments, and dotted arrows indicate potential reactions or data that have been experimentally supported in other systems.
MATERIALS AND METHODS
Fungal strains and growth conditions.
The DH5α strain of Escherichia coli was used for plasmid amplification and grown in Luria-Bertani (LB) medium containing 100 mg/ml ampicillin or 50 mg/ml kanamycin, as required. G. lucidum was used as the wild-type (WT) strain. The WT, CK (empty vector control), NR-silenced, and CaM-silenced strains were cultured at 28°C in potato dextrose broth (PDB) medium.
HS treatments and chemical treatments.
The HS treatments were conducted according to a protocol described previously, with some modifications (14). The strains were inoculated on a potato dextrose agar (PDA) plate that was overlaid with a layer of cellophane and cultured for 5 days at 28°C. The fungal mycelium was then transferred onto a PDA plate with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO), sodium nitroprusside (SNP), ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), and CaCl2 for 30 min before HS treatment (14, 33, 34). The G. lucidum strains were exposed to 42°C for 20 min, and the NO and Ca2+ levels were then evaluated. The mycelium was cultured on PDA plates overlaid with a layer of sterile cellophane for 5 days and then transferred to PDA with cPTIO, SNP, EGTA, and CaCl2 for 30 min before HS treatment. To evaluate the transcriptional expression levels of the NR gene and the CaM gene, 5-day-old G. lucidum strains were heat-stressed at 42°C for 90 min (14).
To detect GA and its mesostate, chemical treatments were conducted according to a previously described protocol, with some modifications (14). The strains were cultured on PDA liquid cultures for 5 days with shaking, exposed to 42°C for 12 h, and then incubated in stable PDA liquid cultures at 28°C until the 7th day (14). In the experiments, the samples were pretreated with cPTIO, SNP, EGTA, and CaCl2 for 30 min prior to HS. In the experiments requiring the readdition of exogenous calcium and SNP, the specimens were supplemented with 2 mM CaCl2 and 500 μM SNP after EGTA or cPTIO pretreatment for 30 min.
Real-time PCR analysis of gene expression.
The levels of gene-specific mRNA expressed by the WT and isolated RNAi transformant strains were assessed using quantitative real-time PCR, according to our previous study (33). Gene expression was evaluated by calculating the difference between the threshold cycle (CT) value of the analyzed gene and the CT value of the 18S rRNA housekeeping gene. Quantitative real-time reverse transcriptase PCR (qRT-PCR) calculations analyzing the relative gene expression levels were performed according to the 2−ΔΔCT method described by Livak and Schmittgen (35). We considered a gene fold change of >2 and P value of <0.01 as reflecting a significant difference in expression (36). The gene fragments were amplified by real-time PCR using the primers shown in Table S1.
NO detection assay.
The fluorescent probe 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA; Sigma) was used to measure the intracellular NO production, as described previously (16). The mycelium was stained with DAF-FM DA for 20 min to visualize NO, and a fluorescence microscope (BX53F; Olympus) was used to detect the fluorescence. We selected all the hyphae in the images for NO quantification, and the average fluorescence intensities of DCFH-DA in the mycelia were analyzed using Zen Lite (Zeiss Software). The average fluorescence intensity was the total value of all hyphal fluorescence intensities divided by the number of all hyphae in the images.
Ca2+ detection assay.
The Ca2+ concentrations were assessed according to a previously described method (33). For the fluorescence assay, the tissues were stained with Fluo-3AM to visualize the free cytosolic Ca2+. Fluo-3AM was loaded into the cells by incubation for 30 min, and the cells were then washed three times with phosphate-buffered saline (PBS). The Fluo-3AM-labeled cells were examined using a fluorescence microscope (BX53F; Olympus). To eliminate the contribution of the fluorescence background, control cells without Fluo-3AM labeling were also imaged under identical conditions. We selected all the hyphae in the photos for Ca2+ quantification, and the average fluorescence intensities of Fluo-3AM in the mycelia were analyzed using Zen Lite (Zeiss Software). The average fluorescence intensity was the total value of all hyphal fluorescence intensities divided by the number of all hyphae in the photos.
Construction of RNAi plasmids and strains.
The construction of a fungal RNAi vector, pAN7-dual, was performed as previously described (13). The glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter and 35S promoter were used to suppress the expression of the NR gene and the CaM gene. The coding region of the NR gene and the CaM gene was amplified by PCR using G. lucidum cDNA as the template and the primers listed in Table S1 in the supplemental material. The RNA interference (RNAi)-silencing vectors pAN7-dual-NRi and pAN7-dual-CaMi were transferred into G. lucidum by electroporation. Dozens of transformants were selected randomly, and qRT-PCR analyses were performed to determine the silencing efficiency of the transformants. Two independent silencing strains with the highest silencing efficiency were selected for further study.
Detection and measurement of GA and its mesostate in the GA biosynthesis pathway.
GAs, squalene, and lanosterol were extracted and measured from fungal mycelia according to a previously described method (37). Ganoderic acid-a was used as the standard (38).
Statistical analysis.
All experimental data shown in this paper were obtained from three independent samples to ensure that the trends and relationships observed in the cultures were reproducible. The error bars in Fig. 1 to 7 indicate the standard deviation (SD) from the means of triplicates. Within each set of experiments, bars with different letters are significantly different at a P value of <0.05, according to Duncan's multiple-range test.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant 81773839 to Ang Ren and grant 31672212 to Liang Shi), the earmarked fund for the China Agriculture Research System (grant CARS-20 to Ming-wen Zhao), the Natural Science Foundation of Jiangsu Province, China (grant BK20171377 to Ang Ren), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant KYZZ16_0383 to Rui Liu).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00043-18.
REFERENCES
- 1.Richter K, Haslbeck M, Buchner J. 2010. The heat shock response: life on the verge of death. Mol Cell 40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Liao X, Lu HL, Fang W, St. Leger RJ. 2014. Overexpression of a Metarhizium robertsii HSP25 gene increases thermotolerance and survival in soil. Appl Microbiol Biotechnol 98:777–783. doi: 10.1007/s00253-013-5360-5. [DOI] [PubMed] [Google Scholar]
- 3.Guyot S, Gervais P, Young M, Winckler P, Dumont J, Davey HM. 2015. Surviving the heat: heterogeneity of response in Saccharomyces cerevisiae provides insight into thermal damage to the membrane. Environ Microbiol 17:2982–2992. doi: 10.1111/1462-2920.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, Chen Q, Wu X, Zhang J, Huang C. 2014. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr Microbiol 69:611–616. doi: 10.1007/s00284-014-0634-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Wisniewski M, Wang W, Liu J, Liu Y. 2014. Heat-induced oxidative injury contributes to inhibition of Botrytis cinerea spore germination and growth. World J Microbiol Biotechnol 30:951–957. doi: 10.1007/s11274-013-1513-z. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Yang ZJ, Guo K, Li Z, Zhou HZ, Wei YL, Li JS, Zhang XJ, Harvey P, Yang HT. 2015. Oxidative damage induced by heat stress could be relieved by nitric oxide in Trichoderma harzianum LTR-2. Curr Microbiol 70:618–622. doi: 10.1007/s00284-014-0764-8. [DOI] [PubMed] [Google Scholar]
- 7.Kong W, Huang C, Chen Q, Zou Y, Zhang J. 2012. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet Biol 49:15–20. doi: 10.1016/j.fgb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Mizushina Y, Hanashima L, Yamaguchi T, Takemura M, Sugawara F, Saneyoshi M, Matsukage A, Yoshida S, Sakaguchi K. 1998. A mushroom fruiting body-inducing substance inhibits activities of replicative DNA polymerases. Biochem Biophys Res Commun 249:17–22. doi: 10.1006/bbrc.1998.9091. [DOI] [PubMed] [Google Scholar]
- 9.Hsu MJ, Lee SS, Lin WW. 2002. Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway. J Leukoc Biol 72:207–216. [PubMed] [Google Scholar]
- 10.Zhao LY, Dong YH, Chen GT, Hu QH. 2010. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr Polym 80:783–789. doi: 10.1016/j.carbpol.2009.12.029. [DOI] [Google Scholar]
- 11.Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, Li C, Wang L, Guo X, Sun Y, Luo H, Li Y, Song J, Henrissat B, Levasseur A, Qian J, Li J, Luo X, Shi L, He L, Xiang L, Xu X, Niu Y, Li Q, Han MV, Yan H, Zhang J, Chen H, Lv A, Wang Z, Liu M, Schwartz DC, Sun C. 2012. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun 3:913. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Fang X, Li M, Mu D, Ren A, Tan Q, Zhao M. 2012. Development of a simple and efficient transformation system for the basidiomycetous medicinal fungus Ganoderma lucidum. World J Microbiol Biotechnol 28:283–291. doi: 10.1007/s11274-011-0818-z. [DOI] [PubMed] [Google Scholar]
- 13.Mu DS, Shi L, Ren A, Li MJ, Wu FL, Jiang AL, Zhao MW. 2012. The development and application of a multiple gene co-silencing system using endogenous URA3 as a reporter gene in Ganoderma lucidum. PLoS One 7:e43737. doi: 10.1371/journal.pone.0043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Ren A, Li MJ, Cao PF, Chen TX, Zhang G, Shi L, Jiang AL, Zhao MW. 2016. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Appl Environ Microbiol 82:4112–4125. doi: 10.1128/AEM.01036-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rand MJ, Li CG. 1995. Nitric oxide as a neurotransmitter in peripheral-nerves—nature of transmitter and mechanism of transmission. Annu Rev Physiol 57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- 16.Marcos AT, Ramos MS, Marcos JF, Carmona L, Strauss J, Canovas D. 2016. Nitric oxide synthesis by nitrate reductase is regulated during development in Aspergillus. Mol Microbiol 99:15–33. doi: 10.1111/mmi.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun CL, Lu LL, Liu LJ, Liu WJ, Yu Y, Liu XX, Hu Y, Jin CW, Lin XY. 2014. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol 201:1240–1250. doi: 10.1111/nph.12597. [DOI] [PubMed] [Google Scholar]
- 18.Zhao MG, Chen L, Zhang LL, Zhang WH. 2009. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767. doi: 10.1104/pp.109.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders D, Pelloux J, Brownlee C, Harper JF. 2002. Calcium at the crossroads of signaling. Plant Cell 14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamler JS, Lamas S, Fang FC. 2001. Nitrosylation, the prototypic redox-based signaling mechanism. Cell 106:675–683. doi: 10.1016/S0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 21.Takata T, Kimura J, Tsuchiya Y, Naito Y, Watanabe Y. 2011. Calcium/calmodulin-dependent protein kinases as potential targets of nitric oxide. Nitric Oxide 25:145–152. doi: 10.1016/j.niox.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Clementi E, Meldolesi J. 1997. The cross-talk between nitric oxide and Ca2+: a story with a complex past and a promising future. Trends Pharmacol Sci 18:266–269. doi: 10.1016/S0165-6147(97)90641-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang WH, Yi XQ, Han AD, Liu TW, Chen J, Wu FH, Dong XJ, He JX, Pei ZM, Zheng HL. 2012. Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J Exp Bot 63:177–190. doi: 10.1093/jxb/err259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González A, Cabrera MDLA, Henriquez MJ, Contreras RA, Morales B, Moenne A. 2012. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol 158:1451–1462. doi: 10.1104/pp.111.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virdi AS, Singh S, Singh P. 2015. Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front Plant Sci 6:809. doi: 10.3389/fpls.2015.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. 2012. CaMKII determines mitochondrial stress responses in heart. Nature 491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy AS, Ali GS, Celesnik H, Day IS. 2011. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23:2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banti V, Mafessoni F, Loreti E, Alpi A, Perata P. 2010. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152:1471–1483. doi: 10.1104/pp.109.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YP, Lee TY, Tanaka A, Charng YY. 2014. Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J 80:14–26. doi: 10.1111/tpj.12611. [DOI] [PubMed] [Google Scholar]
- 30.Schroda M, Hemme D, Muhlhaus T. 2015. The Chlamydomonas heat stress response. Plant J 82:466–480. doi: 10.1111/tpj.12816. [DOI] [PubMed] [Google Scholar]
- 31.Lv WT, Lin B, Zhang M, Hua XJ. 2011. Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156:1921–1933. doi: 10.1104/pp.111.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tidball JG, Wehling-Henricks M. 2014. Nitric oxide synthase deficiency and the pathophysiology of muscular dystrophy. J Physiol 592:4627–4638. doi: 10.1113/jphysiol.2014.274878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu DS, Li CY, Zhang XC, Li XB, Shi L, Ren A, Zhao MW. 2014. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: an essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ Microbiol 16:1709–1728. doi: 10.1111/1462-2920.12326. [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Gong L, Zhang XY, Ren A, Gao T, Zhao MW. 2015. The regulation of methyl jasmonate on hyphal branching and GA biosynthesis in Ganoderma lucidum partly via ROS generated by NADPH oxidase. Fungal Genet Biol 81:201–211. doi: 10.1016/j.fgb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Jiang X. 2017. Thermal resistance and gene expression of both desiccation-adapted and rehydrated Salmonella enterica serovar Typhimurium cells in aged broiler litter. Appl Environ Microbiol 83:e00367-17. doi: 10.1128/AEM.00367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren A, Liu R, Miao ZG, Zhang X, Cao PF, Chen TX, Li CY, Shi L, Jiang AL, Zhao MW. 2017. Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in Ganoderma lucidum. Environ Microbiol 19:566–583. doi: 10.1111/1462-2920.13498. [DOI] [PubMed] [Google Scholar]
- 38.Ren A, Li XB, Miao ZG, Shi L, Jaing AL, Zhao MW. 2014. Transcript and metabolite alterations increase ganoderic acid content in Ganoderma lucidum using acetic acid as an inducer. Biotechnol Lett 36:2529–2536. doi: 10.1007/s10529-014-1636-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.