Abstract
Prevalence of Type 2 diabetes has increased at an alarming rate, highlighting the need to correctly predict the development of this disease in order to allow intervention and thus, slow progression of the disease and resulting metabolic derangement. There have been many recent “advances” geared towards the detection of pre-diabetes, including genome wide association studies and metabolomics. And although these approaches generate a large amount of data with a single blood sample, studies have indicated limited success using genetic and metabolomics information alone for identification of disease risk. Clinical assessment of the disposition index (DI) based on the hyperbolic law of glucose tolerance is a powerful predictor of Type 2 diabetes, but is not easily assessed in the clinical setting. Thus, it is evident that combining genetic or metabolomic approaches for a more simple assessment of DI may provide a useful tool to identify those at highest risk for Type 2 diabetes, allowing for intervention and prevention.
Graphical Abstract
Type 2 diabetes: National and International Burden
Obesity has increased alarmingly in the United States and other westernized countries, and the trend is now worldwide (1). Obesity is the most significant risk factor for Type 2 diabetes, so that the prevalence of Type 2 diabetes has increased alarmingly. It is estimated that 18.8 million in the U.S. have diabetes, with another 7 million undiagnosed. The international burden has also increased, with an estimated over 4 million deaths due to diabetes per year. Thus there is a critical national and international need to prevent Type 2 diabetes. This has become a cause celeb in the United States, with the First Lady, Michelle Obama, leading the charge (2). Yet, it remains unclear how to approach the prevention of diabetes in the U.S. and elsewhere. Obviously the approach is to predict who is at greatest risk of the disease, and then invoke approaches to prevent the disease. In this article, We address the question of the possible role of systems analysis in this laudable and necessary goal.
Systems Analysis in Biology
It is difficult to define systems analysis; it is something like jazz: “I can't define it, but I can tell when I hear it.” Thus, we are free to incorporate a convenient definition; to whit “Understanding biomedical systems by data-based mathematical modeling of their dynamical behavior. The objective of systems biology has been defined as the understanding of network behavior, and in particular their dynamic aspects, which requires the utilization of mathematical modeling tightly linked to experiment (3).” In addition, for convenience, we define two branches of Systems Biology: Hypothesis generating, versus Hypothesis-based. Of course these aforementioned definitions are arbitrary and are proposed merely for purposes of discussion.
Prediction of Type 2 diabetes
Rather recently, there was little emphasis on prediction of disease. It was generally accepted that treatment, particularly with pharmaceuticals, would be limited to treatment of disease, rather than disease risk. Recent developments have changed this perception. Perreault et al. have recently reported that individuals with impaired glucose tolerance are at a significantly lower risk for Type 2 diabetes if they reverted to normal glucose tolerance from pre-diabetes even for a limited time (4). Thus, pre-diabetes is a progressive condition, and it is important to focus efforts on identification of those individuals who are at greatest risk for conversion to full-blown Type 2 diabetes, and intervene early.
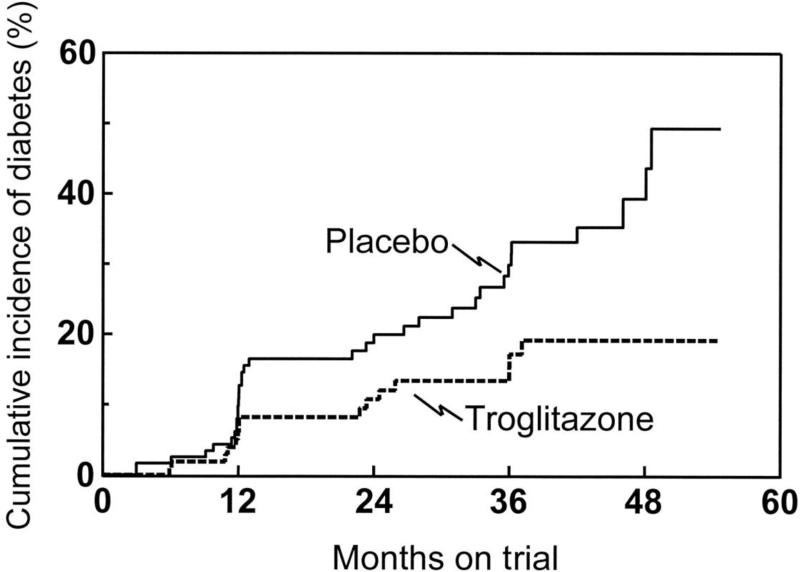
Obesity is the primary risk factor for Type 2 diabetes. 80% of subjects with Type 2 are obese. Because of the panoply of conditions related to obesity (5), the latter has very recently been defined by the American Medical Association as a disease (6). It is very difficult to treat obesity and lifestyle changes (i.e., diet and exercise) usually fail. A few medicines have been approved for treating obesity (7), but the overall effects are limited in effectiveness. Of course, “metabolic (i.e., bariatric)” surgery is very effective to treat obesity and diabetes, but its use is limited to those at high risk, and is unlikely to be indicated except in very high risk situations (8). If obesity cannot be easily treated, is it possible to identify those individuals particularly at risk for diabetes, and are they ways to intervene to prevent the illness? It has been clearly shown in various studies that intervention – lifestyle changes and/or insulin sensitizing medicines – can either delay progression to Type 2 diabetes, or prevent the disease entirely ((9), Figure 1). Can systems analysis help to identify those at risk for Type 2 diabetes? How successful have hypothesis-generating versus hypothesis-based approaches in the effort to detect so-called “prediabetics?”
Figure 1.
Cumulative incidence rates of type 2 diabetes in women with previous gestational diabetes who were treated with either placebo or troglitazone. The rate in the troglitazone group was significantly lower than the rate in the placebo group (P = 0.009). From [9].
Hypothesis-generating approaches
Genetics
There is strong evidence for a genetic basis for Type 2 diabetes from studies in twins and familial aggregation (10). Maturity Onset Diabetes of the Young (MODY) has been clearly attributed to coding mutations in either hepatocyte nuclear factor-1A (HNF1A) or glucokinase (GCK) genes (11). This syndrome accounts for only 1-2% of non-autoimmune Type 2 diabetes. Despite this early success, it has been much more difficult to identify genetic loci for Type 2 diabetes which account for a major fraction of the disease. In fact, approximately 64 loci have been identified from GWAS studies, but these variants have overall a minor effect on diabetes risk (12). In fact, TCF7L2 has the largest identified risk – a factor of about 2 – and other identified variants have less. Therefore there is a good fraction of demonstrated heritability of Type 2 diabetes which remains unaccounted for by known genetics (“missing heritability”), although some may be accounted for by epigenetic changes which occur, possibly in utero. Schmid et al. have reported that adding genetic information does not increase ability to predict Type 2 diabetes beyond that which can be done by clinical risk scores (13). Therefore, it is not at this time appropriate to recommend using genetic information to identify prediabetics beyond what can be gleaned from clinical information. The original optimism regarding the possibility of using genetic analyses to identify those at risk for Type 2 diabetes has not been borne out; however, one very interesting outcome has been the realization that the majority of the variants identified for diabetes risk are associated with insulin secretion or degradation, not with insulin resistance. This has supported the focus on defects or changes in insulin secretion and metabolic clearance of insulin as progenitors of diabetes (see below).
Metabolomics
Recently there has been a flood of information regarding the use of metabolomics to identify those at risk for Type 2 diabetes. The overall concept is that if the approximately 8000 small biological molecules in bodily fluids (blood, urine, CSF etc.) could be accurately measured, they would be useful indicators of and potentially predictors of disease, including diabetes. It is a reasonable assumption that if there are pathways which are particularly important in diabetes development, changes in said pathways would be reflected in the levels of specific biomarkers in bodily fluids including blood. Once specific indicators are identified, it is reasonable to identify the pathways, and possibly the fluxes or metabolic steps within such pathways which might lead to diabetes.
Extensive study of metabolomics patterns have identified a variety of possible risk factors (14-15). These include lipid molecules, including free fatty acids, bile acids, and in particular amino acids – particularly the branched chain amino acids. At present, because a large number of possible risk factors have emerged from metabolomics studies, it is not yet possible to definitively argue that one or more suggested factors will finally be available to confidently predict risk. The use of spectroscopy to measure a large number of factors in individuals at risk of diabetes, as well as those with diabetes, will continue to provide potential metabolic markers. It is not possible at this juncture to identify compounds which will emerge as the greatest predictors. Clearly, however, the metabolomics approach is fundamentally an hypothesis free (or hypothesis generating) approach, as an agnostic perspective is generally taken, in which any and all compounds may be measured and may predict risk—either predictably or surprisingly. Importantly however is the bias in most metabolomics studies in which the metabolic mechanisms potentially related to compounds in blood are most likely to be related to function of tissues prevalent at large mass in the body – for example the liver, muscle and/or adipose tissue. Those tissues are insulin resistant in the prediabetic and diabetic states. We can thereby suggest that the metabolomic approach will identify a metabolic signature which is closely aligned with insulin resistance in the prediabetic state – a condition which is likely to be related also to increased adiposity. It seems less likely that more latent defects in the prediabetic state such as defects in beta-cell function might be harder to detect with the metabolomic approach. Yet, it is the latter defect which may well be the most important and sensitive indicator of prediabetes (16). The potential of the metabolomic approach overall may lie in its putative limitation in detecting beta-cell defects (see below).
Recently, a metabolomics success story has emerged from the work of C. Newgard and others. They specifically identified the branched-chain amino acids as being associated with insulin resistance of obesity in a variety of animal models, as well as in humans (17-19). Insulin resistance is a primarly risk factor for Type 2 diabetes, and therefore it has been suggested that branched chain amino acids can be used as a predictor. In fact, Wang and colleagues exploited the Framingham data base to examine the use of the branched-chains in this context. They examined a limited population in which a fraction of high risk individuals studied converted from a high risk state (obesity, family history) to overt Type 2 diabetes over 12 years. They reported that the observed probability of converting to diabetes was 5-fold greater in those with the lowest versus the highest overall concentrations of branched-chain amino acids. While this number appears compelling, when the same metric was applied to the overall Framingham population, the risk was considerably lower – the odds ratio of converting to Type 2 diabetes was approximately two-fold.
While the metabolomics approach has great potential for identifying those at risk for Type 2 diabetes based upon the measurement of small molecules in blood, it may be limited by the physiological importance of these molecules. In general, branched-chain amino acids are indicators of insulin resistance per se; however insulin resistance is not the only defect leading to Type 2. Genetic studies as well as epidemiological studies (20, 21) have demonstrated convincingly that a beta-cell defect is a critical factor in Type 2 development. Therefore a resistance-based defect may not be able to be a strong predictor of the disease as the influence of the beta-cell may not be reflected in the metabolomic measurement. Whether there is a small molecule or group of molecules which reflect subtle beta-cell defects which may presage diabetes remains to be determined.
Hypothesis-based approaches
Despite the excitement and immense investment in genetics (and epigenetics) and “omic” research (particularly metabolomics) the hypothesis based approaches account for our understanding of the pathogenesis of Type 2 diabetes. If the latter supposition is true, then hypothesis based research should continue to be pursued.
Secretion versus action
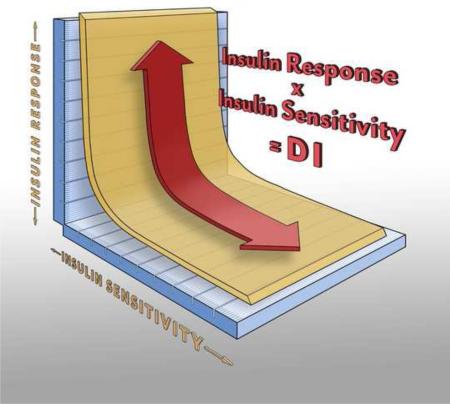
For decades, whether insulin resistance or defects in insulin secretion was responsible for Type 2 diabetes was debated (22, 23). Several decades ago we suggested that this dialectic could be resolved only by considering the relative importance of insulin secretory function in the context of insulin resistance. We considered that in an insulin resistant state, occasioned either normally (e.g., in pregnancy, puberty, normal weight gain) or pathologically (e.g., obesity, infection, PCOS), would be compensated in a defined way by enhanced insulin levels in the blood. In fact, we proposed for the first time that the relationship between insulin sensitivity (the inverse of insulin resistance) and insulin response be expressed quantitatively as the following hypothesis, represented by a quantitative equation (24):
Insulin sensitivity × Insulin Response = the Disposition Index (DI)
In which the DI is considered a characteristic value for a given individual in the normal state. Thus, by this so-called “Hyperbolic Law of Glucose Tolerance (25)” a reduction in insulin sensitivity would be compensated by an increase in insulin sensitivity, such that DI remained relatively constant for a given individual. By this conception, we proposed that an individual at risk for Type 2 diabetes the failure of the pancreatic islets to adequately compensate for insulin resistance would be reflected in a reduced disposition index. Inadequate compensation should be observed in those at risk for diabetes. More recently, it has been demonstrated that although this hyperbolic relationship is maintained across ethnic groups in healthy individuals, there are distinct ethnic differences in the contribution of insulin sensitivity vs insulin response to maintain normal glucose tolerance (Figure 2) (26). It is of interest that the above Eequation represents a specific hypothesis; it was based upon considerations of the ability of a closed-loop system to regulate an important variable, but it was by no means proven initially. However, cross sectional data (27, 28) confirmed the hyperbolic relationship between insulin sensitivity and insulin secretion; in addition Kahn et al. examined the relationship in a population of normal individuals and demonstrated that DI was normally distributed. Of course it was assumed in the original conception that insulin sensitivity changed in response to changes in lifestyle; however it is also possible that hyperinsulinemia per se causes a reflexive change in insulin action at insulin sensitive tissues (muscle, liver and fat) such that insulin level should be the independent variable and insulin action the dependent one (29, 30). At this juncture whether the so-called “insulin resistance syndrome” is driven by oversecretion by the beta cells of the pancreas remains suggested but unproven, but there is evidence that hyperinsulinemia itself can down regulate insulin action. The most likely scenario is that both mechanisms are at play – resulting in a closed loop system regulating the blood glucose which compensates appropriately for insulin resistance or hyperinsulinemia, and resulting in the normal blood glucose concentration.
Figure 2.
Ethnic differences in the relationship between insulin sensitivity and insulin response. From [26]
An important question is the mechanisms underlying the hyperbolic relationship. We have shown that induction of insulin resistance with a moderate elevated fat diet causes a reflexive reduction in metabolic clearance rate of insulin (at the level of the liver) as well as a time-dependent increase in beta-cell sensitivity to glucose. It is reasonable to assume that the signal to the pancreas which is responsible for the increased secretion would be glycemia itself; however we have shown that there is no change in fasting glycemia or in 24-hour glycemia which could be responsible for the increased beta-cell function in an insulin resistant state. We have carefully searched for the signal which provokes the pancreatic response; we have ruled out GLP-1, cortisol, as well as growth hormone. Recently, we discovered that free fatty acids (FFA) increase nocturnally (31), and that the nocturnal increase in FFA is enhanced in the insulin resistant state. In fact, we propose that nocturnal FFA is the signal which enhances beta-cell response in an insulin resistant situation, and that this ability of FFA may be reduced when diabetes risk is elevated.
Diabetes prediction
As discussed, it remains difficult to predict Type 2 diabetes in advance with genetics, and metabolomics is also an imperfect mechanism as changes observed appear to reflect insulin resistance, rather than beta cell function. But the DI reflects the ability of the pancreatic beta cells to compensate for insulin resistance. We expect that this compensatory ability is reduced in those at risk for Type 2 diabetes. Given, this, does the DI provide a means to predict Type 2 diabetes in those at risk for the disease? Population studies have confirmed that the DI value in an individual is an excellent predictor of later conversion to diabetes (21). In a study of members of the Pima Nation by Weyer, et al., individuals with normal glucose tolerance were observed for a 5 year period to determine which developed impaired glucose tolerance, and which developed diabetes (32). As shown in Figure 3, the group with a better DI value had no reduction in glucose tolerance over the observation interval; those with lesser DI (but also normal glucose tolerance) became glucose intolerant and then developed diabetes. Thus, in the Pimas DI was a strong predictor of conversion to Type 2 diabetes. In fact, the risk of conversion to diabetes was 20-fold higher in those in the top decile of DI value compared to the lowest decile of DI. Therefore, DI is a strong predictor of conversion of normal glucose tolerance to diabetes (33); a much stronger predictor than known genetic or metabolomics signals. The ability of the DI to predict diabetes has been confirmed in the “ACT NOW” study, performed by DeFronzo and his colleagues, who reported that in a high risk population, risk of conversion to Type 2 diabetes over a 2.5 year period was greatest for the Disposition Index, calculated from the OGTT (34).
Figure 3.
Changes in insulin response relative to changes in insulin sensitivity in Pima Indian subjects. Those with a higher DI retained normal glucose tolerance (NGT) (nonprogressors) whereas those with a lower DI deteriorated from NGT to impaired (IGT) to diabetic (DIA) (progressors). From [32].
Of course it remains considerably more difficult to estimate DI from a clinical procedure than doing a genetic analysis or measuring analytes from blood. What is needed is a surrogate approach to measure DI from blood samples, or from a simpler clinical procedure than is now available.
Concluding Remarks: Systems Analysis and Diabetes Prediction
Diabetes is increasing nationally and internationally, and is now a highly significant public health problem. Lifestyle changes and pharmaceutical intervention has been demonstrated to at least retard the development of the disease, if not to prevent it altogether. Yet, it is still not possible to identify those individuals most at risk for the illness who deserve the most attention for prevention. Systems analysis in its various guises is being used to address this problem. As we have seen, hypothesis-free approaches such as genetics and “omics” have been proposed as excellent approaches to identify at-risk individuals. Success however, remains to be proven using these approaches. Alternatively, the hypothesis based “law of glucose tolerance” approach, using the disposition index has been proven to be a very powerful (in fact the most powerful) predictor of Type 2 diabetes. But, methods are not yet available to assess DI easily, in a form that can be available in the clinic. Thus, it is an important goal to measure DI easily, either with simple measurement, or possibly using modern methods of “omic” assessment. In this sense, then, hypothesis-free and hypothesis-based methods might come together to identify those at highest risk for Type 2 diabetes mellitus so that intervention and prevention can be used worldwide.
Highlights.
Prediction of Type 2 diabetes allows intervention which slows disease progression.
Genetic and metabolomic approaches have had limited success in diabetes prediction.
Hypothesis-based hyperbolic law of glucose tolerance (DI) predicts Type 2 diabetes.
It is important to easily measure DI in a non-clinical setting.
This may be possible by merging hypothesis-generating and hypothesis-driven approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention National Diabetes Surveillance System [Google Scholar]
- 2.The White House, Office of the Press Secretary National Diabetes Month, 2013 [Press release] 2013 Retrieved from http://www.whitehouse.gov/the-press-office/2013/10/31/presidential-proclamation-national-diabetes-month-2013.
- 3.Timmer Jens. [January 2, 2014];Definition of Systems Biology. 2014 from http://www.zbsa.de/Systems-Biology/defn.
- 4*.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–51. doi: 10.1016/S0140-6736(12)60525-X. [Restoration of normal glucose regulation significantly reduces incidence of diabetes in individuals at high risk for development of Type 2 diabetes, underlining the importance of correctly identifying those with insulin resistance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2009;12:761–5. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollack Andrew. A.M.A. Recognizes Obesity as a Disease. New York Times; Jun 18, 2013. [January 2, 2014]. from http://www.nytimes.com/2013/06/19/business/ama-recognizes-obesity-as-a-disease.html. [Google Scholar]
- 7.Igel LI, Powell AG, Apovian CM, Aronne LJ. Advances in medical therapy for weight loss and the weight-centric management of type 2 diabetes mellitus. Curr Atheroscler Rep. 2012;14:60–9. doi: 10.1007/s11883-011-0221-0. [DOI] [PubMed] [Google Scholar]
- 8.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and Weight in Comparative Studies of Bariatric Surgery vs Conventional Medical Therapy: A Systematic Review and Meta-Analysis. Obes Surg. 2013 doi: 10.1007/s11695-013-1160-3. doi:10.1007/s11695-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of Pancreatic β-Cell Function and Prevention of Type 2 Diabetes by Pharmacological Treatment of Insulin Resistance in High-Risk Hispanic Women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia. 1999;42:139–145. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- 11.Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. SEARCH for Diabetes in Youth Study Group. J Clin Endocrinol Metab. 2013;98:4055–62. doi: 10.1210/jc.2013-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenawalt DM, Sieberts SK, Cornelis MC, Girman CJ, Zhong H, Yang X, Guinney J, Qi L, Hu FB. Integrating genetic association, genetics of gene expression, and single nucleotide polymorphism set analysis to identify susceptibility Loci for type 2 diabetes mellitus. Am J Epidemiol. 2012;176:423–30. doi: 10.1093/aje/kws123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Schmid R, Vollenweider P, Bastardot F, Vaucher J, Waeber G, Marques-Vidal P. Current genetic data do not improve the prediction of type 2 diabetes mellitus: the CoLaus study. J Clin Endocrinol Metab. 2012;97:E1338–41. doi: 10.1210/jc.2011-3412. [It has been suggested that genetic information may add additional identifiers to those at risk for development of diabetes. This study demonstrates that genetic risk scores did not improve clinical assessment of diabetes risk.] [DOI] [PubMed] [Google Scholar]
- 14.Dagogo-Jack S. Metabolomic prediction of diabetes and cardiovascular risk. Med Princ Pract. 2012;21:401–3. doi: 10.1159/000339203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SH, Svetkey LP, Newgard CB. Branching Out for Detection of Type 2 Diabetes. Cell Metab. 2011;13491-2 doi: 10.1016/j.cmet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–30. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newgard CB1, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine Deprivation Increases Hepatic Insulin Sensitivity via GCN2/mTOR/S6K1 and AMPK Pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimas AS, Lagou V, Barker A, Knowles JW, Magi R, Hivert M, et al. Impact of Type 2 Diabetes Susceptibility Variants on Quantitative Glycemic Traits Reveals Mechanistic Heterogeneity. Diabetes. 2014;63:2158–171. doi: 10.2337/db13-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33:2098–103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himsworth H. Diabetes mellitus: its differentiation into insulin-sensitive and insulin insensitive types. Lancet. 1936;227:127–130. doi: 10.1093/ije/dyt203. [DOI] [PubMed] [Google Scholar]
- 23.Cerasi E, Luft R, Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972;21:224–34. doi: 10.2337/diab.21.4.224. [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51:S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 26*.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. doi: 10.2337/dc12-1235. [Measurement of the disposition index in different ethnic groups suggests genetic susceptibility to the onset of Type 2 diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007;56:1489–501. doi: 10.2337/db07-9903. [DOI] [PubMed] [Google Scholar]
- 28.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 29.Bergman RN. Quantitative approaches to pathogenesis of age-related metabolic conditions. In: Goldstein AL, editor. Biomedical Advances in Aging. 229-245, 1990. Plenum Press; New York: [Google Scholar]
- 30.Corkey BE. Diabetes: Have We Got It All Wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35:2432–2437. doi: 10.2337/dc12-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am. J Physiol Endocrinol Metab. 2007;292:E1590–8. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104787-94 doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonsson A, Isomaa B, Tuomi T, Taneera J, Salehi A, Nilsson P, Groop L, Lyssenko V. A variant in the KCNQ1 gene predicts future type 2 diabetes and mediates impaired insulin secretion. Diabetes. 2009;58:2409–13. doi: 10.2337/db09-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD, Gastaldelli A. ACT NOW Study. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care. 2013;36:3607–12. doi: 10.2337/dc13-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]