Abstract
Animal and human research suggests that testosterone is associated with hippocampal structure and function. Studies examining the association between testosterone and either hippocampal structure or hippocampal-mediated cognitive processes have overwhelmingly focused on the effects of testosterone alone, without considering the interaction of other neuroendocrine factors. The aim of the present study was to examine the interactive effects of testosterone and cortisol in relation to hippocampal volume and episodic memory in a sample of late-middle aged men from the Vietnam Era Twin Study of Aging. The average age of participants was 56.3 years (range 51 to 60). Salivary hormone samples were collected at multiple time-points on two non-consecutive at-home days, and an in-lab assessment. Area under the curve with respect to ground measures for cortisol and testosterone were utilized. Significant testosterone-by-cortisol interactions were observed for hippocampal volume, and episodic memory. When cortisol levels were elevated (1 SD above the mean), testosterone levels were positively associated with hippocampal volume and memory performance. However, when cortisol levels were low (1 SD below the mean), testosterone levels were inversely related to hippocampal volume and memory performance. These findings suggest that in context of high cortisol levels, testosterone may be neuroprotective. In contrast, low testosterone may also be neuroprotective in the context of low cortisol levels. To our knowledge this is the first demonstration of such an interaction in a structural brain measure and an associated cognitive ability. These results argue in favor of broadening neuroendocrine research to consider the simultaneous and interactive effects of multiple hormones on brain structure and function.
1. Introduction
Increasing evidence suggests that the primary male androgen testosterone is associated with hippocampal structure and function (Atwi et al., 2014). Although androgen receptors, the primary cytosolic binding sites for testosterone, are distributed widely throughout the central nervous system (Choate et al., 1998), mRNA concentrations in the human hippocampus have been shown to be of the same order of magnitude as concentrations in the prostate, a major site of testosterone action (Beyenburg et al., 2000). Animal studies have found that testosterone promotes hippocampal neurogenesis (Galea et al., 2006), regulates synaptic plasticity in the hippocampus (Harley et al., 2000), and maintains hippocampal volume (Galea et al., 1999). In rats, cognitive abilities regulated by the hippocampus, such as spatial learning, become impaired following gonadectomy, with performance improving following hormone replacement (Kritzer et al., 2001). Testosterone also appears to be neuroprotective with respect to Alzheimer’s disease-related pathology, helping to regulate the accumulation of β-amyloid in cultured hippocampal neurons (Pike, 2001), as well as prevent the formation of tau-related pathology (Papasozomenos and Shanavas, 2002).
Human studies have shown that testosterone levels are positively correlated with hippocampal volume in male and female adolescents (Neufang et al., 2009), as well as with cerebral blood flow within the hippocampus in elderly men (Moffat and Resnick, 2007). Testosterone has been found to have positive associations with hippocampal-mediated cognitive processes in healthy adults, though findings in this area remain mixed (Boss et al., 2014). In middle-aged men, individuals with both low testosterone and at least one copy of the apolipoprotein-E (APOE) ε4 allele were found to have smaller hippocampal volumes relative to individuals with none or only one of these risk factors (Panizzon et al., 2010). Within the same cohort, the correlation between testosterone and verbal memory was found to significantly differ as a function of ε4 status, such that lower testosterone was associated with poorer memory performance in ε4-positive, but not ε4-negative, individuals (Panizzon et al., 2014).
In nearly all areas of neuroendocrine research, studies examining the association among a particular hormone with brain structure and function have been restricted to individual hormone effects, and have not considered the potential roles that other hormones might play in regulating the relationships of interest. The literature on testosterone is no exception to this trend. A hormone that is likely critical to understanding how and the degree to which testosterone is associated with brain structure and function is the glucocorticoid cortisol. Elevated cortisol has been negatively associated with structural aspects of the hippocampus and its related cognitive abilities; however, as with testosterone, findings have at times been inconsistent across both neuroimaging and cognitive studies (Franz et al., 2011; Frodl and O’Keane, 2013; Geoffroy et al., 2012; Lupien and Lepage, 2001; Lupien et al., 2009; Schwabe et al., 2012). Like androgen receptors, the receptors that mediate the effects of cortisol, specifically the glucocorticoid and mineralcorticoid receptors, are prominent in the hippocampus (McEwen et al., 1968), and for all three receptor types the binding to the respective hormone results in alterations of receptor-regulated gene expression (Beato, 1989). The glucocorticoid receptor is particularly relevant to the action of testosterone, as it has been shown to directly interact with the androgen receptor, such that the two receptors have inhibitory effects on the transcriptional activity on one another (Chen et al., 1997). Moreover, in certain tissues glucocorticoid receptors can be upregulated by either knockout of the androgen receptor or castration-induced androgen depletion, as well as downregulated by high testosterone levels (Miyamoto et al., 2007; Silva et al., 2010). These receptor-level interactions provide a mechanism whereby the association of testosterone with hippocampal structure and function might be moderated by cortisol, or vice versa.
We are aware of no studies that have examined the simultaneous or potentially interactive effects of testosterone and cortisol on hippocampal structure and related cognitive function. The interplay of these two hormones, however, has been examined in research on traits such as social aggression, dominance, and leadership. For example, Popma and colleagues found that testosterone was positively associated with overt aggression in adolescent boys, but only in those who had lower cortisol levels, defined as 1 standard deviation or more below the mean (Popma et al., 2007). No relationship was observed in participants with higher cortisol levels (1 standard deviation or more above the mean). Similar findings, in samples consisting of both men and women, have been reported for observer-based ratings of dominance in a leadership task, self-reported empathy, anger response, and risk taking (Mehta et al., 2015). In men with lower cortisol, testosterone was positively associated with activation in the dorsolateral prefrontal cortex in response to an insult, whereas no association was observed in the high cortisol participants.
The aim of the present study was to examine the interactive effects of testosterone and cortisol on hippocampal volume and episodic memory performance. That is, we sought to determine whether associations with testosterone would differ as a function of cortisol level. We predicted that cortisol would moderate the associations of testosterone with hippocampal volume and memory performance, such that these associations would be prominent when cortisol is in a lower (i.e., healthy) range and be abated when cortisol is elevated.
2. Materials and Methods
2.1. Participants
Data were obtained as part of the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of cognitive and brain aging with baseline in midlife (Kremen et al., 2006). VETSA participants are drawn from the larger Vietnam Era Twin Registry, a nationally distributed sample of male-male twin pairs, both of whom served in the United States military at some point between 1965 and 1975 (Goldberg et al., 2002). To be eligible for the VETSA both members of a twin pair had to agree to participate, and be between the ages of 51 and 59 years at the time of recruitment. In total, 1237 men participated in wave 1 of the VETSA. The average age was 55.4 years (SD = 2.5, range = 51 to 60), average education was 13.8 years (SD = 2.1), and participants were predominantly white non-Hispanic (89.7%). Although all VETSA participants served in the military, the majority (~80%) did not experience combat situations during their military careers. Compared to U.S. census data, participants in the VETSA are similar in demographic and health characteristics to American men in their age range (Schoenborn and Heyman, 2009).
VETSA participants traveled to either the University of California, San Diego or Boston University for a daylong series of physical, psychological, and neurocognitive assessments. On rare occasions (2.7% of subjects) project staff traveled to the participants in order to conduct the assessments. Prior to data collection, approval from local institutional review boards was obtained at all participating sites, and all participants provided signed informed consent upon their arrival at the testing site. Beginning in the second and third years of the project, funding was obtained to collect structural neuroimaging (N=526) and endocrine data (N=795) on the remaining eligible participants. Due to incomplete overlap between these two sub-studies, all participants with endocrine data underwent cognitive testing whereas both endocrine and neuroimaging data were available on 445 participants.
2.2. Hormone Collection and Assay
Hormone collection and assay methods have been described in detail elsewhere (Franz et al., 2010; Panizzon et al., 2013). Briefly, saliva samples were collected on two non-consecutive days at home during a participant’s typical week, as well as on the in-lab assessment day. At-home samples were collected approximately two weeks prior to the assessment day. Samples were collected at waking, 30 minutes after waking, 10:00 a.m., 3:00 p.m., and bedtime on all days in order to capture diurnal changes in cortisol levels. Times of sample collections were recorded by the participant, and were later confirmed against data from electronic track caps.
Saliva samples were centrifuged prior to assay at 3000 rpm for 20 minutes to separate the aqueous component from mucins and other suspended particles. Concentrations of cortisol and free testosterone were determined in duplicate using commercial radioimmunoassay kits (Beckman Coulter Inc., formerly Diagnostics Systems Laboratories, Webster, TX; Siemens Medical Solutions Diagnostics, Los Angeles, CA). The least detectable concentrations for the assays were 1.3697 pg/ml for testosterone (intra-assay coefficient of variation = 3.141, inter-assay coefficients of variation = 4.878) and 1.3854 nmol/l for cortisol (intra-assay coefficient of variation = 3.962, inter-assay coefficients of variation = 5.662). Data from one to three individuals were included in each assay batch, and assays were always performed without knowledge of the zygosity of the twin pairs.
Procedures for handling outliers and missing data are described in detail elsewhere (Franz et al., 2010; Panizzon et al., 2013). In brief, individual testosterone and cortisol measurements greater than three standard deviations above the mean waking measurement were set to missing in order to eliminate outlying values. Participants who reported taking testosterone supplements or other medications known to alter testosterone levels were also excluded, as were participants who reported taking medications known to alter cortisol levels. For both testosterone and cortisol values, missing data were imputed if a participant had no more than one missing value on a day. To impute missing data, the full samples’ mean change in each hormone level between the time-point with the missing value and the adjacent time-point was calculated. The mean change in cortisol or testosterone for those two points was then added to or subtracted from the participant’s non-missing time-point. Imputed cortisol values were evenly distributed above and below the means for their respective time-points. Imputed testosterone values, in contrast, were twice as likely to be above the mean for a given time-point as below. The rate of imputation did not differ as a function of hormone collection time, with the exception of the bedtime measurement on the in-lab assessment day, which had the highest rate of imputed data. In total, imputations were made for less than 1% of all available hormone samples.
For the present study we utilized the average area under the curve with respect to ground (AUCG) for testosterone and cortisol averaged across the three assessment days. The AUCG uses values from all collection points in a day and accounts for minor differences in the time between samples by adjusting for the actual time between samples. Given that both hormones have distinct patterns of diurnal variation, the AUCG provides a reasonable measure of total hormonal output across the day (Pruessner et al., 2003). Once data cleaning was complete, testosterone and cortisol AUCG values were available for 741 participants with cognitive data, and 403 participants with MRI data. AUCG measures were transformed (testosterone was square-root transformed, cortisol was log transformed) in order to normalize the distributions and remove potential confounds from the tests of interaction effects.
2.3. Hippocampal Volume Acquisition and Processing
Neuroimaging was performed within 24 hours of the assessment day at either the UCSD Medical Center or the Massachusetts General Hospital (MGH) in Boston. Images were acquired on Siemens 1.5 T scanners. Scanning sequences were specifically designed for use across different scanners and vendors. Sagittal T1-weighted MPRAGE sequences were employed with a TI=1000 ms, TE=3.31 ms, TR=2730 ms, flip angle=7°, slice thickness=1.33 mm, voxel size 1.3×1.0×1.3mm. Raw DICOM MRI scans from both sites were transferred to UCSD for post-processing and quality control.
Hippocampal volumes were obtained using segmentation methods based on the publicly available FreeSurfer software package (Fischl et al., 2002). The semi-automated, fully 3D whole brain segmentation procedure uses a probabilistic atlas and applies a Bayesian classification rule to assign a neuroanatomical label to each voxel. Visual inspection of the hippocampal segmentation was performed by a trained technician to ensure no technical failure of the application or mislabeling of the structure. Participants were also excluded if extreme left-right asymmetry of the hippocampus was noted (greater than 1000 mm3 difference). Data from 26 individuals were omitted following these quality control procedures. Analyses were based on a measure of bilateral (left plus right) hippocampal volume. In order to account for individual differences in head size, an estimate of total intracranial volume (eICV) was derived based on a scaling factor related to the transformation of the full brain mask into atlas space (Buckner et al., 2004). This measure was used as a covariate in all analyses involving hippocampal volume.
2.4. Cognitive Functioning
Episodic memory was assessed with three measures: the California Verbal Learning Test – second edition (CVLT-II) (Delis et al., 2000), the Logical Memory subtest of the Wechsler Memory Scale – third edition (WMS-III) (Wechsler, 1997), and the WMS-III Visual Reproductions subtest. These instruments are well-validated measures of list learning (CVLT-II), story recall (Logical Memory), and design recall (Visual Reproductions). Each instrument was administered according to published instructions, with the exception of the Logical Memory subtest. In the VETSA battery, each of the two stories that make up the subtest were read to the participant only once, whereas the published instructions require two presentations of the second story. We utilized the immediate and delayed free recall scores from each of the WMS-III subtests, and the short and long delay free recall scores from the CVLT-II. In prior work, we showed that there is a general memory factor accounting for the covariance among the three memory tests (Kremen et al., 2014). We therefore created a general memory score based on the first principal component of the six recall measures. Prior to creating the factor score, each measure was centered to a mean of 0 and a variance of 1.0.
2.5. Covariates
All analyses included age, ethnicity (non-Hispanic white versus other), history of hypertension, cardiovascular disease, diabetes, current smoking status (current smoker versus non-smoker), current alcohol use, and self-reported depressive symptoms as covariates. Hypertension (yes/no) was based on whether the average of four blood pressure measurements taken in the morning and the afternoon on the day of testing was greater than or equal to 140 systolic or 90 diastolic, or if the participant currently took anti-hypertensive medication. Cardiovascular disease (yes/no) was based on whether a physician ever diagnosed the participant with a heart attack, heart failure, peripheral vascular disease, stroke, heart surgery, heart catheterization or angioplasty (Carmelli et al., 1994). Diabetes (yes/no) was based on whether a physician ever diagnosed the participant with the condition, or whether they reported taking diabetes-related medication. Current alcohol use was based on consumption during the past two weeks: None = 0; One drink or less on average per day = 1; Greater than one but no more than two drinks on average per day = 2; Two or more drinks on average per day = 3. Depressive symptoms during the week prior to cognitive testing were assessed with the Center for Epidemiologic Studies Depression scale (CES-D) (Radloff, 1977). The analysis of hippocampal volume also included scanner (1 per site), and eICV as additional covariates. The analysis of episodic memory performance included early adulthood general cognitive ability as an additional covariate. Early adulthood general cognitive ability was assessed with the Armed Forces Qualification Test (AFQT, Form 7A) (Bayroff and Anderson, 1963), a 50-minute, 100 item, multiple-choice test that was administered to each VETSA participant at the time of military induction, roughly corresponding to age 20. The AFQT has been shown to correlate highly (r = .84) with widely used measures of general cognitive ability such as the Wechsler Adult Intelligence Scale (McGrevy et al., 1974), and within the VETSA sample has been shown to correlate .73 across a 42-year time interval (Lyons et al., 2017).
2.6. Statistical Analyses
Analyses were conducted using multilevel, mixed linear models in SAS (SAS Proc Mixed, SAS version 9.3), which allowed for the use of data from all available participants while correcting for the non-independence of the observations (i.e., twin clustering). Each hormone assay batch was assigned a unique identification number (referred to as batch ID) so that we could further control for any potential clustering introduced by the laboratory procedures. Both family and batch were entered into the model as random effects. Significant main and interaction effects were determined using the type III test of fixed effects, indicating the unique association of each element of the model independent of the others. Significance level was set at alpha = 0.05 for all analyses. In order to assist with the interpretation of results the outcome measures (i.e., hippocampal volume and episodic memory) and key predictors (i.e., testosterone and cortisol AUCG measures) were standardized to a mean of 0 and a variance of 1.0 prior to analysis.
3. Results
3.1. Sample characteristics
Descriptive characteristics of the VETSA sample, as well as the relative associations of the covariates examined with testosterone and cortisol are presented in Table 1. We observed a positive correlation of .41 (p < .0001) between the testosterone and cortisol AUCG measures (a scatterplot of this association is presented in Supplemental Figure 1). Testosterone was significantly associated with age, cardiovascular disease, and current smoking status. Cortisol was significantly associated with early adult general cognitive ability, current smoking status, alcohol use, and depressive symptoms. Additional descriptive statistics for average hormone levels at each collection time point, as well as the correlations among these values are presented in Supplemental Tables 1 and 2.
Table 1.
Descriptive statistics and relative associations of covariates with hormone AUCG measures
| Association with Hormone AUCG | |||
|---|---|---|---|
| Measure | Value | Testosterone | Cortisol |
| Testosterone AUCG (pg/ml/day) | 1429.64 (471.12) | -- | -- |
| Cortisol AUCG (nmol/l/day) | 90.71 (36.63) | r = .41 (p < .001) | -- |
| Age (years) | 56.34 (2.60) | r = −.14 (p = .001) | r = −.04 (p = .42) |
| Age 20 General Cognitive Ability (Percentile) | 60.35 (22.72) | r = −.06 (p = .26) | r = −.14 (p < .001 ) |
| Ethnicity (% Non-Hispanic White) | 89.5% | t = 1.02 (p = .31) | t = 1.08 (p = .28) |
| Hypertension (% Yes) | 59.8% | t = 1.39 (p = .16) | t = −1.80 (p = .07) |
| Cardiovascular Disease (% Yes) | 17.3% | t = 2.19 (p = .03) | t = 0.26 (p = .80) |
| Diabetes (% Yes) | 11.0% | t = −0.01 (p = .99) | t = −0.23 (p = .82) |
| Current Smoker (% Yes) | 24.0% | t = −2.88 (p < .01) | t = −4.02 (p < .001) |
| Alcohol Use (past 2 weeks) | F = 2.45 (p=.06) | F = 3.01 (p=.03) | |
| None | 34.3% | ||
| ≤1 drink per day | 41.2% | ||
| >1 ≤2 drinks per day | 10.0% | ||
| >2 drinks per day | 14.6% | ||
| CES-D Total Score | 8.07 (7.95) | r = .03 (p = .59) | r = .08 (p = .02) |
Data are presented as mean and standard deviation unless otherwise specified. Total N = 741. Tests of association between the covariates and relative hormones account for the non-independence of the twin data. Degrees of freedom for dichotomous risk factors = 342. Degrees of freedom for Alcohol Use = 3, 340.
3.2. Effects of testosterone and cortisol on hippocampal volume
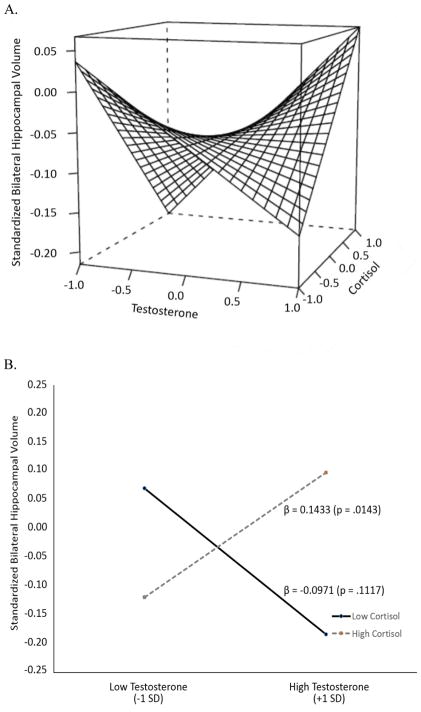
Results for the main and interaction effects of testosterone and cortisol on hippocampal volume are presented in Table 2. Neither testosterone nor cortisol was significantly associated with hippocampal volume. There was, however, a significant interaction effect between the two hormones (F(1,150) = 8.29, p = .0046). The interaction of the two hormone measures is shown in Figure 1A as a three-dimensional saddle graph. In order to simplify the interpretation of the interaction we plotted hippocampal volume at one standard deviation above and below the mean for testosterone (Figure 1B) according to the Aiken and West method (Aiken and West, 1991). Figure 1B does not depict mean differences for distinct groups; rather, it shows the simple slopes for the relationship between testosterone and hippocampal volume when cortisol is high (i.e., 1 SD above the mean), and another slope for the same relationship when cortisol is low (i.e., 1 SD below the mean). In reality these cut-points are arbitrary, as there are an infinite number of these slopes that can be derived. Figures 1A and 1B show that when cortisol levels are higher the relationship between testosterone and hippocampal volume becomes more strongly positive. In contrast, when cortisol levels are lower, the relationship between testosterone and hippocampal volume becomes more strongly negative. The full range of this change in association strength is presented in Supplemental Figure 1.
Table 2.
Main and Interaction Effects of Cortisol and Testosterone on Hippocampal Volume and Episodic Memory
| Estimate | SE | F | DF | p | |
|---|---|---|---|---|---|
| Hippocampal Volume | |||||
| Testosterone | 0.0076 | 0.0452 | 0.03 | 1;150 | .8662 |
| Cortisol | −0.0140 | 0.0427 | 0.11 | 1;150 | .7428 |
| Interaction | 0.1088 | 0.0378 | 8.29 | 1;150 | .0046 |
| Episodic Memory | |||||
| Testosterone | 0.0649 | 0.0372 | 3.05 | 1;311 | .0820 |
| Cortisol | −0.0441 | 0.0397 | 1.44 | 1;311 | .2308 |
| Interaction | 0.0833 | 0.0309 | 7.28 | 1;311 | .0074 |
All analyses control for age, ethnicity, history of hypertension, history of cardiovascular disease, history of diabetes, current smoking status, current alcohol use, and self-report depressive symptoms. The analysis of hippocampal volume also control for scanner, and estimated ICV. The analysis of episodic memory includes age 20 general cognitive ability as an additional covariate. Significant effects are presented in bold font. F and p values represent the type III test of fixed effects.
Figure 1.
Relationship between testosterone and hippocampal volume as a function of cortisol. Panel 1A presents the continuous interaction between the hormone measures. The figure is restricted so as to present the relationships between 1 SD below and 1 SD above the mean for each hormone. Panel 1B presents the simple slopes of the relationship as a function of low cortisol (−1 SD below the mean) and high cortisol (+1 SD above the mean).
3.3. Effects of testosterone and cortisol on episodic memory
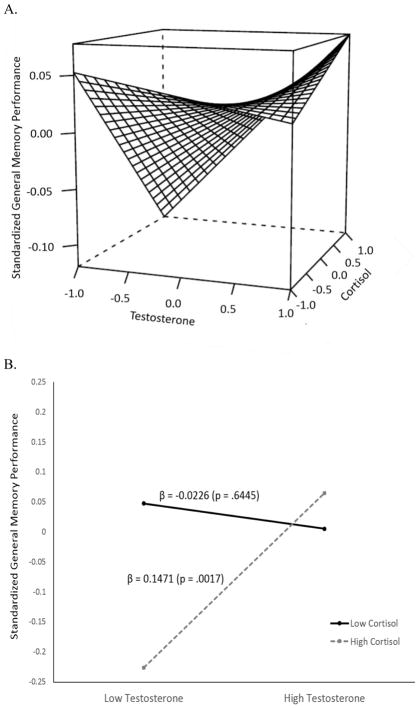
Results for the main and interaction effects of testosterone and cortisol on the general memory factor are also presented in Table 2. Neither hormone was found to have a significant main effect on the general memory factor; however, we observed a significant interaction effect (F(1,311) = 7.28, p = .0075). Figure 2 uses the same approach as Figure 1 to aid in the visualization and interpretation of the testosterone-by-cortisol interaction with respect to the general memory factor. The interaction of the two continuous hormone measures is first presented in Figure 2A as a three-dimensional saddle graph. In Figure 2B we plot the simple slopes for the relationship between testosterone and general memory as a function of cortisol (high cortisol = 1 SD above the mean; low cortisol = 1 SD below the mean). Figure 2 shows that when cortisol levels are higher the relationship between testosterone and memory becomes more strongly positive, and when cortisol levels are lower, the relationship between testosterone and episodic memory weakens, and eventually becomes more strongly negative. The full range of this change in association strength is presented in Supplemental Figure 2.
Figure 2.
Relationship between testosterone and general memory performance as a function of cortisol. Panel 1A presents the continuous interaction between the hormone measures. The figure is restricted so as to present the relationships between 1 SD below and 1 SD above the mean for each hormone. Panel 1B presents the simple slopes of the relationship as a function of low cortisol (−1 SD below the mean) and high cortisol (+1 SD above the mean).
3.4. Alternative interpretation of interactions
It should be noted that while we have presented the observed interaction as cortisol moderating the associations between testosterone, hippocampus, and memory, it is equally valid to consider the moderating effect of testosterone on relationships with cortisol. For example, if we reorganize our hippocampal volume results to consider the association with cortisol as a function of high and low testosterone (see Supplemental Figure 3) we observe a significant negative association when testosterone is low (β = −0.1232, p = .0236), and a trend level positive association when testosterone is high (β = 0.1047, p = .0740). Thus, expected negative associations with cortisol are observed when testosterone approaches a low, unhealthy level. Similarly, our analytic model can itself be reorganized in order to test whether testosterone and hippocampal volume interact to predict cortisol level. With these data, such an interaction is indeed significant (p = .029), and indicates that the positive association between testosterone and cortisol becomes stronger as the size of the hippocampus increases.
4. Discussion
In the present study we demonstrated for the first time a significant testosterone-by-cortisol interaction for both hippocampal volume and episodic memory. For both outcomes we found evidence for a crossover interaction such that different associations were observed with testosterone when cortisol was elevated and when cortisol was low. These results suggest that when cortisol levels are elevated and potentially approach an unhealthy level, higher testosterone levels may help to protect against glucocorticoid-mediated neurotoxicity, thus preserving hippocampal volume and memory performance. In that context, higher testosterone levels, although still within what would be considered a normal range, may be thought of as neuroprotective, as suggested in prior studies (Hammond et al., 2001; Pike, 2001). On the other hand, when cortisol levels are low (healthy range), associations with testosterone go in the opposite direction. In this case, low, rather than high, testosterone may be also neuroprotective. These results may be interpreted from the perspective of either testosterone or cortisol, as there is nothing in our analytic approach that dictates a direction of the moderation effect. Regardless of the perspective taken, however, these results strongly suggest that the interactions between hormones, what may be thought of as the hormonal milieu, is critical when considering any individual hormone associations.
Prior work on the potential interaction between testosterone and cortisol has almost entirely focused on social-behavioral constructs in the context of the “dual hormone hypothesis” (Mehta and Josephs, 2010). The dual-hormone hypothesis suggests that the relationships between testosterone and behavioral outcomes are moderated by cortisol, such that these associations will be observed only when cortisol is low (Mehta and Josephs, 2010). Numerous studies have shown just such an effect for traits ranging from self-reported aggression to risk-taking behavior (Mehta and Prasad, 2015). On a general level, our results are consistent with the dual-hormone hypothesis in that, as predicted, we observed a testosterone-by-cortisol interaction. However, the substantive nature of our interaction is in fact opposite to what the dual-hormone hypothesis would predict. Indeed, in our results the anticipated positive effect of testosterone was only observed when cortisol was elevated. The difference in the nature of the interactions could be due to difference between status/control-seeking behavior and brain/cognitive outcomes, or experimental design differences (e.g., the examination of reactive versus basal hormone levels). Characteristics of the samples should also be considered. Whereas the present results are from a community based sample of middle-aged men, nearly all studies examining the dual-hormone hypothesis have been based on undergraduate volunteers (Mehta and Prasad, 2015). Such dramatic differences in the ages of the samples may have profound effects on the dynamic interplay between hormones and their subsequent effects on outcome measures. The results from the present study suggest that further examination of the interactions between testosterone and cortisol are clearly warranted. Establishing the consistency of the interaction in larger samples, across a wider age range, as well as with regard to a broader array of outcome measures is likely to provide valuable information that can be used to modify the dual-hormone hypothesis.
Although we have primarily interpreted the interactions in this study as cortisol moderating the associations between testosterone, hippocampus, and memory, we stress that it is equally valid to consider the moderating effect of testosterone on cortisol’s relationships with these phenotypes. The dual-hormone hypothesis, in contrast, emphasizes the fact that high cortisol can suppress hypothalamic-pituitary-gonadal (HPG) axis activity (Mehta and Josephs, 2010; Mehta and Prasad, 2015; Viau, 2002), and subsequently conceptualizes cortisol as the dominant factor in the interaction. Under normal functioning the HPG and HPA axes mutually interact with one another at multiple points (Handa et al., 1994). At the molecular level, the androgen and glucocorticoid receptors form heterodimers that translocate to the nucleus and have mutually inhibitory effects on the hormone-dependent transcriptional activity of one another. This heterologous co-regulation of the androgen receptor and glucocorticoid receptor may balance the anabolic actions of testosterone against the catabolic homeostatic actions of cortisol (Chen et al., 1997). It has also been shown that expression of the glucocorticoid receptor can be regulated via the manipulation of androgen levels. Glucocorticoid receptor mRNA and protein levels in the prostate and epididymis become upregulated following the removal of circulating testosterone by gonadectomy, whereas glucocorticoid receptor expression is reduced when circulating androgen levels are increased by exogenous testosterone treatment (Silva et al., 2010). A similar pattern of receptor expression has been found in the CA1 region of the rat hippocampus, where post-gonadectomy administration of dihydrotestosterone, a potent agonist of the androgen receptor, resulted in downregulation of the glucocorticoid receptor mRNA (Kerr et al., 1996). In the present study we observed a positive correlation between testosterone and cortisol (r = .41), one that is consistent with associations from other studies of the two hormones (Mehta and Josephs, 2010; Popma et al., 2007; Zilioli et al., 2014). Such positive associations between the two hormones in relatively healthy populations would suggest a pattern of co-regulation between the hypothalamic-pituitary-adrenal (HPA) and HPG axes, rather than the inhibition of one system by another.
The biological mechanisms that underlie our observed interactions may extend beyond the level of the androgen and glucocorticoid receptors. Both testosterone and cortisol are components of complex biosynthesis pathways that are not represented by our data. Androgen biosynthesis, for example, involves the conversion of DHEA to androstenedione via 3β-hydroxy-steroid dehydrogenase (HSD), which is subsequently converted to testosterone via 17β-HSD (Heberden, 2017). Testosterone itself can then be converted by 5α-reductase to dihydrotestosterone (DHT), or be aromatized to estradiol. Similarly, Cortisol can be converted to cortisone via 11β-HSD and to tetrahydrocortisols and other metabolites via 5α/β-reductases (Cobice et al., 2017; Nixon et al., 2012). These precursory and derivative steroid hormones may have receptor-dependent or receptor-independent effects on neurons in the hippocampus and other brain regions that ultimately modify the association of either testosterone or cortisol with hippocampal structure and function. Our observed interaction effects may also stem from factors unrelated to testosterone or cortisol biosynthesis that are nevertheless impacted by both hormones. Testosterone and cortisol, for example, have both been shown to impact brain-derived neurotrophic factor (BDNF) within the hippocampus; the former having a negative effect on BDNF expression in the hippocampus, while the later negatively regulates BDNF immunoreactivity in the mossy fiber pathway of the hippocampus (Atwi et al., 2016). Testosterone, through its conversion to estradiol, can also result in upregulation of BDNF expression and activation of TrkB receptor signaling in the hippocampus and other brain regions. Additional studies are clearly warranted to elucidate the nature of the testosterone-cortisol interaction, as understanding of this mechanism is likely to improve our understanding of the advantageous and deleterious effects of both hormones.
The present study is not without its limitations. Given the all-male composition of the VETSA sample, we do not know whether similar interaction effects between testosterone and cortisol will be present in women. The VETSA sample is also highly homogenous with respect to ethnicity; thus the generalizability of these results to more diverse populations may be limited. Although the present results did include ethnicity as a covariate, replication of the cortisol-by-testosterone interaction in a more diverse sample is clearly warranted. The cross-sectional nature of the study limits our ability to determine whether the observed interaction effects are long standing, or if they are representative of age-related changes in one or both of the hormones. Finally, we must acknowledge that present study does not assess all hormones relevant to the function of the hippocampus, which limits our ability to determine whether the testosterone-cortisol interaction is due to direct or indirect effects of the hormones. Testosterone, for example, can be can be converted into estradiol, which in turn can regulate the hippocampus via the estrogen receptor (Lu et al., 2004). There is evidence that in women estradiol is proactive against glucocorticoid effects (Ycaza Herrera and Mather, 2015), suggesting that a similar estradiol-cortisol interaction could be identified. Just as alternate mechanisms for androgen action may involve estrogen pathways (Handa et al., 2008), it cannot be ruled out that the results of the present study may stem from additional endocrine mechanisms for which we have not accounted.
Conclusion
The present study demonstrates a significant interaction effect between testosterone and cortisol for hippocampal volume and a composite measure of episodic memory in men. The nature of the interaction was such that when cortisol was elevated, higher testosterone levels were associated with larger hippocampal volume and better memory performance. This would suggest that in the potentially neurotoxic hormonal milieu associated with high cortisol levels, testosterone may be neuroprotective. In contrast, when cortisol levels were low, lower testosterone levels were associated with larger hippocampal volume and better memory. To our knowledge this is the first demonstration of a testosterone-by-cortisol interaction in a structural brain measure and an associated cognitive ability. Overwhelmingly, studies of neuroendocrine associations with brain structure and function have emphasized the effects of individual hormones and have rarely considered the possibility of between-hormone interactions. These results strongly argue in favor of broadening neuroendocrine research to consider the simultaneous and interactive effects of multiple hormones, the broader hormonal milieu, on brain structure and function.
Supplementary Material
Highlights.
Research suggests that testosterone influences hippocampal structure and function.
The interaction of testosterone with other hormones is rarely considered.
We show testosterone-cortisol interactions on hippocampal volume and episodic memory.
Positive associations with testosterone were observed when cortisol was elevated.
This is the first demonstration of such an interaction for hippocampus and memory.
Acknowledgments
This work was supported by National Institute on Aging Grants R01 AG0050595 (to William S. Kremen and Michael J. Lyons), AG022381 and AG022982 (to William S. Kremen), K08 AG047903 (to Matthew S. Panizzon), R03 AG046413 (to Carol E. Franz), as well as a Merit Review grant (5I01BX001566) from the Department of Veterans Affairs (to Richard L. Hauger). This material was, in part, the result of work supported with resources of the Center of Excellence for Stress and Mental Health at the VA San Diego Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken L, West S. Multiple Regression: Testing and Interpreting Interatcions. Sage; London: 1991. [Google Scholar]
- Atwi S, McMahon D, Scharfman H, MacLusky NJ. Androgen Modulation of Hippocampal Structure and Function. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2014 doi: 10.1177/1073858414558065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwi S, McMahon D, Scharfman H, MacLusky NJ. Androgen Modulation of Hippocampal Structure and Function. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2016;22:46–60. doi: 10.1177/1073858414558065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayroff AG, Anderson AA. Technical Research Report 1122. U.S. Army Research Institute; Alexandria, VA: 1963. Development of Armed Forces Qualification Tests 7 and 8. [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beyenburg S, Watzka M, Clusmann H, Blumcke I, Bidlingmaier F, Elger CE, Stoffel-Wagner B. Androgen receptor mRNA expression in the human hippocampus. Neuroscience letters. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- Boss L, Kang DH, Marcus M, Bergstrom N. Endogenous sex hormones and cognitive function in older adults: a systematic review. Western journal of nursing research. 2014;36:388–426. doi: 10.1177/0193945913500566. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Selby JV, Quiroga J, Reed T, Fabsitz RR, Christian JC. 16-year incidence of ischemic heart disease in the NHLBI twin study. A classification of subjects into high and low-risk groups. Annals of Epidemiology. 1994;4:198–204. doi: 10.1016/1047-2797(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Yu G, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. Journal of Biological Chemistry. 1997;272:14087–14092. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- Choate JV, Slayden OD, Resko JA. Immunocytochemical localization of androgen receptors in brains of developing and adult male rhesus monkeys. Endocrine. 1998;8:51–60. doi: 10.1385/ENDO:8:1:51. [DOI] [PubMed] [Google Scholar]
- Cobice DF, Livingstone DEW, McBride A, MacKay CL, Walker BR, Webster SP, Andrew R. Quantification of 11beta-hydroxysteroid dehydrogenase 1 kinetics and pharmacodynamic effects of inhibitors in brain using mass spectrometry imaging and stable-isotope tracers in mice. Biochemical Pharmacology. 2017;148:88–99. doi: 10.1016/j.bcp.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition. The Psychological Corporation; San Antonio, Texas: 2000. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Franz CE, O’Brien RC, Hauger RL, Mendoza SP, Panizzon MS, Prom-Wormley E, Eaves LJ, Jacobson K, Lyons MJ, Lupien S, Hellhammer D, Xian H, Kremen WS. Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: the Vietnam Era twin study of aging. Psychoneuroendocrinology. 2011;36:1040–1052. doi: 10.1016/j.psyneuen.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, Jacobson KC, Levine S, Lupien SJ, Lyons MJ, Prom-Wormley E, Xian H, Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behavior genetics. 2010;40:467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of disease. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Galea LA, Perrot-Sinal TS, Kavaliers M, Ossenkopp KP. Relations of hippocampal volume and dentate gyrus width to gonadal hormone levels in male and female meadow voles. Brain research. 1999;821:383–391. doi: 10.1016/s0006-8993(99)01100-2. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Hertzman C, Li L, Power C. Morning salivary cortisol and cognitive function in mid-life: evidence from a population-based birth cohort. Psychological Medicine. 2012;42:1763–1773. doi: 10.1017/S0033291711002704. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Research and Human Genetics. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. Journal of Neurochemistry. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology and Behavior. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Hormones and behavior. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Heberden C. Sex steroids and neurogenesis. Biochemical Pharmacology. 2017;141:56–62. doi: 10.1016/j.bcp.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ. Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. Journal of neuroendocrinology. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Franz CE, Spoon KM, Vuoksimaa E, Jacobson KC, Vasilopoulos T, Xian H, McCaffery JM, Rana BK, Toomey R, McKenzie R, Lyons MJ. Genetic complexity of episodic memory: a twin approach to studies of aging. Psychology and aging. 2014;29:404–417. doi: 10.1037/a0035962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Hormones and behavior. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lu YP, Zeng M, Swaab DF, Ravid R, Zhou JN. Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer’s disease. Human Pathology. 2004;35:275–280. doi: 10.1016/j.humpath.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Panizzon MS, Liu W, McKenzie R, Bluestone NJ, Grant MD, Franz CE, Vuoksimaa EP, Toomey R, Jacobson KC, Reynolds CA, Kremen WS, Xian H. A longitudinal twin study of general cognitive ability over four decades. Developmental Psychology. 2017;53:1170–1177. doi: 10.1037/dev0000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Weis JM, Schwartz LS. Selective retention of corticosterone by limbic structure in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGrevy DF, Knouse SB, Thompson RA. Personnel Research Division, Air Force Human Resources Laboratory Technical Report, AFHRL-TR-74-25. Brooks Air Force Base; TX: 1974. Relationships among an individual intelligence test and two air force screening and selection tests. [Google Scholar]
- Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Hormones and behavior. 2010;58:898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Prasad S. The dual-hormone hypothesis: a brief review and future research agenda. Current Opinion in Behavioral Sciences. 2015;3:163–168. [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, Carre JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Miyamoto J, Matsumoto T, Shiina H, Inoue K, Takada I, Ito S, Itoh J, Minematsu T, Sato T, Yanase T, Nawata H, Osamura YR, Kato S. The pituitary function of androgen receptor constitutes a glucocorticoid production circuit. Molecular and cellular biology. 2007;27:4807–4814. doi: 10.1128/MCB.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neurobiology of aging. 2007;28:914–920. doi: 10.1016/j.neurobiolaging.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nixon M, Upreti R, Andrew R. 5alpha-Reduced glucocorticoids: a story of natural selection. Journal of Endocrinology. 2012;212:111–127. doi: 10.1530/JOE-11-0318. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, Jacobson K, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley E, Seidman L, Tsuang MT, Xian H, Kremen WS. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology. 2010;75:874–880. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Jacobson KC, Eaves LJ, York TP, Prom-Wormley E, Grant MD, Lyons MJ, McKenzie R, Mendoza SP, Xian H, Franz CE, Kremen WS. Genetic and environmental influences of daily and intra-individual variation in testosterone levels in middle-aged men. Psychoneuroendocrinology. 2013;38:2163–2172. doi: 10.1016/j.psyneuen.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Xian H, Vuoksimaa E, Spoon KM, Mendoza SP, Jacobson KC, Vasilopoulos T, Rana BK, McKenzie R, McCaffery JM, Lyons MJ, Kremen WS, Franz C. Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiology of aging. 2014;36:1778e1771–1778.e1778. doi: 10.1016/j.neurobiolaging.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:1140–1145. doi: 10.1073/pnas.032646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain research. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk CA, Rinne T, van den Brink W, Knol DL, Jansen LM, van Engeland H, Doreleijers TA. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological psychiatry. 2007;61:405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Schoenborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004–2007. National health statistics reports. 2009 [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neuroscience and biobehavioral reviews. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Silva EJ, Queiroz DB, Honda L, Avellar MC. Glucocorticoid receptor in the rat epididymis: expression, cellular distribution and regulation by steroid hormones. Molecular and cellular endocrinology. 2010;325:64–77. doi: 10.1016/j.mce.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of neuroendocrinology. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Third Edition. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Ycaza Herrera A, Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neuroscience and biobehavioral reviews. 2015;55:36–52. doi: 10.1016/j.neubiorev.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S, Ponzi D, Henry A, Maestripieri D. Testosterone, Cortisol and Empathy: Evidence for the Dual-Hormone Hypothesis. Adaptive Human Behavior and Physiology E-pub 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.