Abstract
Enhanced recovery after surgery is a concept initially developed for patients undergoing colorectal surgery but has been adopted by other surgical specialties with similar positive outcomes. The adoption of enhanced recovery after surgery in the obstetric patient population is rapidly gaining popularity. This review highlights perioperative interventions that should be considered in an enhanced recovery after surgery protocol for women undergoing cesarean delivery.
Keywords: Cesarean Delivery, enhanced recovery after surgery
Introduction
Enhanced recovery after surgery (ERAS) is a concept that combines various evidence-based aspects of perioperative care to accelerate patient recovery. It standardizes perioperative management and achieves a reproducible improvement in the quality of care 1. Initial studies on ERAS protocols conducted in colorectal surgery reported a reduction in hospital stay, readmissions, and postoperative complications coupled with improved patient satisfaction 2– 4. Since then, there has been widespread adoption of ERAS protocols in other surgical specialties with similar outcomes reported 5– 8. The specific components of ERAS protocols differ among surgical specialties and institutions, but the core principles remain the same. These principles involve interventions that span the preoperative, intraoperative, and postoperative periods. It addresses the common reasons that delay patient recovery from surgery and prolong hospital stay such as inadequate analgesia, slow return of bowel function, and delayed ambulation 9. There has been slower embrace of the benefits of ERAS in patients undergoing cesarean delivery. However, with increased pressure on maternity services, several centers in Europe have begun implementing ERAS protocols for scheduled cesarean delivery 10, 11, and this concept has recently started to gain popularity in the USA. The aim of this review is to highlight evidence-based perioperative interventions that should be considered as part of an ERAS protocol for scheduled cesarean delivery.
Enhanced recovery pathway for cesarean delivery
Why enhanced recovery for cesarean delivery?
The cesarean delivery rate in the United States is about 32% of all births, with over 1.27 million procedures performed annually 12. The majority of women undergoing cesarean delivery are young and healthy and therefore have the potential for rapid recovery following delivery. Furthermore, being able to care for their newborn provides an added motivation to return to normal physiological function. A study on early discharge following uncomplicated cesarean delivery that pre-dates the concept of ERAS reported higher maternal satisfaction in the early discharge group compared to women in a routine care group 13.
There are already many aspects of current routine perioperative care of the patient undergoing a cesarean delivery that are consistent with components of ERAS. A survey of obstetric anesthesiologists in the UK conducted in 2013 showed that the majority of respondents supported the concept of ERAS for cesarean delivery and most were considering or were in the process of implementing an ERAS protocol at their institutions 10. A similar survey of 36 academic maternity units in the UK conducted in 2015 reported that 50% of respondents had implemented an ERAS protocol and 30% had plans to introduce one 14.
Proposed components for enhanced recovery after cesarean delivery
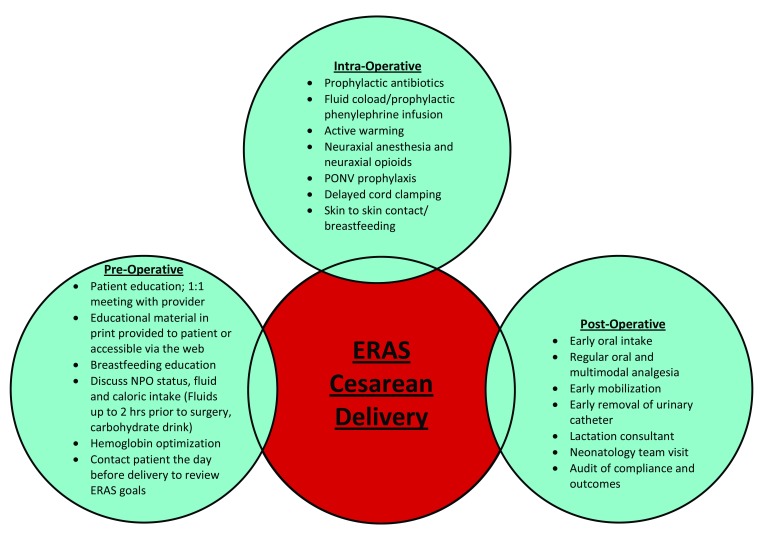
The principles of enhanced recovery cover the entire perioperative care pathway and component interventions occur during the preoperative, intraoperative, and postoperative phases of care 15 ( Figure 1).
Figure 1. Components of Enhanced Recovery Protocol for Cesarean Delivery.
ERAS, enhanced recovery after surgery; NPO, nil per os (nothing by mouth); PONV, postoperative nausea and vomiting.
Preoperative preparation
Patient education
Patient education and counselling and a shared decision-making model are required for the successful implementation of an ERAS program. Studies on ERAS implementation in various surgical specialties have reiterated the need for active participation of the patient in the recovery process and its positive impact on patient outcomes 16. In a recent study focused on patient education to enhance recovery in colorectal surgery, the authors reported that patients wanted to be proactively involved in their recovery process 17. Active patient engagement can be achieved by a comprehensive and timely preoperative education that includes provision of internet-accessible or take-home educational materials allowing patients to be acquainted with the ERAS concepts. Patient education should include information on the procedure and what to expect during surgery, a pain management plan, and goals for early feeding and mobilization. Information should also be provided on breastfeeding, including lactation support services available, length of stay, and the criteria for discharge. Patients can be given a checklist with actions and goals which they can use to keep track of their progress in the recovery process 18.
Nil per os status, preoperative fluid, and caloric intake
Traditionally, patients have been told to fast from midnight before surgery to reduce the risk of pulmonary aspiration. Ultrasonography studies have demonstrated that gastric emptying is normal during pregnancy and is slowed only with the onset of labor 19, 20. The current practice guidelines for obstetric anesthesia from the American Society of Anesthesiologists (ASA) recommend six- to eight-hour fasting for solids and clear oral fluid intake up to two hours before the induction of anesthesia 21. The intake of a high-caloric carbohydrate drink up to two hours before surgery has been shown to reduce preoperative thirst, hunger, and anxiety in patients undergoing abdominal surgery 22. It has also been associated with a reduction in insulin resistance and a higher anabolic state postoperatively 23, 24.
Preoperative hemoglobin optimization
There are inadequate data on the prevalence of iron deficiency anemia in pregnant women in the United States. The National Health and Nutrition Examination Survey (NHANES) from 1999 to 2006 estimated the prevalence of iron deficiency in pregnant women as 18.6% 25. Most women presenting for prenatal care are routinely screened for anemia. However, there are differing opinions among government health agencies and professional associations on the benefits of routine screening and iron supplementation in asymptomatic pregnant women. The Centers for Disease Control and Prevention (CDC) recommend screening for anemia and initiating low-dose iron supplementation for all pregnant women at the first prenatal care visit 26. The American College of Obstetricians and Gynecologists (ACOG) also recommends anemia screening for all pregnant women but treating only those with anemia with supplemental iron 27. On the other hand, the United States Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians (AAFP) conclude that the current evidence is insufficient to recommend for or against routine screening and iron supplementation to prevent adverse maternal and neonatal outcomes 25. At our institutions, pregnant women are routinely screened for anemia and are referred to an anemia clinic for optimization of hemoglobin if anemic or if they have an increased risk of obstetric hemorrhage. Furthermore, preoperative anemia is a significant predictor of severe postpartum anemia, which has been linked to various morbidities such as depression and fatigue 28.
Intraoperative care
Prophylactic antibiotics
Cesarean delivery increases the risk of infection and its related morbidity 5 to 20-fold compared to vaginal delivery 29. Infectious complications lead to hospital readmissions 30 and a significant increase in length of hospital stay. There is compelling evidence that prophylactic antibiotics should be administered to all women undergoing cesarean delivery 29. Traditionally, prophylactic antibiotics have been withheld until cord clamping owing to concern of neonatal exposure to antibiotics. There is, however, conclusive evidence that prophylactic antibiotics administered within 60 minutes before skin incision significantly reduce the incidence of maternal postpartum infection compared to administration after cord clamping 31, 32. The current recommendation is a single dose of a broad-spectrum antibiotic in the non-laboring patient prior to skin incision 33.
Thromboprophylaxis
Pneumatic compression devices are recommended for all women undergoing cesarean delivery and not already receiving pharmacologic thromboprophylaxis 34. The compression devices should be continued until the patient is fully ambulatory. In women with one or more additional risk factors, pharmacological thromboprophylaxis is recommended 35.
Fluids and blood pressure management
One of the core principles of ERAS is the maintenance of a normal fluid balance. In the general surgical population, goal-directed fluid therapy based on physiologic endpoints has been shown to reduce perioperative complications and length of stay 36. The benefits of goal-directed fluid therapy as part of an ERAS protocol are less clear and have generated much debate among clinicians 37– 39. The usefulness of goal-directed fluid therapy has not been investigated in the cesarean delivery population but might be valuable given the likely different hydration status of women presenting for cesarean delivery.
Hypotension occurs commonly in women undergoing cesarean delivery under spinal anesthesia, and it can be detrimental to the mother and the fetus. Hypotension can trigger intraoperative nausea and vomiting (IONV) in the mother and decrease uteroplacental blood flow, which impairs fetal oxygenation. Both fluids and vasopressors have been used to counteract spinal anesthesia-induced hypotension. Fluid loading strategies alone have limited efficacy in reducing the incidence of hypotension 40. However, when used in conjunction with a prophylactic phenylephrine infusion, a rapid crystalloid coload of 2 L was associated with a significant reduction in the incidence of hypotension compared to maintenance fluid administration 41.
Given that the etiology of spinal-induced hypotension is mainly related to peripheral vasodilatation, vasopressors are the mainstay for the management of hypotension. Phenylephrine is currently considered the vasopressor of choice for the management of maternal hypotension induced by neuraxial anesthesia, given its favorable fetal acid–base status and lower incidence of IONV when compared with ephedrine 42– 44. A prophylactic infusion is more effective at reducing the number of hypotensive events as well as decreasing the incidence of nausea and vomiting compared to rescue treatment of established hypotension with phenylephrine boluses 45. Therefore, the recommended strategy as part of an ERAS protocol would be to use a prophylactic phenylephrine infusion initiated at 50 mcg/minute in conjunction with a rapid crystalloid coload of up to 2 L. A low-dose norepinephrine infusion has been investigated as an alternative to phenylephrine in managing hypotension during cesarean delivery. Studies suggest similar efficacy in maintaining blood pressure with a higher heart rate and cardiac output compared to phenylephrine 46– 48.
Temperature management
Maintaining perioperative normothermia in the general surgical population reduces the risk of postoperative wound infection, coagulopathy, blood loss, and transfusion requirement 49, 50. The incidence of hypothermia in women undergoing cesarean delivery under spinal anesthesia is estimated to be >60%. Temperature autoregulation is impaired during spinal anesthesia by the inhibition of vasomotor and shivering responses and a redistribution of heat from the core to the peripheral tissues. Hypothermia associated with spinal anesthesia might be under-appreciated 51. A recent study demonstrated a rapid drop in intestinal temperature by a mean of 1.3 °C during cesarean delivery under spinal anesthesia 52. The median time to the lowest intestinal temperature was one hour after the initiation of spinal anesthesia, and temperature continued to fall in the majority of patients even after completion of the procedure. It took a median of 4.5 hours for intestinal temperature to recover to baseline, and, in 29% of patients, the temperature did not return to baseline during the 8-hour duration of the study. However, patients in this study were not actively warmed. Perioperative hypothermia can be a cause for delayed discharge from the post anesthesia care unit (PACU), which has been correlated with an increased length of stay during implementation of ERAS protocols for cesarean delivery 53.
Hypothermia-related adverse outcomes in women undergoing cesarean delivery have not been adequately examined 51. A meta-analysis of 13 randomized trials by Sultan et al. reported that active warming in women undergoing elective cesarean delivery under spinal anesthesia reduced the maximum fall in temperature and decreased the incidence of hypothermia and shivering when compared with controls not actively warmed 54. Thermal comfort was also improved in patients who had active warming, together with reduced neonatal hypothermia and improved umbilical artery cord pH. Maintaining normothermia can also help facilitate early maternal bonding with the newborn.
The best strategy for active warming is unclear. Most strategies have limited efficacy in isolation, and a combination of preoperative and intraoperative forced air warming with warmed intravenous fluids may be more effective 51 and should be implemented as part of all ERAS protocols.
Neuraxial anesthesia including neuraxial opioids for analgesia
Neuraxial anesthesia (mainly spinal anesthesia) is the anesthetic technique of choice for elective cesarean delivery 55– 57. Neuraxial anesthesia decreases the hypothalamo-pituitary response to surgical stress and has been shown to reduce the duration of postoperative ileus in the general surgical population 58. Neuraxial anesthesia also allows the woman to witness the birth of her child, allows for early skin-to-skin contact with the newborn, and facilitates the presence of a support person in the operating room.
Opioids are usually added to local anesthetic mixture because they improve intraoperative anesthesia, prolong its duration, decrease local anesthesia requirements, and provide postoperative analgesia 59. A lipophilic fast-onset and short-acting opioid such as fentanyl or sufentanil is added for intraoperative effects, and a hydrophilic opioid such as morphine with a prolonged duration of action is added for postoperative analgesia. Neuraxial morphine provides superior analgesia compared to systemic opioid administration. Studies suggest a ceiling in analgesic effect with a dose-related increase in opioid-related side effects, including nausea, vomiting, and pruritus. In a recent meta-analysis, Sultan et al. reported that the odds of nausea or vomiting (OR 0.44 [95% CI 0.27–0.73]) and pruritus (OR 0.34 [95% CI 0.20–0.59]) were lower with smaller doses of intrathecal morphine (50–100 mcg) than with higher doses (>100 mcg) 60, but the time to first request of analgesia was longer by an average of 4.5 hours with the higher doses, with no difference in total postoperative analgesic consumption.
There is wide variability in analgesic requirements after cesarean delivery, and tests such as quantitative sensory testing, hyperalgesia testing, response to local anesthesia skin infiltration, and psychometric evaluation may help identify patients with higher postoperative analgesic requirements 61. However, studies investigating postoperative analgesia use based on the results of these predictive tests are lacking. The preference of the patient for more analgesia versus side effects should also be considered. A study by Carvalho et al. reported that women who preferentially chose a larger intrathecal morphine dose correctly anticipated greater postoperative opioid requirement and more pain compared with women who chose the smaller dose 62.
Postoperative nausea and vomiting prophylaxis
Postoperative nausea and vomiting (PONV) can delay early oral intake, a key objective of ERAS. PONV occurs frequently after cesarean delivery, especially in parturients who received neuraxial opioids 63. The etiology of PONV is multifactorial, and therefore a multifaceted approach for prophylaxis is needed. Combination of anti-emetic agents is more effective in the management of PONV compared to monotherapy; however, studies investigating combination anti-emetic therapy in the obstetric patient population are scarce 64, 65. Use of combination therapy of non-sedating agents such as ondansetron with dexamethasone should be an integral part of an ERAS protocol. Droperidol, an antidopaminergic agent, is also effective for PONV prophylaxis 65. There is, however, a “black box” warning by the Food and Drug Administration (FDA) because of risk of torsade de pointes due to QT prolongation, and this has limited its use in the USA 66.
IONV is common during cesarean delivery under spinal anesthesia 63. Avoidance of hypotension with a prophylactic phenylephrine infusion, administration of metoclopramide, and avoidance of uterine exteriorization and fluid irrigation have been associated with a reduced incidence of IONV 64.
Delayed cord clamping
Delay in clamping of the umbilical cord for at least 30 seconds was initially recommended in preterm newborns because it is associated with a reduction in risk of intraventricular hemorrhage, an increase in hematocrit, and a decrease in need for volume resuscitation 67, 68. However, current data suggest that it may also be beneficial in term infants without evidence of significant harm. A meta-analysis of 15 trials by McDonald et al. reported that delayed cord clamping was associated with higher hemoglobin concentration and iron reserves up to six months after birth compared to early clamping 69. There was, however, a higher risk of jaundice requiring phototherapy in infants who had delayed cord clamping. The current recommendation from ACOG is delayed cord clamping in vigorous term and preterm infants for at least 30–60 seconds after birth 70.
Skin to skin
There are reported benefits for both the newborn and the mother of early skin-to-skin contact. Early skin to skin has been associated with increased rates and duration of breastfeeding 71, 72 and a decrease in maternal anxiety and postpartum depression 73. However, maternal intent to breastfeed may be associated with an increased length of hospital stay 53. If an ERAS protocol for cesarean delivery is to be successful, steps should be taken to support the early initiation of breastfeeding. A concept termed “natural or gentle” cesarean delivery developed about a decade ago seeks to modify some aspects of cesarean delivery so that the woman can have a “natural” experience comparable to a vaginal birth 74. These modifications include using a transparent surgical drape, allowing the mother and her partner to witness the birth, and initiating immediate skin-to-skin contact and breastfeeding after birth. A randomized trial comparing the “natural” cesarean delivery to a traditional cesarean delivery reported a significantly greater rating of birth experience and higher breastfeeding in the “natural” cesarean delivery group 75.
Oxytocin management
A prophylactic low-dose oxytocin infusion (15–18 U/hour) should be commenced to prevent postpartum hemorrhage 76. A low dose reduces the occurrence of adverse effects such as hypotension and myocardial ischemia 77. Carbetocin, a long-acting oxytocin receptor agonist available in Canada and Europe, can also be used as a first-line prophylactic uterotonic instead of oxytocin 78.
Postoperative care
Early oral intake
Traditionally, oral intake has been delayed after abdominal surgery until the return of bowel function is confirmed by bowel sounds or passage of flatus or stools. This is contrary to the current evidence indicating that early oral intake promotes the return of bowel function and early ambulation, decreases the risk of sepsis, reduces the time to breastfeeding, and shortens the length of stay 79– 81.
Regular oral and multimodal analgesia
Provision of adequate postoperative analgesia is an integral component of ERAS protocols, and it assumes even greater importance in women undergoing cesarean delivery. Suboptimal analgesia is associated with delayed functional recovery, delayed mobilization which could increase the risk of thromboembolic complications, poor maternal bonding with the newborn, breastfeeding difficulties, and an increase in the risk of persistent pain and postpartum depression 82, 83. There are multiple complex factors that contribute to postoperative pain, with significant inter-individual variability in pain perception. ERAS protocols recommend a multimodal analgesic regimen using a combination of drugs with different mechanisms of action with the goal of optimizing analgesia, minimizing side effects, and providing opioid sparing 16. This can be achieved through the combination of neuraxial opioid analgesia, oral analgesia, and peripheral nerve blockade. Neuraxial morphine was discussed earlier in this review and is considered the gold standard for post cesarean analgesia. Recommended dosing is 100–150 mcg intrathecal and 3 mg epidural 84, 85. However, supplemental opioid-sparing analgesics are required to optimize the quality of postoperative analgesia and decrease the need for additional rescue oral or intravenous opioids.
Acetaminophen and non-steroidal anti-inflammatory drugs. Acetaminophen has an opioid-sparing effect and provides analgesia with minimal adverse effects or secretion in breast milk 86– 88. An acetaminophen–opioid combination is commonly prescribed for breakthrough pain because of the synergistic effect between the two agents, but scheduled acetaminophen with as-needed opioids is recommended. In a study comparing opioid use in patients on scheduled acetaminophen with as-needed opioids to patients on as-needed acetaminophen plus opioids, the cumulative opioid use was reported to be significantly reduced in patients receiving scheduled acetaminophen 89.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have an opioid-sparing effect of up to 50% 90. Acetaminophen and NSAIDs have an additive analgesic effect and, unless contraindicated, both drugs should be routinely given on a scheduled rather than a pro re nata basis after cesarean delivery 91.
Nerve blocks and wound infiltration. Transversus abdominis plane (TAP) block, local anesthetic wound infiltration, and, recently, quadratus lumborum (QL) block have all been described as adjuvant techniques for analgesia after cesarean delivery. TAP blocks improve postoperative analgesia after cesarean delivery in patients who did not receive spinal morphine but not in those who received intrathecal morphine 92, 93.
There are limited data on the efficacy of local anesthetic wound infiltration in women undergoing cesarean delivery. Local infiltration of NSAIDs might also provide benefit, but the value of wound infiltration in patients receiving spinal morphine with a multimodal analgesic regimen is unclear 94. A recent study comparing spinal morphine to a continuous infusion of ropivacaine into the surgical wound suggested improved analgesia in both groups compared to control, but rescue opioid consumption was lower with spinal morphine 95.
Recent studies have shown that the QL block provides effective analgesia after cesarean delivery and reduces opioid consumption 96, 97. However, the analgesic efficacy of QL block in patients who receive spinal morphine has not been investigated.
In summary, local anesthetic techniques have limited efficacy when used in conjunction with neuraxial morphine but should be considered in patients who do not receive neuraxial morphine or when high postoperative analgesic needs are anticipated. Furthermore, it is not clear if techniques using long-acting liposomal local anesthetics might confer benefit in patients receiving neuraxial morphine.
Early mobilization
Early mobilization improves pulmonary function and tissue oxygenation, improves insulin resistance, reduces risk of thromboembolism, and shortens length of stay 98. Effective postoperative analgesia is a key factor in facilitating early postoperative mobilization. Mobilization goals after cesarean delivery should be discussed during the preoperative patient education.
Early removal of urinary catheter
It is recommended that urinary catheters are removed within 24 hours in ERAS protocols. There are few data on the timing of urinary catheter removal in women who have cesarean delivery under spinal anesthesia. In a published audit of an ERAS protocol for cesarean delivery, urinary catheters were removed 7 hours after the procedure to facilitate early ambulation with no complications reported 99.
Post discharge
Prior to discharge, it must be ensured that the patient has access to a reliable means of communication with the labor and delivery unit, is given the number to call, and knows who to contact if there are any concerns. The patient must be contacted within 24 hours after discharge to assess the wellbeing of the mother and the newborn and to address any questions or concerns.
Barriers to implementation
The potential barriers to successful implementation of an ERAS protocol for cesarean delivery include the discomfort providers feel with change in practice, allocation of resources especially for patient education, post discharge follow up, and the lack of dedicated operating rooms for scheduled cesarean deliveries 15. Goals should be set and targets audited regularly to identify compliance and opportunities for improvement. Coordination with the neonatology team and lactation consultants is also crucial to avoid delays in discharge due to issues related to neonatal tests and evaluation or breastfeeding education.
Conclusion
An enhanced recovery program for cesarean delivery should consist of the best evidence in perioperative care of the parturient. There is wide variability in components of published ERAS protocols for cesarean delivery. Future studies on developing and evaluating the impact of various components are needed.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Dan Benhamou, Department of Anaesthesia and Intensive Care Medicine, Groupe Hospitalier et Faculté de Médecine Paris Sud, Paris, France
Mohamed Tiouririne, Department of Anesthesiology, University of Virginia, Charlottesville, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Adamina M, Kehlet H, Tomlinson GA, et al. : Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149(6):830–40. 10.1016/j.surg.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Anderson AD, McNaught CE, MacFie J, et al. : Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg. 2003;90(12):1497–504. 10.1002/bjs.4371 [DOI] [PubMed] [Google Scholar]
- 3. Kehlet H, Wilmore DW: Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248(2):189–98. 10.1097/SLA.0b013e31817f2c1a [DOI] [PubMed] [Google Scholar]
- 4. Khoo CK, Vickery CJ, Forsyth N, et al. : A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. 2007;245(6):867–72. 10.1097/01.sla.0000259219.08209.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicholson A, Lowe MC, Parker J, et al. : Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–88. 10.1002/bjs.9394 [DOI] [PubMed] [Google Scholar]
- 6. Arsalani-Zadeh R, ElFadl D, Yassin N, et al. : Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98(2):181–96. 10.1002/bjs.7331 [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim MS, Khan MA, Nizam I, et al. : Peri-operative interventions producing better functional outcomes and enhanced recovery following total hip and knee arthroplasty: an evidence-based review. BMC Med. 2013;11:37. 10.1186/1741-7015-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wodlin NB, Nilsson L: The development of fast-track principles in gynecological surgery. Acta Obstet Gynecol Scand. 2013;92(1):17–27. 10.1111/j.1600-0412.2012.01525.x [DOI] [PubMed] [Google Scholar]
- 9. Grocott MP, Martin DS, Mythen MG: Enhanced recovery pathways as a way to reduce surgical morbidity. Curr Opin Crit Care. 2012;18(4):385–92. 10.1097/MCC.0b013e3283558968 [DOI] [PubMed] [Google Scholar]
- 10. Aluri S, Wrench IJ: Enhanced recovery from obstetric surgery: a U.K. survey of practice. Int J Obstet Anesth. 2014;23(2):157–60. 10.1016/j.ijoa.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 11. Benhamou D, Kfoury T: Enhanced recovery after caesarean delivery: Potent analgesia and adequate practice patterns are at the heart of successful management. Anaesth Crit Care Pain Med. 2016;35(6):373–5. 10.1016/j.accpm.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 12. Hamilton BE, Martin JA, Osterman MJ, et al. : Births: Final Data for 2014. Natl Vital Stat Rep. 2015;64(12):1–64. [PubMed] [Google Scholar]
- 13. Brooten D, Roncoli M, Finkler S, et al. : A randomized trial of early hospital discharge and home follow-up of women having cesarean birth. Obstet Gynecol. 1994;84(5):832–8. [PMC free article] [PubMed] [Google Scholar]
- 14. Coates E, Fuller G, Hind D, et al. : Enhanced recovery pathway for elective caesarean section. Int J Obstet Anesth. 2016;27:94–5. 10.1016/j.ijoa.2016.05.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Lucas DN, Gough KL: Enhanced recovery in obstetrics--a new frontier? Int J Obstet Anesth. 2013;22(2):92–5. 10.1016/j.ijoa.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 16. Kalogera E, Dowdy SC: Enhanced Recovery Pathway in Gynecologic Surgery: Improving Outcomes Through Evidence-Based Medicine. Obstet Gynecol Clin North Am. 2016;43(3):551–73. 10.1016/j.ogc.2016.04.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Poland F, Spalding N, Gregory S, et al. : Developing patient education to enhance recovery after colorectal surgery through action research: a qualitative study. BMJ Open. 2017;7(6):e013498. 10.1136/bmjopen-2016-013498 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Tiouririne M: <SOAP Newsletter Winter 2018.pdf>.2018. [Google Scholar]
- 19. Carp H, Jayaram A, Stoll M: Ultrasound examination of the stomach contents of parturients. Anesth Analg. 1992;74(5):683–7. 10.1213/00000539-199205000-00011 [DOI] [PubMed] [Google Scholar]
- 20. Wong CA, McCarthy RJ, Fitzgerald PC, et al. : Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105(3):751–5. 10.1213/01.ane.0000278136.98611.d6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270–300. 10.1097/ALN.0000000000000935 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Hausel J, Nygren J, Lagerkranser M, et al. : A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344–50. 10.1097/00000539-200111000-00063 [DOI] [PubMed] [Google Scholar]
- 23. Soop M, Nygren J, Myrenfors P, et al. : Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(4):E576–83. 10.1152/ajpendo.2001.280.4.E576 [DOI] [PubMed] [Google Scholar]
- 24. Yuill KA, Richardson RA, Davidson HI, et al. : The administration of an oral carbohydrate-containing fluid prior to major elective upper-gastrointestinal surgery preserves skeletal muscle mass postoperatively--a randomised clinical trial. Clin Nutr. 2005;24(1):32–7. 10.1016/j.clnu.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 25. Siu AL, U.S. Preventive Services Task Force: Screening for Iron Deficiency Anemia and Iron Supplementation in Pregnant Women to Improve Maternal Health and Birth Outcomes: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163(7):529–36. 10.7326/M15-1707 [DOI] [PubMed] [Google Scholar]
- 26. Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-3):1–29. [PubMed] [Google Scholar]
- 27. American College of Obstetricians and Gynecologists: ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–7. 10.1097/AOG.0b013e3181809c0d [DOI] [PubMed] [Google Scholar]
- 28. Butwick AJ, Walsh EM, Kuzniewicz M, et al. : Patterns and predictors of severe postpartum anemia after Cesarean section. Transfusion. 2017;57(1):36–44. 10.1111/trf.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Smaill FM, Grivell RM: Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014; (10):CD007482. 10.1002/14651858.CD007482.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Declercq E, Barger M, Cabral HJ, et al. : Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109(3):669–77. 10.1097/01.AOG.0000255668.20639.40 [DOI] [PubMed] [Google Scholar]
- 31. Baaqeel H, Baaqeel R: Timing of administration of prophylactic antibiotics for caesarean section: a systematic review and meta-analysis. BJOG. 2013;120(6):661–9. 10.1111/1471-0528.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bollig C, Nothacker M, Lehane C, et al. : Prophylactic antibiotics before cord clamping in cesarean delivery: a systematic review. Acta Obstet Gynecol Scand. 2018;97(5):521–35. 10.1111/aogs.13276 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. American College of Obstetricians and Gynecologists: ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117(6):1472–83. 10.1097/AOG.0b013e3182238c31 [DOI] [PubMed] [Google Scholar]
- 34. D'Alton ME, Friedman AM, Smiley RM, et al. : National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Anesth Analg. 2016;123(4):942–9. 10.1213/ANE.0000000000001569 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Ducloy-Bouthors AS, Baldini A, Abdul-Kadir R, et al. : European guidelines on perioperative venous thromboembolism prophylaxis: Surgery during pregnancy and the immediate postpartum period. Eur J Anaesthesiol. 2018;35(2):130–3. 10.1097/EJA.0000000000000704 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Miller TE, Raghunathan K, Gan TJ: State-of-the-art fluid management in the operating room. Best Pract Res Clin Anaesthesiol. 2014;28(3):261–73. 10.1016/j.bpa.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 37. Cannesson M, Gan TJ: PRO: Perioperative Goal-Directed Fluid Therapy Is an Essential Element of an Enhanced Recovery Protocol. Anesth Analg. 2016;122(5):1258–60. 10.1213/ANE.0000000000001144 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Joshi GP, Kehlet H: CON: Perioperative Goal-Directed Fluid Therapy Is an Essential Element of an Enhanced Recovery Protocol? Anesth Analg. 2016;122(5):1261–3. 10.1213/ANE.0000000000001233 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Rollins KE, Lobo DN: Intraoperative Goal-directed Fluid Therapy in Elective Major Abdominal Surgery: A Meta-analysis of Randomized Controlled Trials. Ann Surg. 2016;263(3):465–76. 10.1097/SLA.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Morgan PJ, Halpern SH, Tarshis J: The effects of an increase of central blood volume before spinal anesthesia for cesarean delivery: a qualitative systematic review. Anesth Analg. 2001;92(4):997–1005. 10.1097/00000539-200104000-00036 [DOI] [PubMed] [Google Scholar]
- 41. Ngan Kee WD, Khaw KS, Ng FF: Prevention of hypotension during spinal anesthesia for cesarean delivery: an effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103(4):744–50. 10.1097/00000542-200510000-00012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Lee A, Ngan Kee WD, Gin T: A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94(4):920–6, table of contents. 10.1097/00000539-200204000-00028 [DOI] [PubMed] [Google Scholar]
- 43. Ngan Kee WD, Lee A, Khaw KS, et al. : A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107(4):1295–302. 10.1213/ane.0b013e31818065bc [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Ngan Kee WD, Khaw KS, Tan PE, et al. : Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111(3):506–12. 10.1097/ALN.0b013e3181b160a3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Habib AS: A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114(2):377–90. 10.1213/ANE.0b013e3182373a3e [DOI] [PubMed] [Google Scholar]
- 46. Ngan Kee WD, Lee SW, Ng FF, et al. : Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122(4):736–45. 10.1097/ALN.0000000000000601 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Onwochei DN, Ngan Kee WD, Fung L, et al. : Norepinephrine Intermittent Intravenous Boluses to Prevent Hypotension During Spinal Anesthesia for Cesarean Delivery: A Sequential Allocation Dose-Finding Study. Anesth Analg. 2017;125(1):212–8. 10.1213/ANE.0000000000001846 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Vallejo MC, Attaallah AF, Elzamzamy OM, et al. : An open-label randomized controlled clinical trial for comparison of continuous phenylephrine versus norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth. 2017;29:18–25. 10.1016/j.ijoa.2016.08.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Kurz A, Sessler DI, Lenhardt R: Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–15. 10.1056/NEJM199605093341901 [DOI] [PubMed] [Google Scholar]
- 50. Melling AC, Ali B, Scott EM, et al. : Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–80. 10.1016/S0140-6736(01)06071-8 [DOI] [PubMed] [Google Scholar]
- 51. Allen TK, Habib AS: Inadvertent Perioperative Hypothermia Induced by Spinal Anesthesia for Cesarean Delivery Might Be More Significant Than We Think: Are We Doing Enough to Warm Our Parturients? Anesth Analg. 2018;126(1):7–9. 10.1213/ANE.0000000000002604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Du Toit L, van Dyk D, Hofmeyr R, et al. : Core Temperature Monitoring in Obstetric Spinal Anesthesia Using an Ingestible Telemetric Sensor. Anesth Analg. 2018;126(1):190–5. 10.1213/ANE.0000000000002326 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Wrench IJ, Allison A, Galimberti A, et al. : Introduction of enhanced recovery for elective caesarean section enabling next day discharge: a tertiary centre experience. Int J Obstet Anesth. 2015;24(2):124–30. 10.1016/j.ijoa.2015.01.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Sultan P, Habib AS, Cho Y, et al. : The Effect of patient warming during Caesarean delivery on maternal and neonatal outcomes: a meta-analysis. Br J Anaesth. 2015;115(4):500–10. 10.1093/bja/aev325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shibli KU, Russell IF: A survey of anaesthetic techniques used for caesarean section in the UK in 1997. Int J Obstet Anesth. 2000;9(3):160–7. 10.1054/ijoa.1999.0382 [DOI] [PubMed] [Google Scholar]
- 56. Bucklin BA, Hawkins JL, Anderson JR, et al. : Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103(3):645–53. [DOI] [PubMed] [Google Scholar]
- 57. Weiniger CF, Ivri S, Ioscovich A, et al. : Obstetric anesthesia units in Israel: a national questionnaire-based survey. Int J Obstet Anesth. 2010;19(4):410–6. 10.1016/j.ijoa.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 58. Kehlet H: The modifying effect of anesthetic technique on the metabolic and endocrine responses to anesthesia and surgery. Acta Anaesthesiol Belg. 1988;39(3):143–6. [PubMed] [Google Scholar]
- 59. Dahl JB, Jeppesen IS, Jørgensen H, et al. : Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91(6):1919–27. 10.1097/00132586-200010000-00032 [DOI] [PubMed] [Google Scholar]
- 60. Sultan P, Halpern SH, Pushpanathan E, et al. : The Effect of Intrathecal Morphine Dose on Outcomes After Elective Cesarean Delivery: A Meta-Analysis. Anesth Analg. 2016;123(1):154–64. 10.1213/ANE.0000000000001255 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Gamez BH, Habib AS: Predicting Severity of Acute Pain After Cesarean Delivery: A Narrative Review. Anesth Analg. 2017;126(5):1606–1614. 10.1213/ANE.0000000000002658 [DOI] [PubMed] [Google Scholar]
- 62. Carvalho B, Mirza F, Flood P: Patient choice compared with no choice of intrathecal morphine dose for caesarean analgesia: a randomized clinical trial. Br J Anaesth. 2017;118(5):762–71. 10.1093/bja/aex039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Balki M, Carvalho JC: Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth. 2005;14(3):230–41. 10.1016/j.ijoa.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 64. Habib AS, George RB, McKeen DM, et al. : Antiemetics added to phenylephrine infusion during cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2013;121(3):615–23. 10.1097/AOG.0b013e3182839fee [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Wu JI, Lo Y, Chia YY, et al. : Prevention of postoperative nausea and vomiting after intrathecal morphine for Cesarean section: a randomized comparison of dexamethasone, droperidol, and a combination. Int J Obstet Anesth. 2007;16(2):122–7. 10.1016/j.ijoa.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 66. Habib AS, Gan TJ: The use of droperidol before and after the Food and Drug Administration black box warning: a survey of the members of the Society of Ambulatory Anesthesia. J Clin Anesth. 2008;20(1):35–9. 10.1016/j.jclinane.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 67. Committee on Obstetric Practice, American College of Obstetricians and Gynecologists: Committee Opinion No.543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522–6. 10.1097/01.AOG.0000423817.47165.48 [DOI] [PubMed] [Google Scholar]
- 68. Fogarty M, Osborn DA, Askie L, et al. : Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(1):1–18. 10.1016/j.ajog.2017.10.231 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. McDonald SJ, Middleton P, Dowswell T, et al. : Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evid Based Child Health. 2014;9(2):303–97. 10.1002/ebch.1971 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Committee on Obstetric Practice: Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol. 2017;129(1):e5–e10. 10.1097/AOG.0000000000001860 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Bramson L, Lee JW, Moore E, et al. : Effect of early skin-to-skin mother--infant contact during the first 3 hours following birth on exclusive breastfeeding during the maternity hospital stay. J Hum Lact. 2010;26(2):130–7. 10.1177/0890334409355779 [DOI] [PubMed] [Google Scholar]
- 72. Bigelow A, Power M, MacLellan-Peters J, et al. : Effect of mother/infant skin-to-skin contact on postpartum depressive symptoms and maternal physiological stress. J Obstet Gynecol Neonatal Nurs. 2012;41(3):369–82. 10.1111/j.1552-6909.2012.01350.x [DOI] [PubMed] [Google Scholar]
- 73. Moore ER, Anderson GC, Bergman N, et al. : Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2012; (5):CD003519. 10.1002/14651858.CD003519.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith J, Plaat F, Fisk NM: The natural caesarean: a woman-centred technique. BJOG. 2008;115(8):1037–42; discussion 1042. 10.1111/j.1471-0528.2008.01777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Armbrust R, Hinkson L, von Weizsäcker K, et al. : The Charité cesarean birth: a family orientated approach of cesarean section. J Matern Fetal Neonatal Med. 2016;29(1):163–8. 10.3109/14767058.2014.991917 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. George RB, McKeen D, Chaplin AC, et al. : Up-down determination of the ED (90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth. 2010;57(6):578–82. 10.1007/s12630-010-9297-1 [DOI] [PubMed] [Google Scholar]
- 77. Thomas JS, Koh SH, Cooper GM: Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth. 2007;98(1):116–9. 10.1093/bja/ael302 [DOI] [PubMed] [Google Scholar]
- 78. Su L, Chong Y, Samuel M: Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012; (4):CD005457. 10.1002/14651858.CD005457.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guo J, Long S, Li H, et al. : Early versus delayed oral feeding for patients after cesarean. Int J Gynaecol Obstet. 2015;128(2):100–5. 10.1016/j.ijgo.2014.07.039 [DOI] [PubMed] [Google Scholar]
- 80. Huang H, Wang H, He M: Early oral feeding compared with delayed oral feeding after cesarean section: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29(3):423–9. 10.3109/14767058.2014.1002765 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Hsu YY, Hung HY, Chang SC, et al. : Early oral intake and gastrointestinal function after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2013;121(6):1327–34. 10.1097/AOG.0b013e318293698c [DOI] [PubMed] [Google Scholar]
- 82. Hirose M, Hara Y, Hosokawa T, et al. : The effect of postoperative analgesia with continuous epidural bupivacaine after cesarean section on the amount of breast feeding and infant weight gain. Anesth Analg. 1996;82(6):1166–9. 10.1213/00000539-199606000-00011 [DOI] [PubMed] [Google Scholar]
- 83. Eisenach JC, Pan PH, Smiley R, et al. : Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140(1):87–94. 10.1016/j.pain.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Uchiyama A, Nakano S, Ueyama H, et al. : Low dose intrathecal morphine and pain relief following caesarean section. Int J Obstet Anesth. 1994;3(2):87–91. 10.1016/0959-289X(94)90175-9 [DOI] [PubMed] [Google Scholar]
- 85. Palmer CM, Nogami WM, van Maren G, et al. : Postcesarean epidural morphine: a dose-response study. Anesth Analg. 2000;90(4):887–91. 10.1213/00000539-200004000-00021 [DOI] [PubMed] [Google Scholar]
- 86. Elia N, Lysakowski C, Tramèr MR: Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103(6):1296–304. [DOI] [PubMed] [Google Scholar]
- 87. Maund E, McDaid C, Rice S, et al. : Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106(3):292–7. 10.1093/bja/aeq406 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Mathiesen O, Wetterslev J, Kontinen VK, et al. : Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1182–98. 10.1111/aas.12380 [DOI] [PubMed] [Google Scholar]
- 89. Valentine AR, Carvalho B, Lazo TA, et al. : Scheduled acetaminophen with as-needed opioids compared to as-needed acetaminophen plus opioids for post-cesarean pain management. Int J Obstet Anesth. 2015;24(3):210–6. 10.1016/j.ijoa.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 90. Pavy TJ, Paech MJ, Evans SF: The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg. 2001;92(4):1010–4. 10.1097/00000539-200104000-00038 [DOI] [PubMed] [Google Scholar]
- 91. Ong CK, Seymour RA, Lirk P, et al. : Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–9. [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Abdallah FW, Halpern SH, Margarido CB: Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anaesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012;109(5):679–87. 10.1093/bja/aes279 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Mishriky BM, George RB, Habib AS: Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review and meta-analysis. Can J Anaesth. 2012;59(8):766–78. 10.1007/s12630-012-9729-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Adesope O, Ituk U, Habib AS: Local anaesthetic wound infiltration for postcaesarean section analgesia: A systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(10):731–42. 10.1097/EJA.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 95. Lalmand M, Wilwerth M, Fils JF, et al. : Continuous Ropivacaine Subfascial Wound Infusion Compared With Intrathecal Morphine for Postcesarean Analgesia: A Prospective, Randomized Controlled, Double-Blind Study. Anesth Analg. 2017;125(3):907–12. 10.1213/ANE.0000000000001892 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Blanco R, Ansari T, Riad W, et al. : Quadratus Lumborum Block Versus Transversus Abdominis Plane Block for Postoperative Pain After Cesarean Delivery: A Randomized Controlled Trial. Reg Anesth Pain Med. 2016;41(6):757–62. 10.1097/AAP.0000000000000495 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Krohg A, Ullensvang K, Rosseland LA, et al. : The Analgesic Effect of Ultrasound-Guided Quadratus Lumborum Block After Cesarean Delivery: A Randomized Clinical Trial. Anesth Analg. 2018;126(2):559–65. 10.1213/ANE.0000000000002648 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Fearon KC, Ljungqvist O, von Meyenfeldt M, et al. : Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–77. 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 99. Deniau B, Bouhadjari N, Faitot V, et al. : Evaluation of a continuous improvement programme of enhanced recovery after caesarean delivery under neuraxial anaesthesia. Anaesth Crit Care Pain Med. 2016;35(6):395–9. 10.1016/j.accpm.2015.11.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation