Abstract
Introduction
Children undergoing lung transplant are at risk for low bone mineral density (BMD) and fractures. The effect of lung transplantation on bone health in pediatric patients is unknown.
Materials and Methods
We performed a retrospective chart review of all patients ages 2–21 years who underwent lung transplantation at our hospital from January 2000 to January 2015.
Results
51 patients were studied. At the time of transplant evaluation, BMD Z-score was −2.2±1.4, and 59% of patients had low BMD. BMD Z-score declined in the first year after treatment and returned to near-baseline by the third post-transplant year. Fractures occurred in 9 patients (18%) before and 15 patients (29%) after transplant. Bisphosphonate use was associated with improvement in BMD Z-score and lower mortality risk.
Conclusions
Pediatric patients had a high prevalence of low BMD at the time of lung transplant evaluation. BMD Z-scores declined in the year after transplant and returned to the pre-transplant level by the third post-transplant year while remaining below normal levels. Fractures were common at sites associated with significant morbidity. These findings support efforts to optimize bone health before and after pediatric lung transplantation, and future studies are needed to evaluate the role of bisphosphonates in these patients.
Keywords: Bone mineral density, lung transplantation, fracture, pediatric, bisphosphonate
1. Introduction
Individuals with severe lung disease undergoing evaluation for lung transplantation are at risk for low bone mineral density (BMD) and fractures. Risk factors for compromised bone health in this patient population include glucocorticoid use, decreased physical activity, vitamin D deficiency, and poor nutrition (1, 2). Studies in adults have shown that BMD declines even further after solid organ transplantation, particularly in the initial 6–12 months (1–4). In adults, lung transplantation is associated with a 2–5% reduction in BMD and an 18–37% fracture risk in the first year after lung transplant (3, 5, 6). This bone loss is likely multifactorial, related to immunosuppressant medications, immobilization, and other factors (7). Because of this rapid bone loss occurring after solid organ transplant, adults are often treated with medications such as bisphosphonates in order to preserve BMD and prevent post-transplant fracture (2, 8, 9).
The effect of lung transplantation on BMD and fracture risk in children and adolescents is currently unknown. Like adults, children have a high prevalence of low bone density prior to solid organ transplant (10), though there are limited data regarding children with lung disease in particular. Children with end stage pulmonary disease have many of the same risk factors for compromised skeletal health as adults, but they also may experience uniquely pediatric issues such as pubertal delay, compromised growth, and short stature (11). There have been no studies published to date evaluating the natural history of BMD changes after lung transplantation in pediatric patients, and whether lung transplantation improves or worsens skeletal health in children and adolescents is currently unknown. We hypothesized that children and adolescents may experience significant clinical improvement after transplant leading to substantial gains in BMD greater than expected for age, potentially bringing BMD into a more normal range.
Boston Children’s Hospital has one of the largest pediatric lung transplant centers in the United States and routinely monitors BMD of pediatric patients both before and after lung transplantation. The goal of this study was to determine the prevalence of low bone density and fractures in a large cohort of pediatric patients undergoing lung transplant evaluation, identify risk factors for low bone density and fractures in this population, and determine the changes in BMD and BMD Z-score occurring after transplant in this population.
2. Materials and Methods
2.1 Patient Population
This was a retrospective chart review examining all patients ages 2–21 years old who underwent lung transplantation at Boston Children’s Hospital from January 2000 until January 2015. Patients were excluded if they had a solid organ transplant prior to January 2000. The protocol was approved by the hospital institutional review board.
The standard lung transplantation evaluation at our institution includes a pre-transplant clinic visit with full medical evaluation pulmonary function tests, laboratory evaluation including 25-hydroxyvitamin D levels, and assessment of BMD by DXA. As part of routine post-transplant surveillance, DXA scans along with routine laboratory testing are obtained annually in lung transplant patients. The majority, but not all, patients received induction with anti-thymocyte globulin followed by triple immunosuppression therapy with glucocorticoids, mycophenolate, and tacrolimus. In cases with biopsy proven or suspected acute vascular rejection, patients were treated with high dose glucocorticoids, generally 20–30 mg/kg/day for 3 days (with a maximum of 1000mg/day). Over the 15-year period of this study, there were minimal changes in lung transplant protocols at our institution. The most significant change was the addition of induction therapy for most but not all patients included in the study. All patients diagnosed with low BMD are referred to a pediatric endocrinologist, and bisphosphonate treatment is considered on a case-by-case basis.
2.2 Clinical Data Collection
All data were obtained from the electronic medical record by a single physician investigator trained in the interpretation of pediatric DXA scans. Baseline characteristics of patients were recorded from the patients’ clinic visits at the time of the initial transplant evaluation, including results of the DXA scan obtained at the closest time point to the pre-transplant evaluation. The results of each subsequent DXA scan were obtained up to a total of four scans per patient. If a patient had more than four scans, the most recently acquired scan was included as the fourth and final scan in order to capture long-term BMD changes. If the patient required re-transplantation, only the first transplant was considered in the analysis, and all subsequent transplants were included as part of the follow-up period. At the time of each DXA scan, clinical and laboratory data were extracted from the medical record including anthropometric measures, medications (including current glucocorticoid use in mg/kg), and lab results. The number of pathology-confirmed acute or chronic rejection episodes occurring during the time period between DXA scans was determined. Patient charts were electronically searched for all fractures occurring in the pre- or post-transplant period, and fractures were confirmed by radiology report in the chart. If applicable, date and cause of death was recorded.
2.3 Assessment of areal BMD
DXA scans were performed at the Boston Children’s Hospital DXA Center. Since 2000, the original Hologic 4500 model was replaced by a Hologic Discovery A model and ultimately a Hologic Horizon A model, with appropriate cross-calibration studies. Z-scores were determined using standard Hologic reference databases. Given that data were collected over a 15-year period, the recommended sites for DXA imaging of pediatric patients changed over time. Although total body less head (TBLH) and spine are currently the recommended imaging sites in children and adolescents (12), many patients imaged prior to this recommendation in 2013 were scanned at sites that were previously but are no longer are recommended, including the hip and whole body. For the baseline assessment of BMD status, BMD Z-scores were obtained from all imaged sites of each baseline DXA scan (total hip, whole body, TBLH, and/or spine), and the lowest Z-score for each scan was used to characterize bone density status for each patient as low (BMD Z-score −2.0 or below) or normal (BMD Z-score −1.9 or above). For longitudinal analyses, data from the spine and total hip were analyzed because the most data were available at these sites, and BMD changes at other DXA sites (total body and TBLH) were not reported due to the limited number of available scans that included these sites.
2.4 Statistical Analyses
Statistical analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC). For patients older than 20 years, Z-scores for anthropometric measures (height, weight, and BMI) were calculated using the 20 year CDC reference data. To evaluate associations between clinical characteristics and lowest BMD Z-score at baseline, correlations were performed using Pearson correlation coefficient, or Spearman correlation coefficient in the case of non-normally distributed data. Logistic regression was used to determine significant predictors of pre- or post-transplant fractures.
For longitudinal analyses, repeated measures analyses were used to determine change over time in absolute BMD and BMD Z-scores at the total hip and spine, accounting for within- subject correlation using compound-symmetric covariance structure. Scans were categorized into time points based on how many years from transplant they had occurred, and all scans obtained more than five years after transplant (median 5.4 years, range 5–13 years) were combined into a single time point given the small number of available scans. Unlike in adults, in whom BMD would be expected to remain roughly stable until menopause, absolute BMD is expected to increase with time in growing children; therefore, changes in BMD Z-score were reported to capture whether changes in BMD were at, above, or below that expected for age. Number of rejection episodes, height Z-score, and 25(OH)D levels were noted to significantly affect BMD Z-score change on univariate screen; therefore, multivariable analyses were performed adjusting for these variables. Change in BMD Z-score in the subset of patients treated with bisphosphonates was assessed using paired t-tests. Unadjusted Cox regression was used to calculate hazard ratios with 95% confidence intervals (CI) for the effect of fractures or bisphosphonate use on mortality.
Data are reported as mean ± standard deviation (SD) unless otherwise noted, and p values <0.05 are considered statistically significant.
3. Results
3.1 Pre-Transplant Patient Characteristics and Bone Density Results
Patient characteristics at the time of their pre-transplant clinical evaluation are presented in Table 1. A majority of patients had cystic fibrosis (CF) as the etiology of end stage pulmonary disease, followed by pulmonary hypertension and graft vs. host disease (GVHD) secondary to bone marrow transplantation. At the time of transplant, patients were on average 14.7 ± 4.8 years old, with ages ranging 4 to 20 years old, and slightly more than half were female. Patients were shorter than the population average with a mean height Z-score of −1.1 ± 1.4. A majority of patients (71%) reported taking vitamin D supplementation, and mean 25(OH)D level was 29.7 ± 10.6 ng/mL. Fifty-two percent of patients had a 25(OH)D level at or above 30ng/mL at the time of lung transplant evaluation. Three patients had multiple organs transplanted (lung and liver), and five patients required re-transplantation after their original transplant.
Table 1.
Patient Characteristics prior to Lung Transplantation
| Clinical Parameter | Mean ± SD or n (%)
|
||||||
|---|---|---|---|---|---|---|---|
| Entire Cohort (n=51) |
CF (n=31) |
Pulmonary HTN (n=8) |
GVHD (n=7) |
Pulmonary Fibrosis (n=3) |
Other (n=2) |
||
| Gender | Female | 26 (51) | 18 (58) | 4 (50) | 1 (14) | 1 (33) | 1 (50) |
| Male | 25 (49) | 13 (42) | 4 (50) | 6 (86) | 2 (67) | 1 (50) | |
|
| |||||||
| Age at transplant, y | 14.7 ± 4.8 | 15.7 ± 4.4 | 11.9 ± 5.5 | 16.2 ± 2.6 | 14.3 ± 4.8 | 5.6 ± 0.1 | |
|
| |||||||
| Race/Ethnicity | White | 36 (71) | 23 (74) | 4 (50) | 5 (71) | 3 (100) | 1 (50) |
| Black | 2 (4) | 0 | 1 (12.5) | 1 (14) | 0 | 0 | |
| Hispanic | 5 (10) | 3 (10) | 1 (12.5) | 1 (14) | 0 | 0 | |
| Asian | 2 (4) | 0 | 1 (12.5) | 0 | 0 | 1 (50) | |
| Other | 6 (12) | 5 (16) | 1 (12.5) | 0 | 0 | 0 | |
|
| |||||||
| FEV1, % predicted | 32 ± 11 | 35.8 ± 8.6 | — | 19.0 ± 7.3 | 20.5 ± 10.6 | — | |
|
| |||||||
| Height Z-score | −1.1 ± 1.4 | −1.1 ± 1.1 | 0.2 ± 1.3 | −2.0 ± 1.7 | −2.5 ± 0.7 | −3.2 ± 1.2 | |
|
| |||||||
| BMI Z-score | −0.8 ± 1.3 | −1.0 ± 1.1 | 0.4 ± 1.2 | −1.4 ± 1.2 | −0.8 ± 2.0 | −0.5 ± 0.2 | |
|
| |||||||
| Laboratory evaluation | 25(OH)D, ng/mL | 29.7 ± 12.6 | 31.7 ± 9.9 | 17.9 ± 6.8 | 39.3 ± 19.9 | 20.0 ± 15.5 | 23.4 ± 4.7 |
| PTH, ng/mL | 37.2 ± 3.1 | 33.1 ± 19.0 | 81.0 ± 55 | 22.4 ± 34 | 27.8 ± 14 | 30 ± 4.9 | |
| HbA1c, % | 6.3 ± 1.4 | 6.6 ± 1.5 | 5.4 | 5.4 ± 0.8 | 5.7 | — | |
|
| |||||||
| BMD Z-score | Lowest site | −2.2 ± 1.4 | −1.8 ± 1.1 | −1.2 ± 2.2 | −3.4 ± 2.2 | −2.9 ± 0.5 | −2.8 |
| Spine | −1.6 ± 1.1 | −1.7 ± 1.1 | −1.0 ± 2.5 | −2.0 ± 1.0 | −1.4 | −2.2 | |
| Hip | −1.8 ± 1.2 | −1.4 ± 0.8 | −1.0 ± 1.9 | −2.7 ± 2.6 | −2.9 ± 0.5 | −2.8 | |
|
| |||||||
| Patients with Fractures | Pre-transplant | 9 (18) | 4 (13) | 1 (12.5) | 2 (29) | 1 (33) | 1 (50) |
| Post-transplant | 15 (29) | 9 (29) | 3 (37.5) | 1 (14) | 1 (33) | 1 (50) | |
The mean BMD Z-score for all patients who had a baseline DXA scan prior to transplant (n=27) was −2.2 ± 1.4. Fifty-nine percent of patients had low bone density defined as a BMD Z-score at any site below −2.0.Significant predictors of pre-transplant BMD Z-score were height Z-score (r 0.69, p <0.01) and FEV1 (r 0.42, p 0.04). Median time interval between baseline DXA scans and lung transplantation was 0.9 years (range 0.1–3.8 years). Medical indication for transplant, 25(OH)D level, BMI Z-score, hemoglobin A1c, age, and gender were not significant predictors of pre-transplant BMD Z-score.
3.2 Pre-Transplant Fractures
Nine patients (17%) reported a fracture in the 1.3 ± 1.4 years prior to transplant. These fractures involved the vertebrae in six patients, hip in one patient, wrist in one patient, and ankle in one patient. Medical indication for lung transplantation, gender, age, BMI and height Z-scores, and BMD Z-score were not predictive of pre-transplant fracture (p>0.05 for all), though the small number of patients with fractures limits power to detect significant predictors. Four of the nine patients who reported fractures had a pre-transplant DXA scan, and all four of these patients had low bone density based on this scan, with BMD Z-scores ranging −2.9 to −4.9.
3.3 BMD Z-score changes after transplant
Changes in absolute BMD and BMD Z-score at the spine and hip after lung transplantation are presented in Figure 1 and Figure 2, respectively. There was a mild decline in absolute BMD in the first year after transplant, followed by increases in BMD thereafter. BMD Z-score started off low and then declined further in the first year after transplant, indicating that expected gains in BMD did not occur during this time period. This was followed by increases in BMD Z-score to close to baseline by the third post-transplant year. This pattern was significant at the total hip (p=0.017) but not at the spine (p=0.13) (Figure 2a). Height Z-score, 25(OH)D, and number of acute rejection episodes significantly affected BMD Z-score change over time, and multivariable adjustment for these covariates further strengthened this pattern at the hip (p=0.016) and spine (p=0.07). After multivariable adjustment, BMD Z-score at ≥5 years post-transplant was improved compared to baseline but did not normalize (Figure 2b). Age, gender, glucocorticoid dose (mg/kg) at the time of the scan, BMI Z-score, hemoglobin A1c, multiple organ transplant, re-transplantation, and bisphosphonate use were not significant predictors of BMD change on univariate analysis. There was no change in height or BMI Z-scores after transplant (p=0.99 and p=0.15, respectively), suggesting that patients did not experience significant catch-up growth after transplant (data not shown).
Figure 1.
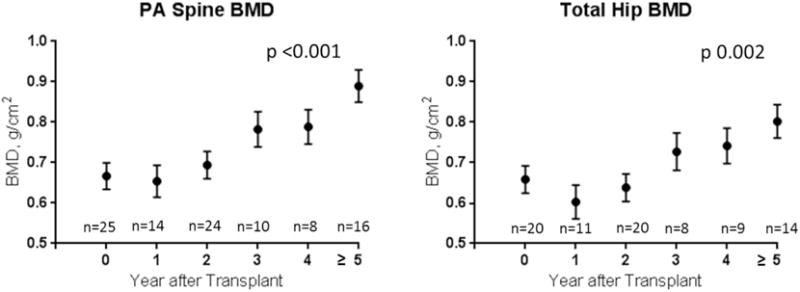
Changes in absolute BMD at the spine and hip after lung transplantation.
Figure 2.
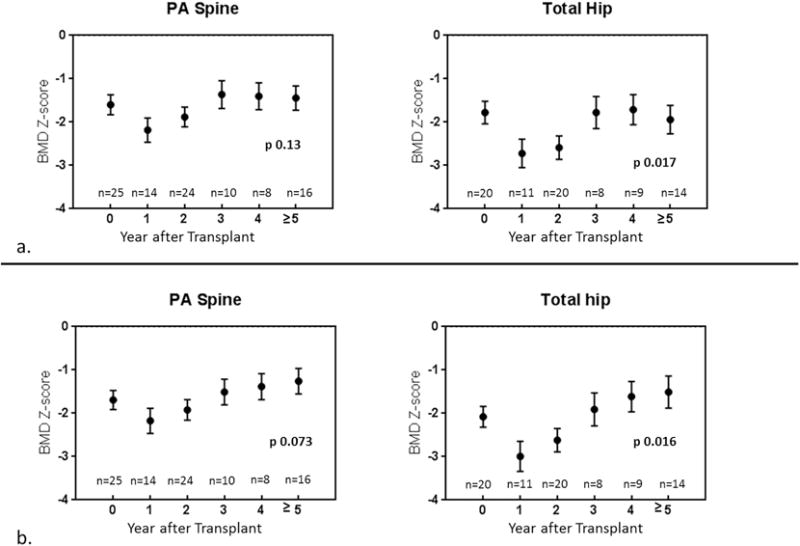
Changes in BMD Z-score at the spine and hip after lung transplantation. Unadjusted results are presented in panel 2a. Multivariable adjusted results after adjustment for 25(OH)D, height Z-score, and number of rejection episodes are presented in panel 2b.
3.4 Fractures after transplant
Fifteen patients (29%) suffered 18 fractures in the post-transplant period, occurring on average 1.6 ± 1.6 years after transplant. These fractures included seven of the spine, three of the hip or pelvis, three of the ribs, three of the upper extremity, and two of the lower extremity. Age, gender, baseline BMD Z-score, indication for transplant, pre-transplant fracture, and baseline height and BMI Z-scores were not significant predictors of post-transplant fracture (p>0.05 for all). Four of the nine patients who had a pre-transplant fracture suffered one or more additional fractures in the post-transplant period.
3.5 Bisphosphonate use
Fourteen patients were treated with bisphosphonates in the peri-transplant period: twelve received alendronate 35mg PO weekly, and two patients received a single dose of pamidronate intravenously. Eight patients started treatment on average 0.4 ± 0.3 years prior to transplant, and six patients started treatment in the post-transplant period approximately 1.5 ± 1.3 years after transplant. The mean duration of treatment for patients on alendronate was 1.9 ± 1.0 years. Indication for bisphosphonate treatment included low BMD alone in eight patients and low BMD plus fracture in six patients. Mean BMD Z-score prior to or at the time bisphosphonate treatment was started was −3.7 ± 1.4. Patients treated with bisphosphonates had a significant increase in lowest BMD Z-score from −3.7 to −2.9 (p=0.04) with treatment; however, six patients incurred fractures despite current or prior bisphosphonate use, all of whom started bisphosphonate treatment in the post-transplant period.
3.6 Mortality
At the time of final data review, 23 patients were still alive (median 5.4 years after transplant, range 2.4–16.4 years), and 28 patients were deceased (median 1.9 years after transplant, range 0–6.2 years). The most common causes of death in these patients were bronchiolitis obliterans and acute respiratory distress syndrome, and none of the deaths were directly related to bone disease. Suffering a fracture in the pre-transplant period was associated with an increased mortality risk of borderline insignificance (HR 2.22, 95% CI 0.92–5.24, p=0.07) whereas use of bisphosphonates before or after transplant was associated with a reduced hazard rate (HR 0.37, 95% CI 0.14–0.98, p=0.046). Occurrence of one or more post-transplant fractures did not have a significant effect on mortality risk.
4. Discussion
Children and adolescents had a high prevalence of low BMD and fractures at the time of lung transplant evaluation. BMD Z-scores declined further in the year after lung transplant and gradually returned to the pre-transplant level by the third post-transplant year, but BMD did not normalize after transplant, even after accounting for height Z-score, vitamin D levels, and rejection episodes. Fractures in the two years after lung transplant were common and occurred at sites associated with significant morbidity. Bisphosphonates may improve BMD and mortality risk in these patients, though the effect on fracture risk is unknown. These findings support the need for increased efforts to optimize bone health in pediatric lung transplant patients, both before and after transplantation.
To our knowledge, this is the first study to evaluate BMD changes and fractures in pediatric lung transplant recipients. The skeletal effects of lung transplantation are much better described in adult patients. The prevalence of low bone density in adults with advanced lung disease prior to lung transplantation has been reported to range from 29–61% (5). Studies suggest that adults experience significant bone loss up to 5% at the hip and spine in the year after transplant, and that this decline in BMD gradually improves over the following year (3, 6). This pattern is similar to what we observed in children, where the first year after transplant led to BMD losses, resulting in further reduction in already low BMD Z-scores. A decline in absolute BMD during childhood and adolescence is notable since BMD should only increase during growth. Like adults, children similarly had gains in BMD and improvement in BMD Z-score in subsequent years after transplant, though not to the normal range. This pattern likely reflects the high dose glucocorticoid use, immobilization, and immunosuppressant treatment in the first year after transplant, which is gradually reduced over time allowing for some degree of recovery of BMD thereafter.
Data evaluating bone density outcomes in children and adolescents receiving other types of solid organ transplants are limited. For example, one study in 13 pediatric patients undergoing cardiac transplantation found that BMD was reduced after transplant, and two patients suffered a vertebral fracture (13). BMD changes in pediatric renal transplant recipients are variable (13–18), likely affected by changes in vitamin D metabolism and hyperparathyroidism after transplant. In contrast, children and adolescents undergoing liver transplantation had low BMD prior to transplant but then experienced significant increases in BMD Z-scores to the normal range in the post-transplant period, likely related to improvements in vitamin D metabolism as well as significant catch-up growth and increases in IGF-1 levels (19–23). Unlike pediatric liver transplant patients, pediatric lung transplant patients in our study did not exhibit significant catch-up growth, and both height and BMD Z-scores remained low after transplant.
In our study, fractures occurring before and after lung transplant were common and occurred at a similar rate as that experienced by adult solid organ transplant recipients (approximately 30%) (24–26). Although even healthy children may be prone to fractures during childhood, in our cohort of patients these fractures occurred at sites that are not typical childhood fracture sites, most frequently involving the spine, hip, or ribs. Fractures at these sites can result in significant morbidity, leading to kyphosis, reduced lung volumes, poor airway clearance, and prolonged immobilization.
The etiology of compromised skeletal health in pediatric lung transplant patients is likely multifactorial, affected by underlying disease status, poor nutrition, decreased physical activity, medication use, and impaired growth. In our study, pre-transplant FEV1 was directly correlated with BMD Z-score, which is consistent with other studies in CF (27–30) and other pulmonary conditions (31, 32). In addition, the number of rejection episodes significantly impacted change in BMD Z-score, which likely reflects the cumulative dose of glucocorticoids patients received on top of standard immunosuppressive doses. Steroid treatment may also impair linear growth and height gain, leading to smaller bone size and increased fragility.
Interpretation of pediatric DXA scans can be confounded by height differences, since smaller bones appear less dense on two dimensional DXA imaging. The patients in this cohort were shorter on average than the general population, and this may contribute to their low BMD. To account for the confounding effect of short stature on BMD Z-score, we adjusted the longitudinal analyses for height Z-scores, and this led to some attenuation in BMD Z-score change, though not to the normal range. The fact that height Z-scores did not improve after transplant suggests that pediatric lung transplant patients may not experience significant catch-up growth, and this may contribute to the lack of BMD normalization. Although shorter height may lead to lower BMD Z-scores, the high fracture rate in these patients suggests that low BMD in this cohort does in fact reflect true bone fragility and is not merely an artifact due to short stature.
This study also presents the largest retrospective review of bisphosphonate use in pediatric solid organ transplant recipients. Although bisphosphonates have been shown to be an effective treatment for glucocorticoid induced osteoporosis and for the prevention of bone loss after solid organ transplant in adults (8, 35–38), data in children are limited. Of the fourteen patients treated with bisphosphonates in this study, BMD Z-scores improved significantly with treatment. However, more than half of these patients fractured despite treatment, which likely reflects the severity of their underlying bone disease. There were no cases of osteonecrosis of the jaw, atypical femur fractures, or other severe adverse events associated with bisphosphonate use in this population, though treatment was typically limited to a relatively short period of approximately two years. These findings support the need for larger, prospective studies in pediatric patients to determine the safety and efficacy of bisphosphonate use in this population.
In this cohort, more than half of patients died within six years of their lung transplant, though several patients remain alive as long as 16 years after transplant. There was a suggestion of a higher risk of mortality in patients who suffered a fracture in the pre-transplant period, though not in the post-transplant period. This may in part reflect the degree of illness and fragility of these patients, predisposing them to fracture even before undergoing transplantation, rather than an effect of the fracture itself on mortality. The fact that bisphosphonate use appeared protective in terms of mortality is interesting and warrants further investigation with a well-designed prospective clinical trial.
The strengths of this study include the relatively large number of pediatric lung transplant recipients studied, though absolute numbers remain small, and the detailed clinical characteristics and densitometry results obtained from the electronic medical record. However, there are also limitations that should be considered. First, the retrospective nature of the study may lend itself to unintended bias by affecting which patients underwent DXA scanning. Although DXA scans were routinely recommended before and after transplant in all patients, not all patients had the recommended scans, likely related to competing medical priorities. In addition, pubertal delay, patient compliance with prescribed bisphosphonate therapy, and physical activity levels before and after transplant could not be assessed retrospectively in this study. Although dose of glucocorticoid at the time of each DXA scan was obtained, specific cumulative steroid exposure for each subject could not be estimated by chart review. Also, although the ISCD recommends the use of height Z-score-adjusted BMD Z-scores in children with delayed maturation or linear growth (12), which can be calculated using the Bone Mineral Density in Childhood Study (BMDCS) reference database (39), a majority of BMD data were extracted from scans not obtained with Hologic software versions 12.1 or higher and therefore could not be used to calculate height Z-score-adjusted BMD Z-scores. In addition, DXA reference populations used for generating Z-scores have changed over this time period, which may affect Z-score calculations. There may also be an era effect due to evolving transplant practices over this time period. Finally, although TBLH and spine are currently the recommended DXA imaging sites in children and adolescents, a majority of patients in this study had total hip and spine measurements as per older ISCD recommendations, and there were not enough data to present change in TBLH BMD. Total hip results should be regarded with caution given the recent ISCD guidelines indicating that this site is not reliable in growing children (12); however, the fact that hip and spine results were consistent with each other is compelling and suggests that these data provide useful insight into skeletal changes post-transplant.
5. Conclusions
Lung transplantation has a detrimental effect on skeletal health in children and adolescents, and these patients are at high risk for fracture before and after transplant. BMD Z-scores fall in the first year after transplantation followed by some degree of recovery; however, BMD Z-scores remain compromised even as long as five or more years after transplant. Future prospective studies will be needed to confirm these findings and to evaluate the role of bisphosphonates in the care of pediatric lung transplant recipients. Increased attention to screening for low bone density and optimizing bone health in children will be important prior to and after lung transplantation.
Highlights.
Children have a high risk of low BMD and fractures before and after lung transplant.
BMD Z-scores fall in the first year after transplant followed by some degree of recovery.
However, BMD Z-scores remain low even as long as five or more years after transplant.
Increased attention to optimizing bone health in these patients will be important.
Acknowledgments
Funding source: This work was supported by NIH K23 DK102600-01A1.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMD
bone mineral density
- BMI
body mass index
- CF
cystic fibrosis
- DXA
dual energy Xray absorptiometry
- FEV1
forced expiratory volume in 1 second
- GVHD
graft versus host disease
- PTH
parathyroid hormone
- TBLH
total body less head
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The authors have no conflicts of interest to disclose.
Authorship Statement
Dr. Putman developed the data collection instruments and database, performed the chart review, performed statistical analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Drs. Simoneau, Boyer, and Haagensen conceptualized and designed the study, assisted with data review and interpretation, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Feldman assisted with database creation, advised and supervised statistical analyses, assisted with data review and interpretation, critically reviewed the manuscript, and approved the final manuscript as submitted.
References
- 1.Shane E, Silverberg SJ, Donovan D, Papadopoulos A, Staron RB, Addesso V, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101(3):262–9. doi: 10.1016/S0002-9343(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 2.Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab. 2005;90(4):2456–65. doi: 10.1210/jc.2004-1978. [DOI] [PubMed] [Google Scholar]
- 3.Spira A, Gutierrez C, Chaparro C, Hutcheon MA, Chan CK. Osteoporosis and lung transplantation: a prospective study. Chest. 2000;117(2):476–81. doi: 10.1378/chest.117.2.476. [DOI] [PubMed] [Google Scholar]
- 4.Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab. 1997;82(5):1497–506. doi: 10.1210/jcem.82.5.3940. [DOI] [PubMed] [Google Scholar]
- 5.Stein E, Ebeling P, Shane E. Post-transplantation osteoporosis. Endocrinology and metabolism clinics of North America. 2007;36(4):937–63. viii. doi: 10.1016/j.ecl.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari SL, Nicod LP, Hamacher J, Spiliopoulos A, Slosman DO, Rochat T, et al. Osteoporosis in patients undergoing lung transplantation. Eur Respir J. 1996;9(11):2378–82. doi: 10.1183/09031936.96.09112378. [DOI] [PubMed] [Google Scholar]
- 7.Epstein S. Post-transplantation bone disease: the role of immunosuppressive agents and the skeleton. J Bone Miner Res. 1996;11(1):1–7. doi: 10.1002/jbmr.5650110102. [DOI] [PubMed] [Google Scholar]
- 8.Shane E, Addesso V, Namerow PB, McMahon DJ, Lo SH, Staron RB, et al. Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med. 2004;350(8):767–76. doi: 10.1056/NEJMoa035617. [DOI] [PubMed] [Google Scholar]
- 9.Aris RM, Lester GE, Renner JB, Winders A, Denene Blackwood A, Lark RK, et al. Efficacy of pamidronate for osteoporosis in patients with cystic fibrosis following lung transplantation. Am J Respir Crit Care Med. 2000;162(3 Pt 1):941–6. doi: 10.1164/ajrccm.162.3.2002051. [DOI] [PubMed] [Google Scholar]
- 10.Chesney RW, Rose PG, Mazess RB. Persistence of diminished bone mineral content following renal transplantation in childhood. Pediatrics. 1984;73(4):459–66. [PubMed] [Google Scholar]
- 11.Saland JM. Osseous complications of pediatric transplantation. Pediatric transplantation. 2004;8(4):400–15. doi: 10.1111/j.1399-3046.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CM, Leonard MB, Zemel BS, International Society for Clinical D 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17(2):219–24. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Daniels MW, Wilson DM, Paguntalan HG, Hoffman AR, Bachrach LK. Bone mineral density in pediatric transplant recipients. Transplantation. 2003;76(4):673–8. doi: 10.1097/01.TP.0000076627.70050.53. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney international. 1998;53(5):1358–64. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 15.Reusz GS, Szabo AJ, Peter F, Kenesei E, Sallay P, Latta K, et al. Bone metabolism and mineral density following renal transplantation. Arch Dis Child. 2000;83(2):146–51. doi: 10.1136/adc.83.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis EN, Floyd-Gimon DM, Berry PL, Wells TG, Seibert J, Belsha C. Risk factors for bone mineral density loss in pediatric renal transplant patients. Pediatric transplantation. 2000;4(2):146–50. doi: 10.1034/j.1399-3046.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 17.Feber J, Cochat P, Braillon P, Castelo F, Martin X, Glastre C, et al. Bone mineral density after renal transplantation in children. J Pediatr. 1994;125(6 Pt 1):870–5. doi: 10.1016/s0022-3476(05)82001-3. [DOI] [PubMed] [Google Scholar]
- 18.Behnke B, Altrogge H, Delling G, Kruse HP, Muller-Wiefel DE. Bone mineral density in pediatric patients after renal transplantation. Clinical nephrology. 1996;46(1):24–9. [PubMed] [Google Scholar]
- 19.D’Antiga L, Moniz C, Buxton-Thomas M, Cheeseman P, Gray B, Abraha H, et al. Bone mineral density and height gain in children with chronic cholestatic liver disease undergoing transplantation. Transplantation. 2002;73(11):1788–93. doi: 10.1097/00007890-200206150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Okajima H, Shigeno C, Inomata Y, Egawa H, Uemoto S, Asonuma K, et al. Long-term effects of liver transplantation on bone mineral density in children with end-stage liver disease: a 2-year prospective study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9(4):360–4. doi: 10.1053/jlts.2001.50038. [DOI] [PubMed] [Google Scholar]
- 21.Argao EA, Balistreri WF, Hollis BW, Ryckman FC, Heubi JE. Effect of orthotopic liver transplantation on bone mineral content and serum vitamin D metabolites in infants and children with chronic cholestasis. Hepatology. 1994;20(3):598–603. [PubMed] [Google Scholar]
- 22.Ulivieri FM, Lisciandrano D, Gridelli B, Lucianetti A, Roggero P, Nebbia G, et al. Bone mass and body composition in children with chronic cholestasis before and after liver transplantation. Transplantation proceedings. 1999;31(5):2131–4. doi: 10.1016/s0041-1345(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 23.D’Antiga L, Ballan D, Luisetto G, Cillo U, Guariso G, Zancan L. Long-term outcome of bone mineral density in children who underwent a successful liver transplantation. Transplantation. 2004;78(6):899–903. doi: 10.1097/01.tp.0000136987.38729.c0. [DOI] [PubMed] [Google Scholar]
- 24.Shane E, Rivas M, Staron RB, Silverberg SJ, Seibel MJ, Kuiper J, et al. Fracture after cardiac transplantation: a prospective longitudinal study. J Clin Endocrinol Metab. 1996;81(5):1740–6. doi: 10.1210/jcem.81.5.8626827. [DOI] [PubMed] [Google Scholar]
- 25.Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. European journal of gastroenterology & hepatology. 2000;12(8):931–5. doi: 10.1097/00042737-200012080-00013. [DOI] [PubMed] [Google Scholar]
- 26.Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357(9253):342–7. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Jaksic M, Fenwick S, Byrnes C, Cundy T. Accrual of Bone Mass in Children and Adolescents With Cystic Fibrosis. J Clin Endocrinol Metab. 2017;102(5):1734–9. doi: 10.1210/jc.2016-3459. [DOI] [PubMed] [Google Scholar]
- 28.Aris RM, Renner JB, Winders AD, Buell HE, Riggs DB, Lester GE, et al. Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med. 1998;128(3):186–93. doi: 10.7326/0003-4819-128-3-199802010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Elkin SL, Fairney A, Burnett S, Kemp M, Kyd P, Burgess J, et al. Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int. 2001;12(5):366–72. doi: 10.1007/s001980170104. [DOI] [PubMed] [Google Scholar]
- 30.Haworth CS, Selby PL, Webb AK, Dodd ME, Musson H, Mc LNR, et al. Low bone mineral density in adults with cystic fibrosis. Thorax. 1999;54(11):961–7. doi: 10.1136/thx.54.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jastrzebski D, Lutogniewska W, Ochman M, Margas A, Kowalski K, Wyrwol J, et al. Osteoporosis in patients referred for lung transplantation. European journal of medical research. 2010;15(Suppl 2):68–71. doi: 10.1186/2047-783X-15-S2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tschopp O, Boehler A, Speich R, Weder W, Seifert B, Russi EW, et al. Osteoporosis before lung transplantation: association with low body mass index, but not with underlying disease. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(2):167–72. doi: 10.1034/j.1600-6143.2002.020208.x. [DOI] [PubMed] [Google Scholar]
- 33.Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Seminars in arthritis and rheumatism. 2014;44(1):47–54. doi: 10.1016/j.semarthrit.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodd C, Lang B, Ramsay T, Alos N, Huber AM, Cabral DA, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis care & research. 2012;64(1):122–31. doi: 10.1002/acr.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford BA, Kam C, Pavlovic J, Byth K, Handelsman DJ, Angus PW, et al. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(4):239–48. doi: 10.7326/0003-4819-144-4-200602210-00005. [DOI] [PubMed] [Google Scholar]
- 36.Pennisi P, Trombetti A, Giostra E, Mentha G, Rizzoli R, Fiore CE. Pamidronate and osteoporosis prevention in liver transplant recipients. Rheumatology international. 2007;27(3):251–6. doi: 10.1007/s00296-006-0196-2. [DOI] [PubMed] [Google Scholar]
- 37.Krieg MA, Seydoux C, Sandini L, Goy JJ, Berguer DG, Thiebaud D, et al. Intravenous pamidronate as treatment for osteoporosis after heart transplantation: a prospective study. Osteoporos Int. 2001;12(2):112–6. doi: 10.1007/s001980170142. [DOI] [PubMed] [Google Scholar]
- 38.Stein EM, Ortiz D, Jin Z, McMahon DJ, Shane E. Prevention of fractures after solid organ transplantation: a meta-analysis. J Clin Endocrinol Metab. 2011;96(11):3457–65. doi: 10.1210/jc.2011-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.https://bmdcs.nichd.nih.gov/zscore.htm.


