Abstract
Obesity and respiratory disorders are major health problems. Obesity is becoming an emerging epidemic with an expected number of over 1 billion obese individuals worldwide by 2030, thus representing a growing socioeconomic burden. Simultaneously, obesity-related comorbidities, including diabetes as well as heart and chronic lung diseases, are continuously on the rise. Although obesity has been associated with increased risk for asthma exacerbations, worsening of respiratory symptoms, and poor control, the functional role of obesity and perturbed metabolism in the pathogenesis of chronic lung disease is often underestimated, and underlying molecular mechanisms remain elusive. This article aims to present methods to assess the effect of obesity on metabolism, as well as lung structure and function. Here, we describe three techniques for mice studies: (1) assessment of intraperitoneal glucose tolerance (ipGTT) to analyze the effect of obesity on glucose metabolism; (2) measurement of airway resistance (Res) and respiratory system compliance (Cdyn) to analyze the effect of obesity on lung function; and (3) preparation and fixation of the lung for subsequent quantitative histological assessment. Obesity-related lung diseases are probably multifactorial, stemming from systemic inflammatory and metabolic dysregulation that potentially adversely influence lung function and the response to therapy. Therefore, a standardized methodology to study molecular mechanisms and the effect of novel treatments is essential.
Keywords: Immunology and Infection, Issue 133, Glucose Tolerance Test, Lung Function, Lung Fixation, Obesity, Airway Resistance, Dynamic Compliance, Chronic Lung Disease
Introduction
According to the World Health Organization (WHO) in 2008, more than 1.4 billion adults, aged 20 and older, were overweight with a body mass index (BMI) greater than or equal to 25; further, over 200 million men and nearly 300 million women were obese (BMI≥30)1. Obesity and metabolic syndrome are major risk factors for a multitude of diseases. While obesity and concomitant increased white adipose tissue mass has been intimately linked to type 2 diabetes2,3, cardio-vascular diseases including coronary heart disease (CHD), heart failure (HF), atrial fibrillation4 and osteoarthritis5, their functional roles in the pathogenesis of respiratory disorders remain poorly understood. However, epidemiological studies have demonstrated that obesity is strongly associated with chronic respiratory conditions, including exertional dyspnea, obstructive sleep apnea syndrome (OSAS), obesity hypoventilation syndrome (OHS), chronic obstructive pulmonary disease (COPD), pulmonary embolism, aspiration pneumonia and bronchial asthma6,7,8,9. Potential mechanisms linking obesity and perturbed metabolism, e.g., insulin resistance and type II diabetes, to the pathogenesis of chronic lung disease not only comprise mechanical and physical consequences of weight gain on ventilation but also induce a chronic subacute inflammatory state10,11. The rise of obesity and lung diseases during the last decade, coupled with the lack of effective preventive strategies and therapeutic approaches, highlights the need to investigate the molecular mechanisms to define new avenues to manage obesity-related lung diseases.
Here, we describe three standard tests, which are important basics to investigate obesity and its impact on lung structure and function in mouse models: (1) intraperitoneal glucose tolerance (ipGTT) (2) measurement of airway resistance (Res) and respiratory system compliance (Cdyn); and (3) preparation and fixation of the lung for subsequent quantitative histological assessment. The ipGTT is a robust screening test to measure glucose uptake, and thus the effect of obesity on metabolism. The simplicity of the method allows good standardization, and therefore the comparability of results between laboratories. More sophisticated methods, such as hyperglycemic clamps or studies on isolated islets, can be used for detailed analysis of the metabolic phenotype12. Here we assess glucose tolerance to define an obesity-associated state of systemic and metabolic disorder as the basis for further studies on a pulmonary outcome. To assess the effect of obesity and metabolic disorder on lung function, we measured airway resistance (Res) and respiratory system compliance (Cdyn). To characterize lung disease, unrestrained as well as restrained methods for assessment of lung function are available. Unrestrained plethysmography in freely moving animals mimics a natural state, reflecting breathing patterns; in contrast, invasive methods, such as input impedance measurement of Res and cDyn in deeply anesthetized mice to assess dynamic lung mechanics, are more accurate13. Since chronic respiratory conditions are reflected by histologic alterations of the lung tissue, proper lung fixation for further analysis is imminent. The choice of the method of tissue fixation and preparation depends on the compartment of the lung which will be studied, for example, conducting airways or lung parenchyma14. Here, we describe a method that allows qualitative and quantitative assessment of the conducting airways to study the effect of obesity on asthma development.
Protocol
All animal procedures were conducted in compliance with protocols approved by local government authorities (Land NRW, AZ: 2012.A424), and were in accordance with the German animal welfare law and the regulations on the welfare of animals used for experiments or for other scientific purposes. Since lung function analysis may affect lung structure and therefore subsequent histological analyzes, the measurement of Res and Cdyn and the preparation and fixation of the lung for histomorphometry should be performed in different animals. However, measurement of Res and Cdyn following ipGTT is possible. Since stress during the ipGTT could interfere with the anesthesia needed for lung function tests, a recovery period of approximately 2 weeks after ipGTT is recommended to allow mice to recover from body weight loss and changes in blood parameters12.
1. Preparation for Intraperitoneal Glucose Tolerance Test (ipGTT)
NOTE: After 12 h of fasting, the complete ipGTT takes approximately 2 h.
Since stress influences blood glucose significantly, ensure that both adaption of mice, as well as training of the scientist, are performed.
Transfer the animals to the experimental area under quiet and stress-free conditions.
Consider the application of a hypercaloric diet to induce obesity in mice. See the discussion section for further advice.
Fast animals for 12 h overnight, without limiting access to water. The next day, after 12 h of fasting, prepare the blood glucose meter according to the manufacturer's protocol (see table of materials) by inserting a new test strip into the test strip port.
Incise the tail tip using sterile scissors, while gently retaining the mouse at its tail, and immediately measure the fasted blood glucose by applying a free-flowing blood drop (minimum sample size 0.5 µL) to the test strip of the blood glucose meter. NOTE: A countdown timer starts on the screen after sufficient application of the blood sample. After 4 s, the test result appears on the screen.
Afterwards, weigh and label the animals individually using color marking.
Administer 2 g glucose/kg body weight via intraperitoneal injection. Ensure that the injection volume is 0.1 mL/10 g body weight (27 G needle and 1 cc syringe).
Subsequently, measure blood glucose after 15, 30, 60, and 120 min by applying a drop of free-flowing blood on a new test strip. NOTE: Blood flow can be increased by gentle massaging of the tail tip-wards. If the tail wound encrusts, clean it using a sterile swab soaked with 0.9% sodium chloride solution.
Allow the animals to rest in their home cages with unlimited access to water between the measurements.
2. Lung Function Analysis to Measure Res and cDyn
NOTE: For undisturbed measurement of Res and cDyn, mice need to be ventilated under deep anesthesia. Stress-free animal handling and proper monitoring of anesthesia are essential. For general instructions using sterile techniques, please review the article by Hoogstraten-Miller et al.15
Calibrate the plethysmograph prior to each set of experiments and prepare the study settings within the software (see Table of Materials).
Prior to surgery, deeply anesthetize animals via intraperitoneal injection of Xylazine (10 mg/kg body weight) and Ketamine (100 mg/kg body weight) (27 G needle and 1 cc syringe). Ensure that the injection volume is 0.1 mL/10 g per body weight. NOTE: Since ketamine has a proper analgesic effect in mice, no additional pain treatment is necessary. The invasive tracheal catheter/plethysmograph procedure takes approximately 5-7 minutes, then data acquisition can begin.
Place the mouse in the supine position on a heating pad to maintain the body temperature.
Cover the eyes with ointment to prevent dryness under anesthesia.
Constantly monitor the depth of anesthesia using the toe pinch-response. NOTE: Additional administration of anesthetic might be necessary to maintain a surgical plane of anesthesia.
Moisten the fur of the surgical area in the thyroid region with 70% ethanol.
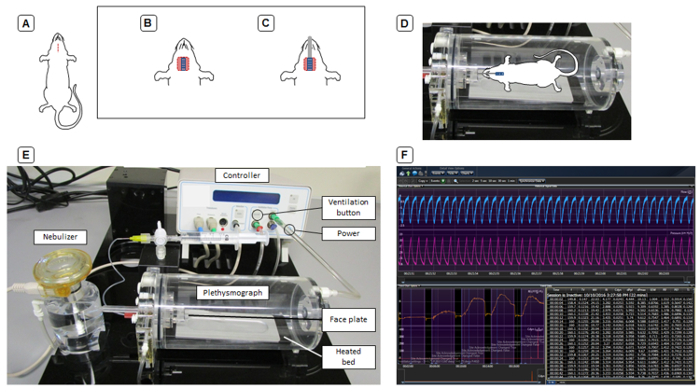
Carefully incise the skin in the midline for approximately 1 cm between the jugular notch of the sternum and the tuber symphyses of the mentum by lifting it with forceps and clipping the skin under visual inspection using blunt scissors (Figure 1A).
Visualize the underlying subcutaneous adipose tissue and thyroid gland.
Expose the trachea by carefully blunt separating both thyroid lobes at the isthmus and dissection of the sternothyroid and sternothyroid muscles (Figure 1B). Be careful not to harm any vessels and cause bleeding, since this can cause adverse effects on the cardiovascular system and ultimately on the measurements.
Subsequently, pass a 4-0 braided surgical suture between the trachea and esophagus using blunt forceps. Carefully incise the trachea close to the larynx between the tracheal cartilages with micro scissors.
Intubate with a tracheal tube (0.04 inch / 1.02 mm diameter) under visual control (Figure 1C). Fix the tube via ligation with the surgical suture to avoid any leak in the system.
Next, move the animal to the heated bed of the body chamber and connect the tracheal tube to the face plate (Figure 1D) and turn on the ventilation by pressing the ventilation button on the front panel of the controller (Figure 1E).
Survey the ventilation by observing the thorax movement contemporaneously with the ventilation rate. To confirm proper placement of the tracheal tube, ensure that both sides of the thorax move simultaneously.
Watch the pressure signal on the computer screen (Figure 1F). Ensure that the ventilation curves are uniform. If this is not the case, detach the animal and check the surgery side. Beware of blood or mucus blocking the tracheal tube. NOTE: For adult animals with a body weight of 20-25 g, the ventilator settings as shown in Figure 2 are suggested in accordance with the manufacturer's recommendations.
To control changes in the trans-pulmonary pressure during ventilation, insert an esophageal tube (0.04 inch / 1.02 mm diameter) into the esophagus at the depth that approximates the levels of the lungs. Watch the screen while placing the tube. Place the tube where maximal pressure deflection and minimal heart artifacts can be seen on the screen.
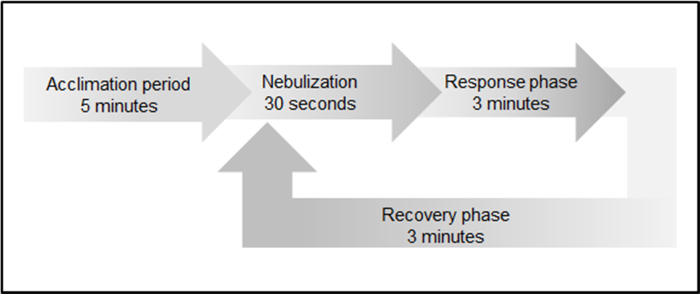
After surgery, prepare the animal for the measurement. Reinject anesthesia via intraperitoneal injection of Ketamine (100 mg/kg body weight) using a 27-G needle and 1 cc syringe. Ensure that the injection volume is 0.1 mL/10 g per body weight. NOTE: To assess bronchial hyperreagibility, nebulize methacholine, a non-selective muscarinic receptor agonist of the parasympathetic nervous system, which induces bronchoconstriction. Data acquisition is performed in four different phases (Figure 3).
Start data acquisition according to the manufacturer´s protocol. NOTE: The software automatically guides users through the acquisition process.
Apply 10 µL of PBS (vehicle) on the nebulizer, and start nebulization after 5 min of acclimation. Next, follow a response phase of 3 min, where Res (cmH2O/mL/s) and cDyn (mL/cmH2O) are measured. At the end, provide a recovery phase of 3 min to the animal prior to the next nebulization.
Follow the software by stepwise application of 10 µL of increasing concentrations of methacholine (2.5 µg/10 µL, 6.25 µg/10 µL, and 12.5 µg/10 µL) on the ventilator.
Once all measurements have been performed and recorded, sacrifice the animal by cervical dislocation.
3. Lung Isolation for Quantitative Histomorphometric Analysis of Adult Mice
Deeply anesthetize the animal via intraperitoneal injection of Xylazine (10 mg/kg body weight) and Ketamine (100 mg/kg body weight) (27 G needle and 1 cc syringe). The injection volume should be 0.1 mL/10 g per body weight. NOTE: After reaching the state of surgical tolerance, the preparation takes approximately 5 min followed by organ perfusion and 30 min for fixation.
Once the animal has reached the state of surgical tolerance (negative toe pinch-response), disinfect the animal with 70% ethanol and fix the animal on a pad with surgical tape.
Sacrifice the animal by cardiac puncture and bleeding. Briefly, open the abdomen with a medial incision through the skin and the peritoneum using blunt scissors.
Locate the diaphragm head wards of the liver, and carefully separate the liver from the diaphragm.
Make a small incision in the diaphragm using blunt scissors, and punctate the left ventricle of the heart with a 20 G needle attached to a 2-mL syringe. Slowly exsanguinate the animal. NOTE: Slow and careful exsanguination is important to prevent the ventricles collapsing due to the negative pressure, inhibiting an undisturbed blood flow.
Dissect the lung by opening the thorax gently through a parasternal incision along the entire length of the rib cage using curved, blunt scissors.
Afterwards, lift the rib cage to expose the pleural cavity (Figure 3C). Remove the thymus to see the heart and lungs. NOTE: Optional injection of the right ventricle, followed by perfusion of the lung vascular system with ice-cold PBS and then with a fixative solution [e.g., 4% (mass/volume) paraformaldehyde (PFA)] is possible. Be aware that there is an increased risk to rupture alveolar septae and adversely affect lung structure using this method.
Dissect the lung by first carefully removing the heart.
Subsequently, pass a 4-0 braided surgical suture between the trachea and esophagus using blunt forceps.
Next, carefully incise the trachea close to the larynx between the tracheal cartilages, intubate with an intravenous cannula (26 G), and inflate the lung by pressure fixation at a constant pressure of 20 cm H2O using fixative agent [e.g., 4% (mass/volume) of PFA].
For PFA fixation, leave the fixative for 30 min at room temperature. Afterwards, ligate the trachea and remove the cannula. Then, excise the lung carefully without harming the tissue, and store it in fixative agent at 4 °C overnight. NOTE: Alternatively, according to the ATS/ETS consensus paper 2.5% GA buffered OsO4, Uracil solution is used for proper tissue stabilization. For further tissue preparation, see the consensus paper by Hsia et al.14
Representative Results
Representative results of intraperitoneal glucose tolerance test (ipGTT) (Figure 4), lung function test (Figure 5), and representative images illustrating hematoxylin and eosin stained lungs (Figure 6).
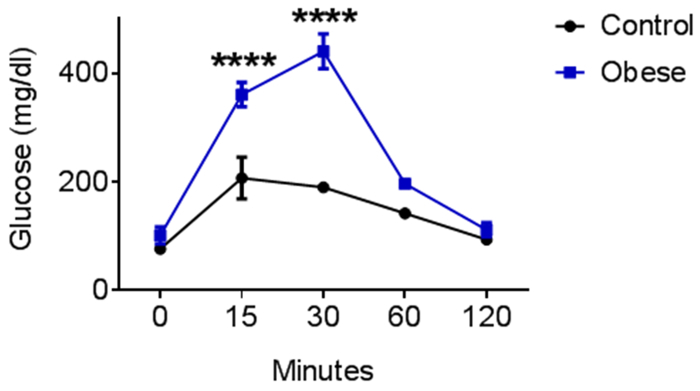
The ipGTT was performed in obese mice (blue) after 7 weeks of high-fat-diet (HFD). Standard diet-fed mice served as controls (black). Obese mice showed increased serum glucose levels 15 and 30 min after intraperitoneal glucose injection, indicating impaired cellular glucose uptake (Figure 4).
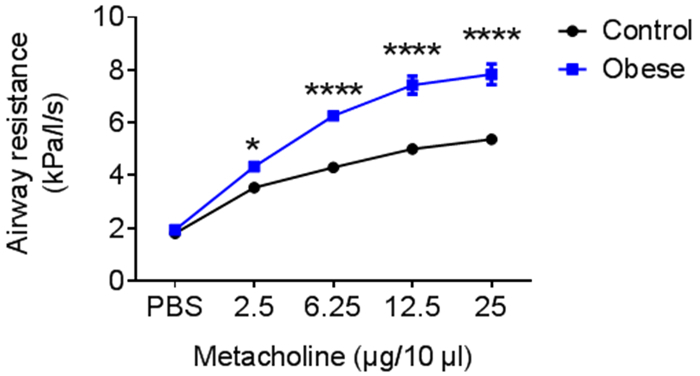
To examine the effect of obesity on lung function, invasive lung function analysis was performed in obese mice (blue) after 7 weeks of high-fat-diet (HFD). Obese mice showed an up to 1.5-fold increase of airway resistance compared to control mice (black) (Figure 5).
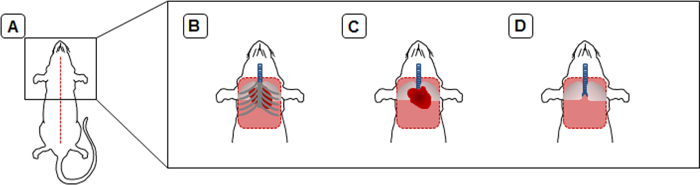
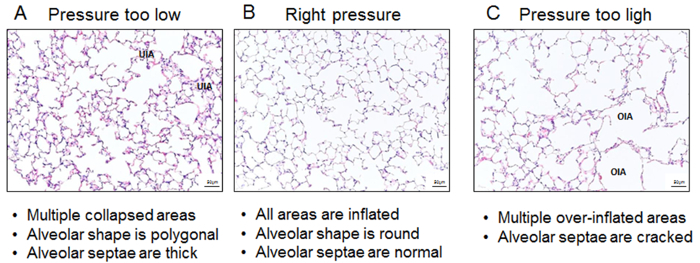
To visualize the effect of pressure fixation during intratracheal instillation of fixatives on lung parenchyma, representative images of hematoxylin and eosin stained lung sections are shown (Figure 6). Too little pressure leads to multiple un-inflated areas, thick alveolar septae, and polygonal shaped alveoli (A), while too much pressure results in over-inflated emphysema-like areas with destructed alveolar septae (C). Application of the appropriate pressure during lung fixation leads to a completely inflated lung with round shaped alveoli (B).
Figure 1: Schematic representation of invasive lung function.(A-C) Steps of tracheotomy. (D) The connection of the animal to the face plate of the plethysmograph. (E) Hardware setup for invasive lung function. (F) Screenshot of data acquisition. Please click here to view a larger version of this figure.
Figure 2: Scheme of data acquisition to assess bronchial hyperreagibility. Data acquisition includes an initial acclimation period (5 min), followed by 30 s of substance nebulization, 3 min of response phase, and 3 min of recovery phase prior nebulization of next substance concentration. Please click here to view a larger version of this figure.
Figure 3: Schematic representation of preparation steps. (A-D) Steps of tracheotomy. (A) Incision of the skin. (B) Situs of the thoracic cavity. (C) View after removal of the rib cage. (D) View after removal of the heart. Please click here to view a larger version of this figure.
Figure 4: Representative intraperitoneal glucose tolerance test (ipGTT). C57Bl/6N mice were fed a high-fat diet for 6-8 weeks; control mice received a standard diet. n = 3; Mean ± SEM; statistical analyses performed were the two-way ANOVA test and Bonferroni posttest. Please click here to view a larger version of this figure.
Figure 5: Representative lung function test. C57Bl/6N mice were fed a high-fat diet for 6-8 weeks; control mice received standard diet. n = 3; Mean ± SEM; statistical analyses performed were the two-way ANOVA test and Bonferroni posttest. Please click here to view a larger version of this figure.
Figure 6: Representative images illustrating hematoxylin & eosin stained lungs. Three different grades of intratracheal inflation: (A) too little pressure, (B) appropriate pressure, and (C) too much pressure. UIA; collapsed area, OIA; over inflated area; Images were taken under 20X magnification. Please click here to view a larger version of this figure.
| Ventilator settings | Spalte1 |
| Max. stroke volume | 0.25ml |
| Max. mouth pressure | 30cmH2O |
| Deep inflation max. volume | 0.5 ml |
| Deep inflation max. pressure | 30cmH2O |
| Rate | 160 breath per minute |
Table 1: Ventilator settings for adult mice. For smaller animals, the ventilation parameters need to be adjusted.
Discussion
This report provides three protocols for three different methods to analyze the impact of obesity on glucose metabolism and pulmonary outcomes. First, the glucose tolerance test offers the opportunity to analyze intracellular glucose uptake and can be indicative of insulin resistance. Second, whole body plethysmography is a technique to measure lung function and is thereby helpful to test the efficacy of novel treatments. Third, a standardized fixation protocol is essential for quantitative morphometric analysis to assess the impact of obesity on structural changes.
Diet-induced obesity in animal research
Significance with respect to existing methods and future applications: To mimic human eating habits, which result in obesity, diet-induced obesity (DIO) models are widely used in rodents. In comparison to the use of genetically modified mice mimicking metabolic disease, DIO models enable analysis of etiology, pathology, and future treatment options. A recent review by Heydemann et al., provides an overview of different murine high-fat diet models in diabetes research16. Specific modifications of nutrient components provide the possibility of future studies of the impact of micro- and macro-nutrients on the molecular mechanisms of obesity, and thereby on lung structure and function.
Modifications and troubleshooting: Various reports show that obesity can be induced by different changes in the composition of dietary nutrients16, knowledge which has, in turn, led to the development of a variety of diets in recent years, for example, diets high in fat or carbohydrate contents or a combination of both diets, which is the so-called Western diet16. In the end, the choice of diet for a study depends on the research question and the aims of the study. Besides controlling for these important factors, the choice of a proper control diet is of utmost importance; otherwise, the interpretation of the results will be limited. Nevertheless, the phenotype is not only caused by the type of diet but is also a result of the feeding period, gender and mouse strain17.
Limitations of the technique: Beside the composition of various nutrients of the diet, the response of the animal and the background strain could account for the results and the phenotype observed by the induction of obesity. For example, it has been shown, that BALB/C mice are more susceptible to liver steatosis compared to C57Bl/6 mice18. Slight genetic variations, e.g., due to genetic drift (C57Bl/6J versus C57Bl/6N), can also affect the susceptibility to obesity19. This highlights the limitations of the technique working with genetically modified mice with different genetic background strains, since even the strain and the vendor from which the mice were obtained may influence the results.
Critical steps within the protocol: For reliable, reproducible, and comparable results between the experimental and control groups, preferably littermates should be used to generate control and experimental mice, environmental factors and stress should be avoided, and control mice should be studied in parallel with experimental mice12.
Intraperitoneal glucose tolerance tests (ipGTT)
Significance with respect to existing methods and future applications: To determine the effect of DIO on murine metabolism, different screening tests exist. The Mouse Metabolic Phenotyping Center (MMPC) Consortium was established to propose standard methods for assessing metabolic phenotypes in mice and has published standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice12. A GTT is a global approach to measure how well the body's cells are able to uptake glucose after ingesting a given amount of sugar, which in turn is indicative of insulin secretion and insulin effect. Differences in serum insulin levels often account for altered glucose tolerance. Therefore, the GTT has become one of the most widely used physiological tests to characterize mouse models of diabetes and obesity. Since the ipGTT is a fast and easy approach, it can be used in future studies as a standardized method to analyze the effect of diet and/or treatment on metabolism.
Modifications and troubleshooting: The GTT is routinely performed following an overnight fast to equalize blood glucose levels in mice20. Since the duration of the fasting period has strong effects on the investigated parameters and is therefore of great importance for the comparison and interpretation of the results, the fasting period should be adapted to the age and body weight of mice. Taking into consideration that mice are nocturnal animals and two-thirds of total daily food intake is consumed overnight, overnight fasting provokes a catabolic state. Thus, the time point of initiation and the duration of fasting have a highly relevant impact on the results, and thus these parameters should be standardized12. Moreover, prolonged fasting depletes liver glycogen stores and therefore reduces variability in baseline blood glucose. In conclusion, since insulin-stimulated glucose utilization in mice is enhanced after prolonged fasting periods21, a 5 to 6 hours fasting duration is recommended to assess insulin action; whereas, overnight fasting is sufficient to test glucose utilization12,20,22. Glucose is normally administered via i.p. injection and the dose of glucose is adjusted to the body weight (usually 1 or 2 g/kg body weight)20,22. In DIO models, body weight is increased, mainly due to a higher fat mass; glucose uptake, however, predominantly occurs in muscle, brain, and liver. Since the amount of these tissues is generally not altered by DIO, obese mice receive a disproportionately high amount of glucose compared to lean mice, which in turn could affect the interpretation of the data and lead to a misdiagnosed glucose intolerance22. For this reason, fasting periods need to be standardized and body composition needs to be considered during interpretation of GTT results23. Therefore, computer tomography (CT) or magnetic resonance imaging(MRI) scans are applicable. For instance, glucose measurements taken by hand-held whole-blood glucose monitors (see Table of Materials) are available, and these monitors are more commonly used than plasma glucose analyzers. Due to the small blood volumes required - typically 5 µL or less - they are more practical than plasma analyzers.
Limitations of the technique: Fasted mice exhibit dependence on duration of fasting and show a significant loss in body weight, body temperature, blood volume, and heart rate as well as changes in serum parameters, such as free fatty acid levels and ketone bodies24. This metabolic stress is triggered by the fact that the housing temperature of mice in animal facilities is standardized to approximately 23 °C, and is therefore below their thermo-neutral temperature of 30 °C25. Prolonged fasting at subthermo-neutral temperatures can result in torpor, characterized by a decrease of the metabolic rate26,27.
Critical steps within the protocol: As mentioned above, for reliable results between the experimental and control groups, littermates should preferably be used during the study and fasting periods, and time points need to be standardized.
Lung function analysis
In this protocol, the plethysmograph directly measures pressure changes that are driving respiration, and the resultant flows in and out of the airways. Flows are measured by a pneumotachograph located in the wall of the plethysmograph. To exclude resistance from the chest wall, airway opening pressure (mouth pressure) and transpulmonary (esophageal tube) pressure are measured and airway resistance and dynamic compliance are calculated. Dynamic compliance is calculated via the difference of the minimum and maximum lung volume divided through the flow.
Significance with respect to existing methods and future applications:Plethysmography is the standard procedure to analyze the mechanical properties of the lung, and can, therefore, be used in future studies to analyze lung pathology and treatment options. To compare groups and studies, a standardized approach is indispensable.
Modifications and troubleshooting: In general, this technique can be classified as unrestrained and restrained whole body plethysmography. Unrestrained methods determine enhanced pause (Penh) and enable analysis of normal breathing patterns, whilst restrained, invasive methods directly measure pressure, flow, or volume. Lung mechanical properties are determined by resistance and elastance; while resistance is calculated as the ratio of the pressure to the flow, elastance reflects the ratio of the pressure to the volume28. In contrast, unrestrained whole-body plethysmography is only measuring the pressure inside the plethysmograph, and therefore a calculation of resistance and elastance is impossible. In 2007, Lundblad et al. have stated that Penh is not the right parameter to measure airway resistance, but represents a nonspecific reflection of the breathing pattern29. Thus, for proper estimation of lung mechanics, invasive plethysmography is indispensable29,30,31.
Since breathing parameters depend on the age and size of mice, ventilation parameters need to be adjusted. For example, the tidal volume is related to body weight and should be set to 10 µL/g body weight with a breathing frequency of 120-250 breaths per minute. When adjusting these parameters, the investigator should take into account that the mean tidal volume is inversely related to the respiratory frequency32. Since spontaneous breathing influences pressure, flow, and volume, the depth of anesthesia has to be monitored and ventilation curves have to be observed constantly. However, anesthesia itself can directly adversely affect lung function. For example, Propofol and Ketamine partly protect against induced airway constriction compared to Thiopental33. Moreover, clinical studies have shown that Ketamine has an anticholinergic effect and can be used as a potential bronchodilator in severe asthma34. Inhalational anesthetics, such as isoflurane in conjunction with pain treatment, are regarded as a controllable alternative to injection anesthesia; however, airway irritation after volatile anesthetics is reported and therefore excludes inhalation anesthetics as an alternative35.
Limitations of the technique: The restrained invasive method to measure lung mechanical properties is due to the necessary tracheotomy, a final procedure, and thereby limits the study to a single-point analysis, without the option to investigate disease progression. To reduce the level of invasiveness, measurement of transfer impedance in conscious animals can be performed, which thereby enables longitudinal studies. However, when measuring respiratory mechanics in non-tracheotomized animals, the resistance of the nose contributes to the total respiratory resistance, and thereby complicates measurements after methacholine provocation13.
Critical steps within the protocol: Here we demonstrate only one method of invasive lung function. Since several established invasive lung function methods exist, standardization within the studies and a detailed description of the method used, groups, and an anesthetic regime in publications is necessary to compare studies.
Lung excision for histomorphometric analysis
Significance with respect to existing methods and future applications: Quantitative histomorphometric analysis can be used to investigate the impact of obesity on lung structure (bronchi and alveoli), to interpret the results obtained by invasive plethysmography, and to study possible treatment options on pulmonary outcome. Data from histological assessments may differ depending on fixative agents and the used fixation procedure.Since it has been shown that obesity has an effect on alveolar and bronchial structure, as well as extracellular matrix and cellular composition, it is necessary to determine an appropriate technique based on the research question in future studies. For unbiased quantitative morphometry, tissue processing following fixation should be performed according to the standards of the American Thoracic Society (ATS)/European Respiratory Society (ERS) for quantitative assessment of the lung structure14.
Modifications and troubleshooting: In 2010 Hsia et al. presented an official statement of the ATS/ERS setting standards for quantitative assessment of the lung structure, which should be taken into consideration prior to lung isolation and fixation14. Beside intratracheal instillation of fixatives, in situ fixation, fixed-volume fixation, or vacuum inflation can be performed to inflate pulmonary tissue36. Inflation, and thereby airspace enlargement, is dependent on fixation procedures and the grade of pressure applied during fixation. High pressure, for example, can lead to alveolar wall rupture and thereby influence the results. Similar to the ventilation parameters, the ideal fixation parameters depend on the age, size, and phenotype of the mice. Fixation can be achieved by chemical or physical means, including chemical agents and/or cryopreservation. Intratracheal instillation of suitable fixatives mimics tissue inflation during breathing, reflecting in vivo conditions, and is therefore widely used37. 20-25 cm above the highest point of the lung is recommended for sufficient pressure, using a wide and short tubing to allow rapid and uniform penetration14. A major objective of fixation is to prevent the degeneration process and to preserve cells and tissue in a "life-like state", while preserving the architectural integrity of the lung parenchyma. In addition, tissue must retain its reactivity to antibodies, stains, and nucleic acid probes. Besides the effect of normal autolysis, adverse effects of tissue processing, including infiltration with hot wax, slicing, and dewaxing, have to be prevented. The choice of fixative as well as further processing of the tissue, e.g. via embedding, can cause tissue shrinkage, swelling, and hardening of various components and lead to artifacts, such as increased autofluorescence38. For example, fixation in 10% buffered formalin and further processing may cause shrinkage of up to 20% - 30% relative to initial volume39. Therefore, Hsia et al. in their 2010 consensus paper of the ATS/ERS recommend the use of 2.5% Glutaraldehyde buffered with osmium tetroxide and uranyl acetate to avoid tissue shrinkage. Lung volume, internal architecture, tissue fine structure, and cell structure was preserved after the airway instillation of this fixative reagent14. Denaturation of proteins and cross-link formation are two major mechanisms which are important in fixation of tissue. Dehydrating compounds or coagulants, such as alcohols or acetone, cause denaturation of proteins, resulting in changes of the tertiary protein structure by destabilizing hydrophobic bindings. Non-coagulant fixing agents like paraformaldehyde or glutaraldehyde chemically react with proteins and form inter-molecular and intra-molecular cross-links. Since several staining procedures, such as hematoxylin and eosin staining, depend on inter-molecular interactions, staining results can be poor, depending on the fixative agent. Antigen-retrieval methods in immunohistochemistry have shown that some of the reactions of fixation are reversible, particularly those of formaldehyde40.
Limitations of the technique: Since the alveolar surface lining is removed by intratracheal instillation of fixatives, the interpretation of, e.g., alveolar microarchitecture, mucus accumulation, or inflammatory cell migration can be manipulated41,42,43.
Critical steps within the protocol: Here we show the intratracheal instillation of 4% PFA as a broad approach to visualize the effect of obesity on pulmonary outcomes. As mentioned above, the protocol must be modified depending on the research question, according to the ATS/ERS recommendations14.
In addition to the previously mentioned parameters, stress is a major factor influencing research results. Therefore, training both mice and the scientist is indispensable. Mice should be adapted to restraining and should be transferred to the experimental area under quiet conditions44. Stress influences metabolism, e.g. as a consequence of stress hormone release, released glucose levels are increased, an effect that can be misinterpreted as impaired glucose tolerance. Stress hormone release also alters susceptibility to anesthetic drugs, specifically, the dose of anesthetics is increased and the time to reach surgical tolerance is prolonged. As mentioned, the increased quantity of anesthetics can influence bronchoconstriction, while stress itself can cause bronchial dilatation.
In summary, this article provides three methods to assess the impact of obesity and metabolism on lung structure and function in mice. All mentioned methods can be transferred to other disease models and rodent species, such as obesity due to genetic modifications or rat models. The application of these techniques can be helpful to define new molecular mechanisms in DIO models with specific gene ablation, or to test new therapeutic approaches to treat/prevent the adverse effects of obesity on chronic lung diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The experiments were supported by the Marga and Walter Boll-Stiftung, Kerpen, Germany; Project 210-02-16 (MAAA), Project 210-03-15 (MAAA) and by the German Research Foundation (DFG; AL1632-02; MAAA), Bonn, Germany; Center of Molecular Medicine Cologne (CMMC; University Hospital Cologne; Career Advancement Program; MAAA), Köln Fortune (Faculty of Medicine, University of Cologne; KD).
References
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- Freemantle N, Holmes J, Hockey A, Kumar S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? International journal of clinical practice. 2008;62:1391–1396. doi: 10.1111/j.1742-1241.2008.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink AMJ, et al. Waist circumference and metabolic risk factors have separate and additive effects on the risk of future Type 2 diabetes in patients with vascular diseases. A cohort study. Diabetic Medicine. 2011;28:932–940. doi: 10.1111/j.1464-5491.2011.03318.x. [DOI] [PubMed] [Google Scholar]
- Oktay AA, et al. The Interaction of Cardiorespiratory Fitness with Obesity and the Obesity Paradox in Cardiovascular Disease. Progress in cardiovascular diseases. 2017. [DOI] [PubMed]
- Azamar-Llamas D, Hernandez-Molina G, Ramos-Avalos B, Furuzawa-Carballeda J. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2017;2017:5468023. doi: 10.1155/2017/5468023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig SM. Pulmonary complications of obesity. The American journal of the medical sciences. 2001;321:249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ. Current views on obesity. The American journal of medicine. 1996;100:230–236. doi: 10.1016/s0002-9343(97)89464-8. [DOI] [PubMed] [Google Scholar]
- Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233–242. doi: 10.1177/1479972308096978. [DOI] [PubMed] [Google Scholar]
- Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. International journal of general medicine. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease models & mechanisms. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94(1985):1297–1306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraten-Miller SL, Brown PA. Techniques in aseptic rodent surgery. Curr Protoc Immunol. 2008;Chapter 1 doi: 10.1002/0471142735.im0112s82. Unit 1 12 11-11 12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydemann A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. Journal of diabetes research. 2016;2016:2902351. doi: 10.1155/2016/2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha GV, Raja Gopal Reddy M, Mahesh M, Vajreswari A, Jeyakumar SM. Male mice are susceptible to high fat diet-induced hyperglycaemia and display increased circulatory retinol binding protein 4 (RBP4) levels and its expression in visceral adipose depots. Archives of physiology and biochemistry. 2016;122:19–26. doi: 10.3109/13813455.2015.1126609. [DOI] [PubMed] [Google Scholar]
- Jovicic N, et al. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS One. 2015;10:e0134089. doi: 10.1371/journal.pone.0134089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine DA, Davis DB. Attention to Background Strain Is Essential for Metabolic Research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016;65:25–33. doi: 10.2337/db15-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, et al. Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. Journal of lipid research. 2005;46:582–588. doi: 10.1194/jlr.M400440-JLR200. [DOI] [PubMed] [Google Scholar]
- Heikkinen S, Argmann CA, Champy MF, Auwerx J. Evaluation of glucose homeostasis. Current protocols in molecular biology. 2007;Chapter 29 doi: 10.1002/0471142727.mb29b03s77. Unit 29B.23. [DOI] [PubMed] [Google Scholar]
- McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297:E849–E855. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab. 2009;9:111–112. doi: 10.1016/j.cmet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci. 2006;26:241–245. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Mead J. Mechanical properties of lungs. Physiological reviews. 1961;41:281–330. doi: 10.1152/physrev.1961.41.2.281. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol. 2002;93:1198–1207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, et al. Penh is not a measure of airway resistance! Eur Respir J. 2007;30:805. doi: 10.1183/09031936.00091307. [DOI] [PubMed] [Google Scholar]
- Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol. 2004;97:286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- Fairchild GA. Measurement of respiratory volume for virus retention studies in mice. Applied microbiology. 1972;24:812–818. doi: 10.1128/am.24.5.812-818.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Wagner EM. Mechanisms of bronchoprotection by anesthetic induction agents: propofol versus ketamine. Anesthesiology. 1999;90:822–828. doi: 10.1097/00000542-199903000-00025. [DOI] [PubMed] [Google Scholar]
- Goyal S, Agrawal A. Ketamine in status asthmaticus: A review. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2013;17:154–161. doi: 10.4103/0972-5229.117048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Ikeda K. Airway irritation produced by volatile anaesthetics during brief inhalation: comparison of halothane, enflurane, isoflurane and sevoflurane. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1993;40:122–126. doi: 10.1007/BF03011308. [DOI] [PubMed] [Google Scholar]
- Braber S, Verheijden KA, Henricks PA, Kraneveld AD, Folkerts G. A comparison of fixation methods on lung morphology in a murine model of emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299:L843–L851. doi: 10.1152/ajplung.00192.2010. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Limacher W, Bachofen H. Electron microscopy of rapidly frozen lungs: evaluation on the basis of standard criteria. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53:516–527. doi: 10.1152/jappl.1982.53.2.516. [DOI] [PubMed] [Google Scholar]
- Rolls G. Process of Fixation and the Nature of Fixatives. 2017.
- Winsor L. Woods A, Ellis R, editors. Tissue processing. Laboratory histopathology. 1994. pp. 4.2-1–4.2-39.
- Pearse A, editor. Histochemistry, theoretical and applied. London: Churchill Livingstone; 1980. [Google Scholar]
- Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiological reviews. 1973;53:419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Bur S, Bachofen H, Gehr P, Weibel ER. Lung fixation by airway instillation: effects on capillary hematocrit. Experimental lung research. 1985;9:57–66. doi: 10.3109/01902148509061528. [DOI] [PubMed] [Google Scholar]
- Bachofen H, Ammann A, Wangensteen D, Weibel ER. Perfusion fixation of lungs for structure-function analysis: credits and limitations. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53:528–533. doi: 10.1152/jappl.1982.53.2.528. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science. 2004;43:42–51. [PubMed] [Google Scholar]


