Abstract
Introduction
Remission induction in antineutrophil cytoplasmic autoantibody (ANCA) vasculitis may be complicated by slow response to treatment and toxicity from glucocorticoids. We describe outcomes with a novel remission induction regimen combining rituximab with a short course of low-dose, oral cyclophosphamide and an accelerated prednisone taper.
Methods
Patients were included in this retrospective study if they had newly diagnosed or relapsing ANCA vasculitis with a Birmingham Vasculitis Activity Score for Wegener Granulomatosis (BVAS-WG) ≥3 and received a standardized remission induction regimen. The primary outcome was complete remission, defined as a BVAS-WG of 0 and a prednisone dose of ≤7.5 mg/d.
Results
We identified 129 patients who met the inclusion criteria, 31% of whom also received plasma exchange (PLEX) for rapidly progressive glomerulonephritis (RPGN) or diffuse alveolar hemorrhage. Seventy percent of patients had myeloperoxidase (MPO)-ANCA and 9% had relapsing disease. Median time to complete remission was 4 months (interquartile range [IQR] 3.9–4.4), and by 5 months 84% of patients were in complete remission. Prednisone was tapered to discontinuation as tolerated, such that the median prednisone dose at 8 months was 0 mg/d (IQR 0–2.5). In patients with RPGN, proteinase 3–ANCA was associated with a greater increase in eGFR at 6 months compared with MPO-ANCA (16 vs. 5.6 ml/min per 1.73m2; P = 0.028). During the year following remission, 1 major relapse occurred over 122 patient-years. Serious infections occurred more frequently in patients receiving PLEX and were associated with increasing age and diffuse alveolar hemorrhage. Four deaths occurred, 3 of which were associated with serious infections.
Conclusion
Combination therapy was efficacious, allowed for rapid tapering of high-dose glucocorticoids and was well tolerated.
Keywords: ANCA vasculitis, cyclophosphamide, remission, rituximab
Antineutrophil cytoplasmic autoantibody (ANCA) vasculitis is a systemic autoimmune disease characterized by small vessel inflammation with a propensity to affect the kidney and respiratory tract.1, 2 Urgent treatment is required to prevent irreversible organ damage.3 Current strategies for remission induction in severe ANCA vasculitis include either rituximab or cyclophosphamide in combination with glucocorticoids.4, 5 The success of these regimens has been limited by the sequelae of prolonged exposure to high-dose glucocorticoids and treatment failure in some patients.2, 3, 4, 6
In an attempt to more rapidly attain remission and minimize exposure to high-dose glucocorticoids, our approach for remission induction evolved into a standardized 3-drug regimen: rituximab, a 2-month course of low-dose, oral cyclophosphamide, and an accelerated glucocorticoid taper. Other regimens combining rituximab and i.v. cyclophosphamide have been previously reported in smaller patient populations in the RITUXVAS trial and in the regimen reported by Mansfield et al. with favorable outcomes.7, 8 Our rationale for this strategy is to target autoantibody-producing plasmablasts and plasma cells with cyclophosphamide and glucocorticoids while simultaneously depleting their precursors with rituximab. In cases of severe rapidly progressive glomerulonephritis (RPGN) or pulmonary hemorrhage, plasma exchange (PLEX) and pulse i.v. glucocorticoids are added to the standardized regimen. We present a retrospective analysis of 129 patients treated with this standardized regimen with a focus on efficacy, risk of relapse, and safety.
Methods
Patients
We performed a retrospective analysis of 129 sequential patients with newly diagnosed or relapsing active ANCA vasculitis treated with a standardized remission induction regimen at the Massachusetts General Hospital Vasculitis and Glomerulonephritis Center from June 2006 to January 2016. Included patients had a positive test for antibodies to proteinase 3 (PR3) or myeloperoxidase (MPO) together with clinical and laboratory features consistent with granulomatosis with polyangiitis, microscopic polyangiitis, or renal-limited vasculitis.9 Active vasculitis was defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS-WG) of ≥3.10, 11 Patients in this study have also been included in other reports addressing different aspects of treatment.10, 12, 13 The study was approved by the Partners HealthCare Human Research Committee and performed in accordance with the Declaration of Helsinki.
Treatment Regimen
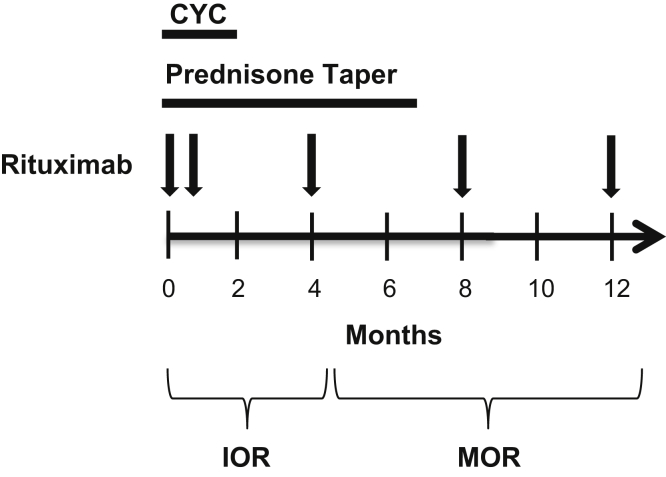
Patients were treated with a 3-drug regimen: rituximab, a 2-month course of oral, low-dose cyclophosphamide, and an accelerated prednisone taper (Figure 1). Rituximab was administered as two 1000-mg i.v. doses separated by approximately 2 weeks. Thereafter, rituximab was administered as one 1000-mg i.v. dose every 4 months for maintenance therapy. Beginning at month 4, B-cell depletion was monitored in most patients before each rituximab dose with flow cytometry by evaluating the population of CD19+CD20+ lymphocytes. B-cell depletion was defined as a CD19+CD20+ population below the level of detection at our laboratory (<0.1% of the lymphocyte pool).
Figure 1.
Treatment regimen. Cyclophosphamide was administered at 2.5 mg/kg daily for 7 days, followed by 1.5 mg/kg daily for 7 weeks. The dose of cyclophosphamide was adjusted for renal function as described in the Methods section. Prednisone was administered at 60 mg daily and tapered to 15 mg by week 5. Thereafter, prednisone was tapered by 2.5 mg monthly. Rituximab (arrows) was administered as 1000 mg i.v. doses separated by approximately 2 weeks, followed by 1 dose every 4 months. CYC, cyclophosphamide; IOR, induction of remission; MOR, maintenance of remission.
Cyclophosphamide was dosed at 2.5 mg/kg daily (maximum 175 mg/d) for 1 week, followed by 1.5 mg/kg daily (maximum 125 mg/d) for 7 weeks. The dose of cyclophosphamide was reduced for impaired renal function as follows: 10% reduction if the estimated glomerular filtration rate (eGFR) was 60 to 90 ml/min per 1.73 m2, 25% if the eGFR was 45 to 59 ml/min per 1.73 m2, 33% if the eGFR was 30 to 44 ml/min per 1.73 m2, 40% if the eGFR was 15 to 29 ml/min per 1.73 m2, and 50% if the eGFR was <15 ml/min per 1.73 m2. In the first month of treatment, prednisone was tapered as follows: 60 mg daily for 1 week, 40 mg daily for 1 week, 30 mg daily for 1 week, and 20 mg daily for 1 week. Thereafter, prednisone was tapered by 2.5 mg monthly, starting at 15 mg daily. Small deviations in prednisone tapering were made at the discretion of the treating physician.
In the setting of severe pulmonary hemorrhage or RPGN, pulse i.v. glucocorticoids and PLEX were added to the standardized regimen. Pulse glucocorticoids were administered as i.v. methylprednisolone at 500 or 1000 mg daily for 3 days. Patients treated with PLEX received 7 treatments over the course of 2 weeks.
During remission induction therapy, patients received prophylaxis for Pneumocystis pneumonia with trimethoprim-sulfamethoxazole. Patients with an allergy to sulfonamides were administered atovaquone. Pneumocystis pneumonia prophylaxis was continued in patients receiving ongoing rituximab therapy until the prednisone dose was ≤5 mg daily. Patients with a positive hepatitis B core antibody were given prophylaxis to prevent hepatitis B reactivation with either entecavir or lamivudine.
Remission
The BVAS-WG was collected prospectively at each visit as part of patient care. Complete remission was defined as a BVAS-WG of 0, a prednisone dose of ≤7.5 mg daily, and the absence of additional immunosuppression.10, 13 Deaths were counted as failures. Patients on the standardized induction regimen would reach a prednisone dose of 7.5 mg at approximately 120 days. Resistant disease was defined as attaining complete remission after 150 days. The proportion of patients in remission (BVAS-WG = 0) and on less than 10 mg of prednisone per day was also examined.4
Relapse
Disease relapse after complete remission was defined as a BVAS-WG >1 or any vasculitis-associated event that prompted an increase in the prednisone dose to >10 mg/d or the addition of any other immunosuppressant.13 A major relapse was defined as a BVAS-WG ≥3 regardless of whether organ-threatening disease existed.13
Renal Outcomes
The eGFR was obtained using the 4-variable Modification of Diet in Renal Disease equation.14 RPGN was defined as the presence of hematuria (>10 red blood cells per high-powered field) and a rise in creatinine of >30% or a reduction in eGFR of 25%.11 Outcomes examined were the change in eGFR at 6 months and renal survival. Patients on dialysis were assigned a GFR of 5 ml/min per 1.73m2 for calculations. Patients who died on dialysis were counted as dialysis dependent.
Serious Adverse Events and Hypogammaglobulinemia
Serious adverse events were defined as events that were life threatening, resulted in hospitalization, caused disability or permanent damage, required urgent treatment to prevent a serious complication, or resulted in death. Infections were considered serious if they required hospitalization or i.v. antibiotics. Serious adverse events were determined by review of electronic flow sheets used for patient management, which keep a record of all adverse events. Serious adverse events from initiation of therapy to attainment of complete remission were included. Serious adverse events occurring in the maintenance phase of treatment in patients receiving this regimen have been previously reported.10, 13 Significant hypogammaglobulinemia was defined as an IgG level <400 mg/dl.10
Statistical Analysis
Baseline characteristics are presented as percentages or medians and interquartile ranges (IQRs), and were compared using the Fisher exact test or Wilcoxon rank-sum test, as appropriate. The Kaplan-Meier method was used to examine time to achieving remission, time to relapse, and renal survival. The analysis was stratified by ANCA serotype and PLEX status. Differences between curves were assessed using the log-rank test. Predictors of resistant disease were evaluated using logistic regression. Poisson regression was used to generate confidence intervals (CIs) and identify predictors of serious infections. Because of the limited number of patients with resistant disease and serious infections, only univariable models were constructed to avoid model overfitting. All analyses were carried out using STATA version 14 (STATA Corp, College Station, TX).
Results
Baseline Characteristics
We identified 129 patients with ANCA vasculitis treated with the standardized remission induction regimen. Baseline characteristics overall and by ANCA serotype are shown in Table 1. Seventy percent of patients had MPO-ANCA and 9% of patients had relapsing disease. Approximately 30% of both MPO- and PR3-ANCA patients were treated with PLEX. Ear, nose, and throat involvement was significantly more common in patients with PR3-ANCA (72%) than with MPO-ANCA (37%) (P < 0.001). RPGN occurred in 61% and 51% of patients with MPO- and PR3-ANCA, respectively.
Table 1.
Baseline characteristics
| Baseline characteristics | Overall (n = 129) | MPO (n = 90) | PR3 (n = 39) | P |
|---|---|---|---|---|
| Age | 64.9 (54.2–75.6) | 66.2 (58.1–78.0) | 57 (49.2–68.6) | 0.01 |
| Male | 55 (42.6) | 38 (42.2) | 17 (43.6) | 0.86 |
| Recurrent disease | 11 (8.5) | 7 (7.8) | 4 (10.3) | 0.73 |
| Initial BVAS-WG | 6 (5–8) | 6 (5–8) | 7 (5–10) | 0.03 |
| PLEX | 40 (31.0) | 28 (31.1) | 12 (30.8) | 0.99 |
| Organ involvement | ||||
| Constitutional | 53 (41.0) | 34 (37.8) | 19 (48.7) | 0.33 |
| Pulmonary | 63 (48.8) | 45 (50.0) | 18 (46.2) | 0.71 |
| DAH | 20 (16) | 17 (18.9) | 3 (7.7) | 0.12 |
| ENT | 61 (47) | 33 (36.7) | 28 (71.8) | <0.001 |
| Renal | 87 (67.4) | 63 (70.0) | 24 (61.5) | 0.41 |
| RPGN | 75 (58.1) | 55 (61.1) | 20 (51.3) | 0.34 |
BVAS-WG, Birmingham Vasculitis Activity Score for Wegener Granulomatosis; DAH, diffuse alveolar hemorrhage; ENT, ear, nose and throat; MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3; RPGN, rapidly progressive glomerulonephritis.
Baseline characteristics are provided for the overall group and stratified by antineutrophil cytoplasmic antibody serotype. Data are presented as median (interquartile range) and n (%).
Remission
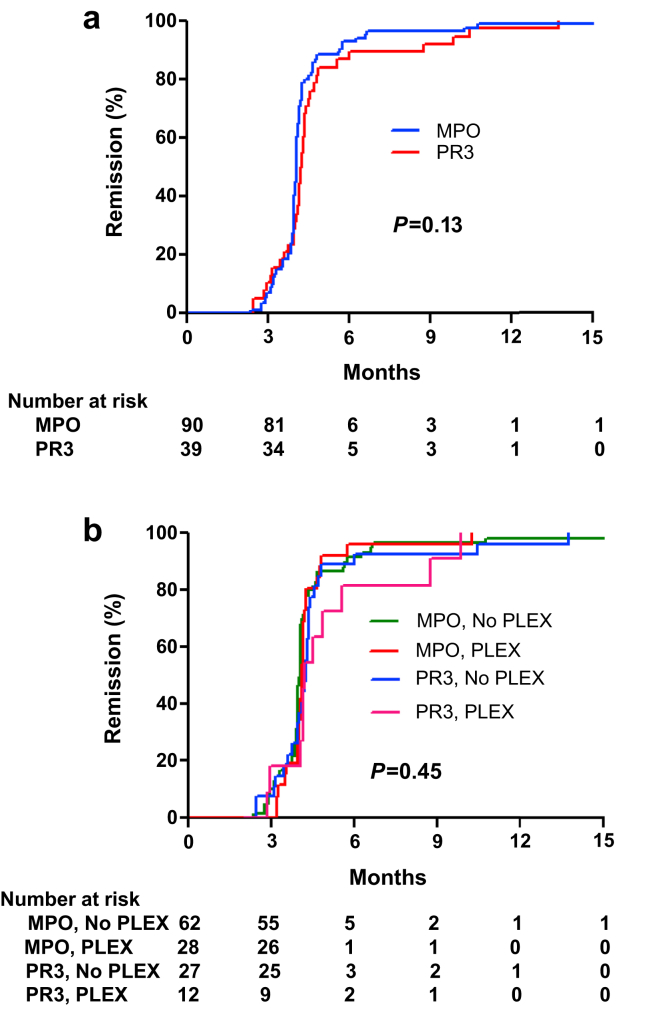
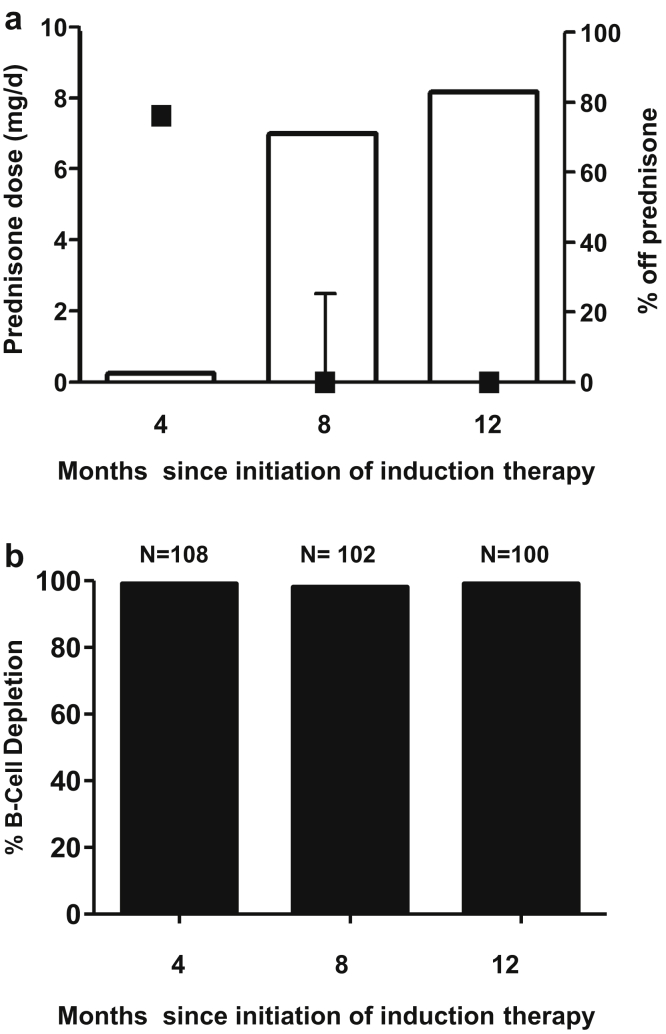
The median time to complete remission (BVAS-WG = 0 and prednisone ≤7.5 mg/d) was 4 months (IQR 3.9–4.4) (Figure 2). There was no difference in time to remission between patents with MPO- and PR3-ANCA (P = 0.13). Likewise, there was no difference in time to remission when taking into account both ANCA serotype and PLEX status (P = 0.44). Prednisone was tapered off except as limited by adrenal insufficiency (Figure 3a). The median (IQR) prednisone dose was 0 mg/d (0–2.5) at 8 months and 0 mg/d (0–0) at 12 months. Continuous B-cell depletion was achieved in >98% of patients during the first year of treatment (Figure 3b).
Figure 2.
Time to complete remission. Shown are Kaplan-Meier curves for time to complete remission stratified by antineutrophil cytoplasmic autoantibody serotype (a) and further by PLEX status (b). MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3.
Figure 3.
Prednisone dose and B-cell depletion during treatment. (a) Squares give the median prednisone dose and interquartile range at each time point (left axis). Bars show the percentage of patients completely off prednisone (right axis). (b) Bars demonstrate percentage of patients with undetectable CD19+CD20+ lymphocytes.
Of the 129 patients treated with the standardized remission induction regimen, 109 (84%) achieved complete remission by 5 months. Sixteen patients had resistant disease (remission at >150 days) and received additional prednisone (n = 16), cyclophosphamide (n = 6), and/or methotrexate (n = 1). For these patients, median time to complete remission was 6.3 months (IQR 5.7–10.4). After the index hospitalization, there were no hospitalizations for vasculitis-related events and no new episodes of glomerulonephritis or diffuse alveolar hemorrhage. By 6 months, 91% of patients had a BVAS-WG of 0 on 10 mg of prednisone or less. All surviving patients ultimately achieved complete remission.
We attempted to identify factors associated with resistant disease (Table 2). In univariable logistic regression, sinus involvement was associated with an increased risk of resistant disease (odds ratio 4.75; 95% CI, 1.60–14.13; P = 0.005). Conversely, a log fall in ANCA titer was associated with a decreased risk of resistant disease.
Table 2.
Predictors of resistant disease
| Variable | OR (95% CI) | P |
|---|---|---|
| Age, per yr | 0.98 (0.95–1.01) | 0.15 |
| Male | 0.55 (0.18–1.68) | 0.29 |
| MPO | 0.70 (0.24–2.09) | 0.53 |
| Sinus | 4.75 (1.60–14.13) | 0.005 |
| Pulmonary | 1.12 (0.39–3.19) | 0.84 |
| RPGN | 0.95 (0.33–2.75) | 0.93 |
| Initial BVAS-WG, per point | 1.04 (0.87–1.23) | 0.67 |
| Relapsing disease | 1.57 (0.31–8.03) | 0.59 |
| PLEX | 1.08 (0.35–3.36) | 0.90 |
| ANCA declinea | 0.24 (0.07–0.81) | 0.02 |
ANCA, antineutrophil cytoplasmic antibody; BVAS-WG, Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis; CI, confidence interval; MPO, myeloperoxidase; OR, odds ratio; PLEX, plasma exchange; RPGN, rapidly progressive glomerulonephritis.
Resistant disease was defined as achieving complete remission greater than 150 days after initiating induction of remission therapy.
ANCA decline refers to a 10-fold reduction in the ANCA titer at 4 months.
Renal Outcomes
In total, 75 patients receiving the induction regimen had RPGN, 39 of whom received PLEX (Table 3). Baseline median eGFR was 18.8 (IQR 10.7–28.2) ml/min per 1.73 m2 and was similar in patients with MPO-ANCA (18.8 [IQR 11.4–28.2] ml/min per 1.73 m2) and PR3-ANCA (18.4 [IQR 8.8–26.7] ml/min per 1.73 m2). After 6 months of treatment, eGFR increased to a median of 28.9 (IQR 18.3–45.7) ml/min per 1.73 m2. The median increase in eGFR was greater among patients with PR3-ANCA (16.1 [IQR 0.0–22.5] ml/min per 1.73 m2) than with MPO-ANCA (5.6 [IQR − 0.4 to 15.6] ml/min per 1.73 m2; P = 0.028).
Table 3.
Renal outcomes for patients with RPGN
| Variable | Overall, n = 75 | MPO-ANCA, n = 55 | PR3 ANCA, n = 20 | P |
|---|---|---|---|---|
| PLEX | 39 (52.0) | 27 (49.1) | 12 (60) | 0.44 |
| Baseline eGFR | 18.8 (10.7–28.2) | 18.8 (11.4–28.2) | 18.4 (8.8–26.7) | 0.54 |
| 6-month eGFR | 28.9 (18.3–45.7) | 25.9 (14.3–44.2) | 37.6 (24.2–50.0) | 0.18 |
| Increase in eGFR at 6 months | 6.8 (0.0–18.1) | 5.6 (−0.4 to 15.6) | 16.1 (0.0–22.5) | 0.028 |
| Required dialysis | 11 (14.8) | 7 (12.8) | 4 (20.0) | 0.47 |
| Dialysis dependenta | 9 (12.0) | 6 (10.9) | 3 (15.0) | 0.45 |
ANCA, antineutrophil cytoplasmic antibody; eGFR, estimated glomerular filtration rate; MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3; RPGN, rapidly progressive glomerulonephritis.
Data are presented as median (interquartile range) and n (%).
Patients who died on dialysis were counted as dialysis dependent.
Nine patients (12%) became permanently dialysis dependent or died on dialysis, and 2 patients transiently required dialysis but subsequently recovered. Among patients who became dialysis dependent, 7 of 9 patients initiated dialysis on the first day of induction therapy, and no patients initiated dialysis after 17 days of therapy. ANCA serotype did not influence renal survival (Supplementary Figure S1).
Relapse After Remission
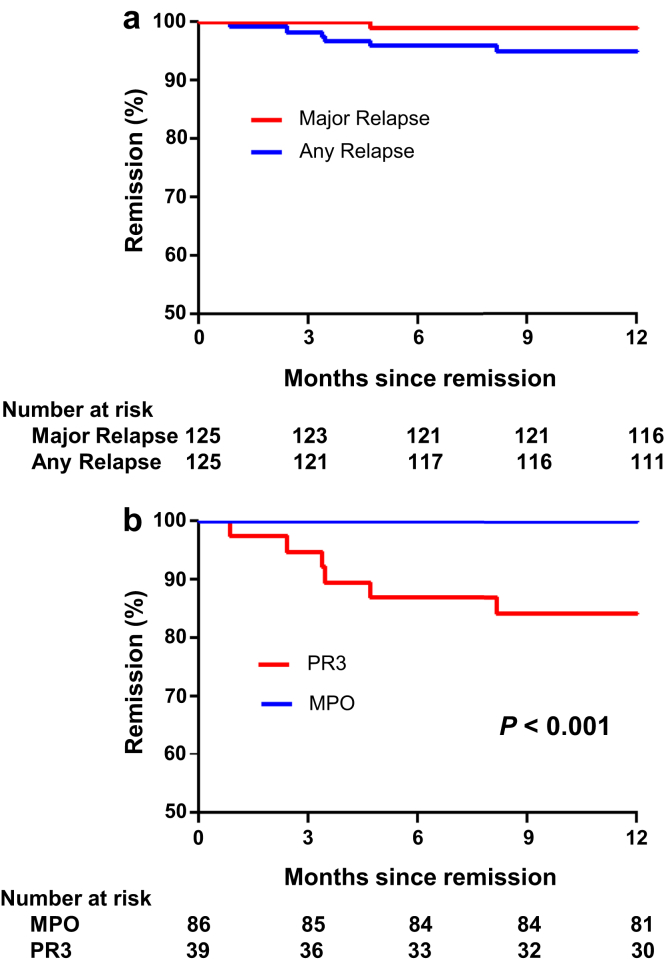
Data are presented for the year following attainment of complete remission. Over a total of 122 years of patient follow-up, 6 relapses occurred (Figure 4a). Only 1 relapse, a recurrent episode of mild scleritis, constituted a major relapse. All relapses occurred in patients with PR3-ANCA (Figure 4b).
Figure 4.
Relapse after complete remission. Shown are Kaplan-Meier curves for time to major relapse and any relapse (a) and time to any relapse by ANCA subtype (b). MPO, myeloperoxidase; PR3, proteinase 3.
Relapses were treated with an increase in prednisone (maximum 20 mg/d), or a decrease in the rate of scheduled prednisone tapering, along with continuation of scheduled rituximab (Supplementary Table S1). The major relapse was successfully treated with an increase in prednisone to 10 mg/d. There were no episodes of RPGN, alveolar hemorrhage, or hospitalizations for vasculitis-related events.
Serious Adverse Events
The total exposure during the induction period for the entire cohort was 580 months. Serious adverse events occurred at a rate of 0.078 events per month (95% CI, 0.057–0.10) and serious infections occurred at a rate of 0.022 events per month (95% CI, 0.012–0.038). Serious adverse events were less common in patients receiving the standardized induction regimen alone (0.059 events per month; 95% CI, 0.038–0.088) compared with patients receiving the standardized induction regimen with PLEX (0.12 events per month; 95% CI, 0.075–0.18; incidence rate ratio 2.0; 95% CI, 1.1–3.7; P = 0.017). Similarly, serious infections occurred less frequently in patients receiving the induction regimen without PLEX (0.017 events per month; 95% CI, 0.007–0.036; versus 0.035; 95% CI, 0.013–0.075; incidence rate ratio 2.0; 95%, CI 0.67–5.96; P = 0.21).
Serious adverse events and serious infections are shown in Table 4. Most serious infections (7 of 13) occurred in the setting of neutropenia. All episodes of neutropenia were successfully treated with subcutaneous filgrastim. The rates of hypogammaglobulinemia overall and stratified by PLEX status are presented in Table 5.
Table 4.
Serious adverse events during induction of remission
| Serious event | IND, n = 89 | IND + PLEX, n = 40 | Overall, n = 129 |
|---|---|---|---|
| Death | 2 | 2 | 4 |
| Isolated neutropeniaa | 3 | 2 | 5 |
| Infection with neutropenia | 3 | 4 | 7 |
| Bacteremia | 1 | 1 | 2 |
| Pneumonia | 1 | 2 | 3 |
| Urinary tract infection | 1 | 0 | 1 |
| Cytomegalovirus | 0 | 1 | 1 |
| Infection without neutropenia | 4 | 2 | 6 |
| Bacteremia | 2 | 0 | 2 |
| Pneumonia | 1 | 2 | 3 |
| Gastroenteritis | 1 | 0 | 1 |
| Myocardial infarction | 2 | 1 | 3 |
| Arrhythmia | 0 | 2 | 2 |
| Hypertension | 0 | 2 | 2 |
| Deep venous thrombosis | 3 | 2 | 5 |
| Stroke | 1 | 0 | 1 |
| Pulmonary embolism | 1 | 0 | 1 |
| Hemoptysis | 1 | 0 | 1 |
| Gastrointestinal bleed | 1 | 1 | 2 |
| Malignancy | 1 | 0 | 1 |
| Tracheal stenosis | 1 | 0 | 1 |
| Renal failure | 0 | 1 | 1 |
| Nephrolithiasis | 1 | 0 | 1 |
| Hyponatremia | 1 | 0 | 1 |
| Falls | 1 | 0 | 1 |
| Seizure | 1 | 0 | 1 |
| Methemoglobinemia | 0 | 1 | 1 |
IND, standard induction regimen; IND + PLEX, standard induction regimen with plasma exchange.
Neutropenia was defined as an absolute neutrophil count <1500 cells per mm3.
Table 5.
Rates of hypogammaglobulinemia during remission induction
| Group | Percent hypogammaglobulinemia (n) |
||
|---|---|---|---|
| 4 months | 8 months | 12 months | |
| IND Alone | 8 (6/78) | 7 (5/73) | 5 (3/63) |
| IND + PLEX | 22 (7/32) | 16 (5/31) | 10 (3/30) |
| Overall | 12 (13/110) | 10 (10/104) | 6 (6/93) |
IND, standard induction regimen; IND + PLEX, standard induction regimen with plasma exchange.
Hypogammaglobulinemia was defined as an IgG level <400 mg/dl.
Chronic myelogenous leukemia was diagnosed in a patient 2 months after initiating therapy. In retrospect, she had an abnormal complete blood count with an absolute monocytosis 5 months prior, and the malignancy was felt to be established before commencing treatment. No other malignancies occurred during the induction period. Four deaths occurred during the induction period (Supplementary Table S2), 3 of which were associated with infections. All deaths occurred in patients aged 74 years or older.
Poisson regression was used to identify risk factors for serious infections (Table 6). There was a trend to an increased rate of infections with PLEX and the presence of RPGN. Only increasing age (incidence rate ratio 1.70 per 10 years; 95% CI, 1.11–2.58; P = 0.01) and the presence of diffuse alveolar hemorrhage (incidence rate ratio 4.97; 95% CI, 1.67–14.79; P = 0.004), however, had a statistically significant association with serious infections.
Table 6.
Risk factors for serious infections
| Variables | Univariable IRR (95% CI) | P |
|---|---|---|
| Age, per 10 yr | 1.70 (1.11–2.58) | 0.01 |
| Male | 0.67 (0.21–2.19) | 0.51 |
| MPO-ANCA | 1.54 (0.42–5.58) | 0.51 |
| Initial BVAS-WG | 1.08 (0.93–1.26) | 0.32 |
| RPGN | 2.71 (0.74–9.86) | 0.13 |
| DAH | 4.97 (1.67–14.79) | 0.004 |
| PLEX | 2.00 (0.67–5.96) | 0.21 |
IRRs were determined using Poisson regression. ANCA, antineutrophil cytoplasmic autoantibody; BVAS-WG, Birmingham Vasculitis Activity Score for Wegener Granulomatosis; DAH, diffuse alveolar hemorrhage; IRR, incidence rate ratio; MPO, myeloperoxidase; PLEX, plasma exchange; RPGN, rapidly progressive glomerulonephritis.
Discussion
This retrospective series of patients with ANCA vasculitis suggests remission induction with combination rituximab, low-dose cyclophosphamide, and glucocorticoids is efficacious. At 5 months, 84% of patients were in complete remission, and all surviving patients ultimately achieved complete remission. The efficacy of therapy was not dependent on ANCA serotype or disease severity. Moreover, the response was durable, with only 1 patient sustaining a major relapse in the first year following complete remission.
The rationale for combining rituximab with cyclophosphamide is based on the differential effect of these medications across the B-cell lineage.10 Rituximab, an anti-CD20 monoclonal antibody, depletes precursors of autoantibody-producing cells, but has little direct effect on ANCA-producing plasmablasts and plasma cells that do not express CD20.15 For this reason, treatment with rituximab alone necessitates prolonged courses of high-dose glucocorticoids to control disease activity and prevent early treatment failure.4, 6 High doses of glucocorticoids are associated with significant adverse effects, including diabetes, osteoporosis, mood disturbances, and an increased risk of infections.16
The addition of cyclophosphamide to target autoantibody-producing plasmablasts and short-lived plasma cells allows for rapid tapering of high-dose steroids. The cumulative dose of prednisone administered in the standardized regimen is 2520 mg over 28 weeks compared with 3500 mg over 20 weeks in the RAVE trial protocol.4 In addition, the duration of exposure to high-dose steroids (≥20 mg/d) is significantly reduced with the current strategy. The daily prednisone dose was reduced to 15 mg by week 5 with the standardized regimen compared with week 11 in the RAVE trial protocol. Given that numerous reports have suggested a graded response of prednisone dose with infectious and other adverse events, the rapid taper of high-dose prednisone likely translates to a lower rate of glucocorticoid-induced adverse events.17, 18, 19 Moreover, when used with rituximab, the dose and duration of cyclophosphamide can be reduced. For a 70-kg man with normal renal function, the total cumulative dose of cyclophosphamide is 6.37 g administered over 8 weeks. In the setting of renal impairment, the dose is reduced as delineated in the Methods section. This dosing regimen administers a lower cumulative dose than conventional regimens used for ANCA vasculitis and reduces cyclophosphamide exposure to a level that minimizes the risk of secondary malignancies and infertility.5, 20, 21, 22
Most patients (87%) attained complete remission without need for alteration of the regimen. Of note, this strategy appeared equally efficacious even in aggressive cases of RPGN and alveolar hemorrhage that were treated with PLEX. Only sinus involvement predicted refractory disease. This may in part reflect the difficulty in clinically differentiating active vasculitis in the upper airways from infection and chronic damage.
Comparison of our results with the endpoints of well-conducted clinical trials can provide a metric of efficacy. In the RAVE trial, 71% of patients in the rituximab arm and 62% of patients in the control group reached the endpoint of remission at 6 months on less than 10 mg per day of prednisone per day.4 In patients treated with combination therapy, 118 patients (91%) were in remission using the same criteria despite inclusion of critically ill patients requiring PLEX. Moreover, after the index hospitalization there were no hospitalizations for vasculitis activity.
Despite including patients with severe renal disease, no patients initiated dialysis more than 17 days after starting induction therapy, suggesting rapid and sustained control of disease activity. Patients with PR3-ANCA had a greater increase in eGFR at 6 months. The favorable renal response in patients with PR3-ANCA may be due to a more chronic and insidious course with resulting fibrosis in some patients with MPO-ANCA.23, 24 Furthermore, patients with PR3-ANCA are more likely to have symptomatic extrarenal manifestations that can lead to earlier diagnosis.25
After attaining complete remission, 1 major relapse occurred in the ensuing year. The major relapse was a mild recurrence of scleritis that resolved with increasing the prednisone dose to 10 mg daily. It is possible the induction regimen influenced the low relapse rate during maintenance therapy. For example, in the CYCLOPS trial, oral cyclophosphamide was associated with a lower risk of relapse after remission than i.v. pulse cyclophosphamide.5, 26 In our case, however, the low relapse rate was likely related to ongoing treatment with rituximab.13, 27 All relapses occurred in patients with PR3-ANCA, consistent with other reports of a greater predisposition to relapse in this subgroup.6, 28 In addition, most patients were treated for de novo rather than relapsing disease. The low percentage of patients with PR3-ANCA and relapsing disease within our cohort likely contributed to the overall low rate of relapse.
Treatment with the standardized induction regimen was generally well tolerated. Importantly, in contrast to randomized trials, patients were not excluded from our analysis due to age, comorbidities, or severe organ-threatening disease. The rate of serious infections in patients not receiving PLEX (0.017 per month) was similar to the number of serious infections in the RAVE trial (0.012 per month).4 As expected, the rate of serious infections was higher in patients who required PLEX due to severe RPGN or pulmonary hemorrhage. In univariable Poisson regression, increasing age and pulmonary hemorrhage had a statistically significant association with serious infection. The increased risk of serious infections with alveolar hemorrhage can likely be attributed to the increased infectious risk with mechanical ventilation and the difficulty in clinically assessing superimposed infection in this population.
Neutropenia was an important serious adverse event, occurring in 8.5% of patients. Furthermore, most serious infections (7 of 13) occurred concomitantly with neutropenia. Episodes of neutropenia were reversible in all cases with subcutaneous filgrastim. The mechanism of neutropenia in this setting is likely multifactorial. Cyclophosphamide can lead to neutropenia in a dose-dependent fashion.29 Despite the absence of CD20 on neutrophils, rituximab can lead to episodes of severe and reversible neutropenia.30, 31 The cause for this phenomenon remains unknown, but autoimmune mechanisms and alterations in hematopoietic growth factors have been postulated.31 It is possible that the combination of rituximab and cyclophosphamide acts synergistically to cause neutropenia. The incidence of neutropenia we observed, however, does not exceed that observed in other cohorts. In a cohort of patients with autoimmune disease treated with rituximab, 23% of patients with ANCA vasculitis developed neutropenia.31 In another retrospective analysis of patients with ANCA vasculitis treated with varying induction regimens, neutropenia occurred in 18% of patients.32
Our study has several important limitations. Namely, the study is retrospective, is derived from a single center, and lacks a comparator group. In addition, 70% of patients had MPO-ANCA, making the results potentially less applicable to patients with PR3-ANCA. Nonetheless, ANCA serotype was not associated with time to remission or resistant disease in patients treated with combination therapy. Strengths of the study include the relatively large size and inclusion of patients across the entire spectrum of disease encountered in clinical practice. In particular, the elderly and patients with severe organ-threatening disease requiring PLEX were included.
Outcomes in patients with ANCA vasculitis have improved over the past 30 years due to earlier diagnosis and improved treatments.33 Despite these improvements, current remission induction regimens are still complicated by early treatment failure and the inability to minimize exposure to high-dose glucocorticoids.6 The ability to reliably and swiftly induce remission in ANCA vasculitis while reducing glucocorticoid toxicity represents an unmet need. In this study, we present evidence that combination therapy with rituximab and cyclophosphamide is highly efficacious, allows for rapid tapering of high-dose glucocorticoids, and has an acceptable side-effect profile. Given the preliminary success of this regimen, additional studies evaluating this strategy are warranted.
Disclosure
JLN has served as a rituximab-specific advisory board member for Genentech and is currently participating in the Genentech-sponsored Rituximab in ANCA-Associated Vasculitis Registry (RAVER) Study. FBC was previously supported by a fellowship grant from Genentech (G-17505). All the authors declared no competing interests.
Acknowledgments
FBC was previously supported by National Institutes of Health grant 5T32DK007540 and a fellowship grant from Genentech (G-17505). ZSW has received funding from a Scientist Development Award from the Rheumatology Research Foundation, a National Institutes of Health (NIH) Loan Repayment Award, and the NIH/NIAMS T32- AR007258.
Footnotes
Figure S1. Renal survival in patients with rapidly progressive glomerulonephritis. Shown are Kaplan-Meier curves for renal survival in patients with rapidly progressive glomerulonephritis stratified by antineutrophil cytoplasmic autoantibody subtype (a) and further by PLEX status (b). MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3.
Table S1. Relapses during the first year following complete remission.
Table S2. Deaths during remission induction.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Renal survival in patients with rapidly progressive glomerulonephritis. Shown are Kaplan-Meier curves for renal survival in patients with rapidly progressive glomerulonephritis stratified by antineutrophil cytoplasmic autoantibody subtype (a) and further by PLEX status (b). MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3.
Relapses during the first year following complete remission.
Deaths during remission induction.
References
- 1.Jennette J.C., Falk R.J., Gasim A.H. Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens. 2011;20:263–270. doi: 10.1097/MNH.0b013e3283456731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman G.S., Kerr G.S., Leavitt R.Y. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 3.Robson J., Doll H., Suppiah R. Damage in the ANCA-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74:177–184. doi: 10.1136/annrheumdis-2013-203927. [DOI] [PubMed] [Google Scholar]
- 4.Stone J.H., Merkel P.A., Spiera R. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot K., Harper L., Jayne D.R. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 6.Miloslavsky E.M., Specks U., Merkel P.A. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2013;65:2441–2449. doi: 10.1002/art.38044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R.B., Tervaert J.W., Hauser T. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 8.Mansfield N., Hamour S., Habib A.M. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant. 2011;26:3280–3286. doi: 10.1093/ndt/gfr127. [DOI] [PubMed] [Google Scholar]
- 9.Jennette J.C., Falk R.J., Bacon P.A. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar F.B., Pendergraft W.F., 3rd, Wenger J. Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG Levels in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2017:691045–691053. doi: 10.1002/art.40032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone J.H., Hoffman G.S., Merkel P.A. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44:912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Rhee E.P., Laliberte K.A., Niles J.L. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol. 2010;5:1394–1400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendergraft W.F., 3rd, Cortazar F.B., Wenger J. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol. 2014;9:736–744. doi: 10.2215/CJN.07340713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hiepe F., Radbruch A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol. 2016;12:232–240. doi: 10.1038/nrneph.2016.20. [DOI] [PubMed] [Google Scholar]
- 16.Huscher D., Thiele K., Gromnica-Ihle E. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 17.Ginzler E., Diamond H., Kaplan D. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum. 1978;21:37–44. doi: 10.1002/art.1780210107. [DOI] [PubMed] [Google Scholar]
- 18.Stuck A.E., Minder C.E., Frey F.J. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 19.del Rincon I., Battafarano D.F., Restrepo J.F. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264–272. doi: 10.1002/art.38210. [DOI] [PubMed] [Google Scholar]
- 20.Talar-Williams C., Hijazi Y.M., Walther M.M. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med. 1996;124:477–484. doi: 10.7326/0003-4819-124-5-199603010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Travis L.B., Curtis R.E., Glimelius B. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin's lymphoma. J Natl Cancer Inst. 1995;87:524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 22.van den Brand J.A., van Dijk P.R., Hofstra J.M., Wetzels J.F. Cancer risk after cyclophosphamide treatment in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9:1066–1073. doi: 10.2215/CJN.08880813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franssen C.F., Gans R.O., Arends B. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int. 1995;47:193–199. doi: 10.1038/ki.1995.23. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin D.S., Neugarten J., Feiner H.D. The existence of a protracted course in crescentic glomerulonephritis. Kidney Int. 1987;31:790–794. doi: 10.1038/ki.1987.67. [DOI] [PubMed] [Google Scholar]
- 25.Franssen C.F., Stegeman C.A., Kallenberg C.G. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–2206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 26.Harper L., Morgan M.D., Walsh M. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71:955–960. doi: 10.1136/annrheumdis-2011-200477. [DOI] [PubMed] [Google Scholar]
- 27.Guillevin L., Pagnoux C., Karras A. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 28.Hogan S.L., Falk R.J., Chin H. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 29.Langford C.A. Cyclophosphamide as induction therapy for Wegener's granulomatosis and microscopic polyangiitis. Clin Exp Immunol. 2011;164(Suppl 1):31–34. doi: 10.1111/j.1365-2249.2011.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voog E., Morschhauser F., Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348:2691–2694. doi: 10.1056/NEJM200306263482620. discussion 2691–2694. [DOI] [PubMed] [Google Scholar]
- 31.Tesfa D., Ajeganova S., Hagglund H. Late-onset neutropenia following rituximab therapy in rheumatic diseases: association with B lymphocyte depletion and infections. Arthritis Rheum. 2011;63:2209–2214. doi: 10.1002/art.30427. [DOI] [PubMed] [Google Scholar]
- 32.Goupil R., Brachemi S., Nadeau-Fredette A.C. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:416–423. doi: 10.2215/CJN.07300712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilhorst M., Wilde B., van Paassen P. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013;28:373–379. doi: 10.1093/ndt/gfs428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Renal survival in patients with rapidly progressive glomerulonephritis. Shown are Kaplan-Meier curves for renal survival in patients with rapidly progressive glomerulonephritis stratified by antineutrophil cytoplasmic autoantibody subtype (a) and further by PLEX status (b). MPO, myeloperoxidase; PLEX, plasma exchange; PR3, proteinase 3.
Relapses during the first year following complete remission.
Deaths during remission induction.