The role of HPV type distribution on the disparity of cervical cancer (CC) incidence between human populations remains unknown. The incidence of CC in the Amazonas State of Venezuela is higher than the national average. In this study, we determined the diversity of known HPV types (the viral agent of CC) in Amerindian and mestizo women living in the Venezuelan Amazonas State. Understanding the ecological diversity of HPV in populations undergoing lifestyle transformations has important implication on public health measures for cervical cancer prevention.
KEYWORDS: diversity, human papillomavirus, lifestyle, oncogenic virus, urbanization
ABSTRACT
Human papillomavirus (HPV), an etiological agent of cervical cancer (CC), has infected humans since ancient times. Amerindians are the furthest migrants out of Africa, and they reached the Americas more than 14,000 years ago. Some groups still remain isolated, and some migrate to towns, forming a gradient spanning urbanization. We hypothesized that, by virtue of their history, lifestyle, and isolation from the global society, remote Amerindian women have lower HPV diversity than do urban women (Amerindian or mestizo). Here we determined the diversity of the 25 most relevant cervical HPV types in 82 Amerindians spanning urbanization (low, medium, and high, consistent with the exposure to urban lifestyles of the town of Puerto Ayacucho in the Venezuelan Amazonas State), and in 29 urban mestizos from the town. Cervical, anal, oral, and introitus samples were taken, and HPVs were typed using reverse DNA hybridization. A total of 23 HPV types were detected, including 11 oncogenic or high-risk types, most associated with CC. Cervical HPV prevalence was 75%, with no differences by group, but Amerindians from low and medium urbanization level had significantly lower HPV diversity than mestizos did. In Amerindians, but not in mestizos, infections by only high-risk HPVs were higher than coinfections or by exclusively low-risk HPVs. Cervical abnormalities only were observed in Amerindians (9/82), consistent with their high HPV infection. The lower cervical HPV diversity in more isolated Amerindians is consistent with their lower exposure to the global pool, and transculturation to urban lifestyles could have implications on HPV ecology, infection, and virulence.
IMPORTANCE The role of HPV type distribution on the disparity of cervical cancer (CC) incidence between human populations remains unknown. The incidence of CC in the Amazonas State of Venezuela is higher than the national average. In this study, we determined the diversity of known HPV types (the viral agent of CC) in Amerindian and mestizo women living in the Venezuelan Amazonas State. Understanding the ecological diversity of HPV in populations undergoing lifestyle transformations has important implication on public health measures for CC prevention.
INTRODUCTION
Cervical human papillomavirus (HPV) infection (1) is a viral infection of the cervical epithelium (2) and the cause of cervical cancer (CC). It is nearly totally sexually transmitted. More than 80% of sexually active women are infected at least once in their lifetime (3), and its prevalence in a population mostly depends on the multiplicity of sexual partners (4). The course of the infection leads to either clearance by the immune system or persistence as an episome in infected cells (5). More than 180 HPV types have been completely sequenced (http://pave.niaid.nih.gov) (6), and around 40 have mucosal tropism (7). The types of HPVs circulating in a population can be defined by geographical and biological interaction among different HPV types and host immunogenic characteristics (e.g., HLA polymorphisms) (8).
Cervical cancer is one of the five deadliest types of cancer among women. As high as 80% of CC cases occur in developing countries (9, 10), with high mortality due to lower preventive medical screening, higher infection by virulent types, or both. In Venezuela, CC is the main cause of female deaths by cancer (11), with an incidence of 29 per 100,000. In the Amazonas State, the incidence is even higher, of 46 per 100,000 (11), consistent with other reports in Amazonian Amerindians (12).
HPV prevalence among Venezuelan women with normal cytologies has been reported to be 22 to 37% (N = 238 and N = 409, respectively) (13), with seven HPV types detected, including 23% HPV18 and 15% HPV16, followed by HPV31, HPV52, HPV45, HPV58, and HPV56 (<0.5%) (14). Among Venezuelan CC patients, the most common types are 68% HPV16 and 12% HPV18 followed by HPV33, -45, -31, -35, -58, -52, -26, -53, and -66 (<6.3%) (13). One of the very few studies in Amerindians in Brazil reported a prevalence of 46% in a population with 5.6% cytological abnormalities with the most common types being HPV16, HPV31, and HPV18 (15).
The evolution of HPV diversity is not well-known, but HPV has infected humans since times that preceded the human migrations out of Africa (16, 17). Amerindian ancestors that populated the Americas 14,000 to 24,000 years ago (18, 19) must have carried HPVs. We hypothesized that, consistent with their isolation and smaller community sizes, traditional Amerindians from remote villages have lower HPV diversity than urban women do. In this work, we compared HPV diversity between Piaroa Amerindians (living in a gradient of urbanization, from rainforest to town) and town mestizos.
RESULTS
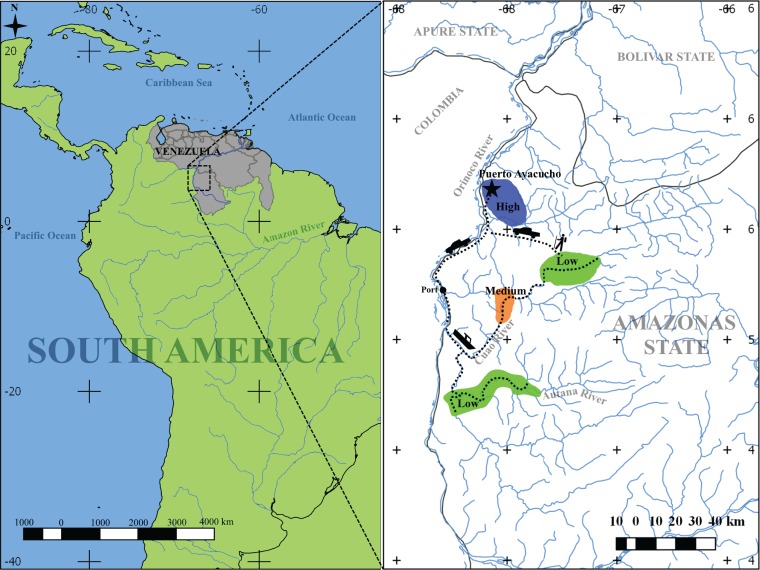
We determined the prevalence and diversity of HPV types in 111 sexually active women in the northern part of the Venezuelan Amazonas State in the Orinoco River basin (Fig. 1). The study included 82 Amerindians living in a spectrum of urbanization (defined as the gradient in lifestyle from traditional to urban), including 24 Amerindians living in traditional villages in the rainforest (low urbanization), 28 living in villages more exposed to non-Amerindians (medium urbanization level), and 30 living in the town capital of the Amazonas State, Puerto Ayacucho, which has a high mestizo population (high urbanization level). We also included 29 mestizos from the town. Surveys were applied to women to determine an individual (subject-based) or village (community-based) urbanization score (see Fig. S1 in the supplemental material; see also the data posted at https://doi.org/10.6084/m9.figshare.5579299.v1). Principal-component analysis (PCA) showed better segregation of subject-based groups (P < 0.003; Fig. S1c and e), than community-based classification (Fig. S1b and d; see also the data posted at https://doi.org/10.6084/m9.figshare.5579299.v1).
FIG 1 .
Diagram of geographic locations included in this study. Sampling was performed at eight locations with different urbanization levels: five locations with low urbanization level (green), one location with medium urbanization level (orange), and two locations with high urbanization (blue). Distances to the urban town were 150 to 210 km (by road and river) for the medium and low urban-level communities. Most communities can be reached only by river; however, some low-level urban communities can be accessed by 1 to 2 days of trekking through the forest. The medium urban level community is located 190 km from an urban location (130 km by river and 60 km by road). The two high urban level communities are located 8 km from each other. The map was generated using Quantum GIS Geographic Information System v. 2.18.14 (https://www.qgis.org/en/site/).
Urban score assignment based on community or subject attributes. (a) Principal-component analysis (PCA) depicting villages based on urban scores. Five villages with low urbanization scores (green), one village with an intermediate score (orange), and two villages with high scores (blue) are shown. Principal component 1 (PC1) separated by urbanization indicator (italic labels), in a gradient from low to high urban level. Urban attribute vectors show directions of community location in the space, with high urban communities placed in the direction of the vectors. The length of the arrow is proportional to the contribution of each urbanization indicator explaining the spatial distribution of women. (b and c) PCA depicting individual urban scores colored by urban groups based on their village (community-based groups) (b) or subject-based groups (c). Community-based medium urbanization and high urbanization groups overlapped their 95% confidence interval (CI95%) ellipses, while low urbanization Amerindians cluster apart from mestizo women (ID refers to Identification Document possession). Subject-based medium urbanization and high urbanization Amerindians groups showed less overlap. The area of each ellipse represents each group distribution with the CI95%. (d and e) Urban index boxplots for community-based groups (d) and subject-based groups (e). Mean urban index comparison indicated significant increase from low to high urbanization Amerindian groups and with mestizos being the highest for both classification approaches (P = 0.000 by ANOVA; P < 0.003 by Tukey’s test for all paired comparisons). Different letters over the boxplots indicate significant differences. P values shown were still significant after Holm correction. (f) Distribution of women by their subject-based urban group (color legend), in each of the community-based urban groups (x axis). The dominant color in each community-based group was concordant with the subject-based urban group, the discordant cases showed heterogeneity within communities reflecting an urbanization transition that occurs at the subject level. (g and h) Linear regression of community versus subject urbanization indices including only Amerindians (g) and for all subjects (66 Amerindians and 24 mestizos) (h). A positive correlation was observed for both cases (linear model, r2 = 0.73, y = 0.636x + 0.123, and slope, P = 0.000 and r2 = 0.56, y = 0.477x + 0.196, slope, P = 0.000, respectively). Download FIG S1, PDF file, 0.9 MB (994.5KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
On the basis of the surveys (see the data posted at https://doi.org/10.6084/m9.figshare.5579299.v1), 77% of the women in the study had never had a cytological screening before. There were no age differences by urbanization level (mean, 28.9 years), use of hormonal contraceptives (uncommon in all groups), or lifetime sexual partners (Table 1 and Table S1). As expected, intestinal protozoa and helminthes were more prevalent in Amerindians than in mestizos (Table 2 and Table S2), and there was a significant increase in Amerindian schooling, sexual contact with mestizo men, smoking, and reduction in parity (number of times a woman has given birth), with urbanization (Table 1 and Table S1). Amerindian women reported practicing only vaginal intercourse, while 28% of mestizo women reported additional practice of oral and/or anal sex.
TABLE 1 .
Demographic characteristics, condition, contraception use, and sexual behavior for 91 womena
| Variableb | Value of variable for: |
P valuec |
||||
|---|---|---|---|---|---|---|
| Amerindians in the following urbanization group: |
Mestizos | |||||
| Low | Medium | High | Amerindians from urbanization groups | Amerindians high vs mestizos | ||
| No. of subjects | 22 | 22 | 23 | 24 | 1.000 (a) | 1.000 (a) |
| Age (yr), mean [range] | 31.1 [12–46] | 31.3 [18–42] | 29.2 [18–44] | 26.7 [17–53] | 0.930 (b) | 0.320 (b) |
| Educational level (%) (n/N) [95% CI] | ||||||
| No studies | 68.2 (15/22) [45.1–85] | 13.6 (3/22) [3.6–34] | 4.3 (1/23) [0.3–24] | 4.1 (1/24) [0.2–23] | 1 × 10−6* (a) | 1.000 (a) |
| Finished elementary school only | 31.8 (7/22) [15–54] | 50.0 (11/22) [31–69] | 17.3 (4/23) [5.7–40] | 16.7 (4/24) [5.5–38] | 0.080 (a) | 1.000 (a) |
| Finished high school | 0.0 (0/22) [0–18.4] | 36.4 (8/22) [18–59] | 78.3 (18/23) [56–92] | 83.3 (20/24) [62–95] | 3 × 10−8* (a) | 0.730 (a) |
| Currently using hormonal contraceptived (%) (n/N) [95% CI] | 4.5 (1/22) [0.2–25] | 0.0 (0/22) [0.0–19] | 4.3 (1/23) [0.2–24] | 29.2 (7/24) [13–51] | 0.600 (a) | 0.060 (a) |
| Parity, mean no. [range] | 5.1 [0–11] | 4.6 [0–13] | 2.0 [1.0–6] | 1.8 [0–8] | 0.003* (b) | 0.730 (b) |
| Currently breastfeeding (%) (n/N) [95% CI] | 72.7 (16/22) [49–88] | 50 (11/22) [31–69] | 39.1 (9/23) [20–61] | 70.8 (17/24) [49–87] | 0.071 (b) | 0.059 (b) |
| Median no. of sexual partners in sexual history [range] | 2.0 [1–4] | 2.5 [1–6] | 2.0 [1–15] | 2.0 [1–25] | 0.850 (c) | 0.210 (c) |
| No. of sexual partners in last 60 days (%) (n/N) [95% CI] | ||||||
| None | 22.7 (5/22) [8.7–46] | 27.3 (6/22) [12–50] | 30.4 (7/23) [14–53] | 16.7 (4/24) [5.5–38] | 0.840 (a) | 0.490 (a) |
| 1 | 77.2 (17/22) [54–91] | 72.7 (16/22) [50–88] | 69.6 (16/23) [47–86] | 79.2 (19/24) [58–92] | ||
| Weekly sexual intercourse frequency (%) (n/N) [95% CI] | ||||||
| ≤1 times | 91.0 (20/22) [69–98] | 72.7 (16/22) [50–88] | 69.6 (16/23) [47–86] | 41.7 (10/24) [23–63] | 0.180 (a) | 0.100 (a) |
| ≥2 times | 9.1 (2/22) [16–31] | 27.3 (6/22) [12–50] | 30.4 (7/23) [14–53] | 58.3 (14/24) [37–77] | ||
| Sexual contact with mestizo (%) (n/N) [95% CI] | 0.0 (0/22) [0.0–19] | 22.7 (5/22) [8.6–46] | 34.8 (8/23) [17–57] | 100 (24/24) [83–100] | 0.012 (a) | 7 × 10−6* (a) |
| Currently smokinge (%) (n/N) [95% CI] | 0.0 (0/22) [0.0–19] | 4.5 (1/22) [0.0–25] | 8.6 (2/23) [1.5–30] | 16.7 (4/24) [5.5–38] | 0.768 (a) | 0.484 (a) |
Demographic characteristics, contraception use, sexual behavior, and other characteristics (variables) are compared for Amerindians in the three subject-based urbanization groups (low, medium, and high) and for urban mestizos.
n/N is the number of women with that characteristic/total number of women in that group. The values for 95% confidence interval (95% CI) are shown in brackets.
The P values comparing the values for Amerindians in the high urbanization group compared to the values for mestizos are shown in the rightmost column. The tests used are shown in parentheses after the P value as follows: (a), χ2 test or Fisher's exact test; (b), t test and ANOVA for two groups or more than two groups; (c), Kruskal-Wallis test. An asterisk indicates that significant differences were reached (P < 0.05) after Holm correction for multiple comparisons.
For nonhormonal contraceptive use, the values were as follows: for Amerindians, zero cases for the low urbanization group, one sterilization for the medium urbanization group, and two sterilizations and one condom use case for the high urbanization group; for mestizos, three condom use cases.
Smoking frequency from 1 to 10 cigarettes daily during 1 or more years.
TABLE 2 .
HPV prevalence, cytological results, intestinal helminthes, and anemia prevalence among subject-based urban groups
| Variable | Value of variable for: |
P valuea |
||||
|---|---|---|---|---|---|---|
| Amerindians in the following urbanization group: |
Mestizos | |||||
| Low | Medium | High | Amerindians from urban groups | Amerindians high vs mestizos | ||
| Prevalence (%) of any HPV typeb (n/N) [95% CI] | 63.6 (14/22) [41–82] | 68.2 (15/22) [45–85] | 78.3 (18/23) [56–92] | 79.2 (19/24) [57–92] | 0.546 | 1.000 |
| HPVb prevalence (%) by age (n/N) [95% CI] | ||||||
| ≤35 years old | 57.2 (8/14) [30–81] | 69.0 (9/13) [39–90] | 75.0 (12/16) [47–92] | 75.0 (15/20) [51–90] | 0.657 | 1.000 |
| >35 years old | 75.0 (6/8) [36–96] | 67.0 (6/9) [31–91] | 85.7 (6/7) [40–100] | 100 (4/4) [40–100] | 0.843 | 1.000 |
| Prevalence (%) of any HPV typeb excluding women with cervical abnormality (n/N) [95% CI] | 60 (12/20) [36–80] | 61.1 (11/18) [36–82] | 77.2 (17/22) [54–91] | 86.6 (19/22) [64–96] | 0.414 | 1.000 |
| Prevalence (%) of any high-risk HPV typeb (n/N) [95% CI] | 54.5 (12/22) [33–75] | 68.2 (15/22) [45–85] | 78.3 (18/23) [56–92] | 62.5 (15/24) [41–80] | 0.237 | 0.389 |
| Prevalence (%) of multiple HPV typesc among HPV-positive women (n/N) [95% CI] | 71.4 (10/14) [42–90] | 66.7 (10/15) [39–87] | 38.9 (7/18) [18–64] | 61.2 (11/19) [36–82] | 0.124 | 0.408 |
| Prevalence (%) of cervical abnormalities (n/N) [95% CI] | 9.1 (2/22) [1.6–31] | 18.2 (4g/22) [6.0–41] | 4.3 (1/23) [0.2–24] | 0.0 (0/22d) [0.0–15] | 0.287 | 0.489 |
| Prevalence (%) of cervical inflammation (n/N) [95% CI] | 100 (22/22) [82–100] | 100 (22/22) [82–100] | 95.7 (22/23) [76–100] | 100 (22/22d) [82–100] | 1.000 | 1.000 |
| Prevalence (%) of intestinal helminthese (n/N) [95% CI] | 75 (15/20) [51–90] | 65 (13/20) [41–84] | 33.3 (5/15) [13–61] | 28.6 (2/7) [5.1–70] | 0.038 | 1.000 |
| Prevalence (%) of anemiaf (n/N) [95% CI] | 27.3 (6/22) [12–50] | 27.3 (6/22) [12–50] | 13.0 (3/23) [3.4–35] | 0.0 (0/24) [0.0–17] | 0.415 | 0.218 |
aThe P values comparing the values for Amerindians in the high urbanization group compared to the values for mestizos are shown in the rightmost column. P value reached significant differences (P < 0.05) after Holm correction for multiple comparisons. The χ2 test or Fisher’s exact test was used.
High-risk HPV detected by the LiPA25 test: HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. Low-risk HPV detected by the LiPA25 test: HPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 68/73, 70, and 74. Note that any incidence in type 68/73 is counted as one HPV type.
More than one HPV from any risk type.
Two cytology results from mestizo group were excluded because of poor-quality smears.
Ascaris lumbricoides, Hymenolepis diminuta, Trichuris trichiura, Enterobius vermicularis, Strongyloides stercoralis, and Ancylostomatidae.
Hemoglobin levels lower than 120 (grams/liter), according to the WHO.
One woman was negative by cytology but positive by biopsy specimen.
Demographic characteristics, contraception, and sexual behavior for 111 women of the study by community-based urbanization groups. Download TABLE S1, PDF file, 0.1 MB (100.7KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cervical HPV prevalence, cytological results, and prevalence of intestinal helminthes and anemia by community-based urbanization groups. Download TABLE S2, PDF file, 0.1 MB (100.4KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
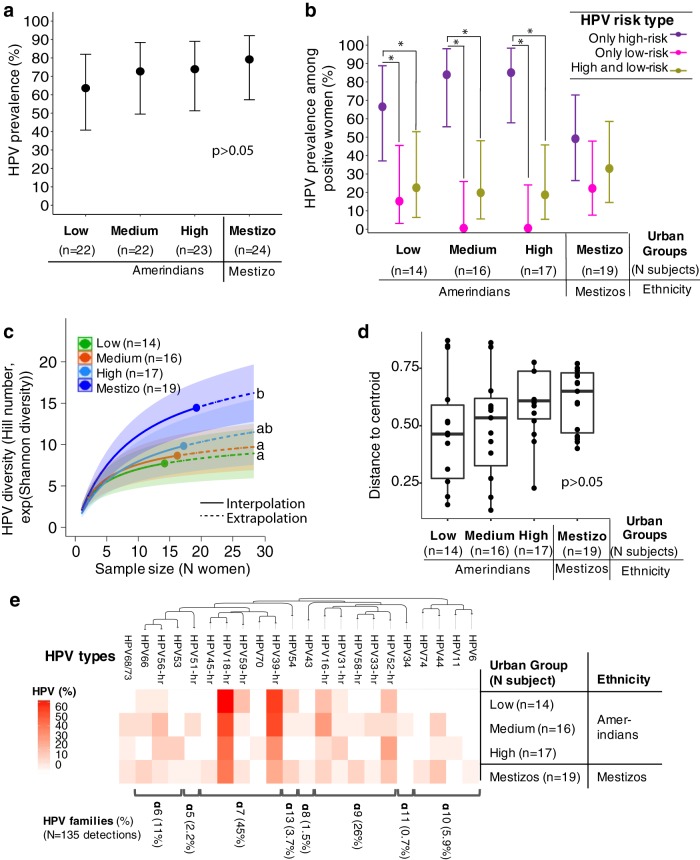
The overall prevalence of cervical HPV in this study was 75% (74%, excluding cervical abnormalities; see below) and did not differ between urban groups (P > 0.05; Table 2, Table S2, Fig. 2a, and Fig. S2a). There was a median of 1 to 2 HPV types per woman (Table 3 and Table S3; not different between groups; P > 0.05), and the differences in the frequency of single or multiple HPV infections were not significant between groups (P > 0.124; Table 2 and Table S2). In Amerindians, but not in mestizos, the prevalence of infections by exclusively high-risk HPVs was higher than infections with exclusively low-risk HPVs or with both HPV risk types (P = 0.007; Fig. 2b and Fig. S2b).
FIG 2 .
Prevalence and diversity of cervical HPV by subject-based urban groups. (a) HPV general prevalence. (b) HPV risk type prevalence. No prevalence differences were found among Amerindian groups (P = 0.540 by χ2 test) or between Amerindians from the high urban group and mestizos (P = 1.000 by χ2 test). Unlike mestizos, Amerindian women showed higher prevalence of only high-risk HPV types in relation to low-risk HPV or both types (P = 0.007 in the log linear model). The circles represent mean prevalence, and the bars show 95% confidence intervals (95% CIs). Prevalence that is statistically significantly (P < 0.050) different is indicated by a bar and asterisk. (c) Shannon diversity (Hill number q = 1) of cervical HPV by urban groups, based on a rarefied/extrapolated sample size of 28 women. Amerindians for low and medium urban groups were significantly less diverse than mestizos. There was a nonsignificant tendency to increasing HPV diversity with urbanization. The solid line curve fraction (interpolation) corresponds to the actual number of women sampled. The dashed line corresponds to the estimated diversity (extrapolation). Curved shaded areas represent the 95% CIs estimated from the bootstrap (50 replications). Significant differences are reached when 95% CIs do not overlap. Different letters indicate significant differences. (d) Beta diversity analysis by urban groups. Median distance to the centroid using Sorensen dissimilarity index. No difference among or within a group’s dispersion was observed (P > 0.05, PERMANOVA and permutation test for homogeneity of multivariate dispersions). (e) Heat map of prevalence of cervical HPV types. HPV18 and HPV39 of the α7 family showed the highest relative proportions. HPV L1 region sequences were used to generate a maximum likelihood tree rooted with theta HPV type (not shown). HPV families and their relative proportions (as a percentage; among only HPV-positive samples) are shown on the right. HPV68 and HPV73 were excluded from the tree, since the LiPA25 kit does not discriminate between these two types.
TABLE 3 .
Cervical HPV alpha, beta, and gamma diversity measuresa
| Diversity measure | Value for diversity measure for the followingb: |
||||
|---|---|---|---|---|---|
| Amerindians in the following urbanization group: |
Mestizos (n = 19) | All individuals (n = 66) | |||
| Low (n = 14) | Medium (n = 16) | High (n = 17) | |||
| Median no. of HPV types per woman [range]c | 2 [1.0–4.0] | 2 [1.0–4.0] | 1 [1.0–4.0] | 2 [1.0–6.0] | 2 [1.0–6.0] |
| No. of high- and low-risk HPV typesd | 11 | 12 | 13 | 18 | 21 |
| No. of high-risk HPV typesd | 7 | 8 | 10 | 11 | 11 |
| No. of low-risk HPV typesd | 5 | 5 | 2 | 7 | 10 |
| Observed richness (Hill no. q = 0) [95% CI] | 13.2 [8.7–17.7] (A) | 13.7 [9.9–17.6] (A) | 15.3 [11.5–19.2] (A) | 19.7[16.0–23.4] (A) | 21.0 [21.4–39.6] |
| Shannon diversitye (Hill no. q = 1) [95% CI] | 8.6 [6.0–11.3] (A) | 9.4 [6.6–11.4] (A) | 10.9 [8.2–13.7] (AB) | 15.5 [11.4–19.6] (B) | 12.6 [13.6–16.0] |
| Simpson diversitye (Hill no. q = 2) [95% CI] | 6.2 [4.1–8.4] (A) | 7.0 [4.1–9.4] (AB) | 8.2 [4.8–11.6] (AB) | 12.4 [8.8–15.9] (B) | 8.7 [8.7–10.9] |
| Mean Sorensen dissimilarity indexf | 0.755 | 0.757 | 0.819 | 0.826 | |
Alpha diversity analysis by urban groups was performed at a rarefaction/extrapolation of 28 women per group and at 66 women among all population (gamma diversity).
The presence of different capital letters within parentheses across groups indicate significant differences based on the non-overlapping of their 95% CI in brackets.
Median comparison was performed with Kruskall-Wallis test. Two comparisons were performed: among Amerindian groups and between Amerindians from high urbanization and mestizos; none were statistically significant.
High-risk HPV detected by the LiPA25 test: HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. Low-risk HPV detected by the LiPA25 test: HPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 68/73, 70, and 74. Note that any incidence of 68/73 is counted as one HPV type.
Shannon diversity refers to exp(Shannon diversity), and Simpson diversity refers to 1/Simpson index.
Sorensen index of dissimilarity. Comparisons were performed with permutation test for homogeneity of multivariate dispersions, based in 99 permutations. No group was significantly different.
Prevalence and diversity of cervical HPV by community-based urban groups. (a) HPV general prevalence. (b) Risk type prevalence. No general differences were found in prevalence by Amerindian groups (P = 0.540 by χ2 test) or between Amerindians from the high urbanization group and mestizos (P = 0.570 by χ2 test). Unlike mestizos, Amerindian women showed higher prevalence of having only high-risk HPV types in relation to low-risk HPV types or both types (*, P < 0.05, log-linear model). Dots represent prevalence, and bars are 95% confidence interval (CI95%). (c) Sample size-based Shannon diversity of cervical HPV by urban groups on an extrapolated 32 women. Amerindians for low and high urbanization groups were significantly less diverse than the mestizo group. There was a nonsignificant tendency of increasing HPV diversity with urbanization (CI95%, P > 0.05). The interpolation (solid line) curve fraction corresponds to the HPV diversity of the actual number of women sampled. The extrapolation fraction (dashed lines) corresponds to the estimated diversity. Curved shaded areas represent the CI95% estimated from the bootstrap (50 replications). Different letters indicate significant differences, reached when CI95% do not overlap. (d) Beta diversity analysis by urban groups. Median distance to centroid, using Sorensen dissimilarity index. No significant difference in variability between or within groups was observed (P > 0.05 by PERMANOVA and permutation test for homogeneity of multivariate dispersions). (e) Heat map of the prevalence of cervical HPV types. HPV18 and HPV39 of the α7 family show the highest relative proportions. HPV L1 region sequences were used to generate a maximum likelihood tree rooted with theta HPV type (not shown). HPV families and their relative proportions (as a percentage; among only HPV-positive samples) are shown on the right. HPV68 and HPV73 were excluded from the tree, since the LiPA25 kit does not discriminate between these two types. Download FIG S2, PDF file, 0.8 MB (812.7KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cervical HPV alpha and gamma diversities among HPV-positive women by community-based urbanization groups. Sample size-based, coverage-based, and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 1, 2, and 3, respectively) at a rarefied/extrapolated sample size of 32 women or 83 women for all populations (gamma diversity) or at a coverage-based level of 0.937 and 0.977, respectively. Shannon and Simpson metrics show mestizo women with a significantly higher HPV diversity than low urbanization Amerindians. Mestizos had a significantly higher Simpson index than any other Amerindian group in the asymptotic estimation. There was a nonsignificant tendency of increasing diversity with urbanization in Amerindians. Download TABLE S3, PDF file, 0.1 MB (76.9KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
A total of 23 HPV types were detected, of which 22 were from the cervix (Table S3). Alpha diversity was significantly higher in mestizos than in Amerindians from the lowest urban levels (based on subject-based groups in Fig. 2c, Table 3, and Table S4; community-based classification in Table S3 and Fig. S2c). The group differences in alpha diversity were mainly due to relative abundance rather than richness of HPV types (Table 3 and Tables S3 and S4). Beta diversity analysis using Sorensen dissimilarity index showed a nonsignificant tendency of increasing with urbanization (Fig. 2d, Fig. S2d, Table 3, and Table S3). For both classification approaches (subject- and community-based urban groups), a hierarchical tree showed that mestizos segregated from Amerindian low and medium urban groups (see the data posted at https://figshare.com/s/9bffb3ea746016f78b4e).
Cervical HPV alpha, beta, and gamma diversities among positive women by subject-based urbanization groups. Coverage-based and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 1, 2, and 3, respectively). Coverage-based analyses for urban groups and mestizos were performed at a coverage level of 0.906, while for the total population, the coverage level was 0.971. Mestizos showed significant higher diversity than Amerindian low urbanization group. At the asymptotic estimation, mestizos showed a significant higher HPV diversity than the Amerindian low urbanization group. There was a nonsignificant tendency of increasing diversity with urbanization in Amerindians. Download TABLE S4, PDF file, 0.1 MB (105.2KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The most common HPV family was α7, followed by α9. HPV18 and HPV39 were the most prevalent cervical types (Fig. 2e and Fig. S2e). Only six cervical HPVs, all of them high-risk types, were shared among women in the four groups (Fig. S3). A comparative analysis of body site HPV distribution in 16 women with at least one body site positive for HPV showed 15 viral types in the cervix (14 women), 6 in the introitus (10 women), 4 anal (7 women), and 7 oral (6 women) (Table S5). The highest HPV prevalence and diversity was found in the cervix (P < 0.050; Table S5), and cooccurrence of any high-risk HPV or HPV18 in different body sites was low (Cohen’s kappa coefficients of ≤0.26 and ≤0.37, respectively; Table S6).
Prevalence of high- or low-risk cervical HPV types among infected women by urbanization groups. HPV18 and HPV39 showed the highest prevalence. Percentages were calculated per urbanization group. Download FIG S3, PDF file, 0.3 MB (267.3KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HPV prevalence and alpha and beta diversity by body site in 18 women, of which 16 are HPV infected in at least one body site. Sample-size-based, coverage-based, and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 0, 1, and 2, respectively) at a rarefied/extrapolated sample size level of 12 women or at a coverage level of 0.854. The cervix had the highest observed richness. Beta diversity with Sorensen dissimilarity index did differ significantly between groups. Download TABLE S5, PDF file, 0.1 MB (106.8KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Congruence of detection of any high-risk HPV or HPV18 across body sites. There was low to no significant agreement in the presence of high-risk HPV in different body sites. Agreement between body sites varied from “less than chance” to “fair.” The highest specificity was observed in any high-risk HPV cooccurrence in anal and introitus sites. The highest sensitivity was observed in HPV18 cooccurrence in cervix and introitus sites. Download TABLE S6, PDF file, 0.1 MB (70.3KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Nine women presented cervical abnormalities, and they were all Amerindians with mostly high-risk HPV infections (Table 2, Table S2, and Table S7; see the data posted at https://doi.org/10.6084/m9.figshare.5579299.v1). The presence of cervical lesions in these women did not significantly change HPV diversity.
HPV types, age, and urban group classification of nine women with cervical abnormalities. Download TABLE S7, PDF file, 0.1 MB (97.6KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Urban groups segregated better using subject-based rather than community-based metrics of classification, likely because villages in transition are heterogeneous in the lifestyles of their individuals. Interestingly, the Amerindian women who lived in the town did not reach the high urban scores of mestizo women, reflecting a certain level of attachment to their traditional lifestyles.
Cervical HPV prevalence in this study is similar to that reported in high-risk populations (20, 21) and higher than in other reports that used the same detection method, in Latin America (37 to 51%) (20, 22), Europe (<20%) (23), and Japan (<20%) (24). This disparity in prevalence may be due to the high prevalence of anemia and intestinal helminthes, which may reduce HPV clearance (25), degree of isolation from the global HPV pool, etc., while for industrialized countries, vaccination is significantly reducing HPV infection (26, 27).
The results of more isolated Amerindians having lower HPV diversity than mestizos confirmed our hypothesis and are consistent not only with the Amerindian’s higher isolation from the global viral pool but also with their lower genetic diversity. Amerindians descend from Asians who migrated east from Africa in successive genetic bottlenecks, thus only a fraction of the population—and gene pools—advanced (19, 28). Across the urbanization gradient, Amerindians become more exposed to mestizos and increase their genetic diversity (mestizaje) as well as their exposure to the global viral pool. However, a previous study in isolated Yanomamis of Brazil (15) reported higher HPV diversity than in more urbanized Macuxi and Wapishana Amerindians. This contradiction might be affected by the HPV detection methods used or by the degree of real isolation of the studied populations. In this study, we used a sensitive hybridization method that recognizes 25 HPV types (29, 30) based on L1 gene, the most conserved region in the HPV genome (31). The sensitivity of the detection method could decrease if there was divergence of the HPV during the isolation of Amerindian groups in the last 12,000 to 24,000 years. However, the probability of new diversity seems low, based on the estimated 200,000 years of evolution for intratypic variation of HPV18 (32). The question of novel variants in Amerindians is beyond the scope of the present study that aimed at characterizing the known HPV types, but future metagenomic studies should address this important question.
In relation to the presence of HPV in multiple body sites, our study shows 33% oral HPV (which is higher than in other reports using the same detection method; e.g., 1.6% in Costa Rica [33]) and 30% anal prevalence (similar to that in other reports [22]). There was low cooccurrence of specific HPV types in different body sites, which might result from epithelial tropism (34, 35) or site-related clearance (36) and may depend on sexual practices, such as nonvaginal sex (37), that are uncommon in our studied population. However, there can be extrasexual HPV transmission, such as self-transmitted to different body sites, or mother-child vertical transmission (38). The fact that the introitus site showed lower HPV prevalence than cervix (24 versus 75%, respectively) has implications when self-sampling is used for sample collection in population cervical HPV screenings.
Understanding the causes underlying the high incidence of CC in Amerindians is of crucial importance for decisions in public health interventions. While the same virulent types circulate among Amerindian and mestizo women, Amerindians showed higher prevalence of infections by the virulent types than infection by low-risk types or both. Amerindians in this study did show high HPV18 and HPV16, common virulent types in other human groups, but they also had high prevalence of a rare high-risk HPV type of the α7 family, HPV39, consistent with reports for Amerindians in the northern United States (39) and Central and South America (40). Its prevalence in this study shows a nonsignificant trend to decrease with urbanization. Regrettably, contemporary HPV vaccines do not include this virulent HPV39 highly prevalent in these populations.
That cervical abnormalities were found only in Amerindians, consistent with the epidemiological evidence of high CC incidence in this human group (12), suggests that infections by only oncogenic HPVs increase the risk of cervical abnormalities; this was reported before for squamous CC (41). Amerindian genetic variations in the immune-relevant HLA-B locus may also increase their susceptibility to colonization by oncogenic types (42, 43). A high prevalence of only oncogenic HPV infections is consistent with the more efficient clearance of low-risk HPVs in relation to high-risk HPVs, which evade immune clearance, producing low virion yields (44, 45), and thus, the factors that sustain the coexistence of different HPV risk types in mestizos are unclear. Coexistence of high- and low-risk HPVs has been associated with higher sexual partner turnover (46), although we did not find differences in the reported number of sexual partners. Definitely, more studies are needed to clarify the relative contribution of lifestyle and host genetic factors to the type of HPV infection and health risks. The results of this study are consistent with the association between high-risk HPVs and increased inflammation and risks of cervical lesions (41, 47), and this is particularly serious in regions with precarious or nonexistent health services (48). Finally, the elimination of high-risk HPV types with the current vaccines is a promising scenario to reduce the dramatically high CC mortality in Venezuelan Amerindians. Studies that follow up the effects of the vaccines on the circulating HPV diversity, using metagenomic approaches (15) will be important for monitoring the evolution of HPV type virulence.
MATERIALS AND METHODS
Experimental design.
This study included young adult, nonpregnant, healthy women from the Venezuelan Amazon. The women were from the following two groups: Piaroa Amerindian from villages in a spectrum of urbanization (from traditional to urban lifestyles) or urban mestizo. All experimental protocols were approved by SA Centro Amazónico de Investigación y Control de Enfermedades Tropicales Simón, Bolívar, Venezuela (SACAICET, IRB 78-2014), and University of Puerto Rico (IRB 1314-163).
Inclusion criteria.
Women included in the study belonged to eight different villages in northern Amazonas State, Venezuela: one urban town, Puerto Ayacucho (state capital), one village in the periurban area, and six villages at the Orinoco Basin on the Sipapo River, Autana River, and Cuao River (Fig. 1). A total of 228 sexually active women attending a health evaluation were invited to participate, and 111 (82 women who self-identified as Amerindians with Piaroa ethnicity, appeared to be Piaroa Amerindians, and also spoke Piaroa language and 29 urban mestizos) aged 12 to 53 years were included in the study. We had received prior approval from the captain/leader to visit the villages. Informed consent was obtained from all participants and/or their legal guardians. Parental consent was requested for women less than 18 years old. Inclusion criteria included women at reproductive age who at the time of recruitment had none of the following: pregnancy, menses, bleeding in the last 24 h, sexually transmitted infection diagnosed in the last 2 months, antibiotics in the last month, vaginal douches in the last 24 h, sexual intercourse in the last 24 h, hysterectomy, diabetes, urinary incontinence, urinary tract infections, and HIV. Individuals excluded from the study (n </it>= 117) were mostly due to recent exposure to antibiotics or antiparasitic drugs (28%), menses (25%), postmenopausal (13%), pregnant (12%), urinary infections (8%), refusing to participate (4%), sexual contact in the last 24 h (3%), hysterectomy (2%), belonging to a different ethnicity (1%), diabetes (1%), and HIV (1%).
Surveys and urban classification.
Each woman received two urbanization indices, one based on her individual exposure to urban practices (subject-based index) and another on her community urban level (community-based index) (see data posted at https://doi.org/10.6084/m9.figshare.5579299.v1). Subject-based surveys included education, identification document (ID) possession, purchasing power, preservation of traditional practices, frequency of mobility to urbanized towns, level of environmental exposure (drinking water treatment, use of shoes, etc.), use and acceptance of Western medicine, and level of adoption of nontraditional diets (see data posted at https://doi.org/10.6084/m9.figshare.5579299.v1). Community-based urbanization survey included access to health, urban services (electricity, telephone, gas, and water), political representation, education, salaries, and language command (Spanish-Piaroa). This village survey was completed with the community captains, schoolteachers, or health workers (see data posted at https://doi.org/10.6084/m9.figshare.5579299.v1).
Categorical variables of the urbanization surveys were transformed into numeric values ranked between 0 and 1, with 1 being the highest level of urbanization (also reflecting the loss of traditional practices). Each indicator component was equally weighted, and its values were averaged using arithmetic means. Community-based groups included 111 women, but subject-based groups included only 91 women due to missing data in the surveys. Urban groups had similar sample sizes (Table 1 and see Table S1 in the supplemental material). Community urban indices were categorized in three levels: low (scores below 0.33; n = 24 women), medium (scores of >0.33 and <0.66; n = 28 women), and high (scores above 0.66; n = 30 women). Subject-based urbanization groups were built first, sorting in ascending order individual women scores and then grouping them in tertiles: the first group corresponds to the low urban group (n = 22 women; scores of 0.22 to 0.37), the second group corresponds to the medium urban group (n = 22 women; scores of 0.40 to 0.55), and the third group corresponds to the high urban group (n = 23 women; scores of 0.56 to 0.77). Mestizo women had a high urban level by both classification approaches (n = 29 to 24, respectively) (scores of 0.70 to 0.93 for subject-based groups).
Clinical history, sexual behavior, contraceptive usage, and hygiene practices were also recorded in a separate clinical survey (see the data posted at https://doi.org/10.6084/m9.figshare.5579299.v1). Surveys were coded without personal identifiers.
Samples.
Swabs were taken by specialized health personnel, from cervix/fornix (referred to as cervix in the text) (N = 111), introitus (N = 18), anal (N = 18), and oral (N = 18) sites. DNA was extracted using Power Soil DNA kit (Mo Bio Laboratories Inc.) according to the manufacturer’s instructions. The main concern about HPV detection methods is obtaining false-negative results, usually after not being able to extract/detect viral DNA in an HPV-positive sample. The Power Soil method involves an aggressive bead beating step and allowing good extraction of the viral DNA. Cervical smears were performed by an obstetrician-gynecologist using an endocervical brush and spatula, and biopsy specimens were taken and treatment was provided if indicated. Papanicolaou’s stain was performed for the cytological analysis. Results were reported according to Bethesda 2001 classification system. A drop of blood was taken from fingers for in situ hemoglobin (using Easylife rapid test in peripheral blood) to detect anemia according to the WHO limits (49). Sera were transported for HIV, syphilis, and hepatitis B and C detection, processed at the Public Health Center of Puerto Ayacucho, Amazonas State, Venezuela. Fecal samples were taken, preserved using iodine-formaldehyde, and microscopically analyzed for the presence of intestinal protozoa and helminthes by microscopic methods.
HPV genotyping.
The approach used in this study, the SPF10 assay that amplifies 60 different known HPV strains with high sensitivity (29, 30) and hybridizes the SPF10 PCR product on the LiPA25, was limited to 25 of the most relevant and prevalent known genotypes. A reverse hybridization method SPF10-PCR-LiPA25 system, version 1 (Labo Biomedical Products, Rijswijk, The Netherlands, based on licensed Innogenetics technology) (50), was used to detect HPV and typing 25 of the most common mucosa HPV types (types 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74). Briefly, 65-bp biotinylated amplicons from the highly conserved L1 gene region were generated using SPF10 primers. Amplified fragments were hybridized with a strip with specific oligonucleotide probes for each of the 25 HPV types. Visualization was performed by adding streptavidin-conjugated alkaline phosphatase to the hybrids formed, yielding a dark precipitate in a particular strip area that determines the specific HPV type. Negative and positive controls were included. We confirmed results of the highly sensitive method for HPV detection using the SPF10 primers (29, 30), repeating a subsample of replicate swabs from 10 women. This is a study performed in a non-HPV-vaccinated population, since HPV vaccines have not been included in the national vaccination program in Venezuela.
Statistical analysis.
Principal-component analysis (PCA) for the villages and for women based on their urbanization indicator values were performed with the ggfortify package (51) in R (52). To visualize the urban groups for both types of classification, 95% confidence interval (95% CI) ellipses were drawn for community-based and subject-based group distributions (Fig. S1). Mean comparisons among urban group scores were performed with analysis of variance (ANOVA) and Tukey’s test as a posthoc test (Fig. S1). Correlations between village- and subject-based urban scores among all populations and only including Amerindians were evaluated by a linear regression (Fig. S1).
Association between prevalence of HPV types, having only a high-risk or low-risk type or both risk types, and comparisons among single and multiple types and among body sites, were performed using log linear models and the contrast package (53) in R version 3.3.2 (52) to compute comparisons of the estimated regression coefficient. Comparisons of means with normal distribution (verified by QQ plot) were performed with ANOVA and Tukey’s test as a posthoc test, and between Amerindians from high urbanization group and mestizo group with Student’s t test. Means with nonnormal distribution were compared using the Kruskal-Wallis test. Proportion comparisons among groups were performed with Pearson’s chi-squared test (χ2 test) with Yates’ continuity correction if needed or Fisher exact test for count data. P values were adjusted for multiple comparisons by the Holm method (54) (Table 1 and Tables S1 and S2). P values of <0.05 were considered statistically significant.
High-risk HPV and HPV18 detection agreement across body site were measured for only 18 women using Cohen’s kappa coefficient (55, 56) and were interpreted as follows: Cohen’s kappa coefficient of <0, less than chance; 0.01 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and 0.81 to 0.99, almost perfect (Tables S5 and S6) (56, 57).
Phylogenetic trees were built based on the HPV L1 region from sequences obtained from the PaVE database (58); MAFFT was used for the nucleotide alignment (59). The maximum likelihood method was used in PhyMLb (60). The tree was rooted with theta HPV type (not shown in the figure) (Fig. 2e and Fig. S2). The hierarchical tree for HPV and urban groups or individual women was built using hclust function from the R base “stats” package by the Spearman method and was visualized together with a heat map plotted with heatmap.2 from gplots (61) and RColorBrewer (62) from R packages.
Alpha and gamma HPV diversity were measured using three of the most typical used Hill’s family of diversity (63–65) numbers or the effective number of types, order (q) 0, 1, and 2 (HPV type observed richness, exponential of Shannon entropy index, and inverse of Simpson concentration index) integrating rarefaction (interpolation) and extrapolation curves following Hsieh et al. (66) approach using iNEXT package (66) in R 3.3.2 version (52). Hill number of order 0 (q = 0) counts all HPV types present in each group, Hill number of order 1 (q = 1) can be interpreted as the number of common HPV types per group, and Hill number of order 2 (q = 2) can be interpreted as the number of dominant HPV types. Alpha diversities were compared using the nonasymptotic and asymptotic analysis for incidence type data. For the nonasymptotic analysis, we compared groups at the same sample size (sample size based) or at the same level of sampling coverage (sample coverage based). The latter measures the proportion of the total number of individuals that belong to the HPV type detected in the sample and has been shown to better evaluate the magnitude of the diversity differences among groups than the traditional sample size-based comparison (64). The asymptotic analysis allowed estimating diversity when the accumulation curves reach the asymptote guided by the Chao2 estimator. Comparisons among groups were performed at the extrapolated diversity values. The 95% CI was estimated from the bootstrap method based on 50 replications. Significant differences were reached when the 95% CIs among groups did not overlap (Fig. 2c, Fig. S2, Table 3, and Tables S3 to S5).
Beta diversity was analyzed with the vegan (67) package in R (52). Beta diversity was measured using the nonparametric permutational multivariate analysis of variance (PERMANOVA) (68) to compared variance between groups with the variance within groups. Sorensen dissimilarity index matrix was built with betadiver function. The model calculates a pseudo F ratio that is tested for significance based on 999 permutations. A more robust analysis for within group dispersion (variance) comparison was performed with the permutation test for homogeneity of multivariate dispersions (10). The betadisper function was used to reduce the distances to the principal coordinate. The method computes the F statistic to compare median distances-to-centroids of each group. P value was generated with the permutest function based on 999 permutations. The plot function was used for the principal-coordinate analysis visualization (Fig. 2d, Fig. S2, and Tables S3 and S4).
Statistics and graphics were also performed using reshape2 (69), ggplot2 (70) and defaults R 3.3.2 version functions (52). The map was generated using QGIS Geographic Information System 2.18.14 (71).
Data availability.
Three data sets containing urbanization survey results and metadata and other data have been deposited in figshare at https://doi.org/10.6084/m9.figshare.5579299.v1.
ACKNOWLEDGMENTS
This work was supported by the Emch Foundation and C&D Research Fund.
We acknowledge the collaboration in the field of Vanessa Ochoa, Yeslin Rivas, José Alayón, María Antonieta Aguilar, Aura Marín, Elvis Sanz, Gabriel Tobar, Carla Tovar, Yseliam Tovar, Lindsay Gómez, Oscar Noya, Damián Ruiz, Aníbal Carrasquel, Luis Abreu, Julieta Hernández, Oriana Vargas, Mercedes Robles, Miguel Ángel Vargas, Rafael López, Karla González, and the personnel at the Department of Pathology, Domingo Luciani Hospital. We thank the Dean for Graduate Studies and Research at the University of Puerto Rico. We thank Yi Cai for her technical support in the lab at NYU. We are grateful to the volunteers, community leaders, and health personnel from the villages.
REFERENCES
- 1.zur Hausen H, Gissmann L, Steiner W, Dippold W, Dreger I. 1975. Human papilloma viruses and cancer. Bibl Haematol 1975:569–571. [DOI] [PubMed] [Google Scholar]
- 2.Jeon S, Allen-Hoffmann BL, Lambert PF. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol 69:2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, Tan T, Halsey N, Jenkins D. 2009. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis 9:347–356. doi: 10.1016/S1473-3099(09)70108-2. [DOI] [PubMed] [Google Scholar]
- 4.Burchell AN, Winer RL, de Sanjosé S, Franco EL. 2006. Epidemiology and transmission dynamics of genital HPV infection. Vaccine 24(Suppl 3):S52–S61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. 2012. The biology and life-cycle of human papillomaviruses. Vaccine 30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins MJ, Sharp R, Macfarlane GT. 2002. Variation in human intestinal microbiota with age. Dig Liver Dis 34(Suppl 2):S12–S18. doi: 10.1016/S1590-8658(02)80157-8. [DOI] [PubMed] [Google Scholar]
- 7.Egawa N, Egawa K, Griffin H, Doorbar J. 2015. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildesheim A, Wang SS. 2002. Host and viral genetics and risk of cervical cancer: a review. Virus Res 89:229–240. doi: 10.1016/S0168-1702(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 11.Negrin LGC. 2006. Aspectos epidemiológicos del cáncer en Venezuela. Rev Venez Oncol 18:269–281. [Google Scholar]
- 12.da Silva CS, Adad SJ, Hazarabedian de Souza MA, Macêdo Barcelos AC, Sarreta Terra AP, Murta EF. 2004. Increased frequency of bacterial vaginosis and Chlamydia trachomatis in pregnant women with human papillomavirus infection. Gynecol Obstet Invest 58:189–193. doi: 10.1159/000079822. [DOI] [PubMed] [Google Scholar]
- 13.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. 2017. Human papillomavirus and related diseases report in Venezuela. Summary Report 27 July 2017 ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre), Barcelona, Spain: www.hpvcentre.net. [Google Scholar]
- 14.Téllez L, Michelli E, Mendoza JA, Vielma S, Noguera ME, Callejas D, Cavazza M, Correnti M. 2015. Persistent infection with high-risk human papilloma viruses: cohort study, Merida, Venezuela. Ecancermedicalscience 9:579. doi: 10.3332/ecancer.2015.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca AJ, Taeko D, Chaves TA, Amorim LD, Murari RS, Miranda AE, Chen Z, Burk RD, Ferreira LC. 2015. HPV infection and cervical screening in socially isolated indigenous women inhabitants of the Amazonian Rainforest. PLoS One 10:e0133635. doi: 10.1371/journal.pone.0133635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho L, Chan SY, Burk RD, Das BC, Fujinaga K, Icenogle JP, Kahn T, Kiviat N, Lancaster W, Mavromara-Nazos P, Labropoulou V, Mitrani-Rosenbaum S, Norrild B, Pillai MR, Stoerker J, Syrjaenen K, Syrjaenen S, Tay SK, Villa LL, Wheeler CM, Williamson AL, Bernard HU. 1993. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J Virol 67:6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, Yamaoka Y, Mégraud F, Otto K, Reichard U, Katzowitsch E, Wang X, Achtman M, Suerbaum S. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeon L, Burke A, Higham T. 2017. Earliest human presence in North America dated to the last glacial maximum: new radiocarbon dates from Bluefish Caves, Canada. PLoS One 12:e0169486. doi: 10.1371/journal.pone.0169486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bortolini MC, Salzano FM, Thomas MG, Stuart S, Nasanen SP, Bau CH, Hutz MH, Layrisse Z, Petzl-Erler ML, Tsuneto LT, Hill K, Hurtado AM, Castro-de-Guerra D, Torres MM, Groot H, Michalski R, Nymadawa P, Bedoya G, Bradman N, Labuda D, Ruiz-Linares A. 2003. Y-chromosome evidence for differing ancient demographic histories in the Americas. Am J Hum Genet 73:524–539. doi: 10.1086/377588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraets DT, Grünberg AW, van der Helm JJ, Schim van der Loeff MF, Quint KD, Sabajo LO, de Vries HJ. 2014. Cross-sectional study of genital carcinogenic HPV infections in Paramaribo, Suriname: prevalence and determinants in an ethnically diverse population of women in a pre-vaccination era. Sex Transm Infect 90:627–633. doi: 10.1136/sextrans-2013-051384. [DOI] [PubMed] [Google Scholar]
- 21.Safaeian M, Herrero R, Hildesheim A, Quint W, Freer E, Van Doorn LJ, Porras C, Silva S, González P, Bratti MC, Rodriguez AC, Castle P, Costa Rican Vaccine Trial Group . 2007. Comparison of the SPF10-LiPA system to the Hybrid Capture 2 Assay for detection of carcinogenic human papillomavirus genotypes among 5,683 young women in Guanacaste, Costa Rica. J Clin Microbiol 45:1447–1454. doi: 10.1128/JCM.02580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro FA, Quint W, Gonzalez P, Katki HA, Herrero R, van Doorn LJ, Schiffman M, Struijk L, Rodriguez AC, DelVecchio C, Lowy DR, Porras C, Jimenez S, Schiller J, Solomon D, Wacholder S, Hildesheim A, Kreimer AR, Costa Rica Vaccine Trial Group . 2012. Prevalence of and risk factors for anal human papillomavirus infection among young healthy women in Costa Rica. J Infect Dis 206:1103–1110. doi: 10.1093/infdis/jis458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenselink CH, Melchers WJ, Quint WG, Hoebers AM, Hendriks JC, Massuger LF, Bekkers RL. 2008. Sexual behaviour and HPV infections in 18 to 29 year old women in the pre-vaccine era in the Netherlands. PLoS One 3:e3743. doi: 10.1371/journal.pone.0003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konno R, Tamura S, Dobbelaere K, Yoshikawa H. 2011. Prevalence and type distribution of human papillomavirus in healthy Japanese women aged 20 to 25 years old enrolled in a clinical study. Cancer Sci 102:877–882. doi: 10.1111/j.1349-7006.2011.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravitt PE, Marks M, Kosek M, Huang C, Cabrera L, Olortegui MP, Medrano AM, Trigoso DR, Qureshi S, Bardales GS, Manrique-Hinojosa J, Cardenas AZ, Larraondo MA, Cok J, Qeadan F, Siracusa M, Gilman RH. 2016. Soil-transmitted helminth infections are associated with an increase in human papillomavirus prevalence and a T-helper type 2 cytokine signature in cervical fluids. J Infect Dis 213:723–730. doi: 10.1093/infdis/jiv498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Karsa L, Arbyn M, De Vuyst H, Dillner J, Dillner L, Franceschi S, Patnick J, Ronco G, Segnan N, Suonio E, Törnberg S, Anttila A. 2015. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res 1:22–31. doi: 10.1016/j.pvr.2015.06.006. [DOI] [Google Scholar]
- 27.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. 2016. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 137:1–9. doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 28.Sudmant PH, Mallick S, Nelson BJ, Hormozdiari F, Krumm N, Huddleston J, Coe BP, Baker C, Nordenfelt S, Bamshad M, Jorde LB, Posukh OL, Sahakyan H, Watkins WS, Yepiskoposyan L, Abdullah MS, Bravi CM, Capelli C, Hervig T, Wee JT, Tyler-Smith C, van Driem G, Romero IG, Jha AR, Karachanak-Yankova S, Toncheva D, Comas D, Henn B, Kivisild T, Ruiz-Linares A, Sajantila A, Metspalu E, Parik J, Villems R, Starikovskaya EB, Ayodo G, Beall CM, Di Rienzo A, Hammer MF, Khusainova R, Khusnutdinova E, Klitz W, Winkler C, Labuda D, Metspalu M, Tishkoff SA, Dryomov S, Sukernik R, Patterson N, Reich D, Eichler EE. 2015. Global diversity, population stratification, and selection of human copy-number variation. Science 349:aab3761. doi: 10.1126/science.aab3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snijders PJ, van den Brule AJ, Meijer CJ. 2003. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol 201:1–6. doi: 10.1002/path.1433. [DOI] [PubMed] [Google Scholar]
- 30.Carozzi FM, Del Mistro A, Confortini M, Sani C, Puliti D, Trevisan R, De Marco L, Tos AG, Girlando S, Palma PD, Pellegrini A, Schiboni ML, Crucitti P, Pierotti P, Vignato A, Ronco G. 2005. Reproducibility of HPV DNA testing by Hybrid Capture 2 in a screening setting. Am J Clin Pathol 124:716–721. doi: 10.1309/84E5-WHJQ-HK83-BGQD. [DOI] [PubMed] [Google Scholar]
- 31.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2007. IARC monographs on the evaluation of carcinogenic risks to humans, vol 90 Human papillomaviruses. World Health Organization International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- 32.Ong CK, Chan SY, Campo MS, Fujinaga K, Mavromara-Nazos P, Labropoulou V, Pfister H, Tay SK, ter Meulen J, Villa LL, Bernard HU. 1993. Evolution of human papillomavirus type 18: an ancient phylogenetic root in Africa and intratype diversity reflect coevolution with human ethnic groups. J Virol 67:6424–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang Kuhs KA, Gonzalez P, Struijk L, Castro F, Hildesheim A, van Doorn LJ, Rodriguez AC, Schiffman M, Quint W, Lowy DR, Porras C, Delvecchio C, Katki HA, Jimenez S, Safaeian M, Schiller J, Solomon D, Wacholder S, Herrero R, Kreimer AR, Costa Rica Vaccine Trial Group . 2013. Prevalence of and risk factors for oral human papillomavirus among young women in Costa Rica. J Infect Dis 208:1643–1652. doi: 10.1093/infdis/jit369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. 2010. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. J Infect Dis 201:1331–1339. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelstein ZR, Schwartz SM, Hawes S, Hughes JP, Feng Q, Stern ME, O’Reilly S, Lee SK, Fu Xi L, Koutsky LA. 2012. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis 39:860–867. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EM, Ritchie JM, Yankowitz J, Wang D, Turek LP, Haugen TH. 2004. HPV prevalence and concordance in the cervix and oral cavity of pregnant women. Infect Dis Obstet Gynecol 12:45–56. doi: 10.1080/10647440400009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernández L, Idris A, Sánchez MJ, Nieto A, Talamini R, Tavani A, Bosch FX, Reidel U, Snijders PJ, Meijer CJ, Viscidi R, Muñoz N, Franceschi S, IARC Multicenter Oral Cancer Study Group . 2003. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 38.Syrjänen S, Puranen M. 2000. Human papillomavirus infections in children: the potential role of maternal transmission. Crit Rev Oral Biol Med 11:259–274. doi: 10.1177/10454411000110020801. [DOI] [PubMed] [Google Scholar]
- 39.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. 2007. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol Oncol 107:236–241. doi: 10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst 87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 41.Sundström K, Ploner A, Arnheim-Dahlström L, Eloranta S, Palmgren J, Adami HO, Ylitalo Helm N, Sparén P, Dillner J. 2015. Interactions between high- and low-risk HPV types reduce the risk of squamous cervical cancer. J Natl Cancer Inst 107:djv185. doi: 10.1093/jnci/djv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watkins DI, McAdam SN, Liu X, Strang CR, Milford EL, Levine CG, Garber TL, Dogon AL, Lord CI, Ghim SH, Troup GM, Hughes AL, Letvin NL. 1992. New recombinant HLA-B alleles in a tribe of South American Amerindians indicate rapid evolution of MHC class I loci. Nature 357:329–333. doi: 10.1038/357329a0. [DOI] [PubMed] [Google Scholar]
- 43.de Araujo Souza PS, Maciag PC, Ribeiro KB, Petzl-Erler ML, Franco EL, Villa LL. 2008. Interaction between polymorphisms of the human leukocyte antigen and HPV-16 variants on the risk of invasive cervical cancer. BMC Cancer 8:246. doi: 10.1186/1471-2407-8-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley M. 2006. Immune responses to human papillomavirus. Vaccine 24(Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. 2007. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 16:709–715. doi: 10.1158/1055-9965.EPI-06-0846. [DOI] [PubMed] [Google Scholar]
- 46.Orlando PA, Gatenby RA, Giuliano AR, Brown JS. 2012. Evolutionary ecology of human papillomavirus: trade-offs, coexistence, and origins of high-risk and low-risk types. J Infect Dis 205:272–279. doi: 10.1093/infdis/jir717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castle PE, Hillier SL, Rabe LK, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Burk RD, Rodriguez AC, Alfaro M, Hutchinson ML, Morales J, Schiffman M. 2001. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol Biomarkers Prev 10:1021–1027. [PubMed] [Google Scholar]
- 48.Kahn JA, Lan D, Kahn RS. 2007. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol 110:87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization, ; Geneva, Switzerland. [Google Scholar]
- 50.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W. 1999. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 37:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Y, Horikoshi M, Li W. 2016. ggfortify: unified interface to visualize statistical result of popular R packages. R J 8:474–485. [Google Scholar]
- 52.R Development Core Team 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 53.Kuhn M. 2016. Contrast: a collection of contrast methods. https://CRAN.R-project.org/package=contrast. [Google Scholar]
- 54.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70. [Google Scholar]
- 55.Cohen J. 1960. A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 56.Viera AJ, Garrett JM. 2005. Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363. [PubMed] [Google Scholar]
- 57.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 58.Van Doorslaer K, Tan Q, Xirasagar S, Bandaru S, Gopalan V, Mohamoud Y, Huyen Y, McBride AA. 2013. The Papillomavirus Episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res 41:D571–D578. doi: 10.1093/nar/gks984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 61.Warnes GR, Ben Bolke LB, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B. 2016. gplots: various R programming tools for plotting data. https://CRAN.R-project.org/package=gplots. [Google Scholar]
- 62.Neuwirth E. 2014. RColorBrewer: ColorBrewer palettes. https://CRAN.R-project.org/package=RColorBrewer. [Google Scholar]
- 63.Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM. 2014. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. doi: 10.1890/13-0133.1. [DOI] [Google Scholar]
- 64.Chao A, Jost L. 2012. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. doi: 10.1890/11-1952.1. [DOI] [PubMed] [Google Scholar]
- 65.Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL, Longino JT. 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21. doi: 10.1093/jpe/rtr044. [DOI] [Google Scholar]
- 66.Hsieh TC, Ma KH, Chao A. 2016. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. doi: 10.1111/2041-210X.12613. [DOI] [Google Scholar]
- 67.Oksanen J, Blanchet FG, Kindt R, Legendre P, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2010. vegan: community ecology package. R package version 2.4-4. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 68.Anderson MJ. 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639. doi: 10.1139/f01-004. [DOI] [Google Scholar]
- 69.Wickham H. 2007. Reshaping data with the reshape package. J Stat Softw 21:1–20. [Google Scholar]
- 70.Wickham H. 2009. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
- 71.QGIS Development Team 2016. QGIS geographic information system, v2.18.14. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Urban score assignment based on community or subject attributes. (a) Principal-component analysis (PCA) depicting villages based on urban scores. Five villages with low urbanization scores (green), one village with an intermediate score (orange), and two villages with high scores (blue) are shown. Principal component 1 (PC1) separated by urbanization indicator (italic labels), in a gradient from low to high urban level. Urban attribute vectors show directions of community location in the space, with high urban communities placed in the direction of the vectors. The length of the arrow is proportional to the contribution of each urbanization indicator explaining the spatial distribution of women. (b and c) PCA depicting individual urban scores colored by urban groups based on their village (community-based groups) (b) or subject-based groups (c). Community-based medium urbanization and high urbanization groups overlapped their 95% confidence interval (CI95%) ellipses, while low urbanization Amerindians cluster apart from mestizo women (ID refers to Identification Document possession). Subject-based medium urbanization and high urbanization Amerindians groups showed less overlap. The area of each ellipse represents each group distribution with the CI95%. (d and e) Urban index boxplots for community-based groups (d) and subject-based groups (e). Mean urban index comparison indicated significant increase from low to high urbanization Amerindian groups and with mestizos being the highest for both classification approaches (P = 0.000 by ANOVA; P < 0.003 by Tukey’s test for all paired comparisons). Different letters over the boxplots indicate significant differences. P values shown were still significant after Holm correction. (f) Distribution of women by their subject-based urban group (color legend), in each of the community-based urban groups (x axis). The dominant color in each community-based group was concordant with the subject-based urban group, the discordant cases showed heterogeneity within communities reflecting an urbanization transition that occurs at the subject level. (g and h) Linear regression of community versus subject urbanization indices including only Amerindians (g) and for all subjects (66 Amerindians and 24 mestizos) (h). A positive correlation was observed for both cases (linear model, r2 = 0.73, y = 0.636x + 0.123, and slope, P = 0.000 and r2 = 0.56, y = 0.477x + 0.196, slope, P = 0.000, respectively). Download FIG S1, PDF file, 0.9 MB (994.5KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Demographic characteristics, contraception, and sexual behavior for 111 women of the study by community-based urbanization groups. Download TABLE S1, PDF file, 0.1 MB (100.7KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cervical HPV prevalence, cytological results, and prevalence of intestinal helminthes and anemia by community-based urbanization groups. Download TABLE S2, PDF file, 0.1 MB (100.4KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Prevalence and diversity of cervical HPV by community-based urban groups. (a) HPV general prevalence. (b) Risk type prevalence. No general differences were found in prevalence by Amerindian groups (P = 0.540 by χ2 test) or between Amerindians from the high urbanization group and mestizos (P = 0.570 by χ2 test). Unlike mestizos, Amerindian women showed higher prevalence of having only high-risk HPV types in relation to low-risk HPV types or both types (*, P < 0.05, log-linear model). Dots represent prevalence, and bars are 95% confidence interval (CI95%). (c) Sample size-based Shannon diversity of cervical HPV by urban groups on an extrapolated 32 women. Amerindians for low and high urbanization groups were significantly less diverse than the mestizo group. There was a nonsignificant tendency of increasing HPV diversity with urbanization (CI95%, P > 0.05). The interpolation (solid line) curve fraction corresponds to the HPV diversity of the actual number of women sampled. The extrapolation fraction (dashed lines) corresponds to the estimated diversity. Curved shaded areas represent the CI95% estimated from the bootstrap (50 replications). Different letters indicate significant differences, reached when CI95% do not overlap. (d) Beta diversity analysis by urban groups. Median distance to centroid, using Sorensen dissimilarity index. No significant difference in variability between or within groups was observed (P > 0.05 by PERMANOVA and permutation test for homogeneity of multivariate dispersions). (e) Heat map of the prevalence of cervical HPV types. HPV18 and HPV39 of the α7 family show the highest relative proportions. HPV L1 region sequences were used to generate a maximum likelihood tree rooted with theta HPV type (not shown). HPV families and their relative proportions (as a percentage; among only HPV-positive samples) are shown on the right. HPV68 and HPV73 were excluded from the tree, since the LiPA25 kit does not discriminate between these two types. Download FIG S2, PDF file, 0.8 MB (812.7KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cervical HPV alpha and gamma diversities among HPV-positive women by community-based urbanization groups. Sample size-based, coverage-based, and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 1, 2, and 3, respectively) at a rarefied/extrapolated sample size of 32 women or 83 women for all populations (gamma diversity) or at a coverage-based level of 0.937 and 0.977, respectively. Shannon and Simpson metrics show mestizo women with a significantly higher HPV diversity than low urbanization Amerindians. Mestizos had a significantly higher Simpson index than any other Amerindian group in the asymptotic estimation. There was a nonsignificant tendency of increasing diversity with urbanization in Amerindians. Download TABLE S3, PDF file, 0.1 MB (76.9KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cervical HPV alpha, beta, and gamma diversities among positive women by subject-based urbanization groups. Coverage-based and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 1, 2, and 3, respectively). Coverage-based analyses for urban groups and mestizos were performed at a coverage level of 0.906, while for the total population, the coverage level was 0.971. Mestizos showed significant higher diversity than Amerindian low urbanization group. At the asymptotic estimation, mestizos showed a significant higher HPV diversity than the Amerindian low urbanization group. There was a nonsignificant tendency of increasing diversity with urbanization in Amerindians. Download TABLE S4, PDF file, 0.1 MB (105.2KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Prevalence of high- or low-risk cervical HPV types among infected women by urbanization groups. HPV18 and HPV39 showed the highest prevalence. Percentages were calculated per urbanization group. Download FIG S3, PDF file, 0.3 MB (267.3KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HPV prevalence and alpha and beta diversity by body site in 18 women, of which 16 are HPV infected in at least one body site. Sample-size-based, coverage-based, and asymptotic diversity analyses were performed for observed richness and Shannon and Simpson diversity (Hill numbers of order q = 0, 1, and 2, respectively) at a rarefied/extrapolated sample size level of 12 women or at a coverage level of 0.854. The cervix had the highest observed richness. Beta diversity with Sorensen dissimilarity index did differ significantly between groups. Download TABLE S5, PDF file, 0.1 MB (106.8KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Congruence of detection of any high-risk HPV or HPV18 across body sites. There was low to no significant agreement in the presence of high-risk HPV in different body sites. Agreement between body sites varied from “less than chance” to “fair.” The highest specificity was observed in any high-risk HPV cooccurrence in anal and introitus sites. The highest sensitivity was observed in HPV18 cooccurrence in cervix and introitus sites. Download TABLE S6, PDF file, 0.1 MB (70.3KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HPV types, age, and urban group classification of nine women with cervical abnormalities. Download TABLE S7, PDF file, 0.1 MB (97.6KB, pdf) .
Copyright © 2018 Vargas-Robles et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Three data sets containing urbanization survey results and metadata and other data have been deposited in figshare at https://doi.org/10.6084/m9.figshare.5579299.v1.