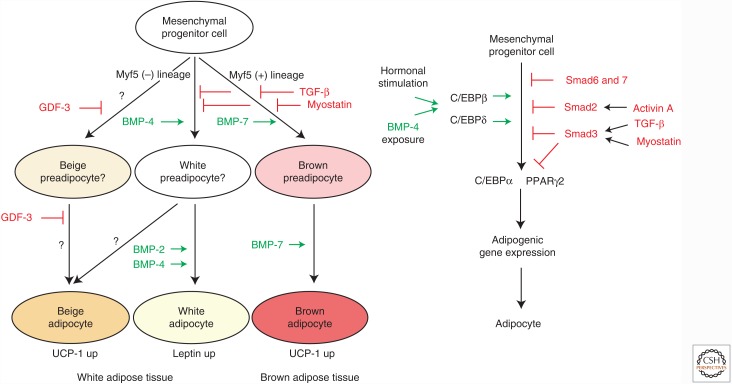
Abstract
Mesenchymal stem cells (MSCs) can differentiate into several lineages during development and also contribute to tissue homeostasis and regeneration, although the requirements for both may be distinct. MSC lineage commitment and progression in differentiation are regulated by members of the transforming growth factor-β (TGF-β) family. This review focuses on the roles of TGF-β family signaling in mesenchymal lineage commitment and differentiation into osteoblasts, chondrocytes, myoblasts, adipocytes, and tenocytes. We summarize the reported findings of cell culture studies, animal models, and interactions with other signaling pathways and highlight how aberrations in TGF-β family signaling can drive human disease by affecting mesenchymal differentiation.
Mesenchymal stem cells (MSCs) are multipotent cells that have the ability for self-renewal and the capacity to progress into several cell lineages, including osteoblasts, chondrocytes, myoblasts, adipocytes, and tenocytes (Friedenstein et al. 1970, 1976; Grigoriadis et al. 1988; Pittenger et al. 1999; Horwitz et al. 2005; Augello and De Bari 2010; Worthley et al. 2015). They contribute to tissue differentiation and regeneration, including maintenance of tissue homeostasis and function, adaptation to altered metabolic or environmental requirements, and repair of damaged tissue (Friedenstein et al. 1970; Grigoriadis et al. 1988; Pittenger et al. 1999; Charge and Rudnicki 2004; Augello and De Bari 2010). MSCs have been isolated from fetal tissues, adult bone marrow, and most connective tissues, including adipose tissue, dental tissues, and skin, as well as from peripheral blood, synovial fluid, and the perivascular compartment (Friedenstein et al. 1970, 1976; Pittenger et al. 1999; Tang et al. 2004; Bartsch et al. 2005; Wagner et al. 2005; Crisan et al. 2008; Morito et al. 2008; Riekstina et al. 2008; Huang et al. 2009a; Ab Kadir et al. 2012; Raynaud et al. 2012). MSCs can, in a first step, commit to specific cell lineages and then, in a second step, progress in differentiation along these lineages. These steps are initiated and regulated through interactions with other cells, in response to mechanical signals, and by extracellular signaling factors. Together, these interactions and signals promote or suppress the expression of cell lineage-specific transcription and survival factors that regulate expression of genes important for the specific cell functions of this lineage (Grigoriadis et al. 1988; Pittenger et al. 1999; Langley et al. 2002; Javed et al. 2008; Karalaki et al. 2009; Wang and Chen 2013; Worthley et al. 2015). For instance, MSC-derived preosteoblasts express early markers of the osteoblast lineage, including type I collagen (encoded by Col1A1 and Col1A2) and alkaline phosphatase (encoded by Alpl), whereas terminally differentiated osteoblasts express genes such as Bglap, encoding osteocalcin (Ocn), and show the capacity to form a mineralized extracellular matrix (Fakhry et al. 2013). Interestingly, and potentially of therapeutical interest, MSCs may also transdifferentiate in culture into cells of ectodermal and endodermal lineages, and express markers of neuronal cells, hepatocytes, or pancreatic cells (Safford et al. 2002; Kanafi et al. 2013; Wang et al. 2014). Furthermore, MSCs can support hematopoietic cells in the bone marrow microenvironment, and exert anti-inflammatory and immunomodulatory effects through interactions with the immune system (Haynesworth et al. 1996; Aggarwal and Pittenger 2005; Li et al. 2005; Carrade et al. 2012; Franquesa et al. 2012; Svobodova et al. 2012).
The commitment of MSCs to certain mesenchymal lineages, and their progression in differentiation along these lineages, is controlled by specific transcription factors. For instance, osteogenic lineage commitment is induced by the expression of runt-related transcription factor 2 (Runx2), a master transcription factor of osteoblastogenesis (Ducy et al. 1997; Komori et al. 1997; Otto et al. 1997; Komori 2010a). Runx2 promotes differentiation of MSCs into preosteoblasts and expression of genes during early stages of osteoblast differentiation, while it inhibits MSC commitment to the adipocyte lineage (Komori 2010b). Further osteoblast differentiation and maturation is then driven by the expression of the transcription factor osterix (Osx, encoded by Sp7), resulting in increased alkaline phosphatase activity and mineralization (Nakashima et al. 2002; Komori 2006). Runx2 is not crucial to promote differentiation into mature osteoblasts, and its expression is reduced later during differentiation (Maruyama et al. 2007; Komori 2010b).
The commitment of MSCs to the adipocyte lineage is induced by expression of the CCAAT/enhancer binding proteins (C/EBPs) β and δ (encoded by Cebpb and Cebpd, respectively) (Cao et al. 1991; Otto and Lane 2005). To allow DNA binding, C/EBPβ requires “activation” by phosphorylation by extracellular signal-regulated kinase (Erk) mitogen-activated protein kinase (MAPK), and glycogen synthase kinase-3β (GSK3β), and, consequently, induces expression of C/EBPα and peroxisome proliferator-activated receptor-γ (PPARγ) (encoded by Cebpa and Pparg, respectively) (Wu et al. 1996; Rosen and MacDougald 2006; Tang and Lane 2012). PPARγ and C/EBPα together regulate genes that are important for the adipocyte phenotype and drive progression of adipocyte differentiation (Tang and Lane 2012). Although PPARγ and C/EBPα are expressed throughout the differentiation process, C/EBPβ expression is down-regulated at later stages (Chen et al. 2016). Interestingly, the key osteogenic and adipogenic transcription factors Runx2 and PPARγ inhibit each other’s expression, and PPARγ also inhibits chondrogenesis (Zhang et al. 2006; Isenmann et al. 2009; Valenti et al. 2011).
MSC differentiation to the chondrogenic lineage requires expression of the key chondrogenic transcription factor SRY-box 9 protein (Sox9, encoded by Sox9), a member of the “high-mobility group box” transcription factor family (Lefebvre and Smits 2005; Quintana et al. 2009). In addition, the transcription factor NK3 homeobox 2 (Nkx3.2, encoded by Nkx3-2) maintains Sox9 expression by blocking the expression of inhibitors of Sox9 transcription, and Sox9 and Nkx3.2 can induce each other’s expression (Zeng et al. 2002; Kozhemyakina et al. 2015). At later stages of differentiation, Sox5 and Sox6, together with Sox9, promote progression to chondrocyte differentiation, but Sox9 expression is reduced in late-stage hypertrophic chondrocytes (Akiyama et al. 2002; Ikeda et al. 2004; Lefebvre and Smits 2005; Kozhemyakina et al. 2015; Liu and Lefebvre 2015).
The key transcription factors for myogenic differentiation are Myf5, Mrf4, MyoD, and myogenin, members of the MyoD family of myogenic regulatory factors (MRFs), which act in cooperation with myocyte enhancer factor (MEF) proteins (Weintraub et al. 1991; Rudnicki et al. 1993; Naya and Olson 1999; Sabourin et al. 1999; Berkes and Tapscott 2005). Myf5, Mrf4, and MyoD are essential for myogenic lineage commitment (Rudnicki et al. 1993; Kassar-Duchossoy et al. 2004), and myogenin together with Mrf4, MyoD, and MEF2 family members, which induce the expression of late muscle-specific genes, drive the progression of myogenic differentiation (Hasty et al. 1993; Naya and Olson 1999; Myer et al. 2001; Berkes and Tapscott 2005).
The key transcription factors that control commitment of MSCs to the tenocyte lineage, and drive progression in differentiation are incompletely understood. Scleraxis (Scx) is a key transcription factor involved in tenocyte lineage selection, and activates the expression of tendon-related genes, while inhibiting osteogenic, chondrogenic, and adipogenic differentiation (Shukunami et al. 2006; Li et al. 2015). However, the exact roles of other transcription factors associated with tendon development, including Six1, Six2, Eya1, Eya2, and Mohawk, have to be elucidated in future studies (Aslan et al. 2008; Jelinsky et al. 2010; Onizuka et al. 2014).
Multiple members of the transforming growth factor-β (TGF-β) signaling family modulate MSC lineage selection and progression of mesenchymal differentiation into specified cells, by controlling the expression and activities of these key transcription factors (Minina et al. 2001; Langley et al. 2002; Huang et al. 2007b; Neumann et al. 2007; Lee et al. 2011; Dorman et al. 2012). TGF-β family signaling is initiated by extracellular ligands that bind at the cell-surface to specific tetrameric transmembrane receptor complexes, consisting of two type II and two type I receptors (Feng and Derynck 2005; Chaikuad and Bullock 2016; Heldin and Moustakas 2016). Ligand binding to the receptor complex induces phosphorylation of the type I receptor I kinase domains by the type II receptors, resulting in the activation of intracellular signaling mediators (Shi and Massagué 2003; Feng and Derynck 2005; Hata and Chen 2016). TGF-β family ligands include TGF-β1, β2, and β3, bone morphogenetic proteins (BMPs), activins, and growth and differentiation factors (GDFs), including myostatin (GDF-8). TGF-β1 and TGF-β3 bind primarily to the TGF-β receptor type II (TβRII), which then activates the ALK-5/TβRI type I receptor (gene name Tgfbr1), whereas TGF-β2 requires binding to betaglycan (also called TGF-β type III receptor) or cooperative binding to TβRII and ALK-5/TβRI to activate the TβRI (Chaikuad and Bullock 2016). Activins bind to the activin type II receptors ActRII (also known as ActRIIA) and ActRIIB, which induce phosphorylation of ALK-4 (ActRIB, gene name ACVR1B), whereas BMPs bind to BMPRII, ActRII, and ActRIIB, which activate ALK-2 (gene name Acvr1), BMPRIA (ALK-3, gene name Bmpr1a), and BMPRIB (ALK-6, gene name Bmpr1b) (ten Dijke et al. 1993; Massagué 1998; Lux et al. 1999). GDFs interact with several of these type II receptors and induce activation of ALK-2, BMPRIA, BMPRIB, or, in the case of myostatin, ALK-4 or TβRI. The activated type I receptor phosphorylates and thereby activates specific Smad proteins in the canonical signaling pathway, which translocate into the nucleus to control target gene transcription (Feng and Derynck 2005; Hata and Chen 2016; Hill 2016). TGF-βs and activins induce phosphorylation of Smad2 and Smad3 by TβRI or ALK-4, whereas BMPs, acting through ALK-2, BMPRIA, or BMPRIB, activate Smad1, 5, and 8 (de Caestecker 2004; Chaikuad and Bullock 2016; Hata and Chen 2016; Xu et al. 2016). These phosphorylated Smads form complexes with the common co-Smad Smad4, translocate into the nucleus, and form either activating or inhibitory transcriptional regulatory complexes (Hill 2016). The inhibitory Smad6 and Smad7 inhibit these signaling cascades, and their expression is stimulated in response to TGF-β family signaling, thus providing a negative feedback loop (Hayashi et al. 1997; Imamura et al. 1997; Miyazawa and Miyazono 2017). Additionally, ligand–receptor binding also activates noncanonical intracellular signaling cascades, such as the Erk1 and Erk2 MAPK, c-Jun amino-terminal kinases (JNKs), and p38 MAPK pathways, as well as the phosphatidylinositol 3-kinase (PI3K)–Akt pathway (Zhang 2009).
In this review, we focus on the effects in cell culture and the in vivo roles of TGF-β family signaling in mesenchymal lineage commitment and differentiation into osteoblasts, chondrocytes, myoblasts, adipocytes, and tenocytes.
TGF-β FAMILY SIGNALING IN OSTEOBLAST DIFFERENTIATION
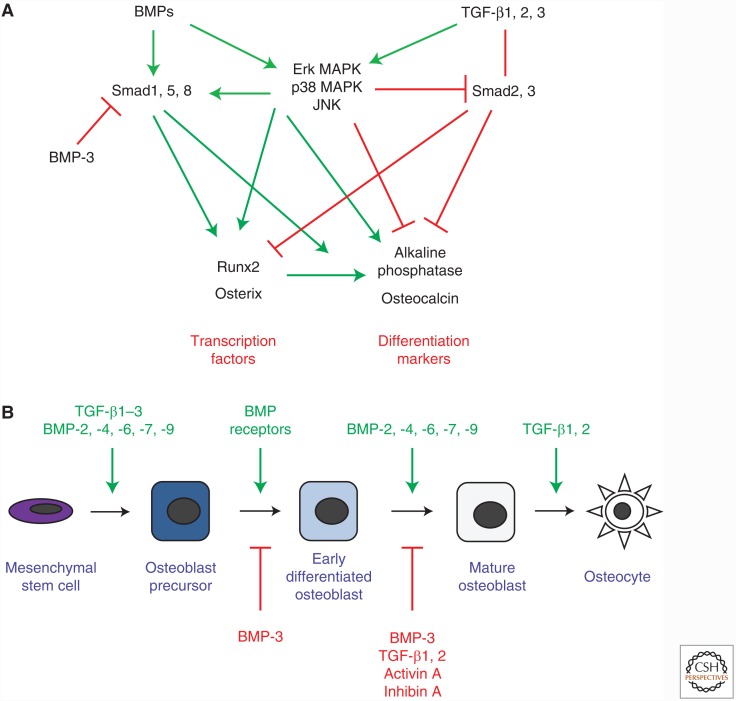
The skeleton functions in physical movement, regulates mineral homeostasis, and secretes endocrine factors (Oldknow et al. 2015). Bone is constantly remodeled in a tightly regulated sequence, coupling bone resorption with bone formation to maintain bone mass (Sims and Vrahnas 2014). Osteoclasts derive from a myeloid lineage and are responsible for bone resorption, whereas osteoblasts mature from a mesenchymal lineage and accomplish bone formation. Osteoblastogenesis occurs in three stages: proliferation, matrix maturation, and mineralization (Huang et al. 2007a). This differentiation process depends on the transcription factors Runx2 and Osx (Ducy et al. 1997; Komori et al. 1997; Otto et al. 1997; Nakashima et al. 2002). Osteoblast development is characterized by the expression of a set of gene expression markers, including alkaline phosphatase early in osteoblast differentiation, and osteocalcin and osteopontin at later stages of differentiation (Huang et al. 2007a). Osteoblasts can progress to become osteocytes, which are enveloped in mineralized bone, have mechanosensory and metabolic functions, and regulate bone remodeling (Bonewald 2011; Nakashima et al. 2011; Komori 2013; Sims and Vrahnas 2014). TGF-β family members, including BMPs, TGF-βs, activins, and inhibins regulate differentiation from early bone marrow stromal cells (BMSCs) to mature matrix-secreting osteoblasts and osteocytes (Fig. 1A,B).
Figure 1.
TGF-β family signaling in osteoblast differentiation. (A) Major intracellular and transcriptional targets of TGF-β and bone morphogenetic protein (BMP) signaling in osteoblastic differentiation. (B) Osteoblasts originate from mesenchymal stem cells. Signaling induced by TGF-β family ligands can inhibit or stimulate lineage selection and progression in differentiation. BMPs, with the exception of BMP-3, mostly promote progression of osteoblast differentiation, whereas activins and inhibins inhibit differentiation, and the TGF-β ligands affect certain stages of osteoblast differentiation. MAPK, mitogen-activated protein kinase; JNK, c-Jun amino-terminal kinase.
BMPs and Osteoblast Differentiation
In cell culture, most BMPs signal through BMPRII and ALK-2, BMPRIA, or BMPRIB to promote osteoblast differentiation (Fig. 1B) (ten Dijke et al. 1994; Chen et al. 1998; Ebisawa et al. 1999; Fujii et al. 1999; Jikko et al. 1999; Suzawa et al. 1999). BMP-2 and -6 potently stimulate, whereas BMP-4 and -7 moderately stimulate osteoblast differentiation, apparent by increased expression and activity of alkaline phosphatase in early osteoblast progenitors, and expression of osteocalcin and osteopontin in differentiated osteoblasts (Yamaguchi et al. 1991; Hughes et al. 1995; Kawasaki et al. 1998; Gori et al. 1999; Cheng et al. 2003; Friedman et al. 2006). Although BMP-2 does not regulate extracellular matrix protein secretion by osteoblasts, it stimulates mineral deposition into the matrix (Yamaguchi et al. 1991; Fromigué et al. 1998; Gori et al. 1999), leading to a higher number of mineralized bone nodules (Chen et al. 1997; Haÿ et al. 1999). BMP-7 also induces highly calcified bone nodules (Chen et al. 2001; Chaudhary et al. 2004), possibly by increasing inositol 1,4,5-trisphosphate (IP3) receptor levels, which increase calcium mobilization and deposition (Bradford et al. 2000). Unlike other BMP ligands, BMP-3 (also called BMP-3A) and BMP-3b (GDF-10) repress osteoblast differentiation, resulting in decreased expression of osteoblastic markers, bone nodule formation, and mineralization (Kokabu et al. 2012; Matsumoto et al. 2012). Whereas BMP-3A seems to signal through ActRIIB, BMP-3b potentially functions through the ActRII and ALK-4 receptors (Kokabu et al. 2012; Matsumoto et al. 2012). BMP ligands regulate osteoblast differentiation through multiple intracellular pathways, including signaling through Smad1, 5, and/or 8, but also noncanonical signaling pathways such as Erk1/2 MAPK, p38 MAPK, and JNK (Fig. 1A). Smad1, 5, and/or 8 signaling promotes osteoblast differentiation. For instance, increased expression of Smad1 in mesenchymal progenitor cells enhances BMP-2-induced expression of alkaline phosphatase (Ju et al. 2000), whereas bone-specific Smad1 inactivation results in reduced BMP signaling and delayed bone development in mice (Wang et al. 2011). In addition, blocking Smad5 activation prevents BMP-2-induced alkaline phosphatase and osteocalcin expression (Nishimura et al. 1998). BMP-4 induces activation of Smad1, 5, and 8 in osteoblastic cell lines, whereas BMP-6 and -7 induce activation of Smad1 and 5, but not Smad8, to promote alkaline phosphatase activity, suggesting differential regulation by individual BMPs (Ebisawa et al. 1999; Aoki et al. 2001). In contrast, the inhibitory BMP-3b blocks BMP-2-induced phosphorylation of Smad1, 5, and 8, and expression of osteoblast genes (Matsumoto et al. 2012). BMP-2 and -7 also regulate osteoblast differentiation by activating the Erk1/2 MAPK pathway (Lou et al. 2000; Xiao et al. 2002), and BMP-2 also acts through the p38 MAPK and JNK pathways. Blocking p38 MAPK or Erk MAPK signaling reduces BMP-2-induced Alp and Ocn expression, whereas inhibiting JNK activation primarily decreases osteocalcin expression (Galléa et al. 2001; Lai and Cheng 2002; Guicheux et al. 2003). In addition, BMP-2-induced activation of p38 MAPK and JNK enhances Smad1 activation and canonical BMP signaling (Nöth et al. 2003; Liu et al. 2011).
At the transcriptional level, BMP-induced Smad signaling targets genes encoding key osteoblastic differentiation factors such as Runx2 and Osx (Fig. 1A) (Lee et al. 2003). Interestingly, osteoblast precursors cultured from Runx2−/− mice do not fully differentiate into osteoblasts, even in the presence of BMP-2, although they still induce alkaline phosphatase and osteocalcin expression (Liu et al. 2007). On the other hand, in cells overexpressing Runx2, anti-BMP-2, -4, and -7 antibodies prevent Runx2 from stimulating Ocn transcription, suggesting that BMP signaling is necessary for Runx2 transcriptional activity (Phimphilai et al. 2006). BMP-2 likely does not directly activate Runx2, but rather induces phosphorylation and acetylation by MAPK signaling, modifications necessary for Runx2 to form a complex with phosphorylated Smad1, 5, and/or 8 to target osteoblast gene promoters (Afzal et al. 2005; Javed et al. 2008, 2009; Jun et al. 2010). BMP-2 also regulates Osx expression through Smad1 activation, and indirectly through other transcription factors including the homeobox protein Msx2, the homeobox protein Alx3, and the DNA-binding protein inhibitors ID-1, -2, and -3, and Runx2- and p38 MAPK-dependent pathways (Ogata et al. 1993; Lee et al. 2003; Peng et al. 2004; Matsubara et al. 2008; Ulsamer et al. 2008; Matsumoto et al. 2013).
BMP signaling during osteoblast differentiation is repressed by BMP inhibitors, and depends on cross-talk with other signaling pathways, including those activated by Wnt, TGF-β, fibroblast growth factor (FGF), Notch, and tumor necrosis factor-α (TNF-α) (Luo 2017). For example, noggin and gremlin antagonize BMPs by preventing their interaction with their receptors. Furthermore, increasing noggin expression in preosteoblastic cells inhibits BMP-2-induced differentiation (Wu et al. 2003), and decreasing noggin expression increases the phosphorylation of Smad1, 5, and 8, the activity of alkaline phosphatase, and osteocalcin and Runx2 expression (Gazzerro et al. 2007; Wan et al. 2007). During BMP-stimulated osteoblast differentiation, noggin and gremlin expression is enhanced in a feedback loop that inhibits BMP signaling (Gazzerro et al. 1998; Abe et al. 2000; Pereira et al. 2000). Activated Wnt–β-catenin signaling increases BMP-2-induced osteoblast differentiation and is required for the induction of alkaline phosphatase expression by BMP-2 (Rawadi et al. 2003), whereas deletion of β-catenin inhibits the response to BMP-2 (Mbalaviele et al. 2005; Salazar et al. 2008; Zhang et al. 2009). Also, Wnt–β-catenin signaling increases the expression and secretion of BMP-2 (Qiu et al. 2010), whereas BMP-2 can antagonize Wnt–β-catenin signaling by increasing the expression of the Wnt inhibitors sclerostin and dickkopf-1 (Dkk1) (Kamiya et al. 2010). TGF-β can antagonize BMP-2-induced osteoblast differentiation by inhibiting the activation of Smad1, 5, and 8, and promote BMP-2 activity by repressing noggin expression (Galléa et al. 2001; de Gorter et al. 2010). Furthermore, studies in cell culture suggest that FGF-2 is required for BMP-2-induced nuclear accumulation of Runx2 and Smad1, 5, and 8 (Sabbieti et al. 2013), which is supported by the observation that BMP-2 does not induce differentiation of Fgf2−/− osteoblast precursor cells (Hanada et al. 1997; Naganawa et al. 2008). Also, Notch signaling increases BMP-2-induced alkaline phosphatase activity and bone nodule formation (Nobta et al. 2005), and by silencing the expression of Hairy/enhancer-of-split related with YRPW motif protein 1 (Hey1), a mediator of Notch signaling, reduces BMP-9-induced osteoblast differentiation (Sharff et al. 2009). In addition, TNF-α acts through either the JNK pathway to inhibit activation of Smad1, 5, and/or 8, and decrease alkaline phosphatase and osteocalcin expression, or the NF-κB pathway to prevent BMP-2-induced Runx2 expression by blocking Smad complexes from binding to DNA (Eliseev et al. 2006; Singhatanadgit et al. 2006; Mukai et al. 2007; Billings et al. 2008; Yamazaki et al. 2009; Hirata-Tsuchiya et al. 2014). Menin, a protein implicated in multiple endocrine neoplasia type I, also interacts with BMP signaling by binding to Smad1 and 5, and enhances BMP-2-induced osteoblast differentiation (Sowa et al. 2003a).
Most BMPs and their receptors are indispensable for development. Insight into the roles of BMPs in osteoblast differentiation in vivo has mainly come from mouse models in which a gene of interest was conditionally inactivated (conditional knockout; cKO) or overexpressed. Mice have been generated in which either BMP-2 or BMP-4 expression was specifically inactivated in the limb using Cre recombinase expressed from the paired mesoderm homeobox protein 1 (Prx1) promoter that is expressed in embryonic limb bud mesenchyme (Prx1-Cre mice). Both models with inactivation of BMP-2 or BMP-4 show normal skeletal development. However, compound inactivation of both Bmp2 and Bmp4 in the limb almost completely abolishes bone formation, suggesting functional redundancy of BMP-2 and BMP-4, and the requirement of at least one of the two BMPs for normal osteogenesis (Bandyopadhyay et al. 2006; Tsuji et al. 2008). Interestingly, the Bmp4 Prx1-Cre cKO mice recover normally from fractures, whereas the corresponding Bmp2 Prx1-Cre cKO mice are unable to initiate fracture healing, suggesting that BMP-2 is necessary for normal differentiation of mesenchymal cells in bone repair (Bandyopadhyay et al. 2006; Tsuji et al. 2008). On the other hand, transgenic mice overexpressing BMP-4 under the control of the early osteoblast-specific Col1a1 promoter develop severe osteopenia and increased osteoclast numbers, suggesting a role for BMP-4 in bone resorption (Okamoto et al. 2006). BMP-4 and -7 may have overlapping functions in the development of the ribs, sternum, and digits, as apparent from the skeletal phenotype of Bmp4+/−; Bmp7+/− mice (Katagiri et al. 1998). Bmp3−/− mice have 50% more trabecular bone than wild-type mice, whereas BMP-3 overexpression under the Col1a1 promoter results in low bone mass and spontaneous fractures in utero, confirming the repression of osteoblast differentiation by BMP-3 seen in cell culture (Daluiski et al. 2001; Gamer et al. 2009). Mice with conditional Bmpr1a deletion in mature osteoblasts, by Cre recombinase-mediated recombination from the osteocalcin 2 promoter (Og2-Cre mice) show decreased bone formation rate, bone size, bone volume per total volume (BV/TV), and osteoblast marker expression at one month after birth; however, by 10 months these mice show increased BV/TV and bone mineral density (BMD) (Mishina et al. 2004). Disruption of Bmpr1a in immature osteoblasts using Cre recombinase expression from the Col1a1 promoter in mice results in an increased bone mass and BMD at late gestation, weaning, and adult stages (Kamiya et al. 2008a,b). Compared with wild-type controls, these mice develop increased tibial trabecular bone volume and decreased osteoclast numbers in response to mechanical loading (Iura et al. 2015). These ostensibly contradictory outcomes may be explained by considering that BMP signaling may promote the expression of sclerostin and Dkk1 in osteoblasts. These two proteins alter the balance of functional expression of RANKL (receptor activator of NF-κB ligand) and osteoprotegerin (OPG) such that osteoclastogenesis and bone resorption are reduced to a greater extent than osteogenesis, thus resulting in increased overall bone mass (Mishina et al. 2004; Kamiya et al. 2008b, 2010). Disruption of Bmpr1a using Cre recombinase expression from the Dmp1 promoter, which drives expression of dentin matrix protein 1 in more mature osteoblasts and osteocytes, results in a dramatic increase of trabecular bone mass in association with reduction of sclerostin expression (Kamiya et al. 2016; Lim et al. 2016). The similar phenotype of mice with immature osteoblast-specific disruption of Acvr1 (encoding ALK-2) using Cre recombinase driven by the Col1a1 promoter further supports the notion that BMP signaling in osteoblasts plays a dual role in promoting osteoblast differentiation to produce bone matrix and supporting osteoclastogenesis to resorb bones (Kamiya et al. 2011). Interestingly, inactivation of Bmpr1a using either Dmp1- or Sp7-induced Cre recombinase expression in mature osteoblasts and osteocytes, or immature osteoblasts, respectively, results in similar increases in bone mass with increased osteoblast numbers despite reduced osteoblast activity (Lim et al. 2016). However, contrary to the mice with osteoblast-specific Bmpr1a inactivation from the Col1a1 promoter, these mice do not show changes in osteoclast numbers or bone resorption (Lim et al. 2016). Additionally, mice with Bmpr2 inactivation under the control of the Prx1 promoter also develop increased bone mass and BMD by 9 weeks of age, but in this case this is the result of increased osteoblast activity with no change in osteoblast numbers (Lowery et al. 2015). This phenotype may be due to the use of the ActRII and/or ActRIIB receptors by the BMPs in the absence of BMPRII as type II receptor. In contrast, global inactivation of Bmpr1b expression does not result in overt bone phenotypes, suggesting common and unique functions of the three type I receptors BMPRIA, BMPRIB, and ALK-2 in osteoblasts (Baur et al. 2000; Yi et al. 2000). Mice that express a cytoplasmically truncated, dominant-negative form of BMPRIB from the Col1a1 promoter have reduced bone mass, suggesting that signaling by BMPRIB plays an important role in physiological osteogenesis (Zhao et al. 2002). Osteoblast-specific Smad1 inactivation using Cre recombinase from the Col1a1 promoter in mice results in osteopenia, providing in vivo evidence for the importance of Smad signaling in osteoblast differentiation (Wang et al. 2011). In addition to regulation of bone mass, BMP signaling also controls bone quality in conjunction with mechanosensing mechanisms (Iura et al. 2015). There are many unanswered questions about the functions of BMP signaling in vivo, and future studies will help us to further understand the context-dependent functions of BMP signaling in osteoblasts.
In humans, BMPs have been implicated in disorders affecting limb development. Mutations in the BMP2 or BMPR1B gene result in autosomal dominant brachydactyly type A2, characterized by hypoplastic middle phalanges of the second and fifth fingers (Dathe et al. 2009). A mutation in the noggin gene (NOG) that prevents normal binding of noggin to BMPs leads to brachydactyly type B, manifested by extreme shortening or complete loss of the distal portions of fingers and toes (Lehmann et al. 2007). A study of a large cohort of Dutch men and women found no increased risk for osteoporosis in subjects with common BMP2 polymorphisms that result in Ser37Ala and Arg190Ser substitutions (Fiori et al. 2006).
TGF-β and Osteoblast Differentiation
The effects of TGF-β1, β2, and β3 on osteoblastic cells are context-, time-, and dose-dependent, and differentially affect certain stages of osteoblast differentiation (Morikawa et al. 2016). Despite some conflicting findings, most experiments suggest that TGF-β promotes proliferation, early differentiation of osteoblast progenitor cells, and matrix production, while inhibiting later differentiation and matrix mineralization (Fig. 1B) (Chen and Bates 1993; Breen et al. 1994; Harris et al. 1994; Janssens et al. 2005). TGF-β ligands act through canonical Smad2 and Smad3 signaling, as well as noncanonical pathways, to regulate osteoblast differentiation (Fig. 1A). Smad2 overexpression in osteoblasts suppresses expression of Runx2 but does not seem to affect Runx2 transcriptional activity. In contrast, increased Smad3 expression reduces Runx2 expression and Runx2 transcriptional activity at early stages of differentiation, but increases Runx2 expression at later stages (Li et al. 1998; Alliston et al. 2001; Kaji et al. 2006). Activated Smad3 inhibits osteoblastic lineage commitment, yet promotes the progression of osteoblast differentiation at earlier stages and increases the expression of alkaline phosphatase, type I collagen, and proteins involved in matrix mineralization (Alliston et al. 2001; Kaji et al. 2006). Noncanonical TGF-β signaling through the Erk MAPK pathway inhibits alkaline phosphatase expression, but promotes collagen synthesis (Sowa et al. 2002; Arita et al. 2011), while signaling through both Erk1 and/or Erk2 and p38 MAPK leads to suppression of osteocalcin expression (Karsdal et al. 2002). As a feedback mechanism, TGF-β can also inhibit transcription of Smad3 through the Erk and JNK MAPK pathways (Sowa et al. 2002). In addition, TGF-β may control the progression of osteoblasts to osteocytes by preventing apoptosis of terminally differentiated cells through the Erk MAPK pathway (Karsdal et al. 2002). Consistent with the inhibitory role of TGF-β in early osteoblast differentiation, inhibition of the TβRI kinase activity increases alkaline phosphatase expression in BMSCs and C2C12 myoblast cells cultured in osteogenic media (Maeda et al. 2004). In addition, TGF-β can also activate a negative feedback loop to inhibit its own signaling via Runx2 to suppress expression of TβRI and thereby reduce the TGF-β responsiveness (Kim et al. 2006).
As in other tissues, TGF-β activation in the skeleton is highly regulated, requiring active TGF-β release from latent complexes and associated proteins that sequester TGF-β in the extracellular matrix. Bone is unique in that more TGF-β is secreted without being attached to latent TGF-β binding proteins (LTBPs) than in other tissues (Dallas et al. 1994). The small leucine-rich proteoglycans biglycan and decorin bind and likely sequester active TGF-β in the extracellular matrix, which is supported by the finding that BMSCs from mice with inactivated biglycan and decorin expression show a higher ratio of active to latent TGF-β than wild-type controls, with impaired osteoblast differentiation (Afzal et al. 2005). Intracellular regulation of TGF-β maturation by E-selectin ligand-1 (ESL-1), a Golgi protein that inhibits TGF-β bioavailability, is required for normal bone development, as mice lacking ESL-1 expression develop severe osteopenia with increased bone resorption and reduced mineralization because of higher TGF-β activity (Yang et al. 2013).
TGF-β signaling interacts with other signaling pathways to affect osteoblast differentiation. In particular, signaling cross-talk between the BMP- and TGF-β pathways seems to play a crucial role (Fig. 1A) (Maeda et al. 2004). For example, BMP-2 can repress TGF-β signaling by repressing the expression and promoting the intracellular relocation of TβRII (Centrella et al. 1995; Chang et al. 2002). In addition, all three TGF-βs can activate the Sost gene, which encodes sclerostin, resulting in inhibition of Wnt signaling in bone (Loots et al. 2012). TGF-β can also stabilize β-catenin through activation of Smad3 and the PI3K pathway (Loots et al. 2012). Furthermore, the observation that TGF-β no longer inhibits BMSC differentiation when β-catenin expression is silenced suggests that TGF-β and Wnt signaling synergize to inhibit osteoblast differentiation (Zhou 2011). In addition, Wnt signaling can increase Tgfbr1, but not Tgfbr2 expression in a β-catenin-independent pathway, thus increasing responsiveness to TGF-β (McCarthy and Centrella 2010). Parathyroid hormone (PTH) signaling interacts with TGF-β signaling by increasing the levels of Smad3, which in turn stabilizes β-catenin and thus enhances TGF-β-induced expression of type I collagen in osteoblasts (Sowa et al. 2003b; Inoue et al. 2009). Furthermore, TβRII phosphorylates the PTH receptor 1 (PTH1R), leading to endocytosis of both receptors, and consequently reduced TGF-β and PTH signaling (Qiu et al. 2010). Inactivating TβRII expression in osteoblasts leads to increased PTH1R levels, resulting in a high trabecular/low cortical bone mass phenotype (Qiu et al. 2010). TGF-β also regulates the expression of many other growth factors. For instance, in BMSCs TGF-β increases transcription of the genes encoding fibroblast growth factor 2 (FGF-2), insulin-like growth factor I (IGF-I), and the extracellular matrix-associated protein connective tissue growth factor (CTGF), which all contribute to collagen matrix production (Kveiborg et al. 2001; Sobue et al. 2002; Arnott et al. 2008). Moreover, TNF-α acts through NF-κB to prevent TGF-β from activating Smad2 and Smad3, similar to its role in inhibiting activation of Smad1, 5, and 8, suggesting an inhibitory function of TNF-α in bone (Mukai et al. 2007).
Modulating the expression of TGF-β signaling components in mouse models has further shown its complex roles in osteoblast differentiation and osteogenesis in vivo. Tgfb1−/− mice, for example, show a remarkable absence of mature osteoblasts and reduced ALP activity, but normal osteoclast numbers and activity (Geiser et al. 1998). These mice have normal bones early in development, but show by 3 months of age a reduced growth and significant bone loss, consistent with impaired osteoblast differentiation (Geiser et al. 1998; Atti et al. 2002). Further studies show that TGF-β, released from bone matrix during osteoclastic bone resorption, induces migration of BMSCs to sites of bone resorption, thereby “coupling” bone resorption with bone formation (Pfeilschifter et al. 1990; Hughes et al. 1992; Tang et al. 2009).
Tgfb2−/− mice have reduced bone size and ossification, as well as limb and rib defects by E18.5, and die perinatally from multiple developmental defects, indicating the importance of TGF-β2 in bone patterning and development (Sanford et al. 1997). In contrast, Tgfb3−/− mice have normal skeletons (Dünker and Krieglstein 2002). Increased expression of biologically active TGF-β2 in differentiated osteoblasts under control of the Bglap promoter results in a dramatic reduction of bone volume with frequent fractures by 1 month of age (Erlebacher and Derynck 1996), and by 7 months severely reduced trabecular bone and thin unmineralized cortical bone. Bone of these mice shows increased osteoclastic resorption, osteoblast activity, osteoprogenitor cell number, and osteocyte density, suggesting that TGF-β2 regulates both osteoclast activity as well as osteoblast differentiation (Erlebacher and Derynck 1996). However, mice overexpressing a dominant-negative form of TβRII have increased bone mass, potentially also because of osteoblast-mediated reduction of osteoclast activity despite normal osteoclast numbers (Filvaroff et al. 1999). Smad3−/− mice show a phenotype similar to that of Tgfb1−/− mice, with reduced bone volume, normal osteoblast, and osteoclast numbers, but impaired osteoblast function, resulting in a decreased bone formation rate (Borton et al. 2001). These Smad3−/− mice also show an increased osteocyte density, similar to mice that overexpress TGF-β2 in mature osteoblasts under control of the Bglap promoter (Borton et al. 2001). Pathologically, increased TGF-β signaling also contributes to the phenotype in osteogenesis imperfecta (OI), a genetic bone dysplasia characterized by brittle bones and increased susceptibility to fractures (Grafe et al. 2014). Mouse models of dominant OI (due to heterozygous mutation in Col1a2) and recessive OI (due to lack of cartilage-associated protein CRTAP that is involved in posttranslational modifications of type I collagen; Crtap−/− mice) both show phenotypes similar to models with increased TGF-β signaling, and treating these mice with a pan-anti-TGF-β antibody improves the bone phenotype (Grafe et al. 2014).
In humans, some TGF-β1 polymorphisms associate with osteoporotic phenotypes. One study found an association between osteoporosis with increased bone turnover and a single-base deletion in intron 8 of TGFB1 that likely affects splicing (Langdahl et al. 1997). Another study found a polymorphism, a T-C polymorphism in the fifth intron, 20 bases upstream of exon 6 that is less common in osteoporotic patients and associates with higher bone mass (Langdahl et al. 2003). Many different mutations in the proregion of TGF-β1, also known as latency-associated peptide (LAP), can cause Camurati-Engelmann disease, an autosomal dominant bone dysplasia that causes osteosclerosis and increased fracture risk (Kinoshita et al. 2000; Campos-Xavier et al. 2001; Wu et al. 2007). Mice generated with these mutations recapitulate the bone dysplasia and show increased levels of active TGF-β1 in the bone marrow, raising the possibility that the mutations affect the ability of TGF-β1 to be sequestered in the extracellular matrix (Tang et al. 2009), and inhibition of TβRI in these mice rescues the bone phenotype and prevents fractures. These findings suggest that modulating TGF-β signaling, and thus altering osteoblast differentiation, could represent a compelling treatment approach for certain bone diseases.
Activins, Inhibins, Follistatin, and Osteoblast Differentiation
The roles of activins and inhibins in osteoblast differentiation have been less well characterized but these TGF-β-related ligands modulate the effects of BMP and TGF-β ligands. For instance, activin A, a homodimer of inhibin βA chains, acts in a similar way as TGF-β in osteoblastic cell culture, and increases proliferation but inhibits differentiation of early osteoprogenitor cells (Fig. 1B) (Centrella et al. 1991; Hashimoto et al. 1992; Ikenoue et al. 1999). Activin A also inhibits mineralization, at least in part by inhibiting the expression of the transcription factor homeobox protein Msx2, even in late osteoblast differentiation (Eijken et al. 2007; Alves et al. 2013). However, when overexpressed noggin inhibits BMP signaling, activin A rescues the progression of osteoblast differentiation, suggesting that it acts through multiple pathways in osteoblastogenesis (Gaddy-Kurten et al. 2002). Mice with inactivated Inhba, which encodes inhibin βA, have severe craniofacial defects, whereas inactivation of Acvr2, which encodes the activin type II receptor ActRII, does not affect skeletal development in most but not all mice. These results suggest that activins may act through a different type II receptor to enact their effects on bone (Matzuk et al. 1995a). Inhibin A, a heterodimer of inhibin α and inhibin βA, also inhibits osteoblast differentiation, reduces alkaline phosphatase activity in early osteoblasts, and suppresses mineralization by mature osteoblasts (Fig. 1B) (Gaddy-Kurten et al. 2002). The finding that inhibin represses osteoblastogenesis even when activin is added suggests that inhibin does not act by competing with activin for the same receptor, but instead might signal through a distinct inhibin-specific receptor (Gaddy-Kurten et al. 2002). Furthermore, inhibin-mediated repression of osteoblastogenesis cannot be rescued with BMP-2, indicating that the inhibitory effect of inhibin is dominant over BMP-2 activity (Gaddy-Kurten et al. 2002). The glycoprotein follistatin, encoded by Fst, binds activins and inhibins and prevents their interaction with their receptors (Nakamura et al. 1990; Harrison et al. 2005; Gordon and Blobe 2008). It is expressed only at very low levels at all stages of osteoblast differentiation, and exogenous follistatin can block activin A functions (Funaba et al. 1996; Gaddy-Kurten et al. 2002). In vivo studies are required to determine if follistatin plays a role in osteogenesis.
TGF-β FAMILY SIGNALING IN CHONDROCYTE DIFFERENTIATION
BMPs and Chondrogenesis
BMP signaling is critical during each stage of chondrogenesis. BMP ligands are expressed in a defined spatiotemporal pattern in the precartilagious mesenchyme, the perichondria, and the growth plates. In particular, Bmp2 and Bmp4 are highly expressed by prehypertrophic and hypertrophic chondrocytes in the growth plates (Feng et al. 2003; Nilsson et al. 2007). Inactivating both Bmp2 and Bmp4 in limb bud mesenchyme results in defective skeletal development (Bandyopadhyay et al. 2006). Inactivating Bmp2 only in chondrocytes, using Cre-mediated recombination from the Col2a1 promoter results in similarly severe skeletal defects, which suggests that BMP-2 is a key ligand for growth plate function (Shu et al. 2011). Disrupting the expression of either BMP type I receptor, through inactivation of Bmpr1a, Bmpr1b, or Acvr1, in the chondrogenic lineage has minor consequences for morphogenetic phenotypes (Baur et al. 2000; Yi et al. 2000; Ovchinnikov et al. 2006; Rigueur et al. 2015). In contrast, compound inactivation of Bmrp1a and Bmrp1b substantially diminishes the size of cartilage primordia by increasing apoptosis (Yoon et al. 2005). These striking results underscore redundancies in some functions of BMP signaling through BMPRIA and BMPRIB during chondrogenesis. Compound inactivation of either Bmpr1a and Acvr1 or Bmpr1b and Acvr1 causes subtle cervical vertebrae abnormalities, suggesting that BMP signal transduction through ACVR1/ALK-2 has a minor role during chondrogenesis (Rigueur et al. 2015). Neural crest-specific deletion of Bmpr1a causes early lethality owing to cardiac malfunction (Stottmann et al. 2004; Nomura-Kitabayashi et al. 2009). However, neural crest-specific deletion of Acvr1 results in craniofacial defects, including mandibular hypoplasia with hypoplastic Meckel’s cartilage (Dudas et al. 2004). In contrast, Meckel’s cartilage persists in Nog−/− mice that lack expression of the BMP inhibitor noggin (Wang et al. 2013b). Inactivation of Bmpr1a expression in the chondrogenic lineage after birth halts long bone growth and reduces Sox9 expression (Jing et al. 2013). Interestingly, the growth plates in these mutants are replaced by bone-like tissue suggesting that BMP signaling through BMPRIA prompts chondrogenic differentiation by regulating Sox9 expression.
Chondrocyte-specific inactivation of Smad1, Smad5, or Smad8 individually results in viable mice. However, compound inactivation of Smad1 and Smad5 results in severe chondrodysplasia that mimics the phenotype of chondrocyte-specific Bmpr1a−/−;Bmpr1b−/− compound mutants (Retting et al. 2009). This stands in contrast to the phenotype of chondrocyte-specific disruption of Smad4 using Col2a1-Cre; these mice have disorganized growth plates and shorter bones, but live for at least several months after birth (Zhang et al. 2005). These observations raise the possibility that Smad4 has only a limited role in mediating the BMP–Smad signaling pathway in chondrocytes. Transcriptional intermediary factor-1γ (TIF1γ; also known as TRIM33) binds Smad2 and Smad3 and allows these complexes to exert distinct functions from Smad4 complexes with Smad2 or Smad3 (He et al. 2006; Xi et al. 2011). Accordingly, conditional compound inactivation of Smad4 and Tif1g results in a more severe phenotype than inactivation of the individual genes, and results in cleft palate, as seen because of epithelium-specific Tgfb3 inactivation (Lane et al. 2015), suggesting that BMP–Smad signaling through TIF1γ/TRIM33 represents an arm of the pathway that does not require Smad4.
After endochondral ossification, a small population of chondrocytes at the end of long bone remains as articular cartilage (Kronenberg 2003). How this subpopulation of chondrocytes is destined to become articular cartilage, differently from the chondrocytes in the growth plate, is not well understood; however, BMP and Wnt signaling activities likely contribute to their differentiation phenotype (Tsumaki et al. 1999; Guo et al. 2004; Pacifici et al. 2005; Spater et al. 2006a,b). Wnt signaling at the interzone, the site of the future joint, is essential for articular cartilage development (Hartmann and Tabin 2001; Guo et al. 2004; Spater et al. 2006a,b). Furthermore, noggin is highly expressed just proximal to the distal proliferating zone (DPZ) to “insulate” BMP signaling from more proximal regions of the growth plate (Ray et al. 2015). This expression pattern of noggin may explain how chondrocytes in the most distal part of cartilage primordia are exposed to a high ratio of Wnt/BMP signaling and specify to become articular cartilage.
TGF-β-activated kinase 1 (TAK1) initiates p38 MAPK and JNK signaling in response to TGF-β, yet is also involved in signaling responses to other types of ligands (Cui et al. 2014). Chondrocyte-specific inactivation of Tak1 results in chondrodysplasia, characterized by delayed formation of secondary ossification centers and absence of elbow and tarsal joints (Shim et al. 2009; Greenblatt et al. 2010a). These mutant mice also have defective cartilage proliferation and maturation (Gunnell et al. 2010). Inactivation of Tak1 in developing limb mesenchyme results in widespread joint fusions (Gunnell et al. 2010). During both embryogenesis and postnatal development, TAK1 signaling promotes the expression of three Sox transcription factors (i.e., Sox5, Sox6, and Sox9) that are essential for the organization of growth plates and articular cartilage development (Gunnell et al. 2010; Gao et al. 2013). In addition to the expected reduction of p38 MAPK and JNK activation, these Tak1−/− mice show decreased Erk MAP kinase activity and decreased activation of the BMP-responsive Smad1, 5, and 8 (Shim et al. 2009). Similarly, decreased Smad1, 5, and 8 activation is observed following osteoblast-specific or neural crest-specific inactivation of Tak1 (Greenblatt et al. 2010b; Yumoto et al. 2013), suggesting that TAK1 controls both BMP-activated Smad and non-Smad pathways in multiple cell types. However, because BMP or TGF-β ligands are not the only ones that initiate TAK1-mediated signaling, these phenotypes do not necessarily result from alterations in BMP- or TGF-β signaling only.
More evidence that BMP signaling plays pivotal roles in chondrogenesis comes from the identification of gene mutations that result in fibrodysplasia ossificans progressiva (FOP). FOP is a rare, autosomal dominant disease characterized by ectopic ossification in soft tissues following even minor trauma. ACVR1 mutations have been identified in all patients diagnosed so far (Shore et al. 2006; Kaplan et al. 2012). The mutation in ACVR1 that results in R206H substitution is believed to affect the interaction of the type I receptor with FKBP12, and confers increased basal signaling activity (Shore et al. 2006). Thus, the R206H substitution in ACVR1/ALK-2 enhances chondrogenesis in micromass culture (Shen et al. 2009b), and chimeric mice with the R206H substitution develop ectopic ossification on blunt injury (Chakkalakal et al. 2012). ACVR1 with the R206H substitution can also respond to activin ligands that normally antagonize BMP signaling through ACVR1 (Hatsell et al. 2015). Administration of an activin A-blocking antibody to mice that express ACVR1 with the R206H substitution prevents formation of FOP-like lesions, which strongly suggests that a broadened ligand specificity because of the mutation contributes to the pathogenesis of FOP (Hatsell et al. 2015). Another substitution, Q207D, renders ACVR1 constitutively active, and conditional transgenic mice that express the Q207D mutant receptor in skeletal muscles, activated by intramuscular injection of Cre recombinase-expressing adenovirus, develop ectopic ossification in combination with inflammation (Fukuda et al. 2006; Yu et al. 2008). Ligand antagonists of the nuclear retinoic acid receptor-γ (RAR-γ) are known for their antichondrogenic action (Pacifici et al. 1980), and administration of RAR-γ agonists was shown to block heterotopic ossification in the Q207D mouse model (Shimono et al. 2011). Together, these observations reinforce the idea that chondrogenic differentiation promoted by aberrantly increased BMP signaling in progenitor cell populations is a critical step for heterotopic ossification.
Enhanced BMP–Smad signaling through BMPRIA in neural crest cells leads to an increase of p53-mediated apoptosis in developing nasal cartilage, resulting in abnormal nasal cavity morphogenesis leading to perinatal lethality (Hayano et al. 2015). In this model, increased levels of p53 protein are observed without increases of p53 gene expression, but are accompanied by decreased MDM2–p53 complex formation and increased complex formation of p53 with Smad1, 5, and 8 (Hayano et al. 2015). MDM2 acts as an E3 ligase promoting proteasomal degradation of p53 (Momand et al. 1992; Kussie et al. 1996; Lai et al. 2001). Together with the observation that association of activated Smad1 with p53 prevents MDM2-mediated p53 degradation (Chau et al. 2012), these results raise the possibility that increased BMP–Smad signaling not only increases the nuclear levels of activated Smad1, 5, and 8, but additionally prevents the MDM2–p53 interaction that leads to activation of apoptotic pathways in chondrocytes at nasal cavity.
During early embryogenesis, the relative timing of Sonic hedgehog (Shh) and BMP signals defines the fate selection of lateral plate mesoderm toward either a chondrogenic or presomitic mesoderm (PSM) fate. Thus, sequential exposure of lateral plate mesoderm to Shh followed by BMP-4 robustly induces chondrogenesis, whereas simultaneous exposure of both Shh and BMP blocks chondrogenesis (Murtaugh et al. 1999, 2001). Shh signaling activates Sox9 expression through activation of Gli2 and Gli3 at the lateral plate mesoderm and at the same time induces the expression of Nkx3.2, which blocks the expression of the GATA4, 5, and 6 transcription factors (Zeng et al. 2002; Daoud et al. 2014). On the other hand, BMP signaling in the PSM blocks Shh-mediated induction of Nkx3-2 and Sox9 through induction of the expression of the GATA4, 5, and 6 transcription factors that suppress Nkx3-2 expression and the expression of Gli transcription factors dependent on the zinc finger protein FOG1 (friend of GATA protein 1, also known as ZFPM1) (Daoud et al. 2014). These results suggest that Shh signaling installs competence in lateral plate mesoderm for BMP-induced chondrogenesis by inducing Nkx3.2 expression.
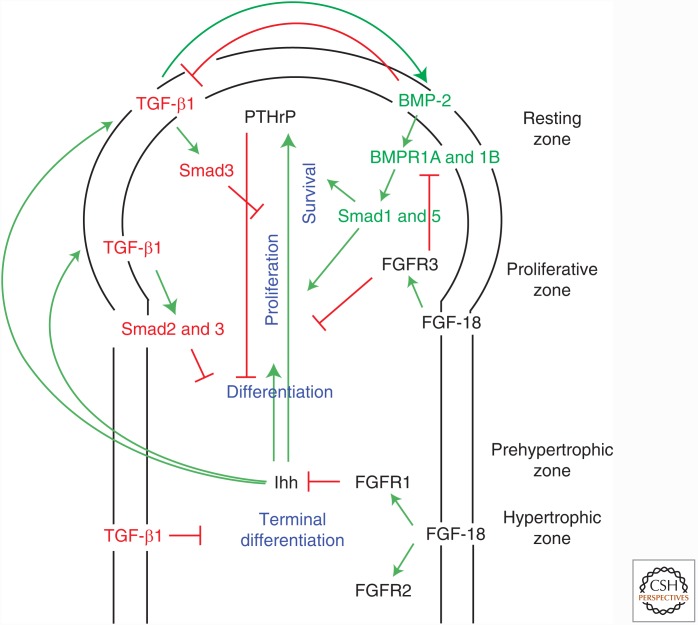
BMP signaling, in conjunction with other signaling pathways, regulates the size and organization of the growth plate. A feedback loop between Indian hedgehog (Ihh) produced in the prehypertrophic and hypertrophic zones and parathyroid hormone related protein (PTHrP) produced in the resting zone plays a critical role in maintaining the columnar height of the growth plate (Fig. 2) (Kronenberg 2003). FGF signaling inhibits proliferation of chondrocytes, whereas BMP signaling stimulates chondrocyte proliferation and differentiation, and inhibits apoptosis of hypertrophic chondrocytes (Fig. 2) (Minina et al. 2001, 2002). BMP signaling induces Ihh expression, and Ihh signaling induces BMP expression, forming a positive feedback loop (Minina et al. 2002). Accordingly, Cre-recombinase-mediated, cartilage-specific conditional inactivation of Smad1 and Smad5 from the Col2a1-promoter results in reduced Ihh expression in the hypertrophic zone (Retting et al. 2009). Activation of BMP signaling in the perichondrium together with activation of hedgehog signaling prompts osteogenic differentiation, whereas BMP signaling alone induces chondrogenic differentiation (Hojo et al. 2013). Pharmacological inhibition of hedgehog signaling results in reduced Smad and p38 MAPK activation in response to BMP-2, and suppresses BMP-2-induced chondrogenesis in micromass culture (Mundy et al. 2015), suggesting that Hedgehog signaling may repress BMP signaling. As with the PSM, the timing of these two signaling stimuli is critical for lineage specification of the cells in perichondrium.
Figure 2.
TGF-β family signaling in chondrocyte differentiation during growth plate development. Regulation of endochondral ossification involves a feedback loop between parathyroid hormone related protein (PTHrP) and Indian hedgehog (Ihh) controlling chondrocyte differentiation, whereas fibroblast growth factor (FGF) signaling represses chondrocyte proliferation. Bone morphogenetic protein (BMP)-2, expressed within perichondrium, promotes the survival and proliferation of chondrocytes through the type I receptors BMPRIA and BMPRIB and subsequent Smad1 and Smad5 activation. FGF-18 signaling through the fibroblast growth factor receptor 3 (FGFR3) receptor further refines chondrocyte proliferation by repressing BMP receptor activity. Ihh expressed in the prehypertrophic zone induces the expression of both TGF-β1 in the perichondrium and PTHrP in the resting zone, respectively. PTHrP participates in this intricate feedback loop by inhibiting differentiation until the proliferating chondrocytes enter the prehypertrophic zone. TGF-β1 represses terminal chondrocyte differentiation in the hypertrophic zone. It is suggested that TGF-β1 induces BMP-2 expression, which then inhibits further TGF-β1 expression.
FGF signaling inhibits Ihh expression in the growth plate (Minina et al. 2002), and increased FGF signaling is observed in growth plates in both Bmpr1a−/−;Bmpr1b−/− and Smad1−/−;Smad5−/− compound mutant mice (Yoon et al. 2005; Retting et al. 2009). These results suggest that diminished BMP signaling in the growth plate leads to imbalanced cross talk between BMP, FGF, and Ihh signaling. Hyperactivated FGF receptor 3 (FGFR3) promotes degradation of BMPRIA through the E3 ubiquitin ligase Smurf1 (Smad ubiquitination regulatory factor-1), thus inhibiting BMP-induced chondrogenesis (Qi et al. 2014). These findings lend credence to the notion that shortened growth plates, found in achondroplasia due to single gain-of-function amino acid substitutions in FGFR3, such as K644E, may result from reduced BMP signaling. Indeed, BMP-2 treatment of metatarsals of Fgfr3K644E mice show increased hypertrophic zone length (Qi et al. 2014).
Growth Differentiation Factors and Joint Formation
The TGF-β family proteins named “growth and differentiation factors” (GDFs) also play critical roles during endochondral ossification and joint formation. Gdf5, which encodes GDF-5/BMP-14, is expressed in precartilaginous mesenchyme and the perichondrium of proximal structures of the limb buds in E12.5 mouse embryos. At later stages, Gdf5 expression localizes to the sites of joint formation. Gdf6, which encodes GDF-6/BMP-13, and Gdf7, which encodes GDF-7/BMP-12, are also expressed in a subset of developing joints (Wolfman et al. 1997; Settle et al. 2003). The spontaneous mutation brachypodism in mice shows abnormal skeletal patterns that are attributed to mutations in Gdf5 (Storm et al. 1994). Gdf5−/− mice show shorter appendicular bones although axial bones are unaffected (Storm et al. 1994; Storm and Kingsley 1996), and abnormal joint formation in the synovial joints of the limb leading to abnormal fusion between particular skeletal elements (Storm and Kingsley 1996). In humans, GDF5 mutations are at the basis of hereditary diseases, such as acromesomelic chondrodysplasia, Hunter–Thompson type (CHTT) and chondrodysplasia, Grebe type (CGT) (Thomas et al. 1996, 1997). These diseases are characterized by shortening of the appendicular skeleton and abnormal joint development resembling the skeletal abnormalities in Gdf5−/− mice. CHTT is due to a missense mutation in GDF5 resulting in total loss of function, whereas CGT is due to a C400Y substitution in GDF-5 that affects dimerization of BMP/GDF ligands (Thomas et al. 1996, 1997). Overexpression of Gdf5 in the chick limb results in larger size of cartilage condensation (Francis-West et al. 1999), suggesting a potential role of GDF-5 in skeletal growth. Chondrocyte-specific expression of Gdf5 in mice also prompts mesenchymal condensations caused by increased cell adhesion and proliferation (Tsumaki et al. 1999, 2002). Increased expression of Gdf5 in chondrocytes restricts expression of joint markers, and promotes overgrowth of cartilage, and thus may cause fusion of adjacent skeletal elements and loss of joints.
Gdf6−/− mice show skeletal phenotypes that are similar to, but distinct from, those of Gdf5−/− mice. Gdf6−/− mice display fusions between specific carpal bones in the wrists and between talus and the central tarsal bones in ankle, coincident with high expression of Gdf6 (Settle et al. 2003). In Gdf6−/− mutants, the process to subdivide larger skeletal precursors into individual skeletal elements does not take place (Settle et al. 2003). Gdf6−/− mice also show loss of coronal sutures, which separate frontal bones from parietal bones in the skull (Settle et al. 2003). In control embryos, the frontal and parietal bones are visible at E14.5 as separate ossification centers; however, one continuous bone is found in the Gdf6−/− embryos (Clendenning and Mortlock 2012). Suture width is reduced in Gdf6+/− embryos, and sutures are absent, accompanied with increased alkaline phosphatase activity, in Gdf6−/− embryos (Clendenning and Mortlock 2012). Fgfr2 is highly expressed in proliferating osteoprogenitors, and its expression is down-regulated as differentiation progresses (Iseki et al. 1999). However, the expression of Fgfr2 that is normally observed in coronal sutures is repressed in the Gdf6−/− embryos (Settle et al. 2003). These results suggest that GDF-6 represses and prevents osteogenic differentiation through expression of Fgfr2 to maintain the suture mesenchyme undifferentiated.
Gdf5−/−;Gdf6−/− mice have a more severe phenotype of bone size and joint formation than either single mutant (Settle et al. 2003). Many limb bones are much smaller or completely missing. The vertebral column of these double mutant mice has a reduction of alcian blue-stained extracellular matrix, suggesting a reduction in cartilaginous extracellular matrix, whereas no overt phenotype is observed in the vertebral column of either mutant (Settle et al. 2003). Gdf7 is also expressed in joint interzones, but unlike Gdf5−/− or Gdf6−/− mice, no overt morphological skeletal phenotypes are found in Gdf7−/− mutant mice (Settle et al. 2001). Although the tibial growth plates of Gdf7−/− mice have a histologically normal columnar structure, their proliferation rate is higher than that of control mice (Mikic et al. 2008). This distinguishes Gdf7−/− mice from Gdf5−/− mice that show a reduced proliferation rate of hypertrophic chondrocytes in the tibial growth plate (Mikic et al. 2004).
GDF-5, like other BMP ligands, interacts with type I and type II receptors to induce activation of Smad proteins (Nishitoh et al. 1996; Nohe et al. 2004). GDF-5 dimers interact with BMPRIA or BMPRIB, albeit preferentially with BMPRIB (Nickel et al. 2005). Bmpr1b is expressed in early cartilage condensations, and is later defined in the digital rays of hands or feet that outline the future digits pattern, and colocalizes with Gdf5 (Kawakami et al. 1996; Zou et al. 1997; Degenkolbe et al. 2014). When Gdf5 expression concentrates in joint interzones, starting at E13.5 in mice, those domains are flanked by Bmpr1b expression (Degenkolbe et al. 2014). In contrast, Bmpr1a is expressed in direct proximity in the interphalangeal regions and surrounding limb epithelium. The limb phenotype of Bmpr1b−/−;Gdf5−/− mice highly resembles that of Gdf5−/− mutants (Baur et al. 2000; Yi et al. 2000), further reinforcing the idea that BMPRIB is the primary receptor for GDF-5 during limb development.
The activities of GDF-5 are counteracted by ligand antagonists, such as the BMP antagonist noggin, encoded by Nog (Merino et al. 1999a). Nog is expressed during chondrogenesis and joint specification, and its expression domains overlap with those of Bmpr1b (Degenkolbe et al. 2014). Nog−/− mice show skeletal abnormalities that include the absence of joints. After the initial condensations of limb mesenchyme, Nog−/− mice show increased recruitment of mesenchymal precursors that subsequently results in overgrowth of cartilage and fusion of neighboring skeletal elements (Brunet et al. 1998). In chick embryos, ectopic expression of BMPs suppresses Gdf5 expression, suggesting that increased BMP signaling in Nog−/− limb bud may lead to decreased GDF-5 levels, which is at the basis of the similar phenotypes of Gdf5−/− limbs (Macias et al. 1997; Merino et al. 1999a).
TGF-β, Chondrogenesis, and Osteoarthritis
TGF-β has potent chondrogenic inductive ability both in cell culture and in vivo. Bovine bone extracts were shown to induce chondrocyte differentiation of embryonic rat muscle mesenchymal cells, and this chondrogenic activity was then identified as TGF-β (Seyedin et al. 1983, 1986). TGF-β ligands and receptors are broadly expressed in skeletal systems, and TGF-β plays a pivotal role during mesenchymal condensation (Kulyk et al. 1989; Tuli et al. 2003; Song et al. 2007). TGF-β further stimulates the expression of cartilage-specific extracellular matrix proteins such as type II collagen and aggrecan (Denker et al. 1995; Blaney Davidson et al. 2007; Shen et al. 2014). TGF-β does not promote chondrogenic differentiation when bone marrow mesenchymal cells are cultured on plastic or type I collagen, but strongly promotes chondrogenic differentiation of cells cultured in Matrigel (Tuli et al. 2003). In the latter case, TGF-β induces Wnt7a expression leading to N-cadherin expression that increases cell–cell contacts that are required for chondrogenic differentiation.
Injection of TGF-β underneath the periosteum results in increased chondrocyte proliferation, differentiation, and formation of cartilage (Joyce et al. 1990; Critchlow et al. 1995; Pedrozo et al. 1999). During endochondral bone formation, the perichondrium is a critical site of TGF-β1 signaling. TGF-β1 treatment of metatarsal bone cultures results in partial reduction of chondrocyte proliferation and chondrogenic differentiation, measured by collagen X expression (Alvarez et al. 2001). These inhibitory effects of TGF-β1 are diminished when the perichondrium is removed before culture (Alvarez et al. 2001). Perichondrium produces and secretes several other growth factors that control chondrocyte differentiation, such as Ihh and Shh, and PTHrP (Kronenberg 2003). Ihh and Shh induce perichondrial TGF-β2 expression, and TGF-β2 induces PTHrP expression in the perichondrium, which then inhibits differentiation into hypertrophic chondrocytes (Lanske et al. 1996; Vortkamp et al. 1996; Serra et al. 1999). However, the inhibitory effect of TGF-β1 on longitudinal bone growth is PTHrP-independent (Serra et al. 1999).
The severe bone defects in mice deficient for Tgfb2 or Tgfb3 underscore the important roles of these TGF-β isoforms during skeletogenesis (Dünker and Krieglstein 2000). Expressing a dominant-negative form of TβRII (dnTgfbr2) in skeletal tissues promotes terminal differentiation of chondrocytes in the growth plate (Serra et al. 1997) and hypertrophy of the articular chondrocytes in the superficial zone, concomitant with loss of proteoglycan, leading to progressive cartilage degradation as seen in osteoarthritis (Serra et al. 1997). Global disruption of Smad3 leads to chondrocyte hypertrophy of articular chondrocytes in the superficial zone and spontaneous joint degeneration (Yang et al. 2001). Treatment of bones from Smad3-deficient mice with TGF-β1 in culture results in partially impaired differentiation and inhibition of cell proliferation (Alvarez and Serra 2004). These findings suggest that Smad3 is the major signaling mediator of TGF-β-induced inhibition of chondrocyte proliferation in growth plate and articular cartilage. Several lines of evidence from mice with tissue-specific inactivation of Tgfbr2 further support the essential roles of TGF-β signaling in normal cartilage development and maintenance of the both growth plate and articular cartilages. Targeted inactivation of Tgfbr2 in undifferentiated limb bud mesenchyme reduces chondrocyte proliferation and accelerates hypertrophic differentiation, but delays terminal differentiation into hypertrophic chondrocytes (Seo and Serra 2007; Spagnoli et al. 2007). In contrast, inactivation of Tgfbr2 specifically using Col2a1-Cre in chondrogenic cells results in defects in the axial skeleton without altering chondrocyte differentiation (Baffi et al. 2004). Cre-mediated inactivation of Tgfbr2 specifically from the Col10a1 promoter in hypertrophic chondrocytes leads to delayed conversion of proliferating chondrocytes into hypertrophic chondrocytes and subsequent terminal differentiation (Sueyoshi et al. 2012). These results suggest that the function of TGF-β signaling depends on the differentiation state of the chondrocytes, and that TGF-β promotes terminal chondrocyte differentiation (Fig. 2).
Postnatal inactivation of Tgfbr2 using the tamoxifen-inducible Col2a1-CreERT2 cassette in chondrogenic cells results in increased Runx2, Mmp13 (encoding matrix metalloproteinase 13), Adamts5 (encoding a disintegrin and metalloproteinase with thrombospondin motif, ADAMTS 5), and Col10 expression in articular cartilage (Chen et al. 2007; Zhu et al. 2008; Shen et al. 2013). These mice show articular cartilage degradation at three months, and loss of the entire articular cartilage with extensive osteophyte formation, resembling osteoarthritis, by six months (Shen et al. 2013). In these mice, the osteoarthritis phenotype is alleviated by compound inactivation of Mmp13, whereas treatment with the MMP13 inhibitor CL82198 also decelerates the progression of the osteoarthritis phenotype (Shen et al. 2013). These observations are consistent with the attenuation of articular cartilage degeneration upon Mmp13 inactivation in a mouse model for medial meniscus destabilization, and provide a potential therapeutic strategy for human osteoarthritis (Little et al. 2009; Wang et al. 2013a; Shen et al. 2014; Ha et al. 2015).
TGF-β and BMP signaling interact functionally with each other during chondrogenic differentiation and growth plate development. Chondrocyte-specific expression of dominant-negative form of TβRI from the Col2a1 promoter results in an elongated growth plate, expanded prehypertrophic zone, and increased chondrocyte proliferation (Keller et al. 2011), supporting the idea that TGF-β signaling is critical for terminal differentiation of chondrocytes. Interestingly, BMP-2 treatment suppresses TGF-β-induced Smad activation, while inducing BMP–Smad signaling, in ATDC5 chondrogenic cells (Fig. 2) (Keller et al. 2011). TGF-β treatment of other cell types, such as C2C12 myoblasts, mouse embryonic fibroblasts, and HepG2 hepatoma cells, also increases BMP signaling (Wrighton et al. 2009). As mentioned already, chondrocyte-specific disruption of Smad4 with Col2a1-Cre results in impaired growth plate organization and dwarfism, but does not cause the lethality that is seen after chondrocyte-specific inactivation of both Smad1 and Smad5 (Zhang et al. 2005). It is possible that inactivation of both Smad signaling pathways in the Smad4-defective mice somewhat compensates for the loss of each Smad signaling branch to lessen the phenotype, although the loss of Smad4 may merely attenuate Smad signaling. Results using ATDC5 cells suggest that TGF-β and BMP signaling interact in chondrocytes to precisely regulate the length of the growth plates by forming a feedback loop similar to Ihh and PTHrP.
Activins and Chondrogenesis
Compared with TGF-β and BMPs, less information is available on the roles of activins in chondrogenesis. Activin A (inhibin βA homodimer), added to limb bud micromass cultures, enhances chondrogenesis by increasing the size of precartilaginous condensations and cartilaginous nodules (Jiang et al. 1993). However, another report describes that activin A inhibits chondrogenic differentiation while inhibin A (inhibin α and inhibin βA heterodimer) stimulates chondrogenesis in limb bud micromass culture (Chen et al. 1993). Implantation of activin-soaked beads into limb mesenchyme induces Bmpr1b expression that in turn increases local BMP signaling and subsequently induces the expression of activin A and TGF-β2, which are both necessary for digit elongation (Merino et al. 1999b). In contrast, implantation of follistatin-soaked beads into the tips of growing digits blocks chondrogenesis and digit formation (Merino et al. 1999b).
As mentioned, Inhba−/− mice and Fst−/− mice show craniofacial abnormalities, including cleft palate (Matzuk et al. 1995b,c). Acvr2−/− mice also display craniofacial abnormalities, including mandibular hypoplasia and defective Meckel’s cartilage, underscoring the role of activin signaling in chondrogenesis (Matzuk et al. 1995a). Although transgenic mice with chondrocyte-specific increase of activin signaling have not been generated, administration of activin A onto the periosteum of parietal bone in newborn rats results in increased thickness of both the periosteal and bone matrix layers (Oue et al. 1994). Transgenic mice that produce human inhibin α, encoded by INHA, display increased bone mass and improved biomechanical properties of the tibia through suppression of activin signaling (Perrien et al. 2007).
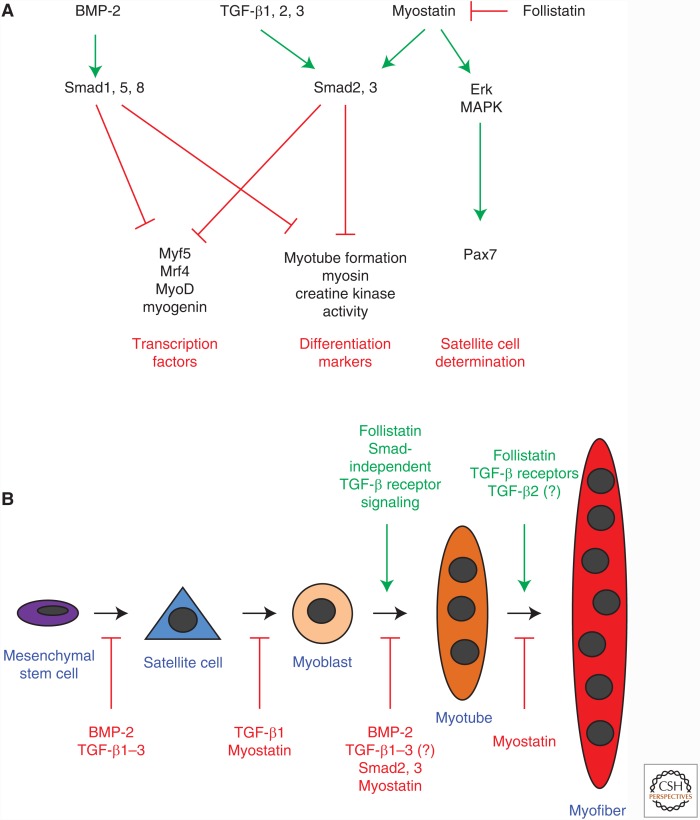
TGF-β FAMILY SIGNALING IN MYOBLAST DIFFERENTIATION
Muscle tissue contributes ∼40% to total body mass in the human body (Huard et al. 2002). Skeletal muscle has a variety of physiological functions, including locomotion, protection of underlying structures, metabolic functions, such as modulating blood glucose levels, and paracrine and endocrine functions (Huard et al. 2002; LeBrasseur et al. 2011; Pedersen and Febbraio 2012). The basic structural units of mammalian skeletal muscle are multinucleated myofibers (Huard et al. 2002). They contain sarcomeres consisting of myosin and actin filaments that facilitate the contractile function (Huard et al. 2002). To repair minor lesions caused by normal daily activity and injury after trauma, skeletal muscle tissue is regenerated in a coordinated process in which local myogenic progenitors, termed satellite cells, are activated (Mauro 1961; Kaji et al. 2006; Karalaki et al. 2009). Although normally quiescent and localized between myofibers, satellite cells migrate to the site of damage, where they proliferate (Charge and Rudnicki 2004; Kaji et al. 2006; Karalaki et al. 2009). Activated satellite cells can then differentiate into myoblasts that can advance to terminal myogenic differentiation to ultimately form new muscle fibers (McLennan and Koishi 2002). In this process, myoblasts fuse with each other or existing myofibers to finally form multinucleated myofibers (Pavlath and Horsley 2003; Charge and Rudnicki 2004; Kaji et al. 2006; Karalaki et al. 2009).
Multiple members of the TGF-β family are involved in coordinating these differentiation processes, including BMPs (Patterson et al. 2010), TGF-β (Massagué et al. 1986; Olson et al. 1986; Filvaroff et al. 1994; Karalaki et al. 2009), and myostatin (Langley et al. 2002; Rios et al. 2004; Amthor et al. 2006; McFarlane et al. 2008; Karalaki et al. 2009). They control the expression of myogenic transcription factors (Vaidya et al. 1989; Martin et al. 1992; Langley et al. 2002), including Pax transcription factors, involved in satellite cell determination and survival (Seale et al. 2000; Olguin and Pisconti 2012), as well as MRFs, which are essential for myoblastic lineage determination and terminal differentiation (Fig. 3A) (Sassoon et al. 1989; Weintraub et al. 1991; Megeney and Rudnicki 1995; Sabourin et al. 1999; Charge and Rudnicki 2004; Beylkin et al. 2006). The final outcomes of TGF-β family signaling on myoblast differentiation is context-dependent, including the cell type and differentiation state (Kollias and McDermott 2008), presence of other regulatory factors (Blachowski et al. 1993; Ewton et al. 1994; Engert et al. 1996; Florini et al. 1996; McLennan and Koishi 2002; Karalaki et al. 2009), and modulation of TGF-β family ligand bioavailability (e.g., through extracellular matrix molecules such as proteoglycans) (Casar et al. 2004; Droguett et al. 2006; Karalaki et al. 2009; Olguin and Pisconti 2012). This section will focus on the effects of BMP-2, TGF-β, and myostatin signaling in myoblast differentiation.
Figure 3.
TGF-β family signaling in myoblast differentiation. (A) Major intracellular and transcriptional targets of TGF-β and bone morphogenetic protein (BMP) signaling in myoblastic differentiation. (B) Satellite cells and myoblasts arise from mesenchymal stem cells. Members of the TGF-β family mostly inhibit myoblast differentiation. However, signaling through TGF-β receptors is required for myotube and myofiber formation. Myostatin inhibits myoblast differentiation, and Smad-independent TGF-β receptor mediated signaling may promote final myoblast differentiation. Erk, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase.
BMPs and Myoblast Differentiation
BMP-2 inhibits myogenic differentiation, and promotes chondrogenic and osteogenic lineage selection and differentiation (Fig. 3B) (Yamaguchi 1995). In cell culture, BMP-2 prevents myogenic differentiation of C2C12 myoblasts, resulting in an almost complete inhibition of myotube formation (Katagiri et al. 1994; Yamaguchi 1995), and induces osteoblastic differentiation, with increased expression of alkaline phosphatase and osteocalcin (Katagiri et al. 1994; Yamaguchi 1995). Similarly, in C26 osteoblast precursors, which can also differentiate into myotubes, addition of BMP-2 reduces myotube formation while promoting osteoblastic differentiation (Yamaguchi et al. 1991; Yamaguchi 1995). Additionally, BMP-2 inhibits myotube formation of primary murine muscle cells (Katagiri et al. 1994; Yamaguchi 1995). At the molecular level, BMP-2 inhibits the expression of MRFs, and, consequently, myogenic lineage selection (Fig. 3A) (Yamaguchi 1995). For instance, MyoD and myogenin expression normally increase during myogenic differentiation of C2C12 cells, but BMP-2 inhibits their expression (Katagiri et al. 1994). In vivo, administration of BMP-2 into muscles of mice results in ectopic bone formation (Wozney et al. 1988; Yamaguchi 1995), supporting the notion that BMP-2 alters muscle stem cell differentiation toward an osteoblastic lineage. Accordingly, increased BMP signaling is associated with FOP, a genetic disorder with progressive ectopic bone formation in muscle, tendons, and other connective tissues (Shafritz et al. 1996). As described already, FOP patients have mutations in the BMP type I receptor ACVR1/ALK-2 that lead to increased basal downstream receptor signaling (Shafritz et al. 1996; Fiori et al. 2006; Shore et al. 2006; Billings et al. 2008; Kaplan et al. 2009), increased signaling in response to BMP (de Gorter et al. 2010), and additional responsiveness to activins (Hatsell et al. 2015; Hino et al. 2015).
TGF-β and Myoblast Differentiation
TGF-β1, β2, and β3 inhibit myoblast proliferation, differentiation, and myotube formation in culture (Massagué et al. 1986; Olson et al. 1986; Yamaguchi 1995), but, in contrast to BMP-2, do not promote osteoblastic differentiation (Fig. 3B) (Katagiri et al. 1994; Yamaguchi 1995; Karalaki et al. 2009). However, while TGF-β can inhibit expression of muscle-specific genes, myotube formation and fusion of myoblasts (Fig. 3A) (Massagué et al. 1986; Olson et al. 1986; Allen and Boxhorn 1987; McLennan and Koishi 2002), it promotes embryonic myoblast differentiation (Cusella-De Angelis et al. 1994). This difference may be explained by intrinsic differences in the embryonic and fetal myoblasts with differential expression of surface molecules and transcription factors, and different sensitivity to TGF-β and other ligands (Biressi et al. 2007). Pharmacological inhibition of the TβRI kinase and silencing of Smad2 and Smad3 using siRNA increase the expression of myogenin in rat myoblasts, supporting the notion that TGF-β signaling partially inhibits early myoblast differentiation (Droguett et al. 2010). Interestingly, silencing of Smad2 and Smad3 also promotes expression of the later differentiation marker myosin and myotube fusion, whereas TβRI inhibition reduces myosin expression and fusion, even when Smad2 and Smad3 expression is silenced (Droguett et al. 2010). Expression of a dominant-negative TβRII also inhibits differentiation and myoblast fusion of C2C12 and rat myoblasts (Filvaroff et al. 1994; Droguett et al. 2010). Together, these findings suggest that TGF-β signaling is required for normal late myoblast differentiation, potentially through Smad-independent mechanisms (Fig. 3B) (Droguett et al. 2010).
The net effects of TGF-β ligands on myoblasts also depend on the presence of other factors such as IGF-I and FGFs (Olson et al. 1986; Cook et al. 1993; Florini et al. 1996; McLennan and Koishi 2002; Karalaki et al. 2009). For example, in primary satellite cells, IGF-I prevents the inhibition of differentiation by TGF-β (Allen and Boxhorn 1989). Additionally, differences of TGF-β receptor expression in myogenic cells in culture may help explain differences in responses. L6 cells, a rodent myoblast cell line, do not express betaglycan and the TGF-β receptor RIIB, an alternatively spliced variant of TβRII that can bind TGF-β2 in the absence of betaglycan, resulting in their inability to respond to TGF-β2 (Lopez-Casillas et al. 1993; Rotzer et al. 2001; McLennan and Koishi 2002).
Mechanistically, TGF-βs inhibit myoblast differentiation by repressing the expression and activities of MRFs, including myogenin and MyoD, through Smad3 activation (Fig. 3A) (Katagiri et al. 1994; Liu et al. 2001, 2004). Thus, inhibition of TβRI signaling using a kinase inhibitor increases expression of MRFs and Pax transcription factors in human embryonic stem cells (Mahmood et al. 2010). Additionally, TGF-β can repress myoblast differentiation by impairing the responsiveness to IGF-I (Schabort et al. 2011), reducing the activity of 2-5A synthetase and double-stranded RNA activated protein kinase (PKR) (Salzberg et al. 1995), as well as reducing miR-24 expression (Sun et al. 2008). Moreover, TβRI expression in rat myotubes is repressed by electrical activity (Ugarte and Brandan 2006), suggesting that muscle activity could promote tissue growth by preventing TGF-β inhibition of myoblast differentiation and fusion.
Despite similar effects on myoblast differentiation in cell culture, TGF-β1, 2, and 3 may have distinct effects on myoblast fusion in vivo (McLennan and Koishi 2002). TGF-β1 levels remain constant during myotube development (McLennan 1993), and Tgfb1−/− mice show normal myotube formation and muscle fiber development (McLennan et al. 2000; McLennan and Koishi 2002). In contrast, TGF-β2 is expressed by myotubes in developing and regenerating muscles, and its levels increase during development (McLennan and Koishi 1994, 1997), suggesting a role in late myoblast differentiation and during initiation of myotube formation in vivo (McLennan et al. 2000; McLennan and Koishi 2002). Consistent with this notion, C2C12 cells that express a dominant-negative form of TβRII cannot fuse when injected in vivo (Filvaroff et al. 1994). After injury, TGF-β1 and TGF-β3 are released from damaged muscle tissue and platelets, and stimulate further TGF-β synthesis by myogenic cells within the regenerating muscles (Assoian and Sporn 1986; Husmann et al. 1996; Karalaki et al. 2009). TGF-β is chemotactic for inflammatory cells, such as neutrophils and macrophages, which secrete pro-inflammatory factors including FGF, TNF, and interleukin 1 (IL-1), and thus induces angiogenesis (Husmann et al. 1996; Karalaki et al. 2009). In addition, other signaling factors are released from the extracellular matrix and vasculature, including IGF-I and hepatocyte growth factor (HGF), and together promote wound healing (Koutsilieris et al. 1997; Karalaki et al. 2009). Although HGF and IGF-I initiate proliferation and promote satellite cell differentiation, TGF-β1 can inhibit satellite cell proliferation and differentiation in a dose-dependent manner (Allen and Boxhorn 1987; Ewton et al. 1994; Bladt et al. 1995; Engert et al. 1996; Dietrich et al. 1999; Yamane et al. 2003; Karalaki et al. 2009). Furthermore, TGF-β1 induces the expression of proteins of the extracellular matrix that surrounds the muscular defect during injury repair, and promotes regeneration of the myofiber basement membrane (Edwards et al. 1987; Streuli et al. 1993; Husmann et al. 1996). By stimulating the expression of these proteins, including collagens and proteoglycans, TGF-β promotes postinjury generation of fibrosis and scar tissue (Massagué et al. 1986; Husmann et al. 1996; Karalaki et al. 2009). As a result, intramuscular application of decorin, which inhibits TGF-β activity in the extracellular matrix, improves muscle healing and prevents muscle fibrosis (Sato et al. 2003; Casar et al. 2004; Karalaki et al. 2009; Ten Broek et al. 2010).
Myostatin and Myoblast Differentiation
Myostatin is a potent repressor of myoblast differentiation required for muscle development, growth and repair, and, thus, of muscle mass (Langley et al. 2002; Rios et al. 2004; Karalaki et al. 2009). Myostatin is expressed by satellite cells and myoblasts during embryogenesis and regeneration of skeletal muscle (Karalaki et al. 2009; Ten Broek et al. 2010), and inhibits myoblast recruitment and differentiation, thereby decreasing the fiber size and number (Fig. 3B) (Kaji et al. 2006; Karalaki et al. 2009; Trendelenburg et al. 2009; Ge et al. 2011). In cell culture, myostatin inhibits proliferation, differentiation, and protein synthesis of rodent myoblasts (Langley et al. 2002; McFarlane et al. 2006; Huang et al. 2007a), and reduces myoblast fusion and creatine kinase activity in human myoblasts (Trendelenburg et al. 2009). Mechanistically, myostatin binds to the type II receptor ActRIIB (and to a lesser extent ActRII) and the type I receptors ALK-4 or TβRI/ALK-5, resulting in Smad2 and Smad3 as well as Erk MAPK activation (Lee and McPherron 2001; Zhu et al. 2004; Yang et al. 2006; McFarlane et al. 2008). Inhibition of Akt signaling through mTOR complex 1 further enhances myostatin-induced Smad activation and potentiates myostatin’s inhibitory effects (Bentzinger et al. 2008; Trendelenburg et al. 2009). Myostatin can also inhibit IGF-I signaling through the PI3K–Akt pathway in myoblasts (Huang et al. 2007a), and stimulate the ubiquitin-mediated proteolysis through a FoxO1-dependent mechanism (McFarlane et al. 2006). At the transcriptional level, myostatin increases Pax7 expression, which requires Erk MAPK activation, and represses the expression of Myf5, myogenin and MyoD, thereby inhibiting satellite cells from progressing in myoblastic lineage differentiation (Fig. 3A) (Amthor et al. 2002; Langley et al. 2002; McFarlane et al. 2008; Trendelenburg et al. 2009; Ten Broek et al. 2010). In addition, myostatin induces expression of the cell cycle inhibitor p21Cip1 to maintain satellite cell quiescence (McCroskery et al. 2003; Kaji et al. 2006). Mice with inactivated myostatin expression show an extensive increase of skeletal muscle mass, muscle hypertrophy and hyperplasia, and increased myofiber size and number (McPherron et al. 1997; Lee and McPherron 2001; Karalaki et al. 2009). Cattle with a homozygous frameshift mutation that removes a conserved sequence of the myostatin protein show doubling of their muscle mass, further supporting a key role of myostatin in repressing muscle mass (Grobet et al. 1997; Kambadur et al. 1997; McPherron et al. 1997). After injury, myostatin is detected in necrotic muscle fibers, but not in regenerating myotubes during maturation and fusion, consistent with the concept that myostatin expression is repressed during muscle repair to facilitate satellite cell recruitment and proliferation (Bradford et al. 2000; Karalaki et al. 2009).
Follistatin, Smads, and Myoblast Differentiation
Follistatin binds not only to activins and inhibins, but also to myostatin and some BMPs, and can inhibit interactions between these TGF-β ligands with their cell surface receptors (Nakamura et al. 1990; Harrison et al. 2005; Gordon and Blobe 2008). Consequently, follistatin can oppose the inhibitory effects of these ligands on myoblast differentiation and promote differentiation as well as myoblast fusion (Fig. 3A, B) (Lee and McPherron 2001; Iezzi et al. 2004; Pisconti et al. 2006). Follistatin administration also antagonizes the pro-apoptotic effects of BMP-7, but remarkably enhances BMP-7-induced Pax3 expression during chick limb development (Amthor et al. 2002). Perhaps, for BMPs that bind to follistatin with low affinity such as BMP-7, follistatin could store and present these BMPs to myogenic cells, and thereby in part promote BMP signaling (Amthor et al. 2002).
In vivo, follistatin overexpression under control of the myosin light chain promoter leads to increased muscle mass in mice (Lee and McPherron 2001), and follistatin overexpression under control of the myosin promoter in zebrafish increases muscle growth by inducing myofiber hyperplasia (Li et al. 2011). It is currently unclear if follistatin acts in vivo mainly through inhibition of myostatin or other TGF-β family ligands (Amthor et al. 2004; Olguin and Pisconti 2012). That follistatin not merely acts through inhibition of myostatin is supported by observations that follistatin overexpression in muscle of myostatin-deficient mice further increases muscle mass, whereas heterozygous loss of follistatin results in reduced muscle mass (Gilson et al. 2009; Lee et al. 2010). Modulation of activin A activity as well as myostatin-independent Smad3 and mTOR signaling are also involved in the in vivo effects of muscle mass regulation by follistatin (Lee et al. 2010; Winbanks et al. 2012).
TGF-βs and myostatin exert their inhibitory effects on myoblast differentiation, at least in part, through activation of Smad signaling. Both Smad3−/− and Smad4−/− mice show defects in satellite cell number and function, myogenic differentiation, and myoblast fusion, and have decreased muscle mass (Ge et al. 2011; Han et al. 2012). Interestingly, Smad3−/− mice show increased levels of myostatin in myoblasts, and myostatin inactivation in Smad3−/− mice improves the muscle phenotype (Ge et al. 2011). In addition, noncanonical pathways and cross-talk with other signaling pathways may also determine the net outcome of TGF-β signaling on myoblast differentiation in vivo (Luo 2017; Zhang 2017).
TGF-β FAMILY SIGNALING IN ADIPOCYTE DIFFERENTIATION
Adipose tissue can be divided into two groups based on function; white adipose tissues (WATs) store energy, and brown adipose tissues (BATs) are thermogenic. WATs and BATs are found at anatomically distinct positions in the body. WATs are located intra-abdominally where they are also called visceral fat, and subcutaneously, whereas BATs are most commonly located in the neck and supraclavicular regions (Hilton et al. 2015). Cold exposure or β-adrenergic stimulation induces brown adipose-like tissues within WATs, a phenomenon known as browning of WATs. These brown fat-like cells in WATs show comparable levels of uncoupling protein-1 (UCP-1) that are critical for thermogenesis similar to classical brown adipocytes in BATs. Although brown adipocytes in BATs are of the Myf5+ lineage, these induced brown adipocytes within WATs are Myf5− like other cells found in WATs. These cells are now thought to constitute a third type of adipocyte referred to as either a “beige” adipocyte or “brite” (brown-in-white) adipocyte (Fig. 4) (Gesta et al. 2007; Wu et al. 2012, 2013; Harms and Seale 2013).
Figure 4.
TGF-β family signaling in adipocyte differentiation. Mesenchymal stem cells give rise to adipocytes under the guidance of a complex signaling network. The Myf5− lineage gives rise to beige and white preadipocytes, and the Myf5+ lineage gives rise to brown adipocytes. Growth and differentiation factor (GDF)-3 inhibits the differentiation of beige preadipocytes from progenitor cells as well as beige preadipocytes into beige adipocytes. Bone morphogenetic protein (BMP)-4 and -7 promote white and brown preadipocyte differentiation, respectively. TGF-β and myostatin (GDF-8) each inhibit both brown and white preadipocyte formation. Enhancer binding proteins are stimulated by BMP-4 and hormonal cues. These DNA-binding proteins promote gene expression indicative of adipocyte maturation, such as the genes encoding CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor-γ2 (PPARγ2), whereas Smad2 and Smad3 signaling, as well as Smad6 and/or Smad7 in the mesenchymal progenitor reduce expression of these genes. UCP-1, uncoupling protein-1.
Undifferentiated MSCs initially differentiate into a preadipocyte stage and then proliferate by mitotic clonal expansion. Hormonal cues stimulate further differentiation by initiating production of C/EBPs β and δ, encoded by Cebpb and Cebpd, respectively (Wu et al. 1996). These transcription factors then activate the expression of PPARγ2 (encoded by Pparg2) and C/EBPα (encoded by Cebpa) leading to growth arrest for terminal differentiation and expression of adipocyte marker genes such as FABP4 (fatty acid-binding protein 4)/aP2 and leptin. At this stage, activation of FoxO1 transcription factor expression activates the cell cycle inhibitor p21CIP1 (Morrison and Farmer 1999; Nakae et al. 2003).
BMPs and Adipogenesis
Many BMPs play dual roles in osteogenesis and adipogenesis of multipotent cell populations (Asahina et al. 1996; Kang et al. 2009). C3H10T1/2 cells are fibroblasts established from late stage embryos of the C3H mouse strain. These cells functionally resemble MSCs and are competent to differentiate into adipocytes, osteoblasts, myoblasts, and chondrocytes (Reznikoff et al. 1973). Among the BMP ligands, BMP-4 has proadipogenic action and directs multipotent C3H10T1/2 cells to the adipocyte lineage (Bowers and Lane 2007; Zamani and Brown 2011). Subcutaneous injection of BMP-4-treated C3H10T1/2 cells results in development of adipose tissues (Fig. 4) (Tang et al. 2004). Genetic engineered expression of a constitutively active form of Bmpr1a or Bmpr1b in C3H10T1/2 cells results in accumulation of lipid and expression of Fabp4, which lends further credence to the notion that BMP signaling in multipotent cell populations favors adipogenic differentiation (Huang et al. 2009b). Interestingly, noggin treatment of A33 cells, a committed preadipocyte line derived from C3H10T1/2 cells, blocks adipocyte differentiation (Bowers et al. 2006), suggesting that autocrine BMP signaling enables competence for adipocyte differentiation.
BMP-7 is also involved in adipogenesis in many populations of multipotent mesenchymal cells including BMSCs (Chen et al. 2001). BMP-7 can induce human MSCs to an adipogenic lineage in high-density micromass culture, a condition that normally induces chondrogenic differentiation (Neumann et al. 2007). Treating brown preadipocytes with BMP-7 markedly increases Ucp1 expression compared with other BMP ligands (Tseng et al. 2008). It is noteworthy that BMP-7 can activate Smad1, 5, and 8 in both brown and white preadipocytes, whereas a robust activation of p38 MAPK is additionally observed in brown preadipocytes (Tseng et al. 2008). These findings indicate roles of BMP-7-induced Smad and p38 MAPK pathway signaling in the regulation of thermogenesis in conjunction with a nuclear coactivator PGC1 (PPARγ coactivator-1). Furthermore, detailed gene expression analyses show that BMP-7 treatment significantly suppresses expression of necdin, PREF1 (preadipocyte factor 1) and Wnt10a, which inhibit brown adipogenesis while increasing the expression of PRDM16 (PRD1-BF1-RIZ1 homologous domain containing 16), a transcription factor that directs the brown fat lineage (Seale et al. 2007). Bmp7−/− mice die at birth, and Bmp7−/− embryos show significant reduction in interscapular BAT mass compared with that of littermate controls (Tseng et al. 2008). Tail vein injection of BMP-7 expressing but not BMP-3 expressing adenovirus specifically induces Prdm16 and Ucp1 expression in BAT, but not other genes involved in energy metabolisms in other tissues, leading to increased body temperature and decreased body mass of the injected mice (Tseng et al. 2008). These results highlight the indispensable roles of BMP-7 in brown adipocyte differentiation and energy expenditure (Fig. 4).
In contrast to the proadipogenic function of BMP-4 to promote white adipocytes, and of BMP-7 to promote brown adipocytes, BMP-2 treatment of BMSCs promotes osteogenic differentiation and inhibits adipogenesis (Gimble et al. 1995; Pereira et al. 2002). Dose-dependent effects of BMP-2 treatment of C3H10T1/2 cells are observed. Low doses of BMP-2 induce adipogenic differentiation, whereas high doses of BMP-2 promote chondrogenic and osteogenic differentiation (Wang et al. 1993; Asahina et al. 1996). Similar to other BMPs, BMP-2 is also able to induce adipogenesis, yielding white adipocytes from committed preadipocytes such as 3T3-L1 and 3T3-F442A (Ji et al. 2000; Rebbapragada et al. 2003). Treatment of preadipocytes with BMP-2 along with rosiglitazone, a PPARγ agonist, further enhances adipogenesis (Sottile and Seuwen 2000). Of note, forced expression of a constitutively active form of either BMPRIA or BMPRIB in 3T3-F442A cells inhibits adipogenesis and stimulates osteogenesis (Skillington et al. 2002). Differences in culture methods or clonal variation of the cells used in each study may explain such discrepancies; however, an alternative interpretation is that cell fate specification of mesenchymal cells through BMP signaling is highly dose-dependent. The differentiation factor NEL-like molecule-1 (NELL1), a secreted protein that can induce bone formation (Ting et al. 1999), can antagonize adipogenesis of the mouse BMSC cell line M2-10B4 and human primary BMSCs, when adipogenesis is induced by BMP-2 (Shen et al. 2016). These findings suggest that, in addition to the dose of BMP-2, additional factors may contribute to cell fate determination of BMSCs. BMP-2 induces both Smad and p38 MAPK signaling in C3H10T1/2 cells. Studies using specific inhibitors reveal that the Smad pathway is important for Pparg expression, whereas p38 MAPK signaling is critical for PPARγ to control gene transcription (Hata et al. 2003). Upon BMP-2 treatment, Schnurri-2 (Shn2) enters the nucleus and directly interacts with Smad1 and Smad4, and with C/EBPα at the Pparg2 promoter to induce Pparg2 expression (Jin et al. 2006). Shn2−/− mice show reduced white fat mass with normal brown fat mass, which suggests that BMP ligands (or at least BMP-2) use the BMP–Smad pathway along with Shn2 to regulate white adipogenesis in vivo (Jin et al. 2006). These findings suggest that the effects of BMP-2 exposure are largely determined by the dose administered as well as the adipocyte differentiation state at the time of administration.
Pioneering work using 2T3 mesenchymal cells have led to the hypothesis that BMP signaling through BMPRIA favors adipogenic differentiation, whereas signaling through BMPRIB favors osteoblastic differentiation (Chen et al. 1998). The experimental strategy was to overexpress dominant-negative forms of receptors, because RNA interference technology to silence gene expression in mammalian cells had not been established; thus, the specificity of the signaling pathways blocked by each dominant-negative receptor form has been a concern. Although body weight is slightly reduced in Bmrp1b−/− mice, no overt phenotypes are found in adipose tissues (Schulz et al. 2013). Studies in humans reveal that increased expression of BMPRIA is associated with visceral and subcutaneous white fat deposits in obese individuals (Bottcher et al. 2009). In mice, the specific disruption of Bmpr1a in Myf5+ lineage cells results in a significant reduction of BAT (Schulz et al. 2013). Subcutaneous WAT and epididymal WAT that chiefly originate from a Myf5− lineage have the expected mass, whereas interscapular WAT and retroperitoneal WAT with subpopulations of cells from the Myf5+ lineage have reductions in size, showing the importance of BMPRIA-induced BMP signaling in adipogenesis (Schulz et al. 2013). Specific disruption of Acvr1/Alk2 in Myf5+ lineage results in a similar yet milder phenotype, whereas compound conditional inactivation of Bmpr1a and Acvr1 in Myf5+ lineage cells results in complete loss of brown adipogenesis. In the mice with inactivated Bmpr1a expression in Myf5+ lineage cells, the severe BAT paucity increases sympathetic input to WAT, and thus enhances beige adipogenesis to promote browning of the WAT (Fig. 4) (Schulz et al. 2013). Although BMP-2 and BMP-4 bind BMPRIA with much higher affinity than BMP-7 (Sebald et al. 2004), the results suggest that BMP-7 binding to BMPRIA is of key importance for brown adipogenesis. These results also reveal a potentially new mechanism to compensate for loss of brown adipocytes, by promoting beige adipogenesis to restore total thermogenic capacity.
TGF-β as a Negative Regulator of Adipogenesis
Unlike the BMPs, TGF-β has inhibitory roles during adipogenesis. TGF-β treatment increases proliferation of 3T3-F442A preadipocytes (Jeoung et al. 1995; Choy et al. 2000), and potently inhibits the adipogenic conversion of 3T3-L1 preadipocytes (Ignotz and Massagué 1985). TGF-β also down-regulates adipose gene expression in fully differentiated TA1 adipocytes (Torti et al. 1989). Transgenic mice expressing TGF-β1 from the rat Pck1 promoter (of the gene encoding phosphoenolpyruvate carboxykinase 1) in several tissues, including WAT and BAT, show severely reduced WAT and BAT (Clouthier et al. 1997), supporting the notion that TGF-β represses adipogenesis.
During adipogenesis the expression levels of Smad2, 3, and 4 are unchanged in 3T3-F442A preadipocytes, whereas those of Smad6 and Smad7 are severely decreased (Choy et al. 2000). Overexpression of Smad2 or Smad3 inhibits lipid accumulation of 3T3-F442A preadipocytes, with Smad3 exerting stronger effect. In contrast, a dominant-negative form of Smad3 is able to suppress the inhibitory function of TGF-β signaling on adipogenesis; however, adipogenesis proceeds normally in the presence of dominant-negative form of Smad2 (Choy et al. 2000). These findings support Smad3 as a TGF-β signaling component that inhibits adipogenic differentiation, with Smad2 playing no discernible role in this process (Fig. 4). Smad3, along with Smad4, associates with C/EBPβ and C/EBPδ resulting in decreased Pparg expression (Choy and Derynck 2003). Smad6 and Smad7, known as inhibitory Smads, block TGF-β family signaling through Smads. However, overexpressing Smad6 or Smad7 in 3T3-F442A preadipocytes yields a strong inhibitory effect on adipogenesis (Choy et al. 2000). In consideration of the finding that inhibitory Smad levels are sharply reduced during adipogenesis, Smad6 and Smad7 may play a distinct role in regulating TGF-β family signaling activity in adipocytes (Fig. 4) (Choy et al. 2000). Because Smad6 inhibits BMP signaling, and Smad7 inhibits both BMP and TGF-β signaling (Hayashi et al. 1997; Imamura et al. 1997), and BMP-4 and BMP-7 stimulate white and brown adipogenesis, respectively (Fig. 4) (Bowers and Lane 2007; Tseng et al. 2008; Zamani and Brown 2011), the inhibitory effect on adipogenesis by Smad6 and/or Smad7 may additionally relate to inhibition of BMP signaling.
Growth Differentiation Factors and Adipogenesis
GDF-3 is primarily expressed in adipose tissue (McPherron and Lee 1993), and a high-fat diet selectively increases Gdf3 expression in WAT (Witthuhn and Bernlohr 2001). Increased expression of Gdf3 in mice by adenoviral-mediated gene transfer results in increased body fat with adipocyte hypertrophy when the mice are fed a high-fat diet, but these mice have normal lipid distribution when on a normal fat diet (Wang et al. 2004). In contrast, Gdf3−/− mice show resistance to diet-induced obesity (Andersson et al. 2008; Shen et al. 2009a). Control mice become obese when fed with a high-fat diet, whereas Gdf3−/− mice accumulate less WAT while consuming a similar amount of food to account for their higher metabolic rate (Shen et al. 2009a). Interestingly, the gene expression profile of WAT in Gdf3−/− mice resembles that of BAT, suggesting that loss of GDF-3 leads to browning of WAT and prompts beige adipogenesis (Fig. 4). GDF-3 binds to the activin type I receptors ActRIB/ALK-4 and ActRIC/ALK-7 along with ActRIIB, and induces activation of Smad2 and Smad3 (Chen et al. 2006; Levine and Brivanlou 2006). Because Gdf3−/− mice experience WAT browning only when placed on high-fat diets, this differentiation phenotype differs from the WAT browning that results from Bmpr1a inactivation in Myf5+ lineage cells (Schulz et al. 2013). These findings suggest that different nutrient conditions regulate GDF-3-induced signaling through ActRIB/C and Smad2 and Smad3 to control beige adipogenesis in WAT.
Myostatin, known as a key negative regulator of skeletal mass, promotes adipogenesis and inhibits myogenesis in C3H10T1/2 multipotent mesenchymal cells (Artaza et al. 2005; Feldman et al. 2006). However, the resulting adipocytes in cell culture are smaller and have a gene expression profile reminiscent of immature adipocytes (Feldman et al. 2006). In contrast, human MSCs cultured with myostatin undergo less adipogenic differentiation, as apparent by decreased expression of key adipogenic genes such as PPARG and CEBPA (Guo et al. 2008). CEBPB expression, which is required for clonal expansion of committed adipocytes (Tang et al. 2003), is not affected by myostatin treatment, suggesting that myostatin-induced inhibition occurs after mitotic clonal expansion during early adipogenic differentiation. These observations are consistent with the inhibition of adipogenic differentiation of 3T3-L1 preadipocytes by myostatin treatment (Kim et al. 2001). These inhibitory effects require Smad3 as in the case of TGF-β (Guo et al. 2008).
Gdf8−/− mice have reduced quantities of adipose tissues, decreased cell size of the remaining adipocytes, and slower metabolic rate than control mice, while maintaining normal body temperature and food intake (Lin et al. 2002; McPherron and Lee 2002; Hamrick et al. 2006). Overexpression of a dominant-negative form of ActRIIB in skeletal muscle from the Myl1 promoter (of the gene encoding myosin light chain 1) results in muscle and adipose phenotypes similar to Gdf8−/− mice (Lee and McPherron 2001). In contrast, expression of the dominant-negative form of ActRIIB only in adipose tissue from an Fabp4 (also named aP2) promoter in mice does not lead to overt changes in either muscle or adipose tissue (Guo et al. 2009). Moreover, myostatin signaling activates both PPARγ and MyoD expression in adipose-derived stem cells, whereas it represses their expression in muscle satellite cells (Zhang et al. 2015). Taken together, these findings suggest that the reduced adiposity of Gdf8−/− mice may be a result of the muscle hypertrophy that increases muscle glucose metabolism and decreases the energy available for lipid storage.
Systemic increase of myostatin levels by injecting myostatin producing cells into athymic nude mice results in a reduced skeletal muscle mass and a dramatic reduction of WATs (Zimmers et al. 2002). Expression of Gdf8 in adipocytes of mice from an Fabp4 promoter decreases adipocyte size and increases resistance to diet-induced obesity (Feldman et al. 2006). Muscle-directed expression of Gdf8 from the Cmk promoter (gene encoding muscle creatine kinase) increases epididymal fat pads in mice (Reisz-Porszasz et al. 2003). These results show a sharp contrast with reduction in WAT resulting from systemic increase in myostatin. A plausible explanation is that adipose and muscle tissues have sensitive but varied dose responses to myostatin signaling. An alternative explanation is that overexpressed myostatin competes with other signaling pathways. Thus, the interaction of myostatin with ActRIIB may inhibit the BMP-7 signaling pathway (Rebbapragada et al. 2003), leading to reduced overall adipogenesis that primarily affects BATs (Singh et al. 2014).
BMP-3b, also known as GDF-10, is expressed at much higher levels than either BMP-3 or BMP-2 in both WATs and BATs (Cunningham et al. 1995; Hino et al. 1996, 2012). BMP-3b inhibits osteoblast differentiation of C2C12 myoblasts by binding to ActRIB/ALK-4 and ActRIIB and consequent Smad3 activation (Matsumoto et al. 2012). siRNA-mediated silencing of Bmp3b in 3T3-L1 preadipocytes increases adipogenic gene expression, apparent from expression of adiponectin and PPARγ, and increased expression of Bmp3b reduces the expression of these genes (Hino et al. 2012). Bmp3b−/− mice lack an apparent developmental phenotype, suggesting compensation for the loss of BMP-3b in vivo by other members of TGF-β family (Zhao et al. 1999). Future research (e.g., using high-fat diet and cold exposure) on Bmp3b−/− mice will inform investigators of the functions of BMP-3b during adipogenesis.
Activin and Energy Expenditure
The effects of activin on adipogenesis in cell culture are similar to those of TGF-β. Activin A promotes proliferation of human adipocyte precursor cells but inhibits early stages of differentiation (Zaragosi et al. 2010). Activin A-treated 3T3-L1 preadipocytes express less PPARγ and CEBPα than untreated controls (Hirai et al. 2005). Silencing of SMAD2 in human adipose progenitor cells overrides the effect of activin A treatment to prompt adipogenesis, suggesting that activin A signaling is mediated largely by Smad2 along with C/EBPβ (Zaragosi et al. 2010). In human adipocytes, the expression of INHBA, which encodes the activin A monomer, is repressed during adipocyte differentiation, whereas the expression of activin B from the INHBB gene remains high (Sjöholm et al. 2006; Carlsson et al. 2009; Zaragosi et al. 2010). A mutant mouse line that has Inhbb introduced into the Inhba locus allows for activin B expression with the same spatiotemporal pattern of activin A (Brown et al. 2000). These mice have much less WAT, elevated metabolic rates, and increased expression of genes necessary for mitochondrial biogenesis and uncoupling (Li et al. 2009). These results suggest that activin signaling controls mitochondrial energy expenditure, in addition to its function in adipocyte differentiation.
TGF-β FAMILY SIGNALING IN TENOCYTE DIFFERENTIATION
Clinically, tendon and ligament injuries are some of the most common musculoskeletal injuries ranging from ankle or wrist sprains and strains to Achilles tendon rupture (Boyer et al. 2005; Towler and Gelberman 2006). These injuries include tendinosis, tendinitis, and paratendinitis, and can be traumatic and inflammatory in nature, or stem from overuse or degenerative etiologies (Maffulli et al. 1998; Asplund and Best 2013). Regardless of the specific condition, severe tendon injuries are challenging to manage as restoration of flexibility and tensile strength at tendon–tendon and tendon–bone interfaces are difficult to replicate with surgical procedures that rely on autograft, allograft, or synthetic materials (Olson et al. 1988; Sabiston et al. 1990; Paulos et al. 1992; Jackson and Simon 2002; Towler and Gelberman 2006; Bagnaninchi et al. 2007). Tendon itself is a highly specialized tissue with type I collagen arranged in hierarchical, longitudinal fibril arrays bound into fascicles by the endotenon, which runs contiguously with the epitenon through which the vasculature, innervation, and lymphatics traverse (Kastelic et al. 1978; Fenwick et al. 2002; Clegg et al. 2007). Embryologically, tendons derive from a specialized compartment of the somite, the syndetome (Brent et al. 2003). A specific marker, the basic helix-loop-helix (bHLH) transcription factor scleraxis (encoded by the Scx gene), is present in tendon progenitor cells and persists in mature tenocytes (Cserjesi et al. 1995; Schweitzer et al. 2001). Although this unique marker is very useful in tenocyte research, the signaling pathways that lead to tenogenic mesenchymal differentiation are still being elucidated.
TGF-β family members play a role in tenocyte differentiation and maintenance of the tenocyte phenotype. Although the transcription factors that guide tenocyte differentiation significantly overlap with those in other musculoskeletal tissues, most research to date on tenogenic lineage differentiation and tendon healing focuses on differences between these factors. These studies also show that certain members of the TGF-β family are protenogenic, whereas others promote cartilaginous, osteogenic, or myogenic differentiation. Specifically, BMP-2, -4, and -7 are antitenogenic, whereas GDF-7 (BMP-12) is the best studied tenogenic BMP (Lee et al. 2011; Yee Lui et al. 2011; Rui et al. 2012b). GDF-5, -6, and -7 (also known as BMP-14, -13, and -12, respectively) have been shown to induce new tendon formation in animal models, and TGF-β promotes retention of Scx expression and maintenance of the tenocyte phenotype (Wolfman et al. 1997; Barsby et al. 2014). Furthermore, Smads are crucial in the gene expression profile resulting from tensile and compressive forces, which are essential for tenocyte formation and regeneration (Maeda et al. 2011).
Similar to myoblast differentiation into myocytes, BMPs play a variety of roles in tenogenesis. BMP-2 promotes tenocyte precursor cell differentiation into osteocytes, chondrocytes, and adipocytes in cell culture with increased production of glycosaminoglycans and expression of aggrecan (Rui et al. 2013; Liu et al. 2014). Furthermore, BMP-2 potently induces osteogenic differentiation of tendon-derived stem cells, which express higher levels of the BMPRIA, BMPRIB, and BMPRII receptors than bone marrow-derived stem cells (Rui et al. 2012a). Although it does not promote tenocyte differentiation, BMP-2 signaling may be important at the tendon–bone junction (Rodeo et al. 1999; Ma et al. 2007; Rui et al. 2012a). Specifically, canonical BMP signaling stimulates ectopic bone formation after tendon transection as there is an increase in downstream Smad1/5/8 phosphorylation and inhibition of BMP signaling mitigates this process (Peterson et al. 2014). Additionally, overexpression in BMP receptor ALK-2 in cells of the scleraxis lineage (thought to mark tendon progenitor cells) leads to ectopic bone at sites of enthesis such as the Achilles tendon (Agarwal et al. 2017). In addition to BMP-2, BMP-4 and BMP-7 were shown to be antitenogenic. In calcifying tendinopathy models, these BMPs induce chondrocyte-like and fibroblast-like differentiation changes in healing tendon (Yee Lui et al. 2011). BMP-7 changes the gene expression profile of tendon tissue to more closely resemble cartilaginous meniscus tissue (Ozeki et al. 2013). Interestingly, these BMPs are found in clinical samples of tendinopathy, but not in healthy tendon (Rui et al. 2013). Furthermore, tendon-derived stem cells from unhealthy or injured tendon are more sensitive to BMP signaling through Smads to induce nontenogenic differentiation (Lui and Wong 2013).
Although BMP-2, -4, and -7 are antitenogenic, other BMPs directly control the differentiation of tenogenic precursors into tenocytes. GDF-5, GDF-6, and GDF-7 have been shown to promote tendon repair and the formation of new tendon tissue (Wolfman et al. 1997; Yu et al. 2007; Lee et al. 2011), and the tenogenic activity of GDF-7 specifically was shown both in cell culture and in vivo (Lou et al. 2001; Wang et al. 2005; Violini et al. 2009; Lee et al. 2011). Of particular interest is the ability of GDF-7 to induce MSCs of nontendon origin (e.g., bone marrow–derived stem cells) to differentiate into cells with tenocyte characteristics, with higher expression of scleraxis and tenomodulin (Lee et al. 2011). In addition, collagen scaffolds seeded with bone marrow-derived stem cells exposed to GDF-7 improve tendon repair in an in vivo model (Lee et al. 2011).
As tendon plays a unique role in the musculoskeletal system, sustaining dynamic ranges of compression and extension while retaining flexibility, physical forces play an essential role in tenocyte differentiation and function. These forces act in conjunction with effects of other members of the TGF-β family. TGF-β is known to maintain scleraxis expression in tendons in response to mechanical forces (Maeda et al. 2011). Specifically, TGF-β3 promotes Scx expression and scleraxis translation, as well as tenocyte differentiation, when combined with tensile and compressive forces (Barsby and Guest 2013; Barsby et al. 2014). TGF-β2 has also been shown to induce tenogenic differentiation of MSCs (Hoffmann et al. 2006; Guerquin et al. 2013). As mentioned, other TGF-β family members including GDF-5, GDF-6, and GDF-7 (those most related to BMPs) can induce new tendon and ligament formation, even at ectopic sites in animal models (Wolfman et al. 1997). Smads, specifically Smad2 and Smad3, are also required in the regulation of tenogenic gene expression that is induced by physical forces acting on tendon tissue (Maeda et al. 2011). Furthermore, Smad8 has been shown to play a role in the tenogenic differentiation of MSCs (Hoffmann et al. 2006).
Although the body of work regarding MSC differentiation to tenocytes is not as robust as that of adipogenic, myogenic, and osteogenic differentiation, it is clear that BMP and TGF-β signaling through Smads plays a leading role. Tendon is a functionally distinct and dynamic musculoskeletal tissue, and its mesenchymal roots are evident in the signaling pathways that share common mediators with other differentiated mesenchymal tissues.
CONCLUDING REMARKS
The effects of TGF-β family signaling in mesenchymal lineage selection and progression in differentiation are complex and time-, dose-, as well as context-dependent. Also, some TGF-β signaling factors differentially affect specific stages during mesenchymal differentiation. For example, TGF-βs promote proliferation and early differentiation of MSCs into osteoblast, chondrocyte, and adipocyte progenitor cells, but inhibit later conversion into mature osteoblasts, chondrocytes, and adipocytes. In contrast, TGF-βs inhibit myoblast proliferation, differentiation, and myotube formation. Similar to TGF-βs, BMP-2 promotes chondrogenic and osteogenic lineage selection and differentiation, and inhibits myogenic differentiation, but, in contrast to TGF-βs, also promotes late osteoblast differentiation and matrix mineralization. We discussed in this review how the final effect of TGF-β family signaling on mesenchymal differentiation also depends on interactions between different TGF-β signaling pathways, and the presence of other signaling molecules such as IGF-I, Wnts, FGFs, and others.
In addition to findings in cell culture, discoveries in animal models and genetic studies in patients during the past decades have greatly extended our understanding of how signaling by the TGF-β family members can drive disease. This includes diseases caused by mutations in genes encoding ligands or receptors of the TGF-β family as well as disorders caused by other primary mechanisms, in which altered TGF-β family signaling contributes to disease. The first group includes, for example, Camurati-Engelmann disease, with mutations in the sequence encoding pro-TGF-β1 (Kinoshita et al. 2000), brachydactyly type A2 with mutations in the BMP2 or BMPRIB genes, brachydactyly type B resulting from NOG mutations (Lehmann et al. 2007; Dathe et al. 2009), acromesomelic chondrodysplasia (Hunter-Thompson type), and chondrodysplasia Grebe type with GDF5 mutations (Thomas et al. 1996, 1997), as well as FOP resulting from mutations in the ACVR1/ALK-2 receptor (Shore et al. 2006). The second group includes genetic as well as acquired disorders, for instance, OI and osteoarthritis, in which increased TGF-β signaling has been identified as a contributing mechanism in mouse models (Zhen et al. 2013; Grafe et al. 2014). Additionally, genetic polymorphisms in genes encoding TGF-β family proteins or their signaling mediators may modify the susceptibility, course, and severity of genetic, acquired or age-related diseases, for instance for the risk of osteoporosis (Langdahl et al. 1997, 2003). Importantly, preclinical studies suggest that pharmacological targeting of TGF-β family members may be beneficial for the treatment of certain diseases. For instance, inhibition of TGF-β ligands with antibodies in mouse models improve the bone and extraskeletal phenotype in OI and attenuate the degeneration of articular cartilage in osteoarthritis (Zhen et al. 2013; Grafe et al. 2014). As another example, inhibition of TGF-β signaling prevents obesity and diabetes mellitus in mice (Yadav et al. 2011), and inhibition of GDF-3 function may represent an additional target for the treatment of obesity (Shen et al. 2009a). Similarly, myostatin antagonists may be of clinical value for the future treatment of muscle wasting conditions, and potentially in muscular dystrophies (Wagner et al. 2002). In the context of cell therapy, understanding the roles of TGF-β family signaling in MSC differentiation will help to realize the potential of MSCs in therapy, ,(e.g., for the repair of cartilage and other connective tissues) (Mobasheri et al. 2009). Ultimately, these encouraging new avenues have to be studied in the human context to ascertain effectiveness and safety.
ACKNOWLEDGMENTS
We thank all the excellent scientists and reviewers in the field for their tremendous and inspirational work. We apologize sincerely to those colleagues whose work we have not cited because of space limitations. We like to thank Patricia Fonseca for editorial assistance. This work was supported by the National Institutes of Health (NIH) Grants 1F31DE024693-01 (S.A.), 1K08GM109105-01 (Be.L.), P01 HD70394 (Br.L.), and R01 DE020843 to (Y.M.). The μCT core at the School of Dentistry, University of Michigan, is supported in part by NIH/NCRR S10RR026475-01. This work was also supported by a Michael Geisman Fellowship from the Osteogenesis Imperfecta Foundation (I.G.), the Plastic Surgery Foundation National Endowment Award (Be.L.), International FOP Association (Y.M., Be.L.), the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (HD024064), and the Rolanette and Berdon Lawrence Bone Disease Program of Texas.
REFERENCES
*Reference is also in this subject collection.
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. 2000. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: Antagonism by noggin. J Bone Miner Res 15: 663–673. [DOI] [PubMed] [Google Scholar]
- Ab Kadir R, Zainal Ariffin SH, Megat Abdul Wahab R, Kermani S, Senafi S. 2012. Characterization of mononucleated human peripheral blood cells. ScientificWorldJournal 2012: 843843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal F, Pratap J, Ito K, Ito Y, Stein JL, van Wijnen AJ, Stein GS, Lian JB, Javed A. 2005. Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J Cell Physiol 204: 63–72. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Loder SJ, Cholok D, Peterson J, Li J, Breuler C, Cameron Brownley R, Hsin Sung H, Chung MT, Kamiya N, et al. 2017. Scleraxis-lineage cells contribute to ectopic bone formation in muscle and tendon. Stem Cells 35: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16: 2813–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. 1987. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-β. J Cell Physiol 133: 567–572. [DOI] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. 1989. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-β, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315. [DOI] [PubMed] [Google Scholar]
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. 2001. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 20: 2254–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Serra R. 2004. Unique and redundant roles of Smad3 in TGF-β-mediated regulation of long bone development in organ culture. Dev Dyn 230: 685–699. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Horton J, Sohn P, Serra R. 2001. The perichondrium plays an important role in mediating the effects of TGF-β1 on endochondral bone formation. Dev Dyn 221: 311–321. [DOI] [PubMed] [Google Scholar]
- Alves RDAM, Eijken M, Bezstarosti K, Demmers JAA, van Leeuwen JPTM. 2013. Activin A suppresses osteoblast mineralization capacity by altering extracellular matrix composition and impairing matrix vesicle production. Mol Cell Proteomics 12: 2890–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Christ B, Rashid-Doubell F, Kemp CF, Lang E, Patel K. 2002. Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243: 115–127. [DOI] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. 2004. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270: 19–30. [DOI] [PubMed] [Google Scholar]
- Amthor H, Otto A, Macharia R, McKinnell I, Patel K. 2006. Myostatin imposes reversible quiescence on embryonic muscle precursors. Dev Dyn 235: 672–680. [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. 2008. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci 105: 7252–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K. 2001. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci 114: 1483–1489. [DOI] [PubMed] [Google Scholar]
- Arita NA, Pelaez D, Cheung HS. 2011. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFβ-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 405: 564–569. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. 2008. Molecular requirements for induction of CTGF expression by TGF-β1 in primary osteoblasts. Bone 42: 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, Meerasahib MF, Gonzalez-Cadavid NF. 2005. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology 146: 3547–3557. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Hauschka PV. 1996. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res 222: 38–47. [DOI] [PubMed] [Google Scholar]
- Aslan H, Kimelman-Bleich N, Pelled G, Gazit D. 2008. Molecular targets for tendon neoformation. J Clin Invest 118: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund CA, Best TM. 2013. Achilles tendon disorders. BMJ 346: f1262. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Sporn MB. 1986. Type β transforming growth factor in human platelets: Release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol 102: 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atti E, Gomez S, Wahl SM, Mendelsohn R, Paschalis E, Boskey AL. 2002. Effects of transforming growth factor-β deficiency on bone development: A Fourier transform-infrared imaging analysis. Bone 31: 675–684. [DOI] [PubMed] [Google Scholar]
- Augello A, De Bari C. 2010. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21: 1226–1238. [DOI] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. 2004. Conditional deletion of the TGF-β type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol 276: 124–142. [DOI] [PubMed] [Google Scholar]
- Bagnaninchi PO, Yang Y, El Haj AJ, Maffulli N. 2007. Tissue engineering for tendon repair. Br J Sports Med 41: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. 2006. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsby T, Guest D. 2013. Transforming growth factor β3 promotes tendon differentiation of equine embryo-derived stem cells. Tissue Eng Part A 19: 2156–2165. [DOI] [PubMed] [Google Scholar]
- Barsby T, Bavin EP, Guest DJ. 2014. Three-dimensional culture and transforming growth factor β3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng Part A 20: 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch G, Yoo JJ, De Coppi P, Siddiqui MM, Schuch G, Pohl HG, Fuhr J, Perin L, Soker S, Atala A. 2005. Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev 14: 337–348. [DOI] [PubMed] [Google Scholar]
- Baur ST, Mai JJ, Dymecki SM. 2000. Combinatorial signaling through BMP receptor IB and GDF5: Shaping of the distal mouse limb and the genetics of distal limb diversity. Development 127: 605–619. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. 2008. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411–424. [DOI] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ. 2005. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16: 585–595. [DOI] [PubMed] [Google Scholar]
- Beylkin DH, Allen DL, Leinwand LA. 2006. MyoD, Myf5, and the calcineurin pathway activate the developmental myosin heavy chain genes. Dev Biol 294: 541–553. [DOI] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. 2008. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res 23: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Tagliafico E, Lamorte G, Monteverde S, Tenedini E, Roncaglia E, Ferrari S, Cusella-De Angelis MG, Tajbakhsh S, Cossu G. 2007. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev Biol 304: 633–651. [DOI] [PubMed] [Google Scholar]
- Blachowski S, Motyl T, Orzechowski A, Grzelkowska K, Interewicz B. 1993. Comparison of metabolic effects of EGF, TGF-α, and TGF-β1 in primary culture of fetal bovine myoblasts and rat L6 myoblasts. Int J Biochem 25: 1571–1577. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768–771. [DOI] [PubMed] [Google Scholar]
- Blaney Davidson EN, van der Kraan PM, van den Berg WB. 2007. TGF-β and osteoarthritis. Osteoarthritis Cartilage 15: 597–604. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. 2011. The amazing osteocyte. J Bone Miner Res 26: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borton AJ, Frederick JP, Datto MB, Wang XF, Weinstein RS. 2001. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J Bone Miner Res 16: 1754–1764. [DOI] [PubMed] [Google Scholar]
- Bottcher Y, Unbehauen H, Kloting N, Ruschke K, Korner A, Schleinitz D, Tonjes A, Enigk B, Wolf S, Dietrich K, et al. 2009. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 58: 2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers RR, Lane MD. 2007. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 6: 385–389. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Kim JW, Otto TC, Lane MD. 2006. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc Natl Acad Sci 103: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer MI, Goldfarb CA, Gelberman RH. 2005. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther 18: 80–85; quiz 86. [DOI] [PubMed] [Google Scholar]
- Bradford PG, Maglich JM, Ponticelli AS, Kirkwood KL. 2000. The effect of bone morphogenetic protein-7 on the expression of type I inositol 1,4,5-trisphosphate receptor in G-292 osteosarcoma cells and primary osteoblast cultures. Arch Oral Biol 45: 159–166. [DOI] [PubMed] [Google Scholar]
- Breen EC, Ignotz RA, McCabe L, Stein JL, Stein GS, Lian JB. 1994. TGFβ alters growth and differentiation related gene expression in proliferating osteoblasts in vitro, preventing development of the mature bone phenotype. J Cell Physiol 160: 323–335. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. 2003. A somitic compartment of tendon progenitors. Cell 113: 235–248. [DOI] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. 2000. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25: 453–457. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. 1998. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280: 1455–1457. [DOI] [PubMed] [Google Scholar]
- Campos-Xavier B, Saraiva JM, Savarirayan R, Verloes A, Feingold J, Faivre L, Munnich A, Le Merrer M, Cormier-Daire V. 2001. Phenotypic variability at the TGF-β1 locus in Camurati-Engelmann disease. Hum Genet 109: 653–658. [DOI] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5: 1538–1552. [DOI] [PubMed] [Google Scholar]
- Carlsson LM, Jacobson P, Walley A, Froguel P, Sjostrom L, Svensson PA, Sjöholm K. 2009. ALK7 expression is specific for adipose tissue, reduced in obesity and correlates to factors implicated in metabolic disease. Biochem Biophys Res Commun 382: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. 2012. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. 2004. Transient up-regulation of biglycan during skeletal muscle regeneration: Delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol 268: 358–371. [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. 1991. Activin-A binding and biochemical effects in osteoblast-enriched cultures from fetal-rat parietal bone. Mol Cell Biol 11: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, Casinghino S, Kim J, Pham T, Rosen V, Wozney J, McCarthy TL. 1995. Independent changes in type I and type II receptors for transforming growth factor β induced by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol Cell Biol 15: 3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chaikuad A, Bullock AN. 2016. Structural basis of intracellular TGF-β signaling: Receptors and Smads. Cold Spring Harb Perspect Biol 8: a022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM. 2012. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res 27: 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Parra M, Ji C, Liu Y, Eickelberg O, McCarthy TL, Centrella M. 2002. Transcriptional and post-transcriptional regulation of transforming growth factor β type II receptor expression in osteoblasts. Gene 299: 65–77. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. 2004. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238. [DOI] [PubMed] [Google Scholar]
- Chau JF, Jia D, Wang Z, Liu Z, Hu Y, Zhang X, Jia H, Lai KP, Leong WF, Au BJ, et al. 2012. A crucial role for bone morphogenetic protein–Smad1 signalling in the DNA damage response. Nat Commun 3: 836. [DOI] [PubMed] [Google Scholar]
- Chaudhary LR, Hofmeister AM, Hruska KA. 2004. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 34: 402–411. [DOI] [PubMed] [Google Scholar]
- Chen TL, Bates RL. 1993. Recombinant human transforming growth factor β1 modulates bone remodeling in a mineralizing bone organ culture. J Bone Miner Res 8: 423–434. [DOI] [PubMed] [Google Scholar]
- Chen P, Yu YM, Reddi AH. 1993. Chondrogenesis in chick limb bud mesodermal cells: Reciprocal modulation by activin and inhibin. Exp Cell Res 206: 119–127. [DOI] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. 1997. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int 60: 283–290. [DOI] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. 1998. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TL, Shen WJ, Kraemer FB. 2001. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem 82: 187–199. [DOI] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. 2006. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133: 319–329. [DOI] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, Chen D. 2007. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis 45: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al. 2016. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ 23: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al. 2003. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85-A: 1544–1552. [DOI] [PubMed] [Google Scholar]
- Choy L, Derynck R. 2003. Transforming growth factor-β inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278: 9609–9619. [DOI] [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R. 2000. Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J Cell Biol 149: 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg PD, Strassburg S, Smith RK. 2007. Cell phenotypic variation in normal and damaged tendons. Int J Exp Pathol 88: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendenning DE, Mortlock DP. 2012. The BMP ligand Gdf6 prevents differentiation of coronal suture mesenchyme in early cranial development. PLoS ONE 7: e36789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier DE, Comerford SA, Hammer RE. 1997. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-β1 transgenic mice. J Clin Invest 100: 2697–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DR, Doumit ME, Merkel RA. 1993. Transforming growth factor-β, basic fibroblast growth factor, and platelet-derived growth factor-BB interact to affect proliferation of clonally derived porcine satellite cells. J Cell Physiol 157: 307–312. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313. [DOI] [PubMed] [Google Scholar]
- Critchlow MA, Bland YS, Ashhurst DE. 1995. The effect of exogenous transforming growth factor-β2 on healing fractures in the rabbit. Bone 16: 521–527. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. 1995. Scleraxis: A basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121: 1099–1110. [DOI] [PubMed] [Google Scholar]
- Cui J, Chen Y, Wang HY, Wang RF. 2014. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother 10: 3270–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham NS, Jenkins NA, Gilbert DJ, Copeland NG, Reddi AH, Lee SJ. 1995. Growth/differentiation factor-10: A new member of the transforming growth factor-β superfamily related to bone morphogenetic protein-3. Growth Factors 12: 99–109. [DOI] [PubMed] [Google Scholar]
- Cusella-De Angelis MG, Molinari S, Le Donne A, Coletta M, Vivarelli E, Bouche M, Molinaro M, Ferrari S, Cossu G. 1994. Differential response of embryonic and fetal myoblasts to TGFβ: A possible regulatory mechanism of skeletal muscle histogenesis. Development 120: 925–933. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF. 1994. Characterization and autoregulation of latent transforming growth factor β (TGFβ) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGFβ-binding protein. J Biol Chem 269: 6815–6821. [PubMed] [Google Scholar]
- Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. 2001. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27: 84–88. [DOI] [PubMed] [Google Scholar]
- Daoud G, Kempf H, Kumar D, Kozhemyakina E, Holowacz T, Kim DW, Ionescu A, Lassar AB. 2014. BMP-mediated induction of GATA4/5/6 blocks somitic responsiveness to SHH. Development 141: 3978–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathe K, Kjaer KW, Brehm A, Meinecke P, Nürnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, et al. 2009. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet 84: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Caestecker M. 2004. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev 15: 1–11. [DOI] [PubMed] [Google Scholar]
- Degenkolbe E, Konig J, Zimmer J, Walther M, Reissner C, Nickel J, Ploger F, Raspopovic J, Sharpe J, Dathe K, et al. 2014. A GDF5 point mutation strikes twice—Causing BDA1 and SYNS2. PLoS Genet 9: e1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gorter DJJ, van Dinther M, Korchynskyi O, ten Dijke P. 2010. Biphasic effects of transforming growth factor β on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res 26: 1178–1187. [DOI] [PubMed] [Google Scholar]
- Denker AE, Nicoll SB, Tuan RS. 1995. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-β1. Differentiation 59: 25–34. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. 1999. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126: 1621–1629. [DOI] [PubMed] [Google Scholar]
- Dorman LJ, Tucci M, Benghuzzi H. 2012. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum 48: 81–87. [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. 2006. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol 25: 332–341. [DOI] [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Santander C, Brandan E. 2010. TGF-β receptors, in a Smad-independent manner, are required for terminal skeletal muscle differentiation. Exp Cell Res 316: 2487–2503. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. 1997. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 89: 747–754. [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. 2004. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121: 173–182. [DOI] [PubMed] [Google Scholar]
- Dünker N, Krieglstein K. 2000. Targeted mutations of transforming growth factor-β genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem 267: 6982–6988. [DOI] [PubMed] [Google Scholar]
- Dünker N, Krieglstein K. 2002. Tgfβ2–/–Tgfβ3–/– double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol 206: 73–83. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. 1999. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci 112: 3519–3527. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. 1987. Transforming growth factor β modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 6: 1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HAP, et al. 2007. The activin A–follistatin system: Potent regulator of human extracellular matrix mineralization. FASEB J 21: 2949–2960. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Schwarz EM, Zuscik MJ, O’Keefe RJ, Drissi H, Rosier RN. 2006. Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NFκB. Exp Cell Res 312: 40–50. [DOI] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. 1996. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Derynck R. 1996. Increased expression of TGF-β2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol 132: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton DZ, Roof SL, Magri KA, McWade FJ, Florini JR. 1994. IGF-II is more active than IGF-I in stimulating L6A1 myogenesis: Greater mitogenic actions of IGF-I delay differentiation. J Cell Physiol 161: 277–284. [DOI] [PubMed] [Google Scholar]
- Fakhry M, Hamade E, Badran B, Buchet R, Magne D. 2013. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells 5: 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Streeper RS, Farese RV Jr., Yamamoto KR. 2006. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci 103: 15675–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Xing L, Zhang JH, Zhao M, Horn D, Chan J, Boyce BF, Harris SE, Mundy GR, Chen D. 2003. NF-κB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J Biol Chem 278: 29130–29135. [DOI] [PubMed] [Google Scholar]
- Fenwick SA, Hazleman BL, Riley GP. 2002. The vasculature and its role in the damaged and healing tendon. Arthritis Res 4: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff EH, Ebner R, Derynck R. 1994. Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-β receptor. Development 120: 1085–1095. [DOI] [PubMed] [Google Scholar]
- Filvaroff E, Erlebacher A, Ye J, Gitelman SE, Lotz J, Heillman M, Derynck R. 1999. Inhibition of TGF-β receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development 126: 4267–4279. [DOI] [PubMed] [Google Scholar]
- Fiori JL, Billings PC, de la Pena LS, Kaplan FS, Shore EM. 2006. Dysregulation of the BMP–p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res 21: 902–909. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. 1996. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. 1999. Mechanisms of GDF-5 action during skeletal development. Development 126: 1305–1315. [DOI] [PubMed] [Google Scholar]
- Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM. 2012. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol 3: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. 1970. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3: 393–403. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. 1976. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4: 267–274. [PubMed] [Google Scholar]
- Friedman MS, Long MW, Hankenson KD. 2006. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem 98: 538–554. [DOI] [PubMed] [Google Scholar]
- Fromigué O, Marie PJ, Lomri A. 1998. Bone morphogenetic protein-2 and transforming growth factor-β2 interact to modulate human bone marrow stromal cell proliferation and differentiation. J Cell Biochem 68: 411–426. [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. 1999. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell 10: 3801–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. 2006. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis 44: 159–167. [DOI] [PubMed] [Google Scholar]
- Funaba M, Ogawa K, Murata T, Fujimura H, Murata E, Abe M, Takahashi M, Torii K. 1996. Follistatin and activin in bone: Expression and localization during endochondral bone development. Endocrinology 137: 4250–4259. [DOI] [PubMed] [Google Scholar]
- Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. 2002. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology 143: 74–83. [DOI] [PubMed] [Google Scholar]
- Galléa S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, et al. 2001. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28: 491–498. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Cox K, Carlo JM, Rosen V. 2009. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Dev Dyn 238: 2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Sheu TJ, Dong Y, Hoak DM, Zuscik MJ, Schwarz EM, Hilton MJ, O’Keefe RJ, Jonason JH. 2013. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J Cell Sci 126: 5704–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Gangji V, Canalis E. 1998. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest 102: 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Economides AN, Canalis E. 2007. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem 282: 31549–31557. [DOI] [PubMed] [Google Scholar]
- Ge X, McFarlane C, Vajjala A, Lokireddy S, Ng ZH, Tan CK, Tan NS, Wahli W, Sharma M, Kambadur R. 2011. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res 21: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser AG, Zeng QQ, Sato M, Helvering LM, Hirano T, Turner CH. 1998. Decreased bone mass and bone elasticity in mice lacking the transforming growth factor-β1 gene. Bone 23: 87–93. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. 2007. Developmental origin of fat: Tracking obesity to its source. Cell 131: 242–256. [DOI] [PubMed] [Google Scholar]
- Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. 2009. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297: E157–E164. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Morgan C, Kelly K, Wu X, Dandapani V, Wang CS, Rosen V. 1995. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem 58: 393–402. [DOI] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. 2008. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta 1782: 197–228. [DOI] [PubMed] [Google Scholar]
- Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL. 1999. Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res 14: 1522–1535. [DOI] [PubMed] [Google Scholar]
- Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, et al. 2014. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 20: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt MB, Shim JH, Glimcher LH. 2010a. TAK1 mediates BMP signaling in cartilage. Ann NY Acad Sci 1192: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al. 2010b. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest 120: 2457–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Heersche JN, Aubin JE. 1988. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: Effect of dexamethasone. J Cell Biol 106: 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, et al. 1997. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17: 71–74. [DOI] [PubMed] [Google Scholar]
- Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, Ruggiu M, Olivera-Martinez I, Robert N, Lu Y, et al. 2013. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest 123: 3564–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. 2003. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res 18: 2060–2068. [DOI] [PubMed] [Google Scholar]
- Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, O’Keefe RJ. 2010. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res 25: 1784–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. 2004. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 18: 2404–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. 2008. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J Biol Chem 283: 9136–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. 2009. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 4: e4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CW, Cho JJ, Elmallah RD, Cherian JJ, Kim TW, Lee MC, Mont MA. 2015. A multicenter, single-blind, phase IIa clinical trial to evaluate the efficacy and safety of a cell mediated gene therapy in degenerative knee arthritis patients. Hum Gene Ther Clin Dev 26: 125–130. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Webb CN, Isales CM. 2006. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int J Obes (Lond) 30: 868–870. [DOI] [PubMed] [Google Scholar]
- Han D, Zhao H, Parada C, Hacia JG, Bringas P Jr, Chai Y. 2012. A TGFβ–Smad4–Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139: 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Dennis JE, Caplan AI. 1997. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res 12: 1606–1614. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. 2013. Brown and beige fat: Development, function and therapeutic potential. Nat Med 19: 1252–1263. [DOI] [PubMed] [Google Scholar]
- Harris SE, Bonewald LF, Harris MA, Sabatini M, Dallas S, Feng JQ, Ghosh-Choudhury N, Wozney J, Mundy GR. 1994. Effects of transforming growth factor β on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 9: 855–863. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM. 2005. Antagonists of activin signaling: Mechanisms and potential biological applications. Trends Endocrinol Metab 16: 73–78. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. 2001. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104: 341–351. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shoda A, Inoue S, Yamada R, Kondo T, Sakurai T, Ueno N, Muramatsu M. 1992. Functional regulation of osteoblastic cells by the interaction of activin-A with follistatin. J Biol Chem 267: 4999–5004. [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. 1993. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364: 501–506. [DOI] [PubMed] [Google Scholar]
- *.Hata A, Chen Y-G. 2016. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8: a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T. 2003. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor γ during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 14: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, et al. 2015. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7: 303ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haÿ E, Hott M, Graulet AM, Lomri A, Marie PJ. 1999. Effects of bone morphogenetic protein-2 on human neonatal calvaria cell differentiation. J Cell Biochem 72: 81–93. [DOI] [PubMed] [Google Scholar]
- Hayano S, Komatsu Y, Pan H, Mishina Y. 2015. Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development 142: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr., Wrana JL, et al. 1997. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89: 1165–1173. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. 1996. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1α. J Cell Physiol 166: 585–592. [DOI] [PubMed] [Google Scholar]
- He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massagué J. 2006. Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGFβ pathway. Cell 125: 929–941. [DOI] [PubMed] [Google Scholar]
- *.Heldin C-H, Moustakas A. 2016. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol 8: a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hill CS. 2016. Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol 8: a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C, Karpe F, Pinnick KE. 2015. Role of developmental transcription factors in white, brown and beige adipose tissues. Biochim Biophys Acta 185: 686–696. [DOI] [PubMed] [Google Scholar]
- Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H, Kangawa K. 1996. cDNA cloning and genomic structure of human bone morphogenetic protein-3B (BMP-3b). Biochem Biophys Res Commun 223: 304–310. [DOI] [PubMed] [Google Scholar]
- Hino J, Miyazawa T, Miyazato M, Kangawa K. 2012. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int J Obes (Lond) 36: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, et al. 2015. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci 112: 15438–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Yamanaka M, Kawachi H, Matsui T, Yano H. 2005. Activin A inhibits differentiation of 3T3-L1 preadipocyte. Mol Cell Endocrinol 232: 21–26. [DOI] [PubMed] [Google Scholar]
- Hirata-Tsuchiya S, Fukushima H, Katagiri T, Ohte S, Shin M, Nagano K, Aoki K, Morotomi T, Sugiyama G, Nakatomi C, et al. 2014. Inhibition of BMP2-induced bone formation by the p65 subunit of NF-κB via an interaction with Smad4. Mol Endocrinol 28: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, et al. 2006. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest 116: 940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo H, Ohba S, Taniguchi K, Shirai M, Yano F, Saito T, Ikeda T, Nakajima K, Komiyama Y, Nakagata N, et al. 2013. Hedgehog-Gli activators direct osteo-chondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J Biol Chem 288: 9924–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. 2005. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7: 393–395. [DOI] [PubMed] [Google Scholar]
- Huang W, Yang S, Shao J, Li YP. 2007a. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12: 3068–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yang S, Shao J, Li YP. 2007b. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12: 3068–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. 2009a. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res 88: 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. 2009b. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci 106: 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. 2002. Muscle injuries and repair: Current trends in research. J Bone Joint Surg Am 84-A: 822–832. [PubMed] [Google Scholar]
- Hughes FJ, Aubin JE, Heersche JN. 1992. Differential chemotactic responses of different populations of fetal rat calvaria cells to platelet-derived growth factor and transforming growth factor β. Bone Miner 19: 63–74. [DOI] [PubMed] [Google Scholar]
- Hughes FJ, Collyer J, Stanfield M, Goodman SA. 1995. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology 136: 2671–2677. [DOI] [PubMed] [Google Scholar]
- Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. 1996. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev 7: 249–258. [DOI] [PubMed] [Google Scholar]
- Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, et al. 2004. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell 6: 673–684. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massagué J. 1985. Type β transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci 82: 8530–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. 2004. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum 50: 3561–3573. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Jingushi S, Urabe K, Okazaki K, Iwamoto Y. 1999. Inhibitory effects of activin-A on osteoblast differentiation during cultures of fetal rat calvarial cells. J Cell Biochem 75: 206–214. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. 1997. Smad6 inhibits signalling by the TGF-β superfamily. Nature 389: 622–626. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Canaff L, Hendy GN, Hisa I, Sugimoto T, Chihara K, Kaji H. 2009. Role of Smad3, acting independently of transforming growth factor-β, in the early induction of Wnt–β-catenin signaling by parathyroid hormone in mouse osteoblastic cells. J Cell Biochem 108: 285–294. [DOI] [PubMed] [Google Scholar]
- Iseki S, Wilkie AO, Morriss-Kay GM. 1999. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development 126: 5611–5620. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Arthur A, Zannettino AC, Turner JL, Shi S, Glackin CA, Gronthos S. 2009. TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stem cell growth and commitment. Stem Cells 27: 2457–2468. [DOI] [PubMed] [Google Scholar]
- Iura A, McNerny EG, Zhang Y, Kamiya N, Tantillo M, Lynch M, Kohn DH, Mishina Y. 2015. Mechanical loading synergistically increases trabecular bone volume and improves mechanical properties in the mouse when BMP signaling is specifically ablated in osteoblasts. PLoS ONE 10: e0141345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DW, Simon TM. 2002. Donor cell survival and repopulation after intraarticular transplantation of tendon and ligament allografts. Microsc Res Tech 58: 25–33. [DOI] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Janssens S, Van Hul W. 2005. Transforming growth factor-β1 to the bone. Endocr Rev 26: 743–774. [DOI] [PubMed] [Google Scholar]
- Javed A, Bae J-S, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, et al. 2008. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem 283: 8412–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Afzal F, Bae J-S, Gutierrez S, Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS, Lian JB. 2009. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2–SMAD complex to promote osteoblast differentiation. Cells Tissues Organs 189: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky SA, Archambault J, Li L, Seeherman H. 2010. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res 28: 289–297. [DOI] [PubMed] [Google Scholar]
- Jeoung DI, Tang B, Sonenberg M. 1995. Mitogenic response to TGF-β in 3T3-F442A cells. Biochem Biophys Res Commun 216: 964–969. [DOI] [PubMed] [Google Scholar]
- Ji X, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T. 2000. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab 18: 132–139. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Yi JR, Ying SY, Chuong CM. 1993. Activin enhances chondrogenesis of limb bud cells: Stimulation of precartilaginous mesenchymal condensations and expression of NCAM. Dev Biol 155: 545–557. [DOI] [PubMed] [Google Scholar]
- Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. 1999. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J Bone Miner Res 14: 1075–1083. [DOI] [PubMed] [Google Scholar]
- Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S. 2006. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell 10: 461–471. [DOI] [PubMed] [Google Scholar]
- Jing J, Ren Y, Zong Z, Liu C, Kamiya N, Mishina Y, Liu Y, Zhou X, Feng JQ. 2013. BMP receptor 1A determines the cell fate of the postnatal growth plate. Int J Biol Sci 9: 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce ME, Roberts AB, Sporn MB, Bolander ME. 1990. Transforming growth factor-β and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110: 2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Hoffmann A, Verschueren K, Tylzanowski P, Kaps C, Gross G, Huylebroeck D. 2000. The bone morphogenetic protein 2 signaling mediator Smad1 participates predominantly in osteogenic and not in chondrogenic differentiation in mesenchymal progenitors C3H10T1/2. J Bone Miner Res 15: 1889–1899. [DOI] [PubMed] [Google Scholar]
- Jun JH, Yoon WJ, Seo SB, Woo KM, Kim GS, Ryoo HM, Baek JH. 2010. BMP2-activated Erk/MAP kinase stabilizes Runx2 by increasing p300 levels and histone acetyltransferase activity. J Biol Chem 285: 36410–36419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji H, Naito J, Sowa H, Sugimoto T, Chihara K. 2006. Smad3 differently affects osteoblast differentiation depending upon its differentiation stage. Horm Metab Res 38: 740–745. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. 1997. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7: 910–916. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. 2008a. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res 23: 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. 2008b. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135: 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. 2010. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res 25: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Kaartinen VM, Mishina Y. 2011. Loss-of-function of ACVR1 in osteoblasts increases bone mass and activates canonical Wnt signaling through suppression of Wnt inhibitors SOST and DKK1. Biochem Biophys Res Commun 414: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Shuxian L, Yamaguchi R, Phipps M, Aruwajoye O, Adapala NS, Yuan H, Kim HK, Feng JQ. 2016. Targeted disruption of BMP signaling through type IA receptor (BMPR1A) in osteocyte suppresses SOST and RANKL, leading to dramatic increase in bone mass, bone mineral density and mechanical strength. Bone 91: 53–63. [DOI] [PubMed] [Google Scholar]
- Kanafi MM, Rajeshwari YB, Gupta S, Dadheech N, Nair PD, Gupta PK, Bhonde RR. 2013. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 15: 1228–1236. [DOI] [PubMed] [Google Scholar]
- Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, et al. 2009. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, et al. 2009. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Chakkalakal SA, Shore EM. 2012. Fibrodysplasia ossificans progressiva: Mechanisms and models of skeletal metamorphosis. Dis Model Mech 5: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalaki M, Fili S, Philippou A, Koutsilieris M. 2009. Muscle regeneration: Cellular and molecular events. In Vivo 23: 779–796. [PubMed] [Google Scholar]
- Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaisse JM, Foged NT. 2002. Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 277: 44061–44067. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. 2004. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431: 466–471. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. 1978. The multicomposite structure of tendon. Connect Tissue Res 6: 11–23. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. 1994. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127: 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. 1998. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet 22: 340–348. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. 1996. BMP signaling during bone pattern determination in the developing limb. Development 122: 3557–3566. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Aihara M, Honmo J, Sakurai S, Fujimaki Y, Sakamoto K, Fujimaki E, Wozney JM, Yamaguchi A. 1998. Effects of recombinant human bone morphogenetic protein-2 on differentiation of cells isolated from human bone, muscle, and skin. Bone 23: 223–231. [DOI] [PubMed] [Google Scholar]
- Keller B, Yang T, Chen Y, Munivez E, Bertin T, Zabel B, Lee B. 2011. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE 6: e16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Liang L, Dean RG, Hausman DB, Hartzell DL, Baile CA. 2001. Inhibition of preadipocyte differentiation by myostatin treatment in 3T3-L1 cultures. Biochem Biophys Res Commun 281: 902–906. [DOI] [PubMed] [Google Scholar]
- Kim KK, Ji C, Chang W, Wells RG, Gundberg CM, McCarthy TL, Centrella M. 2006. Repetitive exposure to TGF-β suppresses TGF-β type I receptor expression by differentiated osteoblasts. Gene 379: 175–184. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Saito T, Tomita H, Makita Y, Yoshida K, Ghadami M, Yamada K, Kondo S, Ikegawa S, Nishimura G, et al. 2000. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat Genet 26: 19–20. [DOI] [PubMed] [Google Scholar]
- Kokabu S, Gamer L, Cox K, Lowery J, Tsuji K, Raz R, Economides A, Katagiri T, Rosen V. 2012. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol Endocrinol 26: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias HD, McDermott JC. 2008. Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104: 579–587. [DOI] [PubMed] [Google Scholar]
- Komori T. 2006. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Komori T. 2010a. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339: 189–195. [DOI] [PubMed] [Google Scholar]
- Komori T. 2010b. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 658: 43–49. [DOI] [PubMed] [Google Scholar]
- Komori T. 2013. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res 352: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764. [DOI] [PubMed] [Google Scholar]
- Koutsilieris M, Reyes-Moreno C, Sourla A, Dimitriadou V, Choki I. 1997. Growth factors mediate glucocorticoid receptor function and dexamethasone-induced regression of osteoblastic lesions in hormone refractory prostate cancer. Anticancer Res 17: 1461–1465. [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E. 2015. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 142: 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423: 332–336. [DOI] [PubMed] [Google Scholar]
- Kulyk WM, Rodgers BJ, Greer K, Kosher RA. 1989. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-β. Dev Biol 135: 424–430. [DOI] [PubMed] [Google Scholar]
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. 1996. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274: 948–953. [DOI] [PubMed] [Google Scholar]
- Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M. 2001. Transforming growth factor-β1 stimulates the production of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in human bone marrow stromal osteoblast progenitors. J Endocrinol 169: 549–561. [DOI] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. 2002. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-β in normal human osteoblastic cells. J Biol Chem 277: 15514–15522. [DOI] [PubMed] [Google Scholar]
- Lai Z, Ferry KV, Diamond MA, Wee KE, Kim YB, Ma J, Yang T, Benfield PA, Copeland RA, Auger KR. 2001. Human mdm2 mediates multiple mono-ubiquitination of p53 by a mechanism requiring enzyme isomerization. J Biol Chem 276: 31357–31367. [DOI] [PubMed] [Google Scholar]
- Lane J, Yumoto K, Azhar M, Ninomiya-Tsuji J, Inagaki M, Hu Y, Deng CX, Kim J, Mishina Y, Kaartinen V. 2015. Tak1, Smad4 and Trim33 redundantly mediate TGF-β3 signaling during palate development. Dev Biol 398: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdahl BL, Knudsen JY, Jensen HK, Gregersen N, Eriksen EF. 1997. A sequence variation: 713-8delC in the transforming growth factor-β1 gene has higher prevalence in osteoporotic women than in normal women and is associated with very low bone mass in osteoporotic women and increased bone turnover in both osteoporotic and normal women. Bone 20: 289–294. [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. 2003. Polymorphisms in the transforming growth factor β1 gene and osteoporosis. Bone 32: 297–310. [DOI] [PubMed] [Google Scholar]
- Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. 2002. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277: 49831–49840. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, et al. 1996. PTH/PTHrP receptor in early development and Indian Hedgehog-regulated bone growth. Science 273: 663–666. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Walsh K, Arany Z. 2011. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab 300: E3–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci 98: 9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. 2003. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun 309: 689–694. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER. 2010. Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24: 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, Maharam ER, Leong DJ, Laudier DM, Ruike T, et al. 2011. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS ONE 6: e17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. 2005. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today 75: 200–212. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Silan F, Goecke TO, Irgang S, Kjaer KW, Kjaergaard S, Mahoney MJ, Morlot S, Reissner C, et al. 2007. A new subtype of brachydactyly type B caused by point mutations in the bone morphogenetic protein antagonist NOGGIN. Am J Hum Genet 81: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. 2006. GDF3 at the crossroads of TGF-β signaling. Cell Cycle 5: 1069–1073. [DOI] [PubMed] [Google Scholar]
- Li J, Tsuji K, Komori T, Miyazono K, Wrana JL, Ito Y, Nifuji A, Noda M. 1998. Smad2 overexpression enhances Smad4 gene expression and suppresses CBFA1 gene expression in osteoblastic osteosarcoma ROS17/2.8 cells and primary rat calvaria cells. J Biol Chem 273: 31009–31015. [DOI] [PubMed] [Google Scholar]
- Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, Mao N. 2005. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res 15: 539–547. [DOI] [PubMed] [Google Scholar]
- Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, Li Z, Shaw C, Graham BH, Brown CW. 2009. Activin signaling: Effects on body composition and mitochondrial energy metabolism. Endocrinology 150: 3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nie F, Yin Z, He J. 2011. Enhanced hyperplasia in muscles of transgenic zebrafish expressing Follistatin1. Sci China Life Sci 54: 159–165. [DOI] [PubMed] [Google Scholar]
- Li Y, Ramcharan M, Zhou Z, Leong DJ, Akinbiyi T, Majeska RJ, Sun HB. 2015. The role of Scleraxis in fate determination of mesenchymal stem cells for tenocyte differentiation. Sci Rep 5: 13149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lim J, Shi Y, Karner CM, Lee SY, Lee WC, He G, Long F. 2016. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in the mouse. Development 143: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. 2002. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun 291: 701–706. [DOI] [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. 2009. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum 60: 3723–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Lefebvre V. 2015. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res 43: 8183–8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R. 2001. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15: 2950–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Kang JS, Derynck R. 2004. TGF-β-activated Smad3 represses MEF2-dependent transcription in myogenic differentiation. EMBO J 23: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Gao Y, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, Shibata Y, Bessho K, Komori T, Yamaguchi A. 2007. BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol 211: 728–735. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Viggeswarapu M, Zheng Z, Titus L, Boden SD. 2011. Activation of c-Jun NH2-terminal kinase 1 increases cellular responsiveness to BMP-2 and decreases binding of inhibitory Smad6 to the type 1 BMP receptor. J Bone Miner Res 26: 1122–1132. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen L, zhou Y, Liu X, Tang K. 2014. Insulin-like growth factor-1 and bone morphogenetic protein-2 jointly mediate prostaglandin E2-induced adipogenic differentiation of rat tendon stem cells. PLoS ONE 9: e85469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. 2012. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone 50: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Casillas F, Wrana JL, Massagué J. 1993. Betaglycan presents ligand to the TGFβ signaling receptor. Cell 73: 1435–1444. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Li S, Manske PR. 2000. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun 268: 757–762. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Burns M, Silva MJ, Manske P. 2001. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res 19: 1199–1202. [DOI] [PubMed] [Google Scholar]
- Lowery JW, Intini G, Gamer L, Lotinun S, Salazar VS, Ote S, Cox K, Baron R, Rosen V. 2015. Loss of BMPR2 leads to high bone mass due to increased osteoblast activity. J Cell Sci 128: 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PP, Wong Y. 2013. Higher BMP/Smad sensitivity of tendon-derived stem cells (TDSCs) isolated from the collagenase-induced tendon injury model: Possible mechanism for their altered fate in vitro. BMC Musculoskelet Disord 14: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K. 2017. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol 9: a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Attisano L, Marchuk DA. 1999. Assignment of transforming growth factor β1 and β3 and a third new ligand to the type I receptor ALK-1. J Biol Chem 274: 9984–9992. [DOI] [PubMed] [Google Scholar]
- Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. 2007. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: A study of rhBMP-2 and noggin. Am J Sports Med 35: 597–604. [DOI] [PubMed] [Google Scholar]
- Macias D, Ganan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. 1997. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development 124: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. 2004. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 23: 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, et al. 2011. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol 21: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N, Khan KM, Puddu G. 1998. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy 14: 840–843. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Harkness L, Schroder HD, Abdallah BM, Kassem M. 2010. Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-β/activin/nodal signaling using SB-431542. J Bone Miner Res 25: 1216–1233. [DOI] [PubMed] [Google Scholar]
- Martin JF, Li L, Olson EN. 1992. Repression of myogenin function by TGF-β1 is targeted at the basic helix-loop-helix motif and is independent of E2A products. J Biol Chem 267: 10956–10960. [PubMed] [Google Scholar]
- Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R, Miyazaki T, Kitaura H, Nakamura K, Fujita T, et al. 2007. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn 236: 1876–1890. [DOI] [PubMed] [Google Scholar]
- Massagué J. 1998. TGF-β signal transduction. Annu Rev Biochem 67: 753–791. [DOI] [PubMed] [Google Scholar]
- Massagué J, Cheifetz S, Endo T, Nadal-Ginard B. 1986. Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci 83: 8206–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T. 2008. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem 283: 29119–29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Otsuka F, Hino J, Miyoshi T, Takano M, Miyazato M, Makino H, Kangawa K. 2012. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol Cell Endocrinol 350: 78–86. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamada A, Aizawa R, Suzuki D, Tsukasaki M, Suzuki W, Nakayama M, Maki K, Yamamoto M, Baba K, et al. 2013. BMP-2 induced expression of Alx3 that is a positive regulator of osteoblast differentiation. PLoS ONE 8: e68774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. 1995a. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 374: 356–360. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. 1995b. Functional analysis of activins during mammalian development. Nature 374: 354–356. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. 1995c. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374: 360–363. [DOI] [PubMed] [Google Scholar]
- Mauro A. 1961. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng SL, Chen D, Civitelli R. 2005. β-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem 94: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M. 2010. Novel links among Wnt and TGF-β signaling and Runx2. Mol Endocrinol 24: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. 2003. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. 2006. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-κB-independent, FoxO1-dependent mechanism. J Cell Physiol 209: 501–514. [DOI] [PubMed] [Google Scholar]
- McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, Sharma M, Kambadur R. 2008. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res 314: 317–329. [DOI] [PubMed] [Google Scholar]
- McLennan IS. 1993. Localisation of transforming growth factor β1 in developing muscles: Implications for connective tissue and fiber type pattern formation. Dev Dyn 197: 281–290. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. 1994. Transforming growth factor-β-2 (TGF-β2) is associated with mature rat neuromuscular junctions. Neurosci Lett 177: 151–154. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. 1997. Cellular localisation of transforming growth factor-β2 and -β3 (TGF-β2, TGF-β3) in damaged and regenerating skeletal muscles. Dev Dyn 208: 278–289. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. 2002. The transforming growth factor-βs: Multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol 46: 559–567. [PubMed] [Google Scholar]
- McLennan IS, Poussart Y, Koishi K. 2000. Development of skeletal muscles in transforming growth factor-β1 (TGF-β1) null-mutant mice. Dev Dyn 217: 250–256. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. 1993. GDF-3 and GDF-9: Two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J Biol Chem 268: 3444–3449. [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. 2002. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Rudnicki MA. 1995. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol 73: 723–732. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Ganan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM. 1999a. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol 206: 33–45. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Ganan Y, Rodriguez-Leon J, Economides AN, Rodriguez-Esteban C, Izpisua-Belmonte JC, Hurle JM. 1999b. Control of digit formation by activin signalling. Development 126: 2161–2170. [DOI] [PubMed] [Google Scholar]
- Mikic B, Clark RT, Battaglia TC, Gaschen V, Hunziker EB. 2004. Altered hypertrophic chondrocyte kinetics in GDF-5 deficient murine tibial growth plates. J Orthop Res 22: 552–556. [DOI] [PubMed] [Google Scholar]
- Mikic B, Ferreira MP, Battaglia TC, Hunziker EB. 2008. Accelerated hypertrophic chondrocyte kinetics in GDF-7 deficient murine tibial growth plates. J Orthop Res 26: 986–990. [DOI] [PubMed] [Google Scholar]
- Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, Vortkamp A. 2001. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128: 4523–4534. [DOI] [PubMed] [Google Scholar]
- Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. 2002. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell 3: 439–449. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada SI, et al. 2004. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem 279: 27560–27566. [DOI] [PubMed] [Google Scholar]
- *.Miyazawa K, Miyazono K. 2017. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 9: a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. 2009. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: Applications in cartilage repair and osteoarthritis therapy. Histol Histopathol 24: 347–366. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69: 1237–1245. [DOI] [PubMed] [Google Scholar]
- *.Morikawa M, Derynck R, Miyazono K. 2016. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8: a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. 2008. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 47: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Morrison RF, Farmer SR. 1999. Role of PPARγ in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18INK4c and p21Waf1/Cip1, during adipogenesis. J Biol Chem 274: 17088–17097. [DOI] [PubMed] [Google Scholar]
- Mukai T, Otsuka F, Otani H, Yamashita M, Takasugi K, Inagaki K, Yamamura M, Makino H. 2007. TNF-α inhibits BMP-induced osteoblast differentiation through activating SAPK/JNK signaling. Biochem Biophys Res Commun 356: 1004–1010. [DOI] [PubMed] [Google Scholar]
- Mundy C, Bello A, Sgariglia F, Koyama E, Pacifici M. 2015. HhAntag, a Hedgehog signaling antagonist, suppresses chondrogenesis and modulates canonical and non-canonical BMP signaling. J Cell Physiol 231: 1033–1044. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Chyung JH, Lassar AB. 1999. Sonic Hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev 13: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Zeng L, Chyung JH, Lassar AB. 2001. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev Cell 1: 411–422. [DOI] [PubMed] [Google Scholar]
- Myer A, Olson EN, Klein WH. 2001. MyoD cannot compensate for the absence of myogenin during skeletal muscle differentiation in murine embryonic stem cells. Dev Biol 229: 340–350. [DOI] [PubMed] [Google Scholar]
- Naganawa T, Xiao L, Coffin JD, Doetschman T, Sabbieti MG, Agas D, Hurley MM. 2008. Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. J Cell Biochem 103: 1975–1988. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs WH III, Arden KC, Accili D. 2003. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 4: 119–129. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. 1990. Activin-binding protein from rat ovary is follistatin. Science 247: 836–838. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. 2002. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. 2011. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Olson E. 1999. MEF2: A transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11: 683–688. [DOI] [PubMed] [Google Scholar]
- Neumann K, Endres M, Ringe J, Flath B, Manz R, Haupl T, Sittinger M, Kaps C. 2007. BMP7 promotes adipogenic but not osteo-/chondrogenic differentiation of adult human bone marrow-derived stem cells in high-density micro-mass culture. J Cell Biochem 102: 626–637. [DOI] [PubMed] [Google Scholar]
- Nickel J, Kotzsch A, Sebald W, Mueller TD. 2005. A single residue of GDF-5 defines binding specificity to BMP receptor IB. J Mol Biol 349: 933–947. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Parker EA, Hegde A, Chau M, Barnes KM, Baron J. 2007. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol 193: 75–84. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. 1998. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem 273: 1872–1879. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. 1996. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem 271: 21345–21352. [DOI] [PubMed] [Google Scholar]
- Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, Shindo H, Yamaguchi A. 2005. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem 280: 15842–15848. [DOI] [PubMed] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. 2004. Signal transduction of bone morphogenetic protein receptors. Cell Signal 16: 291–299. [DOI] [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura K, Samtani R, Lo CW, Mishina Y. 2009. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol 297: H1617–H1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. 2003. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res 291: 201–211. [DOI] [PubMed] [Google Scholar]
- Ogata T, Wozney JM, Benezra R, Noda M. 1993. Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc Natl Acad Sci 90: 9219–9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Yoshikawa H, Tsumaki N. 2006. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res 21: 1022–1033. [DOI] [PubMed] [Google Scholar]
- Oldknow KJ, MacRae VE, Farquharson C. 2015. The endocrine role of bone: Recent and emerging perspectives beyond osteocalcin. J Endocrinol 225: R1–R19. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Pisconti A. 2012. Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions. J Cell Mol Med 16: 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. 1986. Regulation of myogenic differentiation by type β transforming growth factor. J Cell Biol 103: 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EJ, Kang JD, Fu FH, Georgescu HI, Mason GC, Evans CH. 1988. The biochemical and histological effects of artificial ligament wear particles: In vitro and in vivo studies. Am J Sports Med 16: 558–570. [DOI] [PubMed] [Google Scholar]
- Onizuka N, Ito Y, Inagawa M, Nakahara H, Takada S, Lotz M, Toyama Y, Asahara H. 2014. The Mohawk homeobox transcription factor regulates the differentiation of tendons and volar plates. J Orthop Sci 19: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto TC, Lane MD. 2005. Adipose development: From stem cell to adipocyte. Crit Rev Biochem Mol Biol 40: 229–242. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771. [DOI] [PubMed] [Google Scholar]
- Oue Y, Kanatani H, Kiyoki M, Eto Y, Ogata E, Matsumoto T. 1994. Effect of local injection of activin A on bone formation in newborn rats. Bone 15: 361–366. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Selever J, Wang Y, Chen YT, Mishina Y, Martin JF, Behringer RR. 2006. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol 295: 103–115. [DOI] [PubMed] [Google Scholar]
- Ozeki N, Muneta T, Koga H, Katagiri H, Otabe K, Okuno M, Tsuji K, Kobayashi E, Matsumoto K, Saito H, et al. 2013. Transplantation of Achilles tendon treated with bone morphogenetic protein 7 promotes meniscus regeneration in a rat model of massive meniscal defect. Arthritis Rheum 65: 2876–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Cossu G, Molinaro M, Tato F. 1980. Vitamin A inhibits chondrogenesis but not myogenesis. Exp Cell Res 129: 469–474. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. 2005. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today 75: 237–248. [DOI] [PubMed] [Google Scholar]
- Patterson SE, Bird NC, Devoto SH. 2010. BMP regulation of myogenesis in zebrafish. Dev Dyn 239: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos LE, Rosenberg TD, Grewe SR, Tearse DS, Beck CL. 1992. The GORE-TEX anterior cruciate ligament prosthesis. A long-term followup. Am J Sports Med 20: 246–252. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Horsley V. 2003. Cell fusion in skeletal muscle—Central role of NFATC2 in regulating muscle cell size. Cell Cycle 2: 420–423. [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. 2012. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465. [DOI] [PubMed] [Google Scholar]
- Pedrozo HA, Schwartz Z, Robinson M, Gomes R, Dean DD, Bonewald LF, Boyan BD. 1999. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-β1 from the extracellular matrix of growth plate chondrocytes. Endocrinology 140: 5806–5816. [DOI] [PubMed] [Google Scholar]
- Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, et al. 2004. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem 279: 32941–32949. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Economides AN, Canalis E. 2000. Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology 141: 4558–4563. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Delany AM, Canalis E. 2002. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: Correlation with CCAAT-enhancer binding protein expression. Bone 30: 685–691. [DOI] [PubMed] [Google Scholar]
- Perrien DS, Akel NS, Edwards PK, Carver AA, Bendre MS, Swain FL, Skinner RA, Hogue WR, Nicks KM, Pierson TM, et al. 2007. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology 148: 1654–1665. [DOI] [PubMed] [Google Scholar]
- Peterson JR, De La Rosa S, Eboda O, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN, et al. 2014. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med 6: 255ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Wolf O, Naumann A, Minne HW, Mundy GR, Ziegler R. 1990. Chemotactic response of osteoblastlike cells to transforming growth factor β. J Bone Miner Res 5: 825–830. [DOI] [PubMed] [Google Scholar]
- Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. 2006. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, Sartorelli V, Cossu G, Clementi E. 2006. Follistatin induction by nitric oxide through cyclic GMP: A tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol 172: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Qi H, Jin M, Duan Y, Du X, Zhang Y, Ren F, Wang Y, Tian Q, Wang X, Wang Q, et al. 2014. FGFR3 induces degradation of BMP type I receptor to regulate skeletal development. Biochim Biophys Acta 1843: 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. 2010. TGF-β type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol 12: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana L, zur Nieden NI, Semino CE. 2009. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: New paradigms for cartilage tissue engineering. Tissue Eng Part B Rev 15: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. 2003. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18: 1842–1853. [DOI] [PubMed] [Google Scholar]
- Ray A, Singh PN, Sohaskey ML, Harland RM, Bandyopadhyay A. 2015. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development 142: 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud CM, Maleki M, Lis R, Ahmed B, Al-Azwani I, Malek J, Safadi FF, Rafii A. 2012. Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int 2012: 658356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. 2003. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol 23: 7230–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. 2003. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285: E876–E888. [DOI] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. 2009. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136: 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff CA, Brankow DW, Heidelberger C. 1973. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33: 3231–3238. [PubMed] [Google Scholar]
- Riekstina U, Muceniece R, Cakstina I, Muiznieks I, Ancans J. 2008. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 58: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigueur D, Brugger S, Anbarchian T, Kim JK, Lee YJ, Lyons K. 2015. The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development. J Bone Miner Res 30: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios R, Fernandez-Nocelos S, Carneiro I, Arce VM, Devesa J. 2004. Differential response to exogenous and endogenous myostatin in myoblasts suggests that myostatin acts as an autocrine factor in vivo. Endocrinology 145: 2795–2803. [DOI] [PubMed] [Google Scholar]
- Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. 1999. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med 27: 476–488. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. 2006. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896. [DOI] [PubMed] [Google Scholar]
- Rotzer D, Roth M, Lutz M, Lindemann D, Sebald W, Knaus P. 2001. Type III TGF-β receptor-independent signalling of TGF-β2 via TβRII-B, an alternatively spliced TGF-β type II receptor. EMBO J 20: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. 1993. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75: 1351–1359. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PP, Lee YW, Chan KM. 2012a. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int Orthop 36: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui YF, Lui PP, Rolf CG, Wong YM, Lee YW, Chan KM. 2012b. Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc 20: 1409–1417. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PP, Wong YM, Tan Q, Chan KM. 2013. BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res 31: 746–753. [DOI] [PubMed] [Google Scholar]
- Sabbieti MG, Agas D, Marchetti L, Coffin JD, Xiao L, Hurley MM. 2013. BMP-2 differentially modulates FGF-2 isoform effects in osteoblasts from newborn transgenic mice. Endocrinology 154: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiston P, Frank C, Lam T, Shrive N. 1990. Allograft ligament transplantation. A morphological and biochemical evaluation of a medial collateral ligament complex in a rabbit model. Am J Sports Med 18: 160–168. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. 1999. Reduced differentiation potential of primary MyoD–/– myogenic cells derived from adult skeletal muscle. J Cell Biol 144: 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. 2002. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294: 371–379. [DOI] [PubMed] [Google Scholar]
- Salazar VS, Mbalaviele G, Civitelli R. 2008. The pro-osteogenic action of β-catenin requires interaction with BMP signaling, but not Tcf/Lef transcriptional activity. J Cell Biochem 104: 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S, Mandelboim M, Zalcberg M, Shainberg A, Mandelbaum M. 1995. Interruption of myogenesis by transforming growth factor β 1 or EGTA inhibits expression and activity of the myogenic-associated (2'-5’) oligoadenylate synthetase and PKR. Exp Cell Res 219: 223–232. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. 1997. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development 124: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, Buckingham M. 1989. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature 341: 303–307. [DOI] [PubMed] [Google Scholar]
- Sato K, Li Y, Foster W, Fukushima K, Badlani N, Adachi N, Usas A, Fu FH, Huard J. 2003. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 28: 365–372. [DOI] [PubMed] [Google Scholar]
- Schabort EJ, van der Merwe M, Niesler CU. 2011. TGF-β isoforms inhibit IGF-1-induced migration and regulate terminal differentiation in a cell-specific manner. J Muscle Res Cell Motil 31: 359–367. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. 2013. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. 2001. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development 128: 3855–3866. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. 2000. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab 6: 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W, Nickel J, Zhang JL, Mueller TD. 2004. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol Chem 385: 697–710. [DOI] [PubMed] [Google Scholar]
- Seo HS, Serra R. 2007. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol 310: 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. 1997. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Karaplis A, Sohn P. 1999. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J Cell Biol 145: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settle S, Marker P, Gurley K, Sinha A, Thacker A, Wang Y, Higgins K, Cunha G, Kingsley DM. 2001. The BMP family member Gdf7 is required for seminal vesicle growth, branching morphogenesis, and cytodifferentiation. Dev Biol 234: 138–150. [DOI] [PubMed] [Google Scholar]
- Settle SH Jr., Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. 2003. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol 254: 116–130. [DOI] [PubMed] [Google Scholar]
- Seyedin SM, Thompson AY, Rosen DM, Piez KA. 1983. In vitro induction of cartilage-specific macromolecules by a bone extract. J Cell Biol 97: 1950–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin SM, Thompson AY, Bentz H, Rosen DM, McPherson JM, Conti A, Siegel NR, Galluppi GR, Piez KA. 1986. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-β. J Biol Chem 261: 5693–5695. [PubMed] [Google Scholar]
- Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. 1996. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med 335: 555–561. [DOI] [PubMed] [Google Scholar]
- Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, Bi Y, He BC, Huang J, Li X, et al. 2009. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem 284: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JJ, Huang L, Li L, Jorgez C, Matzuk MM, Brown CW. 2009a. Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol Endocrinol 23: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, et al. 2009b. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 119: 3462–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, Yang Y, Im HJ, O’Keefe R, Chen D. 2013. Deletion of the transforming growth factor β receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum 65: 3107–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Li S, Chen D. 2014. TGF-β signaling and the development of osteoarthritis. Bone Res 2: 14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, James AW, Zhang X, Pang S, Zara JN, Asatrian G, Chiang M, Lee M, Khadarian K, Nguyen A, et al. 2016. Novel Wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol 186: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700. [DOI] [PubMed] [Google Scholar]
- Shim JH, Greenblatt MB, Xie M, Schneider MD, Zou W, Zhai B, Gygi S, Glimcher LH. 2009. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J 28: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, et al. 2011. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med 17: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et al. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, et al. 2011. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci 124: 3428–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. 2006. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol 298: 234–247. [DOI] [PubMed] [Google Scholar]
- Sims NA, Vrahnas C. 2014. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch Biochem Biophys 561: 22–28. [DOI] [PubMed] [Google Scholar]
- Singh R, Braga M, Pervin S. 2014. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol 2: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhatanadgit W, Salih V, Olsen I. 2006. Bone morphogenetic protein receptors and bone morphogenetic protein signaling are controlled by tumor necrosis factor-α in human bone cells. Int J Biochem Cell Biol 38: 1794–1807. [DOI] [PubMed] [Google Scholar]
- Sjöholm K, Palming J, Lystig TC, Jennische E, Woodruff TK, Carlsson B, Carlsson LM. 2006. The expression of inhibin βB is high in human adipocytes, reduced by weight loss, and correlates to factors implicated in metabolic disease. Biochem Biophys Res Commun 344: 1308–1314. [DOI] [PubMed] [Google Scholar]
- Skillington J, Choy L, Derynck R. 2002. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol 159: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue T, Gravely T, Hand A, Min YK, Pilbeam C, Raisz LG, Zhang X, Larocca D, Florkiewicz R, Hurley MM. 2002. Regulation of fibroblast growth factor 2 and fibroblast growth factor receptors by transforming growth factor β in human osteoblastic MG-63 cells. J Bone Miner Res 17: 502–512. [DOI] [PubMed] [Google Scholar]
- Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. 2007. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-β1 to induce mesenchymal cell condensation. J Cell Physiol 210: 398–410. [DOI] [PubMed] [Google Scholar]
- Sottile V, Seuwen K. 2000. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett 475: 201–204. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. 2002. Activations of ERK1/2 and JNK by transforming growth factor β negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J Biol Chem 277: 36024–36031. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Canaff L, Hendy GN, Tsukamoto T, Yamaguchi T, Miyazono K, Sugimoto T, Chihara K. 2003a. Inactivation of menin, the product of the multiple endocrine neoplasia type 1 gene, inhibits the commitment of multipotential mesenchymal stem cells into the osteoblast lineage. J Biol Chem 278: 21058–21069. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Iu MF, Tsukamoto T, Sugimoto T, Chihara K. 2003b. Parathyroid hormone–Smad3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor β in osteoblasts. J Biol Chem 278: 52240–52252. [DOI] [PubMed] [Google Scholar]
- Spagnoli A, O'Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, et al. 2007. TGF-β signaling is essential for joint morphogenesis. J Cell Biol 177: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spater D, Hill TP, Gruber M, Hartmann C. 2006a. Role of canonical Wnt-signalling in joint formation. Eur Cell Mater 12: 71–80. [DOI] [PubMed] [Google Scholar]
- Spater D, Hill TP, O’Sullivan R J, Gruber M, Conner DA, Hartmann C. 2006b. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133: 3039–3049. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. 1996. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development 122: 3969–3979. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. 1994. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature 368: 639–643. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. 2004. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development 131: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. 1993. Extracellular matrix regulates expression of the TGF-β1 gene. J Cell Biol 120: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Yamamoto K, Akiyama H. 2012. Conditional deletion of Tgfbr2 in hypertrophic chondrocytes delays terminal chondrocyte differentiation. Matrix Biol 31: 352–359. [DOI] [PubMed] [Google Scholar]
- Sun Q, Zhang Y, Yang G, Chen X, Cao G, Wang J, Sun Y, Zhang P, Fan M, Shao N, et al. 2008. Transforming growth factor-β-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res 36: 2690–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T. 1999. Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology 140: 2125–2133. [DOI] [PubMed] [Google Scholar]
- Svobodova E, Krulova M, Zajicova A, Pokorna K, Prochazkova J, Trosan P, Holan V. 2012. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev 21: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. 2012. Adipogenesis: From stem cell to adipocyte. Annu Rev Biochem 81: 715–736. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. 2003. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci 100: 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. 2004. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci 101: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. 2009. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Broek RW, Grefte S, Von den Hoff JW. 2010. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224: 7–16. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. 1993. Activin receptor-like kinases: A novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 8: 2879–2887. [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. 1994. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem 269: 16985–16988. [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. 1996. A human chondrodysplasia due to a mutation in a TGF-β superfamily member. Nat Genet 12: 315–317. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. 1997. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet 17: 58–64. [DOI] [PubMed] [Google Scholar]
- Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S, et al. 1999. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res 14: 80–89. [DOI] [PubMed] [Google Scholar]
- Torti FM, Torti SV, Larrick JW, Ringold GM. 1989. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor β. J Cell Biol 108: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler DA, Gelberman RH. 2006. The alchemy of tendon repair: A primer for the (S)mad scientist. J Clin Invest 116: 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. 2009. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296: C1258–C1270. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. 2008. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am 90: 14–18. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. 1999. Role of CDMP-1 in skeletal morphogenesis: Promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol 144: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. 2002. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res 17: 898–906. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. 2003. Transforming growth factor-β-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278: 41227–41236. [DOI] [PubMed] [Google Scholar]
- Ugarte G, Brandan E. 2006. Transforming growth factor β (TGF-β) signaling is regulated by electrical activity in skeletal muscle cells. TGF-β type I receptor is transcriptionally regulated by myotube excitability. J Biol Chem 281: 18473–18481. [DOI] [PubMed] [Google Scholar]
- Ulsamer A, Ortuño MJ, Ruiz S, Susperregui ARG, Osses N, Rosa JL, Ventura F. 2008. BMP-2 induces Osterix expression through up-regulation of Dlx5 and its phosphorylation by p38. J Biol Chem 283: 3816–3826. [DOI] [PubMed] [Google Scholar]
- Vaidya TB, Rhodes SJ, Taparowsky EJ, Konieczny SF. 1989. Fibroblast growth factor and transforming growth factor β repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol 9: 3576–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti MT, Garbin U, Pasini A, Zanatta M, Stranieri C, Manfro S, Zucal C, Dalle Carbonare L. 2011. Role of ox-PAPCs in the differentiation of mesenchymal stem cells (MSCs) and Runx2 and PPARγ2 expression in MSCs-like of osteoporotic patients. PLoS ONE 6: e20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. 2009. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. 1996. Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science 273: 613–622. [DOI] [PubMed] [Google Scholar]
- Wagner KR, McPherron AC, Winik N, Lee SJ. 2002. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52: 832–836. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, et al. 2005. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33: 1402–1416. [DOI] [PubMed] [Google Scholar]
- Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, Harland R, Blau HM, Longaker MT. 2007. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem 282: 26450–26459. [DOI] [PubMed] [Google Scholar]
- Wang YK, Chen CS. 2013. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J Cell Mol Med 17: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EA, Israel DI, Kelly S, Luxenberg DP. 1993. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9: 57–71. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Meng Y, Shi Y. 2004. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem Biophys Res Commun 321: 1024–1031. [DOI] [PubMed] [Google Scholar]
- Wang QW, Chen ZL, Piao YJ. 2005. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng 100: 418–422. [DOI] [PubMed] [Google Scholar]
- Wang M, Jin H, Tang D, Huang S, Zuscik MJ, Chen D. 2011. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthritis Cartilage 19: 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D. 2013a. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther 15: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng Y, Chen D, Chen Y. 2013b. Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckel’s cartilage in mice. Dev Biol 381: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang F, Zhao H, Zhang X, Chen H, Zhang K. 2014. Human adipose-derived mesenchymal stem cells are resistant to HBV infection during differentiation into hepatocytes in vitro. Int J Mol Sci 15: 6096–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. 1991. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science 251: 761–766. [DOI] [PubMed] [Google Scholar]
- Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, et al. 2012. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J Cell Biol 197: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn BA, Bernlohr DA. 2001. Upregulation of bone morphogenetic protein GDF-3/Vgr-2 expression in adipose tissue of FABP4/aP2 null mice. Cytokine 14: 129–135. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, et al. 1997. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J Clin Invest 100: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, et al. 2015. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160: 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. 1988. Novel regulators of bone formation: Molecular clones and activities. Science 242: 1528–1534. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Yu PB, Feng XH. 2009. Transforming growth factor β can stimulate Smad1 phosphorylation independently of bone morphogenic protein receptors. J Biol Chem 284: 9755–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR. 1996. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol 16: 4128–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-B, Li Y, Schneider A, Yu W, Rajendren G, Iqbal J, Yamamoto M, Alam M, Brunet LJ, Blair HC, et al. 2003. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest 112: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liang S, Yan Y, Wang Y, Li F, Deng Y, Huang W, Yuan W, Luo N, Zhu C, et al. 2007. A novel mutation of TGFβ1 in a Chinese family with Camurati-Engelmann disease. Bone 40: 1630–1634. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. 2013. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev 27: 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Wang Z, Zaromytidou AI, Zhang XH, Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K, Kaartinen V, et al. 2011. A poised chromatin platform for TGF-β access to master regulators. Cell 147: 1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. 2002. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res 17: 101–110. [DOI] [PubMed] [Google Scholar]
- *.Xu P, Lin X, Feng X-H. 2016. Posttranslational regulation of Smads. Cold Spring Harb Perspect Biol 8: a022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. 2011. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A. 1995. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-β superfamily. Semin Cell Biol 6: 165–173. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. 1991. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol 113: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane A, Amano O, Slavkin HC. 2003. Insulin-like growth factors, hepatocyte growth factor and transforming growth factor-α in mouse tongue myogenesis. Dev Growth Differ 45: 1–6. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fukushima H, Shin M, Katagiri T, Doi T, Takahashi T, Jimi E. 2009. Tumor necrosis factor α represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-κB. J Biol Chem 284: 35987–35995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. 2001. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. 2006. Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res 66: 1320–1326. [DOI] [PubMed] [Google Scholar]
- Yang T, Grafe I, Bae Y, Chen S, Chen Y, Bertin TK, Jiang MM, Ambrose CG, Lee B. 2013. E-selectin ligand 1 regulates bone remodeling by limiting bioactive TGF-β in the bone microenvironment. Proc Natl Acad Sci 110: 7336–7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee Lui PP, Wong YM, Rui YF, Lee YW, Chan LS, Chan KM. 2011. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J Orthop Res 29: 816–821. [DOI] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. 2000. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127: 621–630. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci 102: 5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Bliss JP, Bruce WJ, Walsh WR. 2007. Bone morphogenetic proteins and Smad expression in ovine tendon-bone healing. Arthroscopy 23: 205–210. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, et al. 2008. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto K, Thomas PS, Lane J, Matsuzaki K, Inagaki M, Ninomiya-Tsuji J, Scott GJ, Ray MK, Ishii M, Maxson R, et al. 2013. TGF-β-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J Biol Chem 288: 13467–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N, Brown CW. 2011. Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr Rev 32: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, et al. 2010. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 59: 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. 2002. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev 16: 1990–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. 2009. Non-Smad pathways in TGF-β signaling. Cell Res 19: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Zhang YE. 2017. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 9: a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan X, Li W, Wang Y, Wang J, Cheng X, Yang X. 2005. Smad4 is required for the normal organization of the cartilage growth plate. Dev Biol 284: 311–322. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang M, Lin L, Chen P, Ma KT, Zhou CY, Ao YF. 2006. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose--derived stem cells in vitro and in vivo. Calcif Tissue Int 79: 169–178. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yan Y, Lim YB, Tang D, Xie R, Chen A, Tai P, Harris SE, Xing L, Qin YX, et al. 2009. BMP-2 modulates β-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. J Cell Biochem 108: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Deng B, Wen J, Chen K, Liu W, Ye S, Huang H, Jiang S, Xiong Y. 2015. PPARγ and MyoD are differentially regulated by myostatin in adipose-derived stem cells and muscle satellite cells. Biochem Biophys Res Commun 458: 375–380. [DOI] [PubMed] [Google Scholar]
- Zhao R, Lawler AM, Lee SJ. 1999. Characterization of GDF-10 expression patterns and null mice. Dev Biol 212: 68–79. [DOI] [PubMed] [Google Scholar]
- Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. 2002. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol 157: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al. 2013. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 19: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. 2011. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem 112: 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Topouzis S, Liang LF, Stotish RL. 2004. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 26: 262–272. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. 2008. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreERT2 transgenic mice. Osteoarthritis Cartilage 16: 129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. 2002. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massagué J, Niswander L. 1997. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev 11: 2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]