Abstract
Human first-trimester decidual cells (FTDCs) chemoattract CXCR3-expressing circulating CD56brightCD16− natural killer (NK) cells, which increase uteroplacental blood flow by remodeling spiral arteries and arterioles. This recruitment reflects elevated FTDC expression of NK cell-recruiting induced protein 10 and interferon (IFN)-inducible T-cell-α chemoattractant produced in response to the synergistic effects of tumor necrosis factor α (TNF-α) and IFN-γ stimulation. Decidual macrophages express TNF-α, whereas the cellular origin of IFN-γ is unclear. Therefore, this study aims to identify the cell source(s) of IFN-γ in human first trimester decidua. Immunostaining of decidual sections revealed that both FTDCs and decidual NK (dNK) cells express IFN-γ. Although individual dNK cells express higher IFN-γ levels, the more numerous FTDCs account for greater proportion of total IFN-γ immunostaining. Freshly isolated FTDCs express greater IFN-γ staining than dNK cells as measured by flow cytometry, whereas incubation of dNK cells with documented NK cell activators significantly increases IFN-γ above FTDC levels. Confluent FTDCs intrinsically produce, but paradoxically respond to, exogenous IFN-γ.
Keywords: interferon γ, NK cells, decidual cells
Introduction
Human first-trimester blastocyst-derived extravillous trophoblasts (EVTs) invade the underlying decidua comprised primarily of resident decidual cells (50%) and an immune cell population dominated by decidual natural killer (dNK) cells (˜70%) and macrophages (˜20%).1 Endovascular EVTs invade the tunica media of spiral arteries via either the vessel lumen or the surrounding decidualized stroma. By either route, upon entering the vessel, the trophoblastic epithelial cell adhesion molecule phenotype is transformed into an endothelial cell-like adhesion molecule phenotype.2 The net effect is to produce a low resistance, high capacity conduit that delivers increased uteroplacental blood flow to the developing fetal–placental unit.2,3 Decidual invasion occurs via sequential integrin-mediated binding of EVTs to specific extracellular matrix (ECM) proteins followed by their degradation.4 Shallow EVT invasion elicits incomplete spiral artery remodeling, resulting in reduced uteroplacental blood flow and a hypoxic placenta that secretes elevated levels of soluble antiangiogenic factors into the maternal plasma. The resulting antiangiogenic milieu promotes endothelial cell activation and dysfunction implicated in the later development of preeclampsia (PE).5 Immunostaining of decidual sections revealed that in preeclamptic specimens, decidual cells express significantly higher ECM-degrading matrix metalloproteinases (MMPs) 1, 3, and 9 than gestational age-matched control decidual cells.6 In primary cultures of human first-trimester decidual cells (FTDCs), the potent proinflammatory cytokine, tumor necrosis factor α (TNF-α), significantly enhances steady-state MMP-1, 3, and 9 messenger RNA (mRNA) levels and protein levels, whereas coincubation with interferon γ (IFN-γ) reverses induction of all 3 MMPs.6
Compared with the well-documented requirement for EVTs to complete spiral artery remodeling,2 newer observations indicate that dNK cells mediate an initial trophoblast-independent stage of vascular remodeling by secreting angiogenesis-related factors such as angiopoietin 1 and 2, vascular endothelial growth factor C, and IFN-γ.7 Approximately 80% of dNK cells are CD56brightCD16−, a surface phenotype shared with 10% of peripheral NK (pNK) cells, with 90% of the remaining circulating natural killer (NK) cells displaying a CD56dimCD16+ phenotype.
Our previous study demonstrated that resident decidual cells recruit pNK cells to the decidua. Specifically, incubation of human FTDCs with IFN-γ and either TNF-α or interleukin 1β (IL-1β) synergistically enhances expression of 2 key pNK cell recruiting chemokines, IFN-γ-induced protein 10 (IP-10) and IFN-inducible T-cell-α chemoattractant (I-TAC).8 The dominant role played by IFN-γ in regulating EVT invasion of6 and in recruiting additional pNK cells to the decidua8 stimulated a search for local cellular sources of IFN-γ that could serve as autocrine and/or paracrine effectors in both processes. The current study addressed this question by integrating in situ and in vitro observations of IFN-γ expression by decidual cells and dNK cells, which together comprise approximately 80% of the total cell population in human first-trimester decidua.
Materials and Methods
Immunofluorescent Staining of Decidua for IFN-γ
Decidual specimens were obtained under institutional review board (IRB) approval at Mackay Memorial Hospital (Taipei, Taiwan) from elective terminations of pregnancies between 5 and 8 weeks of gestation without uterine contraction, vaginal bleeding, or evidence of fetal demise. Decidua basalis was evacuated, snapped frozen in liquid nitrogen, and stored at −80°C for future use. Serial sections of OCT-embedded decidual sections were (1) immunostained with mouse antihuman CD56 or antivimentin (Dako, Carpinteria, California) followed by rhodamine-conjugated donkey antimouse antibody (EMD Millipore, Billerica, Massachusetts) and (2) then incubated with rabbit antihuman IFN-γ (Sigma-Aldrich, St Louis, Missouri) followed by the corresponding FITC-conjugated secondary antibody and 4′,6′-diamidino-2-phenylindole (Sigma-Aldrich). Cell IFN-γ immunofluorescent levels were quantified by TissueQuest software (TissueGnostice GmbH, Vienna, Austria).
Cell Isolation
The FTDCs were isolated and cultured as described previously.9 Briefly, uterine decidua was obtained after pregnancy terminations at 6 to 12 weeks gestation under IRB approval at the Beth Israel Medical Center (New York, New York). Cells were purified using Ficoll-Hipaque Plus (GE Healthcare, Piscataway, New Jersey).
Experimental Decidual Cell Incubations
Thawed cells were incubated in basal medium, a phenol red-free 1:1 (v/v) mix of Dulbecco modified Eagle medium (Gibco Life Technologies, Grand Island, New York) and Ham F-12 (Flow Labs, Rockville, Maryland), with 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL fungizone supplemented with 10% charcoal-stripped calf serum (basal medium with serum [BMS]). After 2 additional passages, leukocyte-free confluent cultures were incubated in BMS containing 10−8 mol/L estradiol (E2) + 10−7 mol/L medroxyprogesterone (MPA; Sigma-Aldrich) to mimic the pregnant steroid milieu, and MPA was used in place of progesterone because of its greater stability in culture.10 After 7 days, the cultures were washed twice with Hank balanced salt solution to remove residual serum then switched to a defined medium (DM) consisting of basal medium plus ITS+ (Collaborative Research, Waltham, Massachusetts), 5 µmol/L FeSO4, 50 µmol/L ZnSO4, 1 nmol/L CuSO4, 20 nmol/L Na2SeO3, trace elements (Gibco), 50 µg/mL ascorbic acid (Sigma-Aldrich), and 50 ng/mL epidermal growth factor (Becton-Dickinson, Bedford, Massachusetts), with either vehicle control (0.1% ethanol) or steroids added with or without 1 ng/mL each of TNF-α or IL-1β (R&D Systems, Minneapolis, Minnesota). After 24 hours, conditioned medium supernatants (CMSs) were centrifuged and stored at −80°C. Cells were harvested for protein extraction. Total RNA was extracted from cultured cells following incubation with TNF-α or IL-1β for 6 hours.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA obtained from FTDCs was extracted using a total RNA purification plus kit (Norgen Bioteck, Ontario, Canada). Reverse transcription used SuperScript III First-Strand Synthesis System from Invitrogen (Grand Island, New York). Specific primer sets for IFN-γ or β-actin (Integrated DNA technologies, Coralville, Iowa; Table 1) were used for quantitative polymerase chain reaction (PCR). Quantitation of unknowns was determined and adjusted to quantitative expression of β-actin in the experimental samples. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation.
Table 1.
Primer Sets With Predicted PCR Product Sizes for qRT-PCR.
| Gene | Forward | Reverse | Product Size, BP |
|---|---|---|---|
| β-Actin | 5′-CGTACCACTGGCATCGTGAT-3′ | 5′-GTGTTGGCGTACAGGTCTTTG-3′ | 452 |
| IFN-γ | 5′-GTCCAACGCAAAGCAATACA-3′ | 5′-CTCTTCGACCTCGAAACAGC-3′ | 90 |
Abbreviations: IFN-γ, interferon γ; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Bio-Plex Assay
Bio-Plex assays (Bio-Rad, Hercules, California) measured IFN-γ levels in CMS from leukocyte-free FTDC monolayer cultures according to the manufacturer’s instructions. Eight concentrations of IFN-γ were used to generate a standard curve. Data acquisition and analyses were completed with the Bio-Plex 200 system using Bio-Plex Manager Software v6. Bicinchoninic acid protein assay (Thermo Scientific, Rockford, Illinois) measured total cell protein levels.
Flow Cytometry
First-trimester decidua was digested with 0.1% collagenase IV to prepare single-cell suspensions for flow cytometry. The isolated cells were incubated with or without IL-12 (10 ng/mL) + IL-18 (100 ng/mL) for 24 hours followed by addition of 1 μL/mL of GolgiPlug (106 cells/mL; BD Biosciences, San Jose, California). The cells were then stained with antihuman CD3-V450, CD45-Alexa Fluor 700 (CD45-AF700), CD56-Fluorescein Isothiocyanate (CD56-FITC), vimentin-phycoerythrin (vimentin-PE), and IFN-γ-Allophycocyanin (IFN-γ-APC) (BD Biosciences) at 4°C for 20 minutes for flow cytometric analysis using a BD LSRII and FACSDiva8.0 software gating on IFN-γ+CD3−CD56bright dNK cells and IFN-γ+CD45−vimentin+ decidual cells.
Statistics
The variance and normality of results from immunofluorescent staining, quantitative reverse transcription PCR, Bio-Plex assay, and flow cytometry were first determined. The statistical significance of results with equal variance was then examined by t test. Results with unequal variance that passed or failed normality testing were evaluated by t test assuming unequal variance or Mann-Whitney rank sum test, respectively, with P < .05 considered to be significant.
Results
Expression of IFN-γ by dNK Cells and Decidual Cells in Sections of First-Trimester Human Decidua
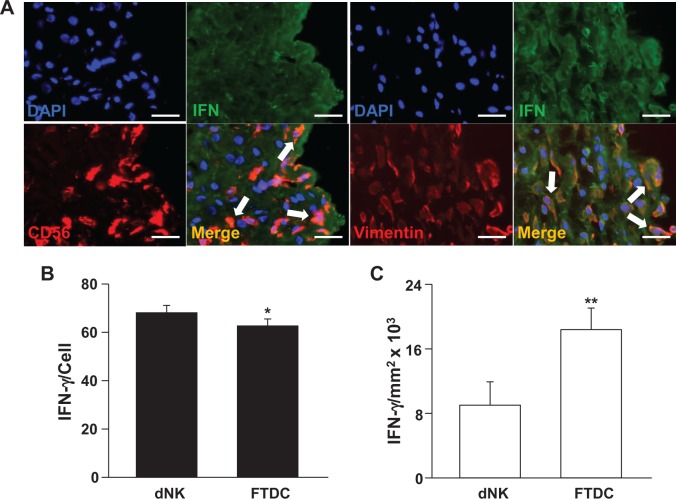
First-trimester decidual sections were stained with antihuman IFN-γ and either CD56 or vimentin antibodies. In Figure 1A, colocalization of either CD56 (red) or vimentin (red) with IFN-γ (green) is indicated by yellow–orange immunofluorescence (merge). Figure 1 indicates that both CD56bright dNK cells and vimentin-positive FTDCs express IFN-γ. Compared with individual FTDCs (Figure 1B), each dNK cell displays statistically significantly higher IFN-γ staining than each FTDC. However, reflecting relatively greater numbers of decidual cells than dNK cells per unit area of decidua, total IFN-γ staining is significantly higher among FTDCs than in dNK cells (Figure 1C).
Figure 1.
Immunoreactive IFN-γ in decidual natural killer (dNK) cells and first-trimester decidual cells (FTDCs) in human decidua. A, First-trimester decidual sections were stained with either antihuman CD56 (red) or antihuman vimentin (red) antibodies and then incubated with rabbit antihuman IFN-γ (green). Staining with antihuman 4′,6′-diamidino-2-phenylindole (DAPI; blue) denotes nuclei. Arrows in merged images indicate yellow-orange immunofluorescence resulting from double staining. B, Ordinate indicates immunoreactive IFN-γ levels/CD56+ cell. C, Ordinate indicates IFN-γ levels/decidual mm2 × 103. The results are reported as mean ± standard error of the mean (SEM). n = 18; *P < .05 and **P < .01. Magnification: 400×; scale bar: 50 μm. IFN-γ indicates interferon γ.
Interferon γ is Produced by Cultured FTDCs in Response to Proinflammatory Stimuli
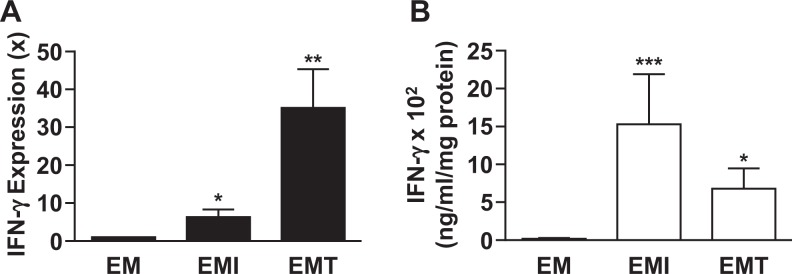
Expression of IFN-γ mRNA and protein was evaluated in confluent monolayer cultures of primary leukocyte-free FTDCs in response to either IL-1β or TNF-α. Compared with control incubations, Figure 2A indicates that steady-state levels of IFN-γ mRNA are significantly elevated during incubations with either IL-1β (6.32 ± 2.01-fold, n = 8, P < .05) or TNF-α (35.14 ± 10.16-fold, n = 8, P < .01). Corresponding increases in secreted IFN-γ protein levels determined by Bio-Plex assay are displayed in Figure 2B. Specifically, IL-1β and TNF-α induced IFN-γ expression by 89- and 40-fold, respectively.
Figure 2.
Effects of IL-1β or TNF-α on IFN-γ mRNA and secreted protein in first-trimester decidual cell (FTDC) monolayers incubated with E2 + MPA. Confluent, leukocyte-free decidual cells were incubated for 7 days in BMS containing 10−8 mol/L E2 + 10−7 mol/L MPA and then switched to DM with corresponding steroids with or without 1 ng/mL each of IL-1β (I) or TNF-α (T) for 6 hours (for mRNA) or 24 hours (for protein). A, The IFN-γ mRNA expression was assessed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR; n = 8). B, The IFN-γ protein levels were measured by Bio-Plex assay in conditioned DM supernatants and normalized to cell protein (n = 6). The results are reported as mean ± standard error of the mean (SEM). *P < .05, **P < .01, and ***P < .005. DM indicates defined medium; E2, estradiol; IFN-γ, interferon γ; IL, interleukin; MPA, medroxyprogesterone; mRNA, messenger RNA; TNF-α, tumor necrosis factor α.
Interferon γ is Expressed by Freshly Isolated dNK Cells and FTDCs
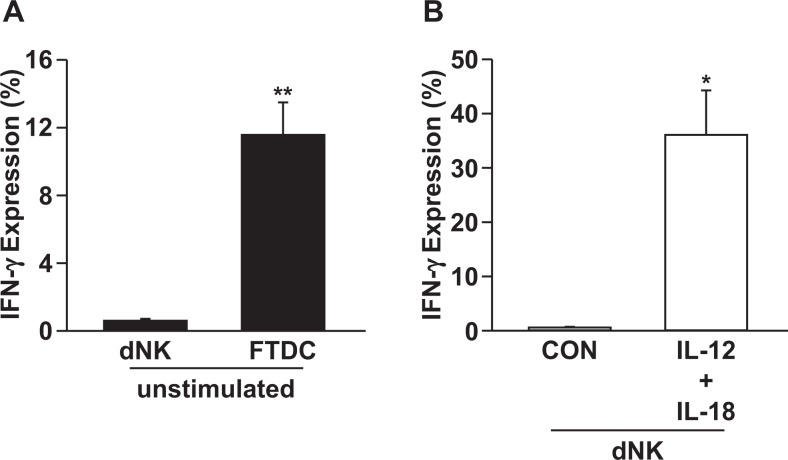
Expression of IFN-γ in freshly isolated FTDCs and dNK cells from first-trimester decidua obtained from elective terminations was evaluated by flow cytometric analysis. Figure 3A indicates that IFN-γ is expressed by 11.57% of FTDCs compared to 0.6% of dNK cells. However, after stimulation with proinflammatory cytokines, IL-12 and IL-18, previously shown to enhance IFN-γ production by NK cells,11 the percentage of dNK cells expressing IFN-γ is increased to 36.07%.
Figure 3.
Flow cytometric analysis of IFN-γ expression in freshly isolated FTDCs and dNK cells from first-trimester decidua. Single-cell suspensions prepared from enzyme-digested fresh decidua were stained with antihuman CD3-V450, CD45-AF700, CD56-FITC, vimentin preeclampsia (PE), and IFN-γ-APC antibodies. A, The IFN-γ expression in dNK cells and FTDCs in steady state. B, The IFN-γ expression in dNK cells treated with IL-12 + IL-18. The results are reported as mean ± standard error of the mean (SEM). n = 3; *P < .05 and ** P < .01. dNK indicates decidual natural killer; FTDC, first-trimester decidual cell; IFN-γ, interferon γ; IL, interleukin.
Discussion
At the human implantation site, the decidua is comprised primarily of resident decidual cells and dNK cells, which account for about 50% and 30% of the total cell population, respectively.1 The origin of dNK cells has been attributed to (1) in situ differentiation of resident endometrial NK cells12,13; (2) self-renewal from a population of local progenitor stem cells14; and (3) trafficking of pNK cells.15 In the circulation, mature NK cells comprise an estimated 5% to 15% of the total lymphocyte population and consist of 2 functionally distinct subsets. A majority CD56dimCD16+ pNK subset (90%), which are cytotoxic, express high levels of killer cell immunoglobulin-like receptors (KIRs) as well as CD57 and usually do not secrete cytokines. In contrast, the absence of CD16 expression by the minority (1%-10%), less mature, CD56brightCD16− pNK cells is consistent with an inability to mediate antibody-dependent cell toxicity.12 These CD56brightCD16− NK cells express low levels of perforin and high levels of the CD94/NKG2 receptor and adhesion-mediating L-selectin.16 They are the major pNK cell source of secreted immunoregulatory cytokines as exemplified by IFN-γ, which are expressed in response to IL-12 acting together with other cytokines or after engagement of either the CD16 (FcγRIIIa) or the NKG2D pNK cell-activating receptors.17
In early human decidua, approximately 80% of dNK cells are CD56bright CD16−,12,13 a unique immune cell subtype that both fosters immune tolerance of the semiallogeneic fetal–placental unit while promoting EVT invasion and spiral artery and arteriole remodeling via expression of vascular endothelial and placental growth factors.12,13,18,19 The dominant subtype of circulating NK cells, CD56dimCD16+ pNK cells, is reported to traffic to the decidua then differentiate into CD56brightCD16− NK cells in response to decidual cell-derived transforming growth factor β.19,20 However, growing evidence now points to the minority circulating CD56brightCD16− pNK cells as the major contributors to the dNK cell population. Their preferential recruitment is suggested by elevated expression of L-selectin, which mediates interactions with vascular endothelium21 by CD56brightCD16− but not by CD56dimCD16+ pNK cells. Recently, our laboratory confirmed that CD56bright CD16− pNK cells express high levels of CXCR3,8 the cognate receptor for both IFN-γ-IP-10 (IP-10/CXCL10) and I-TAC, which are potent NK cell chemoattractants. We also observed that incubation of primary cultures of human decidual cells with IFN-γ and either IL-1β or TNF-α induced a synergistic enhancement of IP-10 and I-TAC mRNA and protein expression. Moreover, we noted that incubation of pNK cells with either IP-10 or I-TAC produced a concentration-dependent increase in expression of CXCR3 and migration of pNK cells that became inhibitory at high concentrations.
In pregnant mice, dNK cell-secreted IFN-γ plays a key role in spiral artery remodeling. Approximately 90% of IFN-γ found in the mesometrial decidua and a transient myometrial structure, mesometrial lymphoid aggregate of pregnancy, that surrounds blood vessels supplying placentae are derived from dNK cells, whereas systemic IFN-γ administration to NK cell-deficient mice promotes spiral arterial modification.22,23 These observations indicate that dNK cell-derived IFN-γ is both necessary and sufficient to mediate normal uterine vascular conversion to low resistance high capacity conduits. As in women, close proximity between dNK cells and blood vessels in mice suggests that dNK cell-derived angiogenic factors augment the effects of IFN-γ to promote normal vascular remodeling.24
Decidualization in women is initiated in the late luteal phase25,26 and continues throughout pregnancy,27 whereas decidualization in mice is initiated following implantation and is short lived.24 Moreover, mouse placenta-derived trophoblasts normally display shallow invasion. In contrast, human EVTs exhibit deep invasion of spiral vessels down to the inner third of the underlying myometrium as they temporarily replace maternal endothelial cells during the process of spiral vascular remodeling to augment uteroplacental blood flow.2,28 These differences limit the mouse as a model for the restricted trophoblast invasion that characterizes PE and intrauterine growth restriction in women.
Unlike the in situ presence of IFN-γ expression in mouse dNK cells,24 detection of IFN-γ expression in human dNK cells requires ex vivo stimulation.29 Thus, staining of freshly isolated decidual leukocyte preparations by intracellular flow cytometry detected only small amounts of IFN-γ with significant levels of IFN-γ evident only after 6-hour incubation with the phorbol 12-myristate 13-acetate.29 This low basal IFN-γ expression by human dNK cells viewed within the context of our previous reports that IFN-γ targets FTDCs to regulate expression of MMPs, and NK cell recruiting chemokines8 stimulated the current comparison of IFN-γ expression between dNK cells and decidual cells. These new observations indicate that both cell types, which together account for an estimated 80% of the cells at the human implantation site, are major contributors to IFN-γ expression. Specifically, (1) immunostaining of first-trimester decidual sections shows that individual CD56bright dNK cells express significantly higher IFN-γ than vimentin-positive decidual cells. However, consistent with their higher numbers, decidual cells account for greater total IFN-γ expression; (2) flow cytometric analysis of freshly isolated cells indicate that under resting conditions, decidual cells express about 19 times greater IFN-γ levels than dNK cells, whereas coincubation of dNK cells with IL-12 + IL-18, which is documented to enhance IFN-γ expression by human and mouse NK cells,11,30 elevated IFN-γ output by these activated dNK cells by more than 60-fold; and (3) in confluent FTDC monolayers incubated with E2 + MPA to mimic the steroid milieu of pregnancy, addition of IL-1β or TNF-α markedly increased IFN-γ mRNA and protein expression.
In the current study, immunostaining of decidual sections and flow cytometry of freshly isolated cells reveal that both FTDCs and dNK cells express IFN-γ. Significantly enhanced IFN-γ expression by dNK cells during coincubation with IL-12 + IL-18 is consistent with observations made on other cytokine-primed human NK cells: (1) after direct contact with Candida albicans following actin-dependent engulfment of fungal cells that elicits degranulation and release of IFN-γ and other cytokines that induce fungal damage31; (2) in Toxoplasma gondii infected dNK cells leading to enhanced dNK cell IFN-γ and NKG2D expression accompanied by increased cytotoxicity32; and (3) identification of IFN-γ as the predominant cytokine produced by activated NK cells involved in antibacterial immune responses.33 Beyond their well-documented pregnancy-supporting roles in promoting both immune tolerance of the semiallogeneic fetal–placental unit and vascular remodeling,8 these observations indicate that activation of the latent cytotoxic machinery in human dNK cells also protects against microbial infections.34
In summary, in the current study immunostaining of first-trimester decidual sections provides the first evidence that human decidual cells express significant levels of IFN-γ in situ. These observations are augmented by the demonstration that both IL-1β and TNF-α significantly enhance IFN-γ protein mRNA and protein expression by confluent cultures of primary human FTDCs. The revelation of endogenous IFN-γ protein expression by human FTDCs complement our previous demonstration that coincubation of decidual cells with IFN-γ and TNF-α synergistically enhances NK cell-recruiting chemokine expression8 while inhibiting aberrant TNF-α-induced MMP-1, 3, and 9 expression.6 Solving the apparent paradox of how FTDCs express endogenous IFN-γ, yet respond to exogenous IFN-γ indicates the importance of future experiments that identify activation of specific signaling pathways resulting from binding of IFN-γ with its membrane-bound IFN-γ receptor 1 leading to heterodimerization with intracellular IFN-γ receptor 2.35
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant 5R01HD056123 from NICHD, NIH (SJH).
References
- 1. Erlebacher A. Immunology of the maternal–fetal interface. Annu Rev Immunol. 2013;31:387–411. [DOI] [PubMed] [Google Scholar]
- 2. Zhou Y, Fisher SJ, Janatpour M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pijnenborg R, Vercruysse L, Carter AM. Deep trophoblast invasion and spiral artery remodelling in the placental bed of the chimpanzee. Placenta. 2011;32(5):400–408. [DOI] [PubMed] [Google Scholar]
- 4. Lockwood CJ, Oner C, Uz YH, et al. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78(6):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci. 2012;122(2):43–52. [DOI] [PubMed] [Google Scholar]
- 6. Lockwood CJ, Basar M, Kayisli UA, et al. Interferon-gamma protects first-trimester decidual cells against aberrant matrix metalloproteinases 1, 3, and 9 expression in preeclampsia. Am J Pathol. 2014;184(9):2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robson A, Harris LK, Innes BA, et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26(12):4876–4885. [DOI] [PubMed] [Google Scholar]
- 8. Lockwood CJ, Huang SJ, Chen CP, et al. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183(3):841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M, Wu ZM, Yang H, Huang SJ. NFkappaB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli. J Clin Endocrinol Metab. 2011;96(8):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arici A, Marshburn PB, MacDonald PC, Dombrowski RA. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64(8):530–534. [DOI] [PubMed] [Google Scholar]
- 11. Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25(6):439–448. [DOI] [PubMed] [Google Scholar]
- 12. Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63(6):434–444. [DOI] [PubMed] [Google Scholar]
- 13. Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59(5):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lynch L, Golden-Mason L, Eogan M, O’Herlihy C, O’Farrelly C. Cells with haematopoietic stem cell phenotype in adult human endometrium: relevance to infertility? Hum Reprod. 2007;22(4):919–926. [DOI] [PubMed] [Google Scholar]
- 15. Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185(7):3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryceson YT, Chiang SC, Darmanin S, et al. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3(3):216–226. [DOI] [PubMed] [Google Scholar]
- 17. Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lash GE, Burton GJ, Chamley LW, et al. IFPA Meeting 2009 workshops report. Placenta. 2010;31 suppl:S4–S20. [DOI] [PubMed] [Google Scholar]
- 19. Keskin DB, Allan DS, Rybalov B, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16-NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104(9):3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allan DS, Rybalov B, Awong G, et al. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40(8):2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaguchi T, Kitaya K, Daikoku N, Yasuo T, Fushiki S, Honjo H. Potential selectin L ligands involved in selective recruitment of peripheral blood CD16(−) natural killer cells into human endometrium. Biol Reprod. 2006;74(1):35–40. [DOI] [PubMed] [Google Scholar]
- 22. Ashkar AA, Black GP, Wei Q, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171(6):2937–2944. [DOI] [PubMed] [Google Scholar]
- 23. Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192(2):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol. 1992;42(3-4):337–344. [DOI] [PubMed] [Google Scholar]
- 26. Bell SC. Decidualization and relevance to menstruation In: D’Arcangues C, Fraser IS, Newton JR, Odlind V, eds. Contraception and Mechanisms of Endometrial Bleeding. Cambridge, United Kingdom: Cambridge University Press; 1990:188. [Google Scholar]
- 27. Lockwood CJ, Murk W, Kayisli UA, et al. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab. 2009;94(6):2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):273–285. [DOI] [PubMed] [Google Scholar]
- 29. Apps R, Sharkey A, Gardner L, et al. Ex vivo functional responses to HLA-G differ between blood and decidual NK cells. Mol Hum Reprod. 2011;17(9):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keppel MP, Saucier N, Mah AY, Vogel TP, Cooper MA. Activation-specific metabolic requirements for NK cell IFN-gamma production. J Immunol. 2015;194(4):1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voigt J, Hunniger K, Bouzani M, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis. 2014;209(4):616–626. [DOI] [PubMed] [Google Scholar]
- 32. Liu X, Zhao M, Yang X, et al. Toxoplasma gondii infection of decidual CD1c(+) dendritic cells enhances cytotoxicity of decidual natural killer cells. Inflammation. 2014;37(4):1261–1270. [DOI] [PubMed] [Google Scholar]
- 33. Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012;18:270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Bouteiller P. Human decidual NK cells: unique and tightly regulated effector functions in healthy and pathogen-infected pregnancies. Front Immunol. 2013;4:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207(10):2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]