Preexisting vacuoles are reprogrammed to give rise to protein storage vacuoles.
Abstract
Protein storage vacuoles (PSV) are the main repository of protein in dicotyledonous seeds, but little is known about the origins of these transient organelles. PSV are hypothesized to either arise de novo or originate from the preexisting embryonic vacuole (EV) during seed maturation. Here, we tested these hypotheses by studying PSV formation in Arabidopsis (Arabidopsis thaliana) embryos at different stages of seed maturation and recapitulated this process in Arabidopsis leaves reprogrammed to an embryogenic fate by inducing expression of the LEAFY COTYLEDON2 transcription factor. Confocal and immunoelectron microscopy indicated that both storage proteins and tonoplast proteins typical of PSV were delivered to the preexisting EV in embryos or to the lytic vacuole in reprogrammed leaf cells. In addition, sectioning through embryos at several developmental stages using serial block face scanning electron microscopy revealed the 3D architecture of forming PSV. Our results indicate that the preexisting EV is reprogrammed to become a PSV in Arabidopsis.
During seed development, protein reserves and minerals are stored in specialized vacuoles called protein storage vacuoles (PSV). PSV are functionally different from lytic vacuoles (LV), which are present in most vegetative plant tissues and function as lysosome-like, degradative organelles (Marty, 1999; De, 2000).
PSV exist in both monocots and dicots but are the main site of storage protein accumulation in dicotyledonous species. In spite of the global importance of PSV as primary repositories for storage proteins, very little is known about their origins. It is debated whether they arise de novo during seed maturation or whether they derive from the vacuoles present in embryo cells, henceforth named embryonic vacuoles (EV), which undergo a functional reprogramming during seed maturation. Whatever the intracellular origin of PSV, a specific PSV developmental program must exist, as the simple overexpression of seed storage proteins in nonseed organs is not sufficient to either induce PSV formation or change the function of the existing LV (Bagga et al., 1992; Frigerio et al., 1998, 2000). PSV have been shown to arise by a de novo mechanism in cotyledons of developing pea (Pisum sativum), and a similar mechanism may operate in Medicago truncatula embryos (Hoh et al., 1995; Frigerio et al., 2008). However, there have been few studies addressing the early stages of PSV formation in Arabidopsis (Arabidopsis thaliana; Mansfield and Briarty, 1992).
In Arabidopsis cells, an EV is present during early embryogenesis (Rojo et al., 2001; D’Ippólito et al., 2017). During the maturation phase of seed development, PSV arise to accumulate storage reserves and ultimately become the only detectable vacuole (Mansfield and Briarty, 1992; Otegui et al., 2006). PSV persist in embryonic cells until seed germination, when reserves are mobilized to provide nutrients for the growing seedling. The PSV-to-LV transition during germination has been studied in tobacco (Nicotiana tabacum) root tip cells: LV were shown to arise by a reprogramming and fusion of PSV (Zheng and Staehelin, 2011). PSV tonoplast markers also are replaced by LV markers in both tobacco and Arabidopsis seedling cells (Gattolin et al., 2011; Zheng and Staehelin, 2011). It is not yet clear whether similar reprogramming mechanisms apply to PSV formation during seed maturation in Arabidopsis.
The study of PSV formation is challenging, as PSV are transient organelles and the EV-to-PSV transition occurs relatively quickly (Mansfield and Briarty, 1992). Genetic approaches have not pinpointed the actual formation of PSV. Mutants of the ESCRT component FREE1/FYVE1 (Gao et al., 2015; Kolb et al., 2015), the SNARE proteins VTI11 and VTI12 (Sanmartin et al., 2007; Zheng et al., 2014), the VAMP727 SNARE complex (Ebine et al., 2008), the δ-subunit of the AP-3 adaptor complex (Zwiewka et al., 2011), and the shoot meristem identity protein TFL1 (Sohn et al., 2007), for example, all show disruptions in protein trafficking to PSV rather than in PSV formation. In addition, vacuoleless mutants are embryo lethal (Rojo et al., 2001; D’Ippólito et al., 2017). Finally, there is a shortage of both EV- and PSV-specific markers, which makes it difficult to identify the PSV unequivocally and to distinguish it from the EV or LV, particularly during developmental transitions such as embryo formation or seed germination, respectively.
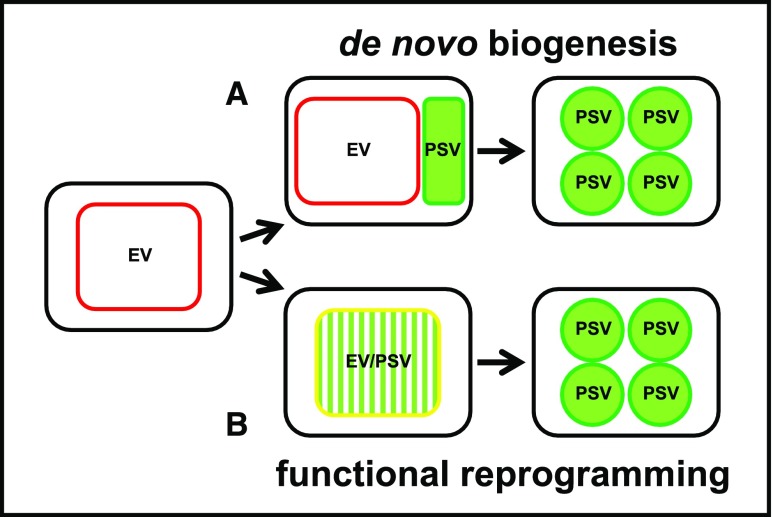
Here, we used a combination of experimental approaches to address the origins of PSV. We produced transgenic Arabidopsis lines expressing fluorescent protein-tagged markers for the tonoplast and the lumen of PSV as well as tonoplast markers for preexisting vacuoles (i.e. EV in embryos and LV in leaves). In addition, we visualized PSV using both fluorescent pH-sensitive stains and inherent PSV autofluorescence. Altogether, these markers were used to observe PSV formation in two experimental systems: embryo cells from maturing seeds and leaf cells reprogrammed toward a seed developmental program by overexpressing LEAFY COTYLEDON2 (LEC2), a master regulator of seed maturation (Stone et al., 2001; Feeney et al., 2013a). These experimental systems were used to explore the two conceptually simplest scenarios of PSV formation (Fig. 1). If PSV arise de novo, it is likely that, as the seed matures, the newly synthesized PSV tonoplast markers will label new compartments, which are separate from the EV. These separate compartments will contain storage proteins (Fig. 1A). If, however, PSV derive from the reprogramming of existing EV, then the PSV membrane markers will label the preexisting tonoplast and will colocalize with the EV tonoplast markers, while storage proteins will appear in the EV lumen (Fig. 1B). We complemented these approaches with immunoelectron microscopy and serial block face scanning electron microscopy (SBF-SEM) to study vacuoles in developing embryos in 3D. Our findings indicate that Arabidopsis PSV arise by the remodeling of preexisting vacuoles rather than by the de novo biogenesis of PSV.
Figure 1.
Hypotheses for PSV formation tested in this work. A, PSV form de novo, briefly coexisting with the EV to eventually become the dominant structure in mature seeds. During the transition, PSV-specific tonoplast markers (green outline) appear alongside the EV tonoplast markers (red outline). B, PSV arise through reprogramming of the EV by the accumulation of seed storage proteins in the EV lumen (green pattern fill). PSV and EV tonoplast markers coexist (yellow outline) while the EV is converted to a PSV.
RESULTS
Timing of PSV Formation
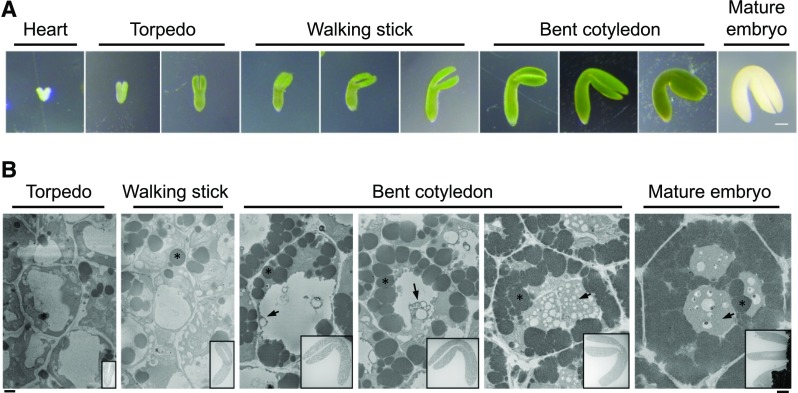
To capture the time during development when PSV, here defined as vacuolar structures containing storage proteins, are formed, we dissected Arabidopsis embryos from developing siliques and assigned them to different stages of maturation (Fig. 2A). The stages we defined here reflect the overall morphology of the embryos after dissection from the ovule: heart for heart-shaped embryos, torpedo for elongated but not yet curved embryos, walking stick for all embryos with cotyledons bent less than 90° relative to the radicle, and bent cotyledon for all further developed green embryos (Fig. 2A). We further divided the walking stick and bent cotyledon stages into early, mid, and late substages. Mature embryos were dissected from dry seeds after imbibing them in water for approximately 3 h.
Figure 2.
Stages assigned to Arabidopsis embryo development and an overview of PSV formation in a representative cell from selected stages. A, Images of heart, torpedo, walking stick, and bent cotyledon embryos dissected from Arabidopsis ovules. The mature embryo was dissected from a dry seed. Bar = 100 µm. B, PSV arise during the maturation phase of embryonic development. Images shows single micrographs from SBF-SEM stacks of embryos at different developmental stages. Deposits of electron-opaque material (arrows) are first observed in the vacuole lumen and along the tonoplast of the EV in early-bent cotyledon embryos. The deposits accumulate and eventually fill the vacuolar lumen in mature seed. Asterisks indicate oil bodies. Bar at left for high-magnification images = 1 µm; bar at right for inset overview images = 100 µm.
For an initial analysis of the embryo ultrastructure, embryos were fixed for transmission electron microscopy (TEM). The EV is clearly visible as a transparent structure in torpedo and walking stick embryos (Fig. 2B). However, at the early-bent cotyledon stage, electron-opaque material begins to appear within the EV, and this material gradually fills the vacuolar lumen in mid- and late-bent cotyledon embryos (Fig. 2B). In agreement with Mansfield and Briarty (1992), we estimate that PSV formation from an empty EV to a full PSV takes approximately 5 d. Therefore, within this time frame, we focused on the transition between late-walking stick and bent cotyledon stages, which encompasses the onset of storage protein deposition. For consistency, we chose to study cotyledon cells for both electron microscopy (EM) and live cell imaging.
During the most critical time of PSV formation, the overall morphology of the bent cotyledon embryos does not change significantly (Fig. 2). Therefore, we rely on PSV characteristics to evaluate the stage of PSV formation. EM shows the gradual accumulation of electron-opaque material in the vacuole and indicates the stage of PSV formation. Likewise, confocal microscopy of fluorescent protein-tagged tonoplast markers reveals vacuole morphologies that characterize forming PSV. With the large numbers of embryos imaged by confocal microscopy, we noted that particular events occurring during the vacuole transition often could not be pinned down to a specific developmental substage, even among bent cotyledon embryos isolated from the same plant. This variability has been observed previously (Mansfield and Briarty, 1992). Consequently, for confocal analysis, we often avoided assigning an event to a particular substage of bent cotyledon embryos.
PSV Tonoplast Markers Colocalize with Tonoplast Markers Labeling the Preexisting EV in Developing Embryos
We used the temporal expression patterns of a panel of known vacuolar markers to identify, and distinguish between, the EV and PSV. As a first step to determine whether PSV are formed de novo or by a reprogramming of the preexisting vacuole, we used confocal microscopy to localize two PSV-specific aquaporins, Tonoplast Intrinsic Protein3;1 (TIP3;1) and TIP3;2 (Gattolin et al., 2011). The expression of both TIP3 isoforms is restricted to seeds and begins in bent cotyledon stage embryos, which makes them suitable PSV markers (Johnson et al., 1990; Jauh et al., 1999; Hunter et al., 2007; Gattolin et al., 2011). To visualize the EV in late-walking stick to early-bent cotyledon embryos, we imaged the 35S-driven expression of known tonoplast proteins: TPK1-GFP (Voelker et al., 2006; Maîtrejean et al., 2011), VHA-a3-mRFP (Brux et al., 2008), and TIP1;1-RFP (Gattolin et al., 2009). These constitutively expressed markers are present on the EV tonoplast before the TIP3 PSV markers are expressed (Fig. 3, A–D); thus, we believe that this is a viable approach to study the EV-to-PSV transition.
Figure 3.
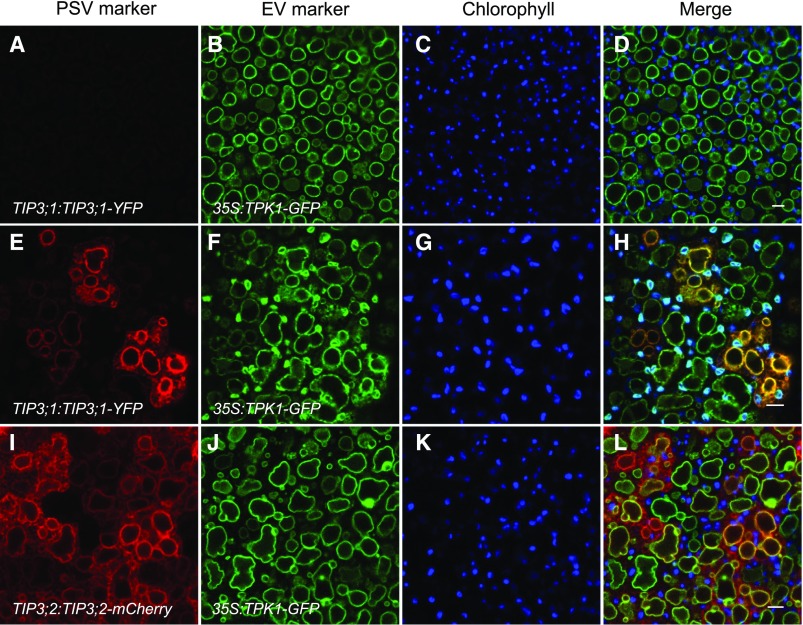
PSV tonoplast markers appear on the preexisting EV tonoplast in bent cotyledon embryos. Embryos constitutively expressing the EV tonoplast marker 35S:TPK1-GFP (green) and the PSV tonoplast markers (red) TIP3;1:TIP3;1-YFP (top and middle rows) or TIP3;2:TIP3;2-mCherry (bottom row) are shown. Chlorophyll autofluorescence is shown in blue. A to D, In early-bent cotyledon embryos, the EV is labeled with TPK1-GFP before PSV markers are expressed. E to L, In late-bent cotyledon embryos, PSV tonoplast markers colocalize with the EV tonoplast marker. Bars = 5 μm.
We imaged embryos in early- and late-bent cotyledon stages expressing either TIP3;1-YFP or TIP3;2-mCherry, under the control of their native promoters, in combination with 35S:TPK1-GFP (Maîtrejean et al., 2011; Fig. 3). Constitutively expressed TPK1-GFP is visible in all bent cotyledon embryos and labels the EV membrane before TIP3;1-YFP or TIP3;2-mCherry markers are observed (Fig. 3, A–D). Once PSV markers begin to appear, they label the same membrane as TPK1-GFP, as indicated by the colocalization of both markers (Fig. 3, E–L). It should be noted that, during embryo development, chlorophyll autofluorescence from plastids often is detectable in the GFP/YFP emission channel. To distinguish our GFP- and YFP-labeled makers from plastids, a separate channel for chlorophyll autofluorescence is shown (Fig. 3, C, G, and K). At no stage were we able to visualize, at least at the resolution and sensitivity of the confocal microscope, TIP3;1-YFP- or TIP3;2-mCherry-labeled structures that were distinct from the EV tonoplast.
Seed Storage Proteins Accumulate in the EV Lumen in Developing Embryos
If the PSV tonoplast markers localize to the EV membrane, we then hypothesized that seed storage proteins also would accumulate in the lumen of the vacuole labeled by both EV and PSV tonoplast markers. Therefore, we studied an Arabidopsis line coexpressing TIP3;2-mCherry and the seed storage protein 2S1 albumin fused to GFP (2S1-GFP), both driven by their endogenous promoters. When first detectable in early-bent cotyledon embryos, 2S1-GFP is located both in punctate structures in the cytosol and within the lumen of the existing vacuoles (Fig. 4, A–D). At the same time, the TIP3;2-mCherry signal labels the tonoplast but also the endoplasmic reticulum (ER) and the plasma membrane, as observed previously (Gattolin et al., 2011). We reasoned that the 2S1-GFP punctate structures could be prevacuolar compartments/multivesicular bodies (PVC/MVB) on account of their size and distribution, as described previously (Miao et al., 2008). Staining with FM4-64 can be used to study membrane trafficking events in plant cells. The dye inserts into the outer leaflet of the plasma membrane and is thought to enter the secretory pathway by endocytosis (Bolte et al., 2004). As FM4-64 is endocytosed, it is transported to the trans-Golgi network/early endosome, where it is sorted to the MVB (Tse et al., 2004; Dettmer et al., 2006; Ebine et al., 2008; Viotti et al., 2010) along with newly synthesized proteins, such as 2S albumins (Otegui et al., 2006; Miao et al., 2008). MVB then fuse with the vacuole to release their contents (Otegui et al., 2006; Ebine et al., 2008; Scheuring et al., 2011). Long-term staining of embryos with FM4-64 revealed the localization of 2S1-GFP-labeled punctate structures to subdomains of larger structures labeled by FM4-64 (Supplemental Fig. S1A). Using the high-resolution Zeiss Airyscan detector, the FM4-64-stained structures that associated with the punctate 2S1-GFP signals appeared brighter than other cellular structures (Supplemental Fig. S1A). Additionally, 2S1-GFP puncta no longer associating with the FM4-64-stained structures were observed to accumulate inside vacuolar lumina (Supplemental Fig. S1B), implying that the 2S1-GFP cargo was delivered to the vacuole. At no point were the 2S1-GFP puncta seen to fuse or accumulate into a separate compartment other than EV lumina. Taken together, these observations suggest that the 2S1-GFP puncta that associate with larger FM4-64-labeled structures are likely to be PVC/MVB.
Figure 4.
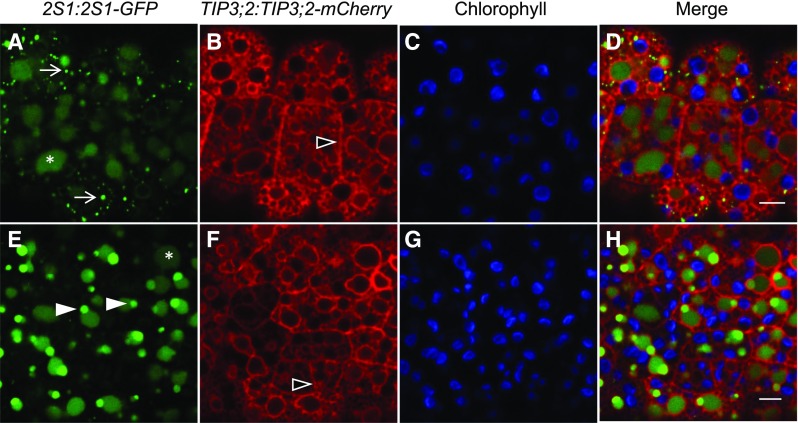
2S1 albumin seed storage proteins accumulate initially in punctate cytosolic structures and ultimately as deposits inside the lumina of forming PSV in bent cotyledon embryos. 2S1-GFP (green) labels small punctate structures (arrows) that accumulate in the EV/PSV lumen (asterisk). The PSV tonoplast marker TIP3;2-mCherry (red) is localized to the ER, tonoplast, and plasma membrane (open arrowheads). Chlorophyll is shown in blue. A to D, 2S1-GFP signal is first observed as small punctate structures in the cytoplasm and accumulates in PSV lumina whose tonoplasts are labeled with TIP3;2-mCherry. E to H, In late-bent cotyledon embryos, the 2S1-GFP signal is observed only in vacuole lumina. Subregions of more intense 2S1-GFP fluorescence are visible in PSV lumina (closed arrowheads). Bars = 5 µm.
In late-bent cotyledon embryos of the Arabidopsis line coexpressing TIP3;2-mCherry and 2S1-GFP, the TIP3;2-mCherry signal becomes more visible on the tonoplast while 2S1-GFP no longer labels punctate structures and is observed only within the lumina of vacuoles (Fig. 4, E–H). Within the vacuoles, subregions of more intense 2S1-GFP signal are observed (Fig. 4, E–H, arrowheads). We also were able to observe the combined arrival of a PSV tonoplast marker and luminal marker to the EV in a triple transgenic line where the EV was labeled by 35S:TIP1;1-RFP (Supplemental Fig. S2). In agreement with our previous results (Fig. 3, E–L), EV (TIP1;1-RFP) and PSV (YFP-TIP3;1) tonoplast markers were located on the same membrane (Supplemental Fig. S2). At the same time, the PSV luminal marker (2S1-GFP) localized to the lumen of vacuoles labeled with both PSV and EV markers. Taken together, our fluorescent protein-tagged PSV markers consistently associate with the vacuole labeled by our EV markers, suggesting that PSV originate from the preexisting EV.
PSV Autofluorescence and pH-Sensitive Fluorescent Dyes Are Detected within the EV
Due to the limited choice of markers available to label EV and PSV, we took advantage of the PSV’s affinity for staining with pH-sensitive fluorescent dyes (Hara-Nishimura et al., 1987; Otegui et al., 2006; Gattolin et al., 2011) and their inherent luminal autofluorescence (Fuji et al., 2007; Hunter et al., 2007; Feeney et al., 2013b) to map the formation of PSV. Bent cotyledon embryos were stained with two acidotropic dyes: Neutral Red (NR) and 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM). At neutral pH, these stains pass freely through membranes in their unprotonated forms, but protonation in acidic compartments reduces their permeability and leads to their accumulation (Dubrovsky et al., 2006; Scheuring et al., 2015).
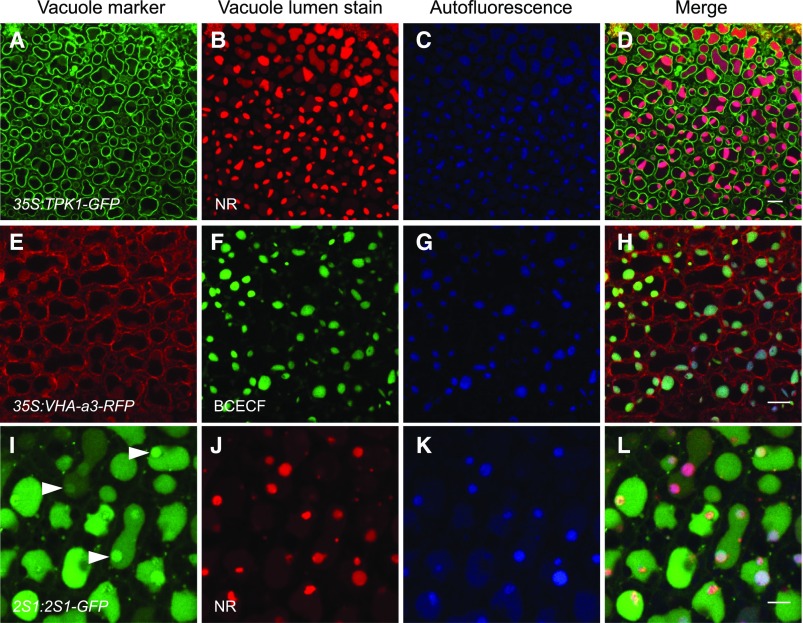
Both stains accumulated and fluoresced within vacuoles or subregions of the vacuoles whose tonoplasts were labeled with constitutively expressed TPK1-GFP and VHA-a3-RFP (Fig. 5, A–H). PSV autofluorescence was associated with the staining patterns (Fig. 5, C, G, and K). To investigate whether the staining patterns are associated with the accumulation pattern of seed storage proteins, bent cotyledon embryos expressing 2S1-GFP were stained with NR. We observed the 2S1-GFP signal to disperse within the entire vacuole lumen (Fig. 5, I–L), as also shown in Figure 4. However, bright areas of NR stain and PSV autofluorescence were both observed to colocalize with areas of more intense 2S1-GFP fluorescence in subregions of the vacuoles (Fig. 5, I–L). These intensely fluorescent 2S1-GFP subregions also were seen without NR staining, as shown in Figure 4.
Figure 5.
Forming PSV are identified by the acidotropic stains NR and BCECF-AM and PSV luminal autofluorescence in bent cotyledon embryos. A to H, NR and BCECF-AM stain vacuoles labeled with the tonoplast markers TPK1-GFP (A) and VHA-a3-RFP (E), respectively. Vacuole lumen autofluorescence (blue) colocalizes with the stains (D and H). I to L, Embryos accumulating 2S1 albumin-GFP were stained with NR. The 2S1-GFP signal fills vacuole lumina, and areas of more intense GFP fluorescence are observed (arrowheads in I). NR stains distinct subregions of the vacuole lumina (J). These NR-stained subregions colocalize with PSV lumen autofluorescence (K) and with areas of intense 2S1-GFP fluorescence (L). Bars = 10 μm (A–H) and 5 μm (I–l).
To further investigate the pattern of staining and the occurrence of PSV autofluorescence, bent cotyledon embryos expressing the PSV-specific tonoplast marker YFP-TIP3;1 under the control of its native promoter were stained with NR. As PSV form from the large, round EV (Supplemental Fig. S3A) and remodel to assume the characteristic mature PSV morphology (Supplemental Fig. S3, B and C), NR staining becomes more prominent. The stain initially accumulates in discrete zones within the vacuolar lumen (Supplemental Fig. S3A) to then eventually stain the entire lumen in late-bent cotyledon embryos (Supplemental Fig. S3C). At no point are the stains observed to accumulate in structures other than vacuoles labeled by EV (Fig. 5, A–H) or PSV (Supplemental Fig. S3) tonoplast markers. A similar pattern is observed for PSV autofluorescence in forming PSV (Supplemental Fig. S3, D–F). Taken together, these data indicate that the accumulation of both acidotropic stains and the inherent PSV autofluorescence associate with the EV reprogramming to become the PSV.
PSV Tonoplast Markers and Seed Storage Proteins Label a Transitioning EV
Our light microscopy observations were corroborated by immunoelectron microscopy to identify where PSV marker proteins begin to accumulate. For embryos prepared for immunogold labeling, the EV is characterized by a large, translucent lumen in torpedo, walking stick, and early-bent cotyledon stage embryos (Fig. 6, A, C, and E). However, unlike routine EM (Fig. 2B), specimens for immunogold labeling were not fixed with osmium. Oil bodies (which, due to the lack of osmium in this preparation, also appear translucent) were differentiated from the EV by their rather uniform size, round shape, and lack of flocculent luminal material.
Figure 6.
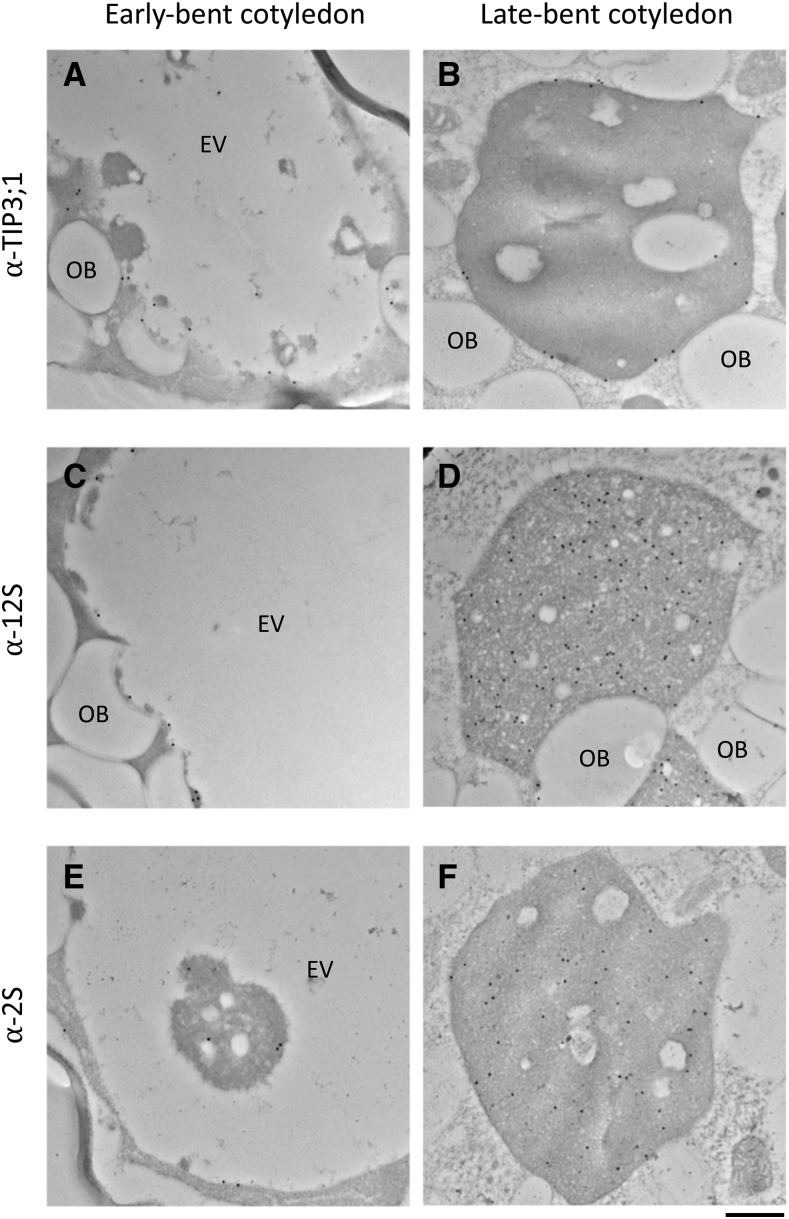
Immunogold labeling reveals the localization of PSV tonoplast aquaporin TIP3;1 and the 2S albumin and 12S globulin seed storage proteins to the EV in bent cotyledon embryo cells. A and B, Anti-TIP3;1 antibody labels the tonoplast of transitioning vacuoles. C and D, Anti-12S globulin antibody labels electron-opaque material accumulating along the luminal side of the tonoplast (C) and the entire PSV lumen in late-bent cotyledon embryos (D). E and F, Anti-2S antibody labels electron-opaque material accumulating along the luminal side of the tonoplast (E) as well as electron-opaque material in the vacuole lumen (F). OB, Oil bodies. Bar = 500 nm.
Antibodies against TIP3;1 as well as 2S albumin and 12S globulin seed storage proteins showed no significant gold labeling in embryos from heart to walking stick stages (Supplemental Fig. S4). In early-bent cotyledon embryos, anti-TIP3;1 antiserum labeled the tonoplast of the large, translucent EV (Fig. 6A), and anti-2S and anti-12S were detected along the inner periphery of the tonoplast and on electron-opaque material within the lumen of the vacuole (Fig. 6, C and E). In late-bent cotyledon embryos, the lumen of the maturing PSV is completely filled with electron-opaque material (Fig. 2B) and shows a high density of gold labeling with anti-TIP3;1, anti-12S, and anti-2S antibodies (Fig. 6, B, D, and F). To allow a quicker visualization of the distribution of the gold particles, we highlighted them on transparent versions of the micrographs (Supplemental Fig. S5). These results support our confocal microscopy observations that suggest that TIP3 isoforms and seed storage proteins appear at the tonoplast and in the lumen of the EV, respectively (Fig. 4; Supplemental Fig. S5).
In addition, the electron-opaque material accumulating along the periphery and dispersed within the EV/PSV lumen was labeled by anti-complex glycan antiserum (Laurière et al., 1989), confirming that at least a proportion of PSV glycoproteins underwent N-glycan processing in the Golgi (Supplemental Fig. S6).
PSV Form through Remodeling of the LV in Leaf Cells Reprogrammed by LEC2
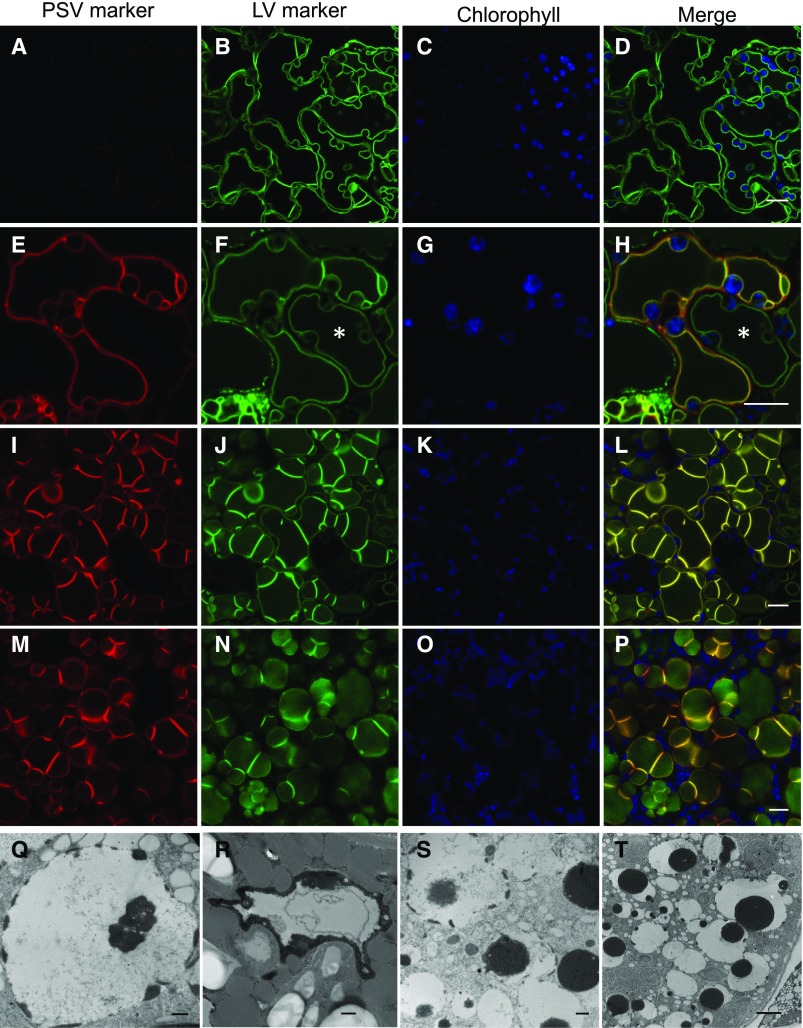
Our data so far indicate that PSV are formed in embryos by the repurposing of the preexisting vacuole. In order to test whether this functional transition could be recapitulated in a system where the only vacuole present is the LV, we investigated the formation of PSV in leaves reprogrammed to follow a seed developmental pathway by the overexpression of LEC2 (Feeney et al., 2013b). LEC2 is a key transcriptional regulator of seed development and, when overexpressed in vegetative tissues, causes cells to change their developmental pathway and acquire characteristics of maturation phase embryos. We showed previously that, in this system, leaf LV are replaced by PSV (Feeney et al., 2013a). However, the details of the LV-to-PSV transition were not explored. Therefore, with a similar strategy to that used for embryos as described above, we imaged leaf cells of Arabidopsis 35S:LEC2-GR lines where the LV was labeled with a constitutively expressed tonoplast marker, TPK1-GFP, and observed the localization of the PSV marker TIP3;1-YFP, expressed under the control of its native, seed-specific promoter (Fig. 7, A–P). After 14 d with dexamethasone (DEX) treatment to induce LEC2 overexpression (Fig. 7, A–D), the PSV marker is not yet detectable while the TPK1-GFP marker labels the LV tonoplast. Between 15 and 20 d with DEX, the PSV marker becomes detectable and is observed to colocalize to the LV marker on the same tonoplast (Fig. 7, E–P), indicating that the LV is reprogrammed to become a PSV. During the LV-to-PSV transition, leaf LV morphology changes from a large central compartment that mirrors the cell’s shape (Fig. 7, A–D) to smaller vacuoles that are more round in shape, resembling PSV (Fig. 7, M–P). In addition, the images show an asynchronous remodeling of tonoplast in neighboring cells; LV highlighted solely with TPK1-GFP lack tonoplast folds (Fig. 7, E–H, asterisks). However, adjacent vacuoles accumulating both TPK1-GFP and TIP3;1-YFP possess brightly fluorescent tonoplast folds, as reported by Feeney et al. (2013a, 2013b).
Figure 7.
PSV form through remodeling of the LV in Arabidopsis leaf cells reprogrammed by LEC2. A to P, Representative images of transitioning vacuoles in LEC2-induced leaf cells at 14 d (A–D), 17 d (E–H), and 20 d (I–P) with DEX. The TIP3;1-YFP PSV tonoplast marker (red) accumulates on the preexisting LV (E, I, and M) in Arabidopsis lines constitutively expressing the tonoplast marker TPK1-GFP (green). Chlorophyll autofluorescence is shown in blue. Asterisks show asynchronous remodeling of the tonoplast in neighboring cells. Bars = 10 μm. Q to T, Electron microscopy of leaf cells 14 d after LEC2 induction with DEX. Electron-opaque PSV material (black) accumulates along the luminal side of the tonoplast and disperses in the vacuole lumen. Bars = 500 nm (Q–S) and 2 μm (T).
In a similar manner to developing embryos, LEC2-induced leaf cells accumulate PSV material in the LV/PSV (compare Fig. 7, Q–T, with Figs. 2B and 6). TEM of leaf cells from 35S:LEC2-GR plants grown in the presence of DEX for 14 d provides a representation of the LV-to-PSV transition (Fig. 7, Q–T). Electron-opaque material accumulates along the inner periphery of the LV tonoplast and is observed to disperse within the vacuole lumen. These electron-opaque deposits were shown previously to contain 12S globulin and 2S albumin seed storage proteins by immunogold labeling (Feeney et al., 2013a).
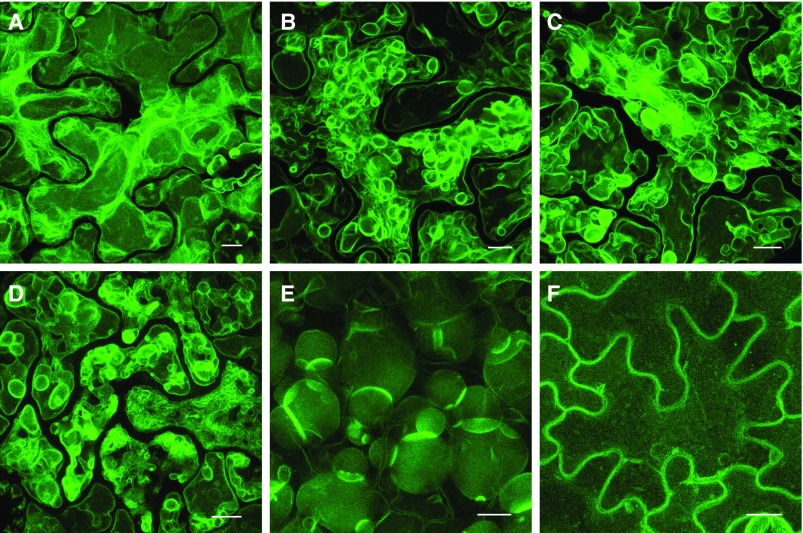
The LV-to-PSV transition occurs over approximately 5 d in LEC2-induced leaf cells, as shown in Figure 7. To visualize the highly dynamic nature of the vacuole remodeling occurring over this time course, optical sections were taken through leaf cell vacuoles of LEC2-induced plants harboring 35S:TPK1-GFP. Maximum intensity projections of representative images are shown in Figure 8. At approximately 14 d on DEX, LV appear normally shaped (Fig. 8A) but possess more transvacuolar strands than control leaf cells without DEX (Fig. 8F). However, as the LV transitions to a PSV, the tonoplast undergoes extensive remodeling (Fig. 8, B–D). At approximately 20 d on DEX, vacuoles are more round in shape and resemble PSV (Fig. 8E). A time series taken from a representative cell during this transition stage reveals fast tonoplast remodeling, compared with minimal remodeling in noninduced cells (Supplemental Fig. S7; Supplemental Movies S1 and S2).
Figure 8.
The tonoplast undergoes extensive remodeling during the LV-to-PSV transition in LEC2-induced leaf cells. Representative images show the progression of tonoplast remodeling during the LV-to-PSV transition at 14 d (A), 17 d (B and C), and 20 d (D and E) with DEX or at 14 d without DEX (F). Arabidopsis 35S:LEC2-GR lines harboring 35S:TPK1-GFP (green) were imaged. Images are maximum intensity projections of Z-stacks taken through leaf epidermal cells. Bars = 10 µm.
PSV Formation Involves Extensive Remodeling of the EV
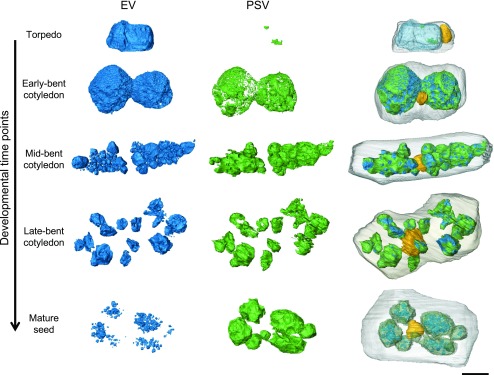
Embryo cells contain one large EV at the torpedo stage. However, in mature embryo cells, there appear to be multiple, smaller PSV present (Fig. 2B). To better understand the EV-to-PSV transition, we used SBF-SEM to obtain 3D data sets of embryos at different developmental stages. By serially imaging the block faces cut at 100-nm intervals through whole cells with high-resolution SEM, we were able to acquire image stacks of entire cells and reconstruct the entire vacuolar system in developing embryos. For comparison, we chose cells in the cotyledon tip of embryos from torpedo, early-, mid-, and late-bent cotyledon stages and from a mature seed (Supplemental Fig. S8). We reconstructed the translucent lumen of the EV, electron-opaque PSV material composed of seed storage proteins (according to our antibody labeling; Fig. 6), the nucleus, and the cell membrane (Fig. 9). For the original data sets and animated reconstructions, see Supplemental Movies S3 to S10.
Figure 9.
Remodeling of the EV to PSV during embryo maturation. The lumen of the EV (blue), electron-opaque PSV luminal material (green), nucleus (yellow), and plasma membrane (gray) were rendered from SBF-SEM image stacks in cotyledon cells of embryos in the torpedo, early-, mid-, and late-bent cotyledon, or mature embryo stages. As electron-opaque material initially accumulates along the periphery of the EV lumen, the vacuole separates into several smaller vacuoles, which are eventually filled with electron-opaque PSV luminal material aside from small translucent areas. The nucleus changes position from the cell cortex to the center of the cell. Bar = 5 μm.
During seed maturation, storage proteins (Fig. 9, green) increasingly accumulate along the periphery of the EV lumen (Fig. 9, blue) and take up an increasing proportion of the vacuolar volume (Supplemental Fig. S9). At the same time, the large EV divides into smaller vacuoles, which are eventually filled with storage protein. In the mature seed, the EV has been replaced by multiple PSV. Electron-opaque PSV lumina possessed small, translucent, and partly crystalline inclusions (Fig. 2B; Supplemental Fig. S9). These inclusions often are identified as globoids (Jiang et al., 2001). To maintain consistency in our reconstructions, these translucent inclusions were considered to be EV (Fig. 9; Supplemental Fig. S9), although we are unable to speculate on their ontology. Additionally, as the embryo matures, the nucleus changes position from the cortex to the center of the cell (Fig. 9, yellow). These snapshots of PSV formation during embryo development, captured in 3D using SBF-SEM, provide further evidence that the PSV originates from the preexisting EV.
DISCUSSION
Our data provide a dynamic picture of PSV formation during seed maturation and indicate that, in Arabidopsis embryo cells, PSV do not arise de novo but result from the repurposing of the preexisting EV. While it has been reported previously that mature seeds only appear to contain PSV (Otegui et al., 2006; Hunter et al., 2007), the events leading to the formation of PSV in Arabidopsis have not been documented previously.
To establish where PSV originate in Arabidopsis embryo cells, we used lines expressing fluorescently labeled vacuolar markers, acidotropic stains, PSV autofluorescence, high-resolution Zeiss Airyscan confocal laser scanning microscopy detection, SBF-SEM, and immunoelectron microscopy. According to our definition of PSV as vacuolar structures that contain seed storage proteins, our data indicate that PSV formation begins between the late-walking stick and early-bent cotyledon stages (Fig. 2B).
PSV formation involves an accumulation of electron-opaque material in the lumen of the preexisting vacuole. The elemental composition of the electron-opaque material accumulating in PSV has been characterized by means of energy-dispersive x-ray spectroscopy analysis by Zheng and Staehelin (2011) and Otegui et al. (2002). The principal components of the spectrum were potassium, calcium, magnesium, as well as phosphorus, which was characterized as phytate (myoinositol-hexakisphosphate). Our immunogold labeling shows that seed storage proteins (Fig. 6; Supplemental Fig. S5) and glycoproteins that have trafficked through the Golgi (Supplemental Fig. S6) also are found within the electron-opaque material. Similarly, the seed storage protein vicilin and barley (Hordeum vulgare) lectin were detected in electron-opaque material in transitioning pea and barley vacuoles (Hoh et al., 1995; Olbrich et al., 2007) as well as 12S globulins and 2S albumins in LV transitioning to PSV in leaf cells reprogrammed by LEC2 overexpression (Feeney et al., 2013a). Zheng and Staehelin (2011) distinguished between vacuoles undergoing PSV-to-LV transitions in tobacco root meristematic cells by the presence of electron-opaque PSV material using TEM. We similarly observed the appearance of electron-opaque deposits during the EV-to-PSV and LV-to-PSV transitions in embryos and leaves reprogrammed by LEC2 overexpression, respectively.
Nascent vacuolar proteins traffic from the ER to their ultimate destination in the EV/PSV. Throughout the vacuolar transition, we observed trafficking of the marker proteins. As PSV form, tonoplast markers, particularly the TIP isoforms, are observed in the ER en route to the tonoplast (Fig. 4; Supplemental Fig. S2). Similarly, the seed storage protein 2S1 albumin-GFP is observed as punctate structures at its earliest time of detection (Fig. 4, A–D; Supplemental Fig. S1). Seed storage proteins are sorted at the Golgi apparatus into dense vesicles that fuse with other small vesicles carrying proteolytic enzymes to give rise to PVC/MVB (Otegui et al., 2006). These organelles function as prevacuolar compartments in the secretory pathway and ultimately fuse with the vacuole (Ebine et al., 2008; Scheuring et al., 2011). Therefore, the punctate 2S1-GFP structures that we observe during early PSV formation are likely to be PVC/MVB, as described previously (Ebine et al., 2008; Miao et al., 2008).
Mature PSV can be identified by the accumulation of acidotropic fluorescent stains and PSV autofluorescence in their lumina (Fuji et al., 2007; Hunter et al., 2007; Feeney et al., 2013b), although we do not yet know what is contributing to PSV autofluorescence. We show here that NR, BCECF-AM, and PSV autofluorescence can be used to identify forming PSV (Fig. 5; Supplemental Fig. S3). Within early PSV lumina, the colocalization of NR, PSV autofluorescence, and 2S1-GFP fluorescence into distinct, brighter areas suggest the existence of subregions in the matrix of the transitioning vacuole during seed filling (Fig. 5, I–L). A similar observation was made in vacuoles of developing pumpkin (Cucurbita maxima) cotyledons stained with NR (Hara-Nishimura et al., 1987). In our study, these distinct areas were first observed to accumulate in the EV/PSV lumen as small, highly fluorescent regions that increased in volume, eventually filling the PSV lumen (Supplemental Fig. S3). Looking at cross sections of our 3D SBF-SEM data sets, we can sometimes observe a similar accumulation of electron-opaque material in one area of the EV, potentially representing such a subregion (Supplemental Fig. S9, early-bent cotyledon). It will be interesting to explore the nature of these EV/PSV subregions and how they relate to the biogenesis of the PSV as a compound organelle (Jiang et al., 2000, 2001).
Once we understood that PSV originate from EV in developing embryos, we documented how a single EV, occupying nearly the entire cell volume, transitions to become a collection of numerous, smaller-sized PSV within the mature embryo cell. SBF-SEM allowed us to image the entire embryo to visualize the 3D organization of cells and tissues and, likewise, the spatial organization of vacuolar structures in those cells. Reconstructions from SBF-SEM data of cotyledon cells from one embryo each of torpedo, early-, mid-, and late-bent cotyledon stages and a mature embryo (Fig. 9; Supplemental Fig. S9) provide snapshots of how EV remodel to form PSV. In mature seeds, PSV exist as a group of separate, individual entities that do not appear to form an interconnected network, as suggested previously by light microscopy data (Hegedus et al., 2015).
The biogenesis of PSV has been a long-standing topic of debate. In cotyledon cells of developing pea embryos, an accumulation of electron-opaque material was observed within a tube-like, membrane-bounded structure surrounding the EV during embryo development. PSV and EV membranes were labeled with anti-TIP3;1 and anti-TIP1;1 antibodies, respectively (Hoh et al., 1995). Altogether, these results supported a de novo mechanism for PSV development. Therefore, if nascent PSV arise separately from the preexisting vacuole, we may anticipate their respective tonoplasts to possess unique TIP isoform markers and, more importantly, seed storage proteins should accumulate in the lumen of the PSV and not within the lumen of the preexisting vacuole. Others have reported TIP1;1-labeled internal membranes in PSV lumina (Gillespie et al., 2005; Bolte et al., 2011). In Arabidopsis embryos, however, we were unable to detect TIP1;1 accumulation on the EV tonoplast (data not shown), and our EV and PSV tonoplast markers appear to colocalize on the same EV membrane (Fig. 3). This is in agreement with previous results in Arabidopsis embryo cells (Otegui et al., 2006; Gattolin et al., 2011) and in PSV forming in leaf cells reprogrammed by LEC2 (Feeney et al., 2013a). Moreover, we consistently observe seed storage proteins accumulating in the lumen of the vacuole labeled by both EV and PSV tonoplast and luminal markers. Therefore, we reason that, in Arabidopsis, PSV arise by a remodeling of the preexisting vacuole.
In Arabidopsis, the presence of adjacent tonoplasts within embryo cells was observed in single sections (Frigerio et al., 2008), potentially suggesting the presence of separate vacuoles. Our study, which covers several developmental time points during PSV biogenesis across whole cellular volumes, shows that extensive membrane remodeling occurs during seed maturation. Therefore, it is possible that the multiple membranes observed at a fixed point may reflect tonoplast remodeling rather than the presence of a separate structure. Upon close inspection of our TEM data, we did not observe a membrane surrounding the storage protein aggregates forming in bent cotyledon embryos or transitioning vacuoles of LEC2-induced leaf cells (Supplemental Fig. S10). In both cases, the tonoplast appears as a clearly defined interface surrounding the outer periphery of the protein aggregates. However, the inner periphery of these aggregates, which faces the vacuole lumen, appears fuzzy and poorly defined, which does not suggest that it is membrane bounded. In addition, immunolabeling of transitioning LEC2-induced leaf vacuoles with an anti-TIP3;1 antiserum shows that the PSV membrane does not enclose the seed proteins but is localized solely to the tonoplast of the preexisting LV (Feeney et al., 2013a; Supplemental Fig. S10). Therefore, our results do not support the hypothesis that the PSV arise by a de novo structure but more strongly support a remodeling mechanism for PSV biogenesis.
This work provides a foundation to further explore the mechanism of PSV biogenesis. During the EV-to-PSV transition, it will be interesting to understand how the EV lumen environment changes to accommodate the influx of seed storage proteins. The overexpression of seed storage proteins targeted to the leaf LV results in degradation of the proteins (Bagga et al., 1992; Frigerio et al., 1998, 2000); however, reprogramming of the LV to a PSV alters the luminal environment to accommodate incoming storage proteins (Feeney et al., 2013a). While it is established that storage proteins are delivered to the nascent PSV by the fusion of MVB (Otegui et al., 2006), additional processes must be at play.
That PSV can arise from both the EV and LV raises some general questions about the relationship between the EV and LV. In lower plants, there is no separation between embryo morphogenesis and postembryonic development (West and Harada, 1993). During the evolution of seed plants, the maturation phase was integrated into embryogenesis to enable plants to interrupt their life cycle (Vicente-Carbajosa and Carbonero, 2005). A network of transcriptional regulators, such as LEC2, are responsible for regulating the activities occurring during this developmental phase (Braybrook and Harada, 2008; Baud et al., 2016), including the formation of PSV (Feeney et al., 2013a, 2013b). Altogether, this suggests that the evolution of seeds has commandeered the existing cellular structures and that, perhaps, the EV and LV are the same vacuole but accommodate a different complement of proteins.
In conclusion, our analysis of the early stages of PSV formation in Arabidopsis suggests that, rather than by a de novo process, PSV arise by functional reprogramming of the EV. While we do not attempt here to elucidate how this change happens, our findings pave the way to understanding the processes underpinning such a developmental change.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) lines used in this study for confocal microscopic analysis were Columbia-0 (Col-0) or lines harboring 35S:TPK1-GFP (Voelker et al., 2006; Maîtrejean et al., 2011), 35S:VHA-a3-mRFP (Brux et al., 2008), 35S:TIP1;1-RFP, TIP3;1:TIP3;1-YFP/TIP1;1:TIP1;1-RFP, TIP3;1:YFP-TIP3;1, and TIP3;2:TIP3;2-mCherry (Gattolin et al., 2009, 2011), and 35S:LEC2-GR (Stone et al., 2008). The 2S1 albumin marker line 2S1:2S1-GFP was modified from Miao et al. (2008). The 2S1-GFP fusion from vector pBI221 (Miao et al., 2008) was PCR amplified with BamHI and EcoRI restriction sites, and the product was ligated into binary vector pCaMterX (Harris and Gleddie, 2001). The 2S1 promoter region was cloned from Col-0 genomic DNA by amplifying 914 bp upstream of the 2S1 coding sequence (At4g27140) with the addition of ClaI and BamHI restriction sites. The 35S promoter was excised from pCaMterX by cutting with ClaI and BamHI and was replaced with the 2S1 promoter region. For experiments, crosses were made for combinations of the above marker lines. For EM studies, Col-0 plants or Wassilewskija-0 plants harboring the 35S:LEC2-GR construct (Stone et al., 2008) were used.
Tissue Culture Conditions and LEC2 Induction
Sterilized seeds were transferred to germination medium consisting of Murashige and Skoog (MS) salts (Sigma-Aldrich) supplemented with full-strength MS vitamins and 0.4 mg L−1 thiamine-HCl, 100 mg L−1 myoinositol, 30 g L−1 Suc, and 0.75% (w/v) agar, pH 5.8. Seeds were stratified for 3 to 4 d at 4°C in the dark and transferred to a growth chamber for germination. The growth chamber was set at 24°C day/22°C night with 70 μmol m−2 s−1 illuminance and with a 16-h-light/8-h-dark photoperiod.
To induce LEC2 overexpression, stratified seeds were allowed to germinate and grow for 7 d on germination medium. Seedlings were then transferred to induction medium composed of MS germination medium supplemented with 30 μm DEX (Sigma-Aldrich). DEX was solubilized in dimethyl sulfoxide. Seedlings were incubated on DEX for up to 21 d before imaging.
Greenhouse Growth Conditions and Embryo Isolation from Siliques
Seedlings germinated in tissue culture were transferred to soil and grown at 20°C day/18°C night with a minimum of 178 μmol m−2 s−1 illuminance and with a 16-h-light/8-h-dark photoperiod for approximately 6 weeks. For confocal imaging, one flower stalk was selected for each line. Siliques (eight to 12) were harvested from the mid to lower region of the flower stalk, closest to the rosette. Using a stereomicroscope, ovules were dissected from siliques and transferred to a 1.5-mL tube. A pestle was used to gently squash the ovules to release embryos. The plant material was then transferred to a petri dish filled with water. Embryos were gently separated from the debris and were classified and sorted according to their developmental stage as described in “Results” and shown in Figure 2A.
Fluorescence and Confocal Laser Scanning Microscopy and Staining
Seeds and seedlings were screened for fluorescence using a Leica MZ FLIII fluorescence stereomicroscope. To observe GFP and YFP fluorescence, a standard GFP filter (excitation, BP480/40 nm; emission, LP510 nm) was used. To observe RFP fluorescence, an RFP filter (excitation, 546/10 nm; emission, LP590 nm) was used.
For confocal microscopy, embryo and leaf samples were observed directly or stained before examination with a Zeiss 880 confocal microscope. All imaging was performed on embryo cotyledon cells. To stain vacuole lumina, embryos were stained for 5 min with 17.5 μm NR (Sigma-Aldrich) or for 30 min with 10 μm BCECF-AM (Molecular Probes). Embryos were washed three times with water before imaging. For long-term staining with FM4-64, embryos were incubated for 1 h in 8 µm FM4-64, washed three times with water, and incubated for a further 2.5 to 3 h in water before imaging. Confocal imaging was performed using a 63× (1.4 numerical aperture) oil-immersion lens. A 405-nm laser line was used to visualize PSV autofluorescence, and 440- to 490-nm emission was collected. A 488-nm laser line was used to excite GFP and BCECF-AM, and emissions were collected as 505 to 540 nm for GFP and 500 to 560 nm for BCECF-AM. A 514-nm laser line was used to excite YFP and FM4-64, and emissions were collected as 525 to 585 nm for YFP and 615 to 645 nm for FM4-64. A 561-nm laser line was used to excite RFP and NR, and emissions were collected as 565 to 640 nm for RFP and 560 to 615 nm for NR. A 630-nm laser line was used to visualize chlorophyll autofluorescence, and 645- to 720-nm emissions were collected. Sequential detection of combinations of the above fluorophores was performed by combining their settings in the frame-scanning mode. Z-stacks were recorded using optimal scan parameters. For Airyscan detection, samples were imaged using a 100× (1.46 numerical aperture) oil-immersion lens. Image processing was performed with Zeiss Zen Lite software. Temporal color coding of time series data was performed using FIJI software.
Immunogold Labeling and TEM
Embryos were isolated from siliques as described above and fixed in 2.5% (v/v) glutaraldehyde and 4% (w/v) paraformaldehyde in 0.1 m sodium phosphate buffer, pH 7.4, at 4°C as described previously (Feeney et al., 2013a). Embryos were dehydrated in a graded ethanol series and then infiltrated in increasing concentrations of LR White resin. Infiltration in pure LR White resin was carried out for 3 d. In the last 24 h of infiltration, 0.5% (w/v) benzoin methyl ether was added as a catalyst for polymerization. Embryos were embedded in flat embedding dishes sealed with Melinex film (Agar Scientific) and polymerized under UV light at −20°C for 24 h followed by 0°C for 24 h.
Specimens were cut into 70-nm-thick sections and collected on nickel mesh grids for immunogold labeling experiments using a PowerTome ultramicrotome (RMC). Sections were blocked with goat normal serum (Aurion) for 30 min followed by 2 h with primary antibodies diluted with dilution buffer (0.2% [v/v] BSA-c [Aurion], 0.05% [v/v] Tween 20, and 1% [w/v] BSA in PBS, pH 7.4). Primary antibodies were rabbit anti-TIP3;1 (1:10; Jauh et al., 1999), rabbit anti-12S globulin (1:500; Shimada et al., 2003), rabbit anti-2S albumin (1:500; Scarafoni et al., 2001), and rabbit anti-complex glycan (1:500; Laurière et al., 1989). Specimens were incubated for 1 h with secondary antibodies diluted 1:10 with dilution buffer. All secondary antibodies were IgGs produced in goats and conjugated to 15-nm gold particles (Aurion). All specimens were stained for 30 min with 4% (w/v) uranyl acetate (UA) and 20 min with lead citrate (Reynolds, 1963) followed by 3 min with 4% (w/v) UA. Specimens were examined with an Hitachi H-7650 transmission electron microscope operating at 100 kV.
Leaves were collected from LEC2-induced plants and were fixed, infiltrated, and embedded in LR Gold resin according to Feeney et al. (2013a). Specimens were cut into 60-nm-thick sections and stained for 10 min with 5% (w/v) UA and for 1 min with lead citrate (Feeney et al., 2013a) and were examined with a CM-10 transmission electron microscope (Philips) operating at 80 kV.
SBF-SEM
Embryos were isolated as described above and fixed in 1% (v/v) glutaraldehyde and 1% (w/v) paraformaldehyde with 2% (w/v) Suc and 2 mm CaCl2 in 0.1 m sodium cacodylate (NaCac) buffer, pH 6.9, for 60 min at room temperature. Embryos were washed in NaCac buffer and then incubated in 1% (w/v) tannic acid in 0.1 m NaCac buffer for 60 min. Embryos were washed with water 3 × 10 min, stained in 1% (w/v) aqueous osmium tetroxide for 2 h at room temperature, washed in deionized water, and dehydrated in a graded ethanol series. Embryos were then infiltrated in gradually higher concentrations of Spurr resin. Infiltration with pure resin was carried out for 3 d, with resin replaced every 24 h. Embryos were embedded in flat dishes, and the resin was polymerized at 70°C for 12 h. Embryos were mounted onto SBF-SEM stubs with conductive adhesive resin as described previously (Kittelmann et al., 2016). Trimmed blocks were gold sputter coated for 30 s (∼20 nm thick). Serial overview images of the entire embryo as well as high-magnification and high-resolution images of the cotyledon tips were collected with a Zeiss Merlin Compact SEM device fitted with a Gatan 3View system. Microscope settings were as follows: 4 kV, 50 Pa at variable pressure mode, and 30-μm aperture. 3View settings were as follows: 100-nm sections, pixel size of ∼0.004 μm, and pixel dwell times of 3 μs for high-magnification images and 2 μs for overview images.
3D Reconstruction
The IMOD software package was used for stack formation, image alignment, trimming, and Gaussian filtering. Rendering of structures of interest was done in Amira Software (FEI) using the magic wand tool to semiautomatically segment the EV and PSV and the brush to manually segment the plasma membrane and nucleus. Labels were modeled using surface generation, and the number of triangles was reduced for visualization. Movies were generated using the Animation tool in Amira.
Accession Numbers
Accession numbers are as follows: AtTPK1, AT5G55630; AtVHA-a3, AT4G39080; AtTIP3;1, AT1G73190; AtTIP3;2, AT1G17810; AtTIP1;1, AT2G36830; At2S1, AT4G27140; and AtLEC2, AT1G28300.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Punctate cytosolic 2S1-GFP-labeled structures associate with FM4-64-stained structures and appear to accumulate in vacuole lumina.
Supplemental Figure S2. Fluorescently labeled PSV tonoplast and lumen markers associate with the preexisting EV in bent cotyledon embryos.
Supplemental Figure S3. NR staining pattern and appearance of PSV autofluorescence in EV/PSV lumina of bent cotyledon embryos.
Supplemental Figure S4. Immunogold labeling of the 2S albumin seed storage proteins in torpedo and early- to mid-walking stick embryos show no significant signal.
Supplemental Figure S5. Highlighted gold particles indicating immunogold labeling with antibodies against TIP3;1, 12S globulins, and 2S albumins in the cells shown in Figure 6.
Supplemental Figure S6. Anti-complex glycan antibody labeling in early- and late-bent cotyledon embryos.
Supplemental Figure S7. Vacuoles exhibit highly dynamic remodeling during the LV-to-PSV transition in LEC2-induced leaf epidermal cells.
Supplemental Figure S8. Regions of interest of selected embryos at different developmental stages imaged with SBF-SEM.
Supplemental Figure S9. Cross-section view through vacuoles in cotyledon cells of embryos in the torpedo, early-, mid-, and late-bent cotyledon, and mature embryo stages.
Supplemental Figure S10. Storage proteins accumulate within the lumen of the preexisting vacuole.
Supplemental Movie S1. Arabidopsis 35S:LEC2-GR line expressing 35S:TPK1-GFP after 18 d with DEX.
Supplemental Movie S2. Arabidopsis 35S:LEC2-GR line expressing 35S:TPK1-GFP after 14 d without DEX.
Supplemental Movie S3. 3D reconstruction of one cell in the cotyledon of a torpedo stage embryo.
Supplemental Movie S4. Orthoslice moving through the image stack of a torpedo stage embryo cotyledon.
Supplemental Movie S5. 3D reconstruction of one cell in the cotyledon of an early-bent cotyledon stage embryo.
Supplemental Movie S6. Orthoslice moving through the image stack of an early-bent cotyledon stage embryo cotyledon.
Supplemental Movie S7. 3D reconstruction of one cell in the cotyledon of a late-bent cotyledon stage embryo.
Supplemental Movie S8. Orthoslice moving through the image stack of a late-bent cotyledon stage embryo cotyledon.
Supplemental Movie S9. 3D reconstruction of one cell in the cotyledon of a mature seed stage embryo.
Supplemental Movie S10. Orthoslice moving through the image stack of a mature stage embryo cotyledon.
Acknowledgments
We thank J. Richardson and I. Hands-Portman for technical support. We are grateful to J. Harada for donating 35S:LEC2-GR seeds, J. Rogers and T. Okita for donating the anti-TIP1;1 and anti-TIP3;1 antibodies, I. Hara-Nishimura for donating the anti-12S antibody, A. Scarafoni for donating the anti-2S antibody, L. Jiang for the 35S:2S1-GFP construct, A. Vitale for the gift of the 35S:TPK1-GFP seeds and the anti-complex glycan antibody, and K. Schumacher for donating 35S:VHA-a3-RFP seeds.
Footnotes
This work was funded by the Leverhulme Trust (grant RPG-327 to L.F.) and the Biotechnology and Biological Sciences Research Council (grants BB/J017582/1 to L.F. and BB/M000168/1 to C.H.).
Articles can be viewed without a subscription.
References
- Bagga S, Sutton D, Kemp JD, Sengupta-Gopalan C (1992) Constitutive expression of the β-phaseolin gene in different tissues of transgenic alfalfa does not ensure phaseolin accumulation in non-seed tissue. Plant Mol Biol 19: 951–958 [DOI] [PubMed] [Google Scholar]
- Baud S, Kelemen Z, Thévenin J, Boulard C, Blanchet S, To A, Payre M, Berger N, Effroy-Cuzzi D, Franco-Zorrilla JM, et al. (2016) Deciphering the molecular mechanisms underpinning the transcriptional control of gene expression by master transcriptional regulators in Arabidopsis seed. Plant Physiol 171: 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Lanquar V, Soler MN, Beebo A, Satiat-Jeunemaître B, Bouhidel K, Thomine S (2011) Distinct lytic vacuolar compartments are embedded inside the protein storage vacuole of dry and germinating Arabidopsis thaliana seeds. Plant Cell Physiol 52: 1142–1152 [DOI] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13: 624–630 [DOI] [PubMed] [Google Scholar]
- Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K (2008) Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De D. (2000) Plant Cell Vacuoles. CSIRO Publishing, Collingwood, Australia [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippólito S, Arias LA, Casalongué CA, Pagnussat GC, Fiol DF (2017) The DC1-domain protein VACUOLELESS GAMETOPHYTES is essential for development of female and male gametophytes in Arabidopsis. Plant J 90: 261–275 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Guttenberger M, Saralegui A, Napsucialy-Mendivil S, Voigt B, Baluska F, Menzel D (2006) Neutral red as a probe for confocal laser scanning microscopy studies of plant roots. Ann Bot 97: 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K, Okatani Y, Uemura T, Goh T, Shoda K, Niihama M, Morita MT, Spitzer C, Otegui MS, Nakano A, et al. (2008) A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell 20: 3006–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney M, Frigerio L, Cui Y, Menassa R (2013a) Following vegetative to embryonic cellular changes in leaves of Arabidopsis overexpressing LEAFY COTYLEDON2. Plant Physiol 162: 1881–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney M, Frigerio L, Kohalmi SE, Cui Y, Menassa R (2013b) Reprogramming cells to study vacuolar development. Front Plant Sci 4: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Hinz G, Robinson DG (2008) Multiple vacuoles in plant cells: rule or exception? Traffic 9: 1564–1570 [DOI] [PubMed] [Google Scholar]
- Frigerio L, Vine ND, Pedrazzini E, Hein MB, Wang F, Ma JKC, Vitale A (2000) Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol 123: 1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, Nishizawa K, Maruyama N, Hara-Nishimura I (2007) Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 19: 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhuang X, Cui Y, Fu X, He Y, Zhao Q, Zeng Y, Shen J, Luo M, Jiang L (2015) Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc Natl Acad Sci USA 112: 1886–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattolin S, Sorieul M, Frigerio L (2011) Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol Plant 4: 180–189 [DOI] [PubMed] [Google Scholar]
- Gattolin S, Sorieul M, Hunter PR, Khonsari R, Frigerio L (2009) In vivo imaging of the tonoplast intrinsic protein family in Arabidopsis roots. BMC Plant Biol 9: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J, Rogers SW, Deery M, Dupree P, Rogers JC (2005) A unique family of proteins associated with internalized membranes in protein storage vacuoles of the Brassicaceae. Plant J 41: 429–441 [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Hayashi M, Nishimura M, Akazawa T (1987) Biogenesis of protein bodies by budding from vacuoles in developing pumpkin cotyledons. Protoplasma 136: 49–55 [Google Scholar]
- Harris LJ, Gleddie SC (2001) A modified Rpl3 gene from rice confers tolerance of the Fusarium graminearum mycotoxin deoxynivalenol to transgenic tobacco. Physiol Mol Plant Pathol 58: 173–181 [Google Scholar]
- Hegedus DD, Coutu C, Harrington M, Hope B, Gerbrandt K, Nikolov I (2015) Multiple internal sorting determinants can contribute to the trafficking of cruciferin to protein storage vacuoles. Plant Mol Biol 88: 3–20 [DOI] [PubMed] [Google Scholar]
- Hoh B, Hinz G, Jeong BK, Robinson DG (1995) Protein storage vacuoles form de novo during pea cotyledon development. J Cell Sci 108: 299–310 [DOI] [PubMed] [Google Scholar]
- Hunter PR, Craddock CP, Di Benedetto S, Roberts LM, Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145: 1371–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11: 1867–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW, Rogers JC (2001) The protein storage vacuole: a unique compound organelle. J Cell Biol 155: 991–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC (2000) Biogenesis of the protein storage vacuole crystalloid. J Cell Biol 150: 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Höfte H, Chrispeels MJ (1990) An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GIpF). Plant Cell 2: 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelmann M, Hawes C, Hughes L (2016) Serial block face scanning electron microscopy and the reconstruction of plant cell membrane systems. J Microsc 263: 200–211 [DOI] [PubMed] [Google Scholar]
- Kolb C, Nagel MK, Kalinowska K, Hagmann J, Ichikawa M, Anzenberger F, Alkofer A, Sato MH, Braun P, Isono E (2015) FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol 167: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurière M, Laurière C, Chrispeels MJ, Johnson KD, Sturm A (1989) Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol 90: 1182–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maîtrejean M, Wudick MM, Voelker C, Prinsi B, Mueller-Roeber B, Czempinski K, Pedrazzini E, Vitale A (2011) Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol 156: 1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG (1992) Cotyledon cell development in Arabidopsis thaliana during reserve deposition. Can J Bot 70: 151–164 [Google Scholar]
- Marty F. (1999) Plant vacuoles. Plant Cell 11: 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li KY, Li HY, Yao X, Jiang L (2008) The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. Plant J 56: 824–839 [DOI] [PubMed] [Google Scholar]
- Olbrich A, Hillmer S, Hinz G, Oliviusson P, Robinson DG (2007) Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiol 145: 1383–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Capp R, Staehelin LA (2002) Developing seeds of Arabidopsis store different minerals in two types of vacuoles and in the endoplasmic reticulum. Plant Cell 14: 1311–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18: 2567–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Gillmor CS, Kovaleva V, Somerville CR, Raikhel NV (2001) VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev Cell 1: 303–310 [DOI] [PubMed] [Google Scholar]
- Sanmartín M, Ordóñez A, Sohn EJ, Robert S, Sánchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104: 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarafoni A, Carzaniga R, Harris N, Croy RR (2001) Manipulation of the napin primary structure alters its packaging and deposition in transgenic tobacco (Nicotiana tabacum L.) seeds. Plant Mol Biol 46: 727–739 [DOI] [PubMed] [Google Scholar]
- Scheuring D, Schöller M, Kleine-Vehn J, Löfke C (2015) Vacuolar staining methods in plant cells. In Estevez JM, ed, Plant Cell Expansion: Methods and Protocols. Springer, New York, pp 83–92 [DOI] [PubMed] [Google Scholar]
- Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, et al. (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Yamada K, Kataoka M, Nakaune S, Koumoto Y, Kuroyanagi M, Tabata S, Kato T, Shinozaki K, Seki M, et al. (2003) Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J Biol Chem 278: 32292–32299 [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueño F, Rojo E, Surpin M, Raikhel NV (2007) The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA 104: 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J, Hsieh TF, Fischer RL, Goldberg RB, Harada JJ (2008) Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: implications for somatic embryogenesis. Proc Natl Acad Sci USA 105: 3151–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98: 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Carbajosa J, Carbonero P (2005) Seed maturation: developing an intrusive phase to accomplish a quiescent state. Int J Dev Biol 49: 645–651 [DOI] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, et al. (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker C, Schmidt D, Mueller-Roeber B, Czempinski K (2006) Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J 48: 296–306 [DOI] [PubMed] [Google Scholar]
- West M, Harada JJ (1993) Embryogenesis in higher plants: an overview. Plant Cell 5: 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Staehelin LA (2011) Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiol 155: 2023–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Han SW, Rodriguez-Welsh MF, Rojas-Pierce M (2014) Homotypic vacuole fusion requires VTI11 and is regulated by phosphoinositides. Mol Plant 7: 1026–1040 [DOI] [PubMed] [Google Scholar]
- Zwiewka M, Feraru E, Möller B, Hwang I, Feraru MI, Kleine-Vehn J, Weijers D, Friml J (2011) The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res 21: 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]