Abstract
Numerous clinical trials suggest that we have reached a limit in our ability to decrease the incidence of coronary heart disease (CHD) and cardiovascular disease (CVD) utilizing the traditional diagnostic evaluation, prevention and treatment strategies for the top five cardiovascular risk factors of hypertension, diabetes mellitus, dyslipidemia, obesity and smoking.
About 80% of heart disease (heart attacks, angina, coronary heart disease and congestive heart failure) can be prevented by optimal nutrition, optimal exercise, optimal weight and body composition, mild alcohol intake and avoiding smoking. Statistics show that approximately 50% of patients continue to have CHD or myocardial infarction (MI) despite presently defined ‘normal’ levels of the five risk factors listed above. This is often referred to as the ‘CHD gap’. Novel and more accurate definitions and evaluations of these top five risk factors are required, such as 24 h ambulatory blood pressure (ABM) results, advanced lipid profiles, redefined fasting and 2 h dysglycemia parameters, a focus on visceral obesity and body composition and the effects of adipokines on cardiovascular risk. There are numerous traumatic insults from the environment that damage the cardiovascular system but there are only three finite vascular endothelial responses, which are inflammation, oxidative stress and immune vascular dysfunction. In addition, the concept of translational cardiovascular medicine is mandatory in order to correlate the myriad of CHD risk factors to the presence or absence of functional or structural damage to the vascular system, preclinical and clinical CHD. This can be accomplished by utilizing advanced and updated CV risk scoring systems, new and redefined CV risk factors and biomarkers, micronutrient testing, cardiovascular genetics, nutrigenomics, metabolomics, genetic expression testing and noninvasive cardiovascular testing.
Keywords: cardiovascular testing, coronary heart disease, nutrition, nutritional supplements
Introduction
Cardiovascular disease (CVD) remains the number one cause of morbidity and mortality in the United States.1 The annual cost (direct and indirect) of treating CVD is approximately $320 billion USD.1 Every one in three deaths is due to CVD with over 2200 US citizens dying from stroke or myocardial infraction (MI) daily.1–4 Clinical studies suggest that a limit has been reached in the ability to reduce CVD.5 About 80% of coronary heart disease (CHD) can be prevented by optimal nutrition, optimal exercise, optimal weight and body fat, mild alcohol intake and avoiding smoking.5 More than 400 CHD risk factors have been defined.1 The three finite responses of the cardiovascular (CV) system to these insults include inflammation, oxidative stress and vascular immune dysfunction. Laboratory measurement of the finite responses allows the physician to ‘backtrack’ and determine the ‘why’ or the genesis of CVD, remove the insult(s) and initiate optimal prevention and treatment methodologies to resolve intermediate downstream abnormalities in order to meet defined clinical, noninvasive testing and laboratory goals. Prevention and treatment strategies must be directed to these finite responses while reducing the allostatic load of the 400 or more CHD risk factors and biomarkers and the intermediate downstream effects1
The traditional diagnostic testing, evaluation, prevention and treatment strategies for the top five CV risk factors: hypertension, diabetes, dyslipidemia, obesity and smoking, have resulted in a ‘CHD gap’.3 Approximately 50% of patients admitted to a hospital with acute coronary syndrome (ACS) or MI have ‘normal’ levels of the top five CHD risk factors.1,4 The ‘cholesterol-centric’ approach to prevent CHD is clearly important, but the other risk factors must be redefined, treated early and aggressively. Simultaneously, the clinician should measure and treat the three finite vascular responses. Advances in the direct assessment of the top five risk factors and the refinement of their definitions are often not utilized by physicians so that optimal prevention and treatment strategies for CHD are not clinically applied.1 Physicians should also evaluate new CV risk scoring systems, novel and redefined CV risk biomarkers (factors), micronutrient testing, CV genetic nutrigenomics, metabolomics, genetic expression testing and noninvasive CV testing that allow nutritional medicine to become personalized and precise.
Revolutionizing the treatment of coronary heart disease
Addressing cell membrane physiology and identification of dysfunction represent the first step in the prevention and treatment of CHD. Cell membranes are the primary barrier between the external milieu (attacked by various hemodynamic or biochemical insults) and the internal cell organelles in the endothelium. The interaction of the various insults with the endothelial vascular receptors [pattern recognition receptors (PRR), nucleotide-binding oligomerization domain (NOD)-like receptors (NLR), toll-like receptors (TLR) and caveolae] determine the signaling transduction into the cell.1 The external insults stimulate these inflammatory vascular receptors directly or through epitopes.1
Any cell membrane insult such as hypertension, alterations in hemodynamics, modified low-density lipoprotein (LDL) cholesterol, glucose, advanced glycosylation end products (AGE), microbes, internal tissue breakdown, toxins, heavy metals, homocysteine or the other CHD risk factors result in a reaction diffusion wave (tsunami effect) throughout the cell membrane that may disrupt cell receptors and the signaling mechanisms with subsequent membrane damage and dysfunction.6,7 Depending on the biological fatty acid makeup of the cell membrane with trans fatty acids (TFAs), saturated fatty acids (SFAs), monounsaturated fatty acids (MUFA), omega 3 fats (polyunsaturated fatty acids, PUFAs), the responses will vary. Fluidity within the cell membrane (MUFA, PUFA) dampens injury at the site of the insult, as well as throughout the rest of the cell membrane. However, stiff cell membranes (SFA, TFA) will exacerbate local and distant cell membrane damage. In addition, a heightened response (metabolic memory) from the inflammatory/immune cascade can create additional cell damage.6,7 The acute response to cell injury is defensive, short lived, regulated and appropriate, but with chronic insults of any type, the inflammatory, oxidative stress and immune responses become dysregulated and dysfunctional to the point that there is damage to the vascular system. In this regard, the blood vessel becomes an ‘innocent bystander’ to its own defense mechanisms that lead to functional then structural CV injury then preclinical CVD and clinical CVD.1 The maladaptation of the renin–angiotensin–aldosterone system (RAAS), sympathetic nervous system (SNS) and the inflammatory/oxidative stress/immune pathways contribute to the vascular dysfunction.1
The CHD risk factors have well-defined clinical goals that indicate the ‘normal’ level at which the risk for CHD is minimal. There exists a continuum of risk of CHD with all risk factors such that a ‘true’ normal level can be somewhat misleading.1,8–12 Normal blood pressure (BP) is defined as 120/80 mm Hg, prehypertension as 121/81–139/89 mm Hg and hypertension is defined as greater than 140/90 mmHg.11,13 Dyslipidemia is defined as a LDL-cholesterol (LDL-C) greater than 100 mg/dl, (depending on the clinical setting) and glucose intolerance at a fasting glucose over 99 mg/dl.1 However, the continuum of risk starts at even lower levels for BP, LDL-C and glucose, as well as for most of the other CHD risk factors.1 The risk for CHD actually starts with a fasting glucose of 75 mg/dl. For each 1 mg/dl increase in fasting blood sugar (FBS), the risk for CHD and MI increases by 1%.1,8–11 The risk for CHD starts at a BP level of 110/70 mm Hg. For every 20/10 mm Hg increase in BP the risk for CHD is doubled.1,11,11 The risk for LDL-C causing a reduction in endothelial nitric oxide (NO) is 60 mg/dl1,12
The concept of ‘translational vascular medicine’ correlates CHD risk factors with the actual presence of vascular disease. Do measured risk factors accurately translate into a vascular pathology that can be evaluated by noninvasive or invasive vascular testing? Conversely, does the absence of measured CHD risk factors accurately define the absence of vascular pathology or of vascular health? Evaluation of this correlation requires more sophisticated technology and advanced risk factor analysis combined with more comprehensive risk factor scoring systems such as those of the Consortium for Southeastern Hypertension Control (COSEHC)12,14 and Rasmussen15 (Tables 1 and 2).
Table 1.
The Consortium for Southeastern Hypertension Control cardiovascular scoring system.
| (1) Absolute risk: probability of an adverse event occurring in an individual within a defined period of time; | |
| (2) Relative risk: probability of an adverse event happening in an individual compared with average or normal individuals sharing similar demographics other than the risk factor. | |
| High CV risk | |
| (1) Relative risk ⩾ 60th percentile | |
| (2) Absolute risk: risk score ⩾ 4 ⩾ 2.3% risk of CV death/5 years | |
| (3) Approximate 60th percentile relative risk scores | |
| • Men Age Range Women | |
| • 29 35–39 18 | |
| • 32 40–44 21 | |
| • 36 45–49 27 | |
| • 40 50–54 31 | |
| • 44 55–59 36 | |
| • 48 60–64 41 | |
| • 53 65–69 45 | |
| • 57 70–74 49 | |
| • Men at age 50 = relative risk ⩾ 60th percentile | |
| • Women at age 60 = relative risk ⩾ 60th percentile | |
| Women (12 CHD risk factors) | |
| • Age (years) | |
| • Cigarette smoking | |
| • Systolic blood pressure (mm Hg) | |
| • Total cholesterol concentration (mg/dl) | |
| • Height (inches) | |
| • Creatinine concentration (mg/dl) | |
| • Homocysteine (µmol/l) | |
| • Prior MI | |
| • Prior stroke | |
| • LVH | |
| • Diabetes | |
| • Nondiabetic, FBS (mg/dl) | |
| Men (17 CHD risk factors) | |
| • Being male | |
| • Age (years) | |
| • Cigarette smoking | |
| • Systolic blood pressure (mm Hg) | |
| • Total cholesterol concentration (mg/dl) | |
| • LDL cholesterol (mg/dl) | |
| • HDL cholesterol (mg/dl) | |
| • Triglyceride (mg/dl) | |
| • Height (inches) | |
| • Creatinine concentration (mg/dl) | |
| • Homocysteine (µmol/l) | |
| • Prior MI | |
| • Family history of MI pre-60 | |
| • Prior stroke | |
| • LVH | |
| • Diabetes | |
| • Nondiabetic, FBS (mg/dl) | |
| Calculated risk score | % dying from CV disease in 5 years |
| 0 | 0.04 |
| 5 | 0.07 |
| 10 | 0.11 |
| 15 | 0.19 |
| 20 | 0.31 |
| 25 | 0.51 |
| 30 | 0.84 |
| 35 | 1.4 |
| 40 | 2.3 |
| 45 | 3.7 |
| 50 | 6.1 |
| 55 | 9.8 |
| 60 | 15.6 |
| 65 | 24.5 |
| High cardiovascular risk with expanded CHD risk factors | |
| Relative risk ⩾ 60th percentile | |
| Absolute risk: risk score ⩾ 40 is a ⩾ 2.3% risk of death in 5 years | |
CV, cardiovascular; MI, myocardial infarction; LVH, left ventricular hypertrophy; FBS, fasting blood sugar; CHD, coronary heart disease; LDL, high-density lipoprotein; HDL, low-density lipoprotein.
Table 2.
Rasmussen cardiovascular scoring system.
(1) Disease score 0–2: no CV events in 6 years;
(2) Disease score 3–5: 5% CV events in 6 years;
(3) Disease score over 6: 15% CV events in 6 years;
(4) Superior to Framingham risk score;
(5) Variables measured: CAPWA, blood pressure at rest and exercise, LV mass by ECHO, microalbuminuria, BNP, retinal score, carotid IMT and ultrasound and EKG.
| Test | Normal | Borderline | Abnormal |
|---|---|---|---|
| Score for each test | 0 | 1 | 2 |
| Large artery elasticity | (age and sex dependent) | ||
| Small artery elasticity | (age and sex dependent) | ||
| Resting BP (mm Hg) | SBP < 130 and DBP < 85 | SBP 130–139 or DBP 85–89 | SBP ⩾ 140 or DBP ⩾ 90 |
| Treadmill exercise BP (mm Hg) | SBP increase < 30 and SBP ⩽ 169 | SBP increase 30–39 or SBP 170–179 | SBP increase ⩾ 40 or SBP ⩾ 180 |
| Optic fundus photography retinal vasculature |
A/V ratio > 3:5 | A/V ratio ⩽ 3:5 or mild A/V crossing changes | A/V ratio ⩽ 1.2 or A/V nicking |
| Carotid IMT | (age and sex dependent) | ||
| Microalbuminuria (mg/mmol) | ⩽0.6 | 0.61–0.99 | ⩾1.00 |
| Electrocardiogram | No abnormalities | Nonspecific abnormality | Diagnostic abnormality |
| LV ultrasound LVMI (g/m2) | <120 | 120–129 | ⩾130 |
| NT-pro BNP (pg/dl) | <150 | 150–250 | >250 |
CV, cardiovascular; CAPWA, computerized arterial pulse wave analysis; LV, left ventricular; ECHO, echocardiogram; BNP, B-type brain natriuretic peptide; IMT, intimal medial thickness; EKG, electrocardiogram; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; A/V, arteriovenous; LVMI, left ventricular mass index; NT-pro BNP, N-terminal prohormone of B-type brain natriuretic peptide.
The endothelium, endothelial function, and endothelial dysfunction
The endothelium is a very thin lining of vascular cells forming an interface between the arterial lumen and the vascular smooth muscle (VSM).1,3,16 Endothelial dysfunction occurs when NO bioavailability is reduced, which leads to inflammation, oxidative stress, immune dysfunction, abnormal growth, vasoconstriction, increased permeability, thrombosis and CHD.1,3,16,17
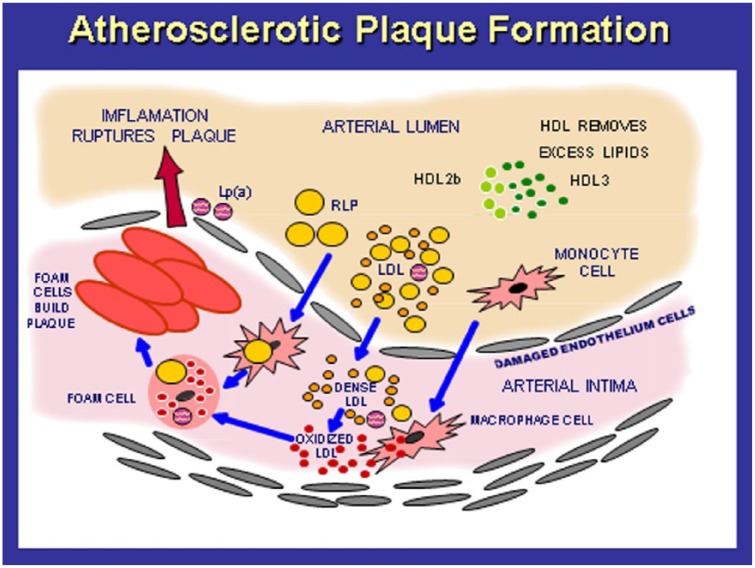
LDL-C has a primary role in the development of atherosclerotic plaque formation starting at birth (Figure 1).18 LDL-C migrates from the blood into the subendothelial layer, attaches to proteoglycans and becomes modified, antigenic and toxic damage-associated molecular patterns primarily via oxidation of LDL (oxLDL) and glycation of LDL (glyLDL) occur.18 The modified LDLs produce numerous cytokines and chemokines that attract monocytes into the subendothelial layer which transform into macrophages. The modified LDLs are taken by scavenger receptors (SRAs) on the macrophage cell membranes, which transform into foam cells then fatty streaks and eventually form a coronary artery plaque (stable plaque or a rupture-prone plaque) that can result in an MI. Approximately 45 different steps exist in this process of dyslipidemia-induced vascular disease that can be interrupted with nutrition, nutritional supplements and drugs.18 Endothelial dysfunction, arterial pathology, cardiac dysfunction and CHD represent a delicate balance of vascular injury (angiotensin II and endothelin) and nitric oxide and vascular repair from endothelial progenitor cells, produced by the bone marrow.1,3,6
Figure 1.
The pathogenesis of atherosclerotic plaque formation.
An increase in LDL particles of small, dense size enter through the endothelial lining to the subintimal area where they are oxidized, taken up by macrophages and induce oxidative stress, inflammation, immune dysfunction and eventually form foam cells, fatty streaks and coronary artery plaques.
LDL, low-density lipoprotein; HDL, high-density lipoprotein; Lp(a), lipoprotein a; RLP, remnant lipoprotein.
The pathophysiology of vascular disease
The primary causes of CHD are the three finite responses that are generated from the large allostatic environmental insults coupled with CV genetics, nutrigenomics, metabolomics, proteomics and gene expression patterns. The three finite vascular responses are:
Oxidative stress: Reactive oxygen species and reactive nitrogen species are increased in the vasculature and kidneys. There exists a concomitant reduction in oxidative defense.1–3
Inflammation: Increased inflammation in the vasculature and kidney can be assessed by measuring inflammatory markers such as high-sensitivity C-reactive protein (hsCRP), leukocytosis with increased neutrophils and decreased lymphocytes, increased levels of interleukins (IL-6 and IL 1B) and tumor necrosis factor alpha (TNF alpha), as well as increased activity of the RAAS in the arteries and the kidney.1–3
Autoimmune dysfunction of both the arteries and kidneys occurs with leukocytosis and involvement of CD4+ (T-helper cells) and CD 8+ (cytotoxic T cells).1–3
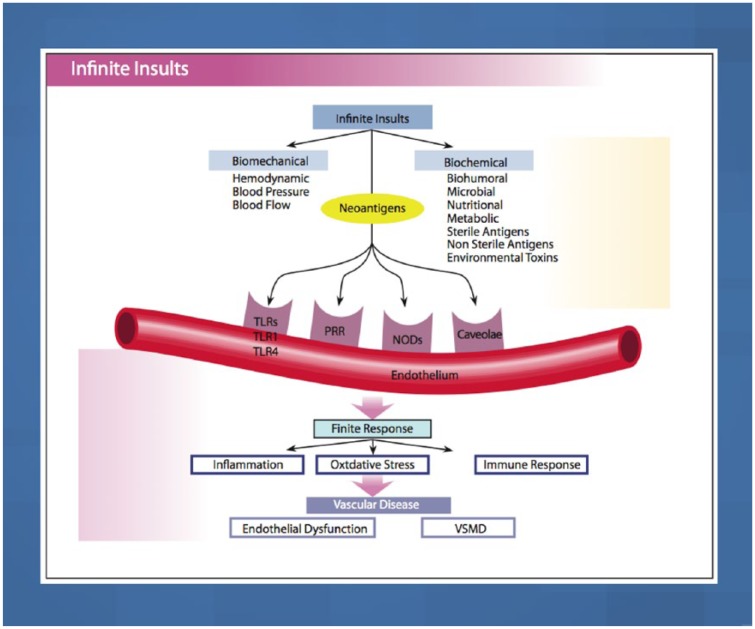
Figure 2 illustrates the interconnection of the external insults and the vascular receptors (PRR, NLR, TLR, caveolae) on the endothelium. These insults are divided into two major categories: biomechanical (BP, pulse pressure, shear stress and oscillatory pressure) within the arterial system and external biochemical factors that include dietary factors, various bio-humoral and metabolic factors, microbes, sterile and nonsterile antigens, and environmental toxins.19
Figure 2.
Biochemical and biomechanical insults interact with vascular receptors, PRRs, NLRs, TLRs and caveolae, to induce the three finite responses of vascular inflammation, oxidative stress and vascular immune dysfunction which lead to endothelial dysfunction, and VSM and cardiac dysfunction, coronary heart disease, myocardial infarction and congestive heart failure.
PRR, pattern recognition receptors; NLR, NOD-like receptors; NOD, nucleotide-binding oligomerization domain; TLR, toll-like receptor; VSMD, vascular smooth muscle dysfunction.
Interrupting the finite pathways
The reduction of the allostatic load, interrupting the insult–vascular receptor interaction to the PPR, NLR, TLR and caveolae, and disruption of the downstream mediators are paramount to a successful prevention and treatment regimen for CVD. Numerous scientifically validated nutritional or dietary components and nutraceutical supplements have great promise in this regard.20 These will be discussed in detail in the treatment section.
Preventing and treating CHD and establishing CV ecology and balance involve utilizing a more complex and logical approach, such as dynamic systems biology, functional and metabolic medicine. As one might expect with a complex network of physiological interactions underlying vascular responsiveness and development of CHD, a single genetic cause has not been identified. Instead, as many as 30 separate loci are associated with MI and CHD. The majority of these involve inflammatory pathways but only a minority of those 30 loci relate the top five CV risk factors.2
Atherosclerosis and endothelial dysfunction
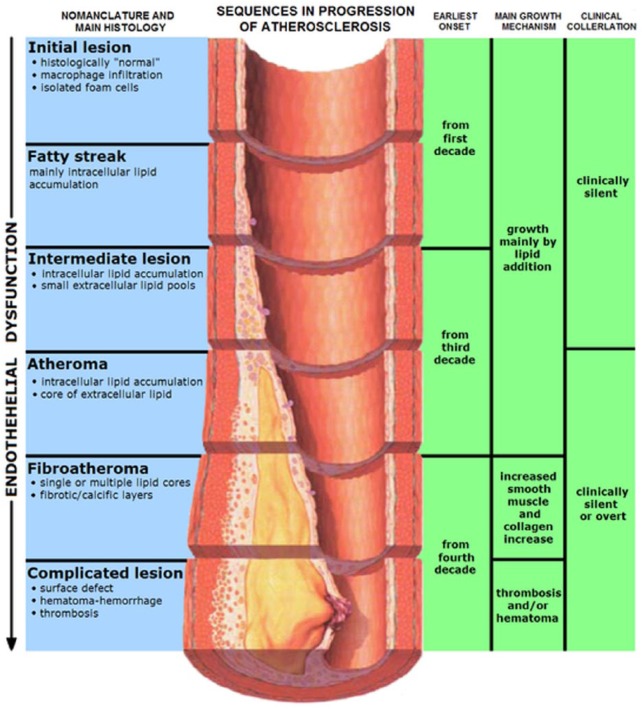
Atherosclerosis and endothelial dysfunction are postprandial diseases.21 The consumption of sodium chloride (NaCl), refined carbohydrates (CHO), sugars, starches and some, but not all, SFAs and TFAs will promote glucotoxicity, triglyceride toxicity, vascular metabolic endotoxemia, inflammation, oxidative stress, and vascular immune dysfunction that may persist long after the initial insult. This may also result in an exaggerated response (metabolic memory) with repeated or chronic dietary insults.6,21 Fortunately, studies have shown that eating a diet rich in low-glycemic foods, reduced refined sugars and starches, low in NaCl and high in potassium and magnesium, high in MUFAs, PUFAs, polyphenols, and antioxidants can help to prevent the postprandial endothelial dysfunction and endotoxemia.18 Early evidence of CHD in the form of fatty streaks in the aorta and coronary arteries has been documented in children in the first and second decades of life and in postmortem exams of teenagers and war victims (Figure 3).1 The CV disease is subclinical for decades prior to any CV events.1,3,16 Endothelial dysfunction is the earliest functional abnormality, followed later by changes in arterial compliance of small resistance arteries and then larger conduit arteries, with loss of elasticity leading to hypertension, VSM hypertrophy, diastolic dysfunction (DD), left ventricular hypertrophy (LVH), congestive heart failure (CHF) and CHD.17
Figure 3.
Atherosclerosis progression.
The earliest abnormality is endothelial dysfunction and loss of nitric oxide bioavailability. Then the IMT increases, followed by fatty streaks, atheroma, fibroatheroma and the complicated lesions with soft or hard plaque that may rupture and induce acute coronary thrombosis and myocardial infarction.
IMT, intimal medial thickness.
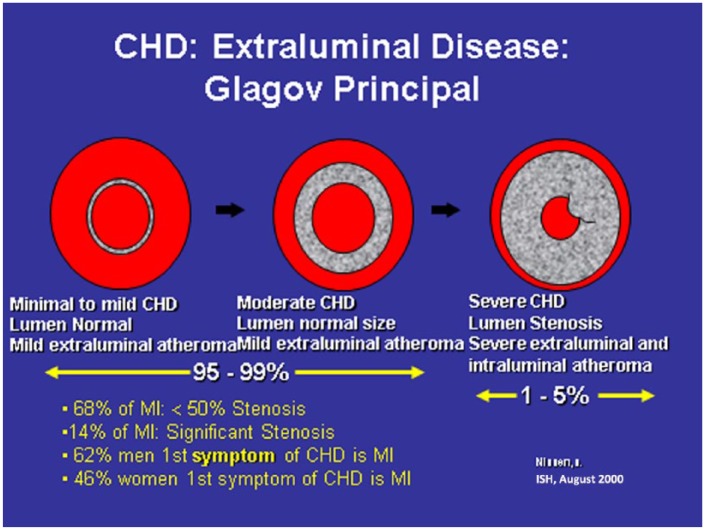
Figure 4 shows the progression from subintimal coronary atherosclerosis to obstructive CHD. The coronary artery on the left is normal, the one in the center demonstrates mild subintimal disease with increased intimal medial thickness (IMT) but a normal and unchanged arterial lumen. This extraluminal disease and inflammation would be captured using computed tomography angiogram (CTA) or intravascular ultrasound (IVUS) but missed by conventional coronary arteriogram (Figure 5). The image on the right in Figure 4 illustrates extensive extraluminal and intraluminal obstructive CHD. Most MIs occur with mild stenosis of the coronary arteries.
Figure 4.
Progression of subintimal coronary atherosclerosis to obstructive coronary heart disease.
In the earliest coronary lesion, there is minimal to no intracoronary luminal plaque formation. As the disease progresses, there is increased subintimal disease but the vascular lumen may still be normal in size on a coronary arteriogram. However, coronary computerized angiograms will demonstrate this extraluminal atheroma. Severe progression ensues with both severe extraluminal and intraluminal atheroma.
CHD, coronary heart disease; MI, myocardial infarction.
Figure 5.
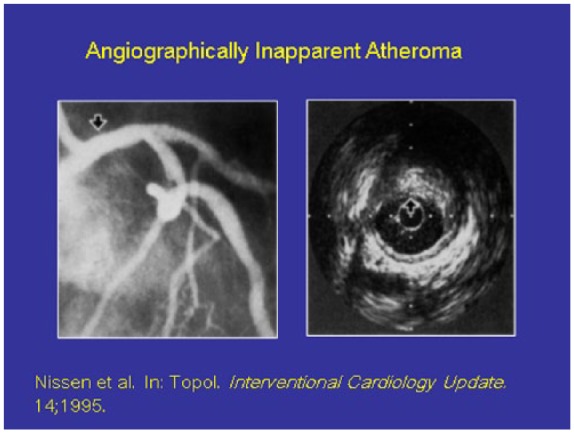
Coronary heart disease that is not detectable by angiogram (left) is evident using intravascular ultrasound (right).
Note the arrow in the left which shows a 100% open lumen in the coronary artery but with intravascular ultrasound there is a large amount of subintimal atheroma present.
Some of the top coronary heart disease risk factors and the coronary heart disease gap
The ‘CHD gap’ is related to incorrect definitions, assessment and treatment of the top five risk factors, the lack of assessment and treatment of the other 395 risk factors, not performing the various noninvasive CV tests, genomic CV individuality and possibly other unknown factors.1
Hypertension
Only a 24 h ABM accurately measures and predicts CHD.1,16 Measurements of nocturnal BP and dipping status (normal is a 10% mean reduction from the daytime BP mean to the night), nondipping status, BP surges, BP load (normal is below 140/90 mm Hg in 15% of the total BP measurements) and BP variability are superior to office BP readings as a predictor of CHD risk.1,16 Excessive dipping is associated with an increased risk of ischemic stroke and reverse dipping is associated with an increased risk of intracerebral hemorrhage (ICH).1,16 Nocturnal blood pressure is more clinically important than daytime blood pressure (a 27/15 mmHg difference is optimal).1,16 Morning blood pressure surges (level and rapidity) increase the risk of ischemic stroke, MI, and LVH.1,16 Hypertension is a marker for vascular endothelial dysfunction with reduced NO bioavailability, but the vascular disease is enhanced in a bidirectional manner with hypertension which leads to greater vascular damage.17 The items that should always be considered when evaluating blood pressure include:16
A normal blood pressure is 120/80 mmHg, but there is a continuum of risk for CHD starting at 110/70 mmHg;
Each increase of 20/10 mmHg doubles CHD risk;
Before age 50, the diastolic blood pressure is a better predictor of CHD risk;
After age 50, the systolic blood pressure (SBP) is a better predictor of CHD risk;
24 h ABM is more accurate than office blood pressure measurements and should be the standard of care for defining blood pressure and CHD risk;
Mercury sphygmomanometers are preferred. Electronic arm units are accurate if done correctly and validated. The wrist or finger monitors are not as accurate and should not be used as the basis for a hypertension diagnosis.
Dyslipidemia
Dyslipidemia should be evaluated using advanced lipid profiles to determine treatment and predict individual CHD risk more accurately.18,22,23 An advanced lipid profile will measure:
Total LDL-C;
LDL-P (LDL particle number) which drives CHD risk;
LDL size (the dense type B LDL is more atherogenic versus large type A LDL);
Modified LDL (oxidized, glycated, glyco-oxidized and acetylated);
Apolipoprotein (APO) B and APO A;
Lipoprotein a [Lp(a)];
Total high-density lipoprotein (HDL);
HDL particle number (HDL-P);
HDL size and HDL mapping (large 2b versus small type 3) or five types of HDL;
Dysfunctional HDL;
Reverse cholesterol transport and cholesterol efflux capacity (CEC);
Myeloperoxidase (MPO);
APO-CIII;
VLDL and triglyceride (TG) total;
Large very low-density lipoprotein (VLDL);
VLDL-P particle number;
Remnant particles.
The primary CHD risk related to LDL-C is LDL-P and APO B particles.12,18,22,23 Dense LDL is also predictive but only if LDL-P is elevated above the normal level.12,18,22,23 oxLDL is also associated with increased CHD. HDL-P is more protective for CHD with larger HDL type 2b being a second important protective mechanism.12,18,22,23 Greater number and size of HDLs are more efficient at reverse cholesterol transport, as well as having numerous other protective effects such as reducing inflammation, oxidative stress and immune dysfunction. It is also important to analyze dysfunctional HDL with MPO.12,18,22,23 Patients who have an HDL of 85 mg/dl or more, often have dysfunctional HDL that may not be protective.22,23 The VLDL, especially large VLDL, triglycerides and remnant particles are very atherogenic and thrombogenic.18
Dysglycemia, insulin resistance and diabetes mellitus
An FBS of over 75 mg/dl increases CHD risk by 1% per increase of 1 mg/dl of FBS and induces endothelial dysfunction.1,8–10 A 2 h glucose tolerance test (GTT) over 110 mg/dl increases CHD risk by 2% per 1 mg/dl over the 110 mg/dl level.1,8–10 The current definition of an abnormal 2 h GTT is >140 mg/dl. Hyperinsulinemia is also an independent risk factor for CHD.1,8–10 Calculating a homeostasis model assessment (HOMA) score will provide additional insight into the clinical presence of insulin resistance. Multiplying the FBS by the insulin level and dividing by 400 will give an excellent estimate of HOMA. A normal HOMA is <1.0, mild insulin resistance is between 1.0 and 2.0 and over 2.0 is severe insulin resistance. Insulin resistance creates inflammation, reduces NO levels, causes endothelial dysfunction and vascular disease through the mitogen-activated protein kinase (MAPK) pathway, which is a hypertensive, inflammatory and proatherogenic pathway.1 On the other hand, the phosphatidylinositol 3-kinase (PI3K) pathway is anti-inflammatory, antihypertensive and antiatherogenic.1
Noninvasive testing
Fortunately, there are many noninvasive tests available to determine CV pathology prior to clinical CHD1 (Table 3). One of the best validated early detection tests for functional abnormalities of the endothelium is the EndoPAT which determines endothelial function and dysfunction.24–26 The EndoPAT measures postocclusion brachial artery hyperemia, which is an excellent indirect measure of NO bioavailability and endothelial dysfunction in the coronary arteries. The EndoPAT predicts accurately the future risk for hypertension, CHD, unstable angina, CVD, CHF, MI, cardiac death, hospitalization, coronary artery bypass graft, stent restenosis, the presence of plaque in the coronary arteries that are rupture prone, pulmonary artery disease (PAD) and cerebrovascular accident (CVA) beyond the Framingham risk scoring (FRS).25–27 In a study of 528 patients with high risk for CV events over 5 years, the EndoPAT reactive hyperemia index (RHI) was measured before and after coronary angiogram.28 The RHI, brain natriuretic peptide (BNP) and CV score by SYNTAX were independent risk predictors for all future CV events such as MI, CV death, unstable angina, ischemic CVA, coronary artery bypass graft (CABG), CHF and PAD.
Table 3.
Noninvasive cardiovascular testing.
| Functional Tests |
| EndoPAT [endothelial dysfunction, augmentation index and heart rate variability (HRV)] |
| CAPWA (computerized arterial pulse wave analysis) |
| HRV: HRV and heart rate recovery time |
| EKG and treadmill test |
| Cardiopulmonary exercise test |
| Magnetocardiography |
| Structural Tests |
| Carotid IMT/duplex (intimal medial thickness) and plaque |
| CT angiogram |
| Coronary artery calcium scoring |
| Cardiac MRI |
| ECHO: rest and exercise |
| Ankle brachial index: rest and exercise |
| Retinal scan and ocular pulse amplitude |
| Other tests |
| Cardiac PET, SPECT |
| FDG-PET/CT: vascular inflammation/plaque/biologic activity |
| PET/CT/F-Na-F for coronary plaque/inflammation/morphology |
| PET/MRI for coronary plaque morphology and inflammation |
| Intravascular ultrasound |
| Coronary angiogram |
CT, computed tomography; EKG, electrocardiogram; FDG-PET, fluorodeoxyglucose–positron emission tomography; IMT, intimal medial thickness; F-Na-F, sodium flouride; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
When RHI was added to FRS, BNP and SYNTAX, the net reclassification index was significantly improved by 27.5% with a significant increase in C-statistic from 0.728 to 0.766. A normal RHI is over 1.67.28
The computerized arterial pulse wave analysis (CAPWA) (CV profiler) also predicts future CHD by measuring large and small arterial compliance.29–32 The C2 compliance identifies the presence of endothelial dysfunction in the microvascular circulation, the very small arterioles and medium-sized arteries (range 4–9 microns). The C1 compliance is a measure of the elastic behavior of the aorta and larger arteries (range 8–17 microns). Lower numbers of C1 and C 2 compliance indicate diseased arteries, arterial stiffness, decreased arterial compliance of the vascular wall and endothelial dysfunction. These are all age and gender adjusted. The CAPWA improves risk stratification beyond usual risk factors, including microalbuminuria (MAU), ECHO and carotid IMT. A low C2 and increased pulse wave velocity predict CHD.
The carotid IMT predicts future risk of CHD and CVA.33–35 Normal values without any plaque present must be adjusted for age and sex. A carotid IMT of <0.6 mm is normal to low risk, 0.6–0.7 mm is moderate risk, and 0.7–0.95 mm is high risk for future CVD. The normal IMT accretion rate (CIMTAR) is <0.016 mm/year. Carotid IMT indicates preclinical atherosclerosis and intimal-medial hypertrophy secondary to vascular smooth muscle and fibrous cell hypertrophy and hyperplasia. Carotid IMT correlates well with CHD risk factors and future CV events such as CHD, MI, transischemic attack (TIA) and stroke. The risk for MI is 1.26 [95% confidence interval (CI) 1.21–1.30] per 1.0 standard deviation (SD) of common carotid artery IMT difference and 1.15 (95% CI 1.12–1.17) per 0.10 mm of common carotid artery IMT difference over 5 years.35 The risk for stroke is 1.32 (95% CI 1.27–1.38) per 1.0 SD common of carotid artery IMT difference and 1.18 (95% CI 1.16–1.21) per 0.10 mm common of carotid artery IMT difference over 5 years.35
Fundus examination of retinal arterioles with SLDF (scanning laser doppler flowmetry) correlates highly with micromyographic biopsies of the medial lumen ratio (MLR) in subcutaneous small arteries. Retinal pathology indicates microvascular disease even after adjustment of renal dysfunction and traditional CVD risk factors. Retinal microvascular endothelial dysfunction assessed with flicker light of retinal veins and arteries is a nitric-oxide-dependent phenomenon and predicts hypertension, as well as CVD and CHD.36–39
Coronary artery calcification (CAC) was associated more strongly than carotid IMT with the risk of incident CHD in 6698 subjects over 5.3 years. The CAC predicted a CHD risk increase of 2.1-fold per 1.0 SD, whereas the carotid IMT predicted a CHD risk increase of 1.3-fold per 1.0 SD.40 CAC progression over 15% annually provides increased CHD risk analysis with a 17-fold increase in CHD. A baseline CAC score predicts CHD risk beyond traditional risk factors and a CAC score of over 300 has a hazard ratio of CHD of 10. A positive CAC increases risk of major cardiac events by 6–35-fold. CAC correlates with traditional risk factors but also with increased oxidative stress, autoantibodies to oxLDL, APO B-immune complexes and glycemic load and index.41–44
The risk of major CV events or death increases in a graded manner with the degree of coronary atherosclerosis as defined by CTA, even in the absence of high-grade coronary artery stenosis. Coronary CTA detects approximately twice as many coronary segments with plaque compared with coronary angiograms. This results in 52% of patients being assigned to a greater risk category.45–47
The multifunction cardiogram (MCG) is a computational electrophysiologic system to detect abnormal stress and strain between the myocardium (visco-elastic solid) and intracardiac blood flow (non-Newtonian fluid at low and intermediate shearing states) from a two-lead (II and V5) resting electrocardiogram (EKG). The MCG detects myocardial ischemia in an 82 s analysis.48–51 This maps the heart’s electrical activity to predict early CHD, ACS, MI and arrhythmias. The sensitivity is 88% and specificity is 88% (range 80–100%) for the early diagnosis of CHD, depending on degree of stenosis in some studies,50,51 but less specific and sensitive in another trial.49
Other noninvasive CV tests include the cardiac exercise ECHO, CEPT (cardiopulmonary exercise testing), exercise treadmill, various cardiac nuclear medicine scans, magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) for coronary plaque and obstruction, the fluorodeoxyglucose–positron emission tomography (FDG-PET)/computed tomography (CT) for arterial inflammation that defines biologic activity, as FDG accumulates in activated immune cells such as macrophages and T cells due to increased glycolysis. It predicts CAC/CHD events. The PET/CT/sodium flouride (F-Na-F) predicts coronary thrombosis, plaque and inflammation and the PET/magnetic resonance (MR) predicts coronary artery plaque and inflammation.52,53
The micronutrient test for functional deficiencies of nutrients (SpectraCell, Houston, TX, US) is valuable for assessing nutritional status and provide a more scientific rationale for nutrition and nutritional supplement treatment for CHD and hypertension.54 This is a lymphocyte assay that measures the status of 28 micronutrients for the previous 6 months. A recent study found that approximately 62% of hypertensive subjects could taper or discontinue pharmacologic therapy utilizing an aggressive micronutrient replacement program in conjunction with other lifestyle changes.54
Treatment
Nutrition
Mediterranean diet: traditional Mediterranean diet
In the 4.8-year primary prevention trial (PREDIMED), the rate of major CV events from MI, CVA or total CV deaths was reduced by 30% with nuts and 30% with extra virgin olive oil (EVOO). The reduction in CVA was 39% (p < 0.003) with a 33% reduction from EVOO and a 46% reduction from nuts. The reduction in MI was 23% (p = 0.25) with a 20% reduction from EVOO and a 26% reduction from nuts. Total CV deaths were reduced by 17% (p = 0.8).55–58 New-onset type 2 diabetes mellitus (T2DM) was decreased by 40% with EVOO and 18% with mixed nuts.58 This reduction was associated with decreases in hsCRP and interleukin-6 (IL-6).
The high content of nitrate (NO3), at an average of 400 mg per day, is converted to nitrite (NO2), which eventually forms NO. Also, the increased amount of omega 3 fatty acids (FAs), good omega 6 FAs and polyphenols (such as quercetin, resveratrol and catechins, in grapes and wine) provide many of the beneficial outcomes in CHD.57 Secondary prevention post MI in the Lyon Heart Study demonstrated significant reductions in all events including cardiac death, nonfatal MI, unstable angina, CVA, CHF and hospitalization at 4 years59 using the Mediterranean-style diet supplemented with alpha-linolenic acid compared with a prudent western diet. Compared with the control, the Mediterranean-style diet demonstrated a 76% lower risk of cardiac death and nonfatal MI during the study period.59 Olive oil was associated with a decreased risk of overall mortality and an important reduction in CVD mortality in a large Mediterranean cohort of 40,622 participants. For each increase in olive oil by 10 grams per day there was a 13% decrease in CV mortality. In the highest quartile of olive oil intake, there was a 44% decrease in CV mortality.60 One of the mechanisms by which the traditional Mediterranean diet (TMD), particularly if supplemented with virgin olive oil at 50 grams per day, can exert CV health benefits is through changes in the transcriptomic response of genes related to CV risk that include genes for atherosclerosis, inflammation, oxidative stress, vascular immune dysfunction, T2DM and hypertension. This includes genes for ADR-B2 (adrenergic beta 2 receptor), IL7R (interleukin 7 receptor), IFN gamma (interferon), MCP1 (monocyte chemotactic protein), TNFα (tumor necrosis factor alpha), IL-6 and hsCRP.56,61–63 In summary, the TMD has been shown to have the following effects:55,56,61–63
Lowers BP;
Improves serum lipids: lowers total cholesterol (TC), LDL, TG, increases HDL, lowers oxLDL and Lp(a), improves LDL size and lowers LDL-P to a less atherogenic profile;
Improves T2DM and dysglycemia;
Improves oxidative defense and reduces oxidative stress: F-2 isoprostanes and 8 hydroxy guanosine;
Reduces inflammation: lowers hsCRP, IL-6, soluble vascular adhesion molecule, soluble intercellular adhesion molecule;
Reduces thrombosis and factor VII after meals;
Improves BNP;
Increases nitrates/nitrites;
Improves membrane fluidity;
Reduces MI, CHD and CVA;
Reduces homocysteine.
Dietary approaches to stop hypertension diets (DASH) (DASH 1 and 2)
The dietary approaches to stop hypertension (DASH) diets reduce BP and CHD. Both DASH 1 and DASH 2 emphasize fruits, vegetables, whole grains, beans, fiber, low-fat dairy products, poultry, fish, seeds and nuts, but limit red meat, sweets, and sugar-containing beverages while increasing the intake of potassium, magnesium, and calcium but with variable restriction in dietary sodium.64,65 Both DASH diets reduced blood pressure within 4 weeks by 10/5 mm Hg or more, which is at least as effective as one antihypertensive medication. In the Nurses Health Study, adherence to the DASH dietary pattern was associated with a lower risk of CHD by 14% in those with the highest adherence to the diet.66
Fats
The role of fats in CHD has been evaluated in 72 clinical trials with over 600,000 patients.67 This included 32 observational studies of FA intake, 17 observational studies of FA biomarkers and 27 randomized clinical trials (RCTs) of FA supplements. Dietary TFA intake increased CHD by 16%, saturated fat intake increased CHD by 2% (worst with palmitic and stearic FA based on circulating FA biomarkers), omega 6 FA increased CHD by 1%, MUFA decreased CHD by 1% and omega 3 FA decreased CHD by 7%.67,68 Intakes of MUFAs and PUFAs are associated with a lower risk of CHD and death, whereas SFA and TFA intakes are associated with a higher risk of CHD. The replacement of SFAs with MUFAs and PUFAs or TFA with MUFAs was inversely associated with CHD.67–69
A large meta-analysis on omega 3 FA70 reviewed 18 RCTs (93,000 subjects) and 16 prospective cohort studies (732,000 subjects) examining eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) from foods or supplements and CHD, including MI, sudden cardiac death, coronary death and angina in primary and secondary prevention. Among RCTs, there was a nonstatistically significant 6% reduction in CHD risk with EPA + DHA. Subgroup analyses of data from RCTs indicated a statistically 14–16% significant CHD risk reduction with EPA+DHA among higher-risk populations, including participants with elevated triglyceride levels over 150 mg/dl and elevated LDL-C above 130 mg/dl. Meta-analysis of data from prospective cohort studies resulted in a statistically 18% significant reduction of CHD for higher intakes of EPA + DHA > 1 g per day and risk of any CHD event. The sudden cardiac death (SCD) rate was reduced by 47%. For those with high TG over 150 mg/dl, doses of omega 3 FA > 1 g per day reduced CHD by 25%. These results and others indicate that EPA + DHA may be associated with a reduction in CHD risk, with a greater benefit observed among higher-risk populations in RCTs and those taking higher doses.
Saturated fatty acids
The relationship of CHD to SFA intake is controversial in published studies, with widely variable results.56,67,71–77 The long-chain FAs (LCFAs) which include C-12–C-18 (lauric, myristic, palmitic and stearic acids) show significant associations with CHD, but the short-chain FAs (SCFAs) which include butyric–capric do not.56,67,71–77 LCFAs induce insulin resistance, dysglycemia, metabolic syndrome, T2DM, dyslipidemia, obesity, thrombosis, endothelial dysfunction, CVAs, CHD and MI, whereas SCFAs do not cause any of these metabolic or medical problems. The replacement of SFA with an isocaloric intake of PUFA, omega 3 FAs, MUFA, a plant-based diet or whole grains reduces the risk of CHD, but an isocaloric replacement of SFA with trans fats (TFAs), omega 6 fatty acids, processed animal fat, refined carbohydrates, starches and high fructose corn syrup (HFCS) increases the risk of CHD.56,67,71–77
Coconut oil
In a meta-analysis of 21 studies with 8 clinical trials and 13 observational studies, coconut oil increased TC and LDL more than PUFA, increased HDL and increased TG with no change in the TC/HDL ratio. There was no change in CV events.78 Coconut oil is 92% SFA, mostly lauric acid which acts mostly like an LCFA not a medium-chain fatty acid (MCFA) or medium-chain triglyceride (MCT), which is C-10 or less (direct portal vein absorption not via micelles and more water soluble). Only 4% of coconut oil is MCT of C-10 or less fatty acids.
Milk and milk products
Recent clinical studies indicate that milk, milk peptide and milk products reduce CHD, diabetes mellitus (DM), CVA and atherosclerosis.79,80 They improve insulin resistance, postprandial hyperglycemia, lower BP [increases NO and improves FMD and endothelial dysfunction (ED)] and decreases inflammation and oxidative stress.79,80
Carbohydrates and sugar substitutes
Sugars, refined carbohydrates, HFCS, starches and TFAs confer more risk for dyslipidemia and CHD than SFA. Omega 3 FAs, MUFAs, fermented foods, fiber, fruit + vegetables, dairy, TMD and the DASH 2 diet reduce CHD.81 In a population-based cohort study of 39,786 participants over 18 years, daily diet soft drink consumption increased the risk of CVA by 21%.82 The National Health Service (NHS) and Health Professioanls Follow-Up Study (HPFUS) showed both sugar-sweetened and low-calorie sodas significantly increased the risk of stroke by 16% per one serving per day and CHD by 20%.83 Sugar substitutes increase the risk for obesity, weight gain, metabolic syndrome, T2DM and CHD. The sugar substitutes interfere with learned responses that normally contribute to glucose and energy homeostasis, kill the microbiome, alter leptin levels and decrease satiety.84,85
Vegetarian diets
Vegetarian diets significantly reduce CVD, CHD and CAC that is proportional to the dietary intake.86–89 In the EPIC Study of 44,561 participants in England and Scotland followed for 11.6 years, the body mass index (BMI), lipids and BP were all reduced in the vegetarian group and had a 32% lower incidence of CHD after adjustment for other CHD risk factors.86 A study of 96,469 Seventh-day Adventist men and women from 2002 to 2007 demonstrated a 12% decrease in total mortality, 15% in vegans, 9% in lacto–ovo vegetarians, 19% in pesco–vegetarian individuals and 8% in semivegetarians, which was primarily related to decreases in CVD.87 In a meta-analysis of nine cohort studies of 222,081 men and women, the overall reduction in CHD risk was 4% for each additional portion of fruit and vegetable intake per day (p = 0.0027) and 7% for each additional serving of fruit (p = 0.0001).89 The association between vegetable intake and CHD risk was heterogeneous (p = 0.0043), more marked for CV mortality (0.74, p < 0.0001) than for fatal and nonfatal MI (0.95, p = 0.0058).89 Dark green leafy vegetables had the most dramatic reduction in CHD risk. Some vegetarian diets may be deficient in many nutrients which require supplemental B12, vitamin D, omega 3 FA, iron, calcium, carnitine, zinc and some high quality amino acids and protein.90 Other studies suggest many other problems, such as decreased sulfur amino acid intake with a low elemental sulfur, increased homocysteine and oxidative stress, lower cysteine (33% of control) and glutathione (63% of controls). In addition, lean muscle mass was 10% lower and there may be an increased risk of subclinical malnutrition and CVD.90
Protein diets
Recent studies show either no correlation or an inverse correlation of grass-fed beef, wild game, organically fed animals and other sources of protein with CHD.91–96 The Paleolithic diet has also shown reductions in total mortality of 23% and CV mortality of 22% in a cohort study of 21,423 participants.91 All meat (including red meat, fish, seafood, poultry) had an inverse relationship to CVD mortality in men in Asian countries.93 Other meta-analyses showed no association between red meat consumption and CHD but found that processed red meat increased risk of hypertension, total mortality, CHD and DM risk.92–96 In the BOLD (Beef in an Optimal Lean Diet Study) trial, a low SFA, heart-healthy diet that contains lean beef elicits a favorable effect on CVD, lipids and lipoprotein risk factors that are comparable with the DASH diet.92 This may be related to certain amino acids in meat versus vegetables, such as the lysine and arginine content.
Specific dietary and nutritional components
Several dietary and nutritional components have been shown to interrupt the inflammatory vascular receptors such as PRRs, NLRs and TLRs.20 These include:
Curcurmin (tumeric) blocks TLR 4, nucleotide-binding oligomerization domain (NOD) 1, and NOD 2;
Cinnamaldehyde (cinnamon) blocks TLR 4;
Sulforaphane (broccoli) blocks TLR 4;
Resveratrol (nutritional supplement, red wine, grapes) blocks TLR 1;
Epigallocatechin gallate (EGCG) (green tea) blocks TLR 1;
Luteolin (celery, green pepper, rosemary, carrots, oregano, oranges, olives) blocks TLR 1;
Quercetin (tea, apples, onion, tomatoes, capers) blocks TLR 1.
A prospective study of 42 participants over 2 years showed a significant reduction in progression of CHD as assessed by electron beam tomography CAC compared with historical controls using a phytonutrient concentrate with a high content of fruit and vegetable extracts. The CAC score increased by 19.6% in treated patients versus 34.7% in controls (p < 0.009).97
Caffeine
The cytochrome P450-CYP1A2 genotype modifies the association between caffeinated coffee intake and the risk of hypertension, CVD, CHD and MI in a linear relationship.98–105 Caffeine is exclusively metabolized by CYP1A2 to paraxanthine, theobromine and theophylline.98 The gene lies on chromosome 15q24.1 and the single nucleotide polymorphism (SNP) is rs7762551 A to C.98 The C SNP decreases enzymatic activity.98 Caffeine also blocks vasodilating adenosine receptors.105 The rapid metabolizers of caffeinated coffee IA/IA allele have lower BP and lower risk of MI.103 Hypertension has a 0.36–0.80 relative risk ratio, with an average reduction in BP of 10/7 mm Hg. MI shows a 17–52% reduction. This SNP represents about 40–45% of the population.98–103 The slow metabolizers of caffeine IF/IF or IA/IF allele have higher BP 8.1/5.7 mm Hg, lasting >3 h after consumption, tachycardia, increased aortic stiffness, higher pulse wave velocity, vascular inflammation and increased catecholamines.98,100 Hypertension risk is increased [1.72–3.00 relative risk (RR)].99 Based on age and consumption, the risk of MI will vary. At age 59 there was a 36% increase in MI with 2–3 cups/day and a 64% increase with 4 cups/day or more. Under the age of 59, MI increased by 24% (1 cup/day), 67% (2 cups/day) and 233% (4 or more cups/day).103,104 This SNP represents about 55–60% of the population.
Nutritional supplements
Numerous nutritional supplements have demonstrated improvement in surrogate endpoints (BP, lipids, glucose, carotid IMT, coronary calcification, etc.). However, there are limited data that nutraceutical supplements reduced hard CV endpoints related to CHD, MI, and CHF. The nutritional supplements that will be discussed below in the paper offer some evidence of surrogate endpoints for CHD and others indicate some improvement in angina, CHD, MI and CHF. Only those supplements with scientific data in these mentioned CV outcomes will be reviewed in this paper. The dose of the supplement will vary depending on prevention or treatment considerations, age, body weight and concomitant medications or other supplements. All nutritional supplements should be obtained from highly certified and reputable nutritional supplement companies that undergo scrutiny with state and federal authorities to assure safety and efficacy standards.
Omega 3 fatty acids
Omega 3 FA supplementation reduces all-cause mortality and MI in primary and secondary prevention trials, as well as many other CV outcomes70,106–120 Omega 3 FAs decrease MI and CHD 18% more with concomitant use of statins,110 reduce stent restenosis,108 CABG occlusion,112,113 plaque formation,114,115 coronary artery calcification,114,115 atherosclerosis,114,115 improve the lipid profile,18,116 lower glucose and improve insulin resistance117–119 and reduce BP.2,4,16,120 The dose prescribed will depend on the condition being treated, as well as age, body weight and use of concomitant medications and other nutritional supplements. It is best to use a balanced formulation with DHA, EPA, gamma linolenic acid (GLA) and gamma–delta tocopherols. This will prevent oxidation in the cell membranes and reduce depletions of the EPA and DHA by GLA or vice versa.18,116,120
D-Ribose
D-Ribose improves cardiomyopathy, systolic and diastolic CHF, acute and chronic CHD and angina, stabilizes and energizes the heart post MI and improves the postoperative ejection fraction in CABG. D-Ribose increases myocardial adenosine triphosphate (ATP) and energetics within 1 h of administration and lasts for about 6–8 h. It is well tolerated, with minor side effects including diarrhea and rarely mild reductions in serum glucose. The dose is 5 g three times daily.121–126
Vitamin K
In an NHS cohort of 72,874 female nurses, there was a 16% RR reduction in CHD from lowest to highest quintile of vitamin K1 (phylloquinone) intake.127 In the HPFUS of 40,087 men, there was a 13–16% RR reduction in CHD.128 The Multi-Ethnic Study of Atherosclerosis (MESA) showed that a low vitamin K1 dietary intake showed increased CAC, especially in those on antihypertensive drugs, by twofold.129 A population-based study of 4807 participants found the incident risk for CHD was reduced by Vitamin K1 and Vitamin K2 (menaquinone). K2 reduced CHD by 57% in the upper versus lower tertile and K2 reduced all-cause mortality by 26% in the upper versus lower tertile. K2 reduced aortic calcification by 52% in the upper versus lower tertile and reduced total mortality by 26%, but there was no association with K1.130 The vitamin-K-dependent matrix Gla protein is a potent inhibitor of the arterial calcification and may become a noninvasive biochemical marker for vascular calcification. Vitamin K2 is considered more important for vascular system health, if compared with vitamin K1.131 The recommended dose of K2 MK7 is at least 100–150 mg per day.
Carnitine
L-carnitine reduced the all-cause mortality by 27%, ventricular arrhythmias by 65% and angina by 40% following an acute MI compared with placebo in 13 controlled trials of 3629 participants.132 There was no change in CHF or recurrent MI. Concerns about the possible effects of carnitine on the microbiome and TMAO (trimethylamine N-oxide) production have to be evaluated against the positive effects in these studies. It is not clear if TMAO has a cause-and-effect relationship with CHD.133 Carnitine is important in the transport of LCFAs of C-12 or greater into the myocardium for beta oxidation of FAs that supply 60% of the ATP for the cardiac myocyte.134
Curcumin
In a study of 121 patients, curcumin reduced MI post CABG from 30% to 13% (p < 0.038) at 4 g per day given 3 days before and 5 days after CABG.135 The hsCRP, malondialdehyde and N-terminal prohormone of B-type brain natriuretic peptide (NT-pro BNP) were also lower. Curcumin significantly attenuated collagen deposition in mice after coronary artery ligation-induced MI and inhibited cardiac fibroblast proliferation and migration and metalloproteinase expression. In addition, there was downregulation of SIRT1 (Sirtuin 1) after MI that was attenuated by curcumin pretreatment, which indicated that the activation of SIRT1 might be involved in the protective action of curcumin.136
Co-enzyme Q10
Co-enzyme Q10 (CoQ10) reduces post-MI reperfusion ventricular arrhythmias, improved LV function and total cardiac death.137,138 In a double blind placebo controlled (DBRPC) trial of 144 subjects with acute MI, CoQ10 at 120 mg per day administered within the first 3 days of an MI resulted in significant improvements in the treated group in all parameters (p < 0.05):138
Angina (9.5% versus 25.3%);
Arrhythmias (9.5% versus 25.3%);
LVF improved (8.2 versus 22.5%);
Total cardiac events and death reduced at 15 versus 30.9% (p < 0.02).
The Q –SYMBIO Trial of CoQ10 and CHF is the most important of all the studies yet published on CoQ10 and heart failure.139 This was a randomized DBRPC trial of 420 patients with CHF New York Heart Association (NYHA) class III and IV over 10 years. Subjects were administered 2 mg/kg CoQ10 per day (100 mg tid) versus placebo plus standard therapy. Serum levels of CoQ10 increased three times above baseline. The primary short-term endpoints were NYHA function class, a 6 min walk test, NT-pro BNP and ejection fraction (EF). There was no difference between groups and nonsignificant change in EF in these short-term primary endpoints. The CoQ10-treated subjects had reduced major adverse cardiac events by 50% (p = 0.003, CI: 32–80) and all-cause mortality by 42%. Major adverse CV events were defined as hospitalization or death due to CHF, CV/MI death, SCD, cardiac transplant or mechanical circulatory support. There was a reduction in CV mortality from 16% to 9% (p = 0.026), all-cause mortality from 18% to 10% (p = 0.018) and reduction in the incidence of hospital stays for CHF (p = 0.033). The NYHA class was significantly improved in the CoQ10 group at 2 years (p = 0.028).
Conclusion
The top five CV risk factors, as presently defined, are not an adequate explanation for the current limited reduction in CHD or the ‘CHD gap’. Proper definition and analysis of the top five risk factors, evaluating the other 395 risk factors and downstream mediators should be included with measurement of the three finite responses of inflammation, oxidative stress and vascular immune dysfunction, micronutrient testing, CV genetics, nutrigenomics, metabolomics, gene expression testing and noninvasive vascular testing. Early detection coupled with aggressive prevention and treatment of all CV risk factors will diminish the progression of functional CV abnormalities, CV structural problems and clinical CVD. If we wish to revolutionize the prevention and treatment of CVD then a new approach should be implemented in the clinical setting. This will be achievable by using a combination of targeted, personalized and precision treatments with optimal nutrition and nutraceutical supplements coupled with optimal exercise, weight and body composition management and a reduction in tobacco use. Approximately 80% of CHD can be prevented with this approach.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
References
- 1. Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in six European countries, Canada and the United States. JAMA 2003; 289: 2363–2369. [DOI] [PubMed] [Google Scholar]
- 2. O’Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med 2011; 365: 2098–2109. [DOI] [PubMed] [Google Scholar]
- 3. Houston MC. Nutrition and nutraceutical supplements in the treatment of hypertension. Expert Rev Cardiovasc Ther 2010; 8: 821–833. [DOI] [PubMed] [Google Scholar]
- 4. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 6. Youssef-Elabd EM, McGee KC, Tripathi G, et al. Acute and chronic saturated fatty acid treatment as a key instigator of the TLR-mediated inflammatory response in human adipose tissue, in vitro. J Nutr Biochem 2012; 23: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Khatib N, Génieys S, Kazmierczak B, et al. Mathematical modelling of atherosclerosis as an inflammatory disease. Philos Transact A Math Phys Eng Sci 2009; 367: 4877–4886. [DOI] [PubMed] [Google Scholar]
- 8. Coutinho M, Gerstein HC, Wang Y, et al. The relationship between glucose and incident cardiovascular events. A meta-regression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999; 22: 233–240. [DOI] [PubMed] [Google Scholar]
- 9. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998; 21: 360–367. [DOI] [PubMed] [Google Scholar]
- 10. Pereg D, Elis A, Neuman Y, et al. Cardiovascular risk in patients with fasting blood glucose levels within normal range. Am J Cardiol 2010; 106: 1602–1605. [DOI] [PubMed] [Google Scholar]
- 11. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 12. Houston MC, Basile J, Bestermann WH, et al. Addressing the global cardiovascular risk of hypertension, dyslipidemia, and insulin resistance in the southeastern United States. Am J Med Sci 2005; 329: 276–291. [DOI] [PubMed] [Google Scholar]
- 13. Ko MJ, Jo AJ, Park CM, et al. Level of blood pressure control and cardiovascular events: SPRINT criteria versus the 2014 hypertension recommendations. J Am Coll Cardiol 2016; 67: 2821–2831. [DOI] [PubMed] [Google Scholar]
- 14. Bestermann W, Houston MC, Basile J, et al. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci 2005; 329: 292–305. [DOI] [PubMed] [Google Scholar]
- 15. Duprez DA, Florea N, Zhong W, et al. Vascular and cardiac functional and structural screening to identify risk of future morbid events: preliminary observations. J Am Soc Hypertens 2011; 5: 401–409. [DOI] [PubMed] [Google Scholar]
- 16. White WB, Gulati V. Managing hypertension with ambulatory blood pressure monitoring. Curr Cardiol Rep 2015; 17: 2. [DOI] [PubMed] [Google Scholar]
- 17. Della Rocca DG, Pepine CJ. Endothelium as a predictor of adverse outcomes. Clin Cardiol 2010; 33: 730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pucci G, Cicero AF, Borghi C, et al. Emerging biologic therapies for hypercholesterolaemia. Expert Opin Biol Ther 2017; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 19. Lundberg AM, Yan ZQ. Innate immune recognition receptors and damage-associated molecular patterns in plaque inflammation. Curr Opin Lipidol 2011; 22: 343–349. [DOI] [PubMed] [Google Scholar]
- 20. Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev 2011; 69: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mah E, Bruno RS. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res 2012; 32: 727–740. [DOI] [PubMed] [Google Scholar]
- 22. Fazio S, Linton MF. High-density lipoprotein therapeutics and cardiovascular prevention. J Clin Lipidol 2010; 4: 411–419. [DOI] [PubMed] [Google Scholar]
- 23. Van der Steeg WA, Holme I, Boekholdt SM, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol 2008; 51: 634–642. [DOI] [PubMed] [Google Scholar]
- 24. Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol 2010; 55: 1688–1696. [DOI] [PubMed] [Google Scholar]
- 25. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004; 44: 2137–2141. [DOI] [PubMed] [Google Scholar]
- 26. Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117: 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoenenberger AW, Urbanek N, Bergner M, et al. Associations of reactive hyperemia index and intravascular ultrasound-assessed coronary plaque morphology in patients with coronary artery disease. Am J Cardiol 2012; 109: 1711–1716. [DOI] [PubMed] [Google Scholar]
- 28. Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc 2013; 2: e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prisant LM, Pasi M, Jupin D, et al. Assessment of repeatability and correlates of arterial compliance. Blood Press Monit 2002; 7: 231–235. [DOI] [PubMed] [Google Scholar]
- 30. Cohn JN, Hoke L, Whitwam W, et al. Screening for early detection of cardiovascular disease in asymptomatic individuals. Am Heart J 2003; 146: 679–685. [DOI] [PubMed] [Google Scholar]
- 31. Nelson MR, Stepanek J, Cevette M, et al. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc 2010; 85: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashimoto J, Ito S. Some mechanical aspects of arterial aging: physiological overview based on pulse wave analysis. Ther Adv Cardiovasc Dis 2009; 3: 367–378. [DOI] [PubMed] [Google Scholar]
- 33. Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep 2009; 11: 21–27. [DOI] [PubMed] [Google Scholar]
- 34. Bots ML, Taylor AJ, Kastelein JJ, et al. Rate of change in carotid intima-media thickness and vascular events: meta-analyses cannot solve all the issues. A point of view. J Hypertens 2012; 30: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 35. Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007; 115: 459–467. [DOI] [PubMed] [Google Scholar]
- 36. Rizzoni D, Porteri E, Duse S, et al. Relationship between media-to-lumen ratio of subcutaneous small arteries and wall-to-lumen ratio of retinal arterioles evaluated noninvasively by scanning laser Doppler flowmetry. J Hypertens 2012; 30: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 37. Ying GS, Maguire M, Pistilli M, et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease [from the Chronic Renal Insufficiency Cohort (CRIC) Study]. Am J Cardiol 2012; 110: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Virdis A, Savoia C, Grassi G, et al. Evaluation of microvascular structure in humans: a ‘state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J Hypertens 2014; 32: 2120–2129. [DOI] [PubMed] [Google Scholar]
- 39. Al-Fiadh AH, Wong TY, Kawasaki R, et al. Usefulness of retinal microvascular endothelial dysfunction as a predictor of coronary artery disease. Am J Cardiol 2015; 115: 609–613. [DOI] [PubMed] [Google Scholar]
- 40. Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008; 168: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi Y, Chang Y, Ryu S, et al. Relation of dietary glycemic index and glycemic load to coronary artery calcium in asymptomatic Korean adults. Am J Cardiol 2015; 116: 520–526. [DOI] [PubMed] [Google Scholar]
- 42. Ahmadi N, Tsimikas S, Hajsadeghi F, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol 2010; 105: 459–466. [DOI] [PubMed] [Google Scholar]
- 43. Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 2004; 24: 1272–1277. [DOI] [PubMed] [Google Scholar]
- 44. Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014; 311: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmermund A, Elsässer A, Behl M, et al. Comparison of prognostic usefulness (three years) of computed tomographic angiography versus 64-slice computed tomographic calcium scanner in subjects without significant coronary artery disease. Am J Cardiol 2010; 106: 1574–1579. [DOI] [PubMed] [Google Scholar]
- 46. Ochs MM, Siepen FA, Fritz T, et al. Limits of the possible: diagnostic image quality in coronary angiography with third-generation dual-source CT. Clin Res Cardiol 2017; 106: 485–492. [DOI] [PubMed] [Google Scholar]
- 47. Rong J, Bai SR, Chen YL, et al. Increased detection of coronary atherosclerosis on 320-slice computed tomographic angiography with burden of cardiovascular risk factors and complications in patients with type 2 diabetes. J Diabetes Complications 2016; 30: 494–500. [DOI] [PubMed] [Google Scholar]
- 48. Kawaji T, Kimura T. The Diagnostic Performance of Multifunction Cardiogram (MCG) in functional myocardial ischemia. Ann Noninvasive Electrocardiol 2015; 20: 508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kawaji T, Shiomi H, Morimoto T, et al. Noninvasive detection of functional myocardial ischemia: multifunction cardiogram evaluation in diagnosis of functional coronary ischemia Study (MED-FIT). Ann Noninvasive Electrocardiol 2015; 20: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kandori A, Ogata K, Miyashita T, et al. Subtraction magnetocardiogram for detecting coronary heart disease. Ann Noninvasive Electrocardiol 2010; 15: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwon H, Kim K, Lee YH, et al. Non-invasive magnetocardiography for the early diagnosis of coronary artery disease in patients presenting with acute chest pain. Circ J 2010; 74: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 52. Greenwood JP, Herzog BA, Brown JM, et al. Prognostic value of cardiovascular magnetic resonance and single-photon emission computed tomography in suspected coronary heart disease: long-term follow-up of a prospective, diagnostic accuracy cohort Study. Ann Intern Med. Epub ahead of print 10 May 2016. DOI: 10.7326/M15-1801. [DOI] [PubMed] [Google Scholar]
- 53. Emami H, Tawakol A. Noninvasive imaging of arterial inflammation using FDG-PET/CT. Curr Opin Lipidol 2014; 25: 431–437. [DOI] [PubMed] [Google Scholar]
- 54. Bucci LR. A functional analytical technique for monitoring nutrient status and repletion. Part 3: clinical experience. Am Clin Lab 1994; 13: 10–11. [PubMed] [Google Scholar]
- 55. Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010; 92: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 56. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 57. Nadtochiy SM, Redman EK. Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 2011; 27: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014; 160: 1–10. [DOI] [PubMed] [Google Scholar]
- 59. De Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999; 99: 779–785. [DOI] [PubMed] [Google Scholar]
- 60. Buckland G, Mayén AL, Agudo A, et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am J Clin Nutr 2012; 96: 142–149. [DOI] [PubMed] [Google Scholar]
- 61. Castañer O, Corella D, Covas MI, et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: a randomized controlled trial. Am J Clin Nutr 2013; 98: 845–853. [DOI] [PubMed] [Google Scholar]
- 62. Konstantinidou V, Covas MI, Sola R, et al. Up-to-date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol Nutr Food Res 2013; 57: 772–783. [DOI] [PubMed] [Google Scholar]
- 63. Corella D, Ordovás JM. How does the Mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. Bioessays 2014; 36: 526–537. [DOI] [PubMed] [Google Scholar]
- 64. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 65. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10. [DOI] [PubMed] [Google Scholar]
- 66. Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008; 168: 713–720. [DOI] [PubMed] [Google Scholar]
- 67. Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014; 160: 398–406. [DOI] [PubMed] [Google Scholar]
- 68. Guasch-Ferré M, Babio N, Martínez-González MA, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr 2015; 102: 1563–1573. [DOI] [PubMed] [Google Scholar]
- 69. Ravnskov U, DiNicolantonio JJ, Harcombe Z, et al. The questionable benefits of exchanging saturated fat with polyunsaturated fat. Mayo Clin Proc 2014; 89: 451–453. [DOI] [PubMed] [Google Scholar]
- 70. Alexander DD, Miller PE, Van Elswyk ME, et al. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc 2017: 92: 15–29. [DOI] [PubMed] [Google Scholar]
- 71. DiNicolantonio JJ, Lucan SC, O’Keefe JH. The evidence for saturated fat and for sugar related to coronary heart disease. Prog Cardiovasc Dis 2016; 58: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Praagman J, Beulens JW, Alssema M, et al. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am J Clin Nutr 2016; 103: 356–365. [DOI] [PubMed] [Google Scholar]
- 73. Chen M, Li Y, Sun Q, et al. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr 2016; 104: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zong G, Li Y, Wanders AJ, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ 2016; 355: i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010; 45: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015; 351: h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruiz-Núñez B, Dijck-Brouwer DA, Muskiet FA. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem 2016; 36: 1–20. [DOI] [PubMed] [Google Scholar]
- 78. Eyres L, Eyres MF, Chisholm A, et al. Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev 2016; 74: 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ballard KD, Bruno RS. Protective role of dairy and its constituents on vascular function independent of blood pressure-lowering activities. Nutr Rev 2015; 73: 36–50. [DOI] [PubMed] [Google Scholar]
- 80. Chrysant SG, Chrysant GS. An update on the cardiovascular pleiotropic effects of milk and milk products. J Clin Hypertens (Greenwich) 2013; 15: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Siri-Tarino PW, Krauss RM. Diet, lipids, and cardiovascular disease. Curr Opin Lipidol 2016; 27: 323–328. [DOI] [PubMed] [Google Scholar]
- 82. Bernstein AM, de Koning L, Flint AJ, et al. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 2012; 95: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eshak ES, Iso H, Kokubo Y, et al. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr 2012; 96: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 84. Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab 2013; 24: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shankar P, Ahuja S, Sriram K. Non-nutritive sweeteners: review and update. Nutrition 2013; 29: 1293–1299. [DOI] [PubMed] [Google Scholar]
- 86. Crowe FL, Appleby PN, Travis RC, et al. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr 2013; 97: 597–603. [DOI] [PubMed] [Google Scholar]
- 87. Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med 2013; 173: 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miedema MD, Petrone A, Shikany JM, et al. Association of fruit and vegetable consumption during early adulthood with the prevalence of coronary artery calcium after 20 years of follow-up: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation 2015; 132: 1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dauchet L, Amouyel P, Hercberg S, et al. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies J Nutr 2006; 136: 2588–2593. [DOI] [PubMed] [Google Scholar]
- 90. Ingenbleek Y, McCully KS. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition 2012; 28: 148–153. [DOI] [PubMed] [Google Scholar]
- 91. Whalen KA, Judd S, McCullough ML, et al. Paleolithic and Mediterranean diet pattern scores are inversely associated with All-Cause and Cause-Specific Mortality in Adults. J Nutr 2017; 147: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Roussell MA, Hill AM, Gaugler TL, et al. Beef in an optimal lean diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr 2012; 95: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee JE, McLerran DF, Rolland B, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr 2013; 98: 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010; 121: 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bellavia A, Larsson SC, Bottai M, et al. Differences in survival associated with processed and with nonprocessed red meat consumption. Am J Clin Nutr 2014; 100: 924–929. [DOI] [PubMed] [Google Scholar]
- 96. Lajous M, Bijon A, Fagherazzi G, et al. Processed and unprocessed red meat consumption and hypertension in women. Am J Clin Nutr 2014; 100: 948–952. [DOI] [PubMed] [Google Scholar]
- 97. Houston MC, Cooil B, Olafsson BJ, et al. Juice powder concentrate and systemic blood pressure, progression of coronary artery calcium and antioxidant status in hypertensive subjects: a pilot study. Evid Based Complement Alternat Med 2007; 4: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Palatini P, Ceolotto G, Ragazzo F, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. Hypertens 2009; 27: 1594–601. [DOI] [PubMed] [Google Scholar]
- 99. Hu G, Jousilahti P, Nissinen A, et al. Coffee consumption and the incidence of antihypertensive drug treatment in Finnish men and women. Am J Clin Nutr 2007; 86: 457–464. [DOI] [PubMed] [Google Scholar]
- 100. Vlachopoulos CV, Vyssoulis GG, Alexopoulos NA, et al. Effect of chronic coffee consumption on aortic stiffness and wave reflections in hypertensive patients. Eur J Clin Nutr 2007; 61: 796–802. [DOI] [PubMed] [Google Scholar]
- 101. Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, et al. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr 2011; 94: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 102. Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Lipidol 2007; 18: 13–19. [DOI] [PubMed] [Google Scholar]
- 103. Cornelis MC, El-Sohemy A, Kabagambe EK, et al. CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006; 295: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 104. Liu J, Sui X, Lavie CJ, et al. Association of coffee consumption with all-cause and cardiovascular disease mortality. Mayo Clin Proc 2013; 88: 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Renda G, Zimarino M, Antonucci I, et al. R8 determinants of blood pressure responses to caffeine drinking. Am J Clin Nutr 2012; 95: 241–248. [DOI] [PubMed] [Google Scholar]
- 106. Finzi AA, Latini R, Barlera S, et al. Effects of n-3 polyunsaturated fatty acids on malignant ventricular arrhythmias in patients with chronic heart failure and implantable cardioverter-defibrillators: a substudy of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Am Heart J 2011; 161: 338–343. [DOI] [PubMed] [Google Scholar]
- 107. Mozaffarian D, Lemaitre RN, King IB, et al. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 2013; 158: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gajos G, Zalewski J, Rostoff P, et al. Reduced thrombin formation and altered fibrin clot properties induced by polyunsaturated omega-3 fatty acids on top of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention (OMEGA-PCI clot). Arterioscler Thromb Vasc Biol 2011; 31: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 109. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther 2009; 16: 326–332. [DOI] [PubMed] [Google Scholar]
- 110. Nozue T, Yamamoto S, Tohyama S, et al. Effects of serum n-3 to n-6 polyunsaturated fatty acids ratios on coronary atherosclerosis in statin-treated patients with coronary artery disease. Am J Cardiol 2013; 111: 6–11. [DOI] [PubMed] [Google Scholar]
- 111. Greene SJ, Temporelli PL, Campia U, et al. Effects of polyunsaturated fatty acid treatment on postdischarge outcomes after acute myocardial infarction. Am J Cardiol 2016; 117: 340–346. [DOI] [PubMed] [Google Scholar]
- 112. Arnesen H. n-3 fatty acids and revascularization procedures. Lipids 2001; 36(Suppl.): S103–S106. [DOI] [PubMed] [Google Scholar]
- 113. Arnesen H, Seljeflot I. Studies on very long chain marine n-3 fatty acids in patients with atherosclerotic heart disease with special focus on mechanisms, dosage and formulas of supplementation. Cell Mol Biol (Noisy-le-grand) 2010; 56: 18–27. [PubMed] [Google Scholar]
- 114. Sekikawa A, Miura K, Lee S, et al. Long chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese men in Japan and white men in the USA: population based prospective cohort study. Heart 2014; 100: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Abedin M, Lim J, Tang TB, et al. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res 2006; 98: 727–729. [DOI] [PubMed] [Google Scholar]
- 116. Jacobson TA, Glickstein SB, Rowe JD, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol 2012; 6: 5–18. [DOI] [PubMed] [Google Scholar]
- 117. Jans A, Konings E, Goossens GH, et al. PUFAs acutely affect triacylglycerol-derived skeletal muscle fatty acid uptake and increase postprandial insulin sensitivity. Am J Clin Nutr 2012; 95: 825–836. [DOI] [PubMed] [Google Scholar]
- 118. García-López S, Villanueva Arriaga RE, Nájera Medina O, et al. One month of omega-3 fatty acid supplementation improves lipid profiles, glucose levels and blood pressure in overweight schoolchildren with metabolic syndrome. J Pediatr Endocrinol Metab 2016; 29: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 119. Sawada T, Tsubata H, Hashimoto N, et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol 2016; 15: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol 2014; 6: 38–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wagner S, Herrick J, Shecterle LM, et al. D-ribose, a metabolic substrate for congestive heart failure. Prog Cardiovasc Nurs 2009; 24: 59–60. [DOI] [PubMed] [Google Scholar]
- 122. Shecterle LM, Terry KR, St Cyr JA. The patented uses of D-ribose in cardiovascular diseases. Recent Pat Cardiovasc Drug Discov 2010; 5: 138–142. [DOI] [PubMed] [Google Scholar]
- 123. Perkowski DJ, Wagner S, Schneider JR, et al. A targeted metabolic protocol with D-ribose for off-pump coronary artery bypass procedures: a retrospective analysis. Ther Adv Cardiovasc Dis 2011; 5: 185–192. [DOI] [PubMed] [Google Scholar]
- 124. Omran H, Illien S, MacCarter D, et al. D-ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur J Heart Fail 2003; 5: 615–619. [DOI] [PubMed] [Google Scholar]
- 125. Pauly DF, Pepine CJ. D-ribose as a supplement for cardiac energy metabolism. J Cardiovasc Pharmacol Ther 2000; 5: 249–258. [DOI] [PubMed] [Google Scholar]
- 126. Bayram M, St Cyr JA, Abraham WT. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther Adv Cardiovasc Dis 2015; 9: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr 2005; 59: 196–204. [DOI] [PubMed] [Google Scholar]
- 128. Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis 2007; 17: 58–62. [DOI] [PubMed] [Google Scholar]
- 129. Shea MK, Booth SL, Miller ME, et al. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013; 98: 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 2004; 134: 3100–3105. [DOI] [PubMed] [Google Scholar]
- 131. Fodor D, Albu A, Poantă L, et al. Vitamin K and vascular calcifications. Acta Physiol Hung 2010; 97: 256–266. [DOI] [PubMed] [Google Scholar]
- 132. DiNicolantonio JJ, Lavie CJ, Fares H, et al. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc 2013; 88: 544–551. [DOI] [PubMed] [Google Scholar]
- 133. Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017; 38: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mingorance C, Rodriguez-Rodriguez R, Justo ML, et al. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nut Review 2011; 69: 279. [DOI] [PubMed] [Google Scholar]
- 135. Wongcharoen W, Jai-Aue S, Phrommintikul A, et al. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol 2012; 110: 40–44. [DOI] [PubMed] [Google Scholar]
- 136. Xiao J, Sheng X, Zhang X, et al. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther 2016; 10: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chello M, Mastroroberto P, Romano R, et al. Protection by coenzyme Q10 from myocardial reperfusion injury during coronary artery bypass grafting. Ann Thorac Surg 1994; 58: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 138. Singh RB, Wander GS, Rastogi A, et al. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc Drugs Ther 1998; 12: 347–353. [DOI] [PubMed] [Google Scholar]
- 139. Mortensen SA, Rosenfeldt F, Kumar A, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail 2014; 2: 641–649. [DOI] [PubMed] [Google Scholar]