Abstract
Metabolic syndrome (MetS) represents a cluster of metabolic abnormalities that include hypertension, central obesity, insulin resistance, and atherogenic dyslipidemia, and is strongly associated with an increased risk for developing diabetes and atherosclerotic and nonatherosclerotic cardiovascular disease (CVD). The pathogenesis of MetS involves both genetic and acquired factors that contribute to the final pathway of inflammation that leads to CVD. MetS has gained significant importance recently due to the exponential increase in obesity worldwide. Early diagnosis is important in order to employ lifestyle and risk factor modification. Here, we review the epidemiology and pathogenesis of MetS, the role of inflammation in MetS, and summarize existing natural therapies for MetS.
Keywords: metabolic syndrome, nutraceuticals, cardiovascular disease, hypertension, obesity, insulin resistance, atherogenic dyslipidemia
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities that includes hypertension, central obesity, insulin resistance, and atherogenic dyslipidemia. MetS is strongly associated with an increased risk of developing atherosclerotic cardiovascular disease (CVD).1 The pathogenesis of MetS involves both genetic and acquired factors that play a role in the final pathway of inflammation that leads to CVD. MetS has become increasingly relevant in recent times due to the exponential increase in obesity worldwide. Early diagnosis is important in order to employ effectively lifestyle and risk factor modification. Pharmaceutical therapy in MetS is aimed at treating the individual components of MetS such as antihypertensives, statins, and metformin. Some natural compounds and dietary elements, also called nutraceuticals, have been shown to have some benefit in the treatment of MetS. Here, we review the epidemiology and pathogenesis of MetS, the role of inflammation in MetS, and summarize existing natural therapies for MetS.
MetS: definitions and epidemiology
Definitions
MetS, also labeled as ‘insulin resistance syndrome’, ‘syndrome X’, ‘hypertriglyceridemic waist’, and ‘the deadly quartet’, is increasingly being recognized as an important cardiovascular risk factor. The diabetes consultation group of the World Health Organization created the first internationally recognized definition of MetS in 1998.1 They defined MetS as the presence of insulin resistance (impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes mellitus) in addition to two of the following risk factors: obesity (waist–hip ratio or body mass index), hyperlipidemia (hypertriglyceridemia, low high-density lipoprotein [HDL] cholesterol), hypertension, or microalbuminuria. Since the initial description of MetS, several iterations of this definition have been proposed (Table 1). MetS defined by any of the above criteria, remains a predictor of atherosclerotic CVD.
Table 1.
Definitions of metabolic syndrome.
| Clinical measure | World Health Organization 19987 |
European Group for the Study of Insulin Resistance 19998 | Adult Treatment Panel III of the National Cholesterol Education Program 200110 | International Diabetes Federation 200511 |
American Heart Association/National Heart, Lung, and Blood Institute 200512 |
|---|---|---|---|---|---|
| Criteria | IR + any other 2 | IR + any other 2 | Any 3 of 5 | Increased WC (population specific) + any other 2 | Any 3 of 5 |
| Insulin resistance | IGT/IFG IR | Plasma insulin > 75th percentile | – | – | – |
| Blood glucose | IFG/IGT/T2DM | IFG/IGT (excludes diabetes) |
⩾ 110 mg/dL (includes diabetes) | ⩾ 100 mg/dL | ⩾ 100 mg/dL (includes diabetes) |
| Dyslipidemia | TG ⩾ 1.69 mmol/L and HDL-C men < 0.90 mmol/L women < 1.01 mmol/L |
TG ⩾ 1.69 mmol/L and HDL-C < 1.01 mmol/L in men and women |
TG ⩾ 1.69 mmol/L HDL-C men < 1.03 mmol/L women < 1.29 mmol/L |
TG ⩾ 1.69 mmol/L or on TG treatment HDL-C men < 1.03 mmol/L women < 1.29 mmol/L Or HDL treatment |
TG ⩾ 1.69 mmol/L or on TG treatment HDL-C men < 1.03 mmol/L women < 1.29 mmol/L Or HDL treatment |
| Blood pressure | ⩾ 140/90 mmHg | ⩾ 140/90 mmHg or on antihypertensive medications | ⩾ 130/85 mmHg or on antihypertensive medications | ⩾ 130/85 mmHg or on antihypertensive medications | ⩾ 130/85 mmHg or on antihypertensive medications |
| Obesity | Waist: hip ratio men > 0.9 women > 0.85 and/or BMI > 30 kg/m2 |
WC men ⩾ 94 cm women ⩾ 80 cm |
WC men ⩾ 102 cm women ⩾ 88 cm |
WC ⩾ 94 cm | WC men ⩾ 102 cm women ⩾ 88 cm |
| Other | Microalbuminuria |
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IR, insulin resistance; T2DM, type 2 diabetes mellitus; TG, triglycerides; WC, waist circumference. (Adapted from the American Heart Association/National Heart, Lung, and Blood Institute report.12)
Epidemiology and gender differences in MetS
The prevalence of MetS varies around the world and often corresponds with the prevalence of obesity. There is a wide variation in prevalence based on age, gender, race/ethnicity, and the criteria used for diagnosis. MetS affects a fifth or more of the population of the USA and about a quarter of the population of Europe. South-east Asia has a lower prevalence of MetS but is rapidly moving towards rates similar to the western world. Beltrán-Sánchez and colleagues reported a decrease in the age-adjusted prevalence of MetS in the USA, from 25% in 2000 to 22.9% between 1999/2000 and 2009/2010 based on National Health and Nutrition Examination Survey (NHANES) data.2
There are also gender- and race-based variations in MetS. Ford and colleagues described the distribution of MetS components in the USA based on gender and race using NHANES data collected between 1988 and 1994.3 The prevalence of MetS in African-American women is 57% higher than in African-American men and 26% higher in Hispanic women compared with Hispanic men. Among the components of MetS, insulin resistance is more common in Hispanics, hypertension in African-Americans, and dyslipidemia in Whites.4 A more recent analysis of the same database by Miller and colleagues estimated that 10.1% of US adolescents have MetS.5 They reported that males have a higher prevalence of MetS compared with females and that Hispanics appear to be at a higher risk.
Pathogenesis of MetS: role of inflammation
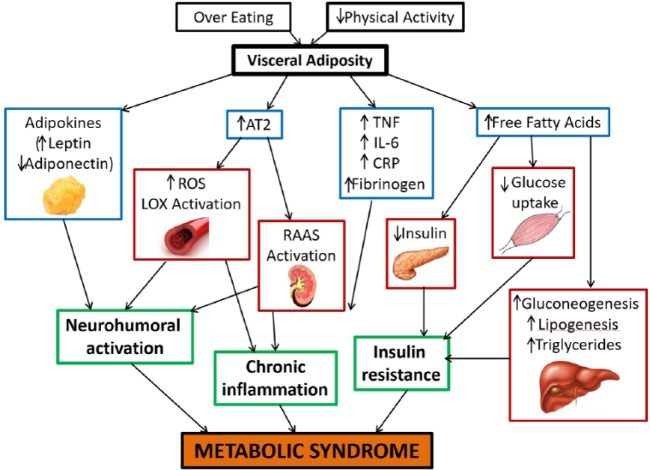
The pathogenic mechanisms of MetS are complex and remain to be fully elucidated. Whether the individual components of MetS represent distinct pathologies or manifestations of a common pathogenic mechanism is still debated. The wide variation in geographic distribution of MetS and the recent ‘catch up’ in the developing world emphasize the importance of environmental and lifestyle factors such as the consumption of excess calories and lack of physical activity as being major contributors. Visceral adiposity has been demonstrated to be a primary trigger for most of the pathways involved in MetS, thus stressing the importance of a high caloric intake as a major causative factor.6 Of all the proposed mechanisms, insulin resistance, neurohormonal activation, and chronic inflammation appear to be the main players in the initiation, progression, and transition of MetS to CVD (Figure 1).
Figure 1.
Pathophysiological mechanisms in metabolic syndrome. AT2, angiotensin II type 2 receptor; CRP, C-reactive protein; IL-6, interleukin 6; LOX, lectin-like oxidized low-density lipoprotein; RAAS, renin-angiotensin-aldosterone system; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Insulin resistance
Insulin resistance-mediated increase in circulating free fatty acids (FFAs) is believed to play a pivotal role in the pathogenesis of MetS. Insulin increases glucose uptake in muscle and liver, and inhibits lipolysis and hepatic gluconeogenesis. Insulin resistance in adipose tissue impairs insulin-mediated inhibition of lipolysis, leading to an increase in circulating FFAs that further inhibit the antilipolytic effect of insulin.7 FFAs inhibit protein kinase activation in the muscle leading to reduced glucose uptake. They increase protein kinase activation in the liver that promotes gluconeogenesis and lipogenesis. The net effect is the creation of a hyperinsulinemic state to maintain euglycemia. Eventually, the compensation fails and insulin secretion decreases. FFAs are also lipotoxic to beta cells of the pancreas causing decreased insulin secretion.8 Insulin resistance also contributes to the development of hypertension due to loss of the vasodilator effect of insulin and vasoconstriction caused by FFAs.9 Additional mechanisms include increased sympathetic activation and sodium reabsorption in the kidneys. Insulin resistance also causes an increase in serum viscosity, induction of a prothrombotic state, and release of pro-inflammatory cytokines from the adipose tissue that contribute to increased risk of CVD.10
Visceral fat deposits contribute to insulin resistance more than subcutaneous fat, as visceral lipolysis leads to an increased supply of FFAs to the liver through the splanchnic circulation. Increase in FFAs leads to increased triglyceride synthesis and the production of apolipoprotein B containing triglyceride-rich very low-density lipoprotein (LDL) in the liver.11 Increase in small dense LDL cholesterol and reduction in HDL cholesterol are indirect effects of insulin resistance caused by altered lipid metabolism in the liver. Visceral adipose tissue is also considered more metabolically active and synthesizes significantly higher amounts of bioactive secretory proteins such as plasminogen activator inhibitor, which promotes a prothrombotic state, and heparin-binding epidermal growth factor like growth factor, which promotes smooth muscle cell proliferation and vascular remodeling.
Neurohormonal activation
The discovery of endocrine and immune properties of adipocytes has provided further mechanistic insights into the development of MetS. Adipokines released from visceral adipose tissue have been shown to be associated with MetS and CVD.12 Leptin is an adipokine that controls energy homeostasis mediated by the hypothalamus and is known to stimulate the immune cells activating the Th1 pathway. Obesity increases leptin levels and higher leptin levels are directly correlated to increased cardiovascular risk. Adiponectin is an anti-inflammatory and anti-atherogenic adipokine and its effects counter those of leptin. Adiponectin has anti-atherogenic properties and it decreases both vascular reactivity and smooth muscle proliferation, and improves plaque stability.13 Adiponectin has been considered a protective factor against the development of diabetes, hypertension, and acute myocardial infarction.14,15 An increase in adipose tissue mass correlates with reduced adiponectin and higher leptin levels, which eventually enhance CVD risk.
Activation of the renin-angiotensin system (RAS) also serves as an important neurohumoral pathway contributing to the development of MetS. Angiotensin II (Ang II), formed as a result of angiotensin-converting enzyme activation, is also produced by adipose tissue. Obesity and insulin resistance are associated with increased production of Ang II.16 Ang II, through activation of the type 1 receptor, activates nicotinamide adenine dinucleotide phosphate oxidase leading to the generation of reactive oxygen species (ROS).17 ROS precipitate a multitude of effects including oxidation of LDL, endothelial injury, platelet aggregation, expression of redox-sensitive transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) on the endothelium and vascular smooth muscle cells.18 RAS, ROS, and LOX-1 have an interrelated positive feedback loop that initiates a vicious cycle of inflammation, endothelial damage, and fibroblast proliferation that contributes to the development of hypertension, dyslipidemia, diabetes, cardiac hypertrophy, and CVD.19
Inflammation: the final common pathway
Activation of various pro-atherogenic pathways in MetS culminates in a final common pathway of inflammation that eventually leads to clinical manifestations of MetS. As described earlier, systemic oxidant stress induced by obesity and insulin resistance leads to increased activation of downstream signaling cascades that cause atherogenesis and tissue fibrosis.
Inflammation plays an important role in the pathogenesis of CVD and various20 inflammatory markers have been shown to be elevated in patients with MetS. Whether these markers play a causative role or are mere bystanders of ongoing inflammation remains controversial.
Tumor necrosis factor alpha
Macrophages within the adipose tissue secrete tumor necrosis factor alpha (TNF-α) and its production increases with increase in adipose tissue mass. TNF-α causes phosphorylation and inactivation of insulin receptors in the adipose tissue as well as in smooth muscle cells, the induction of lipolysis increasing FFA load, and inhibits adiponectin release.21 Elevated serum TNF-α levels are associated with obesity and insulin resistance, both of which are major components of MetS.22
Interleukin 6 and C-reactive protein
Interleukin 6 (IL-6) is a cytokine produced by adipocytes and immune cells and has complex regulatory mechanisms.23 Production of IL-6 increases with increase in body fat and insulin resistance. It acts on the liver, bone marrow, and endothelium, leading to increased production of acute phase reactants in the liver, including C-reactive protein (CRP). Several studies have demonstrated a correlation between high CRP levels and the development of MetS, diabetes, and CVD.24 IL-6 also increases fibrinogen levels resulting in a prothrombotic state. IL6 also promotes adhesion molecule expression by endothelial cells and activation of local RAS pathways.25
Management of MetS
MetS is associated with an increased risk of both atherosclerotic and nonatherosclerotic CVD. Whether the risk is a sum total of its individual components or the clustering of these components induces a synergistic risk is still under debate. Results from a recent meta-analysis by Motillo and colleagues indicated that MetS doubles the risk of CVD outcomes and increases all-cause mortality by 1.5 times.26 Management of MetS involves a dual approach that combines lifestyle changes and pharmacological interventions in an effort to decrease CVD.
Lifestyle modification
As described earlier, MetS results from increased calorie consumption disproportionate to metabolic requirements. Lifestyle modification is imperative in the management of underlying risk factors. Weight reduction and maintenance of ideal body weight are essential preventive and management strategies. The goal of weight reduction is a loss of 7–10% in baseline body weight over a period of 6–12 months as well as a reduction of caloric intake by 500–1000 calories/day. Dietary modification can also regulate other MetS components: low intake of saturated fats, trans fats, cholesterol, sodium, and simple sugars is known to help with dyslipidemia, hyperglycemia and hypertension, for example. Diets high or very low in fat content exacerbate atherogenic dyslipidemia, as such, 25– 35% of daily caloric intake in the form of fat is usually recommended. Judicious use of bariatric surgery has shown benefit in the morbidly obese. Weight reduction helps with improvement in all components of exercise. Exercise increases calorie consumption, aiding weight loss and reducing overall CVD risk: around 30–60 min of moderate intensity exercise and conscious efforts to alter a sedentary lifestyle can be beneficial for the management of MetS.
Pharmacotherapy
Along with modifying the underlying risk factors, pharmacotherapy is another option for the prevention of CVD. Major pharmacological interventions include management of dyslipidemia with statins, decreasing prothrombotic risk with antiplatelet drugs, and the use of insulin sensitizers to decrease the risk of diabetes. There is no single drug therapy for MetS and currently available pharmacotherapy and associated comorbidities necessitate prolonged use of multiple medications, which is challenging for patients due to polypharmacy and reduced compliance. Thus, there is growing interest in the use of naturally occurring compounds in lowering the risk and progression of MetS though their effect on long-term cardiovascular outcomes and long-term compliance is unknown.
Nutraceuticals in the management of MetS
Dietary supplements that provide health benefits in addition to basic nutritional value are termed nutraceuticals. Various natural compounds derived from plant extracts, spices, herbs, and essential oils have demonstrable benefit in the management of patients with MetS (Table 2). As the benefits of these nutraceuticals are still under investigation, these therapies are not recommended as replacement for pharmacotherapies currently used in MetS.
Table 2.
Nutraceuticals in metabolic syndrome.
| Source | Active ingredient | Actions |
|---|---|---|
| Turmeric (Curcuma longa) |
Curcumin | Anti-inflammatory, antioxidant ↑ Insulin sensitivity ↓ Obesity ↓ Leptin and ↑ adiponectin |
| Garlic (Allium sativum) |
Allicin | Anti-inflammatory Antioxidant ↑ Insulin sensitivity ↓ Total cholesterol and triglycerides ↑ Adiponectin levels |
| Cinnamon (Cinnamomum verum) |
Polyphenols | Antithrombotic Anti-inflammatory ↑ Insulin sensitivity Improves fasting blood glucose, blood pressure, and body composition |
| Rhizoma coptidis | Berberine | ↑ Insulin sensitivity ↓ Body weight ↓ Triglycerides ↓ Systolic blood pressure |
| Neem (Azadirachta indica) |
Neem oil | ↑ Insulin secretion ↓ Postprandial hyperglycemia |
| Bergamot orange (Citrus bergamia) |
Bergamot essential oil | Anti-inflammatory and antioxidant effects ↓ Lectin-like oxidized low-density lipoprotein receptor-1 expression ↓ Reactive oxygen species formation |
| Cumin (Cuminum cyaminum) | Cuminaldehyde | ↓ Lipid levels ↓ Blood glucose levels |
| Fenugreek (Trigonella foenum) |
Saponins, galactomannan | ↓ Body weight ↓ Triglycerides and total cholesterol ↑ Insulin sensitivity ↓ Postprandial blood glucose |
| Cardamom (Elettaria cardamomum) |
Terpenine, cineol | ↓ Systolic blood pressure ↓ Platelet aggregation |
| Ginger (Zingiber officinale) |
Gingerols, shogaols, parasols | Anti-inflammatory ↓ Cyclooxygenase-2, ↓ 5-lipoxygenase ↓ Systolic blood pressure |
| Grapes (Vitus vinifera) | Resveratrol, 3,5,4′-trihydroxy-trans-stilbene | ↓ Adipogenesis ↑ Lipolysis ↓ Insulin resistance ↓ Body mass index |
| Onions (Allium cepa) | Quercetin | Anti-inflammatory Antioxidant ↓ Adipogenesis ↑ Lipolysis ↓ Blood glucose, ↓ cholesterol levels |
| Fish oils (Omega fatty acids) | Polyunsaturated fatty acids | ↓ Lipogenesis ↑ Fatty acid oxidation Regulate peroxisome proliferator-activated receptor gamma |
| Broccoli (Brassica oleracea) | Sulforaphrane | Activates nuclear factor erythroid 2-related factor 2 Antioxidant Anti-inflammatory |
Here, we describe the nutraceuticals that have been studied and have shown some benefit in MetS.
Curcumin
Curcumin is a derivative of turmeric (Curcuma longa), a spice commonly used in south-east Asia. The active ingredient, diferuloylmethane, is known to have anti-inflammatory and antioxidative properties. Curcumin has been shown to suppress NF-kB activation, which blunts the inflammatory cascade by reduction in the expression of pro-inflammatory cytokines, downregulates TNF-α expression, and suppresses expression of plasminogen activator inhibitor type-1, which is responsible for a prothrombotic state.27–29 Curcumin also inhibits the Wnt/β-catenin pathway linked to obesity and activates peroxisome proliferator-activated receptor gamma in hepatic stellate cells.30 Curcumin is also known to interrupt leptin signaling thus increasing adiponectin expression. Negative effects on obesity and positive effects on insulin sensitization as well as blunting of the inflammatory pathways serve as beneficial effects in MetS.
Garlic
Garlic (Allium sativum), a commonly used condiment, is also known to have medicinal value because of its antioxidant and antithrombotic properties. Padiya and colleagues found that raw garlic improves insulin sensitivity in fructose-fed rats and may have similar effects in humans.31 Reinhart and colleagues, in a meta-analysis of 29 randomized placebo-controlled trials comparing the effect of garlic on lipid profiles, showed that the garlic intake lowers total cholesterol and triglyceride levels.32 In another study, Gomez-Arbelaez and colleagues demonstrated the effect of aged garlic extract on adiponectin levels in people with MetS. Use of aged garlic extract for 12 weeks was shown to improve adiponectin levels.33 The anti-inflammatory effect of garlic comes from the organosulfur compounds in its derivatives. These compounds have an antioxidant action due to thiol groups that help fight ROS-mediated inflammation making garlic a promising natural therapy for MetS.
Cinnamon
Cinnamon (Cinnamomum verum), derived from a tree bark and used as a spice and flavoring agent, is frequently used in Chinese and Indian traditional medicines. Cinnamon extracts and polyphenols have antithrombotic, insulin-sensitizing, lipid-lowering, anti-inflammatory, and antioxidant properties that are beneficial in MetS. Cinnamon polyphenols have insulin-like activity and several studies have reported improvement in glycemic controls and lipid levels. In a randomized placebo-controlled trial, Ziegenfuss and colleagues demonstrated that the use of an aqueous extract of cinnamon was associated with improvement in fasting blood glucose, blood pressure, and body composition in people with MetS.34 All the mechanistic pathways in this process have yet to be elucidated, however some studies in mouse models indicate that cinnamon extracts can regulate adipocyte gene expression to improve glucose transport (GLUT 4) and insulin signaling.35
Berberine
Berberine is an alkaloid from the plant Rhizoma coptidis and is used in China for its antimicrobial properties, and is known to have antidiabetic properties. Studies in insulin-resistant animals show that the use of berberine improves body weight, triglyceride levels, and insulin sensitivity. Berberine acts through upregulation of genes involved in energy utilization and downregulation of genes involved in lipogenesis.36 It has an insulin-sensitizing action, similar to metformin and thiazolidinediones, mediated by adenosine monophosphate-associated protein kinase activation in adipocytes.37 Studies in humans with MetS have reported a reduction in waist circumference, triglyceride levels, and systolic blood pressure, especially in women.38
Neem
Azadirachta indica, also known as neem or nim tree, is known to have medicinal value. Neem extract is associated with an increased glucose tolerance by reducing intestinal and pancreatic glucosidase activity, which helps improve postprandial hyperglycemia. An increase in glucose-6-phosphate dehydrogenase activity which increases glucose utilization has also been previously observed.39 Furthermore, Neem extracts also help with the regeneration of pancreatic beta cells, improving insulin secretion.
Bergamot oil
Bergamot essential oil is a citrus essential oil derived from Citrus bergamia, and has anticancer, anti-inflammatory, antimicrobial, and antianxiety properties. In the context of MetS, the antioxidant effects of bergamot oil are most significant. Mollace and colleagues studied the effect of the antioxidant component of bergamot oil on LOX-1 expression and free-radical generation on carotid injury in the rat.40 In the study, balloon injury was associated with smooth muscle cell proliferation, neointima formation, ROS generation, and increased LOX-1 expression. Pretreatment of these rats with bergamot essential oil decreased neointima formation, LOX-1 expression, and free-radical generation, reducing the degree of stenosis.40 Other natural compounds such as procyanins, 6-shogoal procyanins, and flavonoids have also been associated with antioxidant properties that may be beneficial for the management of MetS.
Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenol present in plants such as grapes and nuts, and derivatives such as wine. It is an activator of the sirtuin pathway, which regulates several cellular functions related to metabolism, oxidation, and aging. It has favorable effects on cellular energy homeostasis, such as decreasing adipogenesis and increasing lipolysis, through multiple mechanisms, and also inhibits cyclooxygenase with a resulting antioxidant action.41 The use of resveratrol in MetS has been studied in animal models as well as in humans. Clinical studies in patients with insulin resistance and nonalcoholic fatty liver disease have shown promising results.42 In fact, use of resveratrol has shown to improve insulin sensitivity, glucose tolerance, and overall weight and body mass index in patients with MetS.43
Quercetin
Quercetin, a plant-derived flavonoid found in vegetables and fruits such as onions, berries, and tea, has been reported to have anti-inflammatory and antioxidant metabolic effects. Quercetin acts via mitochondrial pathways involved in adipokinesis and lipolysis that affect the development of obesity.44 Rivera and colleagues studied the effect of quercetin in obese Zucker rats. In this study, rats that received quercetin had lower blood pressure, lower cholesterol levels, and lower insulin resistance compared with rats that did not. Higher doses of quercetin also produced an anti-inflammatory effect in the visceral adipose tissue.45 Pfeuffer and colleagues studied the metabolic implications of quercetin use in humans, specifically in men with an apolipoprotein E genotype, and discovered that quercetin improves metabolic parameters such as waist circumference, postprandial blood glucose, and lipids, but also an increase in inflammatory markers such as TNF-α.46
Omega fatty acids
Omega-3 long-chain polyunsaturated fatty acids (PUFAs) have been extensively studied in multiple epidemiological studies, specifically in relation to their protective role in MetS-related diseases.47 Two specific PUFAs, eicosapentaenoic acid and docosahexaenoic acid, found abundantly in fish oils have received wide attention, leading to major societal preventive recommendations.48 PUFAs have been shown to inhibit lipogenesis and induce fatty acid oxidation in liver and adipose tissue via regulation of key transcription factors such as the peroxisome proliferator-activated receptors and sterol regulatory element binding protein.49–52 Despite a favorable impact on various components of MetS, long-term data on prevention of hard cardiovascular endpoints with the use of PUFAs have not been confirmed. However, strong pathophysiological correlates of underlying mechanisms are encouraging and need further study.
Sulforaphane
Another phytochemical, sulforaphane, extracted from vegetables of the Brassica family such as broccoli, has also recently been shown to have potential beneficial effects in MetS, due to its antioxidant and anti-inflammatory properties. Sulforaphane activates nuclear factor erythroid 2-related factor 2, an antioxidant transcription factor. Several animal studies have demonstrated a protective role of sulforaphane against various disorders such as hypertension, hyperlipidemia, and diabetes, all key components of MetS.52–54
Summary
MetS is a global epidemic and an established risk factor for atherosclerotic and nonatherosclerotic CVD. Significant variations exist in the diagnostic criteria and definition of MetS, which represent a temporal change in the understanding of this disease. Various stimuli culminating in a state of chronic inflammation seem to be the main pathophysiological drivers for MetS. Existing therapies to tackle various components of MetS are limited by various factors. Firstly, the existence of only a handful of medications that have been shown to have a convincing effect on long-term outcomes makes the choice of therapy challenging. Secondly, the chronic nature of the components of MetS warrant prolonged and often indefinite use of various medications such as statins, leading to an increased burden of drug-related adverse effects and patient noncompliance. In this context, the development of nutraceuticals that are readily available and with minimal side effects may represent an area of promise in the development of novel therapies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Yogita Rochlani, Division of Cardiovascular Medicine, Westchester Medical Center, Valhalla, NY, USA.
Naga Venkata Pothineni, Division of Cardiovascular Medicine, Central Arkansas Veterans Healthcare System and University of Arkansas for Medical Sciences, 4301 W. Markham St. #532, Little Rock, AR 72205, USA.
Swathi Kovelamudi, Division of Cardiovascular Medicine, Central Arkansas Veterans Healthcare System and University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Jawahar L. Mehta, Division of Cardiovascular Medicine, Central Arkansas Veterans Healthcare System and University of Arkansas for Medical Sciences, Little Rock, AR, USA
References
- 1. Grundy SM, Hansen B, Smith SC, Jr, et al. American Heart Association, National Heart, Lung, and Blood Institute, American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol 2004; 24(2): e19–e24. [DOI] [PubMed] [Google Scholar]
- 2. Beltrán-Sánchez H, Harhay MO, Harhay MM, et al. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol 2013; 62(8): 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287(3): 356–359. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008; 28(4): 629–636. [DOI] [PubMed] [Google Scholar]
- 5. Miller JM, Kaylor MB, Johannsson M, et al. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord 2014; 12(10): 527–532. [DOI] [PubMed] [Google Scholar]
- 6. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011; 18(8): 629–639. [DOI] [PubMed] [Google Scholar]
- 7. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002; 32(Suppl. 3): 14–23. [DOI] [PubMed] [Google Scholar]
- 8. Tooke JE, Hannemann MM. Adverse endothelial function and the insulin resistance syndrome. J Intern Med 2000; 247(4): 425–431. [DOI] [PubMed] [Google Scholar]
- 9. Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003; 52(12): 2882–2887. [DOI] [PubMed] [Google Scholar]
- 10. Juhan-Vague I, Alessi MC, Mavri A, et al. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1(7): 1575–1579. [DOI] [PubMed] [Google Scholar]
- 11. Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 1996; 19(4): 390–393. [DOI] [PubMed] [Google Scholar]
- 12. Wallace AM, McMahon AD, Packard CJ, et al. ; on behalf of the WOSCOPS Executive Committee. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001; 104(25): 3052–3056. [DOI] [PubMed] [Google Scholar]
- 13. Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002; 360(9326): 57–58. [DOI] [PubMed] [Google Scholar]
- 14. Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003; 42(3): 231–234. [DOI] [PubMed] [Google Scholar]
- 15. Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291(14): 1730–1737. [DOI] [PubMed] [Google Scholar]
- 16. Vanecková I, Maletínská L, Behuliak M, et al. Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol 2014; 223(3): R63–R78. [DOI] [PubMed] [Google Scholar]
- 17. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007; 292(1): C82–C97. [DOI] [PubMed] [Google Scholar]
- 18. Gobal F, Deshmukh A, Shah S, et al. Triad of metabolic syndrome, chronic kidney disease, and coronary heart disease with a focus on microalbuminuria: death by overeating. J Am Coll Cardiol 2011; 57(23): 2303–2308. [DOI] [PubMed] [Google Scholar]
- 19. Dai Y, Mercanti F, Dai D, et al. LOX-1, a bridge between GLP-1R and mitochondrial ROS generation in human vascular smooth muscle cells. Biochem Biophys Res Commun 2013; 437(1): 62–66. [DOI] [PubMed] [Google Scholar]
- 20. Pant S, Deshmukh A, Gurumurthy GS, et al. Inflammation and atherosclerosis – revisited. J Cardiovasc Pharmacol Ther 2014; 19(2): 170–178. [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS, Murray DL, Choy LN, et al. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A 1994; 91(11): 4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsigos C, Kyrou I, Chala E, et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism 1999; 48(10): 1332–1335. [DOI] [PubMed] [Google Scholar]
- 23. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83(3): 847–850. [DOI] [PubMed] [Google Scholar]
- 24. Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 2000; 85(9): 3338–3342. [DOI] [PubMed] [Google Scholar]
- 25. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004; 15(11): 2792–2800. [DOI] [PubMed] [Google Scholar]
- 26. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56(14): 1113–1132. [DOI] [PubMed] [Google Scholar]
- 27. Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 2010; 30: 173–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 1995; 270(42): 24995–25000. [DOI] [PubMed] [Google Scholar]
- 29. Pendurthi UR, Rao LV. Suppression of transcription factor Egr-1 by curcumin. Thromb Res 2000; 97(4): 179–189. [DOI] [PubMed] [Google Scholar]
- 30. Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol 2003; 285(1): G20–G30. [DOI] [PubMed] [Google Scholar]
- 31. Padiya R, Khatua TN, Bagul PK, et al. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab (Lond) 2011; 8: 53, 7075–8–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reinhart KM, Talati R, White CM, et al. The impact of garlic on lipid parameters: a systematic review and meta-analysis. Nutr Res Rev 2009; 22(1): 39–48. [DOI] [PubMed] [Google Scholar]
- 33. Gomez-Arbelaez D, Lahera V, Oubina P, et al. Aged garlic extract improves adiponectin levels in subjects with metabolic syndrome: a double-blind, placebo-controlled, randomized, crossover study. Mediators Inflamm 2013; 2013: 285795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziegenfuss TN, Hofheins JE, Mendel RW, et al. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr 2006; 3: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao H, Graves DJ, Anderson RA. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine 2010; 17(13): 1027–1032. [DOI] [PubMed] [Google Scholar]
- 36. Yang J, Yin J, Gao H, et al. Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evid Based Complement Alternat Med 2012; 2012: 363845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006; 55(8): 2256–2264. [DOI] [PubMed] [Google Scholar]
- 38. Perez-Rubio KG, Gonzalez-Ortiz M, Martinez-Abundis E, et al. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2013; 11(5): 366–369. [DOI] [PubMed] [Google Scholar]
- 39. Bhat M, Kothiwale SK, Tirmale AR, et al. Antidiabetic properties of Azardiracta indica and Bougainvillea spectabilis: in vivo studies in murine diabetes model. Evid Based Complement Alternat Med 2009; 2011: 561625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mollace V, Ragusa S, Sacco I, et al. The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J Cardiovasc Pharmacol Ther 2008; 13(2): 120–129. [DOI] [PubMed] [Google Scholar]
- 41. Bremer AA. Resveratrol use in metabolic syndrome. Metab Syndr Relat Disord 2014; 12(10): 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen S, Zhao X, Ran L, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis 2015; 47(3): 226–232. [DOI] [PubMed] [Google Scholar]
- 43. Mendez-del Villar M, Gonzalez-Ortiz M, Martinez-Abundis E, et al. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2014; 12(10): 497–501. [DOI] [PubMed] [Google Scholar]
- 44. Leiherer A, Stoemmer K, Muendlein A, et al. Quercetin impacts expression of metabolism- and obesity-associated genes in SGBS adipocytes. Nutrients 2016; 8(5): 10.3390/nu8050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rivera L, Moron R, Sanchez M, et al. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008; 16(9): 2081–2087. [DOI] [PubMed] [Google Scholar]
- 46. Pfeuffer M, Auinger A, Bley U, et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis 2013; 23(5): 403–409. [DOI] [PubMed] [Google Scholar]
- 47. Wang C, Harris WS, Chung M, et al. n−3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006; 84: 5–17. [DOI] [PubMed] [Google Scholar]
- 48. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114: 82–96. [DOI] [PubMed] [Google Scholar]
- 49. Flachs P, Horakova O, Brauner P, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005; 48: 2365–2375. [DOI] [PubMed] [Google Scholar]
- 50. Guo W, Xie W, Lei T, et al. Eicosapentaenoic acid, but not oleic acid, stimulates beta-oxidation in adipocytes. Lipids 2005; 40: 815–821. [DOI] [PubMed] [Google Scholar]
- 51. Gillies PJ, Bhatia SK, Belcher LA, et al. Regulation of inflammatory and lipid metabolism genes by eicosapentaenoic acid-rich oil. J Lipid Res 2012; 53: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu L, Juurlink BHJ. The impaired glutathione system and its up-regulation by sulforaphane in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens 2001; 19(10): 1819–1825. [DOI] [PubMed] [Google Scholar]
- 53. de Souza CG, Sattler JA, De Assis AM, et al. Metabolic effects of sulforaphane oral treatment in streptozotocin-diabetic rats. J Med Food 2012; 15(9): 795–801. [DOI] [PubMed] [Google Scholar]
- 54. Song M-Y, Kim E-K, Moon W-S, et al. Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicol Appl Pharmacol 2009; 235(1): 57–67. [DOI] [PubMed] [Google Scholar]