Abstract
Aims
Sleep‐disordered breathing (SDB) is associated with arterial stiffness, which may be one of the factors that lead to heart failure (HF). We examined the relationship between pulse wave velocity (PWV) and SDB in patients who have HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).
Methods and results
We measured the apnoea–hypopnoea index (AHI) by polysomnography, echocardiographic parameters, and PWV in 221 HF patients. Age, blood pressure, and PWV were higher in HFpEF (ejection fraction > 50%, n = 70) patients than in HFrEF (ejection fraction < 50%, n = 151) patients. All HF patients were divided into three groups according to AHI: none‐to‐mild SDB group (AHI < 15 times/h, n = 77), moderate SDB group (15 < AHI < 30 times/h, n = 59), and severe SDB group (AHI > 30 times/h, n = 85). Although blood pressure and echocardiographic parameters did not differ among the three groups, PWV was significantly higher in the severe SDB group than in the none‐to‐mild and moderate SDB groups (P = 0.002). When the HFrEF and HFpEF patients were analysed separately, PWV was significantly higher in the severe SDB group than in the none‐to‐mild and moderate SDB groups in patients with HFpEF (P = 0.002), but not in those with HFrEF (P = 0.068). In the multiple regression analysis to determine PWV, the presence of severe SDB was found to be an independent predictor of high PWV in HFpEF (β = 0.234, P = 0.005), but not in HFrEF patients.
Conclusions
Severe SDB is associated with elevated arterial stiffness and may be related to the pathophysiology of HF, especially in HFpEF patients.
Keywords: Pulse wave velocity, Sleep‐disordered breathing, Heart failure with preserved ejection fraction
Introduction
Heart failure (HF) is a common disease, especially in elderly people, and is divided into two types based on cardiac systolic function: reduced ejection fraction (EF) and preserved EF.1 It is recognized that a substantial proportion of HF patients have preserved EF (HFpEF), which has a similarly poor prognosis to HF with reduced EF (HFrEF).2, 3
It is well known that the occurrence of HFpEF has been increasing year by year.2 Crucial pathophysiological conditions in the development of HFpEF include prolonged isovolumic left ventricular (LV) relaxation, slow LV filling, increased diastolic LV stiffness, and LV diastolic dysfunction.4, 5, 6 These pathophysiological characteristics are associated with increased ventricular–arterial stiffness and exaggerated blood pressure response to changes in ventricular loading in HFpEF patients.7, 8, 9 In particular, in HFpEF patients, central aortic stiffness is increased, and arterial stiffness modulates ventricular loading conditions as well as LV diastolic function.10, 11, 12 Aortic stiffness can be assessed by various non‐invasive methods, and aortic pulse wave velocity (PWV), which is one of the most frequently used parameters because it is easily measured, predicts future cardiovascular events, such as stroke and mortality.13, 14 PWV was commonly measured by carotid–femoral and brachial–ankle methods;13 however, in recent years, it has become possible to self‐measure PWV using an ambulatory blood pressure monitoring device.15, 16
Moreover, it is well known that sleep‐disordered breathing (SDB) has an adverse prognostic impact on HF patients, including not only HFrEF but also HFpEF patients.17, 18 Several studies have revealed higher PWV in obstructive sleep apnoea (OSA) patients than in controls and reported nocturnal oxygen desaturation to be associated with high PWV.19, 20
Therefore, the purpose of the present study was to examine the relationship between PWV and SDB in HFpEF and HFrEF patients.
Methods
Study subjects and protocol
This was a cross‐sectional study. We enrolled 221 consecutive HF patients who were hospitalized at Fukushima Medical University Hospital between March 2011 and April 2015 (mean age 64.5 years and 157 men). Symptomatic HF diagnosis was defined by well‐trained cardiologists using the Framingham criteria.21 All HF cases were diagnosed on first admission by attending cardiologists. Patients with acute coronary syndrome and those who were receiving dialysis were excluded. We investigated the patients' backgrounds, including age, gender, New York Heart Association (NYHA) functional class, vital signs on admission, co‐morbidities, laboratory data, and echocardiographic data during hospitalization. Plasma BNP concentrations were measured using a commercially available radioimmunoassay specific to human BNP (Shionoria BNP kit, Shionogi, Osaka, Japan). Estimated glomerular filtration rate was measured using the Modification of Diet in Renal Disease formula. These laboratory and investigation parameters were measured not in patients in an acute phase of HF, but in patients with stable HF immediately before discharge.
Echocardiography was blindly performed by an experienced echocardiographer using standard techniques. Two‐dimensional echocardiographic images were acquired from the parasternal long and short axes, apical long axis, and apical four‐chamber views. The following echocardiographic parameters were investigated: interventricular septum thickness, LV end‐diastolic diameter, LVEF, left atrial volume, early transmitral flow velocity to mitral annular velocity ratio (mitral valve E/e′), inferior vena cava diameter, tricuspid valve regurgitation pressure gradient (TR‐PG), and right ventricular fractional area change (RV‐FAC).22 LVEF was calculated using a modified Simpson's method. We defined HFpEF as ≥50% of LVEF and HFrEF as <50% of LVEF.
Written informed consent was obtained from all study subjects. Our study complies with the Declaration of Helsinki, and the study protocol was approved by the ethical committee of Fukushima Medical University.
Measurement of sleep state
All subjects underwent overnight full polysomnography (PSG) or were examined by portable recording Type III device with the use of standard techniques and scoring criteria for sleep stages and arousals from sleep as previously reported.23, 24 Briefly, PSG was performed using a computerized system (Alice 5, Philips Respironics, Murrysville, PA, USA) that monitored the patient's electroencephalogram, electrooculogram, submental electromyogram, electrocardiogram, and thoracoabdominal motion. Oronasal airflow and arterial oxyhaemoglobin saturation (SPO2) were monitored by an airflow pressure transducer and pulse oximetry, respectively. Some patients used a portable recording Type III device (LS‐300, FUKUDA DENSHI, Tokyo, Japan). Apnoea was defined as the absence of airflow for more than 10 s. Hypopnoea was defined as a >30% reduction in monitored airflow accompanied by a decrease in SPO2 by >3%. The major polysomnographic parameters investigated were the apnoea–hypopnoea index (AHI), central apnoea index, obstructive apnoea index, hypopnoea index, lowest SPO2, and mean SPO2. All HF patients were divided into three SDB groups according to AHI: none‐to‐mild SDB group (AHI < 15 times/h, n = 77), moderate SDB group (15 < AHI < 30 times/h, n = 59), and severe SDB group (AHI > 30 times/h, n = 85). We performed PSG on only inpatients with stable HF.
Measurement of pulse wave velocity
Pulse wave velocity was estimated using a Mobil‐O‐Graph 24 h PWA Monitor (I.E.M. GmbH, Stolberg, Germany), which is the first automated self‐measurement ambulatory blood pressure monitoring device that uses brachial oscillometric blood pressure for a non‐invasive estimation of central blood pressure.15, 16 This device uses a novel transfer function‐like method (ARCSolver algorithm) with brachial cuff‐based waveform recordings, and its measurements of blood pressure, waveform, and PWV have been validated.15, 16, 25, 26 PWV measured by this device has a good correlation with that measured by traditionally used tonometry systems.25, 26 We evaluated PWV in patients with stable HF.
Statistical analysis
Results are expressed as mean ± standard deviation in normally distributed data, and skewed variables are presented as median (interquartile range). Categorical variables are expressed as numbers and percentages, and P values of less than 0.05 were considered statistically significant. The baseline characteristics of the HFpEF patients were compared with those of the HFrEF patients using an unpaired Student's t test for continuous variables and a χ2 test for discrete variables. If data were not distributed normally, the Mann–Whitney U test was used for comparisons. To compare the three SDB groups, we used one‐way ANOVA followed by Tukey's post hoc test. Multivariable regression analysis was used to determine the variables that were significantly related to high PWV. We considered the following to be potential confounding factors that are known to affect PWV in HF patients: older age (65 years or older), gender, body mass index, presence of ischaemic heart disease, hypertension, diabetes, dyslipidaemia, chronic kidney disease, anaemia, and severe SDB. Parameters with statistical significance in the univariate analysis (P < 0.05) were included in the multivariate analysis. Statistical analysis was performed using a standard statistical program package (SPSS Japan, Tokyo, Japan).
Results
Of the study population, 70 patients were found to have HFpEF, and 151 were found to have HFrEF, and we compared baseline clinical characteristics (Table 1). When compared with the HFrEF patients, the HFpEF patients were older (P = 0.004), and a larger proportion were female (P = 0.001). In addition, the HFpEF patients had higher body mass index (P = 0.045), less severe NYHA functional class (P = 0.005), higher systolic blood pressure (P = 0.019), lower prevalence of diabetes mellitus (P = 0.018), higher prevalence of atrial fibrillation (P = 0.027), and lower prevalence of ischaemic heart disease (P < 0.001). Furthermore, PWV was higher (9.79 ± 2.05 vs. 8.73 ± 2.19 m/s, P = 0.001), renal function was better, and plasma BNP levels were lower in the HFpEF patients than in the HFrEF patients (P = 0.028 and P = 0.003, respectively). In echocardiographic data, the LV wall was thicker (P = 0.003) and the LV end‐diastolic diameter was smaller (P < 0.001) in the HFpEF patients than in the HFrEF patients (Table 1).
Table 1.
Comparisons of clinical characteristics between patients with heart failure with preserved ejection fraction and with reduced ejection fraction
| HFpEF (n = 70) | HFrEF (n = 151) | P value | |
|---|---|---|---|
| Age (years) | 68.2 ± 11.1 | 62.7 ± 13.7 | 0.004 |
| Gender (male/female) | 39/31 | 118/33 | 0.001 |
| Body mass index (kg/m2) | 25.1 ± 4.8 | 23.7 ± 4.8 | 0.045 |
| NYHA III and IV (n, %) | 5 (7.1) | 34 (22.5) | 0.005 |
| Systolic BP (mmHg) | 131.5 ± 36.2 | 120.0 ± 32.2 | 0.019 |
| Hypertension (n, %) | 53 (75.7) | 105 (69.5) | 0.344 |
| Diabetes mellitus (n, %) | 22 (31.4) | 73 (48.3) | 0.018 |
| Dyslipidemia (n, %) | 52 (74.3) | 122 (80.8) | 0.271 |
| Anaemia (n, %) | 34 (48.6) | 76 (50.3) | 0.808 |
| CKD (n, %) | 41 (58.6) | 106 (70.2) | 0.088 |
| IHD (n, %) | 8 (11.4) | 54 (35.8) | <0.001 |
| Pulse wave velocity (m/s) | 9.79 ± 2.05 | 8.73 ± 2.19 | 0.001 |
| Blood sample data | |||
| eGFR (mL/min/1.73 m2) | 58.2 ± 17.9 | 50.8 ± 21.8 | 0.028 |
| BNPa (pg/mL) | 216.4 (334.9) | 437.6 (714.4) | 0.003 |
| hs‐CRP (mg/dL) | 1.80 (0.51) | 1.85 (0.78) | 0.732 |
| Echocardiography | |||
| IVST (mm) | 12.6 ± 4.2 | 10.9 ± 2.9 | 0.003 |
| LVEDD (mm) | 46.8 ± 9.4 | 58.8 ± 11.3 | <0.001 |
| LVEF (%) | 61.6 ± 8.8 | 38.3 ± 14.7 | <0.001 |
| E/e′ | 14.7 ± 8.0 | 15.3 ± 9.2 | 0.693 |
| TR‐PG (mmHg) | 31.7 ± 18.1 | 27.4 ± 11.8 | 0.087 |
| Diastolic RV area (mm2) | 16.8 ± 7.4 | 18.8 ± 10.3 | 0.322 |
| RV‐FAC (%) | 43.9 ± 18.5 | 40.7 ± 14.9 | 0.321 |
BNP, B‐type natriuretic peptide; BP, blood pressure; CKD, chronic kidney disease; E/e′, the ratio of early transmitral flow velocity to mitral annular velocity; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hs‐CRP, high‐sensitivity C‐reactive protein; IHD, ischaemic heart disease; IVST, interventricular septum thickness; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association classification; RV, right ventricular; RV‐FAC; right ventricular fractional area change; TR‐PG, tricuspid regurgitation pressure gradient.
Skewed data are reported as median (interquartile range).
In all subjects, 77 had none‐to‐mild SDB (34.8%), 59 had moderate SDB (26.7%), and 85 had severe SDB (38.5%). We compared baseline clinical characteristics among these SDB groups (Table 2). Although age and body mass index correlated with SDB severity (P < 0.001, respectively), no significant differences were observed in gender, NYHA functional class, vital signs, incidence of co‐morbidities, laboratory data, and echocardiographic data among the three groups. Sleep state analysis revealed that AHI, central apnoea index, obstructive apnoea index, hypopnoea index, lowest SPO2, and mean SPO2 worsened with increasing SDB severity. PWV was significantly higher in the severe SDB group than in the none‐to‐mild and moderate SDB groups (9.56 ± 2.38 vs. 8.33 ± 2.38 and 9.24 ± 1.67 m/s, P = 0.002).
Table 2.
Comparisons of clinical characteristics among three sleep‐disordered breathing groups
| None to mild (N = 77) | Moderate (N = 59) | Severe (N = 85) | P value | ||
|---|---|---|---|---|---|
| Age (years) | 60.2 ± 14.8 | 66.0 ± 11.0 | 67.3 ± 12.1 | 0.001 | |
| Gender (male/female) | 49/28 | 44/15 | 64/21 | 0.206 | |
| Body mass index (kg/m2) | 22.9 ± 4.0 | 23.4 ± 3.9 | 25.8 ± 5.6 | <0.001 | |
| NYHA III and IV (n, %) | 13 (16.9) | 12 (20.3) | 14 (16.5) | 0.816 | |
| Systolic BP (mmHg) | 120.5 ± 34.0 | 126.3 ± 36.4 | 124.8 ± 32.1 | 0.571 | |
| Hypertension (n, %) | 51 (66.2) | 40 (67.8) | 67 (78.8) | 0.159 | |
| Diabetes mellitus (n, %) | 32 (41.6) | 28 (47.5) | 35 (41.2) | 0.719 | |
| Dyslipidaemia (n, %) | 58 (75.3) | 46 (78.0) | 70 (82.4) | 0.543 | |
| Anaemia (n, %) | 42 (54.5) | 32 (54.2) | 36 (42.4) | 0.218 | |
| CKD (n, %) | 48 (62.3) | 43 (72.9) | 56 (65.9) | 0.429 | |
| IHD (n, %) | 23 (29.9) | 15 (25.4) | 24 (28.2) | 0.848 | |
| HFpEF/HFrEF | 26/51 | 14/45 | 30/55 | 0.302 | |
| Pulse wave velocity (m/s) | 8.33 ± 2.38 | 9.24 ± 1.67 | 9.56 ± 2.38 | 0.002 | |
| Blood sample data | |||||
| eGFR (mL/min/1.73 m2) | 53.0 ± 23.6 | 51.9 ± 19.2 | 53.7 ± 19.7 | 0.904 | |
| BNPa (pg/mL) | 291.3 (487.6) | 436.4 (652.4) | 230.3 (494.9) | 0.502 | |
| hs‐CRPa (mg/dL) | 0.16 (0.34) | 0.23 (1.57) | 0.18 (0.42) | 0.349 | |
| Echocardiographic data | |||||
| IVST (mm) | 12.0 ± 4.3 | 11.0 ± 2.8 | 11.2 ± 3.0 | 0.259 | |
| LVEDD (mm) | 52.5 ± 12.0 | 55.5 ± 11.5 | 57.0 ± 12.3 | 0.087 | |
| LVEF (%) | 44.8 ± 18.5 | 44.5 ± 16.9 | 46.4 ± 15.9 | 0.792 | |
| E/e′ | 15.9 ± 8.4 | 13.5 ± 6.6 | 15.6 ± 10.5 | 0.394 | |
| TR‐PG (mmHg) | 27.7 ± 14.5 | 30.5 ± 13.8 | 28.6 ± 14.8 | 0.651 | |
| Diastolic RV area (mm2) | 17.5 ± 7.3 | 18.8 ± 14.3 | 18.3 ± 6.4 | 0.841 | |
| RV‐FAC (%) | 45.0 ± 18.1 | 38.3 ± 15.3 | 41.3 ± 14.8 | 0.230 | |
| Measurement of sleep state | |||||
| AHI (/h) | 8.8 ± 3.8 | 22.6 ± 4.1 | 43.1 ± 12.4 | <0.001 | |
| CAI (/h) | 0.9 ± 0.7 | 6.4 ± 5.9 | 12.9 ± 12.4 | <0.001 | |
| OAI (/h) | 1.9 ± 1.7 | 3.4 ± 3.1 | 6.7 ± 6.3 | <0.001 | |
| HI (h) | 6.1 ± 3.5 | 11.3 ± 6.8 | 17.6 ± 15.3 | <0.001 | |
| Lowest SpO2 (%) | 87.8 ± 5.7 | 81.4 ± 11.4 | 76.5 ± 10.6 | <0.001 | |
| Mean SpO2 (%) | 96.8 ± 1.3 | 95.7 ± 2.2 | 94.2 ± 2.7 | <0.001 | |
AHI, apnoea–hypopnoea index; BNP, B‐type natriuretic peptide; BP, blood pressure; CAI, central apnoea index; CKD, chronic kidney disease; E/e′, the ratio of early transmitral flow velocity to mitral annular velocity; eGFR, estimated glomerular filtration rate; HI, hypopnoea index; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hs‐CRP, high‐sensitivity C‐reactive protein; IHD, ischaemic heart disease; IVST, interventricular septum thickness; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association classification; OAI, obstructive apnoea index; RV, right ventricular; RV‐FAC; right ventricular fractional area change; SPO2, arterial oxyhaemoglobin saturation; TR‐PG, tricuspid regurgitation pressure gradient.
Skewed data are reported as median (interquartile range).
We analysed the HFpEF and HFrEF patients separately. Age and body mass index correlated with SDB severity in both the HFpEF and HFrEF patients, and male gender was significantly fewer in the none‐to‐mild SDB group than in the moderate and severe SDB groups in HFpEF patients (P = 0.023) ( Tables S1 and S2 ). No significant differences were observed in vital signs, NYHA functional class, laboratory data, and echocardiographic data among the three SDB groups in both the HFpEF and HFrEF patients ( Tables S1 and S2 ). PWV was significantly higher in the severe SDB group than in the none‐to‐mild and moderate SDB groups in the HFpEF patients (10.67 ± 1.88 vs. 8.77 ± 1.95 and 9.78 ± 1.80 m/s, P = 0.002) (Figure 1 A), but not in the HFrEF patients (8.96 ± 2.43 vs. 8.10 ± 2.56 and 9.08 ± 1.62 m/s, P = 0.068) (Figure 1 B).
Figure 1.
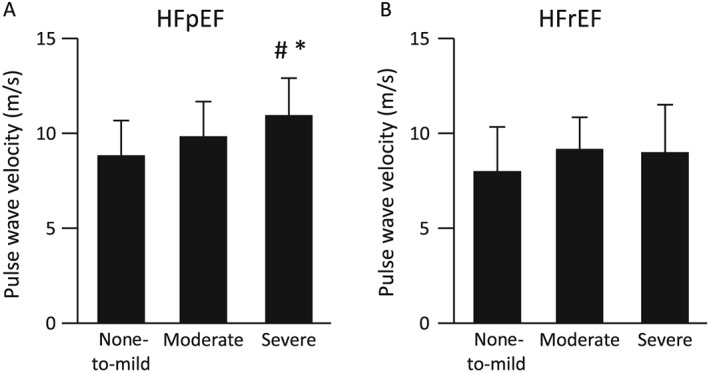
The comparisons of pulse wave velocity in (A) heart failure with preserved ejection fraction (HFpEF) and (B) heart failure with reduced ejection fraction (HFrEF). #P < 0.01 vs. the none‐to‐mild group; *P < 0.05 vs. the moderate group.
The univariate and multivariate regression analyses to determine factors related to high PWV in the HFpEF and HFrEF patients are shown in Table 3. Among considerable clinical risk variables such as age, gender, body mass index, ischaemic heart disease, presence of hypertension, diabetes, dyslipidaemia, chronic kidney disease, anaemia, and SDB, the presence of severe SDB was an independent predictor of high PWV in the HFpEF patients (β = 0.234, P = 0.005), but not in the HFrEF patients.
Table 3.
Multiple regression analysis to determine factors related to pulse wave velocity
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | β coefficient | P value | β coefficient | P value |
| HFpEF patients | ||||
| Age (65 years or older) | 0.714 | <0.001 | 0.549 | <0.001 |
| Gender (male) | 0.100 | 0.409 | — | — |
| Body mass index (over 25) | −0.140 | 0.247 | — | — |
| Ischaemic heart disease | 0.024 | 0.842 | — | — |
| Hypertension | 0.487 | <0.001 | 0.247 | 0.006 |
| Diabetes mellitus | 0.177 | 0.144 | — | — |
| Dyslipidaemia | −0.049 | 0.689 | — | — |
| CKD | 0.167 | 0.167 | — | — |
| Anaemia | 0.092 | 0.450 | — | — |
| Severe SDB | 0.377 | 0.001 | 0.234 | 0.005 |
| HFrEF patients | ||||
| Age (65 years or older) | 0.697 | <0.001 | 0.648 | <0.001 |
| Gender (male) | −0.176 | 0.031 | −0.089 | 0.152 |
| Body mass index (over 25) | −0.109 | 0.183 | — | — |
| Ischaemic heart disease | 0.196 | 0.016 | 0.061 | 0.333 |
| Hypertension | 0.180 | 0.027 | 0.114 | 0.063 |
| Diabetes mellitus | 0.086 | 0.291 | — | — |
| Dyslipidaemia | 0.051 | 0.535 | — | — |
| CKD | 0.256 | 0.001 | 0.044 | 0.479 |
| Anaemia | 0.205 | 0.012 | 0.010 | 0.876 |
| Severe SDB | 0.091 | 0.264 | — | — |
CKD, chronic kidney disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SDB, sleep‐disordered breathing.
Next, we divided each SDB group into two subgroups: patients with central sleep apnoea (CSA)‐dominant sleep disorder and those with OSA‐dominant sleep disorder. Then we examined PWV in each subgroup. As shown in Table 4, in HFpEF patients, PWV was significantly higher in the severe SDB group both with CSA and OSA than in the none‐to‐mild and moderate SDB groups (CSA: 10.25 ± 1.89 vs. 7.40 ± 1.46 and 9.98 ± 1.58 m/s, P = 0.011; OSA: 11.0 ± 1.86 vs. 9.54 ± 1.59 and 9.63 ± 2.05 m/s, P = 0.020), but not in HFrEF patients.
Table 4.
Comparisons of pulse wave velocity in heart failure with preserved ejection fraction and with reduced ejection fraction patients divided into central and obstructive sleep apnoea
| None to mild | Moderate | Severe | P value | |
|---|---|---|---|---|
| HFpEF patients | ||||
| CSA dominant | 7.40 ± 1.46 | 9.98 ± 1.58 | 10.25 ± 1.89 | 0.011 |
| OSA dominant | 9.54 ± 1.59 | 9.63 ± 2.05 | 11.00 ± 1.86 | 0.020 |
| HFrEF patients | ||||
| CSA dominant | 7.23 ± 1.98 | 8.84 ± 1.68 | 8.66 ± 2.71 | 0.076 |
| OSA dominant | 8.48 ± 2.53 | 9.43 ± 1.48 | 9.37 ± 1.66 | 0.325 |
CSA, central sleep apnoea; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; OSA, obstructive sleep apnoea.
Discussion
In the present study, we compared the relationship of arterial stiffness and SDB in HFpEF and HFrEF patients and revealed, with the use of a novel oscillometric PWV measurement device, that arterial stiffness increased according to SDB severity in HFpEF patients, but not in HFrEF patients. There was no inconsistency with previous report, which demonstrated that the plasma BNP level was significantly lower in the HFpEF patients than in the HFrEF patients.27
It is well known that LV diastolic dysfunction plays an essential pathophysiological role in the development of HFpEF. In HFpEF patients, LV diastolic relaxation abnormalities were revealed by pressure–volume analysis and echocardiographic examination.7, 8, 28 Moreover, both ventricular stiffness and arterial stiffness increase with advancing age; further, ventricular–arterial stiffening, compliance, and relaxation abnormalities are common in patients with HFpEF.7, 10, 11, 12 In HFrEF patients, myocardial loss or degeneration and dysfunction play essential pathophysiological roles. A meta‐analysis revealed that low blood pressure and pulse pressure are related to prognosis in HFrEF patients.29 Additionally, LV function is more affected by increased arterial stiffness in HFpEF than in HFrEF patients.
Aortic stiffness can be assessed by various non‐invasive methods such as augmentation index, cardio‐ankle vascular index, and PWV. Of these parameters, aortic PWV is one of the most frequently used because it is easily measured, and there are several reports about the association between HFpEF and PWV. A clinical study with a population of almost 2000 participants demonstrated that PWV was significantly correlated with echocardiographic E/A ratio and was higher in the study's diastolic HF group than in the non‐diastolic HF group.30 Meta‐analysis including 26 studies with 6626 patients investigated the associations between diastolic dysfunction evaluated by echocardiography and arterial stiffness measured by brachial–ankle PWV (baPWV), carotid–femoral PWV, augmentation index, and cardio‐ankle vascular index. They concluded that baPWV showed significantly greater correlation with diastolic function compared with other tonometric techniques, and arterial stiffness measured by arterial tonometry and baPWV is an indicator of diastolic dysfunction.31
Moreover, arterial stiffness increases in OSA patients as severity of SDB increases, and nocturnal oxygen desaturation is associated with high PWV.19, 20, 32, 33 SDB including CSA and OSA occurs frequently in HF and has an adverse prognostic impact on HF patients.17, 18 In the current study, significantly higher PWV was observed in accordance with increased severity of SDB, including CSA and OSA, in the HFpEF patients, but not in HFrEF patients.
Furthermore, this research is significant in terms of the use of a new automated non‐invasive self‐measurement ambulatory blood pressure monitoring device to measure PWV. The Mobil‐O‐Graph 24 h PWA Monitor estimates brachial blood pressure using the oscillometric method,15, 16 and some studies have already reported the reliability of the device by showing good correlation with traditionally used tonometry PWV measurement systems.24, 25
The prevalence of HFpEF has been continually increasing, and established useful pharmacotherapies in HFrEF patients have been ineffective in HFpEF patients.34, 35, 36 Several studies have reported that continuous positive airway pressure therapy decreases blood pressure and PWV in SDB patients with hypertension in both the short‐term and long‐term.37, 38, 39 Additionally, meta‐analysis has demonstrated that continuous positive airway pressure improves aortic stiffness in patients with OSA.40 We previously reported that the reduction of all‐cause mortality in HFpEF patients with SDB after positive airway pressure treatment might be partly due to improvement of aortic stiffness.41, 42 Thus, it is possible to speculate that SDB management using positive airway pressure improves the prognosis of HFpEF patients via a decrease in arterial stiffness, which is one of the important mechanisms underlying HFpEF, as we reported in our previous study.
Study limitations
The current study has several limitations. First, the sample size was small, and the study was conducted in a single centre. Second, HFpEF is common in patients with hypertension, diabetes, obesity, and/or renal dysfunction; however, in the current study, no significant difference was observed in the rate of hypertension between the HFpEF and HFrEF patients. One of the important reasons for this inconsistency may be the diagnostic criteria of hypertension, which was defined as an elevated systolic blood pressure of >140 mmHg, a diastolic blood pressure of >90 mmHg, or when patients had taken antihypertensive drugs. Some of the HFrEF patients had already taken angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers or beta‐blockers for HF treatment. However, echocardiographic results revealed that the HFpEF patients had concentric hypertrophy (increased wall thickness and decreased LV end‐diastolic diameter); therefore, HFpEF patients were more influenced by hypertension compared with HFrEF patients. Third, the cut‐off line between HFpEF and HFrEF is controversial: 40% or 50%. The latest European Society of Cardiology guideline of HF categorizes EF into three groups: HFrEF (EF < 40%), mid‐range EF (EF = 40–50%), and HFpEF (EF > 50%).43 We could not analyse our study subjects according to this classification due to the small sample size. Hence, large‐population and multicentre studies are needed. Moreover, this European Society of Cardiology guideline provides the newest diagnostic criteria of HF,43 but we diagnosed HF using the Framingham criteria.21
Conclusions
The current study demonstrated that severe SDB is associated with elevated atrial stiffness and may be related to the pathophysiology of HFpEF.
Conflict of interest
Satoshi Suzuki and Akiomi Yoshihisa belong to an endowed department (supported by Fukuda Denshi Co., Ltd).
Supporting information
Table S1. Comparisons of clinical characteristics in heart failure with preserved ejection fraction (HFpEF) patients among three SDB groups.
Table S2. Comparisons of clinical characteristics in heart failure with reduced ejection fraction (HFrEF) patients among three SDB groups.
Suzuki, S. , Yoshihisa, A. , Sato, Y. , Watanabe, S. , Yokokawa, T. , Sato, T. , Oikawa, M. , Kobayashi, A. , Yamaki, T. , Kunii, H. , Nakazato, K. , Suzuki, H. , Saitoh, S. , Ishida, T. , and Takeishi, Y. (2018) Association between sleep‐disordered breathing and arterial stiffness in heart failure patients with reduced or preserved ejection fraction. ESC Heart Failure, 5: 284–291. doi: 10.1002/ehf2.12273.
References
- 1. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL, Cardiovascular Health Study Research Group . Importance of heart failure with preserved systolic function in patients > or =65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001; 87: 413–419. [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 4. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 5. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003; 107: 714–720. [DOI] [PubMed] [Google Scholar]
- 8. Borlaug BA, Kass DA. Ventricular–vascular interaction in heart failure. Heart Fail Clin 2008; 4: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimizu T, Yoshihisa A, Kanno Y, Takiguchi M, Sato A, Miura S, Nakamura Y, Yamauchi H, Owada T, Abe S, Sato T, Suzuki S, Oikawa M, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Am J Physiol Heart Circ Physiol 2015; 309: H1123–H1129. [DOI] [PubMed] [Google Scholar]
- 10. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 2009; 15: 658–664. [DOI] [PubMed] [Google Scholar]
- 11. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012; 60: 1455–1469. [DOI] [PubMed] [Google Scholar]
- 12. Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012; 59: 442–451. [DOI] [PubMed] [Google Scholar]
- 13. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H, European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei W, Tölle M, Zidek W, van der Giet M. Validation of the Mobil‐O‐Graph: 24 h‐blood pressure measurement device. Blood Press Monit 2010; 15: 225–228. [DOI] [PubMed] [Google Scholar]
- 16. Wassertheurer S, Kropf J, Weber T, van der Giet M, Baulmann J, Ammer M, Hametner B, Mayer CC, Eber B, Magometschnigg D. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens 2010; 24: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damy T, Margarit L, Noroc A, Bodez D, Guendouz S, Boyer L, Drouot X, Lamine A, Paulino A, Rappeneau S, Stoica MH, Dubois‐Randé JL, Adnot S, Hittinger L, d'Ortho MP. Prognostic impact of sleep‐disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail 2012; 14: 1009–1019. [DOI] [PubMed] [Google Scholar]
- 18. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep‐disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11: 602–608. [DOI] [PubMed] [Google Scholar]
- 19. Drager LF, Diegues‐Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DM, Lorenzi‐Filho G, Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens 2010; 23: 249–254. [DOI] [PubMed] [Google Scholar]
- 20. Chung S, Yoon IY, Lee CH, Kim JW. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration 2010; 79: 363–369. [DOI] [PubMed] [Google Scholar]
- 21. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993; 88: 107–115. [DOI] [PubMed] [Google Scholar]
- 22. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 23. Silber MH, Ancoli‐Israel S, Bonnet MH, Chokroverty S, Grigg‐Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C. The visual scoring of sleep in adults. J Clin Sleep Med 2007; 3: 121–131. [PubMed] [Google Scholar]
- 24. Iwaya S, Yoshihisa A, Nodera M, Owada T, Yamada S, Sato T, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Heart Vessels 2014; 29: 470–477. [DOI] [PubMed] [Google Scholar]
- 25. Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B. Validation of a brachial cuff‐based method for estimating central systolic blood pressure. Hypertension 2011; 58: 825–832. [DOI] [PubMed] [Google Scholar]
- 26. Weiss W, Gohlisch C, Harsch‐Gladisch C, Tölle M, Zidek W, van der Giet M. Oscillometric estimation of central blood pressure: validation of the Mobil‐O‐Graph in comparison with the Sphygmo Cor device. Blood Press Monit 2012; 17: 128–131. [DOI] [PubMed] [Google Scholar]
- 27. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013; 61: 1498–1506. [DOI] [PubMed] [Google Scholar]
- 28. Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular–vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007; 115: 1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jackson CE, Castagno D, Maggioni AP, Køber L, Squire IB, Swedberg K, Andersson B, Richards AM, Bayes‐Genis A, Tribouilloy C, Dobson J, Ariti CA, Poppe KK, Earle N, Whalley G, Pocock SJ, Doughty RN, McMurray JJ, Meta‐Analysis Global Group in Chronic Heart Failure MAGGIC . Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta‐analysis. Eur Heart J 2015; 36: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 30. Kang S, Fan HM, Li J, Fan LY, Miao AY, Bao Y, Wu LZ, Zhu Y, Zhang DF, Liu ZM. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J 2010; 31: 2799–2807. [DOI] [PubMed] [Google Scholar]
- 31. Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta‐analysis. Heart Fail Rev 2015; 20: 291–303. [DOI] [PubMed] [Google Scholar]
- 32. Nagahama H, Soejima M, Uenomachi H, Higashi Y, Yotsumoto K, Samukawa T, Arima T. Pulse wave velocity as an indicator of atherosclerosis in obstructive sleep apnea syndrome patients. Intern Med 2004; 43: 184–188. [DOI] [PubMed] [Google Scholar]
- 33. Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi‐Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2005; 172: 613–618. [DOI] [PubMed] [Google Scholar]
- 34. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 35. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I‐PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 36. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014; 35: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 37. Saito T, Saito T, Sugiyama S, Asai K, Yasutake M, Mizuno K. Effects of long‐term treatment for obstructive sleep apnea on pulse wave velocity. Hypertens Res 2010; 33: 844–849. [DOI] [PubMed] [Google Scholar]
- 38. Kartali N, Daskalopoulou E, Geleris P, Chatzipantazi S, Tziomalos K, Vlachogiannis E, Karagiannis A. The effect of continuous positive airway pressure therapy on blood pressure and arterial stiffness in hypertensive patients with obstructive sleep apnea. Sleep Breath 2014; 18: 635–640. [DOI] [PubMed] [Google Scholar]
- 39. Stein JH, Stern R, Barnet JH, Korcarz CE, Hagen EW, Young T, Peppard PE. Relationships between sleep apnea, cardiovascular disease risk factors, and aortic pulse wave velocity over 18 years: the Wisconsin Sleep Cohort. Sleep Breath 2016; 20: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vlachantoni IT, Dikaiakou E, Antonopoulos CN, Stefanadis C, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta‐analysis. Sleep Med Rev 2013; 1: 19–28. [DOI] [PubMed] [Google Scholar]
- 41. Yoshihisa A, Suzuki S, Yamauchi H, Sato T, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Beneficial effects of positive airway pressure therapy for sleep‐disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol 2015; 38: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshihisa A, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Impact of adaptive servo‐ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep‐disordered breathing. Eur J Heart Fail 2013; 15: 543–550. [DOI] [PubMed] [Google Scholar]
- 43. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of clinical characteristics in heart failure with preserved ejection fraction (HFpEF) patients among three SDB groups.
Table S2. Comparisons of clinical characteristics in heart failure with reduced ejection fraction (HFrEF) patients among three SDB groups.


