Abstract
Lipodystrophy is a rare condition that is often accompanied by one or more metabolic diseases. Here, we report a case of lipoatrophic diabetes induced by juvenile dermatomyositis. Although pioglitazone was not effective for lowering blood glucose levels, our observation suggested that it improved liver function slightly. The effectiveness of metreleptin for lowering blood glucose levels could not be determined, as we administered it in a short period. Liver biopsy showed burned‐out non‐alcoholic steatohepatitis. The present results show that the successful treatment of lipoatrophic diabetes induced by juvenile dermatomyositis requires an early diagnosis and therapeutic intervention.
Keywords: Lipodystrophy, Metreleptin, Pioglitazone
Introduction
Lipodystrophy is a rare condition that is characterized by severe loss of subcutaneous fat tissue from the face, arms and legs; it is often accompanied by metabolic diseases1. We observed a case of generalized lipodystrophy caused by juvenile dermatomyositis, and accompanied by diabetes and liver cirrhosis. Here, we report the effects of treatment with pioglitazone and metreleptin on glycemic control and liver function.
Case Report
A 19‐year‐old man was admitted to the Department of Medicine, Division of Diabetes, Metabolism and Endocrinology, Chiba University Hospital, Chiba, Japan, for close examination and treatment of poorly controlled diabetes. He was diagnosed with juvenile dermatomyositis at 3 years‐of‐age. Immunosuppressive therapy with prednisolone had been initiated, and the dermatomyositis had improved; however, muscle atrophy and joint contracture appeared at 5 years‐of‐age. He was diagnosed with diabetes at age 9 years‐of‐age, and given acarbose at 9 years‐of‐age; metformin was then added at 18 years‐of‐age. Juvenile dermatomyositis completely resolved at 15 years‐of‐age, and the prednisolone was discontinued. However, his blood glucose control did not improve.
On admission, the patient's height was 165 cm, weight was 40.8 kg and body mass index was 15.0 kg/m2. He had no obvious diabetic complications, such as retinopathy or nephropathy, but he lost a considerable amount of subcutaneous fat and peripheral muscle, particularly in his extremities, and his knee joints were contracted (Figure 1). The initial examination findings are shown in Table 1. He also showed decreased levels of adipocytokines (Table 1). A 75‐g oral glucose tolerance test revealed relatively severe insulin resistance (Figure S1). Computed tomography at the umbilical level showed very low amounts of subcutaneous fat tissue (6.4 cm2; Figure S2), but no liver abnormalities. At birth, his weight and appearance were both normal, and he had no family history of lipodystrophy. These findings led to a prior diagnosis of acquired generalized lipodystrophy (AGL) induced by juvenile dermatomyositis.
Figure 1.
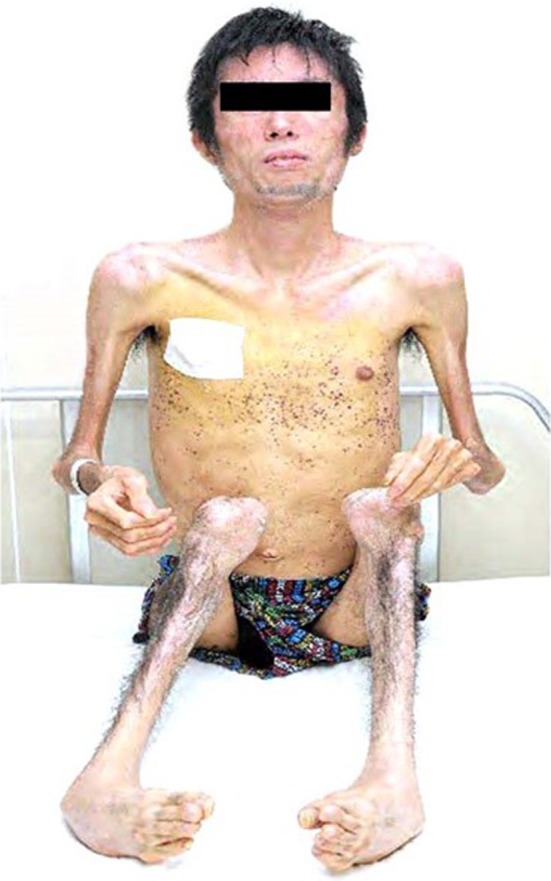
Patient appearance.
Table 1.
Laboratory findings at initial visit and after pioglitazone treatment
| Initial visit | After pioglitazone treatment | |||
|---|---|---|---|---|
| 3 months | 6 months | 18 months | ||
| Bodyweight (kg) | 40.8 | 41.4 | ||
| BMI (kg/m2) | 15.0 | 15.2 | ||
| HbA1c (%) | 8.6 | 8.6 | 9.6 | 8.8 |
| FBS (mg/dL) | 197 | 190 | 205 | |
| IRI (μU/L) | 25.6 | 41.2 | ||
| CPR (ng/mL) | 3.64 | 4.25 | ||
| Total cholesterol (mg/dL) | 206 | 203 | ||
| HDL cholesterol (mg/dL) | 26 | 27 | 28 | 25 |
| LDL cholesterol (mg/dL) | 169 | 164 | 168 | 169 |
| Triglyceride (mg/dL) | 130 | 246 | 196 | 132 |
| AST (U/L) | 61 | 64 | 89 | 44 |
| ALT (U/L) | 132 | 111 | 125 | 101 |
| γGTP (U/L) | 60 | 47 | 60 | 51 |
| Albumin (g/dL) | 4.7 | 4.3 | 4.4 | 4.6 |
| Total bilirubin (mg/dL) | 0.9 | 0.7 | ||
| Platelet (104/μL) | 15.5 | 14.1 | 14.8 | 16.7 |
| Serum creatinine (mg/dL) | 0.12 | 0.10 | 0.14 | 0.17 |
| Fasting leptin (ng/mL) | 2.8 | 3.2 | ||
| Fasting adiponectin (μg/mL) | 2.5 | 2.9 | ||
| Visceral fat area (cm2) | 44.7 | 79.2 | ||
| Subcutaneous fat area (cm2) | 6.4 | 12.0 | ||
| HBsAg | (–) | |||
| HCV antibody | (–) | |||
| Anti‐mitochondrial antibody | <1.5 | |||
| Type IV collagen (ng/mL) | 6.5 | 6.1 | ||
| Hyaluronic acid (ng/mL) | 21 | 11 | ||
| Fibroscan (kPa) | 13.2 | 11.4 | ||
Reference values for type IV collagen, hyaluronic acid, and fibroscan are <6 ng/mL, <50 ng/mL and <7 kPa, respectively. γGTP, gamma‐glutamyltransferase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CPR, C‐peptide reactivity; FBS, fasting blood glucose; HbA1c, glycated hemoglobin; HBsAg, hepatitis B virus surface antigen; HCV, hepatitis C virus; HDL, high‐density lipoprotein; IRI, immunoreactive insulin; LDL, low‐density lipoprotein.
Considering the patient's lipodystrophy and diabetes, he was prescribed 15 mg pioglitazone daily. After receiving pioglitazone for 18 months, his blood glucose and glycated hemoglobin (HbA1c) levels remained unchanged, but his transaminase levels slightly attenuated (Table 1). Changes in transaminase levels and HbA1c from 8 years‐of‐age are also shown in Table S1. Additionally, his levels of type IV collagen, hyaluronic acid and value of Fibroscan® (indicative of liver fibrosis) were all slightly attenuated. Computed tomography scan showed a few changes in the amount of subcutaneous fat after 18 months of pioglitazone treatment (Table 1). As his liver transaminase levels were still abnormal, we therefore carried out a liver biopsy, and diagnosed the patient with type 4 non‐alcoholic fatty liver disease2 (Figure 2; Figure S3).
Figure 2.
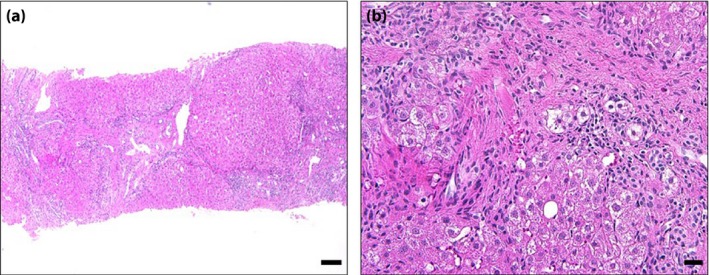
Liver biopsy specimen showed burned‐out non‐alcoholic steatohepatitis. (a) Distortion of hepatic lobular architecture was extensively observed (hematoxylin–eosin stain; lower‐power view). (a,b) Although few lipid droplets (<5%) were observed, burned‐out non‐alcoholic steatohepatitis10 was considered, because pericellular and perivenular fibrosis were prominent, and other causes of liver diseases were ruled out in the present case. Scale bars represent (a) 100 μm and (b) 20 μm.
To determine why pioglitazone did not sufficiently increase the fat mass to reduce blood glucose levels, we obtained subcutaneous fat tissue from the patient's right breast after obtaining his informed consent. We compared the expression levels of adipocyte differentiation markers with those isolated from the subcutaneous fat tissue of a non‐diabetic patient. As shown in Figure S4, adipocyte differentiation markers were severely attenuated in the present patient. We also tried to culture preadipocytes and compare their proliferative activities without success (data not shown), showing that the proliferation and differentiation capacities of the patient's subcutaneous fat tissue were severely attenuated.
As pioglitazone did not improve his blood glucose levels, we administered 0.02 mg/kg metreleptin to control blood glucose. We examined the short‐term effects of metreleptin through a 75‐g oral glucose tolerance test. Compared with baseline levels, the glucose area under the curve decreased by 10.9% after 8 days of metreleptin treatment. Although metreleptin might have lowered his blood glucose levels, this treatment was terminated because of patient non‐compliance due to difficult and painful self‐injections.
Discussion
Juvenile dermatomyositis complicated by lipodystrophy has a prevalence of 10–40%3. The severity of juvenile dermatomyositis is related to lipodystrophy. Particular risk factors for lipodystrophy include panniculitis, calcinosis, joint contracture, and muscle atrophy3. Lipodystrophy leads to the development of metabolic diseases and an average lifetime expectancy of 30–40 years because of metabolic disease complications. The present patient underwent a liver biopsy, but, unexpectedly, the steatohepatitis had already progressed to hepatic cirrhosis, despite his young age. Therefore, it was important to control his hepatic disease to improve his prognosis.
The present case showed a relatively milder phenotype than that of the reported typical AGL (i.e., milder insulin resistance). This is probably because the patient had relatively preserved subcutaneous fat, whereas typical AGL shows a nearly complete absence of adipose tissue.
We examined two different agents (pioglitazone and metreleptin) to improve the patient's blood glucose control. Previous reports suggested that pioglitazone is an effective treatment for lipoatrophic diabetes4, as it promotes preadipocyte differentiation. Previous reports showed that pioglitazone increased fat mass in non‐lipoatrophic body regions in patients with familial partial lipodystrophy4. However, pioglitazone did not reverse subcutaneous fat loss in lipoatrophic body regions5. We administered pioglitazone, with the expectation that it would sufficiently increase the amount of fat tissue to reduce blood glucose levels, but without success. As the patient experienced full‐body fat tissue loss, the number of preadipocytes might have been insufficient to respond to the pioglitazone treatment. Indeed, isolated subcutaneous fat tissue showed severely attenuated levels of adipocyte differentiation markers and proliferation capacity. These results show that the present patient should have been diagnosed and treated with pioglitazone earlier in the course of his disease.
Although pioglitazone was not effective for lowering HbA1c levels, our observation suggested that it improved liver function slightly. His aminotransferase levels attenuated after 18 months of pioglitazone treatment. As we did not carry out a liver biopsy before pioglitazone treatment, we are unable to conclusively show that pioglitazone had favorable effects on his non‐alcoholic steatohepatitis (NASH). However, several reports showed that pioglitazone effectively treats liver disease caused by lipodystrophy6. We resume pioglitazone therapy so that it ameliorates liver function. Furthermore, as none of the markers of liver function predicted the presence of burned‐out NASH in the present case, liver biopsy can be carried out at an early time‐point when patients with lipodystrophy show liver dysfunction.
Metreleptin replacement therapy is a reportedly effective treatment for lipodystrophy7. A 9‐year‐old girl with AGL as a result of active juvenile dermatomyositis was reported to be successfully treated with metreleptin. Not only did her triglyceride and HbA1c levels improve, but also her liver transaminase levels reportedly improved. She developed AGL when she was 7 years‐of‐age, and metreleptin treatment was started after 2 years8. Furthermore, it improves approximately two‐thirds of NASH cases induced by lipodystrophy9, making it superior to pioglitazone treatment. Therefore, metreleptin replacement therapy in the early phase in this case might be effective for lowering blood glucose levels and preventing NASH. Unfortunately, we were unable to continue the metreleptin treatment because of patient non‐compliance. Therefore, transplantation of fat tissue might be a suitable, alternative treatment for similar cases.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | The 75‐g oral glucose tolerance test before treatments.
Figure S2 | Computer tomography image at the umbilical level.
Figure S3 | Liver biopsy specimen showed burned‐out non‐alcoholic steatohepatitis.
Figure S4 | The expression of adipocyte differentiation markers in isolated subcutaneous fat tissue.
Table S1 | Changes in transaminase levels and glycated hemoglobin from 9 years‐of‐age.
Table S2 | Primer sequences for real‐time polymerase chain reaction.
Acknowledgments
We thank Mrs Aki Watanabe and Mrs Naoko Koizumi (Department of Clinical Cell Biology and Medicine, Chiba University Graduate School of Medicine) for their valuable technical assistance.
J Diabetes Investig 2018;9: 632–635
References
- 1. Garg A. Acquired and inherited lipodystrophies. N Engl J Med 2004; 350: 1220–1234. [DOI] [PubMed] [Google Scholar]
- 2. Matteoni CA, Younossi ZM, Gramlich T, et al Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 3. Bingham A, Mamyrova G, Rother KI, et al Predictors of acquired lipodystrophy in juvenile‐onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 2008; 87: 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sleilati GG, Leff T, Bonnett JW, et al Efficacy and safety of pioglitazone in treatment of a patient with an atypical partial lipodystrophy syndrome. Endocr Pract 2007; 13: 656–661. [DOI] [PubMed] [Google Scholar]
- 5. Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab 2008; 10: 1275–1276. [DOI] [PubMed] [Google Scholar]
- 6. Cusi K, Orsak B, Bril F, et al Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016; 165: 305–315. [DOI] [PubMed] [Google Scholar]
- 7. Oral EA, Simha V, Ruiz E, et al Leptin‐replacement therapy for lipodystrophy. N Engl J Med 2002; 346: 570–578. [DOI] [PubMed] [Google Scholar]
- 8. Lebastchi J, Ajluni N, Neidert A, et al A report of three cases with acquired generalized lipodystrophy with distinct autoimmune conditions treated with metreleptin. J Clin Endocrinol Metab 2015; 100: 3960–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Safar Zadeh E, Lungu AO, Cochran EK, et al The liver diseases of lipodystrophy: the long‐term effect of leptin treatment. J Hepatol 2013; 59: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ong J, Younossi ZM, Reddy V, et al Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl 2001; 7: 797–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | The 75‐g oral glucose tolerance test before treatments.
Figure S2 | Computer tomography image at the umbilical level.
Figure S3 | Liver biopsy specimen showed burned‐out non‐alcoholic steatohepatitis.
Figure S4 | The expression of adipocyte differentiation markers in isolated subcutaneous fat tissue.
Table S1 | Changes in transaminase levels and glycated hemoglobin from 9 years‐of‐age.
Table S2 | Primer sequences for real‐time polymerase chain reaction.


