Abstract Abstract
An inventory of the amphibians of the Reserva Ecológica Michelin – REM in southern Bahia, Brazil is presented. Sixty-nine species were recorded during a ten-year sampling period. Amphibians were distributed in two orders (Gymnophiona and Anura), belonging to twelve families [Aromobatidae (1), Bufonidae (3), Centrolenidae (1), Craugastoridae (5), Eleutherodactylidae (3), Hemiphractidae (2), Hylidae (34), Phyllomedusidae (5) Leptodactylidae (7), Microhylidae (4), Odontophrynidae (3) and Caeciliidae (1)]. Fifty per cent of the reproductive modes known for Atlantic forest anurans were recorded. While no threatened species were found at REM, six species are classified as data deficient (DD) by the Brazilian Red List of threatened species and deserve additional attention. Phasmahyla timbo and Vitreorana eurygnatha are listed as endangered in Bahia according to the list of threatened species of the state. Despite a higher diversity of amphibians in the Atlantic forest having been reported for mountainous regions, our results revealed that amphibian richness for lowland forests is also high.
Keywords: Anura, biodiversity, Gymnophiona, inventory, species richness
Introduction
A rapid decline in amphibian populations has been reported worldwide over the past decades (Young et al. 2001), and currently amphibians are considered the most threatened vertebrate group on the planet (Hoffmann et al. 2010). Approximately one-third of all extant species are threatened (Stuart et al. 2008), which is a high rate in comparison to mammals (23%) and birds (12%) (Baillie et al. 2004). The major threats to the group are habitat degradation, fragmentation and destruction (Young et al. 2004, Becker et al. 2007, Loyola et al. 2007), competition from exotic species (Vredenburg 2004), infectious diseases (Daszk et al. 2003) and climate change (Carey and Alexander 2003, Pounds et al. 2006).
Within the Neotropics, Brazil harbours the largest number of described amphibian species worldwide (Segalla et al. 2016). According to the national species conservation status assessment (ICMBio 2014), only 4% of Brazilian amphibians are threatened. However, approximately 17% of Brazilian species are classified as Data Deficient (DD) (ICMBio 2014) and as there are still many gaps in distribution data, the real number of threatened species may be underestimated and much basic biogeographical work remains to be done (Brooks et al. 2004, IUCN 2008).
The Amazon and the Atlantic Forest biomes harbour the greatest species richness in Brazil (Haddad et al. 2013, Jenkins et al. 2015). Particular attention should be paid to the Atlantic Forest, which is considered one of the five most important biodiversity hotspots in the world (Myers et al. 2000, Mittermeier et al. 2011) and has one of the highest levels of amphibian richness and endemism recorded in the country (Morellato and Haddad 2000, Silva and Casteleti 2003, Haddad et al. 2013). According to Haddad et al. (2013) more than half of the country’s species occur in the Atlantic Forest, of which approximately 75% are endemic to the biome. Unfortunately, this biome has been devastated by logging, urbanization, and agricultural development (Ribeiro et al. 2011). Given the high species richness and endemism, the high degree of threat, and the lack of basic biogeographical information for most species, thorough inventories in previously unstudied areas are an essential step for planning future conservation actions (Campos et al. 2017).
Southern Bahia region is unique within the Atlantic Forest, as this area is believed to have been the largest forest refugium in the biome through the Last Glacial Maximum – LGM (Carnaval et al. 2009) and because of this is expected to harbour a rich amphibian fauna. On the other hand, recent studies have suggested that the Atlantic Forest probably expanded during the LGM onto the Brazilian continental shelf and this may have played an important role in the species diversification process (Leite et al. 2016). Until recently the only literature report on amphibian diversity from southern Bahia was a swift survey conducted by Silvano and Pimenta (2003), and although the sampling effort was limited, they recorded significant richness for several areas. During the past decade there has been an increase in studies (e.g., Dias et al. 2014a, b) which have revealed high levels of amphibian richness and endemism. They also highlight the biological importance of this region.
Despite the increasing number of publications on amphibians from Bahia over the past decades, there is still lack of data on amphibian distribution patterns. The increasing number of publications reporting the geographic distribution of several species (e.g., Camurugi et al. 2010, Dias et al. 2011, Mattedi and Pontes 2014, Dias et al. 2014a) and the description of new species corroborate this data (e.g., Napoli et al. 2011, Lourenço-de-Moraes et al. 2012, Teixeira-Jr et al. 2013, Caramaschi et al. 2013, Pontes et al. 2014, Juncá et al. 2015, Dias et al. 2017, Marciano et al. 2017, Vörös et al. 2017). Inventories increase our knowledge of amphibian community composition and allow a better understanding of species diversity patterns (Haddad 1998). These studies also allow a better assessment of species conservation status that is pivotal for developing future conservation plans (Verdade et al. 2012). Our study aims to provide an inventory of amphibian species from the Reserva Ecológica Michelin – REM, a lowland Atlantic Forest site in southern Bahia, northeastern Brazil, known as one of the most biodiverse regions of the world.
Materials and methods
Study area
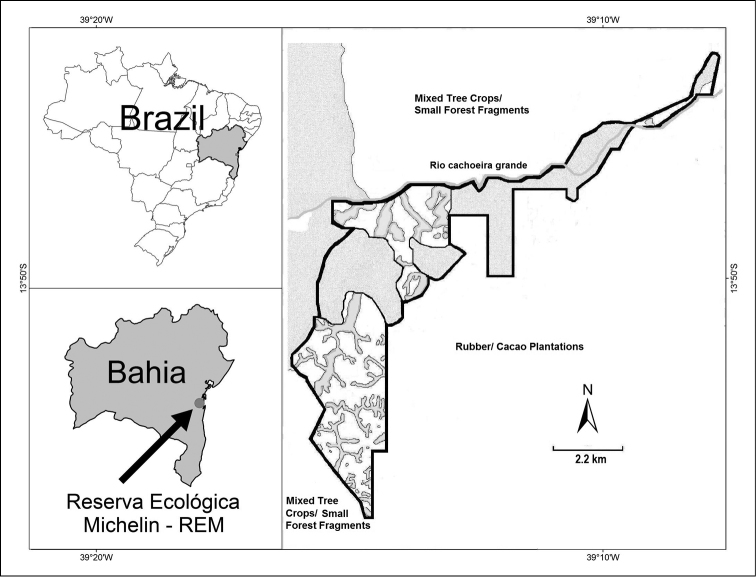
The study was conducted in the Reserva Ecológica Michelin – REM (Figure 1), located in the municipalities of Igrapiúna and Ituberá (13°50'S, 39°10'W), southern Bahia, northeastern Brazil. According to Veloso et al. (1991), the region is characterized as Dense Ombrophilous Lowland Forest. The 3.096 ha reserve supports 1.800 ha of lowland evergreen hill forest distributed in three main fragments (Vila 5/Pancada Grande 625 ha, Pacangê 550 ha, and Luis Inácio 140 ha). The reserve forests have a long history of human disturbance, mostly manioc farming and intensive logging, and the forest is a mosaic of secondary vegetation at different stages of regeneration and primary vegetation logged at varying intensities, with the most intact forests on the steepest slopes and ridge tops. The remainder of the reserve consists of wetlands, small forest fragments and areas with rubber plantations (Hevea brasiliensis) overgrown with pioneer vegetation and enriched with native forest trees (Flesher 2015). The landscape to the east supports rubber, cacao, and banana groves, while to the south, southwest and north, the landscape is one of smallholder properties of mixed tree crops and small forest fragments. A 13.000 ha forest, which is contiguous with the Pacangê forest, lies to the west. The regional landscape (1.000 km²) retains 40% forest cover and a high diversity of agroforestry systems with >60 tree crops planted (Flesher 2006). The average annual rainfall is approximately 2.000 mm with temperatures from 21.7 to 30.8° C (data from REM).
Figure 1.
Map of the Reserva Ecológica Michelin, Bahia State, northeastern Brazil.
Data sampling
Two research teams [(Universidade Estadual de Santa Cruz (UESC) and Universidade Estadual de Feira de Santana (UEFS)] have studied the amphibian community of the reserve for the past ten years. A preliminary inventory carried out between March 2007 and December 2008 revealed 48 anuran species, distributed in ten families (Camurugi et al. (2010)). Between 2010 and 2016 amphibian researchers were continuously active in the reserve, with species diversity recorded through active searching using visual and acoustic cues (Rödel and Ernst 2004) and by opportunistic encounters (i.e., along roads). Between March and December 2015, the terrestrial amphibians were studied using standard pitfall traps (Cechin and Martins 2000, Ribeiro-Júnior et al. 2011), sampling all of the reserve habitats using 24 sets of pitfall traps with 40 cm tall drift fences that included five 30-litre buckets spaced at 8-m intervals, totalling 32 m length. Pitfall traps were kept active during six nights per month, totalling a pitfalls/day effort of 7.200.
All animals were collected according to federal law (ICMBio license #13708-1) and REM protocols. Vouchers were deposited in Bahia at the Museu da Universidade Estadual de Santa Cruz (MZUESC) and Museu de Zoologia da Universidade Estadual de Feira de Santana (MZFS).
Results
Sixty-nine species of amphibians were recorded in the REM: one species of Gymnophiona (Siphonops annulatus – Siphonopidae) and 68 anurans species, belonging to eleven families (Table 1; Figures 2–6). Twenty are new records for the REM, recorded since the study of Camurugi et al. (2010): Frostius erythrophthalmus, Adelophryne cf. pachydactyla, Adelophryne mucronatus, Adelophryne sp., Gastrotheca sp., Gastrotheca recava, Aparasphenodon brunoi, Dendropsophus anceps, Dendropsophus decipiens, Boana exastis, Itapotihyla langsdorffii, Phyllodytes cf. maculosus, Phyllodytes megatympanum, Phyllodytes sp. 2, Phyllodytes wuchereri, Leptodactylus cupreus, Leptodactylus fuscus, Leptodactylus vastus, Dermatonotus muelleri and Siphonops annulatus.
Table 1.
Amphibian species found in the Reserva Ecológica Michelin, southern Bahia, Brazil. ICMBio = Instituto Chico Mendes de Conservação da Biodiversidade; Conservation status: VU = Vulnerable; DD = Deficient Data; LC = Least Concern. Habitat: F = Forest; RP = Rubber plantation. Microhabitat: LL = Leaf litter or understory; SV = Shrub vegetation; S = Streams; TP = Temporary ponds; PP = Permanent ponds; B = bromeliads or epiphytes; C = Canopy; F = Fossorial. Reproductive Modes (sensu Haddad et al. 2013). * = species only found in the inner forests; † = only acoustic record; # only recorded once or twice during the sampling.
| Order/Family/Species | ICMBio | Habitat | Microhabitat | Reproductive modes |
|---|---|---|---|---|
| ANURA | ||||
| Aromobatidae | ||||
| Allobates olfersioides (Lutz, 1925) | VU | F | LL, S | 20 |
| Bufonidae | ||||
| Frostius erythrophthalmus Pimenta & Caramaschi, 2007* | LC | F | SV | ? |
| Rhinella hoogmoedi Caramaschi & Pombal, 2006 | LC | F, RP | LL, S | 1 |
| Rhinella crucifer (Wied-Neuwied, 1821) | LC | F, RP | LL, PP, TP | 1,2 |
| Centrolenidae | ||||
| Vitreorana eurygnatha (Lutz, 1925)* | LC | F | S | 25 |
| Craugastoridae | ||||
| “Eleutherodactylus” bilineatus (Bokermann, 1975)* | LC | F | LL | 23 |
| Haddadus binotatus (Spix, 1824) | LC | F | LL | 23 |
| Pristimantis paulodutrai (Bokermann, 1975) | LC | RP | SV | 23 |
| Pristimantis sp.* | – | F | SV | 23 |
| Pristimantis vinhai (Bokermann, 1975) | LC | F, RP | SV | 23 |
| Eleutherodactylidae | ||||
| Adelophryne cf. pachydactyla Hoogmoed, Borges & Cascon, 1994 | LC | F | LL | 23 |
| Adelophryne mucronatus Lourenço-de-Morais, Solé & Toledo 2012* | LC | F | LL | 23 |
| Adelophryne sp.* | – | F | LL | 23 |
| Hemiphractidae | ||||
| Gastrotheca sp. | – | F | C | 37 |
| Gastrotheca recava Teixeira et al., 2012 | – | F | C | 37 |
| Hylidae | ||||
| Aparasphenodon brunoi Miranda-Ribeiro, 1920* | LC | F | SV | 1 |
| Aplastodiscus cavicola (Cruz and Peixoto, 1985)* | LC | F | S | 5 |
| Aplastodiscus ibirapitanga (Cruz, Pimenta & Silvano, 2003)* | LC | F | S | 5 |
| Aplastodiscus sibilatus (Cruz, Pimenta & Silvano, 2003)* | LC | F | S | 5 |
| Bokermannohyla capra Napoli & Pimenta, 2009* | – | F | S | 2 |
| Dendropsophus anceps (Lutz, 1929) | LC | RB | TP | 1 |
| Dendropsophus branneri (Cochran, 1948) | LC | F, RP | PP, TP | 1 |
| Dendropsophus decipiens (Lutz, 1925) | LC | RB | PP, TP | 24 |
| Dendropsophus elegans (Wied-Neuwied, 1824) | LC | F, RP | PP, TP | 1 |
| Dendropsophus giesleri (Mertens, 1950) | LC | F, RP | TP | 1 |
| Dendropsophus haddadi (Bastos & Pombal, 1996) | LC | F, RP | PP, TP | 24 |
| Dendropsophus minutus (Peters, 1872) | LC | F, RP | TP | 1 |
| Dendropsophus novaisi (Bokermann, 1968) | LC | RP | TP | 1 |
| Dendropsophus aff. oliveirai (Bokermann, 1963) | LC | RB | PP, TP | 1 |
| Boana albomarginata (Spix, 1824) | LC | F, RP | PP, TP | 1 |
| Boana atlantica (Caramaschi & Velosa, 1996) | LC | F, RP | PP, TP | 1,2 |
| Boana crepitans (Wied-Neuwied, 1824) | LC | RB | PP | 4 |
| Boana exastis (Caramaschi & Rodrigues, 2003)* | LC | F | B, C, S | 4 |
| Boana faber (Wied-Neuwied, 1821) | LC | F, RP | PP, TP | 1,4 |
| Boana pombali (Caramaschi, Pimenta & Feio, 2004) | LC | F, RP | PP | 1,2 |
| Boana semilineata (Spix, 1824) | LC | F, RP | PP | 1,2 |
| Itapotihyla langsdorffii (Duméril & Bibron, 1841) | LC | F, RP | TP | 1 |
| Phyllodytes cf. maculosus Cruz, Feio & Cardoso, 2007† | DD | F | B | 6 |
| Phyllodytes melanomystax Caramaschi, Silva & Britto-Pereira, 1992 | LC | F, RP | B | 6 |
| Phyllodytes megatympanum Marciano, Lantyer-Silva & Solé 2017†* | – | F, RP | B | 6 |
| Phyllodytes praeceptor Orrico, Dias & Marciano 2018 | – | F, RP | B | 6 |
| Phyllodytes sp. | – | F, RP | B | 6 |
| Phyllodytes wuchereri (Peters, 1873)†* | LC | F | B | 6 |
| Ololygon argyreornata (Miranda-Ribeiro, 1926)* | LC | F | S | 1 |
| Ololygon strigilata (Spix, 1824)* | DD | F | S | 1,2 |
| Scinax eurydice (Bokermann, 1968) | LC | F, RP | PP, TP | 1 |
| Scinax juncae Nunes & Pombal, 2010 | LC | F, RP | PP | 1 |
| Scinax x-signatus (Spix, 1824) | LC | F, RP | PP, TP | 1 |
| Trachycephalus mesophaeus (Hensel, 1867) | LC | F, RP | TP | 1 |
| Phyllomedusidae | ||||
| Hylomantis aspera (Peters, 1873)* | LC | F | TP | 18 |
| Phasmahyla timbo Cruz, Napoli & Fonseca, 2008* | DD | F | S | 25 |
| Phyllomedusa bahiana Lutz, 1925 | LC | F, RP | TP | 24 |
| Pithecopus nordestinus (Caramaschi, 2006) | LC | F, RP | PP, TP | 24 |
| Pithecopus rohdei (Mertens, 1926) | LC | F, RP | PP, TP | 24 |
| Leptodactylidae | ||||
| Adenomera thomei (Almeida & Angulo, 2006) | LC | RP | LL | 32 |
| Leptodactylus macrosternum Miranda-Ribeiro, 1926 | LC | F, RP | LL, S, PP, TP | 11 |
| Leptodactylus cupreus Caramaschi, Feio & São-Pedro, 2008* | DD | F | LL, TP | 30 |
| Leptodactylus fuscus (Schneider, 1799) | LC | RP | LL, TP | 30 |
| Leptodactylus mystaceus (Spix, 1824) | LC | RP | LL, TP | 30 |
| Leptodactylus vastus Lutz, 1930 | LC | RP | LL, TP | 11 |
| Physalaemus camacan Pimenta, Cruz, & Silvano, 2005 | LC | F, RP | LL, TP | 11 |
| Microhylidae | ||||
| Chiasmocleis cordeiroi Caramaschi & Pimenta, 2003 | DD | F, RP | LL, TP | 1 |
| Chiasmocleis crucis Caramaschi & Pimenta, 2003 | DD | F | LL, TP | 1 |
| Stereocyclops incrassatus Cope, 1870 | LC | F, RP | LL, TP | 1 |
| Dermatonotus muelleri (Boettger, 1885) | LC | RP | TP | 1 |
| Odontophrynidae | ||||
| Macrogenioglottus alipioi Carvalho, 1946* | LC | F | LL, TP | 1 |
| Proceratophrys renalis (Miranda-Ribeiro, 1920) | LC | F | LL, S | 2 |
| Proceratophrys schirchi (Miranda-Ribeiro, 1937)* | LC | F | LL, S | 2 |
| GYMNOPHYONA | ||||
| Caeciliidae | ||||
| Siphonops annulatus (Mikan, 1820) | LC | F, RP | F | |
Figure 2.
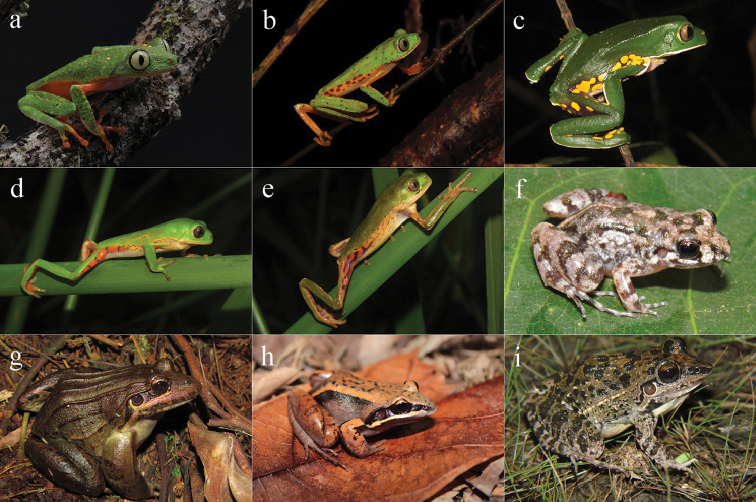
Amphibians from Reserva Ecológica Michelin, Bahia State, Northeastern Brazil. a Allobates olfersioides b Frostius erythrophthalmus c Rhinella hoogmoedi d Rhinella crucifer e Vitreorana eurygnatha f “Eleutherodactylus” bilineatus g Haddadus binotatus h Pristimantis paulodutrai i Pristimantis sp. j Pristimantis vinhai k Adelophryne cf. pachydactyla l Adelophryne mucronatus m Adelophryne sp. n Gastrotheca recava o Gastrotheca sp.
Figure 6.
Amphibians from Reserva Ecológica Michelin, Bahia State, Northeastern Brazil. a Leptodactylus mystaceus b Physalaemus camacan c Chiasmocleis cordeiroi d Stereocyclops incrassatus e Dermatonotus muelleri f Macrogenioglottus alipioi g Proceratophrys renalis h Proceratophrys schirchi i Siphonops annulatus.
Figure 3.
Amphibians from Reserva Ecológica Michelin, Bahia State, Northeastern Brazil. a Aparasphenodon brunoi b Aplastodiscus cavicola c Aplastodiscus ibirapitanga d Aplastodiscus sibilatus e Bokermannohyla capra f Dendropsophus anceps g Dendropsophus branneri h Dendropsophus decipiens i Dendropsophus elegans j Dendropsophus giesleri k Dendropsophus haddadi l Dendropsophus minutus m Dendropsophus novaisi n Dendropsophus aff. oliveirai o Boana albomarginata.
Figure 4.
Amphibians from Reserva Ecológica Michelin, Bahia State, Northeastern Brazil. a Boana atlantica b Boana crepitans c Boana exastis d Boana faber e Boana pombali f Boana semilineata g Itapotihyla langsdorffii h Phyllodytes melanomystax i Phyllodytes praeceptor j Phyllodytes sp. k Ololygon strigilata l Scinax eurydice m Scinax juncae n Scinax x-signatus o Trachycephalus mesophaeus.
Figure 5.
Amphibians from Reserva Ecológica Michelin, Bahia State, Northeastern Brazil. a Hylomantis aspera b Phasmahyla timbo c Phyllomedusa bahiana d Pithecopus nordestinus e Pithecopus rohdei f Adenomera thomei g Leptodactylus macrosternum h Leptodactylus cupreus i Leptodactylus fuscus.
According to the Brazilian federal list (ICMBio 2014), most of the species found in the REM are not threatened (N = 54; 78.2%), except Allobates olfersioides listed as Vulnerable. Approximately 9% (N = 6) are listed as data deficient (DD) and the conservation status of eight species has not yet been defined. Considering the species identified at the species level or as “cf.”, the majority (n = 44; 63.8%) are endemic to the Atlantic Forest biome, and 20.3% (n = 14) are endemic to Bahia. Two species deserve special attention: Phasmahyla timbo and Vitreorana eurygnatha, both considered endangered (EN) in the list of threatened species of the state of Bahia (Bahia 2017).
More than half of the species were recorded in lentic habitats (n = 36; 52.17%), of which 18 species were restricted to temporary ponds, four to permanent ponds, and eleven were found in both habitats (Table 1). Twenty-one species were found on leaf litter, of which two and nine, also occupied streams and temporary ponds, respectively. Eight species were found only in forest streams (Vitreorana eurygnatha, Aplastodiscus cavicola, A. ibirapitanga, A. sibilatus, Bokermannohyla capra, Phasmahyla timbo, Ololygon argyreornata and O. strigilata). Epiphytes and bromeliads were used by species of the genus Phyllodytes. The forest canopy was occupied primarily by Gastrotheca sp. and G. recava, frequently found between two and five meters above ground. Boana exastis was most frequently heard calling from bromeliads in the canopy, but was also spotted along streams. Leptodactylus macrosternum was the species that demonstrated the highest habitat plasticity and was found in streams, permanent and temporary ponds. Only Siphonops annulatus occurred in fossorial habitats. Twenty-nine species were found exclusively inside the forest, ten restricted to open areas and rubber plantations and 31 in both habitats (Table 1).
Taxonomic and nomenclatural remarks concerning Camurugi et al. (2010)
The previous REM checklist of anurans (Camurugi et al. 2010) included several species with taxonomic uncertainties that are revised here. Most of the following species show cryptic patterns that hamper their taxonomic identification. Our analysis was based on adult and larval (whenever possible) morphological characteristics and bio-acoustic parameters.
Based on a study of the phylogenetic relationships within the anuran clade Terrarana (Canedo and Haddad 2012), the species referred as Ischnocnema by Camurugi et al. (2010) have been transferred to Pristimantis, with exception of Ischnocnema bilineata that was relocated as “Eleutherodactylus” bilineatus as incertae sedis. A recent nomenclature review carried out by Dubois (2017) showed that the genus Hypsiboas was erroneously recovered as a Hyla synonym by Faivovich et al. (2005), and that Boana as suggested by Gray (1825) is a valid generic name. Thus, species referred as Hypsiboas in Camurugi et al. (2010) have now been transferred to Boana. Based on molecular data, Duellman et al. (2016) resurrected the generic names Ololygon for the “Scinax catharinae clade” and Phitecopus for the “Phyllomedusa hypochondrialis group”. Thus, Scinax strigilatus and S. argyreornatus from the list by Camurugi et al. (2010) are now referred to as Ololygon strigilata and O. argyreornata. In addition, Phyllomedusa nordestina and P. rohdei are now referred to as Pithecophus nordestinus and P. rohdei. Finally, Phyllomedusa burmeisteri was relocated as P. bahiana based on molecular data sets gathered by Barth et al. (2013) and Brunes et al. (2014).
Ischnocnema aff. ramagii is re-classified as Pristimantis sp. This species is widely distributed in the forests of southern Bahia and is currently being described (Marciano Jr. et al. in prep). Vitreorana sp. was recorded by Camurugi et al. (2010) only in larval form, but adult males were collected and, using morphological and bio-acoustic traits, are identified as Vitreorana eurygnatha. Scinax aff. alter and Chiasmocleis sp. are re-classified as Scinax juncae and Chiasmocleis crucis, respectively. Phyllodytes luteolus as Phyllodytes praeceptor (Orrico et al. 2018). The species formerly classified as Dendropsophus seniculus, Physalaemus signifer and Leptodactylus marmoratus, are now classified as Dendropsophus novaisi, Physalaemus camacan and Adenomera thomei, respectively.
Discussion
The present study increases the amphibian species richness of the REM in more than 30%, increasing the total number of species for the reserve from 48 to 69. The majority (n = 43, 61.4%) of the REM species are endemic to the Atlantic Forest biome (see Haddad et al. 2013). Fourteen of the 69 recorded species (Frostius erythrophthalmus, “Eleutherodactylus” bilineatus, Adelophryne cf. pachydactyla, A. mucronatus, Gastrotheca recava, Hylomantis aspera, Bokermannohyla capra, Phasmahyla timbo, Phyllodytes praeceptor, Phyllodytes wuchereri, Ololygon strigilata, Physalaemus camacan, Chiasmocleis cordeiroi and C. crucis) are also endemic to Bahia (Angulo 2008a, Juncá and Pimenta 2004, Peixoto and Pimenta 2004, Borges-Najosa and Juncá 2004, Lourenço-de-Moraes et al. 2012, Teixeira Jr. et al. 2012, Silvano and Pimenta 2010, Napoli and Pimenta 2009, Angulo 2009, Rodrigues 2006, Juncá and Silvano 2004, Angulo 2008b, Forlani et al. 2013). The results of our study expand the known distribution of Adelophryne mucronatus 60 km to the north (Lourenço-de-Moraes et al. 2012, Dias et al. 2014a). Two species were recorded by collecting only a single individual per species: Aparasphenodon brunoi, a bromeliad species, which had its distribution recently increased from the municipality of Una to the REM (Ruas et al. 2013), and Dermatonotus muelleri, which is a species typically found in open areas..
Although no species has been considered threatened by ICMBio (2014), special attention should be paid to Allobates olfersioides. In a taxonomic review of Allobates from the Atlantic Forest, Verdade and Rodrigues (2007) synonymized the four previously recognized species Allobates olfersioides (Lutz, 1925), A. capixaba (Bokermann, 1967), A. carioca (Bokermann, 1967) and A. alagoanus (Bokermann, 1967) with A. olfersioides. However, in a recent assessment of threatened Brazilian amphibians (Haddad et al. 2016), specialists suggested that only populations from Rio de Janeiro should be recognized as A. olfersioides (U. Caramaschi pers. comm.) while the populations from Bahia should be assigned to A. capixaba or A. alagoanus and classified as Data deficient (DD). A recent acoustic analysis of the Atlantic Forest Allobates agrees with the suggestion of Haddad et al. (2016), arguing that according to the advertisement calls, populations from Bahia can be attributed to A. capixaba or even represent a new species (Forti et al. 2017). However, due to the lack of a recent taxonomic analysis, the species from REM is assigned to A. olfersioides following Verdade and Rodrigues (2007).
The two species classified as endangered (EN) in the list of threatened species of the state of Bahia (Bahia, 2017) occur in streams in the interior of forest fragments. Phasmahyla timbo is restricted to the state of Bahia and known only from the type locality in Serra do Timbó, municipalities of Amargosa and Santa Terezinha (Cruz et al. 2008) and from the Reserva Ecológica Michelin. Vitreorana eurygnatha has been reported from Amargosa (Freitas et al. 2007), Mata de São João, Jandaíra (Tinoco et al. 2008), Camacan and Almadina (Dias et al. 2014a, b), and now also from Igrapiúna, at REM.
Seven species (10.1%) are listed without a specific name or were classified as similar with other species. The formal descriptions of some of them, like Adelophryne sp. have already been submitted for publication. Our results add basic data on the distribution of amphibians from Bahia, and corroborate the data presented by other recent inventory studies conducted in the state (Dias et al. 2014a, b). The amphibian richness from the REM is the second-highest reported from Bahia, ranking only behind that of the RPPN Serra Bonita (80 ssp.), located along an altitudinal gradient of 200 to 950 m a.s.l. (Dias et al. 2014a). Most other Atlantic Forest sites with high amphibian diversity, such as Santa Tereza municipality (92 ssp.), the Parque Natural Municipal Nascentes de Paranapiacaba (80 ssp.), Estação Ecológica de Boracéia (67 ssp.) and Parque Estadual Carlos Botelho (65 ssp.), represent mountainous areas (Heyer et al. 1990, Forlani et al. 2010, Almeida et al. 2011, Trevine et al. 2014). Over the altitudinal gradients, changes in biotic and abiotic features increase the availability of microenvironments which are believed to promote greater species diversity. Vasconcelos et al. (2010) conducted a review in various mountainous ranges in Brazil and found that the increasing amphibian richness is related to the degree of the altitude gradient. Unlike these areas, the REM is inserted in a region classified as Dense Ombrophilous Lowland Forest (Veloso 1991), with maximum altitudes reaching 393 m above sea level.
Despite the lack of distinct altitudinal gradients, the REM is located close to 13.000 ha of forest and nested in a climatically stable region, with high moisture and availability of breeding sites. These diverse habitats provide a high diversity of breeding sites such as temporary and permanent ponds, streams, bromeliads, epiphytes and a dense leaf litter layer. This habitat heterogeneity, high temperatures and rainfall throughout the year create a hot and moist environment, which likely explains the expressive number of species recorded. Additionally, the high abundance of breeding habitats satisfies the reproductive requirements of a large number of species. According to Duellman (1988) reproductive modes play an important role in understanding anuran species diversity. Currently, there are 27 reproductive modes recognized for Atlantic Forest amphibians (Haddad et al. 2013). According to Haddad and Prado (2005), the high diversity of reproductive modes observed for the Atlantic Forest is the result of a successful utilization of the diverse humid microhabitats present in the biome. Fourteen out of the 27 reproductive modes reported for the Atlantic Forest amphibians (~52%) were recorded at the REM.
Another important factor that likely affected the results was the sampling effort. With approximately ten years of sampling this study has the highest sampling effort for northeastern Brazil to date. Long sampling periods are essential for understanding community structure and are necessary for reaching accurate values of diversity for an area. Although the present study shows an increase of 21 amphibian species in comparison to the previous study (Camurugi et al. 2010), we believe that further fieldwork may still reveal new species for the REM. Highest amphibian diversity areas in the Atlantic forest were also the result of long sampling periods. Despite the long sampling periods new species are still found in well sampled regions, as the case of Adelophryne glandulata and Dendropsophus bromeliaceus, recently described from Santa Tereza municipality (Lourenço-de-Moraes et al. 2014, Ferreira et al. 2015). Most long-term amphibian studies have been undertaken in southeastern Brazil, resulting in a much better comprehension of the amphibians of this region within the domain (Rossa-Feres et al. 2011, Campos et al. 2014). On the other hand, the recent increase in studies from other regions of the country (e.g., southern Bahia) is expanding our knowledge for these regions (Dias et al 2014a, b, present study).
Several attempts to understand the processes responsible for the high levels of species diversification of the Atlantic Rainforest have been undertaken. The consensus is that none seem to have acted isolated. The high diversity and endemism of species in southern Bahia has been associated with climate stability and forest conditions during glacial periods (Carnaval et al. 2009). According to these authors, this region was a large Pleistocene climatic refugium for amphibians. Another view of a recent research shows that suitable climatic conditions onto the emerged continental shelf probably expanded the Atlantic Forest during the last glacial period (Leite et al. 2016). Thereby, species could have expanded their distributions during the last glacial period. Thus, these long-term biogeographical processes would have promoted a high level of speciation in southern Bahia. Given the high levels of richness and endemism of amphibians in southern Bahia, future inventories in still un-sampled regions of southern Bahia are expected to recover a high diversity of amphibians, and may result in the discovery of additional new species and expand the ranges of already known species.
Acknowledgements
We thank the Centro de Estudos da Biodiversidade of the Reserva Ecológica Michelin for logistical and financial support, especially to Kevin Flesher, André Santos, Tia Sônia, Ester Paixão and Tipó for their friendship and help during the research. We are also indebted to the colleagues Marcelo Sena, Carlos Augusto Costa, Marcos Lavigne, Diego Generozo, Marcos Vila Nova and Maiara Alves for their help during fieldworks. Furthermore, we wish to thank Maria Lúcia Del-Grande, Diego Santana, Tiago Gomes Santos, and Kevin Flesher for valuable comments on previous drafts of the manuscript. We thank Kevin Flesher and Michelle Pazmino for the English revision of the manuscript. Renato Augusto Martins kindly provided photos of Allobates olfersioides, Adelophryne sp., Gastrotheca recava, Aparasphenodon brunoi, Boana atlantica, Phyllodytes praeceptor, Ololygon strigilata, Hylomantis aspera and Chiasmocleis cordeiroi. MS thanks CAPES/Alexander von Humboldt Foundation for an “experienced researcher” scholarship. CVMM is grateful to FAPESB for fellowships and particular thanks to his wife Fernanda Tonolli for her patience, affection, and eternal loving support.
Citation
Mira-Mendes CB, Ruas DS, Oliveira RM, Castro IM, Dias IR, Baumgarten JE, Juncá FA, Solé M (2018) Amphibians of the Reserva Ecológica Michelin: a high diversity site in the lowland Atlantic Forest of southern Bahia, Brazil. ZooKeys 753: 41–61. https://doi.org/10.3897/zookeys.753.21438
References
- Almeida AP, Gasparini JL, Peloso PLV. (2011) Frogs of the state of Espírito Santo, southeastern Brazil – The need for looking at the ‘coldspots’. Check List 7(4): 542–560. https://doi.org/10.15560/7.4.542 [Google Scholar]
- Angulo A. (2008a) Frostius erythrophthalmus – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Angulo A. (2008b) Physalaemus camacan – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Angulo A. (2009) Phasmahyla timbo – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- BAHIA (2017) Portaria n° 37 de 15 de agosto de 2017. Torna pública a Lista Oficial das Espécies da Fauna Ameaçadas de Extinção do Estado da Bahia. Diário Oficial Bahia, Salvador, BA, Ano · CI · N° 22.240, 16 de ago. 2017.
- Baillie JM, Stuart SN, Hilton-Taylor C. (2004) IUCN Red List of Threatened Species – A Global Species Assessment. IUCN, Gland (Switzerland) and Cambridge (UK), 191 pp. [Google Scholar]
- Barth A, Souza VA, Solé M, Costa MA. (2013) Molecular cytogenetics of nucleolar organizer regions in Phyllomedusa and Phasmahyla species (Hylidae, Phyllomedusinae): a cytotaxonomic contribution. Genetics and Molecular Research 12(3): 2400–2408. https://doi.org/10.4238/2013 [DOI] [PubMed] [Google Scholar]
- Becker CG, Fonseca CR, Haddad CFB, Batista RF, Prado PI. (2007) Habitat Split and the Global Decline of Amphibians. Science 318: 1775–1777. https://doi.org/10.1126/science.1149374 [DOI] [PubMed] [Google Scholar]
- Borges-Najosa D, Juncá FA. (2004) http://www.iucnredlist.org [accessed on 01 January 2015]
- Brooks TM, Bakarr MI, Boucher T, Da Fonseca GA, Hilton-Taylor C, Hoekstra JM, Moritz T, Olivieri S, Parrish J, Pressey RL, Rodrigues AS. (2004) Coverage provided by the global protected area system: Is it enough? Bioscience 54: 1081–1091. https://doi.org/10.1641/0006-3568(2004)054[1081:CPBTGP]2.0.CO;2
- Brunes TO, Alexandrino J, Baêta D, Zina J, Haddad CFB. (2014) Species limits, phylogeographic and hybridization patterns in Neotropical leaf frogs (Phyllomedusinae). Zoologica Scripta 43(6): 586–604. https://doi.org/10.1111/zsc.12079 [Google Scholar]
- Campos FS, Brito D, Solé M. (2014) Diversity patterns, research trends and mismatches of the investigative efforts to amphibian conservation in Brazil. Anais da Academia Brasileira de Ciências 86(4): 1873–1886. https://doi.org/10.1590/0001-3765201420140170 [DOI] [PubMed] [Google Scholar]
- Campos FS, Lourenço-de-Moraes R, Llorente GA, Solé M. (2017) Cost-effective conservation of amphibian ecology and evolution. Science Advances 2017(3): e1602929. https://doi.org/10.1126/sciadv.1602929 [DOI] [PMC free article] [PubMed]
- Camurugi F, Lima TM, Mercês EA, Juncá FA. (2010) Anuros da Reserva Ecológica da Michelin, Município de Igrapiúna, Estado da Bahia, Brasil. Biota Neotropica 10(2): 305–312. https://doi.org/10.1590/S1676-06032010000200032 [Google Scholar]
- Canedo C, Haddad CFB. (2012) Phylogenetic relationships within anuran clade Terrarana, with emphasis on the placement of Brazilian Atlantic rainforest frogs genus Ischnocnema (Anura: Brachycephalidae). Molecular Phylogenetics and Evolution 65: 610–620. https://doi.org/10.1016/j.ympev.2012.07.016 [DOI] [PubMed] [Google Scholar]
- Caramaschi U, Orrico VGD, Faivovich J, Dias IR, Solé M. (2013) A new species of Allophryne (Anura: Allophrynidae) from the Atlantic Rain Forest Biome of eastern Brazil. Herpetologica 69: 480–491. https://doi.org/10.1655/HERPETOLOGICA-D-13-00029 [Google Scholar]
- Carey C, Alexander MA. (2003) Climate change and amphibian declines: is there a link? Diversity and Distributions 9: 111–121. https://doi.org/10.1046/j.1472-4642.2003.00011.x
- Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C. (2009) Stability predicts genetic diversity in the Brazilian Atlantic Forest hotspot. Science 323(5915): 785–789. https://doi.org/10.1126/science.1166955 [DOI] [PubMed] [Google Scholar]
- Cechin SZ, Martins M. (2000) Eficiência de armadilhas de queda (pitfall traps) em amostragem de anfíbios e répteis no Brasil. Revista Brasileira de Zoologia 17: 729–740. https://doi.org/10.1590/S0101-81752000000300017 [Google Scholar]
- Cruz CLG, Napoli MF, Fonseca PM. (2008) A new species of Phasmahyla Cruz, 1990 (Anura: Hylidae) from the state of Bahia, Brazil. South American Journal of Herpetology 3(3): 187–195. https://doi.org/10.2994/1808-9798-3.3.187 [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. (2003) Infectious disease and amphibian population declines. Diversity and Distributions 9: 141–150. https://doi.org/10.3201/eid0506.990601 [Google Scholar]
- Dias IR, Medeiros TT, Vila Nova MF, Solé M. (2014a) Amphibians of Serra Bonita, southern Bahia: a new hotpoint within Brazil’s Atlantic Forest hotspot. ZooKeys 449: 105–130. https://doi.org/10.3897/zookeys.449.7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias IR, Mira-Mendes CV, Solé M. (2014b) Rapid inventory of herpetofauna at the APA (Environmental Protection Area) of the Lagoa Encantada and Rio Almada, Southern Bahia, Brazil. Herpetology Notes 7: 627–637. https://biotaxa.org/hn/article/view/8557/10467 [Google Scholar]
- Dias IR, Medeiros TT, Solé M, Pimenta BVS. (2011) Amphibia, Anura, Hylidae, Bokermannohyla lucianae (Napoli and Pimenta 2003): Distribution extension and geographic distribution map. Check List 7(2): 108–109. https://doi.org/10.15560/7.2.108 [Google Scholar]
- Dias IR, Haddad CFB, Argôlo AJS, Orrico VGD. (2017) The 100th: An appealing new species of Dendropsophus (Amphibia: Anura: Hylidae) from northeastern Brazil. Plos One 12(3): e0171678. https://doi.org/10.1371/journal.pone.0171678 [DOI] [PMC free article] [PubMed]
- Dubois A. (2017) The nomenclatural status of Hysaplesia, Hylaplesia, Dendrobates and relatd nomina (Amphibia, Anura), with general comments on zoological nomenclature and its governance, as well as on taxonomic databases and websites. Bionomina 11: 1–48. [Google Scholar]
- Duellman WE. (1988) Patterns of species diversity in anuran amphibians in the American tropics. Annals of the Missouri Botanical Garden 75: 79–104. https://doi.org/10.2307/2399467 [Google Scholar]
- Duellman WE, Marion AB, Hedges SB. (2016) Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 4104: 1–109. https://doi.org/10.11646/zootaxa.4104.1.1 [DOI] [PubMed] [Google Scholar]
- Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheller WC. (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: a phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History 294: 1–240. https://doi.org/10.5531/sd.sp.12 [Google Scholar]
- Ferreira RB, Faivovich J, Beard KH, Pombal-Jr JPP. (2015) The First Bromeligenous Species of Dendropsophus (Anura: Hylidae) from Brazil’s Atlantic Forest. Plos One 10(12): 1–21. https://doi.org/10.1371/journal.pone.0142893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher KM. (2015) The distribution, habitat use, and conservation status of three Atlantic forest monkeys (Sapajus xanthosternos, Callicebus melanochir, and Callithrix sp.) in an agroforest/forest mosaic in southern Bahia, Brazil. International Journal of Primatology 36(6): 1172–1197. https://doi.org/10.1007/s10764-015-9884-7 [Google Scholar]
- Freitas MA, Silva TFS, Fonseca P. (2007) Geographic distribution. Hyalinobatrachium eurygnathum. Herpetological Review 38: 475–476. [Google Scholar]
- Forlani MC, Mira-Mendes CV, Dias IR, Ruas DS, Tonini JFR, de Sá RO. (2013) The advertisement calls and distribution of two sympatric species of Chiasmocleis (Méhely 1904) (Anura, Microhylidae, Gastrophryninae) from the Atlantic Forest. South American Journal of Herpetology 8(1): 46–51. https://doi.org/10.2994/SAJH-D-12-00027.1 [Google Scholar]
- Forlani MC, Bernardo PH, Haddad CFB, Zaher H. (2010) Herpetofauna of the Carlos Botelho State Park, São Paulo State, Brazil. Biota Neotropica 10(3): 265–309. https://doi.org/10.1590/S1676-06032010000300028 [Google Scholar]
- Haddad CFB. (1998) Biodiversidade dos Anfíbios no Estado de São Paulo. In: Joly CA, Bicudo CEM. (Eds) Biodiversidade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX. FAPESP, São Paulo, 17–26.
- Haddad CFB, Prado CPA. (2005) Reproductive Modes in Frogs and Their Unexpected Diversity in the Atlantic Forest of Brazil. BioScience 55(3): 207–217. https://doi.org/10.1641/0006-3568(2005)055[0207:RMIFAT]2.0.CO;2
- Haddad CFB, Toledo LF, Prado CPA, Loebmann D, Gasparini JL, Sazima I. (2013) Guide to the Amphibians of the Atlantic Forest: Diversity and Biology. Anolisbooks, São Paulo, 544 pp. [Google Scholar]
- Haddad CFB, Segalla MV, Bataus YSL, Uhlig VM, Batista FRQ, Garda A, Hudson AA, Cruz CAG, Strüsmann C, Brasileiro CA, Silvano DL, Nomura F, Pinto HBA, Amaral IB, Gasparini JLR, Lima LP, Martins MRC, Hoogmoed MS, Colombo P, Valdujo PH, Garcia PCA, Feio RN, Brandão RA, Bastos RP, Caramaschi U. (2016) Avaliação do Risco de Extinção de Allobates olfersioides (A. Lutz, 1925). Processo de avaliação do risco de extinção da fauna brasileira. ICMBio. http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/estado-de-conservacao/7498-anfibios-allobates-olfersioides.html [accessed on 18 July 2017]
- Heyer WR, Rand AS, Cruz CAG, Peixoto OL, Nelson CE. (1990) Frogs of Boracéia. Arquivos de Zoologia 31: 231–410. [Google Scholar]
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, Carpenter KE, Chanson J, Collen B, Cox NA, Darwall WR. (2010) The impact of conservation on the status of the world’s vertebrates. Science 330(6010): 1503–1509. https://doi.org/10.1126/science.1194442 [DOI] [PubMed] [Google Scholar]
- ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade] (2014) Lista de espécies ameaçadas. http://www.icmbio.gov.br/portal/faunabrasileira/lista-de-especies [accessed on 18 July 2017]
- IUCN (2008) An Analysis of Amphibians on the 2008 IUCN Red List. http://www.iucnredlist.org [accessed on 01 January 2015].
- Jenkins CN, Alves MAS, Uezu A, Vale MM. (2015) Patterns of vertebrate diversity and protection in Brazil. Plos One 10(12): 1–13. https://doi.org/10.1371/journal.pone.0145064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncá FA, Silvano D. (2004) Scinax strigilatus – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Juncá FA, Pimenta B. (2004) Ischnocnema bilineata – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Juncá FA, Napoli MF, Nunes I, Mercês EA, Abreu RO. (2015) A new species of the Scinax ruber clade (Anura, Hylidae) from the Espinhaço Range, northeastern Brazil. Herpetologica 71: 299–309. https://doi.org/10.1655/HERPETOLOGICA-D-14-00032 [Google Scholar]
- Leite YLR, Costa LP, Loss AC, Rocha RG, Batalha-Filho H, Bastos AC, Quaresma VS, Fagundes V, Paresque R, Passamani M, Pardini R. (2016) Neotropical forest expansion during the last glacial period challenges refuge hypothesis. PNAS 113(4): 1008–1013. https://doi.org/10.1073/pnas.1513062113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço-De-Moraes R, Solé M, Toledo LF. (2012) A new species of Adelophryne Hoogmoed and Lescure 1984 (Amphibia: Anura: Eleutherodactylidae) from southern Bahia, Brazil. Zootaxa 3441: 59–68. [Google Scholar]
- Lourenço-De-Moraes R, Ferreria RB, Fouquet A, Bastos RP. (2014) A new diminutive frog species od Adelophryne (Amphibia: Anura: Eleutherodactylidae) from the Atlantic Forest, southeastern Brazil. Zootaxa 3846: 348–360. https://doi.org/10.11646/zootaxa.3846.3.2 [DOI] [PubMed] [Google Scholar]
- Loyola RD, Kubota U, Lewinsohn TM. (2007) Endemic vertebrates are the most effective surrogate for identifying conservation priorities among Brazilian ecoregions. Diversity and Distributions 13: 389–396. https://doi.org/10.1111/j.1472-4642.2007.00345.x [Google Scholar]
- Marciano Jr E, Lantyer-Silva ASF, Solé M. (2017) A new species of Phyllodytes Wagler, 1830 (Anura, Hylidae) from the Atlantic Forest of southern Bahia, Brazil. Zootaxa 4238: 135–142. https://doi.org/10.11646/zootaxa.4238.1.11 [DOI] [PubMed] [Google Scholar]
- Mattedi C, Pontes R. (2014) Range extension and upated distribution of Hypsiboas pardalis (Spix, 1824) (Anura, Hylidae). Herpetology Notes 7: 791–795. doi: https://biotaxa.org/hn/article/viewFile/9376/11095 [Google Scholar]
- Mittermeier CG, Turner WR, Larsen FW, Brooks TM, Gascon C. (2011) Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC. (Eds) Biodiversity Hotspots: Distribution and Protection of Priority Conservation Areas. Springer-Verlag, Berlin, 3–22. https://doi.org/10.1007/978-3-642-20992-5_1
- Morellato P, Haddad CFB. (2000) Introduction: the Brazilian Atlantic Forest. Biotropica 32: 786–792. https://doi.org/10.1111/j.1744-7429.2000.tb00618.x [Google Scholar]
- Myers N, Mittermeyer RA, Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. https://doi.org/10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Napoli ME, Pimenta BVS. (2009) A new species of the Bokermannohyhla circumdata group (Anura: Hylidae) from the coastal forests of Bahia, northeastern Brazil. Copeia 2009: 674–683. https://doi.org/10.1643/CH-08-224 [Google Scholar]
- Napoli MF, Caramaschi U, Cruz CAG, Dias IR. (2011) A new species of flea-toad, genus Brachycephalus Fitzinger (Amphibia: Anura: Brachycephalidae), from the Atlantic rainforest of southern Bahia, Brazil. Zootaxa 2739: 33–40. https://doi.org/10.11646/zootaxa.2739.1.3 [Google Scholar]
- Orrico VGD, Dias IR, Marciano-Jr E. (2018) Another new species of Phyllodytes (Anura: Hylidae) from the Atlantic Forest of northeastern Brazil. Zootaxa 4407: 101–110. https://doi.org/10.11646/zootaxa.4407.1.6 [DOI] [PubMed] [Google Scholar]
- Peixoto O, Pimenta B. (2004) Ischnocnema vinhai – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Pontes RC, Caramaschi U, Pombal JP. (2014) A remarkable new glass frog (Centrolenidae: Vitreorana) from the northeast Atlantic forest, Brazil. Herpetologica 70: 298–308. https://doi.org/10.1655/HERPETOLOGICA-D-13-00024 [Google Scholar]
- Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sánchez-Azofeifa GA, Still CJ, Young BE. (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439: 161–167. https://doi.org/10.1038/nature04246 [DOI] [PubMed] [Google Scholar]
- Ribeiro MC, Martensen AC, Metzger JP, Tabarelli M, Scarano F, Fortin MJ. (2011) The Brazilian Atlantic Forest: a shrinking biodiversity hotspot. In: Zachos FE, Habel JC. (Eds) Biodiversity hotspots: distribution and protection of conservation priority areas. Springer, Heidelberg, 405–434. https://doi.org/10.1007/978-3-642-20992-5_21
- Ribeiro-Júnior MA, Rossi RV, Miranda CL, Ávila-Pires TCS. (2011) Influence of pitfall trap size amd design on herpetofauna and small mammal studies in a Neotropical Forest. Zoologia 28(1): 80–91. https://doi.org/10.1590/S1984-46702011000100012 [Google Scholar]
- Rödel MO, Ernst R. (2004) Measuring and monitoring amphibian diversity in tropical forests. An evaluation of methods with recommendations for standardization. Ecotropica 10(1): 1–14. [Google Scholar]
- Rodrigues MT. (2006) Phyllodytes wuchereri IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Rossa-Feres DC, Sawaya RJ, Faivovich J, Giovanelli JGR, Brasileiro CA, Schiesari L, Alexandrino J, Haddad CFB. (2011) Anfíbios do Estado de São Paulo, Brasil: conhecimento atual e perspectivas. Biota Neotropica 11(1a): 1–19. https://doi.org/10.1590/S1676-06032011000500004
- Ruas DS, Mira-Mendes CV, Del-Grande ML, Solé M. (2013) Aparasphenodon brunoi Miranda-Ribeiro, 1920 (Anura: Hylidae): Distribution extension and geographic distribution map for Bahia state, Brazil. Check List 9(4): 858–859. https://doi.org/10.15560/9.4.858 [Google Scholar]
- Segalla MV, Caramaschi U, Cruz CAG, Grant T, Haddad CFB, Garcia PCA, Berneck BVM, Langone J. (2016) Brazilian Amphibians: List of Species. Herpetologia Brasileira 5(2): 34–46. [Google Scholar]
- Silva JMC, Casteleti CHM. (2003) Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal C, Câmara IG. (Eds) The Atlantic Forest of South America: biodiversity status, threats, and outlook. Center for Applied Biodiversity Science and Island Press, Washington, DC, 43–59.
- Silvano DL, Pimenta B. (2010) Agalychnis aspera – IUCN Red List of Threatened Species. Version 2015.4. http://www.iucnredlist.org [accessed on 01 January 2015]
- Silvano DL, Pimenta B. (2003) Diversidade de anfíbios na Mata Atlântica do Sul da Bahia. In: Prado PI, Landau EC, Moura RT, Pinto LPS, Fonseca GAB, Alger K (Eds) Corredor de Biodiversidade na Mata Atlântica do Sul da Bahia CD-ROM, Ilhéus, IESB/CI/CABS/ UFMG/UNICAMP
- Stuart SN, Hoffmann M, Chanson JS, Cox NA, Berridge RJ, Ramani P, Young BE. (2008) Threatened Amphibians of the World. Lynx Edicions (Barcelona), IUCN (Gland), Conservation International (Arlington), 134 pp. [Google Scholar]
- Teixeira-Jr M, Vechio FD, Recoder RS, Carnaval ACOQ, Strangas M, Damasceno RP, Sena MA, Rodrigues MT. (2012) Two new species of marsupial tree-frogs genus Gastrotheca Fitzinger, 1843 (Anura, Hemiphractidae) from the Brazilian Atlantic Forest. Zootaxa 3437: 1–23. [Google Scholar]
- Teixeira-Jr M, Recoder RS, Amaro RC, Damasceno RP, Cassimiro J, Rodrigues MT. (2013) A new Crossodactylodes Cochran, 1938 (Anura: Leptodactylidae: Paratelmatobiinae) from the highlands of the Atlantic Forests of southern Bahia, Brazil. Zootaxa 3702(5): 459–472. https://doi.org/10.11646/zootaxa.3702.5.5 [DOI] [PubMed] [Google Scholar]
- Tinôco MS, Browne-Ribeiro HC, Cerqueira R, Dias MA, Nascimento IA. (2008) Habitat change and the amphibians conservation in the Atlantic Forest of Bahia, Brazil. Froglog 89: 1–3. [Google Scholar]
- Trevine V, Forlani MC, Haddad CFB, Zaher H. (2014) Herpetofauna of Paranapiacaba: expanding our knowledge on a historical region in the Atlantic forest of southeastern Brazil. Zoologia (Curitiba) 31(2): 126–146. https://doi.org/10.1590/S1984-46702014000200004 [Google Scholar]
- Vasconcelos TS, Santos TG, Haddad CFB, Rossa-Feres DC. (2010) Climatic variables and altitude as predictors of anuran species richness and number of reproductive modes in Brazil. Journal of Tropical Ecology 26: 423–432. https://doi.org/10.1017/S0266467410000167 [Google Scholar]
- Veloso HP, Rangel-Filho ALR, Lima JC. (1991) Classificação da vegetação brasileira, adaptada a um sistema universal. IBGE, Rio de Janeiro, 124 pp. [Google Scholar]
- Verdade VK, Rodrigues MT. (2007) Taxonomic Review of Allobates (Anura, Aromobatidae) from the Atlantic Forest, Brazil. Journal of Herpetology 41(4): 566–580. https://doi.org/10.1670/06-094.1 [Google Scholar]
- Verdade VK, Valdujo PH, Carnaval AC, Schiesari L, Toledo LF, Mott T, Andrade GV, Eterovick PC, Menin M, Pimenta BVS, Nogueira C, Lisboa CS, De Paula CD, Silvano DL. (2012) A leap further: the Brazilian amphibian conservation action plan. Alytes 29: 27–42. [Google Scholar]
- Vörös J, Dias JR, Solé M. (2017) A new species of Phyllodytes (Anura: Hylidae) from the Atlantic Rainforest of southern Bahia, Brazil. Zootaxa 4337(4): 584–594. https://doi.org/10.11646/zootaxa.4337.4.9 [DOI] [PubMed] [Google Scholar]
- Vredenburg VT. (2004) Reversing introduced species effects: Experimental removal of introduced fish leads to rapid recovery of declining frog. Proceedings of the National Academy of Sciences 101(20): 7646–7650. https://doi.org/10.1073/pnas.0402321101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BE, Lips KR, Reaser JK, Ibanez R, Salas AW, Cedeno JR, Coloma LA, Ron S, Marca E, Meyer JR, Munoz A, Bolanos F, Chaves G, Romo D. (2001) Population declines and priorities for amphibian conservation in Latin America. Conservation Biology 15: 1213–1223. https://doi.org/10.1111/j.1523-1739.2001.00218.x [Google Scholar]
- Young BE, Stuart SN, Chanson JS, Cox NA, Boucher TM. (2004) Joyas que están desapareciendo: El estado de los anfibios en el Nuevo Mundo. Nature Serve Arlington, Virginia, 53 pp. [Google Scholar]