Abstract
In leaves, the light reactions of photosynthesis support fatty acid synthesis but disagreement exists as to whether this occurs in green oilseeds. To address this question, simultaneous measurements of the rates of CO2 and O2 exchange (CER and OER, respectively) were made in soybean (Glycine max L.) fruits. The imbalance between CER and OER was used to estimate the diverted reductant utilization rate (DRUR) in the equation: DRUR = 4 × (OER + CER). This yielded a quantitative measure of the rate of synthesis of biomass that is more reduced per unit carbon than glucose (in photosynthesizing tissues) or than the substrates of metabolism (in respiring tissues). The DRUR increased by about 2.2-fold when fruits were illuminated due to a greater increase in OER than decrease in CER. This characteristic was shown to be a property of the seed (not the pod wall), to be present in fruits at all developmental stages, and to reach a maximal response at relatively low light. When seeds were provided with 13CO2, light reduced 12CO2 production but had little effect on 13CO2 fixation. When they were provided with 18O2, light stimulated 16O2 production but had no effect on 18O2 uptake. Together, these findings indicate that light stimulates fatty acid synthesis in photosynthetic oilseeds, probably by providing both ATP and carbon skeletons.
In oilseeds of plants such as soybean (Glycine max L. Merr.), fatty acids account for 20% (w/w) or more of the seed weight, yet relatively little is known about the environmental and physiological factors that regulate the rate of FAS during seed development.
Studies of the source of ATP and the reducing power for FAS have mostly been done with isolated plastids from root and leaf tissues. In chloroplasts from leaves, FAS is light dependent, light regulated, and can occur without the addition of ATP and reducing equivalents (Browse et al., 1981; Liedvogel and Bauerle, 1986; Roughan and Ohlrogge, 1996; Sasaki et al., 1997; Eastmond and Rawsthorne, 1998). In contrast, isolated plastids from roots are able to provide their own reducing power for FAS, but have an absolute requirement for an external source of ATP (Kleppinger-Sparace et al., 1992; Qi et al., 1995; Xue et al., 1997) or at least a means to make ATP through the dihydroxyacetone phosphate shuttle system (Kleppinger-Sparace et al., 1992). Similar results have been found in amyloplasts from developing wheat and maize endosperm (Mohlmann and Neuhaus, 1997) and in amyloplasts from cauliflower floral buds (Mohlmann et al., 1994).
The picture is slightly more complicated in photosynthetic oilseeds. In the dark, FAS in photosynthetic oilseed plastids was similar to that in nonphotosynthetic plastids in that there was an absolute requirement for ATP (Gupta and Singh, 1996; Eastmond and Rawsthorne, 1998) or at least a means of making ATP such as through the dihydroxyacetone phosphate shuttle (Gupta and Singh, 1996). However, many oilseed plastids contain chlorophyll, so light may play an important role in FAS, as shown for leaf plastids. Indeed, plastids from developing cotyledons of linseeds were found to carry out FAS at low light levels (15–20 μmol m−2 s−1) without the addition of ATP or a reductant (Browse and Slack, 1985). More recently (Fuhrmann et al., 1994; Aach and Heise, 1998), studies with isolated Brassica napus embryos showed that FAS was stimulated by light, and others have suggested that the light reactions of photosynthesis are able to provide ATP and a reductant for FAS (Eastmond et al., 1996; Asokanthan et al., 1997).
Others have come to different conclusions. King et al. (1998) measured the rate of photosynthetic O2 evolution of isolated B. napus embryos and compared this with the amount and activity of Rubisco. Since the total activity of Rubisco under saturating CO2 was greater than the measured rate of O2 evolution, and since developing seeds are thought to have an elevated CO2 concentration within the tissue due to high rates of respiration and a barrier to the diffusion of CO2, they proposed that the photosynthetic light reactions would be used to fix CO2. Other investigators have questioned the importance of photosynthesis within the seed, since the light levels are highly attenuated by the pod wall and seed coat (Browse and Slack, 1985; Eastmond et al., 1996; Eastmond and Rawsthorne, 1998). Using light levels of 300 μmol m−2 s−1, Eastmond and Rawsthorne (1998) found that plastids from isolated B. napus embryos had similar rates of FAS in the light and dark when provided with ATP, and that the light-dependent rates of FAS were about 5-fold less than the ATP-dependent rates. Thus, it appeared that light had little contribution to FAS.
These studies used isolated embryos, plastids, or enzyme activities, making it difficult to offer reliable predictions concerning the effects of light on the FAS of an intact embryo, seed, or entire fruit. The large reductant demand for FAS raises the possibility that the imbalance between CO2 or O2 production and O2 or CO2 uptake could provide the basis for a noninvasive assay for FAS in developing oilseeds. Such measurements have been limited by the availability of instrumentation capable of quantifying low differentials in O2 concentration in a gas stream that has passed by a fruit. Recently, we reported on the design and performance of a differential O2 gas analyzer that was capable of measuring microliter per liter differentials in O2 concentration against a background of air (21% or 210,000 μL L−1 O2) (Willms et al., 1997).
The present study incorporates a differential oxygen analyzer and an IR gas analyzer to monitor the OER and CER of soybean fruit during development and under a variety of light and dark conditions. The results were used to calculate the DRUR in the equation: DRUR = 4 × (OER + CER), a quantitative measure of the rate of synthesis of biomass that is more reduced per unit carbon than Glc (in photosynthesizing tissues) or than the substrates of metabolism (in respiring tissues). Since fatty acids are more reduced per unit carbon than carbohydrate, the DRUR of intact soybean fruits should increase in the light if light stimulates FAS, but would not be affected if light is not important to seed metabolism or is only involved in promoting photosynthetic CO2 fixation to carbohydrate.
MATERIALS AND METHODS
Plant Culture
Soybean (Glycine max L. Merr. cv Maple Arrow) seeds were sown into 4-L plastic pots containing either a soil mixture (Sunshine Mix 1, Fisons Horticulture, Vancouver) or a silica sand:perlite (67:33) mix, and inoculated with Bradyrhizobium japonicum USDA 16. Plants were grown in a controlled-environment cabinet (model PGV 36, Conviron, Winnipeg, Manitoba, Canada) with a light irradiance of 500 μmol m−2 s−1 at plant height, a 16-h day/night cycle, and a temperature of 20°C. Plants were watered with one-half-strength nutrient solution (Walsh et al., 1987) supplemented with 0.5 mm KNO3 for the first 14 d of growth (sand-perlite-grown plants) or with 5 mm KNO3 throughout plant growth (soil-grown plants).
Gas Exchange Measurements
To measure the gas exchange rates of developing soybean fruit, an open-flow gas exchange system was set up in which gas was supplied either from compressed air drawn from outside the building (typically 20.9% O2, 380–400 μL L−1 CO2) or from a mixture of synthetic compressed air (19%–21% O2/N2 balance) and pure CO2 to give a final concentration of approximately 350 to 500 μL L−1 CO2 in the gas stream. The flow rate of air through the cuvette was maintained at a constant rate throughout an experiment at a value between 35 and 60 mL min−1, and the effluent gas stream was dried using a column of magnesium perchlorate before being supplied to an analysis system that contained a differential O2 sensor and an IR CO2 analyzer, as well as other sensors for absolute pressure, absolute O2 concentration, differential pressure between a reference and sample gas stream, and temperature of the differential O2 sensor (Willms et al., 1997).
Operation and calibration of the differential O2 sensor and IR CO2 analyzer were carried out as described previously (Willms et al., 1997). The measured voltage values for differential O2 and absolute CO2 were converted to pascals, corrected for variations in temperature, differential pressure, and drift to yield values for the Pa of O2 and CO2 differentials, respectively, between the incoming and effluent gas streams flushing the cuvette. The measured differences in CO2 concentration between the reference gas stream and the gas stream leaving the cuvette were converted to the CER (in micromoles per gram DW per hour) using the following equation:
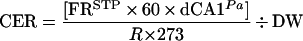 |
1 |
where FRSTP is the flow rate of gas through the cuvette in millililters per minute at STP; dCA1Pa is the difference in CO2 concentration between the effluent gas stream from the cuvette and the reference gas stream; R is the gas constant (8.31451 Pa m3 K−1 mol−1); and 273 is the temperature in kelvins of 0°C (STP). Positive values for CER denote production, whereas negative values indicate CO2 fixation.
A similar equation could not be used to calculate the OER (micromoles per gram DW per hour), since any imbalance in the CER and OER of a tissue would result in a net gas exchange that could have a significant volumetric effect on the O2 partial pressure in the effluent gas from the cuvette (Willms et al., 1997). For example, if the CER was more positive than the OER was negative, more gas would leave the cuvette than enter it, and the O2 concentration in the effluent gas would be diluted. Because of the high concentration of O2 in air (21%), the volumetric effect would significantly alter the observed differential O2 concentration. To calculate the O2 concentration differential associated with only biological gas exchange, the following equation was used:
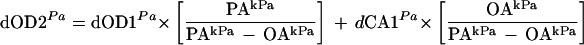 |
2 |
where dOD1Pa is the O2 concentration differential (in pascals) corrected for differential pressure, temperature, and drifts in the OD sensor baseline; dOD2Pa is the dOD1Pa corrected for changes in volume of the sample gas stream; PAkPa is the atmospheric pressure (in kilopascals); and OAkPa is the absolute O2 concentration (in kilopascals).
Finally, OER was calculated as:
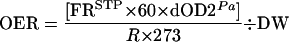 |
3 |
Calculation of Gas Exchange Parameters
GEQ was calculated as:
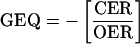 |
4 |
where values for CER and OER are positive for production and negative for consumption. Therefore, GEQ is equivalent to respiratory quotient in the dark, but is used in the present study to describe fruit gas exchange in both the dark and the light.
The values for GEQ are only useful in providing qualitative information on the use of reducing power in plant tissues. To obtain a more quantitative measure of how much reducing power is diverted to sinks other than those associated with the production or metabolism of carbohydrate, the term DRUR (in micromoles per electron per gram DW per hour) was coined and defined as:
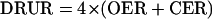 |
5 |
Since gas production is positive and consumption is negative, and since four electrons are associated with each O2 generated (or CO2 fixed) in photosynthesis or with each CO2 produced (or O2 consumed) in respiration, this equation converts the difference in the gas exchange to the rate of synthesis of biomass that is more reduced per unit carbon than Glc (in photosynthesizing tissues) or than the substrates of metabolism (in respiring tissues).
Effect of Light and Dark on Single, Attached Fruits
At 20 to 40 DAF, single soybean fruits (approximately 0.2–0.4 g DW) were enclosed in a clear, acrylic cuvette that was divided lengthwise and had a removable conical head so that it could fit over an intact fruit. The two sides of the cuvette and the junction between the cuvette and the stem were made gas tight with a flexible sealant (Qubitac, Qubit Systems, Kingston, Canada), and the cuvette was connected to an open-flow gas exchange system that measured O2 and CO2 exchange as described above. The chamber was flushed with about 40 mL min−1 of gas, and the presence of leaks were detected by venting a gas mixture of 20% CO2 in N2 around the cuvette and watching for changes in the response of the CO2 or O2 analyzers. To maintain fruit temperature, the cuvette was equipped with an outer chamber that was flushed with temperature-controlled water (23°C ± 2°C).
Measurements of CO2 and O2 exchange in a single, attached fruit was made throughout a dark-to-light (600 μmol m−2 s−1, metal halide lamp) transition and after 60 min in the light, gas exchange was monitored through a light to dark transition. Aluminum foil was used to protect the remainder of the plant from the heat of the metal halide lamp.
The gas exchange of seeds and pod walls was examined by cutting open two fruits, removing the seeds, and separately measuring the gas exchange of the seeds and pod walls in the dark and light. To separate the effects of wound respiration from seed excision, prior to separating the pod wall and seeds, the gas exchange of the two fruits was measured when the pod walls were cut open but the seeds were not removed.
To assess changes in gas exchange throughout the light period, gas exchange of intact, attached fruit was monitored at the end of the dark period, throughout the 16-h light period (400 μmol m−2 s−1, model E8, Conviron), and then once more in the dark.
Ontogenetic Effects on Light Saturation and DRUR in Excised Soybean Fruit
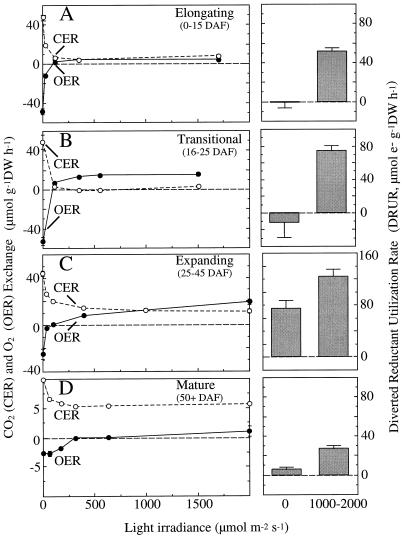
The effect of light on fruit gas exchange was measured at four different fruit developmental stages. In the elongation stage (approximately 0–15 DAF), the pod walls were small but rapidly growing (fruit length <25 mm; fruit width <1.8 mm), whereas in the transition stage (approximately 16–25 DAF), the fruit elongation had ended and the seeds were begin to expand (fruit length >45 mm; 1.8< fruit width <3.0 mm). In the expanding fruit (approximately 25–45 DAF), the seeds were rapidly expanding (fruit width >4.0 mm), whereas in the mature fruit (approximately 50+ DAF), the seeds were fully expanded, yellowing, and beginning to dry.
To increase the accuracy of the gas exchange measurements, at least five fruits of the same developmental stage were detached and enclosed within a flat, rectangular acrylic chamber that was about 5.5 mm thick, with adjustable end walls that could be used to minimize the gas volume around the fruit. The stems of the excised fruit were immersed in a N-free nutrient solution placed in the bottom of the cuvette, which was submerged within a temperature-controlled water bath. Light was supplied from a projector (Carousel 600H, Kodak) with a 350-W bulb and was measured using a photometer (model LI-185B, LI-COR). Light (0 or 100–2,000 μmol m−2s−2) was controlled by either covering the cuvette with aluminum foil (dark) or by attenuating the light with various layers of cheesecloth. Fruit temperature was controlled by immersing the cuvette in a water bath and maintaining the temperature of the water (23°C ± 2°C).
Soybean fruits were initially exposed to darkness for at least 5 min, and were then exposed to a specific irradiance for 17.5 min. After each light treatment, the fruit were returned to the dark for 14 min. This timing ensured that steady-state gas exchange measurements were made, while avoiding complications associated with extended periods of fruit excision. After four or five light levels, the fruits were returned to the initial irradiance levels. The final light irradiance used was the same as the initial one, so that changes in gas exchange due to detachment and enclosure within the cuvette could be quantified.
Each light curve trial consisted of either 20 fruit harvested from three plants (youngest, elongating fruit) or five fruit harvested from one plant (all other fruit growth stages). In total, three trials were completed for each developmental stage, and between four and eight different irradiances were used in each trial. After the experiment, fruit dimensions were recorded and fruits, pod walls, and seeds were weighed and dried.
MS
CO2 Isotope Measurements
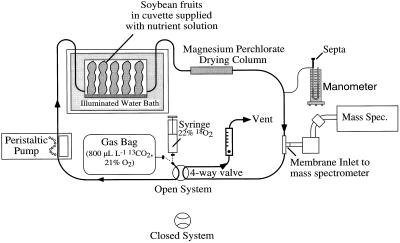
The uptake of 13CO2 and the evolution of 12CO2 in 24- to 45-d-old fruits (three replicates), pod walls (three replicates), and seeds (four replicates) were measured within an open-flow system in which the cuvette was submerged in a temperature-controlled water bath (Fig. 1). Immediately after excision, fruits were placed in the cuvette and darkened. Gas was supplied from a 4-L plastic bag that contained between 800 and 1500 μL L−1 13CO2 in 12CO2-free air, and gas exchange was monitored using a membrane inlet mass spectrometer (model MM 14-80SC, VG Gas Analysis, Middlewich, UK; Mir et al., 1995) for about 7 min in the dark before being exposed to light (400 μmol m−2 s−1) for 20 to 25 min and then dark.
Figure 1.
Gas exchange system used for MS. A four-way valve within the system could be set for either open-system measurements of 12CO2 and 13CO2 exchange or closed-system measurements of 16O2 and 18O2 exchange. Drawing is not to scale.
To calculate specific activity, the concentration of 13CO2 and 12CO2 in the gas stream supplied to the fruit was measured before and after each experiment. The specific activity was used to estimate gross CO2 evolution and fixation. Gross CO2 evolution was used as an estimate of respiration, and gross CO2 uptake was used as an estimate of photosynthetic CO2 fixation. The fruits were then removed from the cuvette and separated into pod walls and seeds; each component was then returned separately to the cuvette, where 12CO2 and 13CO2 exchange were measured in the dark, then in the light, and finally in the dark again. Fresh weight and DW were taken at the end of the experiment.
O2 Isotope Measurements
Consumption of 18O2 and evolution of 16O2 in 24- to 45-d-old soybean seeds (five replicates) were placed in a 30-mL cuvette, and gas exchange was measured using a closed gas analysis system, since the mass spectrometer was unable to detect small changes in O2 concentration against the large background concentration (approximately 21 kPa O2) in air. Cuvettes and the temperature control setup were the same as that used in the measurement of CO2 isotope exchange, except a water-based manometer was added to the system to monitor pressure changes throughout the experiment and to test for leaks. A mixture of 22% 18O2, 15% Ar, and a balance of N2 was prepared in a 60-mL glass syringe and flushed through the pump, cuvette, and tubing before the four-way valve was switched to make a closed system (Fig. 1). The pump was turned on, gas exchange of seeds was measured for about 35 min in the dark and 26 min in the light, and then the seeds were returned to the dark.
The total volume of the closed gas exchange system (typically about 150 mL, including the cuvette and seeds) was determined by closing the system and then either injecting or removing known quantities of air and recording the pressure changes using the manometer.
Statistical Analyses
All statistical analyses were carried out by Student's t test (P < 0.05). Unless otherwise stated, the sample size was three.
RESULTS
CO2 and O2 Exchange of Soybean Fruits in the Dark and Light
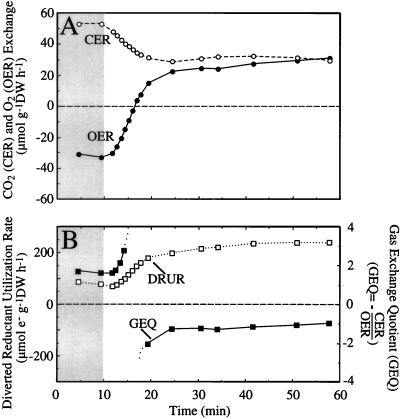
In a 40-d-old soybean fruit (0.407 g DW) in the dark, the CER was 53 μmol CO2 g−1 DW h−1 and the OER was −31 μmol O2 g−1 DW h−1, resulting in a GEQ of 1.71 and a DRUR of 88 μmol electrons g−1 DW h−1 (Fig. 2). When the lights were turned on, the CER decreased by 22.1 μmol CO2 g−1 DW h−1, whereas the OER increased by 59.3 μmol O2 g−1 DW h−1, thereby resulting in simultaneous CO2 and O2 evolution. Since light altered the OER to a much greater extent than it did the CER, the resultant DRUR increased 2.7-fold, from 88 to 240 μmol electrons g−1 DW h−1 (Fig. 2B). The gas exchange rates in the light were relatively stable over the period of 15 to 45 min, so that after 45 min in the light, the GEQ was −0.95 and the DRUR was 246 μmol electrons g−1 DW h−1.
Figure 2.
OER (•) and CER (○) of a single, attached, 40-d-old soybean fruit (0.407 g DW) during a dark to light (600 μmol photons m−2 s−2, metal halide lamp) transition (A) and calculated values for GEQ and DRUR of the fruit (B). Shaded areas indicate dark conditions.
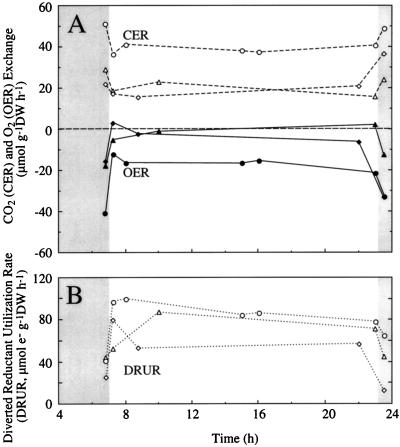
To determine whether the gas exchange response was maintained throughout the light period, attached soybean fruits (25–40 DAF) were placed in a cuvette for the measurement of CO2 and O2 exchange. In the dark, CER ranged from 50.9 to 21.9 μmol CO2 g−1 DW h−1, and OER values ranged from −40.6 to −15.7 μmol O2 g−1 DW h−1 (Fig. 3A). The GEQ was 1.43 ± 0.11 (data not shown). Following a dark-to-light transition, CER decreased by 11.7 ± 0.85 μmol CO2 g−1 DW h−1, whereas the OER increased by 21.0 ± 0.79 μmol O2 g−1 DW h−1, resulting in an increase in DRUR of 41 ± 2.16 μmol electrons g−1 DW h−1 (Fig. 3). The CERs and OERs were relatively constant over the subsequent 16-h photoperiod and, following a light-to-dark transition, CER, OER, and DRUR returned to values that were similar to those observed during the previous dark period. Although this experiment was similar to that reported previously (Fig. 2) in that light altered the OER to a much greater extent than it affected the CER, there were few fruits that showed simultaneous O2 and CO2 exchange. In the light, the positive values for GEQ ranged from +1.9 to infinity and the negative values ranged from − infinity to −6.1 (data not shown), reflecting the fact that the OER was very low compared with CER.
Figure 3.
OER and CER (A) and the DRUR (B) of attached soybean fruits. ○, 31 d old, DW = 0.42 g; ▵, 40 d old, DW = 0.71 g; ⋄, 24 d old, DW = 0.29 g) throughout one 16-h day. Light was 400 μmol m−2 s−2. Shaded areas indicate dark conditions.
CO2 and O2 Exchange in Excised and Dissected Fruits
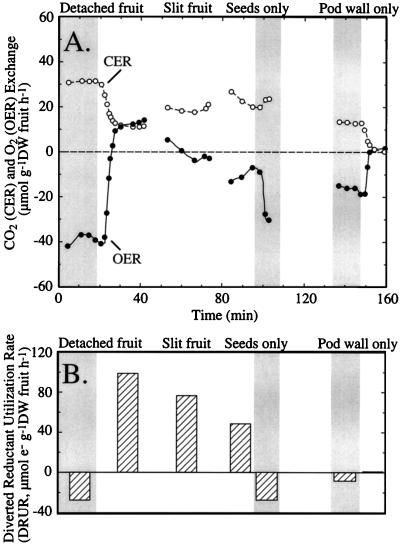
To identify which part of the fruit (pod wall or seeds) was responsible for the greater effect of light on OER than CER, a 40-d-old soybean fruit (0.4814 g DW) was excised and placed in a cuvette, and gas exchange was measured in the dark and light before and after the fruit was separated into its component parts. In freshly excised fruits in the dark, the CER was less than the OER (Fig. 4A), resulting in a GEQ of 0.82 and a negative DRUR (−27.8 μmol electrons g−1 DW fruit h−1) (Fig. 4B). However, as observed previously, the dark-to-light transition had a greater effect on OER than on CER, resulting in a large increase in the DRUR to 99.2 μmol electrons g−1 DW fruit h−1.
Figure 4.
OER (•) and CER (○) of a whole, detached soybean fruit (37–40 d old; 0.481 g DW), slit fruit, seeds, and pod wall in the dark and light (600 μmol photons m−2 s−2, metal halide lamp) (A) and the DRUR bar graph (B). Shaded areas indicate dark conditions.
When the pod wall of the fruit was slit down the dorsal side, the CER increased from 11.5 μmol g−1 DW fruit h−1 to 18.7 μmol g−1 DW fruit h−1, the OER decreased from 13.4 to −2.22 μmol g−1 DW fruit h−1, and the DRUR was reduced to 71.4 μmol electrons g−1 DW fruit h−1. When soybean seeds were isolated from the pod wall and gas exchange was measured on them alone, the light-to-dark transition had a minor effect on CER (approximately 1.9 μmol CO2 g−1 DW fruit h−1) and a major effect on OER (20.8 μmol O2 g−1 DW fruit h−1) (Fig. 4A); consequently, the DRUR decreased from 48.8 μmol electrons g−1 DW fruit h−1 (GEQ = 2.28) in the light to −27.18 μmol electrons g−1 DW fruit h−1 in the dark (GEQ = 0.78) (Fig. 4B). The gas exchange of pod walls (DW = 0.1924) in the dark produced a GEQ of 0.86 and a DRUR of −8.62 μmol electrons g−1 DW fruit h−1. However, in the light, there was virtually no net gas exchange.
When the DRUR for excised seeds and pod walls were summed, the total DRUR in the light (48.8 μmol electrons g−1 DW fruit h−1) was only about one-half of that measured in whole fruits (99.2 μmol electrons g−1 DW fruit h−1), and the DRUR in the dark (−35.80 μmol electrons g−1 DW fruit h−1) was more negative than that in whole fruits (27.8 μmol electrons g−1 DW fruit h−1) (Fig. 4B).
Light Response Curves for CO2 and O2 Exchange
In the light response experiment, young, elongating fruit exposed to the dark had GEQ values close to unity, and a DRUR of only −0.98 ± 5.32 μmol electrons g−1 DW fruit h−1 (Fig. 5A). In transitional fruit, the GEQ was less than one (i.e. 0.94 ± 0.09) and the DRUR was negative (i.e. −11.88 ± 17.26 μmol electrons g−1 DW fruit h−1) (Fig. 5B), whereas in the expanding and mature fruit, GEQ values were greater than one (i.e. 1.74 ± 0.32, and 1.13, respectively), resulting in positive DRUR values (74.4 ± 12.10 and 4.52 ± 0.30 μmol electrons g−1 DW fruit h−1, respectively) (Fig. 5, C and D).
Figure 5.
Light response curves for OER (•) and CER (○) in soybean fruits of different ages are shown on the left, and the DRUR for fruits from each age group in the dark and light are shown on the right. Values are means ± se for those measurements where n = 3. Each sample was composed of five to six fruits from one plant, except for the youngest age group, which were composed of 20 fruits from three plants.
In all fruit, increasing the irradiance resulted in a larger increase in OER than a decrease in CER, thereby resulting in a significant (P < 0.05) increase in DRUR compared with that in the dark treatment (Fig. 5, right panels). Note that the DW specific gas exchange rates of the mature fruit in the dark were only about 25% of those in the other fruit. The increase in DRUR from dark to light was significant (P < 0.05) for elongating (DRUR in light = 51.6 μmol electrons g−1 DW h−1) and mature (>50-d-old) fruit (DRUR in light = 27.0 μmol electrons g−1 DW h−1), and a tendency for greater DRUR was observed in the transition (P = 0.056, DRUR in light = 74.2 μmol electrons g−1 DW h−1) and in expanding fruit (P = 0.15, DRUR in light = 122.96 μmol electrons g−1 DW fruit h−1).
The irradiance level that was required to support 90% of the maximal change in CER and OER ranged from about 170 μmol m−2 s−1 in the elongating fruits to about 1000 μmol m−2 s−1 in the expanding fruits. In all cases, CER and OER were similar in their light response within fruit of a given age (Fig. 5).
In this study, fruit were exposed to the highest irradiance at the beginning of the experiment and, after measurements of CER and OER at the various light levels, the high-light treatment was repeated at the end of the experiment, approximately 3 h later. Although the OER and CER measurements at the end of the experiment were not significantly different (P < 0.05) from those at the beginning, the DRUR values did decrease significantly (P < 0.05) in the elongating and transitional fruits (data not shown). The results (Fig. 5) for the high light levels were based on the initial measurements of CER and OER.
The Contribution of Respiration and Photosynthesis to the CO2 Exchange of Soybean Fruits in the Light
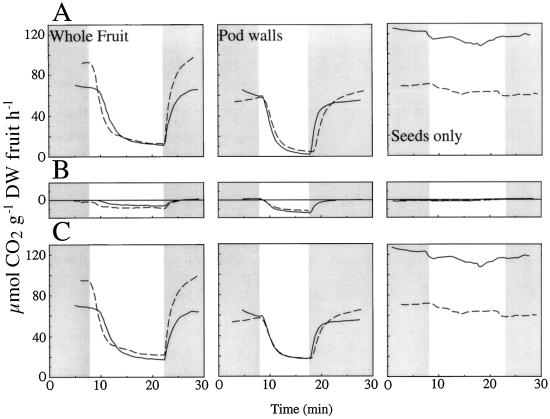
In whole fruits the sum of 12CO2 and 13CO2 exchange resulted in a gas exchange trace (Fig. 6A) that was similar to that obtained using an IR gas analyzer (Figs. 2–5) in that exposure to light resulted in a decrease in the rate of CO2 production but no net CO2 fixation. This effect of light on net CO2 exchange was primarily associated with the pod wall; as shown previously (Fig. 4), light had little effect on the CO2 exchange of seeds (Fig. 6).
Figure 6.
Net CO2 exchange in expanding (30- to 40-d-old) soybean fruits (A) calculated from measurements of 13CO2 uptake (B) and 12CO2 production (C) in the dark and in the light (1,000 μmol m−2 s−2). Each line represents a replicate assay involving five whole fruits, and the pod walls and seeds from the same five fruits. Data were corrected for specific activity of 12CO2 and 13CO2. Ambient CO2 was constant throughout a run but varied from 813 to 890 μL L−1 between tissues measured. Shaded areas indicate dark conditions.
The isotopic CO2 exchange, corrected for specific activity, provided information on the relative contribution of photosynthesis and respiration to net CO2 exchange. In whole fruits an increase in photosynthetic CO2 fixation accounted for only 12.5% of the decrease in CO2 evolution from dark to light (Fig. 6B); 87.5% of the decrease in CO2 evolution appeared to be due to a decline in CO2 production (Fig. 6C). Still, 12CO2 evolution in the light was about 2.6-fold greater than 13CO2 fixation. Similarly, in the pod wall the relative rates of CO2 fixation and CO2 evolution were similar in the light, but the decrease in CO2 evolution from dark to light was primarily due to a 69% decrease in CO2 evolution (Fig. 6C).
The Contribution of Respiration and Photosynthesis to O2 Exchange of Soybean Seeds in the Light
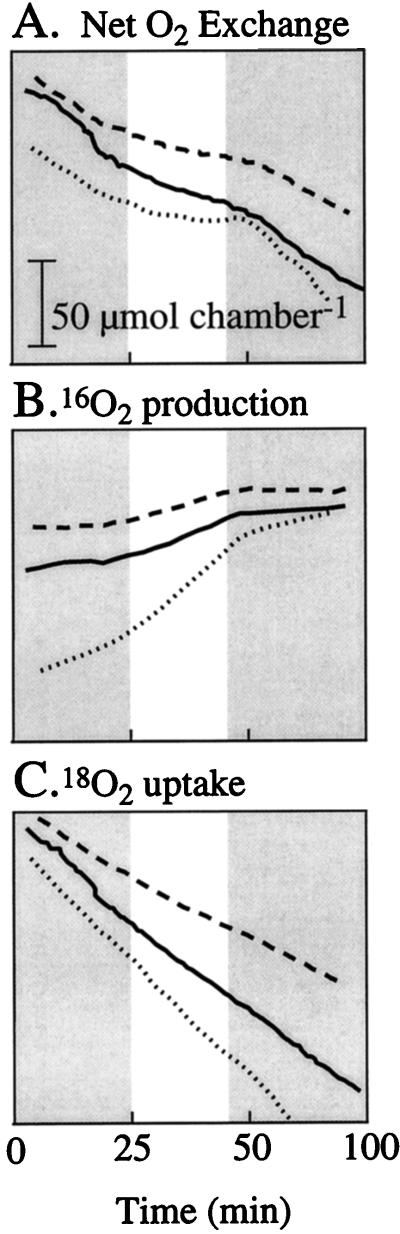
Rates of 16O2 evolution and 18O2 uptake in soybean seeds (24–45 DAF) were measured against an ambient O2 background of 22% 18O2 in a closed gas exchange system using a mass spectrometer. The sum of 16O2 and 18O2 exchange rates revealed that O2 uptake decreased in the light (Fig. 7A). This is similar to what was observed previously in seeds (Fig. 4), where net O2 exchange was measured with the differential O2 sensor. Measurements of 16O2 evolution and 18O2 uptake revealed that the decrease in net O2 uptake in seeds in the light was associated more with an increase in photosynthetic O2 evolution than with a decrease in respiratory O2 consumption (Fig. 7, B and C).
Figure 7.
Changes in net O2 (A), 16O2 (B), and 18O2 concentration (C) in a closed system (approximately 150 mL) containing expanding (30- to 40-d-old) soybean seeds (dashed line, 0.976 g DW, n = 18; solid line, 1.219 g DW, n = 18; dotted line, 0.680 g DW, n = 15) exposed to either dark or light (1,000 μmol m−2 s−2) conditions. An approximate scale (μmol chamber−1) for all panels is provided in A. The initial O2 concentration was approximately 22% 18O2. Shaded areas indicate dark conditions.
DISCUSSION
The DRUR
The DRUR term coined in this paper provides a quantitative measure of the rate of synthesis of biomass that is more reduced per unit carbon than Glc (in photosynthesizing tissues) or than the substrates of metabolism (in respiring tissues). Therefore, in a tissue that is photosynthesizing and producing only Glc, organic acids, or fatty acids, the DRUR would be zero, negative, or positive, respectively. In a respiring tissue using Suc (or Glc or starch) as a substrate, oil synthesis or nitrate reduction to ammonia would give a positive DRUR, whereas organic acid synthesis would give a negative DRUR. If the respiring tissue were using oil as a substrate and making cellulose, the products would be less reduced than the substrates and the DRUR would be negative.
DRUR provides a real-time, noninvasive measurement of biosynthetic processes within plant tissues, and offers a new tool for the study of plant metabolism and regulation. The ability to calculate DRUR values requires precise, simultaneous measurements of low CER and OER, a capability made possible by the recent development of a differential O2 analyzer (Willms et al., 1997).
Gas Exchange and Reductive Biosynthesis of Attached Fruits in the Dark and Light
In the dark, intact, attached soybean fruits evolved CO2 at rates 1.5 ± 0.14 (n = 4) times higher than they consumed O2, resulting in DRUR values of 50.9 ± 14.4 μmol electrons g−1 DW h−1 (n = 4). Such positive DRUR values are consistent with tissues synthesizing compounds that are more reduced (per unit carbon) than the substrates that they are using. For example, FAS from phloem-supplied Suc would be expected to have a positive DRUR.
When the same fruits were exposed to light, the CER decreased slightly whereas the OER changed dramatically (i.e. about 2.14 ± 0.21 times the change in CER, n = 4). This occasionally resulted in the simultaneous evolution of O2 and CO2 in the light and thus a negative GEQ.
To our knowledge, simultaneous O2 and CO2 evolution have not been reported for any plant tissue. Such gas exchange would be indicative of highly reductive biosynthesis, since the net production of both O2 and CO2 is associated with the generation of reducing power that is not used for CO2 fixation or O2 uptake. The observed change in DRUR from 50.9 μmol electrons g−1 DW h−1 in the dark to 113.3 ± 41.8 μmol electrons g−1 DW h−1 (n = 4) in the light was consistent with this interpretation, and demonstrated a 2.2-fold, light-stimulated increase in reductive biosynthesis. These findings were also consistent with previous leaf studies in which FAS was shown to be stimulated by light (Stumpf et al., 1967; Browse et al., 1981; Sauer and Heise, 1983).
Fruit Excision and Reductive Biosynthesis
To obtain more precise measurements of gas exchange in fruit, multiple fruits were excised and enclosed in a gas exchange system so that larger gas exchange differentials could be obtained. Compared with intact fruits, excision resulted in a larger change in CER than OER, and therefore a decline in DRUR, in many cases resulting in negative values (Figs. 4B and 5B) compared with the positive DRUR obtained in attached fruits (Figs. 2 and 3). Moreover, following excision, a gradual decline in DRUR was observed over 3 h in elongating and transition fruit, but not in those from expanding or mature fruit (data not shown). Fruit excision eliminates the phloem supply of carbohydrate to the fruit, which may reduce DRUR by either stopping reductive biosynthesis or causing the tissue to switch to more reduced substrates for carbon metabolism (e.g. fatty acids).
Despite the adverse effects of excision on DRUR in fruits in the dark, the light stimulation of DRUR was similar in intact (Figs. 2 and 3) and excised (Figs. 4 and 5) fruits. In excised fruits, light caused changes in OER 1.70 ± 0.31 (n = 4) times more than it altered CER, resulting in DRUR values in the light (87.02 ± 15.5 μmol electrons g−1 DW h−1, n = 4) that were much higher than those obtained from the same fruit in the dark (7.98 ± 22.9 μmol electrons g−1 DW h−1, n = 4). These findings supported our conclusion that excised organs could be used to further explore the underlying properties of light-stimulated reductive biosynthesis in soybean fruit.
Contribution of Pod Wall and Seed to Net Fruit Gas Exchange
To determine the contributions of the pod wall and seed to net fruit gas exchange, particularly DRUR, control experiments were carried out to test the effects of cutting the fruit (i.e. wounding) and separating the pod wall from the seeds. In the light, fruit wounding caused a slight decrease (to 77.5% of initial) in DRUR, an increase in CO2 evolution, and a decrease in O2 evolution such that net O2 consumption was observed (Fig. 4). Despite these changes in gas exchange, DRUR in the light of the wounded fruit was still greater than that in the dark (Fig. 4B).
Separate gas exchange measurements of seeds and pod walls in the light and dark revealed that the large change in DRUR between light and dark primarily occurs within the seeds, not in the pod walls (Fig. 4B). In the light, seed DRUR was positive and similar to that measured in wounded whole fruit. When the seeds were exposed to dark, rates of O2 consumption increased substantially, but CO2 evolution increased only slightly, resulting in a negative DRUR for seeds. In contrast, O2 and CO2 exchange in pod walls were approximately equal and opposite in the dark, and decreased to almost no net gas exchange in the light (Fig. 4A). Thus, the flow of reductant observed in whole fruits was mainly due to seeds, the change in DRUR from dark to light was due primarily to a change in net O2 consumption within the seeds, and the effects of fruit excision on DRUR were due to changes in seed metabolism.
These results support the suggestion that the large increase in DRUR in fruits following the light-to-dark transition is due to reductive biosynthesis, since it is in the seeds that high amounts of oil are synthesized. The source of the reductant could either come from respiratory processes where CO2 is evolved without O2 consumption or from photosynthetic electron production without CO2 fixation.
To determine the source of reductant (photosynthetic O2 evolution or respiratory CO2 evolution), and to clarify further the gas exchange patterns of soybean fruits and seeds, MS measurements of 12CO2 evolution and 13CO2 uptake were performed on fruits, seeds, and pod walls, and MS measurements of 18O2 consumption and 16O2 uptake were performed on seeds only.
Contribution of Respiration and Photosynthesis to the Gas Exchange of Soybean Fruits, Pod Walls, and Seeds
In whole fruits and pod walls the transition from dark to light caused a large decrease in 12CO2 evolution and a small increase in 13CO2 uptake (Fig. 6). In seeds only, light induced only a small decrease in 12CO2 production and had very little effect on 13CO2 fixation, a finding consistent with the small effect of light on CER (Fig. 4). These findings suggest that CO2 fixation plays only a minor role in accounting for the observed changes observed in whole-fruit CER, the primary role factor being a light-induced decrease in CO2 evolution in the pod wall.
In the light, photosynthetic 16O2 evolution was observed in seeds when exchanges of 18O2 and 16O2 were monitored in a closed system (Fig. 7). Rates of 18O2 consumption did not noticeably change from dark to light (Fig. 7C) and were greater than rates of 16O2 evolution (Fig. 7B). Therefore, net O2 consumption in seeds decreased from dark to light, similar to measurements of net O2 exchange in seeds made by the respiratory quotient/photosynthetic quotient analyzer (Figs. 4 and 7A).
These findings suggest that in seeds, the extra reducing power that flowed to reductive biosynthesis in the light was generated through photosynthetic O2 evolution, and that relatively little of the reducing power from this O2 evolution was coupled to CO2 fixation. The net CO2 exchange observed in whole fruits in the light reflects net CO2 evolution from the pod wall and seed, with some photosynthetic CO2 fixation by the pod wall. Although gross O2 exchanges were not measured in pod walls, O2 exchange of pod walls would be expected to mirror CO2 exchange, because DRUR was attributed to seeds and not the pod wall and the GEQ was near unity (Fig. 4). Therefore, the net O2 evolved from whole fruits in the light also results from a decrease in respiratory O2 consumption and the occurrence of photosynthetic O2 evolution by the pod wall.
It is important to note that these findings must be interpreted with caution since the tissues being studied were large and dense, resulting in the potential for large concentration gradients between the outside air and the sites of CO2 and O2 consumption within the tissue. Consequently, our estimates of CO2 fixation from 13CO2 uptake and O2 consumption from 18O2 uptake probably underestimate the actual values.
The Effect of Fruit Development and Light on Reductive Biosynthesis
Light stimulated simultaneous O2 and CO2 evolution and increased the rates of reductive biosynthesis in fruit of all age groups, but the degree of light stimulation of DRUR and the rate of reductant flow in the light and dark differed depending on fruit age. Maximal rates of light-stimulated reductant flow occurred at low light intensities (generally less than 500 μmol m−2 s−2) compared with what is required to saturate photosynthesis in typical C3 leaves (600 μmol m−2 s−2) (Nobel, 1991). This may partly reflect the low level of light that soybean fruits receive within the canopy.
Light had a much greater stimulatory effect on DRUR in young fruit in which the dark DRUR was zero or negative than in expanding fruit in which the dark DRUR was high (Fig. 5). This may indicate that light has a more important role in regulating reductive biosynthesis in young fruit than in rapidly expanding fruit.
Expanding fruit showed higher rates of DRUR in both dark and light compared with other age groups. This is the stage in fruit growth where the seeds are most rapidly expanding, and where there would be the maximal rate of synthesis of fatty acids. A positive dark DRUR in expanding fruit differs from a previous experiment with excised fruit (Fig. 4) in which excision resulted in a negative DRUR in the dark. However, in this light curve experiment, the dark gas exchange values of the fruit were averages of intermittent dark periods that were sandwiched between light periods. Brief (15-min) exposures to light may have allowed for accumulation of recent photoassimilates that could then be a source of carbohydrate during the dark.
DRUR and Oil Synthesis
To assess the theoretical relationship between DRUR and oil synthesis, calculations were made of the CER and OER associated with Suc conversion into a fatty acid consisting of 30% oleic acid and 70% linoleic acid (data not shown). CER (32 mmol CO2 g−1 oil) was calculated to be much higher than OER (6 mmol O2 g−1 oil), resulting in a theoretical DRUR of 106 mmol electrons g−1 oil. Given that developing soybean fruits (20–40 DAF) synthesize fatty acids at approximately 4.9 mg−1 oil fruit d−1 (Holden et al., 1994), the theoretical DRUR would be about 522 μmol electrons fruit−1 d−1. Assuming a fruit DW of 0.4 g, the average DRUR would be about 54 μmol electrons g−1 DW h−1, a value similar to, or on the low end of the range of, the DRUR values measured in the present study (Figs. 2–5). This fit between the observed and theoretical DRUR values is consistent with the DRUR being a measure of FAS in soybean fruit.
Four other pieces of evidence support the suggestion that DRUR reflects FAS. First, the light-induced shift in DRUR occurred in seeds, the site of FAS, not in the pod wall. Second, DRUR was greatest in rapidly expanding fruit, the stage of fruit development when rates of FAS are highest (Rubel et al., 1972; Dornbos and McDonald, 1986). Third, FAS is the predominant reductive pathway occurring in oilseeds, as virtually all nitrogen is phloem-supplied as amino compounds (Layzell and LaRue, 1982), so the fruit would be free of the large reductant costs associated with NO3− reduction or N2 fixation. Finally, the observed increase in DRUR in the light complements studies done on isolated chloroplasts from leaves and on intact B. napus embryos, which showed that FAS is stimulated by light (Liedvogel and Bauerle, 1986; Fuhrmann et al., 1994; Roughan and Ohlrogge, 1996; Sasaki et al., 1997; Aach and Heise, 1998).
Analysis of the biochemical pathways for Suc conversion to fatty acids (data not shown) indicated that most of the reductant needed for FAS from acetyl-CoA could be met by the catabolism of Suc to acetyl-CoA. The imbalance between CER and OER (and thus the DRUR) arises from CO2 production by pyruvate decarboxylase. This being the case, what does light do to stimulate reductive biosynthesis in soybean seeds? Two options come to mind: ATP synthesis and CO2 fixation.
ATP may be generated through cyclic phosphorylation, which enhances the conversion of carbohydrate into fatty acids. This would not account for the observed light-stimulated 16O2 production in seeds (Fig. 7), but it would be consistent with experimental results showing that plastids can provide most of the reductant needed for FAS (Kleppinger-Sparace, 1992; Fuhrmann et al., 1994; Mohlmann et al., 1994; Qi et al., 1995; Mohlmann and Neuhaus, 1997; Xue et al., 1997). In contrast, only photosynthetic plastids can supply the ATP required (Liedvogel and Bauerle, 1986; Roughan and Ohlrogge, 1996).
Photosynthetic O2 evolution could generate the reductant needed to fix CO2 into carbon skeletons for FAS. This would be consistent with the observed light stimulation of DRUR (Figs. 2–5) and the enhanced 16O2 production (Fig. 7), but not with the lack of 13CO2 fixation (Fig. 6). Perhaps the dense tissue of the seed prevented the 13CO2 from reaching the carboxylation sites, resulting in an underestimate of this process. This proposal would be supported by the work of King et al. (1998), who suggested that the primary purpose of seed photosynthesis is to re-fix CO2.
It is likely that photosynthesis provides both reductant and ATP in support of FAS, but further studies will be needed to determine the relative importance of each in the light stimulation of reductive biosynthesis in soybean seeds.
CONCLUSIONS
The results of this study support and expand the conclusions of various authors (Fuhrmann et al., 1994; Aach and Heise, 1998) by demonstrating that light stimulates reductive biosynthesis within intact soybean fruits. In every experiment, including fruits of all ages and attached or detached, the DRUR measured in the light was significantly greater than that measured in the dark. This finding clearly shows that the products of metabolism in the light are—like fatty acids—more reduced than carbohydrate (per unit carbon), and the rate of biosynthesis is greater in the light than it is in the dark.
These conclusions do not support the proposal of Eastmond and Rawsthorne (1998), who suggested that light contributes little to FAS within intact fruit. Since this study was done using isolated plastids, it is possible that the organelles may have different physiological characteristics than embryos within intact fruit.
ACKNOWLEDGMENTS
The authors thank John Glew for designing the fruit cuvettes, Fayak Negm for technical assistance, and Dr. D.T. Canvin for valuable discussions and the use of supplies.
Abbreviations:
- CER
CO2 exchange rate
- DAF
days after flowering
- DRUR
diverted reductant utilization rate
- DW
dry weight
- FAS
fatty acid synthesis
- GEQ
gas exchange quotient
- OER
O2 exchange rate
- STP
standard temperature and pressure
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada with a research grant to D.B.L. and a postgraduate fellowship to J.R.W.
LITERATURE CITED
- Aach H, Heise K-P. On the compartmentation of triacylglycerol synthesis in developing seeds of Brassica napus. Bot Acta. 1998;111:123–129. [Google Scholar]
- Asokanthan PS, Johnson RW, Griffith M, Krol M. The photosynthetic potential of canola embryos. Physiol Plant. 1997;101:353–360. [Google Scholar]
- Browse JA, Roughan PG, Slack CR. Light control of fatty acid synthesis and diurnal fluctuations of fatty acid compositions in leaves. Biochem J. 1981;196:347–354. doi: 10.1042/bj1960347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse JA, Slack CR. Fatty-acid synthesis in plastids from maturing safflower and linseed cotyledons. Planta. 1985;166:74–80. doi: 10.1007/BF00397388. [DOI] [PubMed] [Google Scholar]
- Dornbos DL, Jr, McDonald MB., Jr Mass and composition of developing soybean seeds at five reproductive stages. Crop Sci. 1986;26:624–630. [Google Scholar]
- Eastmond P, Kolacna L, Rawsthorne S. Photosynthesis by developing embryos of oilseed rape (Brassica napus L.) J Exp Bot. 1996;47:1763–1769. [Google Scholar]
- Eastmond PJ, Rawsthorne S. Comparison of the metabolic properties of plastids isolated from developing leaves or embryos of Brassica napus L. J Exp Bot. 1998;49:1105–1111. [Google Scholar]
- Fuhrmann J, Johnen T, Heise K-P. Compartmentation of fatty acid metabolism in zygotic rape embryos. J Plant Physiol. 1994;143:565–569. [Google Scholar]
- Gupta R, Singh R. Fatty acid synthesis by isolated leucoplasts from developing Brassica seeds: role of nucleoside triphosphates and DHAP-shuttle as the source of energy. J Biosci. 1996;21:819–826. [PubMed] [Google Scholar]
- Holden MJ, Norman HA, Britz SJ. Spectral quality during pod development affects omega-6-desaturase activity in soybean seed endoplasmic reticulum. Physiol Plant. 1994;91:346–351. [Google Scholar]
- King SP, Badger MR, Furbank RT. CO2 refixation characteristics of developing canola seeds and silique wall. Aust J Plant Physiol. 1998;25:377–386. [Google Scholar]
- Kleppinger-Sparace KF, Stahl RJ, Sparace SA. Energy requirements for fatty acid and glycerolipid biosynthesis from acetate by isolated pea root plastids. Plant Physiol. 1992;98:723–727. doi: 10.1104/pp.98.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell DB, LaRue TA. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982;70:1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel B, Bauerle R. Fatty-acid synthesis in chloroplasts from mustard (Sinapis alba L.) cotyledons: formation of acetyl coenzyme A by intraplastidic glycolytic enzymes and a pyruvate dehydrogenase complex. Planta. 1986;169:481–489. doi: 10.1007/BF00392096. [DOI] [PubMed] [Google Scholar]
- Mir NA, Salon C, Canvin DT. Inorganic carbon-stimulated O2 photoreduction is suppressed by NO2− assimilation in air-grown cells of Synechococcus UTEX 625. Plant Physiol. 1995;109:1295–1300. doi: 10.1104/pp.109.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlmann T, Neuhaus HE. Precursor and effector dependency of lipid synthesis in amyloplasts isolated from developing maize and wheat endosperm. J Cereal Sci. 1997;26:161–167. [Google Scholar]
- Mohlmann T, Scheibe R, Neuhaus HE. Interaction between fatty-acid and starch synthesis in isolated amyloplasts from cauliflower floral buds. Planta. 1994;194:492–497. [Google Scholar]
- Nobel PS. Physiochemical and Environmental Plant Physiology. San Diego: Academic Press; 1991. [Google Scholar]
- Qi Q, Kleppinger-Sparace KF, Sparace SA. The utilization of glycolytic intermediates as precursors for fatty acid biosynthesis by pea root plastids. Plant Physiol. 1995;107:413–419. doi: 10.1104/pp.107.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan PG, Ohlrogge JB. Evidence that isolated chloroplasts contain an integrated lipid-synthesizing assembly that channels acetate into long-chain fatty acids. Plant Physiol. 1996;110:1239–1247. doi: 10.1104/pp.110.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel A, Rinne RW, Canvin DT. Protein, oil, and fatty acid in developing soybean seeds. Crop Sci. 1972;12:739–741. [Google Scholar]
- Sasaki Y, Kozaki A, Hatano M. Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase. Proc Natl Acad Sci USA. 1997;94:11096–11101. doi: 10.1073/pnas.94.20.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer A, Heise K-P. On the light dependence of fatty acid synthesis in spinach chloroplasts. Plant Physiol. 1983;73:11–15. doi: 10.1104/pp.73.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf PK, Brooks J, Galliard T, Hawk JC, Simoni R (1967) Biosynthesis of fatty acids by photosynthetic tissues of higher plants. In TW Goodwin, ed, Biochemistry of Chloroplasts, Vol 2. Academic Press, New York
- Walsh KB, Vessey JK, Layzell DB. Carbohydrate supply and N2 fixation in soybean. Plant Physiol. 1987;85:137–144. doi: 10.1104/pp.85.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms JR, Dowling AN, Dong Z-M, Hunt S, Shelp B, Layzell DB. The simultaneous measurement of CO2 and O2 exchange in biological systems. Anal Biochem. 1997;254:272–282. doi: 10.1006/abio.1997.2416. [DOI] [PubMed] [Google Scholar]
- Xue LG, McCune LM, Kleppinger-Sparace KF, Brown MJ, Pomeroy MK, Sparace SA. Characterization of the glycerolipid composition and biosynthetic capacity of pea root plastids. Plant Physiol. 1997;113:549–557. doi: 10.1104/pp.113.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]