Schizophrenia usually emerges in late adolescence or early adulthood (1, 2). If cognitive deterioration occurs, it generally appears early and with relatively stable impairment over the next 5–10 years. Later in the illness, psychotic symptoms may become less intense, and there can be modest improvement in functional later in life (3–5). We present a case whose symptom onset and initial course of illness were typical (although early development was abnormal) but he evidenced notable treatment resistance. In his mid-30’s he developed progressive dementia. As this is atypical for schizophrenia, we conducted more detailed investigation.
History of illness
A 41 year-old man (EF) participated in a precision medicine study of highly treatment resistant schizophrenia. Study procedures were ethically approved, EF’s legal guardian provided written informed consent, and the protocol allowed for return of results and subject recontact.
Table 1 gives milestones for EF’s life events, development, and course of illness. EF was born at term as a breech presentation, with hernia, cleft palate, and anteverted auricles. He was delayed in some developmental milestones, and needed extra support in school. At age 21, he developed psychotic symptoms, and resided in a psychiatric hospital or in a supported living facility nearly continuously for the next 20 years.
Table 1.
Life course chart for EF
| Age | Life event | Clinical notes |
|---|---|---|
| 0 | Birth | Breech birth, hernia, cleft palate, protuberant ears |
| 3 months | Surgery | Two herniorrhaphies |
| 6 months | Adopted | |
| 4 years | Delayed milestones Surgery | Preschool staff did not view him as ready for kindergarten compared to peers Surgery to correct cleft palate |
| Grades K-5 | Elementary school | Learning disability; “warm, sociable” |
| Grades 6–8 | Middle school | Special education classes. IQ measured at 80, dyslexia, auditory processing problems. Surgery to correct protuberant ears. |
| 14–19 years | High school | Graduated 2 years behind grade level; angry, possible substance misuse |
| 20 years | College | Brief college attendance. Few friends, abused by peers, possible substance misuse |
| 21 years | Psychotic episodes resulting in 2 admissions to community psychiatric hospital (14 days each) and day treatment | Delusions of strangers surrounding house with intent to do physical harm, auditory hallucinations, hitting mother, throwing objects. GAF 20–25 Medications: perphenazine, lithium |
| 21–22 years | 3 inpatient admissions into university psychiatry unit for psychosis (1 month each) | Intense paranoia, auditory hallucinations, aggressive threats, suicidal ideation. GAF 10–20. Brain MRI: unremarkable. Thrombocytopenia Medications: perphenazine, olanzapine, clozapine |
| 22–27 years | Long-term state inpatient hospitalization | Admission GAF 20 due to severe paranoia, auditory hallucinations. Poor initial response to clozapine, but good response with adequate dose and duration. Obesity. No EPS and normal gait noted on annual medical/neurologic exam Thrombocytopenia Medications: clozapine, risperidone, fluoxetine |
| 27–29 years | 24-hour supervised community living program | Few delusions or auditory hallucinations; EF actively involved in discharge processing; vocational training recommended as part of therapy program. GAF ~ 60 Medications: clozapine, ziprasidone |
| 29 years | Acute psychotic episode, medication abruptly stopped. 7 month inpatient admission | Extensive bruising, fetal position, incontinent, incoherent, randomly swinging fists Thrombocytopenia Medications: clozapine |
| 29–41 years | 24-hour supervised community living program | Initially relatively functional: able to do most self-care, worked in sheltered environment, participated in group outings, could play video games. Decline in function beginning in early 30’s. By his late 30’s, marked cognitive impairment, impaired gait, incontinence, incoherence, randomly swinging fists, language limited to grunting, required 24 hour 1:1 care. GAF 10–20. Loss of speech prominent at age 37. Thrombocytopenia (workup unrevealing) Medications: clozapine, lorazepam (agitation), PRN temazepam, melatonin (sleep) |
| 37 years | Psychiatric consultation (second opinion) | Prompted by decline in function compared to early 30s: decline in activities of daily living (had done most self-care independently, e.g. personal hygiene, making meals); worsening performance in sheltered work environment; lessened interest in activities that had previously been of interest. Staff had initiated plan to try to correct lessened verbal output (e.g., from lengthy responses to queries to very brief answers). On exam: halting/listing gait; inconsistent responses to questions; bizarre behavior (e.g., standing in center room in “almost catatonic state”). Occasionally incontinent of stool and urine. Brain MRI: poor quality due to movement, read as unremarkable. Thrombocytopenia, hypocalcemia |
| 39 years | Admission to inpatient neurology unit of a major academic center | Cognitive decline now severe. No expressive language, minimal or no comprehension. Evaluated for frontotemporal dementia. Brain MRI without contrast degraded due to motion, study terminated prematurely as EF unable to cooperate but read as no gross intracranial mass or disproportionate atrophy. No movement disorder noted. Discharged back to community living program. |
| 41 years | Enrolled in precision medicine study | Review of all records, genetic testing, examination by behavioral neurologist. |
| 41 years | Death | Autopsy (cause of death lobar pneumonia), neuropathology. |
The initial psychiatric presentation (age 21) included elaborate delusion of strangers surrounding his family home threatening to do harm. At one point, the police were called when he was striking relatives and destroying furniture. He experienced persistent auditory hallucinations of multiple distinct voices making derogatory comments. He was often observed talking to himself and laughing inappropriately. Given data available in the initial year of illness (age 21–22), EF met DSM-5 criteria for schizophrenia given: (A) continuous presence of delusions, hallucinations, thought disorder, and bizarre behavior; (B) marked impairment in function; (C) continuous illness for >6 months; (D) absence of clinically significant manic or depressive episodes; (E) no significant drug use or (at the time) known potentially causal medical condition; and (F) psychotic symptoms were new and prominent despite a history of developmental delay. Clinical response to typical antipsychotics was inadequate due to persistent positive symptoms, and EF received clozapine early in his illness (age 21). Despite medication adherence, EF’s response to clozapine was inadequate, and he was transferred to a state psychiatric hospital (age 22–27). Augmentation of clozapine with risperidone (6) led to an improved clinical response, indicated by discharge to a supervised community living program (ages 27–29). During this period, EF was warmly sociable, consistently denied auditory hallucinations and delusions, usually made good eye contact, evidenced a sense of humor, was active in therapeutic groups, followed professional sports, and enjoyed his work in a sheltered workshop. EF was considering vocational education or community college, and this was judged reasonable by his treatment team.
At age 29, EF’s medications were abruptly discontinued leading to marked deterioration (aggression, paranoid delusions, and hallucinations). Despite resumption of a previously effective medication regimen, EF required a lengthy inpatient stay before clinical improvement was adequate to allow discharge back to the supervised community living program. Per those who knew him, he never returned to his prior baseline. Beginning in his early 30’s, EF experienced cognitive decline which was unequivocal by age 35. In the five years before examination, his condition had deteriorated to the point where he had lost all capacity for meaningful communication and sat silently in a chair for hours, occasionally swinging his arms and grunting.
Due to this unusual presentation, we requested evaluation by a behavioral neurologist (ML) because of profound cognitive decline.
Past medical history
Myopia, asthma, chronic obstructive pulmonary disease, resolved obesity, obstructive sleep apnea, idiopathic thrombocytopenia, hypocalcemia, psoriasis, seborrheic dermatitis, reflux esophagitis, dyslipidemia, kyphoscoliosis, and constipation. Surgeries included two herniorrhaphies, cleft palate correction, and reduction of protuberant ears.
Family history
No confident psychiatric or neurological family history was available.
Investigations
Brain MRI performed when EF was in his mid-twenties was reported as normal. Another at age 40 was confounded by movement artifact, but was interpreted to show general volume loss without lobar predominance and left lens dislocation. Laboratory investigations were notable for thrombocytopenia and hypocalcemia.
Neurological examination
Functional Status
EF’s caretakers had noted a marked decline in cognition and function beginning when he was in his mid-30’s, to the point where he required continual 1:1 assistance. He insidiously became nonverbal except for occasional grunting. He became disengaged from external stimuli, sat in a chair for hours, and did not spontaneously initiate any purposeful activities. He appeared unable to recognize family. He was unable to use utensils and ate with his hands. He needed assistance in dressing and bathing, and was incontinent of bowel and bladder.
Physical Examination
His height was 173 cm and weight 71.7 kg (body mass index 24.0 kg/m2). He had mild scoliosis, and long, thin hands and feet. Facial features included a long tubular nose with bulbous tip, narrow palpebral fissures, and anterverted auricles with extra cartilage. He was edentulous (due to dental caries), and had a small mouth with a crowded oral cavity. He had dermatitis and dry skin on his cheeks and forehead. The remainder of the examination was unremarkable.
Mental Status Examination
He was mostly awake, but dozed briefly several times during the interview and examination. Engagement with the environment was limited. EF was non-verbal and did not respond to commands or to his name, but he did orient to voice. He did not appear to be responding to visual or auditory hallucinations.
Neurological Examination
EF’s right pupil was round and reactive to light. The left cornea was opacified with an unreactive pupil, and he did not blink to threat on the left. He did not pursue moving objects in his visual field. There was no gaze deviation, and no obvious facial asymmetry or ptosis. He showed very brief orienting responses to loud noises.
A hyperkinetic movement disorder was evidenced by multiple components: oro-lingual-buccal dyskinesia; nearly continuous right upper extremity choreoathetosis mingled with repetitive semi-purposeful grabbing movements; and posturing of the left upper extremity. Hyperkinetic movements ceased when he was asleep. There were no adventitious movements of the lower extremities. Muscle tone was increased in the right upper extremity (likely because it was constantly in motion). Tone was normal in other extremities. He did not participate in strength testing but there was no obvious focal weakness. Plantar responses were flexor, and deep tendon reflexes were unremarkable. He withdrew to touch in all extremities. He was unable to stand independently from a seated position. His gait was wide-based and ataxic with a choreiform component.
Genetic analyses
A peripheral venous blood sample was collected, and genomic DNA extracted using standard methods. Assays included genotyping with Illumina InfiniumOmniExpressExome-8 array (v1.3) and whole-genome sequencing (Illumina HiSeq X Ten, PCR-free library preparation, 150 bp paired-end reads, 30x coverage). All genetic assays were done per manufacturer’s protocols. Sequence alignment and variant calling were performed using the GenomeAnalysis Toolkit. Copy number variation in the SNP array data was analyzed using PennCNV (7).
Using the SNP array data, we identified a large, one-copy deletion on chromosome 22 from 18.66–21.70 Mb (hg19) that was also present in the whole genome sequencing data. This finding was consistent with 22q11 deletion syndrome (22q11DS), and its presence was confirmed with array comparative genome hybridization and fluorescent in situ hybridization on an independent blood sample in a CLIA-certified lab.
As the neurological examination showed profound, early-onset dementia with choreoathetoid movements, we carefully examined the sequence data aligning to exon 1 of the huntingtin gene (HTT). There was a large “insertion” adjacent to the HTT exon 1 CAG repeat known to be causal for Huntington’s Disease, suggesting misalignment of sequencing reads due to the presence of many CAG repeats over that in the hg19 genome reference. A triplet-primed PCR assay followed by fragment analysis in a CLIA-certified lab revealed a 48 copy CAG allele (pathogenic range ≥40 copies). An HTT CAG repeat of this size is “full penetrance” for Huntington’s Disease.
Death and Pathological Examination
EF died about three weeks after the neurological examination. He appeared to staff to be in his usual state of health, but died suddenly during routine daily care. An autopsy was performed by a county medical examiner and the cause of death was recorded as lobar pneumonia. There was no report of a large embolism in an artery to the lung, heart, or brain.
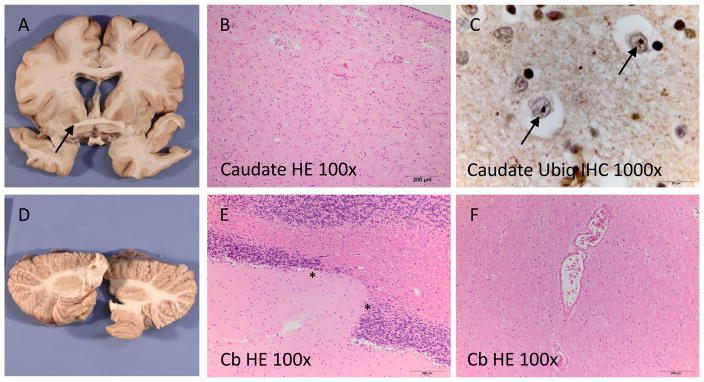
EF’s brain was removed, sectioned, fixed in formalin, and sent to clinical neuropathologists for evaluation (Figure 1). Gross inspection of the brain revealed mild leptomeningeal fibrosis and mild cortical atrophy in the parietal region with focal thinning of the corpus callosum. There were no obvious abnormalities of the gyral pattern and no grey matter heterotopias. The deep grey nuclei showed mild flattening of the head of the caudate nucleus and atrophy and discoloration of the globus pallidus. Mild dilation of the ventricular system was also seen. The putamen, amygdala, left hippocampus, posterior thalamus and cerebellum were normal. The brainstem was not available for evaluation. On histological examination, moderate chronic neurodegenerative changes were seen in the basal ganglia, which were most marked in the caudate nucleus. Small round ubiquitin immunoreactive neuronal intranuclear inclusions were present in the basal ganglia and neocortex, consistent with abnormal accumulation of expanded huntingtin protein. Tau, TDP-43 and alpha-synuclein immunohistochemistry were unremarkable. Thorough evaluation of multiple tissue blocks for developmental abnormalities revealed only subtle changes of uncertain significance including mildly ectatic vessels in the cerebral and cerebellar white matter, focal thinning of the cerebellar granular cell layer, and focal periventricular nodular ependymitis.
Figure 1.
Neuropathological findings: The basal ganglia showed mild atrophy of the caudate nucleus and brown discoloration of the globus pallidus (A). On microscopy, mild chronic neurodegenerative changes (B) and ubiquitin immunoreactive intranuclear inclusions (arrows in C) were observed. The cerebellum was subtly globular in shape (D) and showed focal thinning of the cerebellar cortical granular cell layer (between asterisks in E). Ectatic vessels as shown in (F) were seen focally in the deep white matter. Cb: cerebellum, HE: hematoxylin and eosin, Ubiq: ubiquitin, IHC: immunohistochemistry.
The neuropathological diagnoses were Huntington’s Disease and minor vascular and structural abnormalities of uncertain significance.
Discussion
EF’s early development was abnormal and showed multiple indications of a genetic syndrome like 22q11DS (e.g., cleft palate, dysmorphic features, and developmental delay). EF’s initial psychiatric presentation was consistent with idiopathic schizophrenia but treatment resistance was notable. He subsequently developed profound dementia with a hyperkinetic movement disorder. Genetic analysis, clinical evaluation, and neuropathology provided definite diagnoses of 22q11DS and Huntington Disease.
To the best of our knowledge, this is the only reported co-occurrence of 22q11DS and Huntington’s Disease. The prevalence of the 22q11DS is around 1/4,000 live births (8–11), and a pathogenic trinucleotide expansion in HTT occurs in approximately 1/10,000 live births (12). These genetic variants are on different chromosomes, and we are not aware of a mutational mechanism that could predispose to both events. If these were independent events, the probability of co-occurrence is around 1/40 million, or under 10 similar cases in the United States. The co-occurrence of multiple genetic syndromes (so-called “blended” phenotypes) has been reported in ~4% of individuals referred for clinical genetic testing (13, 14), particularly in people like EF with multisystem disease.
22q11DS is caused by a hemizygous deletion (i.e., the presence of one instead of two copies) of a region on chromosome 22 (15). The deleted region is usually ~3 million bases (chr22:18.6–21.7 Mb, hg19), contains over 50 genes, and is a de novo event in ~90% of cases (9, 16). 22q11DS has been observed world-wide because the fine structure of this region increases chances of its occurrence (the deleted region is flanked by low copy repeat regions that predispose to non-allelic homologous recombination). The clinical presentation of 22q11DS is variable, and can include developmental delay, intellectual disability, cardiac anomalies, palatal defects, immunodeficiency, thrombocytopenia, and hypocalcemia (17). The neuropsychiatric concomitants of 22q11DS are diverse. It is the strongest known genetic risk factor for schizophrenia (prevalence 0.3% in cases, genotypic relative risk >20) (18, 19). While 25–30% of individuals with 22q11DS will develop psychosis, it is also associated with autism, attention-deficit/hyperactivity disorder, pervasive developmental delay, and early-onset Parkinson’s disease (20).
Huntington’s Disease is a dominantly inherited neurodegenerative disorder (21). Most cases (~90%) are inherited (22). It is caused by a mutation in the first exon of the huntingtin gene (HTT), where three DNA bases (cytosine-adenine-guanine or CAG) are repeated multiple times (23, 24). The number of CAG repeats is normally 11–35 but if there are ≥40 CAG repeats, Huntington’s Disease virtually always occurs as the resulting protein becomes selectively toxic to neurons in the caudate, putamen, and deep layers of frontal and parietal cortex (25, 26). Key manifestations of Huntington’s Disease include chorea, cognitive decline/dementia, and psychiatric symptoms (27). Although choreiform movements are its diagnostic hallmark, cognitive and psychiatric manifestations often predate motor signs (28, 29). Although mood symptoms are common, psychotic symptoms occur in 6–25% of cases with Huntington’s Disease (27, 30–32).
With the benefits of hindsight and genotype-driven review of all available medical records, it is apparent that EF manifested multiple physical (e.g., hernia, cleft palate, facial dysmorphism, thrombocytopenia, and hypocalcemia), developmental (developmental delay, lowered IQ), and psychosis strongly suggestive of 22q11DS (33). EF subsequently experienced an early-onset progressive dementia due to Huntington’s Disease. The onset of cognitive decline in Huntington’s Disease is usually in the mid-40’s but can be earlier in those with longer CAG repeats as with EF (34). EF had dyskinetic as well as choreiform movements in the context of prolonged antipsychotic use which may be why Huntington’s Disease was not suspected earlier. EF’s chronic psychotic symptoms during most of his third decade support a diagnosis of schizophrenia. Informed clinicians disagree as to whether the presence of 22q11DS is sufficient to negate the DSM-5 “E” criterion (“not attributable to…another medical condition”) as it is a probabilistic but not deterministic risk factor. In either case, Huntington’s Disease led to a profound neurocognitive disorder which had an overwhelming impact during his last decade of life.
We speculate that EF was impacted both independently and interactively by this extremely rare combination of genetic insults. EF’s abnormal development and predisposition to schizophrenia can be reasonably attributed to 22q11DS. The early onset and rapidly progressive dementia were certainly due to pathologically-confirmed Huntington’s Disease. These two features of EF’s clinical course were probably independent given their distinctive ages of onset. We speculate that these two genetic conditions may have interacted, particularly later in his illness. Brain regions jointly impacted by Huntington’s Disease, schizophrenia, and antipsychotic treatment converge on a limited set of neuronal cell types (e.g., medium spiny neurons of the ventral striatum and cortical pyramidal neurons) (25, 35). This could have been most evident in EF’s atypical chorea where an overt movement disorder may have been masked. The combination of a hypokinetic movement disorder (22q11DS increases the risk of early-onset Parkinson disease, associated with overactivity in the indirect striatopallidothalamocortical pathway, which predisposes to hypokinesia) (36) with a hyperkinetic movement disorder (due to Huntington’s Disease, associated with underactivity of this pathway) as well as the extrapyramidal side effects of antipsychotic treatment may have resulted in the observed atypical motoric presentation.
Conclusions
Given that the diagnostic yield of genetic testing for copy number variants (like 22q11DS) in people with schizophrenia is on the order of 2–3% (37), we advocate broader and even routine testing. Many psychiatrists evaluate people with a new-onset psychotic disorder with tests to exclude rare causes of psychosis (e.g., brain imaging, endocrine, metabolic, viral exposure, and autoimmune studies) although the diagnostic yield is considerably lower than evaluating genomic structural variants. Psychiatrists need not become experts in genetic testing, but should have sufficient familiarity and working knowledge of medical genetics in order to identify patients with signs and symptoms suggestive of a genetic condition that would benefit from referral. Psychiatrists can work closely with specialists in medical genetics to identify which patients should be tested, what clinical circumstances warrant closer evaluation, how to interpret results and incorporate them into clinical care, and how to convey important results to patients in a way that augments and informs the clinical process.
Certain clinical features raise the index of suspicion for the presence of a cryptic copy number variant in people with schizophrenia. Many copy number variants associated with schizophrenia also increase risk for childhood psychiatric disorders including intellectual disability, autism spectrum disorder, attention-deficit/hyperactivity disorder, and pervasive developmental delay. Because these copy number variants change the dosages of many genes in every cell, clinical features can include multi-system abnormalities. For example, as a group, copy number variants linked to schizophrenia are also associated with craniofacial dysmorphisms (e.g., cleft palate or a typical facial appearance), macro- or microcephaly, neurological disorders (e.g., epilepsy), congenital heart disease, immune and hematological dysfunction (e.g., thrombocytopenia and thymic aplasia), and renal disease. However, these additional somatic features can be subtle or absent, underscoring the importance of having a low threshold for screening for copy number variants.
In many health care systems, a clinician can order a “post-natal whole genome copy number variation by array comparative genomic hybridization”. A peripheral venous blood sample is the source of DNA for this test. The test can take several weeks. If abnormalities are found, the report will list the nature and significance of the finding and usually point to additional resources for the clinical and patient. Referral to clinical genetics may be indicated in order to ensure optimal care. Many copy number variants associated with schizophrenia have medical comorbidities. For instance, clinical management of people with 22q11DS requires care across medical specialties (17). In EF’s case, there was extensive clinical investigation of long-standing thrombocytopenia (deemed “idiopathic” in life), and hypocalcemia was untreated.
Identifying copy number variants may become therapeutically important. Multiple academic groups and companies are pursuing therapies for people with psychiatrically important structural variants. Although there are no approved medications, the situation could change in the next decade and knowing which patients have a large structural variant could prove important therapeutically. This can also be important to families in order to connect them to support networks that are available for many rare genetic syndromes. Knowledge of recurrence risk can also be important for reproductive planning.
As genomic evaluation becomes more common clinically, phenotype associated with “classic” genetic disorders will likely expand, and additional features may be recognized as part of a syndrome (including neuropsychiatric conditions). This may be particularly important for people with long-standing psychiatric disorders who develop new signs and symptoms (e.g., dementia and a movement disorder as with EF). Although individually rare, dozens of single gene disorders can initially present with a clinical portrait confusable with idiopathic psychosis (38), and many are detected only when new neurological or medical symptoms develop.
This is by no means a unique recommendation, but a strong case can be made for the rigorous and structured re-evaluation of complex cases like EF. In routine clinical care, important therapeutic and diagnostic decisions are often constrained by a lack of data. Systematic reassessment of medical records, psychiatric and medical re-examination, consideration of a broader differential diagnosis, and diagnostic testing can lead to new etiologic insights.
In summary, this case report illustrates the value of genetic testing in psychiatry. What we call schizophrenia is a complex disorder caused by a heterogeneous disease process. The diagnosis and treatment of some proportion of people with schizophrenia will be influenced by the presence of rare, mechanistically potent variation.
Acknowledgments
The authors thank Dale Adair, MD, Medical Director, Office of Mental Health and Substance Abuse Services, Commonwealth of Pennsylvania, PA for his encouragement and support for this project. The research reported here was supported by the NIMH (K01 MH108894), and we thank the NIMH for support in sample collection and processing. Sequencing and SNP genotyping were performed by the SNP&SEQ Technology Platform (Uppsala, Sweden) within the National Genomics Infrastructure Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. Phlebotomy services were provided by Marice Davis and Kelly Bingaman.
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter WT, Jr, Buchanan RW. Schizophrenia. The New England journal of medicine. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 3.Ganev K. Long-term trends of symptoms and disability in schizophrenia and related disorders. Soc Psychiatry Psychiatr Epidemiol. 2000;35:389–395. doi: 10.1007/s001270050255. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2:340–350. doi: 10.1016/S2215-0366(15)00003-6. [DOI] [PubMed] [Google Scholar]
- 6.Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, Shaughnessy RA. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162:130–136. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, Hakonarson H, Bucan M. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17:1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14:3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezenas Du Montcel S, Mendizabai H, Ayme S, Levy A, Philip N. Prevalence of 22q11 microdeletion. J Med Genet. 1996;33:719. doi: 10.1136/jmg.33.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warby SC, Visscher H, Collins JA, Doty CN, Carter C, Butland SL, Hayden AR, Kanazawa I, Ross CJ, Hayden MR. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet. 2011;19:561–566. doi: 10.1038/ejhg.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balci TB, Hartley T, Xi Y, Dyment DA, Beaulieu CL, Bernier FP, Dupuis L, Horvath GA, Mendoza-Londono R, Prasad C, Richer J, Yang XR, Armour CM, Bareke E, Fernandez BA, McMillan HJ, Lamont RE, Majewski J, Parboosingh JS, Prasad AN, Rupar CA, Schwartzentruber J, Smith AC, Tetreault M, Innes AM, Boycott KM Consortium FC, Care4Rare Canada C. Debunking Occam’s razor: Diagnosing multiple genetic diseases in families by whole-exome sequencing. Clin Genet. 2017 doi: 10.1111/cge.12987. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, Veeraraghavan N, Hawes A, Chiang T, Leduc M, Beuten J, Zhang J, He W, Scull J, Willis A, Landsverk M, Craigen WJ, Bekheirnia MR, Stray-Pedersen A, Liu P, Wen S, Alcaraz W, Cui H, Walkiewicz M, Reid J, Bainbridge M, Patel A, Boerwinkle E, Beaudet AL, Lupski JR, Plon SE, Gibbs RA, Eng CM. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick JA, Jr, DiGeorge AM. Congenital absence of the thymus. Am J Roentgenol Radium Ther Nucl Med. 1968;103:32–37. doi: 10.2214/ajr.103.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Bassett AS, Chow EW. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biological psychiatry. 1999;46:882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A, Vorstman J. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159:332–339. e331. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, Cichon S, Edenberg HJ, Faraone SV, Gelernter J, Mathews CA, Nievergelt CM, Smoller JW, O’Donovan MC Psychiatric Genomics Consortium. Psychiatric Genomics: An Update and an Agenda. Am J Psychiatry. doi: 10.1176/appi.ajp.2017.17030283. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntington G. On Chorea. Medical and Surgical Reporter of Philadelphia. 26:4. 18972. [Google Scholar]
- 22.Houge G, Bruland O, Bjornevoll I, Hayden MR, Semaka A. De novo Huntington disease caused by 26–44 CAG repeat expansion on a low-risk haplotype. Neurology. 2013;81:1099–1100. doi: 10.1212/WNL.0b013e3182a4a4af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wexler NS, Conneally PM, Housman D, Gusella JF. A DNA polymorphism for Huntington’s disease marks the future. Arch Neurol. 1985;42:20–24. doi: 10.1001/archneur.1985.04060010026009. [DOI] [PubMed] [Google Scholar]
- 24.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 25.Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, Squitieri F, Lin B, Bassett A, Almqvist E, et al. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994;330:1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- 26.Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan JC, Snell RG, Tyler A, Houlihan GD, Fenton I, Cheadle JP, Lazarou LP, Shaw DJ, Harper PS. Molecular analysis and clinical correlations of the Huntington’s disease mutation. Lancet. 1993;342:954–958. doi: 10.1016/0140-6736(93)92002-b. [DOI] [PubMed] [Google Scholar]
- 28.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 30.Brooks DS, Murphy D, Janota I, Lishman WA. Early-onset Huntington’s chorea. Diagnostic clues. The British journal of psychiatry : the journal of mental science. 1987;151:850–852. doi: 10.1192/bjp.151.6.850. [DOI] [PubMed] [Google Scholar]
- 31.Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241–246. doi: 10.1111/j.1600-0447.1994.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 32.Caine ED, Shoulson I. Psychiatric syndromes in Huntington’s disease. Am J Psychiatry. 1983;140:728–733. doi: 10.1176/ajp.140.6.728. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence S, McDonald-McGinn DM, Zackai E, Sullivan KE. Thrombocytopenia in patients with chromosome 22q11.2 deletion syndrome. J Pediatr. 2003;143:277–278. doi: 10.1067/S0022-3476(03)00248-8. [DOI] [PubMed] [Google Scholar]
- 34.Langbehn DR, Hayden MR, Paulsen JS. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:397–408. doi: 10.1002/ajmg.b.30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skene NG, Bryois J, Badden TE, Breen G, Crowley JJ, Gaspar HA, Giusti-Rodríguez P, Hodge RD, Miller JA, Munoz-Manchado AB, O’Donovan MC, Owen MJ, Pardinas AF, Ryge J, Walters JTR, Linnarsson S, Lein ES, Sullivan PF, Hjerling-Leffler J. Brain cell types and the genetic basis of schizophrenia. doi: 10.1038/s41588-018-0129-5. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butcher NJ, Kiehl TR, Hazrati LN, Chow EW, Rogaeva E, Lang AE, Bassett AS. Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurol. 2013;70:1359–1366. doi: 10.1001/jamaneurol.2013.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CNV Working Group of the Psychiatric Genomics Consortium, Schizophrenia Working Groups of the Psychiatric Genomics Consortium. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2016;49:27–35. [Google Scholar]
- 38.Demily C, Sedel F. Psychiatric manifestations of treatable hereditary metabolic disorders in adults. Ann Gen Psychiatry. 2014;13:27. doi: 10.1186/s12991-014-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]