Abstract
Aggression is an innate behavior that helps individuals succeed in environments with limited resources. Over the past few decades, neurobiologists have identified neural circuits that promote and modulate aggression; however, far less is known regarding the motivational processes that drive aggression. Recent research suggests that aggression can activate reward centers in the brain to promote positive valence. Here, we review major recent findings regarding neural circuits that regulate aggression, with an emphasis on those regions involved in the rewarding or reinforcing properties of aggressive behavior.
Keywords: aggression, reward circuit, cell-type specific, motivation
Introduction
Aggression is an innate social behavior that helps individuals defend their territories, secure resources and increase the probability of successful mating. However, inappropriate aggressive reactions to provocation, aggression out of context, or insensitivity to non-threatening social signals can have devastating consequences for individuals and society [1]. Excessive irritability or aggression is a common symptom across neuropsychiatric disorders, including PTSD, Alzheimer’s disease, depression, schizophrenia and substance abuse, which negatively affects the quality of life for patients and their caregivers. Despite the health risk that excessive aggressive behavior poses on individuals and society, treatment choices are limited and largely ineffective [2–4].
Growing evidence shows that in some cases, aggression is rewarding and hence a self-perpetuating behavior similar to substance use [5–11]. In fact the term “appetitive aggression” has been used to describe aggression as a positive reinforcer for more than 4 decades [12]. For example, a recent clinical study showed that individuals who demonstrate highly aggressive responses to provocation in a behavioral aggression task have greater activity of the nucleus accumbens (NAc), a primary site in the brain mediating reward behavior [13]. Another study in adolescents with conduct disorder (CD), which is characterized by high levels of aggression, found that while viewing images of people in painful situations that normally prompt empathic responses in control participants, CD subjects showed increased activity of the NAc [14]. Together these studies highlight a potentially important role for motivational processes in aggressive behavior.
To understand the mechanisms underlying the neurobiology of aggression, we depend on animal models that recapitulate essential components of aggressive behavior such as aggression-seeking, initiation, and reward/reinforcement (see Box 1). For more than a century, neurobiologists have investigated brain regions that contribute to the initiation of aggressive behavior by employing electrical brain stimulation and lesion-induced suppression in animal models [15,16]. However, exploration into the neural circuits that modulate the positive valence of aggression and aggression-seeking behavior is relatively understudied. Moreover, novel tools that allow for increased spatial and temporal control over neural activity have aided our ability to dissect the complex cell types and microcircuitry involved in aggression. In this review, we focus on the major recent findings regarding brain areas and cell types regulating aggression in rodent models, with special attention to neural circuits involved in the positive valence of aggressive behavior in aggressive individuals.
Box 1. Behavioral tests to evaluate aggression and aggression-seeking behavior.
Resident intruder test (RI)
In this paradigm, experimenters place a conspecific individual (intruder) in the home cage of a test animal (resident), which usually instigates a territorial fight if both are male, sexually experienced, conspecifics. The resident-intruder interaction is recorded and later can be scored for aggressive (attack latency, number of bites, time spend attacking, tail rattling, etc.) and non-aggressive (social grooming, licking, sniffing, etc.) behaviors.
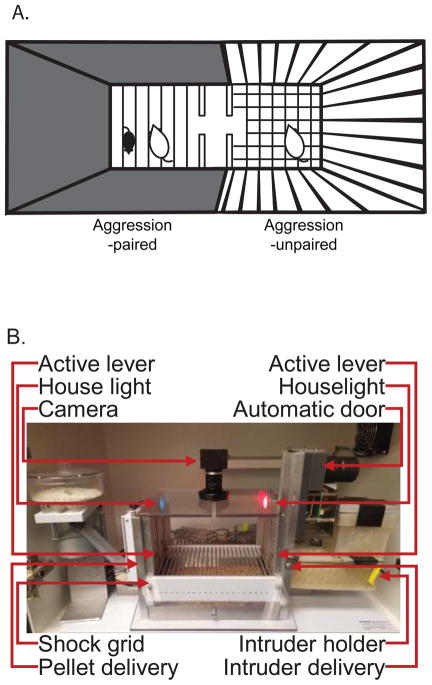
Aggression Conditioned Place Preference (Figure 2A)
[52,55] Here, animals are exposed to a two-compartment apparatus in which compartments are distinguishable through sensory cues such as floor texture and visual patterns on walls. Following an initial pre-test for baseline compartment preference, the animal is trained (generally for 2 or 3 days) to associate one compartment with an intruder conspecific and one compartment with no stimulus through repeated conditioning sessions. During the test the animal is free to explore both sides. The amount of time spent in a side during this test session is used as a measure of the rewarding or aversive salience of the stimulus. This method has been used to measure the rewarding properties of aggression in Syrian hamsters, rats, and mice in the laboratory for more than 2 decades [44].
Operant aggression-seeking box (Figure 2B)
[5,56] Here, an operant conditioning chamber (Skinner box) is equipped with levers or nose-poke sensors (or both) is modified to allow an aggressor animal receive a submissive conspecific to fight with if properly activate certain mechanisms (or follow certain sequence of nose-poking and lever-pressings). In some experimental cases, the experimenter sets a fixed ratio of responses (nose pokes or lever presses) to rewards (access to the conspecific). To measure the motivation of an animal to engage in aggressive behavior, the experimenter can progressively increase the ratio of lever presses/nose pokes to access the target animal.
New insights into hypothalamic control of aggression and aggression-seeking behavior
The involvement of the hypothalamus in the initiation and execution of aggression has been shown extensively across multiple mammalian species (For an extensive review on the topic see [17]). However, recent investigations utilizing cell-type specific manipulations of neurons within the medial hypothalamus have more finely dissected the complex microcircuitry of this sexually-dimorphic brain region (Figure 1). Interestingly, these studies have revealed a complex network of partially overlapping neuronal ensembles controlling aggressive, sexual, and even fear-related behaviors. In the following section, we summarize these studies and discuss their implications for our understanding of the role of the medial hypothalamus in various aspects of aggression-related behaviors.
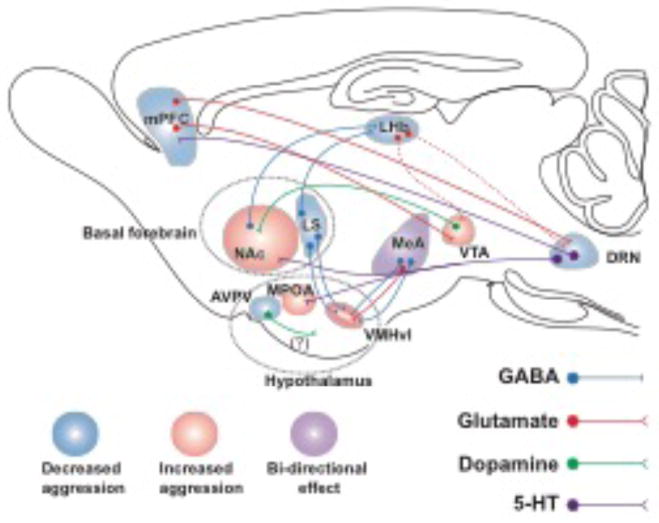
Figure 1. Neural Circuitry of Aggression.
Schematic map of the brain areas and their neuronal connection that are involved in aggressive behavior in mice reviewed in this paper. Areas that stimulate aggression are shaded in “pink” and areas that suppress aggression are shaded in “blue”. Medial amygdala (MeA) is shaded in “violet” and since it can both stimulate and suppress aggression depending on neuronal output that is optogenetically stimulated. AVPV: anteroventral periventricular nucleus of hypothalamus. DRN: dorsal raphe nucleus. LHb: lateral habenula. MeA: Medial amygdala. MPOA: medial preoptic area. NAc: nucleus accumbens. VMHvl: ventrolateral area of ventromedial hypothalamus. VTA: ventral tegmental area. (Dashed line denotes neuronal pathways that have been identified but not studied in the context of aggression or aggression-seeking behavior. The question mark [?] reflects the fact that although highly possible, dopaminergic nature of these neurons yet to be proven).
Ventromedial hypothalamus-ventrolateral area (VMHvl)
In the interest of space, we will highlight recent advances using new genetic, optogenetic or chemogenetic tools to precisely dissect the microcircuitry of the VMHvl in aggression. Lin and colleagues previously reported a spatially defined network of neurons in the hypothalamic attack area (HAA) within the VMHvl that control aggression [18]. Optogenetic stimulation of these cells in male mice initiates immediate attack behavior toward social targets including both male and female mice as well as inanimate objects. To further define this neuronal population they conducted a follow up study, which showed that the estrogen receptor-1 (Ers1)-expressing neurons in this subdivision of hypothalamus are both necessary and sufficient to initiate an attack. Interestingly, activation of the same neuronal population with relatively lower frequency stimulation triggers mounting behavior [19]. This observation shows the subtlety of activation patterns of the same neuronal network in modulating aggression and sexual behavior. Despite the established role of VMHvl in initiation and execution of attack, it was not clear if this area of the brain is involved in reward or reinforcement-related aspects of aggression. In a recent study, Falkner at al. used an operant aggression-seeking paradigm (described in Box 1) and in-vivo calcium imaging to show that the activity of VMHvl neurons is increased during aggression-seeking behavior and that activation of these neurons is both necessary and sufficient to promote aggression-seeking behavior [20]. Although this study clearly shows the role of VMHvl neurons in aggression-seeking, it remains unclear whether the Ers1+ neuronal population, which controls initiation of attack behavior, also regulates aggression-seeking.
A recent study by Sakurai et al. [21] investigated VMHvl neuronal ensembles that modulate fear and aggressive behaviors. In this study they developed and applied a new technique known as Capturing Activated Neuronal Ensembles (CANE) by generating a “knock-in” transgenic mouse line that upon activation of c-Fos, promotes transient expression of an avian specific receptor, dsTVA. The transient expression of dsTVA allows the attachment and entry of an engineered EnvAM21-coated-lentiviral vector (CANE-LV) during a short time window (1.5–2h). Co-injection of CANE-LV expressing Cre-recombinase (CANE-LV-Cre), and an AAV vector expressing a Cre-dependent fluorescent protein (AAV-FLEX-GFP) 1–2h after an aggressive social encounter labels aggression activated neurons. To define fear-activated neurons, they exposed the same mouse to a fear-inducing social encounter after a 10–14 day delay. The c-Fos staining of fear-activated neurons reveals that a different neuronal ensemble in the VMHvl is activated after a fear-inducing event since there is a small overlap (~10–20) between the two labeled group of neurons. The authors were then able to delineate a casual role for hypothalamic neuronal ensembles in fear by optogenetic stimulation of the neurons activated during fear-inducing social interaction.
Medial preoptic area (MPOA) and anteroventral periventricular nucleus (AVPV)
The MPOA is another hypothalamic area that historically has been studied for its pro-aggressive role and reproductive behavior [22]. More recently, Nakata et al. studied the role of estrogen receptor β (ERβ) in MPOA in intermale aggression by using an shRNA knock-down strategy [23]. They show the necessity of ERb during the prepubertal period in MPOA for expression of intermale aggressive behavior in adulthood in mice. McHenry et al. [24] showed that ΔFosB, a truncated splice variant of the transcription factor FosB, which is known to control natural and drug-induced reward is increased in the MPOA of rats after mating. To investigate the functional role of this transcription factor in MPOA in controlling mating behavior, the authors expressed an adeno-associated virus encoding ΔFosB (AAV-ΔFosB) in the MPOA, which impaired copulation and induced female-directed aggression in male mice. The anteroventral paraventricular nucleus (AVPV) is another subregion of the hypothalamus in mice that is involved in male aggression. Scott et al [25] identified a group of tyrosine hydroxylase-expressing (TH+) neurons in this region. Ablation of these neurons increases aggressive behavior, whereas optogenetic simulation of them suppresses it. The presence of TH in these neurons and their intrinsic electrophysiological properties suggest that they are dopaminergic, but it was not directly confirmed in the study. These experiments further emphasize the intricacy of hypothalamic neurocircuitry and the role of specific neuronal populations in the modulation of aggressive behavior.
Limbic regions involved in aggression
It has been hypothesized that brain structures such as amygdala, and medial prefrontal cortex (mPFC) that are historically considered part of the limbic system play a critical role in mediating attention to emotionally salient events that allows an organism to respond to threat and adapt in a way that ensures survival [26]. Thus, when an organism deems a situation as threatening, limbic structures are thought to guide reactive aggression in order to protect oneself or its resources [27,28]. Though a large historical literature exists describing a role for the limbic system in aggression, below we highlight recent advances that provide new insights into the specific cell types and circuits in limbic brain structures mediating aggression (Figure 1).
Medial amygdala (MeA)
MeA neurons receive dense chemosensory cues from the vomeronasal organ [29] and relay signals to multiple regions including VMHvl. Furthermore, their activity increases during innate social behaviors such as fighting and mating [30]. The MeA contains heterogeneous populations of neurons that are glutamatergic and GABAergic. Using optogenetic approaches, Hong et al. [31] directly showed that stimulation of MeA GABAergic neurons elicits aggressive behavior, whereas stimulation of MeA glutamatergic neurons suppresses aggressive behavior. In line with this finding, another recent study [32] showed that Aromatase+ neurons within the MeA are necessary for both intermale and maternal aggressive behavior in mice. These neurons express GAD1 and thus are purported to be inhibitory. However, it is not clear whether these GAD+/aromatase+ are the same GABAergic neurons that initiate aggressive behavior via modulating VMHvl.
Prefrontal cortex
The prefrontal cortex plays a modulatory role in aggression and has been shown to inhibit aggression in both cats and rodents [33,34]. Further evidence in humans suggests that patients suffering from neuropsychiatric conditions marked by loss of frontal control often exhibit heightened aggression or irritability [35]. More recent evidence to support this hypothesis comes from a study by Takahashi et al. [36] that showed optogenetic stimulation of excitatory neurons of the mPFC inhibits the intensity of aggressive behavior in a resident intruder test, whereas inhibition of the same cells intensified aggression toward an intruder mouse. Another recent study [37] showed that when placed in post-weaning social isolation, a model of escalated aggression, rats exhibit structural deficits in the mPFC, including reduced thickness, but higher activity in mPFC cells compared to control rats. It has been proposed that the mPFC helps with appropriate evaluation and interpretation of threat, and these data may suggest that such processes may be ineffective under conditions of chronic social isolation. Together, these findings highlight a complex role for the mPFC beyond merely inhibiting aggression and that further experiments will be needed to understand the dynamic role of mPFC processes in aggressive social behavior.
Latral septum (LS)
Lesions of the LS have been associated with increased aggressive behavior, a phenomenon known as “septal rage” in most mammalian species. In a recent study, Wong et al [38] showed that projections from LS to VMHvl modulate aggression such that optogenetic stimulation of GABAergic neurons of the LS to the VMHvl suppresses attack behavior. These findings suggest that LS exerts an inhibitory influence on neurons of VMHvl that mediate initiation of aggressive behavior. On a microcircuit level, the authors used in vivo single-unit recording in VMHvl to grouped neuronal ensembles according to their responses during aggression. They found groups of neurons that show excitation during attack (attack-excitation) and groups that show inhibition during attack (attack-inhibition). Stimulation of GABAergic input from the LS changes the balance of excitation within the VMHvl in favor of attack-inhibited cells. This study provides the most detailed mechanistic explanation for the inhibitory role of LS on aggression and helps to further explain the phenomena of “septal rage”.
Brainstem regions involved in aggression
Dorsal raphe nucleus (DRN)
The role of the serotonergic system in aggression has long been established both in animal models and clinical studies [39], but perhaps the most direct evidence for its involvement in aggression comes from a recent study by Niederkofler et al [40] that suppressed release of serotonin (5HT) from serotonergic terminals by cell-type specific expression of tetanus toxin, which increased inter-male aggression. Further characterization of these DRN cells showed that at least two sub-populations of 5HT neurons modulate aggressive behavior; dopamine receptor type 1 (Drd1) and type 2 (Drd2) expressing serotonergic neurons. Silencing both of these populations results in increased aggressive behavior. Notably, however, these two sub-populations differentially project to distinct areas of the brain play distinct roles in shaping aggressive social behavior (predominantly Drd1) or processing sensory stimuli (predominantly Drd2). Drd1-expressing 5HT neurons project mostly into rostral areas such as NAc (shell), hippocampus, and ventromedial hypothalamus; while Drd2-expressing 5HT neurons project largely to more caudal regions such as dorsolateral geniculate nucleus, lateral lemniscus, and superior olivary complex. Both subtypes innervate VTA, lateral hypothalamus, medial septum and preoptic area.
Another recent study by Nautiyal and colleagues [41] cleverly investigated the necessity of serotonin receptor 5HT1-B in forebrain neurons for aggressive behavior throughout development. Using a tetracycline-controlled-transcriptional activation system, this study showed that: 1) knocking down of 5-HT-1B receptors in the whole brain induces highly aggressive behavior in mice, especially during early-life period (p0–p60). This aggressive phenotype cannot be rescued by expression of the receptor in adulthood (p60 and above). 2) Permanent lifelong conditional knock down of the 5HT-1B postsynaptic receptors in the forebrain produces a similar aggressive phenotype, while 3) conditional knock out of the 5HT1-B presynaptic receptors in DRN does produce the highly aggressive phenotype. These findings clearly show the necessity of 5HT-1B receptors in forebrain structures for modulating aggressive behavior in response to serotonin input during the development.
Mesolimbic reward circuitry and motivational aspects of aggression
To date, most published studies on aggression have focused on attack initiation models described above. However, it is clear from both human and rodent models of aggression that there is a motivational component to aggression whereby some individuals actually seem to enjoy subordinating or “bullying” others [10,42]. Thus, recent studies have begun to interrogate neural circuits classically involved in control of drug and natural reward behaviors to determine whether they play a role in aggression (Figure 1). In this section, we will highlight a few recent manuscripts that provide novel insights into how reward-related brain regions control aggressive behavior.
Ventral Tegmental area
Dopaminergic neurons of the VTA comprise the mesolimbic dopamine system that is implicated in motivation and reward behavior [43]. More than 20 years ago, increased activity of dopaminergic neurons of the VTA was shown to be associated with aggression in female Syrian hamsters [44]. Moreover, these highly aggressive animals showed increased preference for an intruder-paired context compared to non-aggressive animals, suggesting that they find aggression rewarding to some degree. Only recently, Yu et al [45] provided more direct evidence for the involvement of dopaminergic neurons of the VTA in aggressive behavior. They showed that optogenetic stimulation of dopaminergic neurons of the VTA increased aggressive behavior in male mice interacting with another male. However, this finding does not explain whether dopaminergic activity affects the motivation to exhibit aggressive behavior. Future studies utilizing cell-type specific optogenetic approaches can determine whether dopaminergic neurons of VTA control the rewarding effects of aggression.
Nucleus accumbens
Dopaminergic neurons of the VTA project to the NAc, a major hub within the brain reward system that integrates positive and negative stimuli [46]. Several imaging studies show activation of NAc during aggression-provoking tasks in human subjects [13,27,47]. Using c-Fos mapping as a surrogate measure of neuronal activity, Nehrenberg et al. [48] showed greater activity following aggressive social interactions in the NAc of an aggressive resident mouse. A similar study in rats showed higher c-Fos immunoreactivity and greater release of dopamine in the NAc following aggression, which were blocked by administration of the Dopamine D2 receptor antagonist, haloperidol [49]. While these studies confirmed a functional role for NAc in mediating aggression, they did not address the role of the NAc in the motivation to exhibit aggressive behavior. Earlier work by Couppis and Kennedy [50] demonstrated that pharmacological blockade of dopaminergic D1 and D2 receptors in the NAc reduces operant responding for access to an intruder that can be attacked. Expression of ΔFosB in the NAc has been widely associated with rewarding properties of drugs of abuse and mating behavior [51]. More recently our group showed that ΔFosB, is increased in the NAc of aggressive mice compared to non-aggressive mice suggesting it may play a role in aggression reward (H. Aleyasin, S. Golden, et al: Abstract No. 67.05, 2016 San Diego, CA: Society for Neuroscience, 2016). Further studies are needed to test this hypothesis. In addition, 95% of the neurons in the NAc are GABAergic medium spiny neurons (MSN), which express either D1 or D2 dopamine receptors, however, they play opposing roles in the control of positive and negative reward. It will be important to determine how D1 versus D2 NAc MSNs control aggression intensity and aggression-seeking behavior.
Lateral habenula
The lateral habenula is a major hub connecting key nodes within the reward circuit to signal positive and negative valence. Although little is known about its role in aggressive behavior, a recent study by our group confirmed GABAergic neurons from basal forebrain regions including the NAc, lateral septum and diagonal band project directly to LHb [52]. Utilizing optogenetic strategies, we found that stimulation of these GABAergic terminals suppresses LHb firing and increases the intensity of aggressive behavior, whereas optogenetic inhibition attenuates aggressive behavior. Moreover, to study the role of these projection neurons in the rewarding component of aggressive behavior, we utilize a modified conditioned place preference protocol, described in Box 1, to measures the valence of inter-male social interaction. Activation of basal forebrain projections to the LHb during the test session increases preference for the aggression-associated context in non-aggressive mice, which naturally avoid such context, whereas inhibition of these neurons during the test reduces preference or promotes aversion in aggressor mice. These studies provided the first direct evidence that LHb neurons modulate the valence of aggressive social interactions and aggression-seeking behavior. Further analysis using fiber photometry to monitor neuronal activity of the LHb in real-time shows a sharp decrease in activity when the aggressor subordinates the intruder. Moreover, the intensity of the initial intruder-evoked neuronal activity decreases in aggressors as they successively win in an RI test. This seems to parallel the increase in the intensity of attack observed across days (M Flanigan, H Aleyasin, et al: Abstract No. 453.02, 2016. San Diego, CA: Society for Neuroscience, 2016). These studies clearly implicate LHb activity in aggressive behavior (For an extensive review on the topic please see Flanigan et al [53]). It is also known that LHb neurons send dense projections throughout the reward circuit including midbrain monoaminergic nuclei involved in reward and motivation, such as VTA and DRN, that have not been studied in the context of aggression yet (shown in dashed lines in figure 1). Future studies are required to elucidate the role of LHb and its outputs to monoaminergic nuclei to modulate aggression-associated reward.
Conclusion
While aggression has evolved to protect resources and resolve competition, excessive or pathological aggression poses serious negative risks to society. Despite this, aggression remains relatively understudied compared to other innate and emotional behaviors like fear and reward [54]. Classic lesion and electrical stimulation methods have informed us of the broad importance of various brain nuclei in promoting aggression, but these studies have been limited by poor spatial resolution. The recent emergence of cell-type and circuit-specific manipulations, viral tracing, and in vivo recording methods has allowed for a more precise dissection the neuronal ensembles controlling aggressive behavior. Using methods such as optogenetics, chemogenetics, virus-mediated tracing, and fiber photometry, researchers have delineated neuronal subtypes in the hypothalamus, the limbic system, and the mesolimbic reward system that play roles in distinct behavioral processes like aggression initiation, defensive behavior, and aggression-seeking. The continued development and application of methods to parse translationally relevant aspects of aggression is particularly important for deriving a basic understanding of the mechanisms involved in human psychopathology involving excessive violence and aggression. Ultimately, it is critical that we move beyond frontline treatment strategies aimed largely at patient containment to develop rationally designed treatments that target true disease mechanisms underlying aggression.
Summary
Here we present a summary of various studies that show how brain reward regions, including the NAc, promotes aggression-seeking behavior via negative modulation of the activity of the LHb. We also provide a description of how emotion-regulating brain areas such as lateral septum and medial amygdala influence VMHvl, the hypothalamic attack area, to modulate initiation and execution of aggressive behavior. Our evolving understanding of the cell-type specific neuronal activity within key microcircuits of the brain’s reward regions is helping to shed new light on how aggression is controlled in a range of social and emotional contexts.
Figure 2.
Behavioral tests to evaluate aggression-seeking behavior: A: Aggression conditioned place preference apparatus. B: Operant aggression-seeking box (Courtesy of Dr Sam Golden and Dr. Yavin Shaham, NIH).
Acknowledgments
The preparation of this manuscript and research from our laboratory are supported in part by Mental Health grants RO1 MH090264, P50 MH096890 (SJR), 905 P50 AT008661-01 (SJR) and and F31 MH111108-01A1 (MEF). We thank Dr. Aki Takahashi, Dr. Sam A Golden, and Dr. Lena Khibnik for their invaluable advice and help with the preparation of the manuscript.
Footnotes
Conflict of interest statement
Authors have no conflict of interest to declare.
References and recommended reading
Papers of particular interest, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70:653–662. doi: 10.4088/JCP.08m04150. [DOI] [PubMed] [Google Scholar]
- 3.Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15:1351–1371. doi: 10.1017/S146114571100201X. [DOI] [PubMed] [Google Scholar]
- 4.Khushu A, Powney MJ. Haloperidol for long-term aggression in psychosis. Cochrane Database Syst Rev. 2016;11:CD009830. doi: 10.1002/14651858.CD009830.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, Shaham Y. Compulsive Addiction-like Aggressive Behavior in Mice. Biol Psychiatry. 2017;82:239–248. doi: 10.1016/j.biopsych.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, Russo SJ, Shaham Y. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2017;16:44–55. doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinsberger M, Sommer J, Kaminer D, Holtzhausen L, Weierstall R, Seedat S, Madikane S, Elbert T. Perpetuating the cycle of violence in South African low-income communities: attraction to violence in young men exposed to continuous threat. Eur J Psychotraumatol. 2016;7:29099. doi: 10.3402/ejpt.v7.29099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobach A, Schaal S, Elbert T. Combat high or traumatic stress: violent offending is associated with appetitive aggression but not with symptoms of traumatic stress. Front Psychol. 2014;5:1518. doi: 10.3389/fpsyg.2014.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May ME. Aggression as positive reinforcement in people with intellectual disabilities. Res Dev Disabil. 2011;32:2214–2224. doi: 10.1016/j.ridd.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Porges EC, Decety J. Violence as a source of pleasure or displeasure is associated with specific functional connectivity with the nucleus accumbens. Front Hum Neurosci. 2013;7:447. doi: 10.3389/fnhum.2013.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer J, Hinsberger M, Elbert T, Holtzhausen L, Kaminer D, Seedat S, Madikane S, Weierstall R. The interplay between trauma, substance abuse and appetitive aggression and its relation to criminal activity among high-risk males in South Africa. Addict Behav. 2017;64:29–34. doi: 10.1016/j.addbeh.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasa OAE. Aggression: Appetite or aversion?—An ethologist’s viewpoint. Aggr Behav. 1976;2:213–222. [Google Scholar]
- 13.Chester DS, DeWall CN. The pleasure of revenge: retaliatory aggression arises from a neural imbalance toward reward. Soc Cogn Affect Neurosci. 2016;11:1173–1182. doi: 10.1093/scan/nsv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic response in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol. 1928;84:490–515. [Google Scholar]
- 16.Levison PK, Flynn JP. The objects attacked by cats during stimulation of the hypothalamus. Anim Behav. 1965;13:217–220. doi: 10.1016/0003-3472(65)90037-0. [DOI] [PubMed] [Google Scholar]
- 17.Toth M, Fuzesi T, Halasz J, Tulogdi A, Haller J. Neural inputs of the hypothalamic “aggression area” in the rat. Behav Brain Res. 2010;215:7–20. doi: 10.1016/j.bbr.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 2016;19:596–604. doi: 10.1038/nn.4264. The study demonstrates activation of neurons of hypothalamic attack area in VMHvl during an operant aggression-seeking task. Optogenetic stimulation of these neurons accelerated and chemogenetic inhibition suppressed aggression seeking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, Fu M, Han BX, Wang F. Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social- Fear Circuit. Neuron. 2016;92:739–753. doi: 10.1016/j.neuron.2016.10.015. They developed and used of a novel technology to parse out different neuronal ensembles in hypothalamic attack area in VMHvl that are separately activated in response to social-fear and aggressive interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert DJ, Walsh ML, Gorzalka BB, Mendelson S, Zalys C. Intermale social aggression: suppression by medial preoptic area lesions. Physiol Behav. 1986;38:169–173. doi: 10.1016/0031-9384(86)90151-4. [DOI] [PubMed] [Google Scholar]
- 23.Nakata M, Sano K, Musatov S, Yamaguchi N, Sakamoto T, Ogawa S. Effects of Prepubertal or Adult Site-Specific Knockdown of Estrogen Receptor beta in the Medial Preoptic Area and Medial Amygdala on Social Behaviors in Male Mice. eNeuro. 2016:3. doi: 10.1523/ENEURO.0155-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHenry JA, Robison CL, Bell GA, Vialou VV, Bolanos-Guzman CA, Nestler EJ, Hull EM. The role of DeltafosB in the medial preoptic area: Differential effects of mating and cocaine history. Behav Neurosci. 2016;130:469–478. doi: 10.1037/bne0000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–522. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- 26.Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan G, Preston-Campbell RN, Moeller SJ, Steinberg JL, Lane SD, Maloney T, Parvaz MA, Goldstein RZ, Alia-Klein N. Reward vs. Retaliation-the Role of the Mesocorticolimbic Salience Network in Human Reactive Aggression. Front Behav Neurosci. 2016;10:179. doi: 10.3389/fnbeh.2016.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69:1153–1159. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Cadiz-Moretti B, Abellan-Alvaro M, Pardo-Bellver C, Martinez-Garcia F, Lanuza E. Afferent and efferent projections of the anterior cortical amygdaloid nucleus in the mouse. J Comp Neurol. 2017;525:2929–2954. doi: 10.1002/cne.24248. [DOI] [PubMed] [Google Scholar]
- 30.Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- *31.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. The study identifies a glutamatergic and a GABAergic projection from the dorsal subdivision of medial amygdala to the hypothalamic attack area in VMHvl that differentially modulate aggression. Stimulation of glutamatergic neurons stops aggression and triggers self-grooming behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger EK, Burke KJ, Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10:453–462. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel A, Edinger H, Koo A. Suppression of attack behavior in the cat by the prefrontal cortex: role of the mediodorsal thalamic nucleus. Brain Res. 1977;127:185–190. doi: 10.1016/0006-8993(77)90392-4. [DOI] [PubMed] [Google Scholar]
- 34.Kolb B, Nonneman AJ. Frontolimbic lesions and social behavior in the rat. Physiol Behav. 1974;13:637–643. doi: 10.1016/0031-9384(74)90234-0. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One. 2014;9:e94657. doi: 10.1371/journal.pone.0094657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biro L, Toth M, Sipos E, Bruzsik B, Tulogdi A, Bendahan S, Sandi C, Haller J. Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: relationship with species-typical and deviant aggression. Brain Struct Funct. 2017;222:1861–1875. doi: 10.1007/s00429-016-1312-z. [DOI] [PubMed] [Google Scholar]
- *38.Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, Chang BC, Bernstein H, You X, Feng JE, et al. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr Biol. 2016;26:593–604. doi: 10.1016/j.cub.2015.12.065. The study identifies lateral septum (LS) inhibitory neurons directly project to VMHvl to suppress attack-excited neurons. Optogenetic stimulation of this pathway suppresses aggressive behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo D, Patrick CJ, Kennealy PJ. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and its Comorbidity with other Clinical Disorders. Aggress Violent Behav. 2008;13:383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niederkofler V, Asher TE, Okaty BW, Rood BD, Narayan A, Hwa LS, Beck SG, Miczek KA, Dymecki SM. Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior. Cell Rep. 2016;17:1934–1949. doi: 10.1016/j.celrep.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ, Gardier AM, Blanco C, Hen R, Ahmari SE. Distinct Circuits Underlie the Effects of 5-HT1B Receptors on Aggression and Impulsivity. Neuron. 2015;86:813–826. doi: 10.1016/j.neuron.2015.03.041. The study demonstartes the necessity of postsynaptic serotonergic receptor 5-HT1B in the forebrain during development for modulation of aggressive behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May ME, Kennedy CH. Aggression as positive reinforcement in mice under various ratio- and time-based reinforcement schedules. J Exp Anal Behav. 2009;91:185–196. doi: 10.1901/jeab.2009.91-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranaldi R. Dopamine and reward seeking: the role of ventral tegmental area. Rev Neurosci. 2014;25:621–630. doi: 10.1515/revneuro-2014-0019. [DOI] [PubMed] [Google Scholar]
- 44.Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 45.Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Gingrich JA, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry. 2014;19:688–698. doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gan G, Sterzer P, Marxen M, Zimmermann US, Smolka MN. Neural and Behavioral Correlates of Alcohol-Induced Aggression Under Provocation. Neuropsychopharmacology. 2015;40:2886– 2896. doi: 10.1038/npp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nehrenberg DL, Sheikh A, Ghashghaei HT. Identification of neuronal loci involved with displays of affective aggression in NC900 mice. Brain Struct Funct. 2013;218:1033–1049. doi: 10.1007/s00429-012-0445-y. [DOI] [PubMed] [Google Scholar]
- 49.Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety--involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37:1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl) 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 51.Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. The study identifies a functional GABAergic projection from the basal forebrain (BF) to the lateral habenula (LHb) that bi-directionally controls the valence of aggressive interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flanigan M, Aleyasin H, Takahashi A, Golden SA, Russo SJ. An emerging role for the lateral habenula in aggressive behavior. Pharmacol Biochem Behav. 2017;162:79–86. doi: 10.1016/j.pbb.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 55.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 56.Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology (Berl) 2002;163:459–466. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]