Abstract
The Toll pathway is a central regulator of antifungal immunity in insects. In mosquitoes, the Toll pathway affects infections with the fungal entomopathogen, Beauveria bassiana, which is considered a potential mosquito biopesticide. We report here the use of B. bassiana strain I93–825 in Anopheles gambiae to analyze the impact of Toll pathway modulation on mosquito survival. Exposure to a narrow dose range of conidia by direct contact decreased mosquito longevity and median survival. In addition, fungal exposure dose correlated positively and linearly with hazard ratio. Increased Toll signaling by knockdown of its inhibitor, cactus, decreased survivorship of uninfected females, increased mosquito survival after low dose B. bassiana exposure, but had little effect following exposure to higher doses. This observed tradeoff could have implications for development of B. bassiana as a prospective vector control tool. On the one hand, selection for small increases in mosquito immune signaling across a narrow dose range could impair efficacy of B. bassiana. On the other hand, costs of immunity and the capacity for higher doses of fungus to overwhelm immune responses could limit evolution of resistance.
Keywords: innate immunity, host-pathogen interaction, insect vector, Beauveria bassiana, entomopathogenic fungus
1. Introduction
Control of mosquito vectors continues to be contingent on widespread use of chemical insecticides through insecticide treated bed nets (ITNs) and indoor residual spraying (IRS). However, resistance to widely used public health insecticides is spreading within many vector populations, jeopardizing the effectiveness of these control tools (Benelli and Beier, 2017; Hemingway and Ranson, 2000; Ranson et al., 2011; Ranson and Lissenden, 2016). In light of these circumstances, research into the development of alternative vector control measures has been a topic of continuing interest (Barreaux et al., 2017; Hemingway et al., 2016; Kamareddine, 2012; Thomas et al., 2012). Entomopathogenic fungi have displayed promise as a means to provide such alternative control measures as novel biopesticides (Blanford et al., 2005; Scholte et al., 2005).
Fungal strains of species such as Beauveria bassiana and Metarhizium anisopliae are virulent to Anopheles gambiae, the major vector of malaria in sub-Saharan Africa (Kikankie et al., 2010; Mnyone et al., 2009; Scholte et al., 2003). These fungi are attractive as potential vector control agents as they have been shown to reduce factors related to mosquito physiology that affect disease transmission such as feeding propensity (Blanford et al., 2011; Howard et al., 2010b; Scholte et al., 2006), fecundity (Blanford et al., 2011; Scholte et al., 2006), flight (Blanford et al., 2011), host seeking (George et al., 2011), and vector competence (Blanford et al., 2005), as well as maintaining efficacy against both insecticide-resistant and non-resistant mosquito strains (Blanford et al., 2011; Farenhorst et al., 2009; Howard et al., 2010a). In addition, the use of these agents has the added benefit of horizontal transmission, as seen by transmission through mating from infected males to uninfected females, affecting a population larger than originally exposed (García-Munguía et al., 2011). Formulations of infective conidia can be stable for long periods of time and remain effective (Blanford et al., 2012a), allowing them to be produced in large batches, shipped, and stored for later use. These infective conidia can be applied in a similar fashion as chemical insecticides through IRS (Heinig et al., 2015; Mnyone et al., 2010), or by application to a variety of substrates that can target host searching mosquitoes (Sternberg et al., 2016, 2014), or resting sites for blood-fed mosquitoes (Farenhorst et al., 2008; Lwetoijera et al., 2010; Mnyone et al., 2012; Scholte et al., 2005).
B. bassiana conidia attach to insect cuticles, where they germinate and penetrate this external barrier and proliferate within the mosquito (reviewed in Mascarin and Jaronski, 2016). This infection can progress in a matter of 3 to 14 days before host death, with both fungal dose and isolate virulence playing an important role in the time of death (Bell et al., 2009; Blanford et al., 2012b, 2011; Farenhorst and Knols, 2010; Heinig and Thomas, 2015; Mnyone et al., 2009; Valero-Jiménez et al., 2014). However, insects have the ability to evolve an increased tolerance and/or resistance to pathogens, including entomopathogenic fungi. For example, laboratory-based selection experiments in the greater wax moth, Galleria mellonella, lead to specific resistance to B. bassiana through continuous exposure to sublethal doses of conidia in a mere 25 generations (Dubovskiy et al., 2013). The mechanism of enhanced resistance in such a short period of time was linked to augmentations of front line defenses such as cuticle strength, phenoloxidase activity, and antimicrobial peptide (AMP) expression, highlighting the critical role that immune activation state can have on the survivability to fungal infections (Dubovskiy et al., 2013).
The Toll pathway has an important role in antifungal immunity within insects and, thus, alterations in basal activation of this pathway can influence an insect’s ability to resist B. bassiana (Shin et al., 2005). The Toll pathway is characterized by an extracellular protease cascade and an intracellular signal transduction pathway (reviewed in Valanne et al., 2011). This pathway culminates in the cleavage and degradation of a major pathway inhibitor, Cactus, whereby the NF-κB transcription factor, REL1, is released to translocate into the nucleus and affect gene transcription. Several pathogen killing mechanisms are controlled by the Toll pathway, including AMPs, transcriptional upregulation of components of the complement-like pathway, as well as negative regulators of the melanization cascade (Frolet et al., 2006).
Changes in Toll pathway activation, indeed, have been shown to affect a mosquito’s ability to overcome a B. bassiana infection. Previous studies show that increased expression levels of REL1 boosts basal immunity and positively affected the ability of Aedes aegypti to withstand B. bassiana infections (Bian et al., 2005; Shin et al., 2006, 2005). Therefore, the Toll pathway could constitute a selection target for resistance to B. bassiana. However, the intersection between Toll signaling and B. bassiana exposure dose is less clear. In this study, we describe a trade-off between activation of Toll immune signaling and survival in the context of exposure of An. gambiae to various doses of B. bassiana conidia.
2. Materials and Methods
2.1 Mosquito rearing and maintenance
The An. gambiae G3 strain was reared at standard rearing conditions (An et al., 2011). Adult females 2–4 days old (n = 35 per treatment per replicate) were separated, placed in experimental cups (straight-walled paper cans, 1 pint volume, Neptune Paper Products Inc., Fort Lee, NJ, USA), and fed sugar water (8% D-fructose, 2.5 mM 4-aminobenzoic acid, Sigma-Aldrich, St. Louis, MO, USA) ad libitum (Beier et al., 1994).
2.2 Fungal immune challenge
B. bassiana strain I93-825 conidia were formulated in a mix of mineral oils (80:20 Isopar:Ondina) as described previously (Blanford et al., 2005) with the conidia concentration adjusted to 1.24 × 109 conidia/ml. The resulting conidia formulation was spread on 15 cm filter paper (VWR, Radnor, PA, USA) with volumes adjusted to 4 ml using 80:20 Isopar:Ondina. For all experiments, the following exposure doses were used: (i) low dose = 7.02 × 106/cm2, medium dose = 1.4 × 107/cm2, high dose = 2.81 × 107/cm2. An oil-only formulation was used as the negative control. After drying for 1 day at room temperature, the filter papers were adhered to WHO exposure cones (WHO Collaborating Centre, Universiti Sains Malaysia (USM), Penang, Malaysia), which were modified through the lateral insertion of a 1-inch, mesh-covered straw to allow airflow during mosquito aspirations. Adult females were aspirated into the modified WHO exposure cones through the top of the cone and forced to rest on filter paper for 30 min at room temperature. After exposure, mosquitoes were returned to the experimental cups and kept using the standard rearing conditions cited above. The effects of fungal exposure dose on survival of naive mosquitoes were tested using six biological replicates, with 35 adult female mosquitoes used for each dose and replicate. The effects of dsGFP injection, REL1knockdown (kd) and Cactuskd on female mosquito survival across the fungal exposure dose range were tested using three biological replicates, with 35 adult mosquitoes used for each dose and replicate.
2.3 Mortality analysis
After fungal exposure, survival was monitored daily until mortality reached 100 %. The resulting data were analyzed and graphed using Kaplan–Meier and compared using the log-rank (Mantel–Cox) test and Hazard Ratios (HRs). Lethal time 50 (LT50, the time point after exposure where 50 % of mosquitoes in a given treatment had died) was evaluated statistically using one-way or two-way ANOVA followed by Tukey’s multiple comparisons post-test. All statistical analyses were performed using GRAPHPAD PRISM software v.6 (GraphPad Software Inc., La Jolla, CA, USA).
2.4 Total RNA extraction
Female mosquitoes were flash-frozen in liquid nitrogen and stored at −80 °C. Samples were homogenized in 200 μl Trizol (Ambion, Life Technologies, Carlsbad, CA, USA), and total RNA was extracted using a final volume of 1 ml Trizol according to the manufacturer’s instructions. Pellets were air dried and suspended in 100 μl RNase-free water (Fisher Scientific, Waltham, MA, USA). RNA was further purified with an RNeasy mini kit (Qiagen, Valencia, CA, USA) using the manufacturer’s protocol and eluted in 50 μl RNase-free water. RNA integrity was verified by agarose gel electrophoresis and concentration determined by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA).
2.5 cDNA synthesis
An. gambiae cDNA was synthesized from 100 ng purified total RNA with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), using oligo(dT) and random hexamer primers, in a total reaction volume of 20 μl, following the manufacturer’s protocol.
2.6 dsRNA synthesis
DNA templates for dsRNA synthesis were generated by two rounds of PCR from cDNA of 3–4 day-old female sugar-fed mosquitoes. The first-round PCR was performed in a 25 μL total reaction volume with 100 ng of cDNA as template, using the following primer sets for REL1, Cactus, and GFP (T7 5′ extension is underlined). AGAP009515_REL1_F, 5′-GAATTAATACGACTCACTATAGGGAGAATCA ACAGCACGACGATGAG-3′; AGAP009515_REL1_R, 5′-GAATTAATACGACTCACTATAGGGAGATCGAAAAAGCGCA CCTTAAT-3′; AGAP007938_Cactus_F, 5′-GAATTAATACGACTCACTATAGGGAGAGTCCG CTCTACACATCAGCA-3′; AGAP007938_Cactus_R, 5′-GAATTAATACGACTCACTATAGGGAGACCGTTCGGGTTAATGATGAC-3′; GFP_F, 5′-TAATACGACTCACTATAGGGAGTTC ACCTTGATGCCGTTC-3′; GFP_R,5′-TAATACGACTCACTATAGGGCACATGAAGCAGCA CGACTT-3′. Resulting PCR products were purified by gel extraction (QIAquick Gel Extraction Kit; Qiagen, Hilden, Germany) and eluted in 30 μl Milli-Q® purified water. The second-round PCR was performed using 1 μl of first-round PCR product as template in a 50 μl reaction volume using the following T7 primer: T7_F/R, 5′-TAATACGACTCACTATAGG G-3′. The second-round PCR product was precipitated with 1 volume of isopropanol and the resulting pellet resuspended in 50 μl Milli-Q purified water. DsRNA synthesis and purification was performed with the AmpliScribe T7-Flash transcription kit (Epicentre, Madison, WI, USA) using 1 μg of second-round purified PCR product following manufacturer’s protocol, and the dsRNA after final precipitation was resuspended in RNase-free water at a concentration of 3.0 μg/μL.
2.7 dsRNA injection
Adult females (n = 35 per treatment and replicate) were anaesthetized under a constant flow of CO2 (5 L/min) using a benchtop Flowbuddy™ regulator (Genesee Scientific, San Diego, CA, USA). Mosquitoes were injected with 69 nl of dsRNA solution (210 ng total/mosquito) under the wing base using a nanoinjector (Nanoject II, Drummond Scientific, Broomall, PA, USA). Injected females were kept at standard rearing conditions (see above) until exposed to B. bassiana conidia 3 days post injection.
2.8 Real Time-quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA extraction and template cDNA was prepared from female mosquitoes (n = 8 per treatment and replicate) as described in sections 2.4 and 2.5 above. RT-qPCR was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) according to the manufacturer s protocol with 2 μl of 1:2 diluted cDNA as template for each 20 μL volume reaction. RT-qPCRs were executed on the StepOne Plus RT-PCR System and analyzed with the StepOne software 2.0 (Life Technologies, Carlsbad, CA, USA) with the following amplification protocol: an initial cycle of 5 min at 95 °C, 40 cycles of 15 s at 95 °C, 1 min at 59 °C and 1 min at 72 °C (detection). Primers were as follows: rpS7_F, 5′-CGCTATGGTGTTCGGTT CC-3′; rpS7_R, 5′-TGCTGCAAACTTCGG-3′; REL1_kd_F 5′-TCAACAGATGCCAAAAGAG GAAAT-3′; REL1_ kd_R, 5′-CTGGTTGGAGGGATTGTG-3′; Cactus_ kd_F, 5′-AATCTGGGCCTGATGGACA-3′; Cactus_ kd_R 5′-ACTGCCAGGTGCAGTTGAGT-3′.
To test for potential changes in transcription upon fungal infection, female mosquitoes (n = 8 per treatment and biological replicate) were exposed to fungal conidia (see section 2.2 above), and flash frozen in liquid nitrogen at 1, 2, 4, and 6 days post exposure (dpe). RT-qPCR was used to determine expression of An. gambiae REL1 (AGAP009515) and Cactus (AGAP007938) transcripts relative to the housekeeping gene 40S ribosomal Protein S7 (rpS7, AGAP010592). Fold change was assessed using a modification of the delta delta threshold cycle (ΔΔCt) method (Pfaffl, 2001), taking into account primer efficiencies (Fig. S1). RpS7 expression was used as reference and unexposed treatments as calibrator conditions. RT-qPCRs were performed with three technical replicates using cDNA templates collected from four (REL1) and five (Cactus) biological replicates.
To test for gene knockdown after dsRNA treatment, female mosquitoes (n = 8 per treatment and replicate) were collected 3 days post dsRNA injection. Fold change in expression was assessed using a modification of the ΔΔCt method (Pfaffl, 2001) method, taking into account the primer efficiencies (Fig. S1). RpS7 expression was used as reference and dsGFP treatments as calibrator conditions. RT-qPCRs were performed in triplicate with three biological replicates.
3. Results
3.1 Time course of fungal-induced mortality
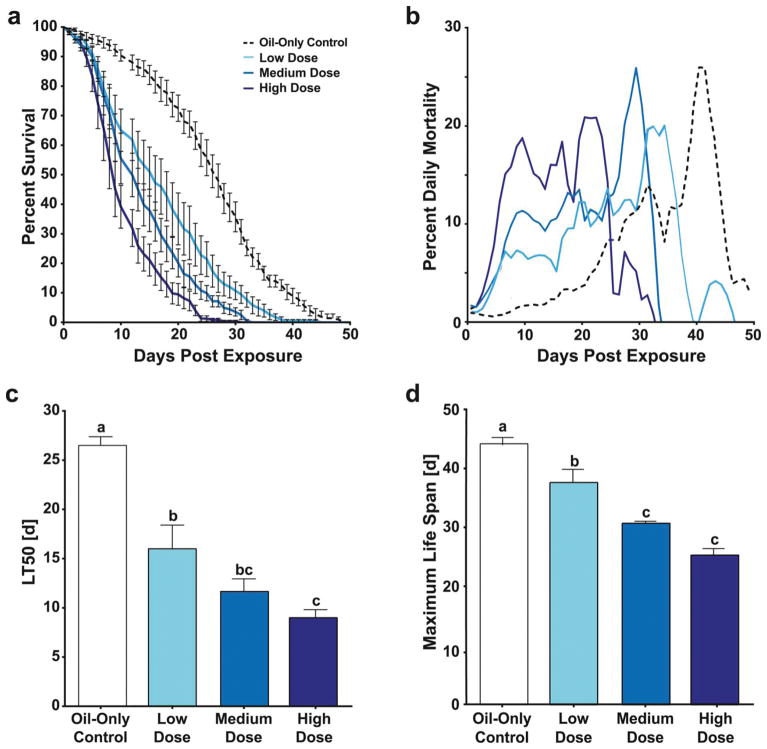
To determine the impact of B. bassiana strain I93-825 on mosquito mortality, female An. gambiae mosquitoes were exposed to a high dose of B. bassiana conidia (2.81 × 107/cm2 filter paper), and the number of dead mosquitoes per treatment was recorded daily until all mosquitoes in the experiment were dead. The resulting survival curves of all treatment groups were sigmoidal (Fig. 1A; individual biological replicates Fig. S2). Oil-only control treated mosquitoes had an average maximum life span of 44.1 ± 1.3 (SE) days and a LT50 of 26.5 ± 0.9 (SE) days. Daily percent mortalities were below 5 % for the first three weeks of data collection, resulting in an average of 70 % survival within this treatment at 21 days (Fig. 1B).
Figure 1. Survival following exposure to B. bassiana I93-825 in adult, female An. gambiae.
(a) Survival curves and corresponding (b) daily mortalities of mosquitoes after exposure to B. bassiana strain I93-825 at indicated doses. (c) Comparison of LT50 and (d) maximum mosquito lifespan after B. bassiana exposures. Lettering denotes statistically significant differences between treatments (One-Way ANOVA with Tukey’s post test, P < 0.05). Data are the combination of six biological replicates (Fig. S2), and are presented as mean ± 1 SEM.
Mosquitoes exposed to the high conidial dose of B. bassiana were nine times more likely to die as compared to oil only controls (log-rank test, P < 0.0001; Hazard Ratio [HR] = 9.035). Their LT50 (9.0 ± 0.8 days) and maximum lifespan (25.5 ± 1.4 days) were significantly shorter as those observed for oil-only controls (Fig. 1C, D; One-way ANOVA P < 0.0001; Tukey’s posttest P < 0.0001 for both). Daily mortality after high dose fungal exposures was strongly elevated between 6–25 dpe, with an average of 17 % daily mortality across this time interval (Fig. 1B). In comparison, oil-only control treatments did not reach this percent daily mortality until 40 dpe (Fig. 1B).
3.2 Dose dependence of the mosquito-killing phenotype
To investigate whether the life-shortening phenotype of B. bassiana was dose-dependent, we assessed survival after exposure to low (7.02 × 106/cm2 filter paper) and medium (1.4 × 107/cm2 filter paper) doses. Comparison of the survival curves among all four treatment groups revealed significant differences (log rank test; df = 3, χ2 = 314.5, P < 0.0001), with each fungal exposure concentration significantly decreasing mosquito survival over time (Fig. 1A, individual biological replicates Fig. S2). HRs of conidia-exposed mosquitoes as compared to oil-only controls positively correlated with dose (Table 1, R2 = 0.9973; P = 0.0013). Interestingly, while fungal exposure dose strongly affected the amplitude of increase in daily mortality, the onset of increased mortality rate remained the same, and was first observed between 7–8 dpe for all fungal treatments (Fig. 1B). LT50 of fungal treatments decreased with increasing conidial dose (one-way ANOVA, df = 3, F = 26.71, P < 0.0001 with Tukey’s multiple comparison test for pairwise analyses; Fig. 1C). Likewise, maximum mosquito lifespan negatively correlated with B. bassiana dose (one-way ANOVA, df = 3, F = 26, P < 0.0001 with Tukey’s multiple comparison test for pairwise analyses, Fig. 1D). Both, LT50 and maximum lifespan correlated negatively with dose, and both relationships fitted a linear regression model, with R2 of 0.7966 and 0.9343, respectively.
Table 1.
Hazard Ratios of mosquitoes exposed to increasing conidial doses
| Fungal exposure dose | Hazard Ratio* (mean ± 1 SE) |
|---|---|
| Low | 2.183 ± 0.2767 |
| Medium | 2.901 ± 0.3525 |
| High | 3.702 ± 0.4542 |
as compared to oil-only treatment
3.3 Transient increase in expression of Toll pathway components after fungal infection
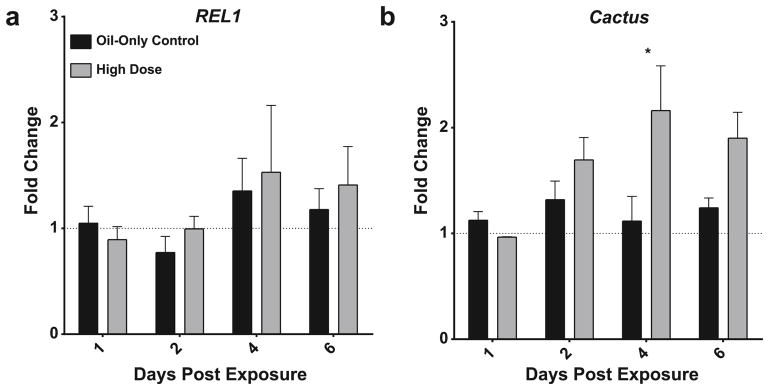
To determine whether B. bassiana infection in An. gambiae affects the Toll pathway, we initially tested for changes in the expression profiles of REL1 and Cactus using RT-qPCR. Transcript levels of REL1 following exposure to a high dose of B. bassiana conidia were determined by RT-qPCR with primer sequences specific to the 5′ sequence common to both REL1 alternative splice isoforms at 1, 2, 4, and 6 dpe.
REL1 expression did not change after B. bassiana exposure as compared to oil-only treatments, and in addition remained steady during the time course of the experiment. Thus, neither infection nor age of the sampled mosquitoes impacted REL1 transcript levels (Fig. 2A; individual biological replicates are shown in Fig. S3; Two-way ANOVA, treatment, df = 1, F = 0.4599, P = 0.4434; time, df = 3, F = 0.9656, P = 0.5117; interaction, df = 3, F = 0.2424, P = 0.8650). Cactus expression also remained constant over time in oil-only treated mosquitoes. However, Cactus expression increased significantly and transiently after fungal exposure, with a maximum two-fold increase observed at 4 dpe (Fig. 2B; Two-way ANOVA, treatment, df = 1, F = 11.54, P = 0.0043; time, df = 3, F = 1.363, P = 0.2947; interaction, df = 3, F = 2.87, P = 0.0740; Sidak’s multiple comparison test, P = 0.0099).
Figure 2. Time course of REL1 and Cactus expression following B. bassiana exposure.
Relative expression of An. gambiae REL1 and Cactus transcripts after exposing female mosquitoes to a high dose of B. bassiana strain I93-825. Graphs depict mean transcript levels at 1, 2, 4, and 6 dpe for oil only controls (black) and high dose, B. bassiana-exposed mosquitoes (grey). RT-qPCR results were analyzed using rpS7 as the reference gene and untreated mosquitoes collected at 0 dpe as calibrator condition. Statistically significant differences are indicated by asterisks (Wilkoxon signed-rank test, Sidak’s post test, P < 0.05). Data are presented as mean ± 1 SEM from four (REL1) and five (Cactus) biological replicates; individual replicates are shown in Fig. S3.
3.4 REL1kd decreases survivorship following fungal challenge in a dose-dependent manner
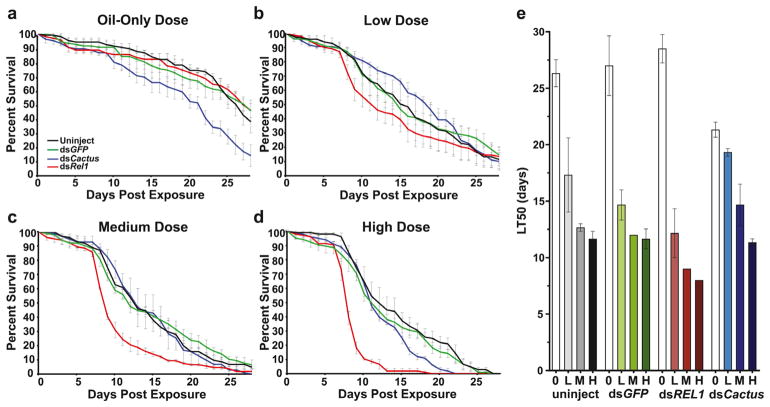
To investigate whether Toll signaling is activated after B. bassiana exposure and does limit B. bassiana-induced pathology, we used an RNAi-based silencing approach to inhibit the Toll pathway by depleting REL1 prior to fungal exposure (Fig. 3, S4 for percent knockdown, S5 for individual replicates). Mosquitoes were injected with dsGFP or dsREL1 and exposed three days post injection to no (oil-only control), low, medium, and high doses of B. bassiana conidia.
Figure 3. Survival of female dsREL1- or dsCactus-injected mosquitoes following exposure to B. bassiana I93-825.
(a–d) Survival curves of mosquitoes after exposure to B. bassiana strain I93-825 at indicated doses. At each dose, curves represent survival over time of the following treatment groups: black, no injection; green, dsGFP-injected; blue, dsCactus-injected; red, dsREL1-injected. (e) LT50 after B. bassiana exposures following no injection (uninject), dsGFP-, dsREL1-, and dsCactus injection, respectively, after exposure to oil only control (O), low (L), medium (M), and high (H) dose exposures. Data were combined from three biological replicates (Fig. S5) and are presented as mean ± 1 SEM.
Both, the fungal dosage, as well as dsRNA injection treatment significantly affected LT50 (Two-way ANOVA, Table 2). In the absence of fungal infection, injection of dsREL1 did not change mosquito survival as compared to dsGFP-injected mosquitoes or uninjected controls (log-rank test, dsREL1/uninjected P = 0.2145; dsGFP/uninjected, P = 0.3881; see Table 3 for HRs).
Table 2.
Two-way ANOVA, dsGFP vs. dsREL1 vs. dsCactus LT50
| ANOVA | df | F | P Value |
|---|---|---|---|
| Interaction | 6 | 6.773 | 0.0003 |
| Dose | 3 | 85.2 | < 0.0001 |
| dsRNA | 2 | 3.549 | 0.0447 |
| Residual | 24 |
Table 3.
Impact of dsRNA injection on hazard ratios with and without fungal exposure
| Treatment | Oil-Only* | Low* | Medium* | High* | |
|---|---|---|---|---|---|
| dsGFP | Uninjected | 0.729 ± 0.599 | 1.018 ± 0.224 | 1.020 ± 0.177 | 0.873 ± 0.598 |
| dsREL1 | Uninjected | 0.723 ± 0.244 | 1.061 ± 0.250 | 1.753 ± 0.167 | 2.848 ± 0.371 |
| dsCactus | Uninjected | 1.733 ± 0.312 | 1.124 ± 0.405 | 1.010 ± 0.346 | 1.349 ± 0.497 |
| dsREL1 | dsGFP | 0.911 ± 0.461 | 1.361 ± 0.208 | 1.745 ± 0.291 | 2.641 ± 0.602 |
| dsCactus | dsGFP | 2.131 ± 1.187 | 1.071 ± 0.140 | 1.217 ± 0.302 | 1.325 ± 0.237 |
| dsREL1 | dsCactus | 2.147 ± 0.705 | 0.784 ± 0.227 | 0.586 ± 0.049 | 0.386 ± 0.046 |
median ± ½ range
As observed previously, in all treatment groups, mosquito survival decreased with increasing dose of conidia. However, neither injection nor the presence of non-specific dsRNA significantly altered mosquito survival curves with HRs at each dose close to 1 (Fig. 3, Table 3). Daily mortalities of dsGFP-injected mosquitoes were comparable to those of uninjected mosquitoes at all fungal exposure doses (Fig. S6). LT50 also remained similar between dsGFP-treated and uninjected mosquitoes regardless of conidial dose (Two-way ANOVA, treatment, df = 1, F = 0.4695, P = 0.5030; conidial dose, df = 3, F = 31.77, P < 0.0001; interaction, df = 3, F = 0.3192, P = 0.8113), as did maximum life span. Likewise, after low dose exposure, dsREL1-survival curves were not significantly different from dsGFP-injected survival curves (log-rank test, P = 0.1728) or uninjected control curves (log-rank test, P = 0.2471) (Fig. 3B).
However, after exposure to the medium and high dosages of conidia, dsREL1-treated mosquitoes experienced statistically significantly decreased survivorship when compared to dsGFP-treated or uninjected mosquitoes (Fig. 3C, D; log-rank test, all P < 0.0001). This decrease was largely a consequence of increased daily mortality beginning at 8 dpe in dsREL1-injected mosquitoes. DsREL1 injection affected the amplitude of increase in daily mortality, e.g. the percent daily mortality at 8 dpe was four-fold higher in dsREL1-injected compared to uninjected mosquitoes (Fig. S6). Again, the onset of this phenotype remained the same, and was first observed between 7–8 dpe for all dsRNA treatments at all fungal doses (Fig. 3).
DsREL1-injected mosquitoes exposed to higher doses of B. bassiana resulted in higher likelihood of mortality when compared to uninjected controls (HR dsREL1-injected low dose = 1.061 ± 0.251, HR dsREL1-injected medium dose = 1.753 ± 0.167, HR dsREL1-injected high dose = 2.848 ± 0.371). After a low dose exposure, LT50 decreased from 14.67 dpe in dsGFP-injected control mosquitoes to 12.2 dpe after dsREL1-injection (Fig. 3E). DsREL1-injected mosquitoes had consistently lower LT50s when compared to dsGFP-injected controls exposed to the same amount of fungus (medium dose: dsGFP-injected = 12.0 dpe, dsREL1-injected = 9.0 dpe; high dose: dsGFP-injected = 11.7 dpe, dsREL1-injected = 8.0 dpe) (Fig. 3E).
3.5 Cactus knockdown increases survivorship after low dose fungal challenge
To determine if increased basal activity of the Toll pathway can limit B. bassiana-induced pathology, we used RNAi to induce the intracellular Toll signaling cascade prior to fungal exposure through the depletion of cactus (Fig. 3, S5 for individual replicates). Mosquitoes were injected with dsCactus or dsGFP as a control treatment and subsequently exposed three days post injection to no (oil-only control), low, medium, and high doses of B. bassiana conidia.
DsCactus injection induced a 33 % decrease in Cactus transcripts (Fig. S4). In the absence of infection, Cactus knockdown was detrimental to mosquito survival, as dsCactus-depleted mosquitoes had overall significantly decreased survival rates compared to both uninjected and dsGFP-injected controls (Fig. 3A; log-rank test, P < 0.0001), with HRs of 1.7 and 2.1, respectively (Table 3). Cactus knockdown increased daily mortality, with Cactus-depleted mosquitoes experiencing a consistently higher percent mortality than all other treatments between 8 to 29 dpe, peaking first at 12 dpe and later at 27 dpe (Fig. S6A).
However, dsCactus-depleted mosquitoes had better survival rates after low dose exposure to B. bassiana as compared to both, uninjected and dsGFP-injected controls (Fig. 3D). DsCactus-depleted mosquitoes consistently displayed lower daily percent mortalities than all other treatments from 5 dpe to 17 dpe, peaking at a later stage of infection at 26 dpe (Fig. S6B). In addition, dsCactus-injected treatments also had significantly increased medium survival compared to dsGFP-injected treatments at low dose (Student’s t-test, P = 0.0274). While we did see increases in variability between biological replicates at low dose exposures, dsCactus-injected mosquitoes consistently fared better than dsGFP-injected mosquitoes, particularly prominent within the third biological replicate (Fig S5).
The dsCactus-dependent, enhanced survival observed at low dose exposures was lost after mosquitoes were exposed to a medium or high dose of B. bassiana (Fig. 3C, D). Here, dsCactus- and dsGFP-injected mosquitoes had overlapping survival curves (Fig. 3C, D), and the HR of dsCactus-depleted mosquitoes as compared to controls was close to 1 (Table 3).
4. Discussion
This study investigated the activation state of Toll immune signaling and its consequences on survival following exposure of An. gambiae to various doses of B. bassiana conidia. We find that exposure to B. bassiana across a narrow dose range has far reaching effects on mortality in these insects. Additionally, we find that decreasing the basal activation of the Toll signaling pathway through RNAi knockdown of REL1 leads to severe and significant decreases in survivorship following infection with B. bassiana. Conversely, knockdown of a key negative regulator of Toll signaling, Cactus, revealed that an increased basal level of Toll activation is beneficial following low dose exposures to this fungus. Interestingly, as fungal dosage increased, any benefits gained through preemptive Toll activation were lost. Together this study highlights the impact fungal exposure dose has on immune system activation and infection outcome in this vector species.
We find that exposure doses over a small range of conidial concentrations can have large effects on the LT50 in An. gambiae. While the highest conidial density utilized in this study was merely four fold higher than the lowest dose (2.81 × 107 vs. 7.02 × 106 conidia/cm2), we observed a strong inverse linear relationship between exposure dose and LT50. Similar relationships between B. bassiana dose and LT50, albeit using log-fold changes in dose, have been reported previously in mosquitoes, including An. gambiae and Anopheles stephensi (Bukhari et al., 2010; Dong et al., 2012; Mnyone et al., 2009), as well as the beetle Agelastica alni (Sonmez et al., 2017). However, the relationship between dose and LT50 seems to be highly variable and dependent not only on experimental conditions and the B. bassiana strain tested (Bukhari et al., 2010; Heinig et al., 2015; Mnyone et al., 2009), but also on insect species and sex (Bukhari et al., 2010; Taylor et al., 2007). Future studies will have to show whether the strong impact on LT50 of An. gambiae over a narrow dose range of B. bassiana is influenced by any or all of these factors or is a general feature of B. bassiana infection dynamics in An. gambiae.
The Toll pathway is a well-known critical negative regulator of fungal infections in mosquitoes (Shin et al., 2005). We were therefore surprised to find that at the lowest dose used in this study this immune signaling pathway does not seem to be activated by B. bassiana. This notion is supported by the following two observations: First, at low dose, dsREL1 injection does not decrease mosquito survival, suggesting that expression of anti-fungal immune factors does not occur or only occurs to levels that do not alter infection outcome. Second, knockdown of Cactus, which activates the intracellular Toll signaling cascade, even in the absence of immune challenge (Frolet et al., 2006), does increase mosquito survival rates, demonstrating the impact Toll signaling has on limiting B. bassiana induced pathology. Possible mechanisms that may contribute to this indirectly observed lack of Toll activation at low dose exposure are mosquito immune system evasion or suppression by B. bassiana. Both mechanisms are commonly employed by arthropod pathogens, e.g. Plasmodium spp. utilize both to escape nitration, encapsulation, and melanization responses (Boëte et al., 2004; Lambrechts et al., 2007; Michel et al., 2005; Molina-Cruz et al., 2015; Ramphul et al., 2015). B. bassiana is no exception, employing a variety of methods to evade host immunity, including utilization of hyphal bodies that evade immune recognition through a lack of antigenic surface compounds (Pendland et al., 1993). Beauveria is also capable of modulating insect responses to suppress a host’s immune defenses using different molecular classes of proteases and toxins (reviewed in Joop and Vilcinskas, 2016). Our data further suggest that if immune evasion/suppression is at play, it is less effective after exposure to higher doses of B. bassiana. The Toll pathway was indeed activated and limited the pathology induced by fungal infection, as evidenced by decreased survivorship after dsREL1 injection. Intriguingly, at these doses, Cactus knockdown did not impact infection outcome, suggesting that the Toll pathway was activated to its maximum level, and the loss of enhanced survival in Cactus knockdown treatments is due to overwhelming B. bassiana infection in these treatments.
A potentially significant caveat to the notion that our data suggest immune suppression/evasion by B. bassiana is the incomplete knockdown we observed for Cactus as well as REL1. While the percent knockdown reported herein is similar to levels previously reported by other investigators (Frolet et al., 2006; Garver et al., 2009), we cannot rule out that further decrease of REL1 and Cactus transcript levels may have decreased mosquito survival at low fungal doses and increased survival at higher fungal dose exposures, respectively. While efficacy of gene knockdown is notoriously difficult to translate to level of protein reduction and phenotypic penetrance (Scott et al., 2013), these technical limitations of RNAi may be overcome in future studies using technological advances in mosquito genetic manipulation, (Li et al., 2018, 2017; O’Brochta et al., 2011).
Previous studies using the same methodology employed by us have shown that in adult, female An. stephensi mosquitoes, B. bassiana load slowly increases over time after exposure to conidia increases in mosquitoes (Bell et al., 2009). Using the same strain as employed in this current study, Bell et al. observed initial increase in load at the time where survival rates decreased, suggesting that increased pathology is tightly linked to fungal growth. The mechanisms underlying the pathology induced by B. bassiana are numerous and include enzymes capable of utilizing and depleting host hemolymph of sugars and toxins such as beauvericin capable of killing host cells (reviewed in Valero-Jiménez et al., 2016).
While our data confirm that even transient genetic manipulation of the Toll pathway has strong impact on pathology of B. bassiana infection, it is currently unclear whether these effects are due to altered disease tolerance or pathogen resistance (Medzhitov et al., 2012; Raberg et al., 2009). Tolerance, the ability to survive higher loads of the pathogen, to B. bassiana infection has thus far not been observed in insects (reviewed in Lu and St. Leger, 2016). Indeed, An. gambiae mosquitoes depleted of TEP1 succumb to the infection more quickly paired with increased fungal loads, favoring the notion that the melanization immune response limits fungal growth and, thus, increases resistance. Interestingly, TEP1 expression is increased in Cactus-depleted An. gambiae (Frolet et al., 2006), providing a possible explanation for the increased survivorship we observed in Cactus-depleted mosquitoes exposed to low levels of conidia. However, this is likely not the only mechanism underlying the increased resistance to infection. Cactus depletion in adult An. gambiae changes the expression of 3 % of the protein coding genes (Garver et al., 2009), including the expression of gambicin, one of the two antifungal peptides known in An. gambiae (Vizioli et al., 2001, 2000).
An overzealous immune system can have detrimental effects on fitness, due to trade-offs in resource allocation as well as pathology induced by immune byproducts (recently reviewed by Schwenke et al., 2016). This trade-off is exemplified in our experimental system, as Cactus knockdown, and thus increased Toll signaling, increases survivorship in the presence of infection, while in the absence of infection leads to higher mortality. Indeed, reduced longevity was previously observed in An. gambiae females after Cactus knockdown (Garver et al., 2009). In addition, Cactus depletion from mosquitoes as well as D. melanogaster has additional phenotypic consequences, including melanotic tumor formation, (Frolet et al., 2006; Qiu et al., 1998), proliferation of and shifts in hemocyte subpopulations, (Qiu et al., 1998; Ramirez et al., 2014), and increased lipid droplet presence in mosquito midguts (Barletta et al., 2016). While increased melanization in a Cactus-depleted background is likely to contribute to the mosquito’s ability to fight B. bassiana infection (Yassine et al., 2012), increased melanization also reduces longevity in absence of infection (An et al., 2011). In addition, constitutive activation of the Toll pathway inhibits Akt signaling and leads to depletion of nutrient stores (DiAngelo et al., 2009), which may contribute to the decreased longevity in Cactus-depleted mosquitoes.
Nevertheless, after low fungal exposure dose, we observed increased longevity in adult female mosquitoes, suggesting that under our experimental conditions the fitness cost of a constitutively activated Toll pathway is at least partially rescued by the mosquito’s increased ability to fight infection. A more detailed analysis of fitness parameters, including biting frequency and population growth parameters will allow the quantification of the fitness tradeoffs between increased basal levels of immunity and the ability to overcome B. bassiana infection suggested by the data presented herein. In how far this observed trade-off may have implications for the development of B. bassiana as a prospective vector control tool is currently unclear. On the one hand, selection for small increases in mosquito immune signaling across a narrow dose range could impair efficacy of B. bassiana. On the other hand, costs of immunity, the capacity for higher doses of fungus to overwhelm immune responses, coupled with the ability to deliver such doses in the field (Heinig et al., 2015), as well as the use of B. bassiana in an integrated vector management approach (Sternberg and Thomas, 2017) is likely to limit evolution of resistance. The potential impact of increased basal immunity on B. bassiana infection outcome can be experimentally addressed in future studies that recapitulate abiotic field conditions and genetic variation in the mosquito host, along with a spectrum of available fungal strains (Kim et al., 2013; Pava-Ripoll et al., 2008; Peng and Xia, 2015; Xie et al., 2015).
Supplementary Material
Highlights.
Beauveria bassiana’s ability to kill mosquitoes varies across a narrow dose range.
Exposure dose has an impact on immune system activation and infection outcome.
Altering the Toll pathway changes mosquito longevity after fungal exposure.
Boosted Toll signaling reveals a trade-off between mosquito immunity and survival.
Acknowledgments
We thank all members of the Michel lab for their help with mosquito colony maintenance. The authors would like to thank the College of Veterinary Medicine Core Facilities at Kansas State University for the use of equipment for RT-qPCR-based quantifications.
Funding
This work is supported by the National Institutes of Health grant number R01AI095842 and through USDA-ARS Specific Cooperative Agreement 58-5430-4-022 (both to K.M.) and by National Institutes of Health grant number R01AI110793 (to M.B.T.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies. This is contribution 18-202-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An C, Budd A, Kanost MR, Michel K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell Mol Life Sci. 2011;68:1929–1939. doi: 10.1007/s00018-010-0543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta ABF, Alves LR, Nascimento Silva MCL, Sim S, Dimopoulos G, Liechocki S, Maya-Monteiro CM, Sorgine MHF. Emerging role of lipid droplets in Aedes aegypti immune response against bacteria and Dengue virus. Sci Rep. 2016;6:19928. doi: 10.1038/srep19928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreaux P, Barreaux AMG, Sternberg ED, Suh E, Waite JL, Whitehead SA, Thomas MB. Priorities for broadening the malaria vector control tool kit. Trends Parasitol. 2017;33:763–774. doi: 10.1016/j.pt.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier MS, Pumpuni CB, Beier JC, Davis JR. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- Bell AS, Blanford S, Jenkins N, Thomas MB, Read AF. Real-time quantitative PCR for analysis of candidate fungal biopesticides against malaria: Technique validation and first applications. J Invertebr Pathol. 2009;100:160–168. doi: 10.1016/j.jip.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G, Beier JC. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. doi: 10.1016/j.actatropica.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Bian G, Shin SW, Cheon HMM, Kokoza V, Raikhel AS. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci. 2005;102:13568–13573. doi: 10.1073/pnas.0502815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Chan BHK, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- Blanford S, Jenkins NE, Christian R, Chan BHK, Nardini L, Osae M, Koekemoer LL, Coetzee M, Read AF, Thomas MB. Storage and persistence of a candidate fungal biopesticide for use against adult malaria vectors. Malar J. 2012a;11:354. doi: 10.1186/1475-2875-11-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Jenkins NE, Read AF, Thomas MB. Evaluating the lethal and pre-lethal effects of a range of fungi against adult Anopheles stephensi mosquitoes. Malar J. 2012b;11:365. doi: 10.1186/1475-2875-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Shi W, Christian R, Marden JH, Koekemoer LL, Brooke BD, Coetzee M, Read AF, Thomas MB. Lethal and pre-lethal effects of a fungal biopesticide contribute to substantial and rapid control of malaria vectors. PLoS One. 2011;6:e23591. doi: 10.1371/journal.pone.0023591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boëte C, Paul REL, Koella JC. Direct and indirect immunosuppression by a malaria parasite in its mosquito vector. Proc R Soc L. 2004;271:1611–1615. doi: 10.1098/rspb.2004.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari T, Middelman A, Koenraadt CJM, Takken W, Knols BGJJ. Factors affecting fungus-induced larval mortality in Anopheles gambiae and Anopheles stephensi. Malar J. 2010;9:22. doi: 10.1186/1475-2875-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Morton JCJ, Ramirez JL, Souza-Neto JA, Dimopoulos G. The entomopathogenic fungus Beauveria bassiana activate Toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem Mol Biol. 2012;42:126–132. doi: 10.1016/j.ibmb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovskiy IM, Whitten MMA, Yaroslavtseva ON, Greig C, Kryukov VY, Grizanova EV, Mukherjee K, Vilcinskas A, Glupov VV, Butt TM. Can insects develop resistance to insect pathogenic fungi? PLoS One. 2013;8:e60248. doi: 10.1371/journal.pone.0060248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst M, Farina D, Scholte E, Takken W, Hunt RH, Coetzee M, Knols BGJ. African water storage pots for the delivery of the entomopathogenic fungus Metarhizium anisopliae to the malaria vectors Anopheles gambiae ss and Anopheles funestus. Am J Trop Med Hyg. 2008;78:910–916. [PubMed] [Google Scholar]
- Farenhorst M, Knols BGJ. A novel method for standardized application of fungal spore coatings for mosquito exposure bioassays. Malar J. 2010;9:27. doi: 10.1186/1475-2875-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst M, Mouatcho JC, Kikankie CK, Brooke BD, Hunt RH, Thomas MB, Koekemoer LL, Knols BGJ, Coetzee M. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc Natl Acad Sci. 2009;106:17443–17447. doi: 10.1073/pnas.0908530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-κ B-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- García-Munguía AM, Garza-Hernández Ja, Rebollar-Tellez Ea, Rodríguez-Pérez Ma, Reyes-Villanueva F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasit Vectors. 2011;4:24. doi: 10.1186/1756-3305-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Blanford S, Domingue MJ, Thomas MB, Read AF, Baker TC. Reduction in host-finding behaviour in fungus-infected mosquitoes is correlated with reduction in olfactory receptor neuron responsiveness. Malar J. 2011;10:219. doi: 10.1186/1475-2875-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig RL, Paaijmans KP, Hancock PA, Thomas MB. The potential for fungal biopesticides to reduce malaria transmission under diverse environmental conditions. J Appl Ecol. 2015;52:1558–1566. doi: 10.1111/1365-2664.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig RL, Thomas MB. Interactions between a fungal entomopathogen and malaria parasites within a mosquito vector. Malar J. 2015;14:22. doi: 10.1186/s12936-014-0526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Shretta R, Wells TNC, Bell D, Djimdé AA, Achee N, Qi G. Tools and Strategies for Malaria Control and Elimination: What Do We Need to Achieve a Grand Convergence in Malaria? PLoS Biol. 2016;14:e1002380. doi: 10.1371/journal.pbio.1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AFV, Koenraadt CJM, Farenhorst M, Knols BGJ, Takken W. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar J. 2010a;9:168. doi: 10.1186/1475-2875-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AFV, N’Guessan R, Koenraadt CJM, Asidi A, Farenhorst M, Akogbéto M, Thomas MB, Knols BG, Takken W. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasit Vectors. 2010b;3:87. doi: 10.1186/1756-3305-3-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joop G, Vilcinskas A. Coevolution of parasitic fungi and insect hosts. Zoology. 2016;119:350–358. doi: 10.1016/j.zool.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Kamareddine L. The biological control of the malaria vector. Toxins (Basel) 2012;4:748–767. doi: 10.3390/toxins4090748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikankie CK, Brooke BD, Knols BGJ, Koekemoer LL, Farenhorst M, Hunt RH, Thomas MB, Coetzee M. The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malar J. 2010;9:71. doi: 10.1186/1475-2875-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Choi JY, Lee JH, Park J Bin, Fu Z, Liu Q, Tao X, Jin BR, Skinner M, Parker BL, Je YH. Bumblebee venom serine protease increases fungal insecticidal virulence by inducing insect melanization. PLoS One. 2013;8:e62555. doi: 10.1371/journal.pone.0062555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Morlais I, Awono-Ambene PH, Cohuet A, Simard F, Jacques JC, Bourgouin C, Koella JC. Effect of infection by Plasmodium falciparum on the melanization immune response of Anopheles gambiae. Am J Trop Med Hyg. 2007;76:475–480. [PubMed] [Google Scholar]
- Li M, Akbari OS, White BJ. Highly efficient site-specific mutagenesis in malaria mosquitoes using CRISPR. G3 Genes, Genomes, Genet. 2018;8:653–658. doi: 10.1534/g3.117.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bui M, Yang T, Bowman CS, White BJ, Akbari OS. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc Natl Acad Sci. 2017;114:E10540–E10549. doi: 10.1073/pnas.1711538114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HL, St Leger RJ. Insect immunity to entomopathogenic fungi. Adv Genet. 2016;94:251–285. doi: 10.1016/bs.adgen.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Lwetoijera DW, Sumaye RD, Madumla EP, Kavishe DR, Mnyone LL, Russell TL, Okumu FO. An extra-domiciliary method of delivering entomopathogenic fungus, Metharizium anisopliae IP 46 for controlling adult populations of the malaria vector, Anopheles arabiensis. Parasit Vectors. 2010;3:18. doi: 10.1186/1756-3305-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarin GM, Jaronski ST. The production and uses of Beauveria bassiana as a microbial insecticide. World J Microbiol Biotechnol. 2016;32:177. doi: 10.1007/s11274-016-2131-3. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Budd A, Pinto S, Gibson TJ, Kafatos FC. Anopheles gambiae SRPN2 facilitates midgut invasion by the malaria parasite Plasmodium berghei. EMBO Rep. 2005;6:891–897. doi: 10.1038/sj.embor.7400478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyone LL, Kirby MJ, Lwetoijera DW, Mpingwa MW, Knols BGJ, Takken W, Russell TL. Infection of the malaria mosquito, Anopheles gambiae, with two species of entomopathogenic fungi: effects of concentration, co-formulation, exposure time and persistence. Malar J. 2009;8:1–12. doi: 10.1186/1475-2875-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyone LL, Kirby MJ, Lwetoijera DW, Mpingwa MW, Simfukwe ET, Knols BGJ, Takken W, Russell TL. Tools for delivering entomopathogenic fungi to malaria mosquitoes: effects of delivery surfaces on fungal efficacy and persistence. Malar J. 2010;9:246. doi: 10.1186/1475-2875-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyone LL, Lyimo IN, Lwetoijera DW, Mpingwa MW, Nchimbi N, Hancock PA, Russell TL, Kirby MJ, Takken W, Koenraadt CJ. Exploiting the behaviour of wild malaria vectors to achieve high infection with fungal biocontrol agents. Malar J. 2012;11:87. doi: 10.1186/1475-2875-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci U S A. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brochta DA, Alford RT, Pilitt KL, Aluvihare CU, Harrell RA. piggyBac transposon remobilization and enhancer detection in Anopheles mosquitoes. Proc Natl Acad Sci. 2011;108:16339–16344. doi: 10.1073/pnas.1110628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava-Ripoll M, Posada FJ, Momen B, Wang C, St Leger R. Increased pathogenicity against coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae) by Metarhizium anisopliae expressing the scorpion toxin (AaIT) gene. J Invertebr Pathol. 2008;99:220–226. doi: 10.1016/j.jip.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Pendland JC, Hung SY, Boucias DG. Evasion of host defense by in vivo-produced protoplast-like cells of the insect mycopathogen Beauveria bassiana. J Bacteriol. 1993;175:5962–5969. doi: 10.1128/jb.175.18.5962-5969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Xia Y. Integration of an insecticidal scorpion toxin (BjαIT) gene into Metarhizium acridum enhances fungal virulence towards Locusta migratoria manilensis. Pest Manag Sci. 2015;71:58–64. doi: 10.1002/ps.3762. [DOI] [PubMed] [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc B Biol Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JL, Garver LS, Brayner FA, Alves LC, Rodrigues J, Molina-Cruz A, Barillas-Mury C. The role of hemocytes in A. gambiae antiplasmodial immunity. J Innate Immun. 2014;6:119–128. doi: 10.1159/000353765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: A eorsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Scott JG, Michel K, Bartholomay LC, Siegfried BD, Hunter WB, Smagghe G, Zhu KY, Douglas AE. Towards the elements of successful insect RNAi. J Insect Physiol. 2013;59:1212–1221. doi: 10.1016/j.jinsphys.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte EJ, Knols BGJ, Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J Invertebr Pathol. 2006;91:43–49. doi: 10.1016/j.jip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Ng’habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BGJ. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Njiru BN, Smallegange RC, Takken W, Knols BGJ. Infection of malaria (Anopheles gambiae ss.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J. 2003;2:29. doi: 10.1186/1475-2875-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenke RA, Lazzaro BP, Wolfner MF. Reproduction–immunity trade-offs in insects. Annu Rev Entomol. 2016;61:239–256. doi: 10.1146/annurev-ento-010715-023924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Bian GW, Raikhel AS. A toll receptor and a cytokine, Toll5A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J Biol Chem. 2006;281:39388–39395. doi: 10.1074/jbc.M608912200. [DOI] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Bian G, Cheon HM, Yu JK, Raikhel AS. REL1, a homologue of Drosophila Dorsal, regulates Toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- Sonmez E, Kocacevik S, Sevim A, Demirbag Z, Demir I. Efficacy of entomopathogenic gungi against the alder leaf beetle Agelastica alni (L.) (Coleoptera3: Chrysomelidae) Acta Zool Bulg. 2017;69:575–581. [Google Scholar]
- Sternberg ED, Ng’Habi KR, Lyimo IN, Kessy ST, Farenhorst M, Thomas MB, Knols BGJ, Mnyone LL. Eave tubes for malaria control in Africa: Initial development and semi-field evaluations in Tanzania. Malar J. 2016;15:447. doi: 10.1186/s12936-016-1499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg ED, Thomas MB. Insights from agriculture for the management of insecticide resistance in disease vectors. Evol Appl. 2017 doi: 10.1111/eva.12501. https://doi.org/10.1111/eva.12501. [DOI] [PMC free article] [PubMed]
- Sternberg ED, Waite JL, Thomas MB. Evaluating the efficacy of biological and conventional insecticides with the new “MCD bottle” bioassay. Malar J. 2014;13:499. doi: 10.1186/1475-2875-13-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Kimbrell Da, Kiger J, Natzle J, Tobin S. Host immune response and differential survival of the sexes in Drosophila. Fly (Austin) 2007;1:197–204. doi: 10.4161/fly.5082. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Godfray HCJ, Read AF, van den Berg H, Tabashnik BE, van Lenteren JC, Waage JK, Takken W. Lessons from agriculture for the sustainable management of malaria vectors. PLoS Med. 2012;9:e1001262. doi: 10.1371/journal.pmed.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Rämet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Valero-Jiménez CA, Wiegers H, Zwaan BJ, Koenraadt CJM, van Kan JAL. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. 2016;133:41–49. doi: 10.1016/j.jip.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Valero-Jiménez Ca, Debets AJM, van Kan JAL, Schoustra SE, Takken W, Zwaan BJ, Koenraadt CJM. Natural variation in virulence of the entomopathogenic fungus Beauveria bassiana against malaria mosquitoes. Malar J. 2014;13:479. doi: 10.1186/1475-2875-13-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Charlet M, Lowenberger C, Blass C, Muller HM, Dimopoulos G, Hoffmann J, Kafatos FC, Richman A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol Biol. 2000;9:75–84. doi: 10.1046/j.1365-2583.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Hoffmann Ja, Kafatos FC, Müller HM, Dimopoulos G. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad Sci. 2001;98:12630–12635. doi: 10.1073/pnas.221466798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Zhang YJ, Zhai XM, Zhao JJ, Peng DL, Wu G. Expression of a scorpion toxin gene BmKit enhances the virulence of Lecanicillium lecanii against aphids. J Pest Sci (2004) 2015;88:637–644. [Google Scholar]
- Yassine H, Kamareddine L, Osta Ma. The Mosquito Melanization Response Is Implicated in Defense against the Entomopathogenic Fungus Beauveria bassiana. PLoS Pathog. 2012;8:e1003029. doi: 10.1371/journal.ppat.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.