Abstract
The objective of present study was to assess the safety and efficacy of nanocurcumin as an anti-inflammatory and antioxidant agent in adults with amyotrophic lateral sclerosis (ALS). We conducted a 12-month, double-blind, randomized, placebo-controlled trial at a neurological referral center in Iran. Eligible patients with a definite or probable ALS diagnosis were randomly assigned to receive either nanocurcumin (80 mg daily) or placebo in a 1:1 ratio. A computerized random number generator was used to prepare the randomization list. All patients and research investigators were blinded to treatment allocation. The primary outcome was survival, and event was defined to be death or mechanical ventilation dependency. Analysis was by intention-to-treat and included all patients who received at least one dose of study drug. A total of 54 patients were randomized to receive either nanocurcumin (n = 27) or placebo (n = 27). After 12 months, events occurred in 1 patient (3.7%) in the nanocurcumin group and in 6 patients (22.2%) in the placebo group. Kaplan–Meier analysis revealed a significant difference between the study groups regarding their survival curves (p = 0.036). No significant between-group differences were observed for any other outcome measures. No serious adverse events or treatment-related deaths were detected. No patients withdrew as a result of drug adverse events. The results suggest that nanocurcumin is safe and might improve the probability of survival as an add-on treatment in patients with ALS, especially in those with existing bulbar symptoms. Future studies with larger sample sizes and of longer duration are needed to confirm these findings.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0606-7) contains supplementary material, which is available to authorized users.
Keywords: Amyotrophic lateral sclerosis, ALS, Nanocurcumin, Curcumin, Clinical trial
Introduction
Amyotrophic lateral sclerosis (ALS) is a highly progressive and lethal neurodegenerative disorder. It is also a motor neuron disease characterized by involvement of both upper and lower motor neurons [1]. Currently, the disease is considered incurable and the only approved treatment, riluzole, has a mild effect on survival time [2].
Based on site of disease onset, patients are mainly categorized into “spinal onset” or “bulbar onset” groups. Bulbar symptoms, such as dysarthria and dysphagia, are reported to be the initial presentation of disease in 30% of patients [3]. The site of onset is a major prognostic factor for disease outcome; patients with bulbar-onset ALS are reported to have a worse prognosis than the others [4]. Moreover, emergence of bulbar symptoms worsens the prognosis in any patient diagnosed with ALS [5, 6].
The exact etiology of ALS is unknown. However, current evidence suggests that oxidative stress, mitochondrial dysfunction, and neuroinflammation may be pathologically involved [7]. Previous studies have indicated that curcumin, the principal component of turmeric, might have a beneficial effect on neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and ALS, through its anti-inflammatory and antioxidant properties [8]. Studies on the animal model of spinal and bulbar muscular atrophy (SBMA), another motor neuron disease commonly confused with ALS, demonstrated that administration of curcumin compounds to the mouse model of SBMA resulted in significant improvement of motor function and increased the survival time [9, 10].
There is a vast need for randomized clinical trials to assess the beneficial effects of curcumin in human subjects with neurodegenerative disorders. We conducted a 12-month, double-blind, randomized, placebo-controlled clinical trial to evaluate the potential of nanocurcumin as an add-on treatment in sporadic cases of ALS. To the best of our knowledge, this is the first clinical trial of nanocurcumin in patients with ALS.
Methods
Study Design and Patients
This was a pilot, 12-month, double-blind, randomized, placebo-controlled, parallel group trial of nanocurcumin as an add-on treatment in patients with ALS. This study was conducted in a neurological referral center affiliated with the Tehran University of Medical Sciences.
Consecutive male and female patients with an age range of 18 to 85 years and definite or probable diagnosis of ALS (according to the revised version of El Escorial criteria) were assessed for eligibility. Patients with familial ALS or any first-degree relatives with ALS; severe respiratory dysfunction (assisted ventilation > 8 h/day); ongoing pregnancy or breastfeeding; severe renal, liver, or heart dysfunction; and prominent cognitive impairment (e.g., dementia, major psychiatric disorders) were excluded from the study. The protocol of this study was approved by the ethical committee of Tehran University of Medical Sciences, and all patients provided signed informed consent before inclusion in the study. This trial was registered in the Iranian Registry of Clinical Trials (IRCT2015062411424N3).
Intervention
To improve the oral bioavailability of curcumin, we used SinaCurcumin oral capsules (Exir Nano, Tehran, Iran) in this study. SinaCurcumin is a registered product from curcuminoids in Iran (IRC: 1228225765). Curcuminoids are dietary polyphenols extracted from the dried rhizomes of Curcuma longa L. (turmeric) and comprise curcumin, desmethoxycurcumin, and bisdemethoxycurcumin, which are together known as the C3 complex. Each soft gelatin capsule of SinaCurcumin contains 80 mg curcuminoids as nanomicelles.
The encapsulation efficiency of curcuminoids in nanomicelles is almost 100%. The mean diameter of nanomicelles is around 10 nm, according to dynamic light scattering. The curcuminoid content and size distribution of nanomicelles remains constant for at least 24 months. The oral absorption of SinaCurcumin is at least 50 times more than the conventional powder of curcumin in mice [11].
Randomization and Blinding
Eligible patients were randomly assigned in a 1:1 ratio to receive either oral-capsule nanocurcumin 80 mg (SinaCurcumin) daily, or placebo. A computerized random number generator was used to prepare the randomization list in 2 groups. All patients and research investigators were blinded to treatment allocation. Placebo capsules were provided by the same company as nanocurcumin and were perfectly matched in size, shape, odor, and color.
Procedures
After taking a detailed medical history and thorough clinical examination, patients who fulfilled the revised El Escorial World Federation of Neurology criteria for probable or definite ALS diagnosis were included for further electrodiagnostic and laboratory evaluations (creatine phosphokinase, complete blood count, liver enzymes, and blood urea and creatinine) to confirm the diagnosis and eligibility. Patients with normal renal and liver function tests (LFTs) were considered eligible to receive the treatment. All patients received 50 mg riluzole twice daily during the study. Antacid was prescribed for all patients to avoid the gastrointestinal side effects of curcumin. Lung function tests were requested for patients with respiratory complaints, and any indication for mechanical ventilation resulted in exclusion from the study.
Scores for the ALS Assessment Questionnaire-40, revised ALS Functional Rating Scale (ALSFRS-R), and manual muscle testing (MMT) were calculated for all patients at baseline and the end of study according to their scoring systems [12–15]. Nerve conduction velocity studies were performed by an expert neurologist (A.T.) at baseline and the study endpoint. In addition to follow-up visits, all patients were contacted at least every 3 months after randomization to monitor adverse drug effects, appearance of new symptoms, and survival status (even in patients who discontinued the treatment). We lost our contact with 2 patients in the active drug group (at months 4 and 9) and 3 patients in the placebo group (at months 6, 9, and 10). LFTs were requested 3 months after drug initiation and after the appearance of any possible drug adverse effects (AEs).
Outcome Measures
The primary outcomes were safety and survival, and event was defined to be death or mechanical ventilation dependency. Secondary outcomes were the rate of decline in functional assessments [ALSFRS-R score, MMT score, and compound muscle action potential (CMAP) amplitude] and percentage of change in disease progression rate.
Statistical Analysis
SPSS version 23.0 (IBM, Armonk, NY, USA) and GraphPad Prism 6.0 (GraphPad Inc., La Jolla, CA, USA) were used for the data analysis. Survival curves were obtained for each group and compared with log-rank statistics. The hazard ratio (HR) for the treatment group was calculated by Cox regression, and age, duration of disease, baseline ALSFRS-R score, and baseline progression rate were defined as the covariates. All participants who received at least 1 dose of assigned treatment were included in the survival analysis. Secondary outcomes were analyzed in the intention-to-treat population. Change from baseline ALSFRS-R score, MMT score, progression rate, and CMAP amplitudes of the median, ulnar, tibial, and common peroneal nerves were assessed with general linear models. Disease progression rates were calculated at baseline () and study endpoint (). According to the acquired values for disease progression rate at baseline, patients were defined to have slow (< 0.5), intermediate (0.5–1.0), and fast (> 1.0) progressive disease [16]. The baseline demographic and clinical features of the patients in each group were compared using the χ2 test and Student’s t test.
Results
From June 2015 to March 2016, 54 patients were randomized to receive either nanocurcumin (n = 27) or placebo (n = 27) (Fig. 1). All participants had received at least 1 dose of the allocated drug and were therefore included in the survival analyses. The median (interquartile range) duration of drug intake in patients who discontinued the study was 5.5 (3–6) months. The baseline characteristics of the patients are shown in Table 1. Except for age and MMT, demographic and clinical characteristics of the both groups were perfectly matched at baseline.
Fig. 1.
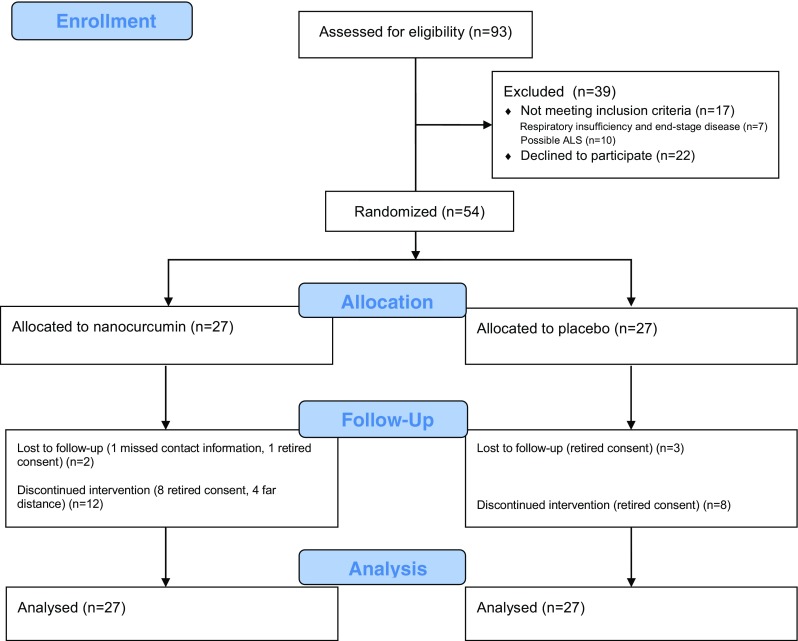
CONSORT flow diagram. ALS = amyotrophic lateral sclerosis
Table 1.
Patient baseline characteristics
| Nanocurcumin (n = 27) | Placebo (n = 27) | p-value | |
|---|---|---|---|
| Sex | |||
| Male | 21 (78) | 18 (67) | 0.544 |
| Female | 6 (22) | 9 (33) | |
| Mean ± SD age (y) | 51.5 ± 13.1 | 58.5 ± 9.6 | 0.030 |
| Mean ± SD age of disease onset (y) | 49.2 ± 13.1 | 56.2 ± 9.5 | 0.028 |
| Mean ± SD BMI* | 25.4 ± 3.9 | 23.7 ± 4.5 | 0.237 |
| Onset | |||
| Bulbar | 6 (22) | 2 (7) | 0.250 |
| Spinal | 21 (78) | 25 (93) | |
| Bulbar symptoms | 18 (67) | 12 (44) | NA |
| Dysarthria | 11 | 11 | |
| Dysphagia | 14 | 9 | |
| Drooling | 10 | 8 | |
| Median (range) disease duration (months) | 24 (8–60) | 24 (5–84) | 0.599 |
| Mean ± SD ALSFRS-R | 36 ± 8.1 | 32.6 ± 8.2 | 0.134 |
| Progression rate | |||
| Slow | 15 (56) | 12 (44) | 0.128 |
| Intermediate | 11 (41) | 9 (33) | |
| Fast | 1 (4) | 6 (22) | |
| Median (range) ALSQ40 | 72 (16–256) | 67 (9–142) | 0.993 |
| Mean ± SD MMT | 108.2 ± 13 | 98.85 ± 15 | 0.019 |
| Mean ± SD FVC† | 86.8 ± 19.5 | 71 ± 30.2 | 0.118 |
Data are n (%) unless otherwise indicated
BMI = body mass index; ALSFRS-R = Revised Amyotrophic Lateral Sclerosis (ALS) Function Rating Scale; ALSQ40 = ALS Assessment Questionnaire-40; MMT = manual muscle testing; FVC = forced vital capacity
*BMI value available for 18 patients in the nanocurcumin group and 16 patients in the placebo group
†FVC available from 14 patients in the nanocurcumin group and 13 patients in the placebo group
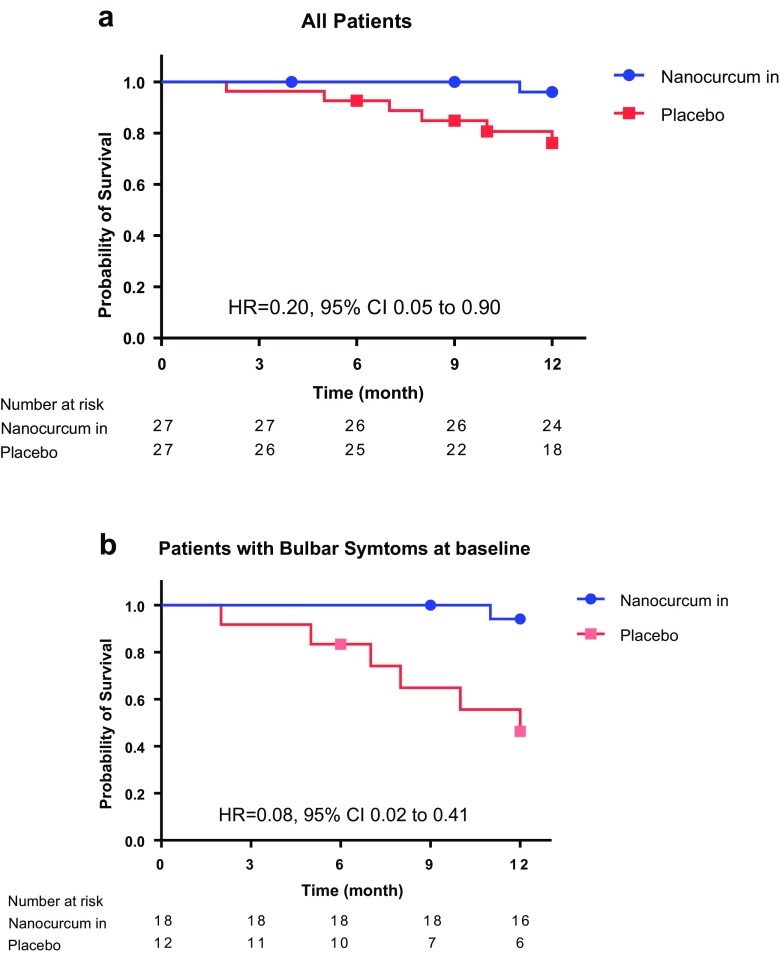
During the follow-up period, 7 events (5 deaths, 1 mechanical ventilation dependency in the placebo group, 1 mechanical ventilation dependency in the nanocurcumin group) occurred. All patients who experienced the event had bulbar symptoms at the baseline visit (Appendix S1). Kaplan–Meier analysis revealed a significant difference between the study groups regarding their survival curves [p = 0.036, HR = 0.20, 95% confidence interval (CI) 0.05–0.90] (Fig. 2). Further survival analysis was done following stratification by site of disease onset, presence of bulbar symptoms at baseline, severity of the disease, and progression rate (Table 2).
Fig. 2.
Kaplan–Meier plots. (a) Survival probability for all patients and (b) patients with bulbar symptoms at baseline. HR = hazard ratio; CI = confidence interval
Table 2.
Subgroup survival analysis
| Nanocurcumin | Placebo | p-value | |
|---|---|---|---|
| Overall events (survival probability; SE) | Overall events (survival probability; SE) | ||
| Disease onset | |||
| Bulbar | 1 (0.83; 0.15) | 1 (0.50; 0.35) | 0.275 |
| Spinal | 0 | 5 (0.79; 0.08) | 0.032 |
| Bulbar symptoms at baseline | |||
| Yes | 1 (0.94; 0.06) | 6 (0.46; 0.15) | 0.002 |
| No | 0 | 0 | – |
| Disease severity | |||
| ALSFRS-R ≥ 33 | 0 | 4 (0.73; 0.11) | 0.025 |
| ALSFRS-R < 33 | 1 (0.89; 0.11) | 2 (0.81; 0.12) | 0.579 |
| Progression rate | |||
| Slow | 0 | 1 (0.92; 0.08) | 0.280 |
| Intermediate | 1 (0.90; 0.1) | 3 (0.65; 0.17) | 0.121 |
| Fast | 0 | 2 (0.67; 0.19) | 0.545 |
ALSFRS-R = Revised Amyotrophic Lateral Sclerosis (ALS) Function Rating Scale
Cox regression analysis with adjustment for prognostic factors (age, duration of disease, first ALSFRS-R, and progression rate) was conducted to calculate the HRs. The difference in survival between groups was not significant after stratification by site of disease onset (HR = 0.049; 95% CI 0.002–1.034; p = 0.053); however, after stratification according to the presence of bulbar symptoms at baseline, it reached significance (HR = 0.031; 95% CI 0.003–0.380; p = 0.007).
The last mean (SE) ALSFRS-R score after adjustment for the baseline value was 31.12 (1.16) in the nanocurcumin group and 29.54 (1.13) in the placebo group (p = 0.371). The rate of deterioration in ALSFRS-R did not differ significantly between the treatment groups (p = 0.297) (Table 3). The mean (SE) progression rate during the follow-up period (adjusted for the baseline value) was 0.284 (0.123) in the nanocurcumin group and 0.380 (0.121) in the placebo group (p = 0.589).
Table 3.
Primary and secondary outcomes (intention-to-treat population)*
| Nanocurcumin | Placebo | p-value | |
|---|---|---|---|
| Mean change from baseline (95% CI) | Mean change from baseline (95% CI) | ||
| ALSFRS-R (%) | –0.6 (–11.6 to 10.4) | –9.1 (–19.3 to 1.0) | 0.297 |
| Progression rate (%) | 2.3 (–29.3 to 34.0) | 36.8 (4.2 to 69.3) | 0.162 |
| MMT (%) | |||
| Total score | –17.0 (–21.2 to –12.9) | –19.3 (–23.6 to –15.1) | 0.470 |
| Upper limbs | –21.8 (–27.7 to –15.9) | –23.9 (–30.2 to –17.7) | 0.645 |
| Lower limbs | –4.5 (–37.9 to 28.8) | 6.1 (–27.7 to 39.8) | 0.674 |
| CMAP amplitude (%) | |||
| Median | –42.1 (–83.2 to –1.0) | –32.3 (–71.4 to 6.8) | 0.727 |
| Ulnar | –23.7 (–69.6 to 22.2) | –41.2 (–94.8 to 12.5) | 0.576 |
| Tibial | –30.1 (–56.3 to –3.9) | –31.7 (–56.6 to –6.9) | 0.922 |
| Common peroneal | –51.0 (–82.6 to –19.3) | –42.8 (–84.9 to –0.7) | 0.763 |
CI = confidence interval; ALSFRS-R = Revised Amyotrophic Lateral Sclerosis (ALS) Function Rating Scale; MMT = manual muscle testing; CMAP = compound muscle action potential
*Univariate general linear model with adjustment for the covariates was used for the analysis. Negative values mean deterioration except for the progression rate; positive values mean deterioration
Total MMT scores did not differ statistically between the treatment groups at month 12 (p = 0. 470). We also calculated the upper and lower limbs scores, which indicated no significant difference between the 2 groups (p > 0.05) (Table S1). The mean decline in MMT scores in each group is shown in Table 3.
The mean rates of decline in CMAP amplitudes of the median, ulnar, tibial, and common peroneal nerves were calculated and compared among the patients of each group, which indicated no significant difference between them (p > 0.05) (Table 3).
When the baseline characteristics of the patients with and without bulbar symptoms at baseline visit were compared, no significant differences in age, sex, body mass index, duration of disease, and MMT scores of the patients were found (data not shown). However, the ALS Assessment Questionnaire-40 score was significantly higher and ALSFRS-R score significantly lower among patients with bulbar symptoms at baseline visit (Table S2). Moreover, the baseline disease progression rate was statistically higher in these patients (Table S2).
No serious AE or treatment-related death was recorded during the follow-up period. The only reported AEs were itching (1 patient in the nanocurcumin group), increased muscle fasciculation (1 patient in the nanocurcumin group), and a mild increase in LFTs (2 patients in the placebo group and 1 in the nanocurcumin group). None of these adverse events was the cause of withdrawal.
Discussion
The results of this double-blind, placebo-controlled trial indicated that nanocurcumin significantly improved the survival probability of patients with ALS over the 12-month period of this study. Other outcome measures, including the ALSFRS-R score, muscle strength, and CMAP amplitude decrements did not significantly differ between the nanocurcumin and placebo groups.
Although previous studies have reported that curcumin is a neuroprotective agent and might improve motor function [10, 17], we did not find any significant impact of nanocurcumin on functional measures. One reason for this finding could be the relatively progressed disease in most of the included patients. Perhaps if treatment was initiated at the very first stage of the disease it could be more efficient. The short duration of therapy and dose-related efficacy of curcumin are other potential reasons for the nonsignificant influence of nanocurcumin on patients’ functional scores. Moreover, we guess that nanocurcumin efficiency is mostly related to its beneficial effects on bulbar symptoms. Unfortunately, ALSFRS total score is not the best choice for evaluating bulbar function in patients with ALS, and more sensitive tools are needed [18].
Bulbar symptoms are not the exclusive feature of bulbar-onset disease and most patients with other types of ALS eventually experience these symptoms too [19, 20]. Bulbar symptoms have a significantly negative impact on patients’ quality of life and increase the risk of aspiration and malnutrition and therefore lead to the reduced probability of survival among those affected [5]. In this study, bulbar-onset ALS was documented only in 8 patients (14.8%), which is less frequent than previous reports from other epidemiological studies [21]. Bulbar symptoms were detected in 47.8% of patients with spinal-onset disease and 55.6% of all included patients. We found that disease progression is quicker among patients who had bulbar symptoms at the baseline visit and these patients are also less functional than those without these symptoms. Moreover, in all patients who experienced the event, bulbar symptoms were noted at baseline. All of these findings point to the poor prognosis of the disease in patients with bulbar symptoms. Here, we demonstrated that the survival rate was significantly higher in the group of patients with bulbar symptoms at baseline who had received nanocurcumin. Because no event had occurred in the group of patients without bulbar symptoms, we could not evaluate the efficacy of this treatment among them. The probability of survival was also higher in recipients of nanocurcumin who had spinal-onset ALS.
Evidence from pathological studies points to the critical role of oxidative stress in the development and progression of the neurodegenerative process in ALS [22]. It has been shown that treatment with antioxidants such as vitamin E and co-enzyme Q10 could slow disease progression in animal models of ALS [23, 24]. However, clinical trials did not prove the significant therapeutic effects of these antioxidants in human patients [22, 25, 26]. Perhaps the low concentration of these agents in the cerebrospinal fluid and plasma of human subjects could be a reason for this nonsignificant result [27].
Curcumin is a powerful scavenger of reactive oxygen species, and it also enhances the endogenous antioxidant response [28]. It has also been demonstrated that curcumin might be able to initiate mitophagy in cells with damaged and swollen mitochondria [29]. All these features make curcumin an excellent antioxidant that is even more potent than vitamin E [30, 31]. However, the low bioavailability of curcumin might limit its health benefit [32]. This problem can be solved by using more soluble forms of this agent, such as nanocurcumin, which is also more potent.
Protein aggregation is a characteristic finding in many neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and ALS [33]. Animal studies have suggested that curcumin can cross the blood–brain barrier and modulate aggregation of misfolded proteins such as β-amyloid, tau, α-synuclein, and prion [8]. It is believed that protein aggregation is a consequence of existing oxidative stress in ALS [34]. Neural TAR DNA binding protein 43 inclusions and superoxide dismutase 1 aggregation are common proteinopathies among patients with ALS [35, 36]. It has been shown that curcumin protects neural cells from toxicity induced by mutant TAR DNA binding protein 43 and reduces its fragments and enhances the antioxidant ability of it [37, 38]. Likewise, protein aggregation and oxidative damage were suppressed secondary to antioxidant modulation of curcumin in SBMA [10].
No AEs were reported in the patients of the present trial. This finding highlights the safety and tolerability of nanocurcumin in human subjects. In line with the present study, many other published clinical trials have reported that curcumin is a safe and efficient treatment in a variety of inflammatory and noninflammatory disorders [39]. However, curcumin may cause abdominal pain in some patients, which may result in discontinuation of the treatment [40]. To avoid abdominal discomfort, we prescribed antacids for all patients included in this trial, which we believe is a reason for the absence of AEs in this study.
Here, we studied patients with a wide range of disease durations and disabilities, which is a strength of this study. A relatively comprehensive evaluation of the included patients is another strength of the present study. However, some major limitations should be noted. The small sample size is the first and the most important limitation of this study. Also, despite randomization, patients in the placebo group were a little older than those in the treatment group. As age is an important prognostic factor for ALS survival, we tried to reduce the risk of bias and improve the power of this study by applying age adjustment for all outcome analyses. The high rate of loss to follow-up is another limitation of the study. The high dropout rate is a significant issue in ALS clinical trials, especially those with longer follow-up periods. Similar to the present trial, many previous studies reported that difficulty attending the clinic owing to severe disabilities or long distances, as well as lack of interest in continuing the trial, are the major causes of loss to follow-up [41–43]. We tried to minimize the dropout rate by following our patients via regular telephone calls; however, this does not fully protect our study from a biased result. Finally, owing to the short duration of the follow-up period, we could not assess the long-term efficacy of nanocurcumin in our patients.
Taken together, the above findings suggest that nanocurcumin is safe and might be an effective add-on treatment in patients with ALS. It might improve the probability of survival, especially in patients with existing bulbar symptoms who were shown to have a poorer disease prognosis. However, owing to the small sample size and high dropout rate, future trials with larger sample sizes and longer durations of follow-up are needed to confirm the results. Monitoring disease progression and bulbar symptoms in patients who receive nanocurcumin at the earliest stage of the disease might better reveal the therapeutic potential of nanocurcumin.
Electronic supplementary material
(XLSX 9 kb)
Manual muscle testing scores (DOCX 17 kb)
Characteristics of patients at baseline (DOCX 15 kb)
(PDF 883 kb)
Acknowledgements
Dr. Jaafari is founder and head of board member of ExirNanosina Company. All the other authors declare no competing interests. This study was funded by Tehran University of Medical Sciences. We thank Tehran University of Medical Sciences (TUMS) for supporting this manuscript. We also thank the Exir Nano company for providing the study drugs free of charge. This trial was Dr. Ahmadi’s postgraduate thesis toward the Iranian Board of Neurology.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Contributor Information
Elmira Agah, Email: e-agah@student.tums.ac.ir.
Abbas Tafakhori, Email: a_tafakhori@sina.tums.ac.ir.
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 3.Talman P, Duong T, Vucic S, et al. Identification and outcomes of clinical phenotypes in amyotrophic lateral sclerosis/motor neuron disease: Australian National Motor Neuron Disease observational cohort. BMJ Open Rep. 2016;6(9):e012054. doi: 10.1136/bmjopen-2016-012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5-6):310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366–374. doi: 10.1038/ncpneuro0853. [DOI] [PubMed] [Google Scholar]
- 6.Turner M, Al-Chalabi A. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology. 2002;59(12):2012–2013. doi: 10.1212/WNL.59.12.2012-a. [DOI] [PubMed] [Google Scholar]
- 7.Gordon PH. Amyotrophic lateral sclerosis: pathophysiology, diagnosis and management. CNS Drugs. 2011;25(1):1–15. doi: 10.2165/11586000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Monroy A, Lithgow GJ, Alavez S. Curcumin and neurodegenerative diseases. BioFactors. 2013;39(1):122–132. doi: 10.1002/biof.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z, Chang YJ, Yu IC, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 10.Bott LC, Badders NM, Chen KL, et al. (2016) A small-molecule Nrf1 and Nrf2 activator mitigates polyglutamine toxicity in spinal and bulbar muscular atrophy. Hum Mol Genet 25 (10):1979-1989. d [DOI] [PMC free article] [PubMed]
- 11.Rahimi HR, Jaafari MR, Mohammadpour AH, et al. Curcumin: reintroduced therapeutic agent from traditional medicine for alcoholic liver disease. Asia Pac J Med Toxicol. 2015;4(1):25–30. [Google Scholar]
- 12.Great Lakes ALS Study Group A comparison of muscle strength testing techniques in amyotrophic lateral sclerosis. Neurology. 2003;61(11):1503–1507. doi: 10.1212/01.WNL.0000095961.66830.03. [DOI] [PubMed] [Google Scholar]
- 13.Franchignoni F, Mora G, Giordano A, Volanti P, Chio A. Evidence of multidimensionality in the ALSFRS-R Scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013;84(12):1340–1345. doi: 10.1136/jnnp-2012-304701. [DOI] [PubMed] [Google Scholar]
- 14.Jenkinson C, Fitzpatrick R, Brennan C, Bromberg M, Swash M. Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: the ALSAQ-40. J Neurol. 1999;246(Suppl. 3):Iii16–21. doi: 10.1007/BF03161085. [DOI] [PubMed] [Google Scholar]
- 15.Shamshiri H, Eshraghian MR, Ameli N, Nafissi S. Validation of the Persian version of the 40-item amyotrophic lateral sclerosis assessment questionnaire. Iran J Neurol. 2013;12(3):102–105. [PMC free article] [PubMed] [Google Scholar]
- 16.Steinacker P, Feneberg E, Weishaupt J, et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry. 2016;87(1):12–20. doi: 10.1136/jnnp-2015-311387. [DOI] [PubMed] [Google Scholar]
- 17.Sanivarapu R, Vallabhaneni V, Verma V. The potential of curcumin in treatment of spinal cord injury. Neurol Res Int. 2016;2016:9468193. doi: 10.1155/2016/9468193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green JR, Yunusova Y, Kuruvilla MS, et al. Bulbar and speech motor assessment in ALS: challenges and future directions. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7-8):494–500. doi: 10.3109/21678421.2013.817585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen A, Garrett CG. Otolaryngologic presentations of amyotrophic lateralsclerosis. Otolaryngol Head Neck Surg. 2005;132(3):500–504. doi: 10.1016/j.otohns.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 20.da Costa FA, Mourao LF. Dysarthria and dysphagia in amyotrophic lateral sclerosis with spinal onset: a study of quality of life related to swallowing. Neurorehabilitation. 2015;36(1):127–134. doi: 10.3233/NRE-141200. [DOI] [PubMed] [Google Scholar]
- 21.Shamshiri H, Fatehi F, Davoudi F, et al. Amyotrophic lateral sclerosis progression: Iran-ALS clinical registry, a multicentre study. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(7-8):506–511. doi: 10.3109/21678421.2015.1074698. [DOI] [PubMed] [Google Scholar]
- 22.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48(5):629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Gurney ME, Cutting FB, Zhai P, et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39(2):147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 24.Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998;95(15):8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graf M, Ecker D, Horowski R, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm (Vienna) 2005;112(5):649–660. doi: 10.1007/s00702-004-0220-1. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann P, Thompson JLP, Levy G, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify Phase III. Ann Neurol. 2009;66(2):235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappert EJ, Tangney CC, Goetz CG, et al. Alpha-tocopherol in the ventricular cerebrospinal fluid of Parkinson's disease patients: dose-response study and correlations with plasma levels. Neurology. 1996;47(4):1037–1042. doi: 10.1212/WNL.47.4.1037. [DOI] [PubMed] [Google Scholar]
- 28.González-Reyes S, Guzmán-Beltrán S, Medina-Campos ON, Pedraza-Chaverri J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid Med Cell Longev. 2013;2013:801418. doi: 10.1155/2013/801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Leung AW, Luo J, Xu C. TEM observation of ultrasound-induced mitophagy in nasopharyngeal carcinoma cells in the presence of curcumin. Exp Ther Med. 2012;3(1):146–148. doi: 10.3892/etm.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Aragon S, Benedi JM, Villar AM. Modifications on antioxidant capacity and lipid peroxidation in mice under fraxetin treatment. J Pharm Pharmacol. 1997;49(1):49–52. doi: 10.1111/j.2042-7158.1997.tb06751.x. [DOI] [PubMed] [Google Scholar]
- 31.Sreejayan RMN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49(1):105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 32.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 33.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 34.Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762(11-12):1051–1067. doi: 10.1016/j.bbadis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61(5):427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 36.Redler RL, Dokholyan NV. The complex molecular biology of amyotrophic lateral sclerosis (ALS) Prog Mol Biol Transl Sci. 2012;107:215–262. doi: 10.1016/B978-0-12-385883-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia NK, Srivastava A, Katyal N, et al. Curcumin binds to the pre-fibrillar aggregates of Cu/Zn superoxide dismutase (SOD1) and alters its amyloidogenic pathway resulting in reduced cytotoxicity. Biochim Biophys Acta. 2015;1854(5):426–436. doi: 10.1016/j.bbapap.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Duan W, Guo Y, Xiao J, et al. Neuroprotection by monocarbonyl dimethoxycurcumin C: ameliorating the toxicity of mutant TDP-43 via HO-1. Mol Neurobiol. 2014;49(1):368–379. doi: 10.1007/s12035-013-8525-4. [DOI] [PubMed] [Google Scholar]
- 39.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 41.Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2013;5:CD005229. doi: 10.1002/14651858.CD005229.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1-2):133–137. doi: 10.1016/S0022-510X(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 43.Paillisse C, Lacomblez L, Dib M, Bensimon G, Garcia-Acosta S, Meininger V. Prognostic factors for survival in amyotrophic lateral sclerosis patients treated with riluzole. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(1):37–44. doi: 10.1080/14660820510027035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 9 kb)
Manual muscle testing scores (DOCX 17 kb)
Characteristics of patients at baseline (DOCX 15 kb)
(PDF 883 kb)