Abstract
The development of high throughput SNP genotyping technologies has improved the genetic dissection of simple and complex traits in many species including cats. The properties of feline 62,897 SNPs Illumina Infinium iSelect DNA array are described using a dataset of over 2,000 feline samples, the most extensive to date, representing 41 cat breeds, a random bred population, and four wild felid species. Accuracy and efficiency of the array’s genotypes and its utility in performing population-based analyses were evaluated. Average marker distance across the array was 37,741 Kb, and across the dataset, only 1% (625) of the markers exhibited poor genotyping and only 0.35% (221) showed Mendelian errors. Marker polymorphism varied across cat breeds and the average minor allele frequency (MAF) of all markers across domestic cats was 0.21. Population structure analysis confirmed a Western to Eastern structural continuum of cat breeds. Genome-wide linkage disequilibrium ranged from 50–1,500 Kb for domestic cats and 750 Kb for European wildcats (Felis silvestris silvestris). Array use in trait association mapping was investigated under different modes of inheritance, selection and population sizes. The efficient array design and cat genotype dataset continues to advance the understanding of cat breeds and will support monogenic health studies across feline breeds and populations.
Subject terms: Genomics, Genotype
Introduction
Feral and owned cats are collectively referred to as “domestic cats”. Over 88 million domestic cats live in homes in the USA alone1,2, and are valued companions, providers of vermin control, and important biomedical models3. The domestic cat, Felis silvestris catus, represents one of the ~41 species in the family Felidae4–6 with the extant species having a common ancestor ~11 million years ago7,8. Previous archeological and genetic research has suggested the modern domesticated cat descends from at least one wildcat progenitor subspecies, Felis silvestris libyca, around 10,000 years ago9–11.
Agricultural development is thought to be the key event that initiated and influenced the domestication of the cat11–13. The availability of grains and other food sources in and around areas of human settlements resulted in substantial rodent population expansion, which in turn attracted the natural predator, the progenitor of the domestic cat, from the wildcat population. Over time, individual cats with temperaments suitable for co-habitation with human populations became isolated from the wild counterparts and evolved into the semi-domesticated cat of today. In spite of their rapid spread and isolation from the progenitor populations, domestic cats have remained remarkably similar to their felid cousins (Felis silvestris subsp.) in form and behavior12,14 and these wild populations have remained widespread across the Old World.
The establishment of cat breeds from domesticated and tamed free-roaming cat populations is a relatively recent event. Many domesticated animal species such as cattle, goats, pig, dog, and horse, were selected for traits of economic value such as meat, milk, drought tolerance, endurance, strength, protection, hunting ability, speed and metabolic efficiency from the onset of their domestication15,16. All these desired qualities are the products of hundreds to thousands of years of selective breeding12,13. However, the domestic cat breeds were selectively bred primarily for aesthetically pleasing traits such as coat color, length, and texture, most of which occurred only in the past 150 years17,18. Between 40 and 55 different cat breeds are currently recognized for standardized phenotypic characteristics by worldwide cat fancy associations, including the Cat Fanciers’ Association19, The International Cat Association20, the Governing Council of the Cat Fancy21, Federation International Feline22, and the World Cat Federation23,24. Due to inbreeding, many cat breeds harbor heritable diseases that are important biomedical models for human health (http://omia.angis.org.au/home/)3,25. However, owned random-bred and un-owned or semi-owned feral cats represent the overwhelming majority of cats in the world26.
The continued development and progress of genetic resources for humans have transformed the field of genetics and accelerated the rate of scientific discovery27–29. Similarly, genetic resources for the domestic cat have methodically and systematically been developed, which include somatic cell hybrid panels30,31, radiation hybrid maps32–39, genetic linkage maps40–44, and the sequencing of the cat genome45–48. Feline genome sequencing efforts to date have included: (1) a 1.9x draft sequence as a representative of the family Felidae45, (2) additional light sequencing (~1X coverage) of six individuals from several breeds and an African wild cat (Felis silvestris cafra) for SNPs discovery47, (3) high throughput sequencing of four pooled samples from each of six different domestic cat breeds, wildcats, as well as the reference cat genome46 and (4) a high-resolution SNP array-based linkage map that supported the assembly of Felis_catus v8.048. The SNPs discovered via these sequencing efforts were used to construct an Illumina Infinium iSelect 63K DNA cat array. The produced array contains 62,897 variants that enable genome-wide case–control association studies and population-based investigations for cats rather than focusing only on pedigree analysis and candidate gene-based approaches.
Using an extensive dataset of over 2,000 cats genotyped using the feline SNP array, this study had two main objectives: firstly, to evaluate the array’s accuracy and efficiency for genome-wide genotyping that included validation tests of (1) remapping SNP physical positions to the newest cat genome assembly, (2) SNP genotyping rate, (3) SNP Mendelian inheritance, and (4) allelic variability across breeds, and secondly, to test the reliability of the array’s genotype data for population based analyses. The population-based analyses included assessments of (1) genetic diversity, (2) population structure, (3) linkage disequilibrium, and (4) association mapping.
Results
DNA array properties
Using the early assembly of the cat genome45 and the improved assembly by re-sequencing47 (FelCat4 (Felis catus 5.8)), ~10 million polymorphic variants, were submitted for design to produce a low density Illumina Infinium iSelect DNA array. SNPs that represented all known cat phenotypes and diseases at the time were submitted, as well as SNPs unique to a single assayed wildcat (Felis cafra)47. The final design (n = 62,897) included 59,469 autosomal SNPs, 2,724 X-linked SNPs (Supplementary Table 1), wildcat-specific SNPs (n = 4,240) and 126 SNPs representing trait-specific or disease-specific loci. A complete list of wildcat SNPs is provided in Supplementary Data File 1 and all other SNPs on the array in Supplementary Data File 2.
Remapping array SNPs to the Felis Catus 8.0 cat genome assembly
The array variants were previously remapped to cat assembly 6.249,50. For the 62,897 SNP positions, 62,193 (~99%) were identified in the Felis_catus_8.0 genome assembly, including 2,724X chromosome markers. The remaining 704 variants were assigned to chromosome 20, representing unknown chromosome locations (Supplementary Data File 3). Unmapped sequences were manually inspected and most had only partial alignments to the reference. The final SNP map maintained the same order as the remapping to cat assembly Felis_catus_6.249. The SNP positions are presented as IDs on the array and a map position for both cat genome assemblies is presented in Supplementary Data File 4. The array average marker distance is 37,741 bp, with a range of an average 36,699 bp between markers on chromosome D2 to an average 46,697 bp between markers on the chromosome X (Supplementary Table 1 and Supplementary Figure 1). The largest gap was detected on chromosome B2 (~3.2 Mb) followed by two markers on chromosome B1 (at ~3 Mb and ~2.5 Mb, respectively). The number of gaps >100 Kb is 1540 comprising 232 Mb, the number of gaps >500 Kb is 20 comprising ~26 Mb (Supplementary Data File 3).
Animals
Table 1 presents the 47 breeds, populations and familial cat groups represented by the 2,078 DNA samples genotyped on the 63K cat array. The dataset included domestic cats (n = 1,570) from 41 breeds and two cross-breed pedigrees49,51, two lions (Panthera leo), two tigers (Panthera tigris), nine leopard cats (Prionailurus bengalenesis), and 60 European wildcats (Felis silvestris ssp). Three breeds were sampled from familial lineages for pedigree studies, including Birman52, Lykoi, and Tennessee Rex. The samples from a mixed-breed research colony of known matings were included to support segregation analyses of the SNPs49. Each sample had a genotyping rate ≥90%. Complete genotype data is found in Supplementary Data File 5. The eight cats used for SNP discovery were included in these analyses46. The multi-dimensional scaling (MDS) clustering (see below) suggested the sample provided and identified as a Burmese (Pixel) was switched with the sample identified as a Cornish Rex (Tipper)47.
Table 1.
Population statistics and linkage disequilibrium (LD) estimates of cat breeds and populations.
| Breed Name | Symbol | No.* | No. LD SNPs† | LD (kb) | % Mono | MAF | HO | FIS |
|---|---|---|---|---|---|---|---|---|
| Abyssinian | ABY | 41* | 36251 | 1050 | 26.6 | 0.16 | 0.20 | 0.06 |
| American Curl | ACURL | 25* | 42704 | 200 | 17.5 | 0.19 | 0.25 | 0.03 |
| American Shorthair | ASH | 2 | — | — | — | — | — | — |
| American Wirehair | WIR | 9 | — | — | 35.4 | 0.16 | 0.25 | −0.12 |
| Asian | Asian | 3 | — | — | — | — | — | — |
| Bengal | BEN | 98* | 41053 | 350 | 11 | 0.18 | 0.23 | 0.05 |
| Birman | BIR | 296* | 34068 | 1450 | 17.1 | 0.15 | 0.2 | 0.05 |
| Bombay | BOM | 11 | — | — | 20.5 | 0.2 | 0.25 | 0.07 |
| British Shorthair | BSH | 22 | 40503 | 250 | 19.9 | 0.18 | 0.24 | 0.02 |
| Burmese | BUR | 106* | 32131 | 700 | 30.6 | 0.14 | 0.17 | 0.12 |
| Chartreux | CHR | 7 | — | — | 33.7 | 0.17 | 0.16 | −0.09 |
| Cornish Rex | CREX | 11 | — | — | 27.5 | 0.17 | 0.23 | 0.03 |
| Devon Rex | DREX | 21 | 39562 | 500 | 19.6 | 0.18 | 0.22 | 0.09 |
| Egyptian Mau | EGY | 10 | — | — | 26.3 | 0.18 | 0.25 | −0.04 |
| Havana Brown | HAV | 1 | — | — | — | — | — | — |
| Japanese Bobtail | JBOB | 13 | — | — | 20.2 | 0.2 | 0.27 | −0.016 |
| Khao Manee | MANEE | 5 | — | — | 41.8 | 0.15 | 0.22 | −0.08 |
| Korat | KOR | 6 | — | — | 55.5 | 0.11 | 0.17 | −0.1 |
| Kurillian Bobtail | KBOB | 1 | — | — | — | — | — | — |
| LaPerm | PERM | 66* | 45805 | 100 | 7.5 | 0.2 | 0.27 | 0.009 |
| Lykoi | LYK | 27 | — | — | 23.1 | 0.19 | 0.28 | −0.12 |
| Maine Coon | MCOON | 54* | 43748 | 150 | 12.3 | 0.19 | 0.25 | 0.025 |
| Manx | MANX | 8 | — | — | 20.4 | 0.2 | 0.28 | −0.02 |
| Munchkin | MUNCH | 40* | 47557 | 50 | 9.1 | 0.21 | 0.29 | −0.007 |
| Norwegian Forest cat | NFC | 15 | — | — | 15.1 | 0.2 | 0.27 | 0.03 |
| Ocicat | OCI | 5 | — | — | 37.7 | 0.16 | 0.24 | −0.1 |
| Oriental | ORI | 56* | 35398 | 300 | 20.1 | 0.16 | 0.2 | 0.046 |
| Persian | PER | 153* | 41893 | 150 | 11.4 | 0.18 | 0.23 | 0.07 |
| Peterbald | PBALD | 31* | 38776 | 300 | 22.2 | 0.17 | 0.24 | −0.05 |
| Ragdoll | RAG | 51* | 42927 | 250 | 10.4 | 0.19 | 0.25 | 0.05 |
| Russian Blue | RBLUE | 6 | — | — | 32.8 | 0.17 | 0.22 | 0.04 |
| Scottish Fold | SFOLD | 150* | 43182 | 150 | 8.1 | 0.2 | 0.25 | 0.05 |
| Selkirk Rex | SREX | 22 | 42131 | 150 | 17.6 | 0.19 | 0.25 | 0.016 |
| Siamese | SIA | 66* | 33711 | 400 | 26.5 | 0.15 | 0.19 | 0.063 |
| Siberian | SIR | 51* | 47587 | 50 | 7.1 | 0.21 | 0.28 | 0.007 |
| Singapura | SIN | 4 | — | — | — | — | — | — |
| Somali | SOM | 6 | — | — | 32.9 | 0.17 | 0.23 | −0.005 |
| Sphynx | SPH | 26 | 42551 | 200 | 18 | 0.19 | 0.25 | 0.03 |
| Tennessee Rex | TREX | 21 | — | — | — | — | — | — |
| Turkish Angora | ANG | 4 | — | — | — | — | — | — |
| Turkish Van | VAN | 20 | 47820 | 50 | 11.7 | 0.2 | 0.27 | 0.026 |
| Total Breeds | 41 | 1570 | ||||||
| Domestic | DOM | 262* | 50544 | <50 | 2.2 | 0.22 | 0.27 | 0.096 |
| Wildcat | FSI | 60 | 13059 | 750 | 36.2 | 0.05 | 0.06 | 0.24 |
| Colony | Colony | 139 | — | — | 10.9 | 0.2 | 0.27 | 0.0015 |
| Oriental/Toygers | HYD | 34 | — | — | 22.2 | 0.17 | 0.24 | −0.07 |
| Big Wild Cats | BIGW | 4 | — | — | — | — | — | — |
| Asian Leopard Cats | ALC | 9 | — | — | 94 | 0.008 | 0.012 | −0.05 |
| Total cats | 47 | 2078 |
*Sample size reduced to 25 most unrelated cats within a breed or population except for the wildcats for LD estimates. No LD was estimated for populations less than 20 individuals or the pedigree populations (Colony, TREX, LYK, and HYD), including 21 populations represented by 597 individuals.
†SNP number does not include X chromosome SNPs.
The reported values for MAF, observed heterozygosity, and inbreeding coefficient are means.
Genotyping accuracy, Mendelian errors and summary statistics
The comprehensive dataset of cats (n = 2,078) had a genotyping rate >90%. The array’s SNPs (n = 62,897) were evaluated for genotyping quality. Only ~1% (n = 625) of the SNPs were missing ≥10% of genotypes and were therefore excluded from downstream analysis (Supplementary Data File 6). The remaining SNPs were examined for Mendelian segregation using 86 trios from the research colony cross-breed pedigree49. All samples represented in the trios exhibited Mendelian errors in ≤2% of the markers, supporting familial relationships. Marker-specific Mendelian errors were identified for 232 SNPs, and each showed ≥10% errors (Supplementary Data File 7). Eleven of the SNPs with Mendelian errors also had a genotyping rate ≤90% across all samples, therefore, the total SNPs excluded were 846, leaving 62,051 SNPs for downstream analyses. The SNPs with Mendelian errors were assigned to the “unknown” chromosome (Chr 20) for a future potential use. Considering that X–linked SNPs could be located either in the pseudo-autosomal region where males could be heterozygous, X chromosome SNPs (n = 160) showed male heterozygote genotypes (errors) in ≥10% of 52 males within the trios (Supplementary Data File 8).
The feline array had an average SNP genotype call rate of ~99% in the 2,039 (98%) samples. Twenty cats were genotyped in replicas, including four samples replicated from the same DNA aliquot but genotyped on different arrays, one sample as a whole genome amplification, two samples represented by tumor tissue of the genotyped cat, and 13 samples replicated as part of separate studies from different DNA aliquots. SNP mismatches between repeated samples were calculated after removing SNPs with a genotyping rate ≤90% and after removing SNPs with Mendelian errors. The average mismatch between samples repeated from the same aliquot of DNA was 0.14%, ranging from 0 to 0.55%. However, the sample with the highest mismatches was a commonly used cat cell line (CCL-94; ATCC). The whole genome amplified DNA had 2.62% mismatches from the non-amplified DNA sample. The two samples represented by the tumor versus non-tumor tissue had 0.69% and 1.06% mismatches. The replicated samples from different DNA aliquots had an average of 0.48% mismatches, ranging from 0.07% to 0.85% (Supplementary Table 2).
After removing SNPs with a genotyping rate of ≤90%, all markers were evaluated for minor allele frequency (MAF) across all samples. None of the SNPs with low genotyping rates were of wildcat origin. Only 752 SNPs were monomorphic in all genotyped individuals, with the highest number of monomorphic SNPs on chromosome A1 (n = 89) and the lowest on chromosome E3 (n = 11) (Supplementary Data File 9). Additionally, 7,813 markers displayed a 0 < MAF ≤ 0.05, including, 2,628 markers with a 0 < MAF ≤ 0.01 (Supplementary Table 3). Overall, 59,423 SNPs (95%) on the cat array displayed high quality genotypes, proper Mendelian inheritance, and polymorphism across cat populations.
Four wild felids that were genotyped represent the most distant lineage from the domestic cat, including two lions and two tigers both from the Pantherine lineage4–6. These felids also had a per individual genotyping rate ≥90% and over ≥90% SNPs were successfully genotyped in the four wild felids combined. The Pantherine cats (BIGW, n = 4) exhibited very low polymorphism and only 1,754 SNPs were polymorphic. No genotypes were obtained from 3,733 SNPs for the large wild felids (BIGW). Asian Leopard cats (ALC, n = 9) were polymorphic for 3,547 SNPs. The European wildcats (n = 60) possessed a considerably higher number of polymorphic markers (n = 40,445). For the wildcat-specific SNPs, 2,576 of 4,240 (61%) were polymorphic with a MAF ≥ 0.05 within the domestic cats. In the Pantherine, 116 (2.7%) wildcat-specific SNPs were polymorphic.
Cat population structure analyses
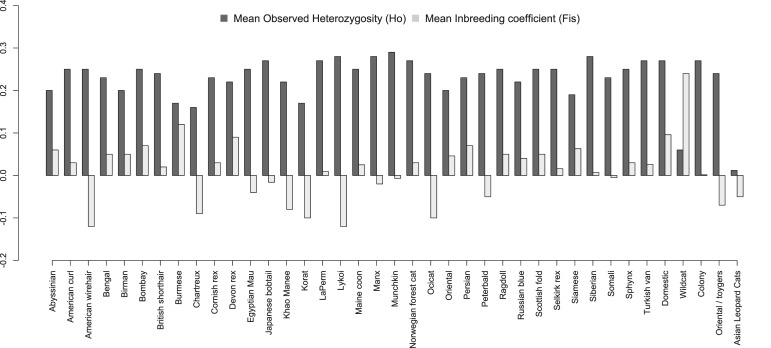
Breed-specific population summaries are presented in Table 1, Fig. 1. The average MAF across breeds and populations (excluding non-domestic cats) was 0.21. The LaPerm, Lykoi, Manx, Munchkin, and Siberian breeds had a slightly higher percentage of SNP heterozygosity compared to other breeds. Depending on cat breed, the percent of monomorphic SNPs were as low as 7% in the LaPerm cats (n = 4,659) and as high as 50% in Korat cats (n = 34,542). The mean MAF ranged from 0.11 in Korats and a high 0.22 for random bred cats, while the observed heterozygosity ranged from 0.16 for Burmese and Korats to 0.28 for Siberians. The population with the lowest number of monomorphic markers was the domestic shorthair population, which is believed to most closely mimic random bred cats, with only 1,410 non-informative markers (2%). The inbreeding coefficient (FIS) for the cat populations ranged from −0.12 for the Lykoi and American Wirehair breeds to 0.12 for the Burmese. Random bred domestic cats had an (FIS) of 0.096.
Figure 1.
Summary of population genetics of cat breeds and populations. Random bred cats have the highest measures of genetic variation whereas several breeds have critically low genetic variation, such as Burmese and Birman. Breeds that have been developed more recently from random bred populations, such as Siberians and Munchkins, have high diversity, as well as breeds continually pulled from random bred populations, such as the Manx cats from the Isle of Man. Note: Domestic group represents random bred samples where as Oriental/Toyger is pedigree.
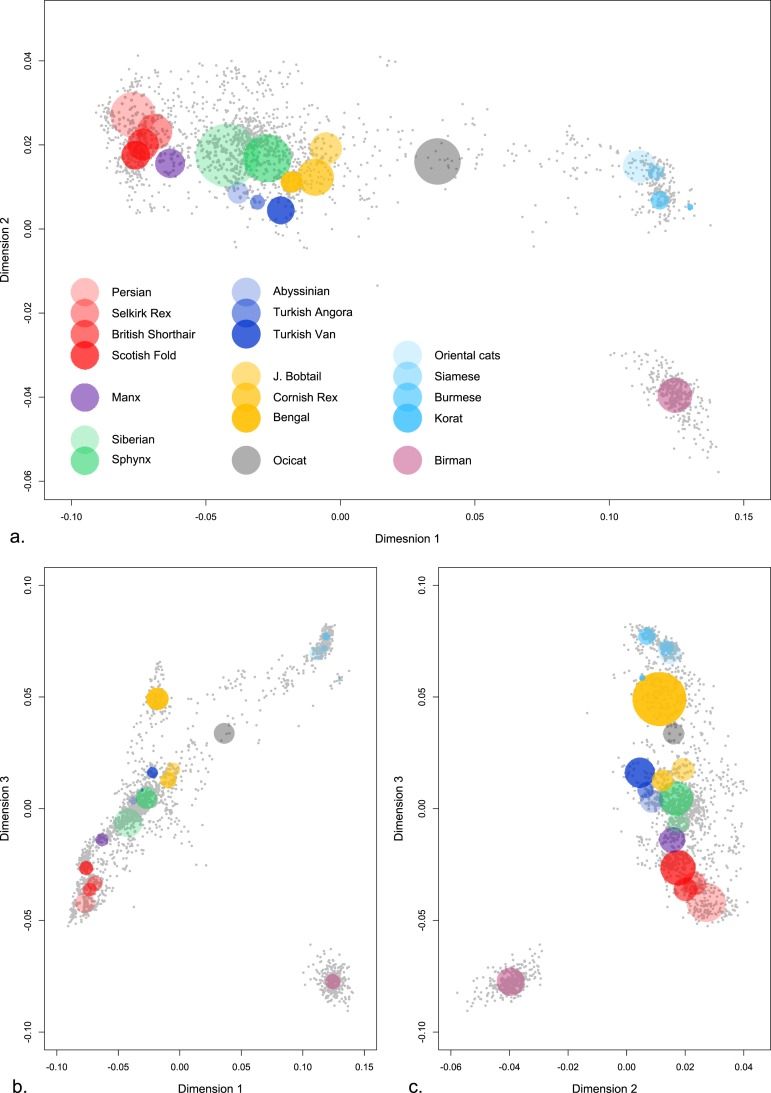
To visualize the relationship within and among cat breeds, all of 2,078 cats were assessed for population structure by multi-dimensional scaling (MDS). The MDS was performed using the 62,272 SNPs that had a call rate ≥90%. To illustrate breed structure, key breeds are highlighted in Fig. 2. Domestic cats were interspersed across all populations but a clear Western-Eastern distribution of the breeds was observed (Supplementary Figure 2a–c). Cat breeds with eastern origins, such as, Oriental, Siamese, Burmese, Korat and Birman, clustered at one side of the MDS plot, whereas, at the opposite extreme of the plot, the Persian breed family including, Persian, Selkirk Rex, British Shorthair, and Scottish Fold, clustered tightly at the opposite extreme of the plot. The Eastern-Western divide was observed on every combination of dimensions. The majority of the breeds clustered towards the Persian breeds (Fig. 2). Cats with Mediterranean origins, such as Turkish Angora, Turkish Van and potentially Abyssinians, formed groupings midway between the Eastern-Western origin breeds.
Figure 2.
Multi-dimensional scaling of cat breed genetic structure. Plots of the genetic distances between individual domestic cats in three dimensions (C1 vs. C2, C2 vs. C3, C1 vs. C3). Gray dots represent individual cats and collectively show the overall distribution of populations. Selected breeds are highlighted by a colored circle where each colored circle corresponds to a population. The positions of the circles and the sizes are drawn to qualitatively distinguish between popular cat breeds (see materials and methods). (a) dimension 1, (b) dimension 2, (c) dimension 3. The Birman breed (light purple) consistently is a highly distinctive population. Asian breeds (light blues) are highly distinct from Western breeds (reds). Ocicat (grey) are a breed developed by crossing Abyssinians with Siamese and are intermediate in the gradation of cat breeds. The MDS of each population is presented in Supplementary Figure 4.
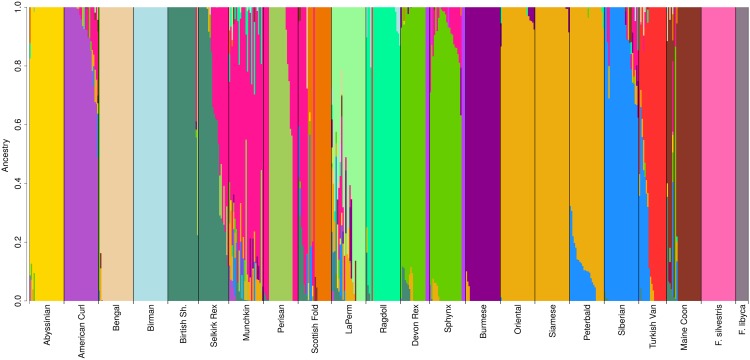
Additionally, the cat breed population structure was investigated using the Bayesian fastSTRUCTURE53 analysis. Approximately 99.99% of the genetic variation (K∅c statistic in fastSTRUCTURE) among the twenty cat breeds and two wild cat (F. silvestris and F. libyca) was explained by a K = 19 (Fig. 3). The ancestry profiles of the cat breeds follow a similar pattern as the MDS (see above) where Eastern breeds such as Oriental, Siamese, and Peterbald shared over 60% of their ancestry assignment to a common cluster. Similarly, the closely related Western breeds, British Shorthair and Selkirk Rex, displayed a clear-shared ancestry, including sharing of Persian lineages that are also common to the Scottish Fold and the Munchkin breeds. Breeds that were developed within the past 30 years, such as LaPerm and Munchkin, showed higher levels of admixture when compared to older established breeds, such as, Birman and Burmese.
Figure 3.
Population structure plot (K = 19) of twenty cat breeds and two wildcat populations. faststructure was used to examine the same cat populations as described for the MDS analyses. Cat breeds with the same colors indicate admixture and shared ancestry/cross-breeding. For examples, Peterbalds are derived from Siamese and Oriental lines and several breeds have been developed from Persian lineages, such as Munchkins and Scottish Fold, to obtain brachycephalic head structure.
Linkage disequilibrium
The genome-wide extent of linkage disequilibrium (LD) was measured using the squared correlation coefficient (r2) between pairs of autosomal SNPs on each chromosome, independently. Only SNPs with MAF ≥ 0.05 were included in the analysis for each breed, separately, therefore the number of markers varied between breeds (Table 1). Initially, the LD estimates were compared across five subpopulations of random bred cats (n = 10, 25, 50, 100 and 200 samples). The greatest difference in the r2 estimates was observed between a sample size of 10 and 25 (Supplementary Figure 3 and Supplementary Table 4). Therefore, further LD analyses and the Bayesian structure analyses were conducted on only populations with ~25 unrelated individuals.
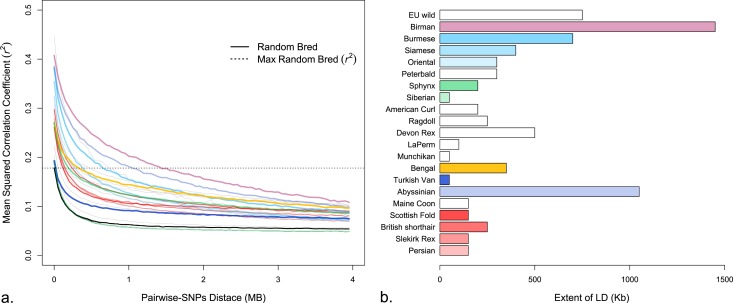
The genome-wide LD was estimated for twenty cat breeds, random bred cats and the European wildcat population (Fig. 4a and Table 1). As a measure of the extent of LD and to allow cross-population comparison, the maximum r2 value for the domestic cat (DOM) population was used as the cutoff point and the r2 value of comparison. Genome-wide LD among cat breeds ranged from 50 Kb in Munchkin, Siberian and Turkish van to a maximum of ~1,500 Kb in Birman cats. (Table 1, Fig. 4b and Supplementary Table 4). In general, Eastern breeds, which include Birman, Burmese, and Siamese, exhibited a larger extent of LD (1450, 700 and 400 Kb, respectively). The Persian family of breeds, which includes Persian, Selkirk Rex, British Shorthair and Scottish Fold, showed an intermediate extent of LD 150–250 Kb with little variation among the breeds. The Siberian, Munchkin, and Turkish van breeds displayed the lowest levels of LD at 50 Kb. The European wildcat population displayed an LD of 750 Kb.
Figure 4.
Genome-wide estimate of linkage disequilibrium (LD) of cat breeds. (a) Decay of LD (r2) at different bins of inter-SNP distances. LD decay of selected population is shown as a color (see (b) for key to colors) and remaining populations are shown in gray. Solid black decay line corresponds to the random bred population, to which all breed populations are compared. Horizontal dotted line represents the maximum of r2 value in random bred population and the point of comparison between populations (the point of LD < 50 Kb). (b) Extent of LD (Kb) where the r2 value reaches that of random bred population.
Genome-wide association analyses
To evaluate the power of the feline array for localizing traits via association analyses in cats, four aesthetic traits were chosen based on sufficient phenotypic documentation in the dataset. Of the four traits, three are inherited in an autosomal recessive fashion, specifically coat color loci Dense54, Color55–57, and the fur type Long58,59, and the X-linked Orange coloration locus43,60. Causative variants of the three autosomal traits were previously identified and were included on the array. The causative variant of X-linked Orange color is still unknown. The presence of the three phenotypic SNPs on the array allowed measuring the power of association under different population conditions (size or heterogeneity), in the presence or absence of artificial selection and allowed a comparison of association of the causative variant and adjacent SNPs. The SNPs associated with each trait and Pgenome values after permutation testing are presented in Table 2. The SNPs with the highest association to the traits are presented in Supplementary Table 5. All association studies remained genome-wide significant after permutation testing. Genomic inflation values are reported in Table 2.
Table 2.
Genome – wide associations to determine power of the cat DNA array.
| GWAS | MOI | Cases | Controls | ʎ | Chr. | Position | Haplotype length | P raw | P genome | SNPs post mperm |
|---|---|---|---|---|---|---|---|---|---|---|
| Dense *,† | AR | 33 | 81 | 1.06 | C1 | 218,100,114 | NA | 1.30e−20 | 0.00001 | 1 |
| - Burmese | AR | 30 | 56 | 1.46 | C1 | 218,200,114 | ~150 Kb | 3.79e−16 | 0.00002 | 15 |
| - Birman | AR | 60 | 41 | 1.24 | C1 | 218,100,114 | ~60 Kb | 8.08e−20 | 0.00002 | 13 |
| Long hair* | AR | 32 | 22 | 1.17 | B1 | 140,077,554 | ~150 Kb | 8.20e−10 | 0.00010 | 2 |
| Color (cs)* | AR | 21 | 28 | 1.41 | D1 | 46,341,460 | ~1 Mb | 2.00e−9 | 0.00040 | 10 |
| Orange | X | 24 | 69 | 1.11 | X | 107,777,134 | ~1.5 Mb | 1.20e−20 | 0.00002 | 7 |
*Causative variant is present on the array. †The power to detect Dense was first considered for random bred cats and then for breeds in which the trait is under selection.
Autosomal recessive trait in the random bred population
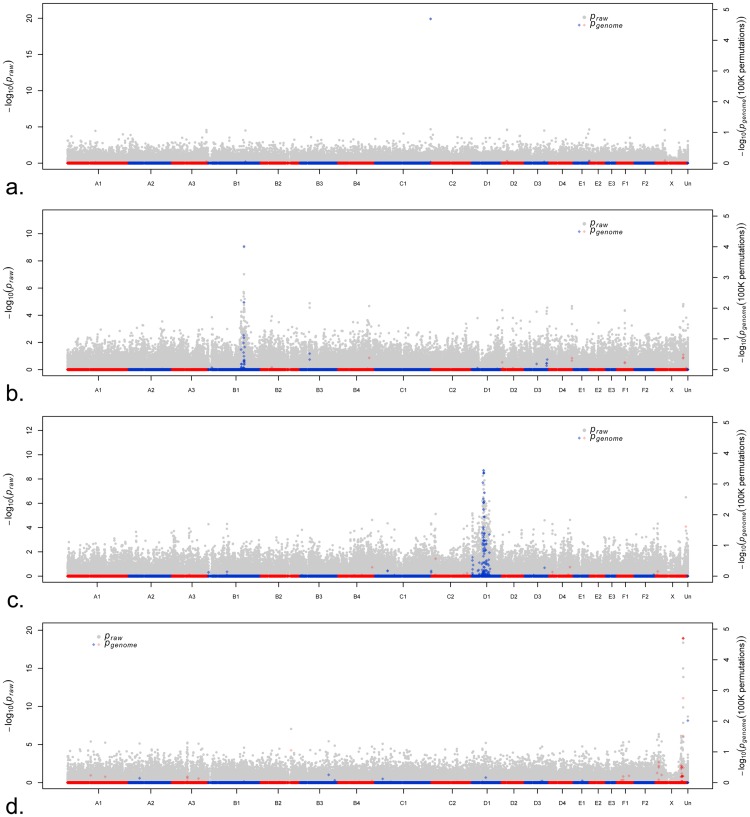
Thirty-three cases and 81 controls of domestic cats were selected for the association of Dense (a.k.a Dilute coat color), a trait not under selection in random bred cats (Table 2 and Supplementary Table 5). A single significant SNP, located on chromosome C1 at position 218,100,114, was associated with the phenotype (raw Pvalue = 1.3e−20), which is the causal variant within Melanophilin (MLPH)54 (Fig. 5a). For the closest SNP to the MLPH causative variant to show a significant association, the number of samples would need to be increased from 114 to 427 when using the current density array (Supplementary Figure 4a). The flanking SNPs were 39 and 22 Kb from the causal SNP.
Figure 5.
Illustrative genome-wide association analyses for four phenotypic traits in the domestic cats. Manhattan plots of the association analyses where x-axis represents chromosomes, gray dots and left y-axis represent raw P-values of the association, and red/blue dots and right y-axis represent the permuted P-values. (a–c) Remapping of three autosomal recessive traits (Dense, Long, and Color (cs allele), respectively) and (d) X – linked Orange using different populations. (a) Only the causal SNP for Dilute is associated in random bred cats on cat chromosome C1. (b) Several SNPs are associated with the long hair phenotype on chromosome B1 in LaPerm, a newer breed but with little selection for the trait. (c) Several SNPs are associated for the cs allele in Color on chromosome D1 in Persians, one of the oldest breeds where the coloration has some positive selection. (d) GWAS of Orange, an X-linked trait, suggesting a critical region for the locus.
In comparison to the Dense association in random bred cats without selection, 30 cases and 56 controls were used to perform the same GWAS within the Burmese breed and 60 cases and 41 controls within the Birman breed with selection for the trait. Several SNPs detected association together with the causal variant (raw Pvalue = 3.79e−16 in Burmese and raw Pvalue = 8.08e−20 in Birman). While the analysis with random bred samples showed an association only with the causative variant, Burmese exhibited a ~150 Kb haplotype block (position 218,100,114–218,250,626) and Birman had a ~60 Kb haplotype block (position 218,060,712–218,122,590) across all cases. This comparison showed how an association analysis within a breed with positive selection for a trait is likely to be more successful than in a random bred population. Furthermore, while achieving an association of the closest marker with the causative variant in random bred cats requires significantly increasing the number of samples or markers, an association can be detected in Burmese even when reducing the number of samples from 101 to 37 (13 cases and 24 controls); the most significantly associated SNP remained statistically significant (Pvalue = 1.05e−6) after permutations (Pgenome 0.009). In Burmese, a unique haplotype of ~4.5 Mb containing the causal variant for the phenotype was detected across all cases while in Birman a unique haplotype of ~200 Kb containing the causal variant was identified in all cases. The SNP composition of the Birman and Burmese haplotypes were different, including within the 200 Kb haplotype that is within the 4.5 Mb haplotype of the Burmese.
The Chartreux, Russian blue, and Korat breeds are fixed for the variant in Dense and the region of homozygosity for these cats extended 190 Kb in Chartreux and Russian blue and 280 Kb in Korat.
Autosomal recessive trait in a breed, without selection
The LaPerm breed is characterized by its curly coat texture and comes in both longhair and shorthair varieties20. However, only the curly coat texture is consistently selected in the breed while the longhair variant is not under selection. LaPerm breed displayed low LD (100 Kb) and high polymorphism (7.5% monomorphic SNPs). Thirty-two cases (longhair) and 22 controls (shorthair) of the LaPerm breed were selected to perform a GWAS for the longhair trait. (Table 2, Supplementary Table 5). The most common causative variant for longhair is in fibroblast growth factor 5 (FGF5)58,59, which is located on chromosome B1 (at position 140,077,554 of the 6.2 genome assembly)46. The FGF5 causative variant was the most significantly associated with the hair length phenotype (raw Pvalue of 8.2e−10), in addition to several other adjacent SNPs (Fig. 5b and Supplementary Figure 4b). For the closest SNP to the causative variant within FGF5 to have similar association power, the number of samples would need to be marginally increased from 54 to 66 cats.
Breeds fixed for longhair include Maine Coon, Norwegian Forest Cat, Persian, Ragdoll, Siberian and Turkish Angora. The regions of homozygosity surrounding FGF5 in these breeds flanked the causal variant for longhair on the array by 382 Kb, while the length of the haplotype block in the LaPerm breed was 150 Kb.
Autosomal recessive trait in a breed and under selection
Pointed cats have a variant at the Color (c) locus within Tyrosinase (TYR) and have a darker coat color on the ears, face, paws and tail55. Using pointed (cscs) Persian cats (a.k.a. Himalayans) as cases and non-pointed Persian cats as controls (Table 2 and Supplementary Table 5), many significantly associated SNPs were identified on chromosome D1 near position 46,341,460 (Fig. 5c and Supplementary Figure 4c). The power of SNPs in the TYR region to detect association was very similar to that of the causative variant due to complete linkage between markers. To obtain the same power as the causative variant using adjacent linked SNPs, the number of samples would need to be increased from 49 to 50. The length of the haplotype block containing the variant in the Himalayan cases was 1 Mb. Points are fixed for cs allele in Siamese and Birman, and the cb allele in Burmese and the haplotype block is 430 Kb, 480 Kb and 4.2 Mb, respectively.
X-linked trait in a cross - breed analysis
The X-linked Orange coloration43 was localized using cases (24) and controls (69) from multiple breeds from the dataset as previously described in Gandolfi et al.61. Orange was localized to the X chromosome by allelic association (most significantly associated SNPs at positions 107,777,134 and 107,994,240 with raw Pvalue = 1.8e−19 and 4.3e−19, respectively), and by Cochran-Mantel-Haenszel test (CMH) to position 107,777,134 with raw Pvalue = 4.4e−5 (Table 2, Fig. 5, Supplementary Table 5 and Supplementary Figure 5). The associated markers reside in the same linkage region identified previously43. A haplotype for Orange was evaluated by exporting 5 Mb of genotypes, from position 105 Mb to position 109 Mb of the X chromosome (107 SNPs). A haplotype block was detected from position 106,241,242 to position 107,745,900 (~1.5 Mb) of the X chromosome (Supplementary Figure 6). The haplotype block contains 12 genes, listed in Supplementary Table 6.
Discussion
Low-density genotyping arrays are available for a variety of species. The design of the feline array benefitted from the results and outcomes from the designs for dog62, cow63, pig64 and horse65. At the time of SNP selection the cat genome assembly was not as robust as these other species, however, the selection of widely diverse cat breeds and domestic cats from diverse regions of the world supported the identification of >10 million SNPs for array design47. The final array contains ~63K variants, the highest number of SNPs when compared to the first-generation equine (54.6K), canine (49.6K) and bovine (58.3K) arrays63,65,66. This low density array is highly suitable for Mendelian trait analyses, particularly in cat breeds.
The position of the SNPs was based on the feline genome assembly FelCat 4 (Felis catus 5.8). After SNP remapping to the latest feline genome assembly FelCat 8.0 (Felis catus 8), only 704 SNPs (1.1%) remained unassigned, a significant improvement from remapping to cat Felis_Catus_6.2 by Alhaddad et al.49, where 6,893 SNPs had unknown locations. Marker coverage on the X chromosome is not as robust, likely due to the complexity of the X chromosome and the high density of repetitive sequences67. The feline inter-marker average distance of 37.7 Kb is equivalent to cattle63 and denser than the horse array, which has a ~43 Kb inter-marker distance65. The cat, cow, and horse genomes (2.64 Gb, 2.70 Gb, 2.42 Gb, respectively) are roughly equivalent in size. Although the feline genome assembly contains several gaps (~40 Mb) and unplaced scaffolds46, the inter-marker distances suggest balanced and slightly better coverage of the cat genome than for other species with early lower density arrays. However, the 20 gaps >500 Kb in the cat SNPs is higher than horse, with only 12 gaps >500 Kb and cow, where the highest gap between SNPs is <350 Kb63,65.
The cat array demonstrates a very low number of SNPs with low genotyping rate (625 SNPs, <0.01%) across ~2,000 samples, a low number of SNPs with Mendelian errors (n = 232, 0.004%), leaving 62,051 robust SNPs for downstream analysis. The number of SNPs excluded for low genotyping rate and Mendelian transmission errors is lower than that of cow and horse (0.09% and 0.05%, respectively)63,65. Thus, exclusion of ~1K SNPs for the array analysis is comparable to other first-generation arrays63,65.
Moreover, the presence of duplicate controls confirms the high reproducibility of the genotypes, with a negligible number of errors between replicates from the same aliquot of DNA. Slightly higher mismatch rates were observed in tumor versus genomic DNA and a cell line, both likely due to somatic mutation heterogeneity. The error rate between WGA samples and the original sample was 2.62%. Thus, excluding SNPs from analyses with a MAF ≤ 0.03 instead of the typical 0.05 may be acceptable. The removal of poor quality SNPs did not significantly affect mismatch rates. The mismatch rate is 10-fold higher than reported in cattle63.
The average MAF was variable across breeds, ranging from 0.11 for Korats to 0.22 for random bred cats. The average MAF of domestic cat populations was 0.18, which is lower than cows (0.26)63 and horses (0.24)65. Specifically, 2,628 SNPs (~4%) showed a MAF < 0.01 across all samples. This observed MAF is lower than other species, and is likely due to inclusion of SNPs that were specific to one wildcat species.
Although a Burmese cat was used as part of SNP sequencing discovery panel47, the percentage of monomorphic SNPs was the highest, at ~31%. For Burmese, the low number of polymorphic SNPs confirms the high inbreeding coefficient in the breed and inbreeding history68,69. A high number of monomorphic SNPs were observed in the large wild felids of the genus Panthera (lions and tigers; 94%), which is consistent with previous reports8. Even with limited numbers of polymorphic SNPs on the array for large wild felids, the remainder of polymorphic SNPs can be used for conservation and zoo management applications. A substantial number of SNPs (63.8%) are informative for European wildcats. These thousands of polymorphic markers may be useful for population and conservation studies, especially in wildcat subspecies70. However, the cat 63K array is unlikely to be useful for disease mapping studies in distant wild felids.
The MDS clustering and Structure analyses confirmed the known origins of the cat breeds and their relationships68,69. The cat breeds displayed a continuum on the MDS plots, however, three main clusters are observed representing cat breeds with Western, Central and Eastern origins. The Western breeds were represented mainly by the Persian family71, clustering in the second and third dimension as well, confirming a strong Persian genetic influence in British shorthair, Selkirk Rex and Scottish Fold, and in agreement with previous STR and SNP based studies71,72. Previously unstudied breeds, such as American Curls and Peterbalds demonstrated their Western and Eastern origins, respectively.
Breeds with Eastern origins (Birman, Havana Brown, Khao Manee, Korat, Oriental Shorthair, Peterbald, Siamese and Singapura) are found at the opposite end of the MDS and showed shared ancestry. The Birman cats are strongly clustered but genetically distinct from other Eastern breeds. The difference between the Birman clustering compared to results from previous study61 may be explained by the presence of a high number of related individuals that belong to mainly two big pedigrees of Birman cats. The Abyssinian breed clustered with the central origin breeds in the MDS that includes only domestic cats, specifically with Siberian in the 2nd and 3rd dimension. However, the close clustering with Siberian cats does not reflect the historical development of the breed. In previous studies, the Siberian breed was suggested to be genetically distinct from the other breeds61,68. The cross-bred Ocicat, an Abyssinian and Siamese hybrid, clustered in between the central and Asian breeds, showing both the European and/or Asian genetic influences69,73.
The present study represents the genome-wide LD estimation in cats and is in overall agreement with the previously reported estimates using selected regions73. The greatest difference of LD estimates (r2 values) was found between 10 and 25 samples of random bred individuals. As a result, LD was calculated for breeds and populations represented by at least 20 individuals. Eight breeds (Abyssinian, Birman, Burmese, Maine Coon, Persian, Siamese, Siberian and Turkish Van) and random bred cats displayed LD estimates that were similar to previously published results73. In contrast, a substantial difference in LD is evident for Abyssinian and Birman cats, where the LD was 10 and 7-fold higher, respectively, using genome-wide data. A significant difference was also observed in Siamese, where the LD was estimated at almost twice as long (400 Kb vs 230 Kb) as detected in the previous study. The discrepancy in LD estimates for these breeds is likely related to the size of region and number of SNPs used. Overall, Eastern breeds tended to have higher levels of LD (Birman, Burmese, Oriental shorthair, Peterbald and Siamese) relative to central and Western breeds.
The short LD of some cat breeds can be explained by (1) a large breeding population, such as Persian and Persian-derived breeds, (2) limited selection, whereby several possible coat colors are permitted (American Curl, LaPerm and Maine Coon), and (3) active outbreeding strategy (Munchkin), or random bred based breeds (Siberian). Persian and Persian-derived cats showed very similar levels of LD, as well as in Eastern breeds, such the Oriental Shorthair, which was used in the development of the Peterbald. The random bred population showed very low levels of LD, and breeds such as Munchkin, Siberian and Turkish Van displayed a haplotype structure similar to the random bred population, which is consistent with their breed history. Haplotypes length and LD levels also reflect the number of successful GWAS conducted in several cat breeds49,72,74,75.
The main application of a high-density array is the localization of simple Mendelian diseases and traits of interest. Using the presence of phenotypic SNPs on the feline array, several association scenarios were conducted and the power of the array was examined by comparing the p-values and LD of genotyped phenotypic SNPs (causative) to that of the surrounding SNPs. The first scenario was a GWAS for the recessive Dense54 trait that is not under selection using 114 random bred cats. As expected, the association identified only the causative variant (c.83delT in Melanophilin (MLPH)), and association analyses using random bred samples will require a denser array or a larger number of samples. When the same trait was analyzed using two breeds (Burmese and Birman) where the trait is under selection only in certain lines, a large haplotype block was associated using substantially fewer samples (n = 37) compared with random bred cats.
The second scenario using LaPerm cats identified a significant association of the most common FGF5 variant (c.475A > C) for Long fur length58. The LaPerm breed is defined by and selected for curly coat texture but exists in longhair and shorthair varieties20. Despite the absence of positive selection for the variant, along with low LD, and high polymorphism within the breed, a significant association was detected with SNPs linked to the FGF5 variant. Clearly, GWAS using cat breeds with traits under selection is more efficient than studies within random bred cats.
The third scenario analyzed the association of the Color mutation c.940G > A within Tyrosinase (TYR)55,56. The TYR variant is under positive selection in Himalayan cats, which have low LD and low inbreeding. A significant association was detected by multiple SNPs linked to the genotyped TYR variant and a haplotype block is shared among Himalayan cats.
The fourth scenario localized and refined the region of the unknown X-linked Orange locus43,60. The association analysis across breeds refined the region of association to a 1.5 Mb haplotype block. The region contains twelve genes, and after visual inspection of the genes and their function, a candidate was not apparent. Additional mapping efforts are required to refine the position of the locus and to identify candidate causal variant(s). This analysis, in addition to its contribution to refining the region of Orange, illustrates the efficiency of performing association analysis of X-linked traits, in random bred cats with no selection for the trait.
Array success and applications
Preliminary predictions of the strength of population structuring and high LD in dog breeds suggested only 5,000 to 30,000 SNP markers were required to achieve complete coverage of the dog genome76, compared to an estimated 200,000 to 500,000 SNP markers in humans77, making GWAS in dogs both cheaper and easier to conduct77,78. Considering both the Illumina Canine SNP20 and the Affymetrix Canine V 2.0 Platinum Panel array, many GWAS in canines have been conducted with ~30 cases and controls. More complex traits79,80 obviously require more samples and hence the development of higher density arrays. Transmission distortion testing (TDT) has been successful with only 7–13 discordant sib-pairs in canine studies81,82. The feline array has also proven its utility within breeds and supported the genetic dissection of simple49,61,72,75,83,84 and complex traits52,85,86. The array clearly shows significant association power for traits under selection or recessive traits. Examples of successful GWAS for diseases include the frontonasal dysplasia in Burmese84, congenital myasthenic syndrome in Devon Rex83, and hypokalemia in Burmese75. Identifying the curly hair variant of Selkirk Rex and the variant for folded ears in Scottish Fold are examples of dominant traits that are under positive selection72,74. A comparable number of cases and controls have been used in these cat studies with minimal cases required for studies in the breeds with the highest LD, such as the Burmese. Many cat breeds are younger in breed development, such as Siberians, or still represent indigenous populations, such as the Manx cats on the Isle of Man, hence an association study in breeds with low LD more likely requires a higher number of samples or a denser array to provide a statistically significant association while analyses of random bred populations likely requires a significantly denser array.
Beyond the successful GWAS approaches presented here and published before, the feline SNP array enabled (1) the development of a high density linkage map48 that has supported the newer genome assembly, (2) an understanding of genetic variation within and between cat breeds61,72, (3) high resolution descriptions of genomic consequences of the selective sweeps61,84, and (4) a more fully refined comparative model for human biomedical research83,84.
Materials and Methods
Data availability
All data generated in the project is available in Supplementary information files included in the article for download.
Ethical statements
Sampling of cats for this study was approved by the Animal Care and Use Committee (ACUC) of the University of California, Davis (protocol # 16991) and the University of Missouri (Protocol # 7808) and samples were collected in accordance with the guidelines and regulations. Samples were acquired by specialists in the field, such as veterinarians, or voluntarily donated by owners and breeders.
SNP selection for array design
SNPs were identified from one cat of each breed representing American Shorthair, Cornish Rex, European Burmese, Persian, Ragdoll and Siamese, as well as one South African wildcat (Felis silvestris cafra)47. The re-sequencing efforts identified over three million polymorphisms with 964K common SNPs suitable for the design of a domestic cat genotyping array and 849K SNPs were likely to have an informative minor allele frequency >5% across cat breeds. Additional SNPs were identified from four pooled individuals representing six breeds, including Birman, Egyptian Mau (n = 1), Japanese Bobtail, Maine Coon (n = 5), Norwegian Forest cat and Turkish Van. Random bred cats with Eastern and Western origins, as well as two Felis silvestris and two Felis libyca, also assisted SNP identification47. Over nine million SNPs were identified from the deep re-sequencing of the cat genome.
A preliminary build of the cat genome, (FelCat 4, Felis Catus 5.8), was used to estimate spacing between SNPs. After exclusion of SNPs based on minor allele frequency (<0.25), near or within a sequence repeat, within a duplicated region, or with more than two alleles, approximately 1 million SNPs were submitted to Illumina for design of the DNA array. A vast majority of the SNPs have a one bead assay design and were mainly targeted as single copy, intergenic and intronic SNPs.
Remapping array SNPs to the newest 8.0 cat genome assembly
To determine the exact coordinate of each variant in Felis_catus_8.0, the following analyses were performed. For each SNP, 100 bp of upstream and downstream sequence was aligned to Felis_catus_8.0 using the program blat87. The entire Felis_catus_8.0 reference sequence was used in the alignment rather than performing multiple alignments with separate chromosome sequences. The program was run in default mode to generate alignments, with a minimum of 11 bp of matching sequence to initiate an alignment (tileSize = 11) and at least 90% matching bases required (minIdentity = 90). The number of tile matches was 2 (minMatch = 2), the minimum score was 30 (minScore = 30), and the size of the maximum gap between tiles in a clump was 2 (maxGap = 2). The best matches were selected to determine the location of each pair of sequences (e.g., [upstream/downstream]) in the assembly and coordinates obtained. The remapped map file is available in Supplementary Data 2, which contains original SNP position and array identification number, the Felis_catus_6.2 position and the Felis_catus_8.0 position.
Animals
A dataset comprised of 2,078 samples from 47 different groups/populations were genotyped on the Illumina Infinium iSelect cat array (Illumina, San Diego) as previously described75. The individuals from most populations were selected with minimal relationships based on pedigree analysis for case-control analysis or population studies (Supplementary Figure 5). The Birman52, Lykoi, and Tennessee Rex breeds, as well as the Oriental/Toyger pedigree and colony cross-breed groups51, contained related individuals. The research colony cats were used for the segregation analyses49. PLINK88 was used to obtain the genotyping rate for each sample. Coat color, texture and fur length information were available for the majority of the samples genotyped.
Genotyping accuracy, Mendelian errors and summary statistics
Quality control analyses for SNPs data were conducted using PLINK88. A dataset comprised of 2,078 samples were genotyped on the Illumina Infinium iSelect SNP array. SNPs with genotyping rate >90% across the dataset were identified using the command–geno 0.1.
A multi-generational cross-bred pedigree comprised of 86 trios (100 individuals – 52 males and 48 females) was used to determine marker-specific significant Mendelian errors49. Using the function–mendel, percent Mendelian errors per individual sample and per SNP were estimated. SNPs exhibiting ≥10% Mendelian errors were reported as significant errors. The distribution of SNPs with errors was investigated for each chromosome. Male-specific Mendelian errors or SNPs located in the pseudo-autosomal region of the X chromosome were determined by examining heterozygous X-chromosome genotypes in males (n = 52). SNPs exhibiting 10% or more of an X-chromosome in the males were reported as likely pseudo-autosomal SNPs.
Genotypic differences between replicates were analyzed for 20 samples. The genotypes of the original and the replicate samples were determined to be identical using the function (identical) in R base. The number of instances where a mismatch was detected were counted and presented. The number of discordant genotypes for each duplicated sample was determined across all SNPs (n = 62,897), after removing SNPs missing 10% genotypes (n = 62,272), and after removing SNPs missing 10% genotypes and with Mendelian errors (n = 62,051).
For each population independently, the following summary statistics were calculated using PLINK88, the function–freq was used to calculate (1) the number of monomorphic SNPs, and (2) the mean and standard deviation of minor allele frequency (MAF). (3) The mean and standard deviation of observed were obtained using the function–hardy. The number and frequency of all polymorphic SNPs (n = 62,272) for a dataset containing all domestic cat breeds combined was determined using the PLINK function (–freq). The numbers of SNPs within different minor allele frequencies bins are reported.
Population inbreeding and structure analysis
The observed heterozygosity and the inbreeding coefficient were both calculated per individual using–het command in PLINK (v1.9) and the mean of the values for each population were reported.
To depict the genetic relationships between populations and individuals within each population, pairwise genetic distances between all individuals in the dataset were calculated in Plink88 using the–genome function. The genetic distances obtained were used to generate a multi-dimensional scaling of the genetic distances between individuals (using the command–mds-plot). Three dimensions were used to visualize the genetic population structure of breeds. Each population was plotted in relation to all other populations in three combinations of dimensions (C1 vs C2, C1 vs C3 and C2 vs C3). The entire dataset was plotted and open circles were used to show the position of the populations. The circles represent a qualitative depiction of the position of a population and drawn as follows. For each population (A), the position of the circle was determined by mean (dimension1), mean (dimension2), whereas the radius of the circle was chosen using the largest of the standard deviations of (dimension 1 or 2). Each of the three combinations of dimensions (C1 vs C2, C1 vs C3, C2 vs C3) was plotted separately.
Additionally, the utility of the array data in identifying levels of population admixture was examined via fastSTRUCTURE53 (version 1.0). To reduce the effects of uneven sample sizes between populations89, only unrelated samples from twenty breed and two wildcat populations (n = 519), which are equal in size (see populations used for LD analysis below) were used in the analysis. The autosomal SNPs of all samples were used and SNPs with a MAF less than 0.01 (n = 1198) were removed, which resulted in 57,690 SNPs to be used in the analysis. fastSTRUCTURE53 was run to determine the genomic contribution of K (K = 1–20) hypothetical populations. Two outputted metrics were considered to determine the appropriate values of K: (1) the K that maximizes the log-marginal likelihood lower bound and (2) the minimum value of K that accounts for 99.99% cumulative ancestry.
Selection of unrelated samples and linkage disequilibrium analysis
To unbiasedly measure the genome-wide extent of linkage disequilibrium (LD) in cat breeds, a number of criteria were considered including, (1) the LD statistic, (2) number of individuals per breed, (3) degree of relatedness among individuals within a breed, and (4) the statistical point (r2 value) of comparison between breeds. The pairwise squared correlation coefficient (r2) was used as a measure of LD between any two autosomal markers on the same chromosome as previously described73. To assess the effects of sample size on the measure of the extent of LD, a dataset of domestic random-bred (DOM) cats (n = 270) was examined by randomly selected (without replacement) individuals to represent five populations of different sample sizes (specifically, 10, 25, 50, 100, and 200 individuals). For each of the DOM subgroups, r2 were calculated, as described above. The effect of the sample size was measured by comparing the r2 values between the five subgroups.
As an outcome of the assessment of the effects of sample size on LD measure (see results), only the breeds represented by 20–30 unrelated individuals were used in the LD analysis. To ensure unbiased measure of LD due to relatedness, the individuals representing each breed were selected based on the lowest identity by descent (IBD) values. IBD values were obtained using the command–genome using PLINK88. For each population independently, r2 was calculated for autosomal markers that exhibited (MAF ≥ 0.05) and analyses were performed using Haploview90.
Pairwise r2 estimates between autosomal markers on the same chromosome were jointly categorized into distance bins of 50 Kb. The range of distances between markers included in the estimation of the extent of LD was 50 Kb–4 Mb. In each distance bin, the mean LD estimate was used as the representative of the statistic. The decay of LD was determined by connecting the statistic r2 mean at every distance bin. To objectively report the extent of LD, the maximum value of r2 found in the random bred population was used as the r2 value of comparison. This r2 value represented lack of LD or extent of LD smaller than 50 Kb that is seen in the random bred population (DOM).
Remapping of known coat colors loci using GWAS
Three autosomal recessive traits in cats: Dense coloration54, Long hair58,59 and points allele (cs) for the Color locus55,56 and one sex-linked trait, the Orange coloration locus43,60,91,92, were analyzed. The number of cats used in each analysis is listed in Table 2. For the recessive traits, the case-control associations (−assoc) were performed with PLINK88 using subsets of samples from the available 2,078 sample dataset. The GWAS to localize Dense was performed on three different datasets: random bred cats, Burmese and Birman. Haplotypes for the locus were identified by exporting genotypes from position 216 Mb to position 221 Mb of chromosome C1 and analyzed visually. Haplotypes were exported for each trait using PLINK88 5 Mb 5′ and 3′ of each causal variant and visually inspected. Haplotypes for Dense were exported in Chartreaux, Korat and Russian Blue, haplotypes for Color were exported in Birman, Burmese and Siamese and haplotypes for Long fur were exported for Maine Coon, Norwegian Forest cat, Persian, Ragdoll, Siberian and Turkish Angora in the sample sets used for the GWAS associations or on the available cats in the dataset.
For the X-linked Orange association, samples from different breeds were selected. Two analyses were conducted for the cross-breed Orange association. The first analysis was performed using chi-square tests for allelic association with individuals from different breeds (–assoc) and the second analysis accounts for population stratification by applying the Cochran-Mantel-Haenszel (CMH) test (–mh). Cats were clustered for the CMH test on the basis of the pair-wise population concordance (PPC) test, with a p-value of 0.01 set for merging individuals (–cluster, –ppc 0.01). Only samples and markers with a genotyping rate >90% and markers with MAF ≥ 0.05 were selected for each association analysis independently. Genomic inflation in the association measures of the p-values was evaluated by calculating the genomic inflation factor (ƛ) using PLINK88 (–adjust). To determine significance, multiple testing correction was accomplished with 100,000 permutation using PLINK88 (–mperm). T-max permuted p-values were considered genome-wide significant at p < 0.05. A Manhattan plot of the genome-wide p-values and permuted p-values were generated using a custom R script. The haplotype for the Orange locus was explored by exporting genotypes from position 105 Mb to positon 109 Mb of the X chromosome and then analyzed visually.
Considering the presence of the causative markers of the three phenotypes on the array, the power of association using the array was calculated by measuring the LD (squared correlation coefficient – r2) between the causative variants and nearby SNPs. The LD between the closest SNP and the causative was used to calculate the power of the current array to detect SNPs density and the sample size needed to detect a significant association93.
Electronic supplementary material
Acknowledgements
Funding for this project was provided by the Office of Research Infrastructure Programs/OD R24OD01092, the Winn Feline Foundation (grant numbers: D12FE-505, D12FE-506, D12FE-507, D12FE-508, D12FE-509, D12FE-551, D12FE-552, D12FE-557, D12FE-558, D12FE-559, D12FE-560, D12FE-562, MT-08–001, W09-008, W10-015, W12-022), the Cat Health Network (grant numbers: D12FE-508, D12FE-510, D12FE-553, D12FE-556), the George and Phyllis Miller Feline Health Fund, Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis (grant numbers: 2008-06-F, 2008-36-F, 2009-07-F/M, 2009-39-F/M, 2011-51-F/M), the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health, the Academy of Finland, and the Jane Aatos Erkko foundation, Biocentrum Helsinki. We appreciate the contribution of the numerous cat breeders who have provided DNA samples.
Author Contributions
B.G., H.A., L.A.L. conceived the idea, planned the experiments, and wrote the manuscript. L.A.L. provided the experimental supplies, samples and/or data was provided by B.W.D., N.H.D., E.I.G., B.H., J.H., C.R.H., H.L., M.L., R.M., K.M.M., J.C.M., W.J.M., N.C.P., C.R., R.S., G.D.S., W.C.W., W.M. Data analysis was performed by B.G., H.A., M.A., E.C.K., M.J.M., S.M.N., J.E.D. Laboratory experiments were performed by B.G., H.A., L.H.B., E.K.C., J.C.G., R.A.G., M.J.H., J.D.K., C.B.P. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Barbara Gandolfi and Hasan Alhaddad contributed equally to this work.
Change history
3/12/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Change history
6/4/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Contributor Information
Hasan Alhaddad, Email: hhalhaddad@gmail.com.
Leslie A. Lyons, Email: lyonsla@missouri.edu
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25438-0.
References
- 1.APPMA. National Pet Owner’s Survey. (American Pet Product Manufacturing Association 2008).
- 2.AVMA. US Pet Ownership and Demographics Sourcebook. (American Veterinary Medical Association 2007).
- 3.Nicholas, F. W., Brown, S. C. & Le Tissier, P. R. Online Mendelian Inheritance in Animals, OMIA, http://omia.angis.org.au (1998).
- 4.Sunquist, M. S. F. Wild Cats of the World. (University of Chicago Press 2002).
- 5.Nowak, R. M. Walker’s Mammals of the World. (The Johns Hopkins University Press 1999).
- 6.Kitchener, A. C. et al. A revised taxonomy of the Felidae: The final report of the Cat Classification Task Force of the IUCN Cat Specialist Group. Cat News (2017).
- 7.Johnson WE, et al. The Late Miocene radiation of modern Felidae: A genetic assessment. Science. 2006;311:73–77. doi: 10.1126/science.1122277. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Davis BW, Eizirik E, Murphy WJ. Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae) Genome research. 2016;26:1–11. doi: 10.1101/gr.186668.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigne JD, et al. First wave of cultivators spread to Cyprus at least 10,600 y ago. P Natl Acad Sci USA. 2012;109:8445–8449. doi: 10.1073/pnas.1201693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driscoll CA, et al. The Near Eastern origin of cat domestication. Science. 2007;317:519–523. doi: 10.1126/science.1139518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottoni C, et al. The palaeogenetics of cat dispersal in the ancient world. Nature Ecology & Evolution. 2017;1:0139. doi: 10.1038/s41559-017-0139. [DOI] [Google Scholar]
- 12.Clutton-Brock, J. A. A Natural History of Domesticated Mammals. (Cambridge University Press 1987).
- 13.Hemmer, H. Domestication: The Decline of Environmental Appreciation. (Cambridge University Press 1990).
- 14.Dobney K, Larson G. Genetics and animal domestication: new windows on an elusive process. Journal of Zoology. 2006;269:261–271. [Google Scholar]
- 15.Larson G, Fuller DQ. The Evolution of Animal Domestication. Annual Review of Ecology, Evolution, and Systematics. 2014;45(115):136. [Google Scholar]
- 16.Wiener P, Wilkinson S. Deciphering the genetic basis of animal domestication. Proc Biol Sci. 2011;278:3161–3170. doi: 10.1098/rspb.2011.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crystal Palace - Summer concert today Cat Show on July 13. Penny Illustrated Paper, Amusement:510, July 08, 11 (1871).
- 18.In New York Times (New York 1881).
- 19.CFA. Cat Fanciers’ Association, http://www.cfa.org/ (2017).
- 20.TICA. The International Cat Association, http://www.tica.org/ (2017).
- 21.GCCF. The Governing Council of the Cat Fancy, http://www.gccfcats.org (2017).
- 22.FIFe. Federation Internationale Feline, fifeweb.org/ (2017).
- 23.Morris, D. Cat breeds of the world, (Penguin Books, 1999).
- 24.WCF. World Cat Federation, http://www.wcf-online.de/WCF-EN/ (2017).
- 25.Lyons LA. DNA mutations of the cat: the good, the bad and the ugly. J Feline Med Surg. 2015;17:203–219. doi: 10.1177/1098612X15571878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louwerens M, London CA, Pedersen NC, Lyons LA. Feline lymphoma in the post-feline leukemia virus era. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2005;19:329–335. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. American journal of human genetics. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury MJ, et al. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in medicine: official journal of the American College of Medical Genetics. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 29.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. The New England journal of medicine. 2012;366:489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien SJ, et al. Comparative gene mapping in the domestic cat (Felis catus) The Journal of heredity. 1997;88:408–414. doi: 10.1093/oxfordjournals.jhered.a023127. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien SJ, Haskins ME, Winkler CA, Nash WG, Patterson DF. Chromosomal mapping of beta-globin and albino loci in the domestic cat. A conserved mammalian chromosome group. The Journal of heredity. 1986;77:374–378. doi: 10.1093/oxfordjournals.jhered.a110264. [DOI] [PubMed] [Google Scholar]
- 32.Bach LH, et al. A high-resolution 15,000 (rad) radiation hybrid panel for the domestic cat. Cytogenet Genome Res. 2012;137:7–14. doi: 10.1159/000339416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menotti-Raymond M, et al. Radiation hybrid mapping of 304 novel microsatellites in the domestic cat genome. Cytogenet Genome Res. 2003;102:272–276. doi: 10.1159/000075762. [DOI] [PubMed] [Google Scholar]
- 34.Menotti-Raymond M, et al. Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus) The Journal of heredity. 2003;94:95–106. doi: 10.1093/jhered/esg008. [DOI] [PubMed] [Google Scholar]
- 35.Murphy WJ, Menotti-Raymond M, Lyons LA, Thompson MA, O’Brien SJ. Development of a feline whole genome radiation hybrid panel and comparative mapping of human chromosome 12 and 22 loci. Genomics. 1999;57:1–8. doi: 10.1006/geno.1998.5695. [DOI] [PubMed] [Google Scholar]
- 36.Murphy WJ, et al. A radiation hybrid map of the cat genome: implications for comparative mapping. Genome research. 2000;10:691–702. doi: 10.1101/gr.10.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy WJ, Sun S, Chen ZQ, Pecon-Slattery J, O’Brien SJ. Extensive conservation of sex chromosome organization between cat and human revealed by parallel radiation hybrid mapping. Genome research. 1999;9:1223–1230. doi: 10.1101/gr.9.12.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis BW, et al. A high-resolution cat radiation hybrid and integrated FISH mapping resource for phylogenomic studies across Felidae. Genomics. 2009;93:299–304. doi: 10.1016/j.ygeno.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy WJ, et al. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics. 2007;89:189–196. doi: 10.1016/j.ygeno.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menotti-Raymond M, et al. A genetic linkage map of microsatellites in the domestic cat (Felis catus) Genomics. 1999;57:9–23. doi: 10.1006/geno.1999.5743. [DOI] [PubMed] [Google Scholar]
- 41.Sun S, Murphy WJ, Menotti-Raymond M, O’Brien SJ. Integration of the feline radiation hybrid and linkage maps. Mammalian genome: official journal of the International Mammalian Genome Society. 2001;12:436–441. doi: 10.1007/s003350010289. [DOI] [PubMed] [Google Scholar]
- 42.Cooper MP, Fretwell N, Bailey SJ, Lyons LA. White spotting in the domestic cat (Felis catus) maps near KIT on feline chromosome B1. Anim Genet. 2006;37:163–165. doi: 10.1111/j.1365-2052.2005.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grahn RA, et al. Localizing the X-linked orange colour phenotype using feline resource families. Anim Genet. 2005;36:67–70. doi: 10.1111/j.1365-2052.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 44.Menotti-Raymond M, et al. An autosomal genetic linkage map of the domestic cat, Felis silvestris catus. Genomics. 2009;93:305–313. doi: 10.1016/j.ygeno.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontius JU, et al. Initial sequence and comparative analysis of the cat genome. Genome research. 2007;17:1675–1689. doi: 10.1101/gr.6380007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montague MJ, et al. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc Natl Acad Sci USA. 2014;111:17230–17235. doi: 10.1073/pnas.1410083111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullikin JC, et al. Light whole genome sequence for SNP discovery across domestic cat breeds. BMC genomics. 2010;11:406. doi: 10.1186/1471-2164-11-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, et al. A High-Resolution SNP Array-Based Linkage Map Anchors a New Domestic Cat Draft Genome Assembly and Provides Detailed Patterns of Recombination. G3. 2016;6:1607–1616. doi: 10.1534/g3.116.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alhaddad, H. et al. Genome-wide association and linkage analyses localize a progressive retinal atrophy locus in Persian cats. Mammalian genome: official journal of the International Mammalian Genome Society (2014). [DOI] [PMC free article] [PubMed]
- 50.Willet CE, Haase B. An updated felCat5 SNP manifest for the Illumina Feline 63k SNP genotyping array. Anim Genet. 2014;45:614–615. doi: 10.1111/age.12169. [DOI] [PubMed] [Google Scholar]
- 51.Keating MK, et al. Characterization of an Inherited Neurologic Syndrome in Toyger Cats with Forebrain Commissural Malformations, Ventriculomegaly and Interhemispheric Cysts. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2016;30:617–626. doi: 10.1111/jvim.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golovko L, et al. Genetic susceptibility to feline infectious peritonitis in Birman cats. Virus Res. 2013;175:58–63. doi: 10.1016/j.virusres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raj A, Stephens M, Pritchard JK. Faststructure: variational inference of population structure in large SNP data sets. Genetics. 2014;197:573–589. doi: 10.1534/genetics.114.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishida Y, et al. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics. 2006;88:698–705. doi: 10.1016/j.ygeno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Lyons LA, Imes DL, Rah HC, Grahn RA. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus) Animal Genetics. 2005;36:119–126. doi: 10.1111/j.1365-2052.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Kuntzel A, Eizirik E, O’Brien SJ, Menotti-Raymond M. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the Albino and Brown loci. Journal of Heredity. 2005;96:289–301. doi: 10.1093/jhered/esi066. [DOI] [PubMed] [Google Scholar]
- 57.Imes DL, Geary LA, Grahn RA, Lyons LA. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim Genet. 2006;37:175–178. doi: 10.1111/j.1365-2052.2005.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drögemüller C, Rüfenacht S, Wichert B, Leeb T. Mutations within the FGF5 gene are associated with hair length in cats. Animal Genetics. 2007;38:218–221. doi: 10.1111/j.1365-2052.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 59.Kehler JS, et al. Four independent mutations in the feline Fibroblast Growth Factor 5 gene determine the long-haired phenotype in domestic cats. Journal of Heredity. 2007;98:555–566. doi: 10.1093/jhered/esm072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt-Kuntzel A, et al. A domestic cat X chromosome linkage map and the sex-linked orange locus: mapping of orange, multiple origins and epistasis over nonagouti. Genetics. 2009;181:1415–1425. doi: 10.1534/genetics.108.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandolfi Barbara, Alhaddad Hasan, Affolter Verena K., Brockman Jeffrey, Haggstrom Jens, Joslin Shannon E. K., Koehne Amanda L., Mullikin James C., Outerbridge Catherine A., Warren Wesley C., Lyons Leslie A. To the Root of the Curl: A Signature of a Recent Selective Sweep Identifies a Mutation That Defines the Cornish Rex Cat Breed. PLoS ONE. 2013;8(6):e67105. doi: 10.1371/journal.pone.0067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaysse Amaury, Ratnakumar Abhirami, Derrien Thomas, Axelsson Erik, Rosengren Pielberg Gerli, Sigurdsson Snaevar, Fall Tove, Seppälä Eija H., Hansen Mark S. T., Lawley Cindy T., Karlsson Elinor K., Bannasch Danika, Vilà Carles, Lohi Hannes, Galibert Francis, Fredholm Merete, Häggström Jens, Hedhammar Åke, André Catherine, Lindblad-Toh Kerstin, Hitte Christophe, Webster Matthew T. Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping. PLoS Genetics. 2011;7(10):e1002316. doi: 10.1371/journal.pgen.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matukumalli LK, et al. Development and characterization of a high density SNP genotyping assay for cattle. Plos One. 2009;4:e5350. doi: 10.1371/journal.pone.0005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramos Antonio M., Crooijmans Richard P. M. A., Affara Nabeel A., Amaral Andreia J., Archibald Alan L., Beever Jonathan E., Bendixen Christian, Churcher Carol, Clark Richard, Dehais Patrick, Hansen Mark S., Hedegaard Jakob, Hu Zhi-Liang, Kerstens Hindrik H., Law Andy S., Megens Hendrik-Jan, Milan Denis, Nonneman Danny J., Rohrer Gary A., Rothschild Max F., Smith Tim P. L., Schnabel Robert D., Van Tassell Curt P., Taylor Jeremy F., Wiedmann Ralph T., Schook Lawrence B., Groenen Martien A. M. Design of a High Density SNP Genotyping Assay in the Pig Using SNPs Identified and Characterized by Next Generation Sequencing Technology. PLoS ONE. 2009;4(8):e6524. doi: 10.1371/journal.pone.0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCue Molly E., Bannasch Danika L., Petersen Jessica L., Gurr Jessica, Bailey Ernie, Binns Matthew M., Distl Ottmar, Guérin Gérard, Hasegawa Telhisa, Hill Emmeline W., Leeb Tosso, Lindgren Gabriella, Penedo M. Cecilia T., Røed Knut H., Ryder Oliver A., Swinburne June E., Tozaki Teruaki, Valberg Stephanie J., Vaudin Mark, Lindblad-Toh Kerstin, Wade Claire M., Mickelson James R. A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies. PLoS Genetics. 2012;8(1):e1002451. doi: 10.1371/journal.pgen.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bannasch D, et al. Localization of canine brachycephaly using an across breed mapping approach. Plos One. 2010;5:e9632. doi: 10.1371/journal.pone.0009632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller JL, et al. Independent specialization of the human and mouse X chromosomes for the male germ line. Nat Genet. 2013;45:1083–1087. doi: 10.1038/ng.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lipinski MJ, et al. The ascent of cat breeds: Genetic evaluations of breeds and worldwide random-bred populations. Genomics. 2008;91:12–21. doi: 10.1016/j.ygeno.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurushima JD, et al. Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations. Anim Genet. 2013;44:311–324. doi: 10.1111/age.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oliveira R, et al. Toward a genome-wide approach for detecting hybrids: informative SNPs to detect introgression between domestic cats and European wildcats (Felis silvestris) Heredity (Edinb) 2015;115:195–205. doi: 10.1038/hdy.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filler S, et al. Selkirk Rex: Morphological and Genetic Characterization of a New Cat Breed. Journal of Heredity. 2012;103:727–733. doi: 10.1093/jhered/ess039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gandolfi, B. et al. A splice variant in KRT71 is associated with curly coat phenotype of Selkirk Rex cats. Sci Rep-Uk3 (2013). [DOI] [PMC free article] [PubMed]
- 73.Alhaddad Hasan, Khan Razib, Grahn Robert A., Gandolfi Barbara, Mullikin James C., Cole Shelley A., Gruffydd-Jones Timothy J., Häggström Jens, Lohi Hannes, Longeri Maria, Lyons Leslie A. Extent of Linkage Disequilibrium in the Domestic Cat, Felis silvestris catus, and Its Breeds. PLoS ONE. 2013;8(1):e53537. doi: 10.1371/journal.pone.0053537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gandolfi B, et al. A dominant TRPV4 variant underlies osteochondrodysplasia in Scottish fold cats. Osteoarthritis Cartilage. 2016;24:1441–1450. doi: 10.1016/j.joca.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Gandolfi B, et al. First WNK4-Hypokalemia Animal Model Identified by Genome-Wide Association in Burmese Cats. Plos One. 2012;7:e53173. doi: 10.1371/journal.pone.0053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersson L. Genome-wide association analysis in domestic animals: a powerful approach for genetic dissection of trait loci. Genetica. 2009;136:341–349. doi: 10.1007/s10709-008-9312-4. [DOI] [PubMed] [Google Scholar]
- 77.Starkey MP, Scase TJ, Mellersh CS, Murphy S. Dogs really are man’s best friend–canine genomics has applications in veterinary and human medicine! Brief Funct Genomic Proteomic. 2005;4:112–128. doi: 10.1093/bfgp/4.2.112. [DOI] [PubMed] [Google Scholar]
- 78.Sutter NB, et al. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome research. 2004;14:2388–2396. doi: 10.1101/gr.3147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilbe M, et al. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42:250–254. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]
- 80.Dodman NH, et al. A canine chromosome 7 locus confers compulsive disorder susceptibility. Mol Psychiatry. 2010;15:8–10. doi: 10.1038/mp.2009.111. [DOI] [PubMed] [Google Scholar]
- 81.Wiik AC, et al. A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in standard wire-haired dachshund. Genome research. 2008;18:1415–1421. doi: 10.1101/gr.074302.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seppälä Eija H., Jokinen Tarja S., Fukata Masaki, Fukata Yuko, Webster Matthew T., Karlsson Elinor K., Kilpinen Sami K., Steffen Frank, Dietschi Elisabeth, Leeb Tosso, Eklund Ranja, Zhao Xiaochu, Rilstone Jennifer J., Lindblad-Toh Kerstin, Minassian Berge A., Lohi Hannes. LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs. PLoS Genetics. 2011;7(7):e1002194. doi: 10.1371/journal.pgen.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gandolfi B, et al. COLQ variant associated with Devon Rex and Sphynx feline hereditary myopathy. Animal Genetics. 2015;46:711–715. doi: 10.1111/age.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lyons LA, et al. Aristaless-Like Homeobox protein 1 (ALX1) variant associated with craniofacial structure and frontonasal dysplasia in Burmese cats. Dev Biol. 2016;409:451–458. doi: 10.1016/j.ydbio.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pedersen NC, Liu HW, Gandolfi B, Lyons LA. The influence of age and genetics on natural resistance to experimentally induced feline infectious peritonitis. Vet Immunol Immunop. 2014;162:33–40. doi: 10.1016/j.vetimm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bertolini, F. et al. Evidence of selection signatures that shape the Persian cat breed. Mammalian genome: official journal of the International Mammalian Genome Society (2016). [DOI] [PubMed]
- 87.Kent WJ. BLAT - The BLAST-like alignment tool. Genome research. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puechmaille SJ. The program structure does not reliably recover the correct population structure when sampling is uneven: subsampling and new estimators alleviate the problem. Mol Ecol Resour. 2016;16:608–627. doi: 10.1111/1755-0998.12512. [DOI] [PubMed] [Google Scholar]
- 90.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 91.Bamber Ruth C., Herdman E. Catherine. The inheritance of black, yellow and tortoiseshell coat-colour in cats. Journal of Genetics. 1927;18(1):87–97. doi: 10.1007/BF03052604. [DOI] [Google Scholar]
- 92.Doncaster, L. On the inheritance of tortoiseshell and related colours in cats. Proceedings of the Cambridge Philosophical Society, 35–38 (1904).
- 93.Pritchard JK, Przeworski M. Linkage disequilibrium in humans: Models and data. American journal of human genetics. 2001;69:1–14. doi: 10.1086/321275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in the project is available in Supplementary information files included in the article for download.