Abstract
Background
In Rwanda, pneumonia and diarrhea are the first and second leading causes of death, respectively, among children under five. Household air pollution (HAP) resultant from cooking indoors with biomass fuels on traditional stoves is a significant risk factor for pneumonia, while consumption of contaminated drinking water is a primary cause of diarrheal disease. To date, there have been no large-scale effectiveness trials of programmatic efforts to provide either improved cookstoves or household water filters at scale in a low-income country. In this paper we describe the design of a cluster-randomized trial to evaluate the impact of a national-level program to distribute and promote the use of improved cookstoves and advanced water filters to the poorest quarter of households in Rwanda.
Methods/Design
We randomly allocated 72 sectors (administratively defined units) in Western Province to the intervention, with the remaining 24 sectors in the province serving as controls. In the intervention sectors, roughly 100,000 households received improved cookstoves and household water filters through a government-sponsored program targeting the poorest quarter of households nationally. The primary outcome measures are the incidence of acute respiratory infection (ARI) and diarrhea among children under five years of age. Over a one-year surveillance period, all cases of acute respiratory infection (ARI) and diarrhea identified by health workers in the study area will be extracted from records maintained at health facilities and by community health workers (CHW). In addition, we are conducting intensive, longitudinal data collection among a random sample of households in the study area for in-depth assessment of coverage, use, environmental exposures, and additional health measures.
Discussion
Although previous research has examined the impact of providing household water treatment and improved cookstoves on child health, there have been no studies of national-level programs to deliver these interventions at scale in a developing country. The results of this study, the first RCT of a large-scale programmatic cookstove or household water filter intervention, will inform global efforts to reduce childhood morbidity and mortality from diarrheal disease and pneumonia.
Trial registration
This trial is registered at Clinicaltrials.gov (NCT02239250).
Keywords: Cluster randomized controlled trial, Rwanda, Improved stoves, Acute respiratory infection, Household water treatment, Diarrhea
Abbreviations: ARI, acute respiratory infection; CHW, community health worker; DBSS, dried blood spot samples; HAP, household air pollution; H-PEM, Harvard Personal Exposure Monitor; ICCM, Integrated Community Case Management of Childhood Illness; IMCI, Integrated Management of Childhood Illness; MFI, mean fluorescence intensity; MOH, Rwanda Ministry of Health; MOLG, Rwandan Ministry of Local Government; RCT, randomized controlled trial
1. Introduction
Environmental contamination at the household level is a major cause of death and disease among rural populations in low-income countries. Household air pollution (HAP) contributes to acute lower respiratory infection in children under five, and among adults is a significant risk factor for hypertension, ischemic heart disease, chronic obstructive pulmonary disease, and lung cancer [19], [39], [14]. Unsafe drinking water is the leading cause of diarrheal disease [32]. HAP and unsafe drinking water rank 7th and 8th among risk factors for the global burden of disease [18]. Collectively, pneumonia and diarrhea are responsible for an estimated 6.9 million deaths annually [7].
These environmental hazards are common among impoverished rural inhabitants of sub-Saharan Africa, the vast majority of whom cook with biomass fuels on traditional stoves and rely on unsafe water supplies [9]. In Rwanda, where more than half the population is living below the poverty line and more than a third in extreme poverty, 98.1% of rural householders cook with biomass, mainly on open three-stone fires; and only 7.6% have water on their premises [30]. After HIV/AIDS, the leading causes of death in Rwanda are ALRI (20%) and diarrhea (12%) [46].
Despite clear evidence that HAP is an important risk factor for respiratory and cardiovascular disease, evidence for the health impact of improved cookstoves that can be deployed at scale among vulnerable populations is limited [38]. Although trials are currently underway to explore the effectiveness of various improved cookstove types, these are all limited scale efficacy trials [17], [8], [42]. Further, doubts about the potential of any biomass stove to achieve WHO indoor air quality targets have shifted much of the focus to clean cooking fuels such as LPG, ethanol and electricity, although supply chain limitations currently render these options impractical in most rural settings [51], [54], [55].
There is strong evidence that household-based water filters are effective in preventing diarrhea [15]. The actual protective effect, however, is likely to vary by setting, season, and the extent to which water is a dominant transmission pathway. As evidence also suggests that even occasional consumption of untreated water vitiates the potential health impact, correct and consistent use is essential [10]. The up-front cost of household filters, together with the need to establish supply chains for consumables, has limited the extent to which they have been scaled up among vulnerable populations, particularly in rural settings. Like improved cookstoves, there has been no large-scale effectiveness trial to assess the impact of household water filters promoted programmatically.
In an effort to reduce the disease burden in rural Rwanda, the Rwanda Ministry of Health (MOH) partnered with the social enterprise DelAgua Health to distribute and promote the use of improved cookstoves and advanced water filters to the poorest 25% of households nationally, beginning in Rwanda's Western Province. The project, know as “Tubeho Neza” or “Live Well”, earns revenue through carbon credits under the United Nations Clean Development Mechanism (CDM), a program authorized by the Kyoto Protocol that provides credits to the implementer based on a formula that includes population coverage and use [22].
Prior to initiation of the Tubeho Neza program, the implementer first undertook a pilot intervention to all 1943 households in 15 rural villages [4]. At this time we conducted a five-month RCT among 566 households in three of these pilot villages to assess the impact of the water filter on fecal indicator bacteria in household drinking water and the impact of the stove on fine particulate mater (PM2.5) in reported cooking areas [56]. Overall, the intervention was associated with a 97.5% reduction in mean fecal indicator bacteria (Williams means 0.5 vs. 20.2 TTC/100 mL, p < . .001) and a 48% reduction of 24-h PM2.5 concentrations in the cooking area (0.485 mg/m3 and 0.267 mg/m3, p = 0.005). The reduction was 37% for those cooking indoors (p = 0.08) and 73% for those cooking outdoors (p < 0.001) [56].
Based on the results from the pilot study, the Rwanda MOH and the implementer elected to proceed with the roll out of the intervention throughout Western Province. However, due to funding constraints, only approximately 100,000 of the 140,000 eligible households in Western Province could be included in the initial implementation. Consequently, it was decided that receipt of the program would initially be limited to households within randomly allocated sectors (groups of villages that generally correspond with catchment areas for primary-care clinics) within Western Province, with the remainder of households scheduled to receive the program the following year. This provided us the unique opportunity to conduct a population-level RCT to assess the impact of the program on health outcomes using records maintained at health facilities and by community health workers (CHW). In addition, we randomly selected a representative sample of households (n = 1580) in the control and intervention areas to conduct intensive data collection in order to assess coverage, use, environmental exposures, and additional health outcome measures. This paper provides details on the evaluation of the Tubeho Neza program, including the design of the study, the study setting and population, primary and secondary outcomes, and other details concerning the methods to be followed.
2. Methods
2.1. Setting
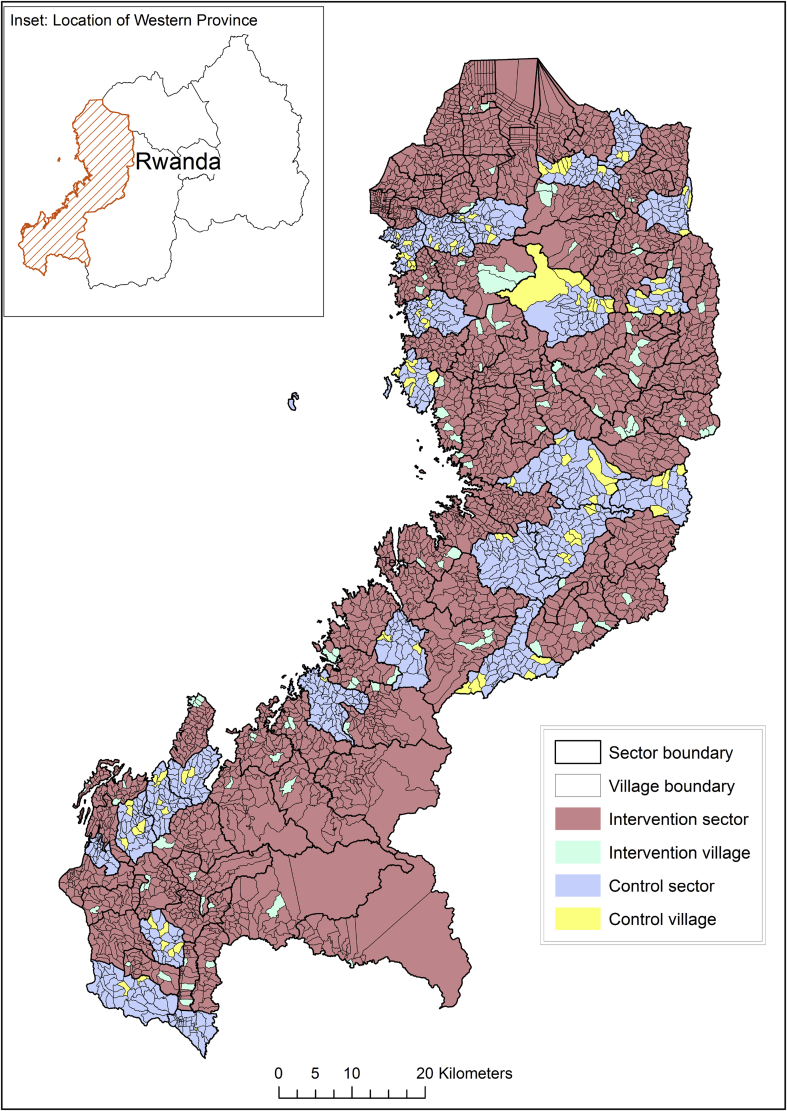
The study is located in Western Province, Rwanda, a predominantly rural province with a total population of roughly 2.5 million (Fig. 1). The Western Province is 87.8% rural, and the main source of energy for cooking is firewood (88.6%), followed by charcoal (8.3%). Most households in Western Province report their main source of water is from protected sources (26.8% public tap, 39.8% protected spring/well, 4.5% piped water on premises), although 16.3% of households obtain from an unprotected spring/well and 9.4% from surface water [30].
Fig. 1.
Map of the study area. Western Province, Rwanda.
2.2. Study design
The study design is a cluster-randomized trial with sectors (administratively defined areas containing an average of 40 villages) as the unit of randomization. Each of the 96 sectors in Western Province have been randomized to either control or intervention status (Fig. 1). The primary study outcomes are diarrhea and acute respiratory infection (ARI) among children under five residing in households eligible to receive the program. Clinician-diagnosed episodes of diarrhea and ARI among all households in the study area will be assessed from records maintained by health facilities and village-level CHW health records that have been made available for use in this study by the Rwanda MOH.
In conjunction with the assessment of clinical outcomes across the entire population of program recipients (referred to as the “sector-level” study), we are conducting intensive data collection in a random sample of households with children <5 residing in the study area. This nested design permits examination of outcomes in addition to those maintained in health records, such as intervention uptake and use and the effect of the intervention on exposure to HAP and fecally contaminated drinking water. It also permits us to collect self-reported information on respiratory disease and diarrhea (to learn about cases that may not be reported to CHWs and clinics) and to assess a variety of objective measures related to health status (e.g. blood pressure, oximetry, inflammatory biomarkers, enteric pathogen antibodies) among household members. We refer to this intensive data collection in a sample of the larger RCT population as the “village-level” study in sections to follow in order to differentiate these efforts from the primary assessment of outcomes across the entire study population.
2.3. Eligibility criteria
All households in Western Province designated as Ubudehe category 1 or 2 were eligible to receive the intervention from the implementer. Ubudehe category is a government-defined household economic status classification and Ubudehe categories 1 and 2 roughly constitute the poorest 25% of the country. Ubudehe category is determined by community members based on classifications outlined by the Rwandan Ministry of Local Government (MOLG), and households categorized as Ubudehe 1 and 2 receive free medical and other assistance through government programs [57]. All children <5 in Western Province who reside in households that are categorized as Ubudehe 1 and 2 are included in active surveillance of diarrhea and pneumonia cases at local health centers and by village CHW's. These records are collected by research staff, in collaboration with the Rwandan MOH, and no additional contact with the study population is required for the sector-level study.
2.4. Randomization
The choice of sector as the unit of randomization was made in collaboration with the program implementers and the Rwandan MOH. We used a database maintained by the Rwanda MOLG that contains the Ubudehe categorization of all households (subsequently referred to as the 'Ubudehe database') to generate a count of eligible households in each sector and determined that a 3:1 allocation of intervention to control sectors in Western Province would meet the implementer's programmatic goal of reaching approximately 100,000 households during the initial roll-out of the program. Sectors were randomly assigned to intervention and control arms by the research team using a computer-generated randomization stratified by district to ensure equal distribution of the intervention across the seven districts of Western Province. Neither the implementers nor the Rwanda MOH were involved in the randomization process.
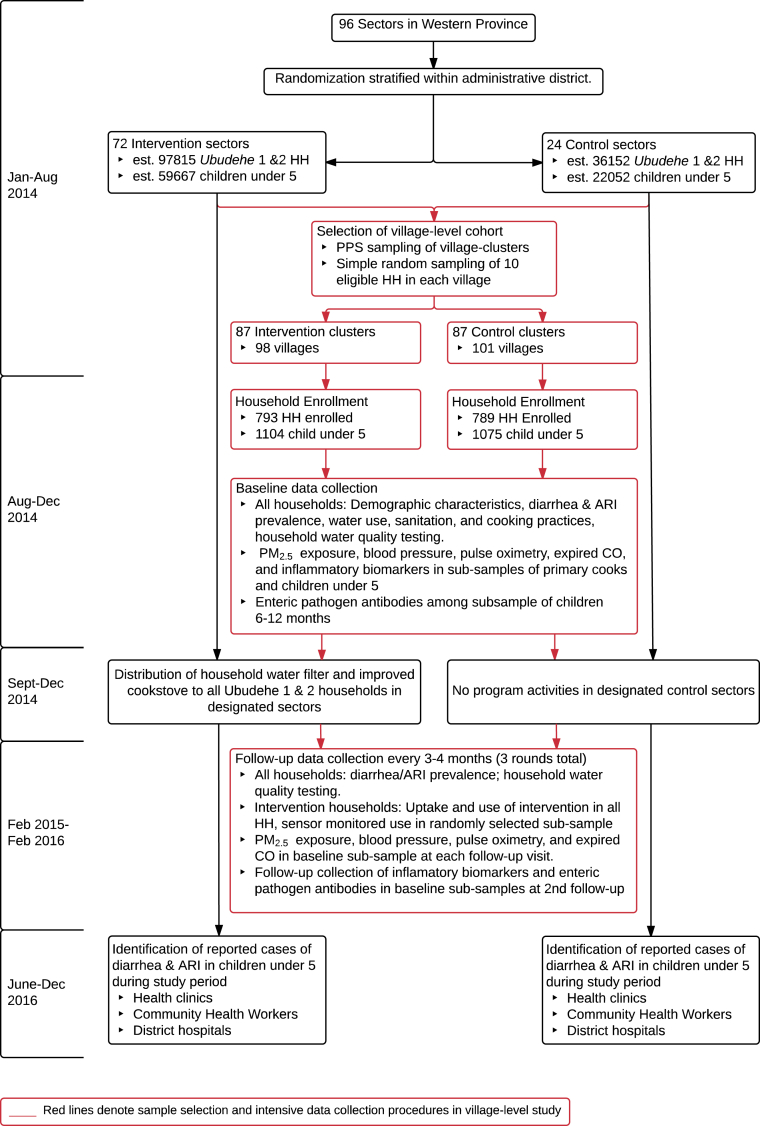
Randomization resulted in 72 designated intervention sectors and 24 designated control sectors, with a total of 97,815 eligible households in the intervention sectors and 36,152 eligible households in the control sectors. Sector-level census data [30] indicate that randomization achieved good balance between the arms in regards to population-level sociodemographic characteristics, water source type, and fuel use (Table 1). It is intended that all eligible households in the 72 sectors randomly assigned to the intervention group received the intervention during the Phase 2 implementation in late 2014, while eligible households in the control sectors will receive the intervention after the 1-year surveillance period (Fig. 2).
Table 1.
Census-derived sociodemographic characteristics, water source type, and fuel use in intervention and control sectors. Western Province, Rwanda, 2012.
| Characteristics | Intervention (n = 72 sectors) |
Control (n = 24 sectors) |
||
|---|---|---|---|---|
| Mean or count | Std dev or % | Mean or count | Std dev or % | |
| Total population | 1,854,751 | 100.0 | 616,488 | 100.0 |
| Female | 978,973 | 52.8 | 323,821 | 52.5 |
| Children under five | 280,442 | 15.1 | 94,663 | 15.4 |
| Rural population | 1,625,681 | 87.6 | 544,246 | 88.3 |
| Mean people per square kilometresa | 691.6 | 602.5 | 683.9 | 392.0 |
| Household socioeconomic characteristics | ||||
| Mean size of household | 4.5 | 0.3 | 4.5 | 0.2 |
| Own house | 350,485 | 85.8 | 117,668 | 87.2 |
| Walls – sundried brick | 283,030 | 69.3 | 95,933 | 71.1 |
| Walls – wood/mud | 92,864 | 22.7 | 29,343 | 21.7 |
| Roof – iron sheets | 193,053 | 47.3 | 53,279 | 39.5 |
| Roof – local tiles | 211,544 | 51.8 | 80,427 | 59.6 |
| Owns TV | 18,870 | 4.6 | 5052 | 3.7 |
| Owns mobile phone | 196065 | 48.0 | 62,511 | 46.3 |
| Owns radio | 230,630 | 56.5 | 75,937 | 56.3 |
| Household energy use | ||||
| Main cooking source – charcoal | 35,971 | 8.8 | 9123 | 6.8 |
| Main cooking source – wood | 360,637 | 88.3 | 121,274 | 89.8 |
| Main lighting source – candle | 42,629 | 10.4 | 15,065 | 11.2 |
| Main lighting source – kerosene | 148,098 | 36.3 | 47,274 | 35.0 |
| Main lighting source – electricity | 51,203 | 12.5 | 13,088 | 9.7 |
| Water source type | ||||
| Piped to compound | 19,315 | 4.7 | 5011 | 3.7 |
| Public tap | 111,950 | 27.4 | 33,868 | 25.1 |
| Protected spring/well | 157,405 | 38.5 | 59,181 | 43.8 |
| Surface water | 39,144 | 9.6 | 11,942 | 8.8 |
| Household sanitation | ||||
| Toilet type – Private pit latrine | 340,813 | 83.4 | 116,054 | 86.0 |
| Toilet type – Shared pit latrine | 43,885 | 10.7 | 12,747 | 9.4 |
| Toilet type – Uses bush | 6129 | 1.5 | 1337 | 1.0 |
| Rubbish disposal – bush, farm or river | 140,101 | 34.3 | 39,817 | 29.5 |
Note: Census data includes all households in study sectors-i.e. both eligible (Ubudehe 1 & 2) and ineligible (Ubudehe 3 to 6 households.
Based on mean values in each sector.
Source: Ref. [30] Fourth Population and Housing Census, Rwanda, 2012, Main Indicators Report, Kigali: National Institute of Statistics of Rwanda
Fig. 2.
Flow diagram of the study. Note: HH = households; ARI = acute respiratory infection; CO = carbon monoxide.
2.5. Village-level study
2.5.1. Eligibility criteria for village-level study
All villages within the study area (N = 3617) were eligible for selection as intensive data collection sites, excluding the 10 villages that were previously included in pilot intervention activities. Enrollment in this village-level cohort was limited to Ubudehe 1 and 2 households with at least one child 4 years of age or younger residing in the home the majority of the year. We limited enrollment to households with a child ≤4 years of age in order to ensure that at least one child per household remained under the age of five for the duration of the study. However, at each visit we collect all outcome data on any additional children present in the household that are currently under the age of five.
2.5.2. Sampling strategy for village-level study
The households selected for intensive data collection were randomly sampled from the study area using a stratified, two-stage design of the type commonly used in nationally-representative surveys in developing countries [45]. Prior to sampling for the village-level study, we estimated the number of eligible households in each village in Western Province using data provided by the Rwandan government and other sources. To ensure that a sufficient number of eligible households were present in each primary sampling unit (PSU), each village in the study area containing fewer than 12 eligible households was randomly paired with a geographically contiguous village in an iterative process using geographic information system software. This yielded 2715 PSUs, of which 2080 consisted of single villages and the remaining 635 were composed of groups of two or more geographically adjacent villages. We then conducted probability proportional to size random sampling of PSUs, stratified by treatment allocation. We oversampled from the control sectors to yield a 1:1 ratio of intervention to control clusters. This resulted in 87 clusters in each arm, with 101 villages in the control group and 98 villages in the intervention group. In stage 2, households were sampled from each PSU in using simple random sampling (Fig. 2). Our target enrollment was 10 households per PSU. The implementer and the Ministry of Health are blinded to the identity and location of villages selected for intensive data collection.
2.5.3. Enrollment in village-level study
Following household selection, trained study staff located the selected households with the assistance of the village CHW. The primary point of household contact was the primary cook. In order for a household to be enrolled into the study, at least one child four years of age or younger had to reside at the house the majority of the year, the primary cook had to be 16 years of age or older, and the primary cook had to give informed written consent. If the primary cook was not present at a first attempted visit of the household, another visit was attempted the same day and a final attempt the following day. If the selected household was not present in the village or if contact was unsuccessful after the repeated attempts, study staff attempted to enroll an alternate household selected by the field supervisor from the list of eligible households in the PSU using simple random sampling. The final sample enrolled in the village-level study consisted of 1582 households. Baseline data collection was conducted in each household immediately following enrollment.
2.6. Intervention
The program implementation under study is branded “Tubeho Neza” which means to “Live Well” in Kinyarwanda. The Tubeho Neza program includes the free distribution of the Vestergaard Frandsen LifeStraw Family 2.0 household water filter and the EcoZoom Dura high efficiency wood cookstove (Fig. 3) with associated community and household education and behavior change messaging to all households in Ubudehe categories 1 and 2 in Western Province. The program was implemented by the UK-based social enterprise DelAgua Health in collaboration with the Rwanda MOH, and is primarily funded through a pay-for-performance model enabled by revenues from the generation and sale of carbon credits under the United Nations Clean Development Mechanism, a program authorized by the Kyoto Protocol. Carbon credits are earned through the reduction of fuel wood from improved stove efficiency and by reduced levels of boiling under a “suppressed demand” construct [22]. To earn credits, the implementer must demonstrate actual use of the intervention hardware through repeated audits one or more times per year.
Fig. 3.
LifeStraw Family 2.0 household water filter (left) and EcoZoom Dura high-efficiency wood cookstove (right).
Large-scale program activities in Western Province began in September 2014, and reached over 101,000 households and nearly 458,000 recipients by December 23, 2014. Approximately 820 DelAgua and MOH trained Community Health Workers conducted community distributions and household education. In community meetings, CHWs and DelAgua supervisors conducted public health focused skits and demonstrations, collected household information, and distributed the cookstoves and water filters. Following distribution, CHWs perform household level education, focusing on correct and consistent adoption of the products. Educational tools are utilized including a picture based flipbook and interactive poster to demonstrate benefits related to health as well as livelihood and environmental benefits. Throughout the lifetime of the project, CHWs visit households approximately bi-yearly to perform further household level education activities with on-going community level engagement. Repair and replacement of products is managed by DelAgua through district level facilities with problems being reported primarily through a dedicated call center phone line and communications with local officials. Further details of the delivery of the interventions and associated program activities are contained in a separate process evaluation paper [5].
2.7. Primary outcomes
The primary outcomes of the sector-level study are the incidence of clinically-reported ARI (all types, severe pneumonia/severe illness and non-severe pneumonia) and diarrhea (including severe persistent diarrhea, persistent diarrhea and bloody diarrhea) among children under five in Ubudehe 1 and 2 categories during the 12 months of follow-up, as defined by the Rwandan Integrated Community Case Management of Childhood Illness (ICCM) diagnostic criteria [36], which are based on the World Health Organization Integrated Management of Childhood Illness (IMCI) [52].
Outcomes will be identified from data collected routinely by health authorities including outpatient and inpatient registers maintained at health posts, health centers, and district hospitals, as well as CHW-maintained registers. Together with the Rwandan MOH, health registers in these health facilities as well as CHW registers were modified to include name of the head of household as well as Mutuelle insurance number of patient or household member. The Rwanda MOH sent letters to all health facilities in the study area explaining the modification and encouraging compliance, and study staff have visited every health facility in order to explain the changes and assess staff fidelity in recording this information in the clinical registers. To differentiate clinic and CHW-reported cases among Ubudehe 1 & 2 households from those in other Ubudehe categories, we will use this information to link health records with a MOLG database containing the Ubudehe categorization of every household in the study area. Notably, this is the database that is used by the implementer to determine household eligibility to receive the intervention. We will also confirm receipt of the intervention by checking health records in the intervention sectors against a list of recipient households provided by the implementer. While health clinicians at health facilities may not be fully aware of patient Ubudehe status nor what sectors in their region have received the intervention and so may be blinded during the health assessment process, CHWs are aware of the allocation of patients during symptom assessment as they are based at the village level. At the end of the follow-up period, non-blinded researchers will screen all health facility and CHW registers and extract data on cases occurring in eligible households.
2.8. Secondary outcomes: intensive data-collection households
We define as secondary outcomes all of those assessed during intensive data collection in the sample of households enrolled in the village-level study. We completed a baseline survey in these households prior to program implementation and are conducting three follow-up visits, at approximately four-month intervals, during the 12-month study period. During these visits, we are assessing the prevalence of caregiver and field staff reported diarrhea and ARI among all children under five in the household (N = 2179), testing household drinking water quality, and evaluating uptake and use of the intervention, as well as collecting extensive survey data on sociodemographic characteristics, cooking practices, water source and treatment practices, and hygiene and sanitation practices among all of the households. In addition, sub-samples of the village-level cohort have been randomly selected for measurement of personal particulate matter (PM2.5) exposure, blood pressure, pulse oximetry, expired carbon monoxide, inflammatory biomarkers, and enteric pathogen antibodies. Details regarding all of these measures are provided in the sections following.
2.8.1. Reported diarrhea and ARI
Among all households enrolled in the village-level study, reported data on diarrhea and respiratory symptoms are collected from the primary caretaker for each child under five at the time of the interview that permanently lived in the household. A 7-day recall period is used for both conditions, with additional follow-up questions to determine length and severity of illness in the event of a positive report and to identify cases for which care was sought at a health facility or from a CWH. Regarding diarrhea, we use the WHO definition of three or more loose or watery stools in 24 h [50]. We define ARI as reported illness with cough accompanied by rapid breathing or difficulty breathing. In addition, caregivers are asked about other symptoms (fever, constant cough, blocked/runny nose, wheezing/stridor) in order to examine the impact of the intervention on specific respiratory symptoms and to construct and test more restrictive definitions of ARI. Lastly, the primary cook is assessed for reported diarrhea and respiratory symptoms at each visit. Toothache, unlikely to be related to either intervention device, was used as a negative control symptom to assess potential reporting bias [26].
2.8.2. Symptom identification using the IMCI methodology
To overcome some of the limitations of the self-reported health data, field staff trained in WHO IMCI methods will assess all children between 2 months and 5 years of age for diarrhea and ARI at baseline and at each follow-up visit. All field staff underwent one-week office and clinic-based IMCI training from a Rwanda MOH community health nurse trainer in diarrhea and ARI sign and symptom recognition including video for fast breathing, chest indrawing and stridor. Field staff were also trained on the classification of illness severity and the identification of children requiring referral to CHW or health centers. Field staff were instructed to remove any clothing covering the chest of the child for the assessment of stridor, chest indrawing and the counting of breaths. Respiratory rate is measured for one minute using a timer in a calm, rested child. Fast breathing is defined as 50 or 40 breaths per minute or more in children 2–12 months and 1–5 years respectively.
These staff-assessed signs and symptoms are combined using IMCI criteria to produce three definitions of ARI (severe pneumonia or very severe disease, pneumonia and no pneumonia (cough or cold) and three definitions for diarrhea (diarrhea with severe dehydration, diarrhea with some dehydration and diarrhea with no dehydration) according to IMCI guidelines.
Additionally, field staff assess objective indicators of nutritional status (mid-upper arm circumference, palmar pallor and edema) in all children undergoing IMCI assessment. While there is no study that directly links to cookstove exposure, these nutritional indicators can be affected by both acute and chronic illnesses [4] and so could be impacted by an intervention targeting diarrheal and respiratory infections, the most common illnesses among children under five in Rwanda.
2.8.3. Cardiovascular function, blood oxygenation and carbon monoxide exposure
A growing body of evidence indicates that exposure to indoor air pollution is a major contributor to cardiovascular disease in low and middle-income countries [18], [31], [6]. To assess the impact of the intervention on cardiovascular function, we are collecting objective measures of blood pressure among primary cooks in a sub-sample of 348 households. This includes all households randomly selected for personal particulate matter exposure measurement (described below) in 112 total village clusters as well as two additional, randomly selected households in the 62 other village clusters in our study. Diastolic and systolic blood pressure is measured using a validated automated Omron-705CP blood pressure device (Omron Corp, Tokyo, Japan). After five minutes of quiet rest, three seated, consecutive blood pressure measures one-minute apart are taken according to standard recommendations [58] For all participants who undergo blood pressure testing, we are collecting data on previous history and current medication for hypertension, diabetes, heart disease and kidney disease, diet, physical activity, height, weight, and waist circumference.
Carbon monoxide is a key product of incomplete combustion and carboxyhemoglobin (COHb) may therefore be an indicator of recent exposure to household air pollution. COHb levels exceeding 2.5% have been deemed unsafe by the WHO, and excess COHb levels (>5%) are associated with neurobehavioral factors, impaired vision and decreased alertness [43]. To better understand the relationship between COHb level and personal PM2.5 exposure and to assess whether the intervention is associated with measurable reductions in COHb, we are measuring COHb saturation among all participants selected for personal particulate matter exposure measurement. Pulse oximetry is used to measure COHb saturation (SpCO), oxygenated hemoglobin (SpO2), and pulse using a Rainbow-SET Rad-57 Pulse CO-Oximeter (Masimo Inc., Irvine, California, USA). Pulse oximetry is performed by field staff in accordance with manufacturers recommendations for use.
Expired CO may also be an indicator of recent exposure to household air pollution [24], [31]. In households selected for personal particulate matter exposure measurement as well as households selected for blood pressure measurements, expired CO (ppm and %COHb) is measured using a MicroCO (CareFusion Corp, San Diego, California, USA). Measurements are conducted outside away from any smoke sources. Primary cooks and the selected child under five are instructed to hold their breaths for as close to 20 s as possible and exhale for up to 5 s. Given variable ability for primary cooks and children to hold their breath, the number of seconds is recorded and may be adjusted for in analyses. In households with personal exposure monitoring, pulse oximetry and expired CO measurements are taken directly after the 48-hour exposure monitoring.
2.8.4. Inflammatory biomarkers
Recognizing the potential contribution of biomarker data, there are increasing calls for biomarker data to be incorporated into future clean cookstove trials [28], [35]. Inflammatory biomarkers related to cooksmoke exposure, ALRI and cardiovascular disease (including interleukin (IL)-1β, IL-6, IL-8, IL-10, tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP)) will be assessed among households selected for personal particulate matter exposure, with a target of 2 households per cluster. Enumerators collected capillary blood on Whatman 903 Protein-Saver cards (Sigma-Aldrich, St. Louis, MO). These dried blood spot samples (DBSS) were dried for a minimum of 4 h after collection at room temperature prior to placing them in sealed plastic bags with desiccant. Samples remained at room temperature for a maximum of 7 days before placing them into storage in a −20C freezer [37]. CRP will be measured through ELISA, using paired capture (anti-CRP monoclonal antibody) and detection antibodies. All other cytokines (IL-1β, IL-6, IL-8, IL-10 and TNF-α), in addition to specific cardiovascular disease markers such as sVCAM, sICAM and sCD40L will be measured through multiplex immunoassay with a dual-laser FACSCalibur flow cytometer (BD Biosciences, Franklin Lake, NJ) in Rwanda's National Reference Laboratory. This semi-quantitative method will yield mean fluorescence intensity (MFI) values that correlate with the concentration of the cytokines and disease markers in the blood sample. Analysis of DBSS through flow cytometry is dependent on validation of this particular multiplex assay with DBSS.
2.8.5. Enteric pathogen antibodies
Serological assays that assess antibody production against various enteric pathogens can provide a far more objective measure of exposure to enteric infections than reported diarrhea or diarrhea diagnosed using clinical indices in the field [16], [40] characterized the age-specific seroprevalence of antibodies against various enteric pathogens, such as Escherichia coli, Norovirus, Cryptosporidium parvum and Helicobacter pylori and hepatitis A virus (HAV) and found the steepest increase in antibody acquisition against antigens such as E. coli heat-labile enterotoxin (ETEC-LT) and norovirus capsid proteins between 6 and 18 months of age. In addition, antibody acquisition against C. parvum surface 27kD surface antigen began to increase steadily after 12 months of age, with seroprevalence peaking and leveling out around 18–24 months of age. Together with other studies [11], [23], [25], [44], [47] that have demonstrated marked age-specific prevalence of antibodies against these pathogens between 6 and 36 months-old, the appropriate age range to assess seroconversion against WASH improvements likely lies within the 6 to 18 month-old age range.
To avoid the influence of maternal antibodies in our analyses [40], children no younger than 6 month-old were eligible for this study. To capture the time period in which children with waning maternal antibody have not fully weaned, children 6-12 month-old were enrolled at baseline and had capillary blood drawn by heelstick or fingerstick. After 6–9 months of follow up (between June and September 2015), these children were visited again for a follow-up capillary blood sample. All blood samples will be preserved on TropBio (Sydney, Australia) filter discs. Seroconversion against G. intestinalis VSP1-5, C. parvum Cry17 and Cry27, HAV, E. histolytica lectin adhesion antigen (LecA), enterohemorrhagic E. coli heat-labile enterotoxin (ETEC-LT), Salmonella lipopolysaccharide (LPS) Group B and D, norovirus, Campylobacter spp. and Vibrio cholerae will be compared between intervention and control groups using multiplex immunoassay technology on the Luminex xMAP platform, as described by Lammie et al. [59].
2.8.6. Drinking water quality
Drinking water samples are tested for thermo-tolerant coliforms (TTC) at baseline and the first two waves of follow-up among all of the households enrolled in the village-level study. Field staff collect the water sample from the water container identified by the household respondent as being that used mainly for drinking by children under five. If this is other than directly from the intervention filter, a second sample is taken directly from the filter if it contained water. All water samples are collected in sterile Whirl- Pak bags (Nasco, Fort Atkinson, WI) containing a tablet of sodium thiosulphate to neutralize any halogen disinfectant. Samples are placed on ice and processed within 6 h of collection to assess levels of TTC. Microbiological assessment is performed using the membrane filtration technique [1] on membrane lauryl sulphate medium (Oxoid Limited, Basingstoke, Hampshire, UK) using a DelAgua field incubator (Robens Institute, University of Surrey, Guilford, Surrey, UK). For quality assurance, a negative control and two replicates are conducted in each batch of analysis.
2.8.7. Personal particulate matter exposure
Within 56 randomly selected intervention and 56 randomly selected control clusters, two households were randomly selected to undergo personal particulate matter exposure measurements, for a target enrollment of 224 households. Based on data from our pilot studies, we calculate that this sample size is sufficient to detect a 20% reduction in PM2.5. To participate, the household had to have a healthy child 1.5–4 years old that could support wearing the exposure monitoring equipment which weighed approximately 1 kg, and the primary cook could not be pregnant or a current smoker.
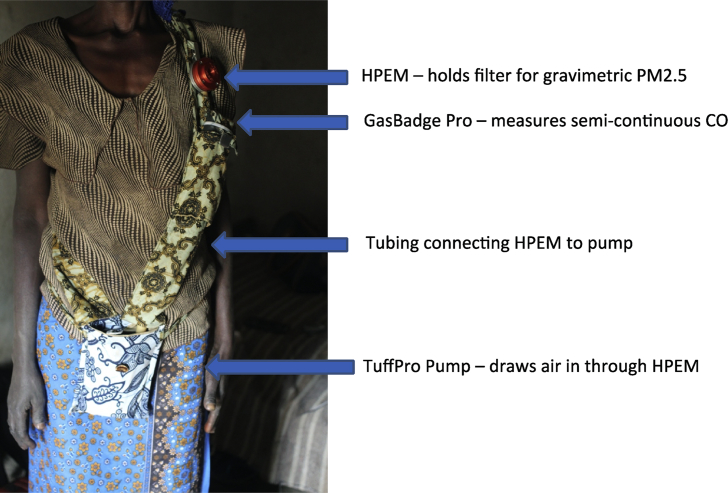
Personal exposure of the primary cook and child under four to particulate matter less than 2.5 microns in diameter (PM2.5) is assessed using integrated gravimetric measurements. The particulate matter is collected on pre-weighed 37-mm diameter PTFE Teflo filters with 0.2-μm pore size and support ring (Teflo, Pall Life Sciences, Port Washington, New York, USA), back supported by Whatman drain disks (Whatman GE Life Sciences, Pittsburgh, Pennsylvania, USA). During the 48-hour exposure measurement period, the sample is collected using a Harvard Personal Exposure Monitor (H-PEM) impactor with a D50 cut of 2.5 μm (BGI, Cambridge, Massachusetts, USA) connected by latex rubber tubing to a Casella TuffPro™ (Casella Measurement, Bedford, UK) low-flow pump set to 1.8 litres per-minute flow at one-minute intervals for 48 h. Flow is calibrated in the household immediately before and after the 48-hour monitoring period using a Challenger (BGI, Cambridge, Massachusetts, USA).
For both primary cooks and children, the H-PEM containing the PTFE filter is affixed within the breathing zone (between chest-level and mouth) in a diagonal chest strap with a pouch that held the pump for cooks, and on the shoulder-strap of a small backpack for children (Fig. 4). Participants are instructed to wear the side-pouch or backpack at all times for a 48-hour period, except during breastfeeding, bathing, sleeping or other activities as necessary, in which case the monitoring equipment is to be kept within 1 meter of the individual. To assess compliance of wearing the monitoring equipment, an unannounced spot check approximately 24 h into the monitoring period is conducted. Additionally, a HOBO Pendant® data logger (Onset, Bourne, Massachusetts, USA) set to record light sensor readings at a 1-minute resolution is affixed to the monitoring equipment of cooks and children in order to qualitatively assess compliance during daylight hours. After the 48-hour monitoring period the filters are kept refrigerated at 4 °C or lower until being processed and post-weighed.
Fig. 4.
Personal particulate matter monitoring device worn by primary cooks. Note: HPEM=Harvard Personal Environmental Monitor, CO = carbon monoxide.
Filters are weighed before and after deployment at Emory University. Filters are conditioned inside of their respective petri dishes overnight in a desiccator (BelArt Products, Wayne, NJ) with lithium chloride desiccant inside a safety hood for an optimal temperature of 20–23 °C [and relative humidity between 30 and 40%]. Immediately prior to each measurement, each filter was passed across an electrostatic bar and placed on a microbalance (Cole-Parmer). Measurements are stabilized for a minimum of 15 s and are performed twice for each filter. In the event that two measurements differ by more than 5 μg, a third measurement is taken. The mean of all measurements for any given filter will be used for analysis.
2.8.8. Uptake and use
Household uptake and use of the water filter and improved cookstove is assessed through a combination of self-report, direct observation by trained field staff, and in randomly selected households, sensor-recorded observations. At each visit, participants in the village-level study are asked to identify the main drinking water container in the household and report on usage and maintenance of the water filter. To assess the degree of exclusive use of the water filter, respondents are asked to report the frequency of consumption of non-filtered water in and outside the home. The field staff documents whether the water filter is present in the home and records potential indicators of use, such as whether the filter contained water at the time of the visit, whether the filter looked clean, whether it was placed on a convenient place, or had a cloth on top of it. Similarly, the respondent is asked to identify all cook stoves ever used in the household and report their current frequency of use. Objective indicators of use are collected on each stove, including the current location of the stove, whether it is in use or warm to the touch, or whether there is presence of ash or smoke marks on the stove.
In addition to household surveys and observations, which are known to present risks of reporting bias and cause reactivity [53], [3], adoption and frequency of usage is assessed through the use of sensor based monitoring in a randomly selected sub-sample of intervention households for a 30-day period. In the pilot study, we evaluated the correlation of sensors on these filters and stoves against household surveys and found that sensor-collected data estimated use to be lower than conventionally-collected data both for water filters (approximately 36% less water volume per day) and cook stoves (approximately 40% fewer uses per week) [41].
2.9. Sample size calculation
Sample size calculations for the sector-level study were based on the two primary outcomes of health center or CHW reported diarrhea and ARI among children under five. In accord with recently published regional and national estimates [48], [49], [33]), we assume a baseline diarrhea incidence of 3.1 episodes per child-year and ARI incidence of 0.42 episodes per child-year among the study population. Analysis of data from the 2010 DHS survey in Rwanda suggest that medical care is sought for 32% of diarrhea episodes and 34% of ARI episodes among children in the lowest socioeconomic quintile in Western Province, resulting in an estimated incidence of clinic/CHW reported diarrhea and ARI of 0.99 and 0.14 respectively. The sector-level intra-cluster correlation coefficients were estimated using simulation [12]. The resulting ICC values were quite low for both diarrhea (0.01) and ARI (0.004), which was anticipated given the large average cluster size. With an estimated 59,667 Ubudehe 1 & 2 children under five in the intervention sectors and 22,052 Ubudehe 1 & 2 children under five in the control sectors, an average of cluster size of 851 Ubudehe 1 & 2 children under five per sector, 80% power, a 70% match rate of clinic records to the Ubudehe database, a design effect of 9.5 for diarrhea and 4.4 for ARI, and α = 0.05 (two-sided test), the sector-level sample size is sufficient to detect a relative risk difference of 15% for clinician-reported diarrhea and 20% for clinician-reported ARI.
The sample size for the village-level study target the primary health outcomes of seven-day period prevalence of caregiver-reported diarrhea and ARI. Based on survey data from 2010 DHS Rwanda (13.98% 14-day prevalence of diarrhea among children under five in the lowest wealth quintile in Western Province) and data from our pilot studies (14.2% 7-day prevalence among Ubudehe 1 and 2 children in Western Province), we estimated the baseline prevalence of diarrhea among children in the control group to be 12%. In regard to respiratory illness among children in Western Province, data from the 2010 DHS Rwanda survey indicate that the baseline prevalence of cough with rapid breathing or difficulty breathing among children under five in Western province was 14.34% in the lowest wealth quintile. We chose this definition of ARI (cough with rapid breathing or difficulty breathing) as it is roughly comparable to the clinical definition of pneumonia (reported illness with cough and observed tachypnea) that is the standard for health clinics and CHW's in the study area. Similar to diarrhea, we took a conservative approach relative to the DHS figures and assume a 12% prevalence of ARI in the control group.
The sample size for the village-level study was selected to achieve sufficient power to detect clinically significant differences in the prevalence of caregiver-reported diarrhea and ARI. For both diarrhea and respiratory illness, we calculated the required sample size necessary to detect a 25% reduction in 7-day period prevalence. This effect size is both clinically significant and consistent with previous studies examining the impact of household water treatment on diarrhea [60] and improved cook stoves on respiratory illness [61].
In addition to a baseline prevalence of 12%, our calculation assumes 10 children per village, a total of three post-baseline observations per child, a within-child (repeated measures) ICC of 0.05, a within-village ICC of 0.02, 15% loss to follow-up, 80% power, and a significance level α = 0.05 (two-sided test). Estimates of within-child and within-village correlation are derived from our previous studies and simulation modeling. Based on these assumptions, we calculated that we required a minimum of 87 village clusters in each arm to detect a relative difference in prevalence of 25% or greater [20].
2.10. Data management and analysis
Clinic data will be extracted from paper-based IMCI, maternity and outpatient patient registers maintained at public health facilities and entered into a secure digital database. CHW patient-level data will be extracted from paper-based sick child encounter forms. Additionally, individual-level patient identifiers and case histories may be examined in health facility registers and CHW reporting forms in order to identify Ubudehe category and more fully characterize health outcomes and care-seeking behavior. A smart phone application is used by field-staff to administer the village-level surveys and data collected in this manner are synced and uploaded to a secure server.
The statistical analysis of all primary health outcomes in the study will be done on an intention-to-treat basis. Baseline data analysis will be conducted to characterize the study population and examine imbalances between treatment arms. The incidence of health center/CHW reported diarrhea and ARI in the control and intervention sectors will be compared using random-effects Poisson regression to account for the sector-level clustering and models will be adjusted for stratified randomization by district [21]. The effect of the intervention on the prevalence of caregiver-reported diarrhea and ARI in the village-level cohort will be assessed with mixed-effects log-binomial regression. Secondary outcomes from the village-level study will analyzed using generalized linear mixed models with the appropriate link function and distribution family (e.g. negative binomial for TTC counts). Analyses of the village-level sample will be adjusted for the multiple levels of clustering resulting from the study design. Analysis of secondary outcomes from the intensive data collection households will be conducted on an intention to treat basis, with per protocol sensitivity analyses.
2.11. Ethics and trial registration
The study has been reviewed by the Ethics Committee of London School of Hygiene and Tropical Medicine, the Institutional Review Board of Emory University, and the Rwanda National Ethics Committee. This trial is registered with Clinical Trials.gov (Registration No. NCT02239250).
3. Discussion
This research seeks to build on prior and existing research in multiple ways. First, previous and ongoing trials of HAP are smaller-scale, research-driven efficacy studies designed to assess the impact of improved cook stoves under controlled conditions. Our study is an effectiveness trial—a health impact evaluation of an intervention as actually delivered at scale. Though our results should be limited to the setting in which it is being delivered, they should be representative of what can be expected when the intervention is delivered programmatically.
Second, because Tubeho Neza program is funded through revenue earned through the generation and sale of carbon credits, it creates a mechanism for the free distribution of improved stoves and advanced water filters on a large scale. While both stoves and filters have at least a 3-year useful life, they have a comparatively high up-front cost (estimated $35 for stoves and $40 for filters by the time they reach households) that has limited the potential for governments and others to distribute them without charge to remote populations. High levels of coverage in a given population may increase the protective effect of the intervention by reducing the overall exposure of air pollution and fecal contamination in the community. This may also translate into a protective effect on non-adopters—a type of “herd immunity” that has been postulated but not shown by these environmental interventions.
Third, because carbon credits are awarded on the basis of intervention use and not just coverage, the implementer has a strong incentive to optimize use. Such pay-for-performance programs may address stove “stacking”—the continued use of traditional stoves together with the intervention stove—a well-known problem with improved stove interventions [29], [34]. They may also increase adherence of household water treatment that is necessary to achieve health benefits [10], [62]. Careful monitoring of actual use in the village-level sub-study, using self-reports as well as remotely monitored sensors, should provide strong evidence of patterns of use over the one-year follow up period.
Fourth, this is the first study evaluating the health effects of a large-scale intervention that combines improved cook stoves and household water treatment. In this way, the intervention is addressing pneumonia and diarrhea, the two major killers of young children both in Rwanda and many other low-income countries.
Fifth, while the LifeStraw 2.0 water filter used in this program meets the WHO's “most protective” standards [50], the EcoZoom stove falls below the Tier 3 performance level recommended by the Global Alliance for Clean Cooking and others. In fact, very few biomass stoves meet this standard; what is required is the use of clean fuels such as LPG or electricity [51], [52]. Evidence on the performance level of stoves that is necessary to achieve any health gains is still somewhat limited, however, and modeling does suggest that biomass stoves—especially those that are portable and can be used out-of-doors—are capable of reducing exposure to levels associated with improvements in health [63]. By assessing personal level exposure and health outcomes in the village-level sub-study, we hope to add additional data points on the dose-response curve and help determine whether biomass stoves—the only choice that is available to remote populations that are beyond the supply chain for clean fuels—can not only reduce expenditures for fuel and green house gas emissions, but also prevent disease.
Finally, our study methods combine a large-scale trial at the clinical catchment level with a nested trial at the village level. This allows us to use clinic-based diagnoses of primary and secondary outcomes, avoiding some of the bias associated with self-reported conditions and focusing on the more serious cases that present at clinics. At the same time, the village-level sub-study allows us to carefully document coverage and use of the intervention, as well as their impact on exposure to HAP and fecally contaminated drinking water.
Funding
This study is funded by DelAgua Health, a for-profit company that implements the intervention in Rwanda in conjunction with the Rwanda Ministry of Health. The funders have no role in study design, randomization, data collection and analysis, or decision to publish.
Competing interests
We have the following interests: This study was funded by DelAgua Health, a for-profit social enterprise that implements the program in Rwanda in conjunction with the Rwanda Ministry of Health. Evan Thomas and Christina Barstow, co-authors of this article, are compensated consultants to DelAgua Health, and are in charge of overseeing the implementation of the program in Rwanda.
Acknowledgements
We thank all of the participants who contributed to this study. We also thank the field staff for their diligent and tireless efforts in conducting the village-level data collection.
References
- 1.APHA, AWWA, WEF . American Public Health Association (APHA); Washington, DC, USA: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 3.Arnold B.F., Khush R.S., Ramaswamy P., Rajkumar P., Durairaj N., Ramaprabha P., Colford J.M. Reactivity in rapidly collected hygiene and toilet spot check measurements: a cautionary note for longitudinal studies. Am. J. Trop. Med. Hyg. 2015;92(1):159–162. doi: 10.4269/ajtmh.14-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barstow C.K., Ngabo F., Rosa G., Majorin F., Boisson S., Clasen T., Thomas E.A. Designing and piloting a program to provide water filters and improved cookstoves in Rwanda. PloS One. 2014;9(3):e92403. doi: 10.1371/journal.pone.0092403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barstow C., Nagel C., Clasen T., Thomas E. Process Evaluation and Assessment of Use of a Large Scale Water Filter and Cookstove Program in Rwanda. BMC Public Health. 2016 doi: 10.1186/s12889-016-3237-0. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner J., Schauer J.J., Ezzati M., Lu L., Cheng C., Patz J.A., Bautista L.E. Indoor air pollution and blood pressure in adult women living in rural China. Environ. Health Perspect. 2011;119(10):1390. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutta Z.A., Das J.K., Walker N., Rizvi A., Campbell H., Rudan I., Black R.E. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381(9875):1417–1429. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 8.Boamah E.A., Asante K.P., Ae-Ngibise K.A., Kinney P.L., Jack D.W., Manu G., Wylie B.J. Gestational Age Assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): ultrasound capacity building, fetal biometry protocol development, and ongoing quality control. JMIR Res. Protoc. 2014;3(4) doi: 10.2196/resprot.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonjour S., Adair-Rohani H., Wolf J., Bruce N.G., Mehta S., Prüss-Ustün A., Smith K.R. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environ. Health Perspect. 2013;121(7):784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J., Clasen T. High adherence is necessary to realize health gains from water quality interventions. PLoS One. 2012;7(5):e36735. doi: 10.1371/journal.pone.0036735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brussow H., Sidoti J., Link H., Hoang Y., Barclay D., Dirren H., Freire W. Age-specific prevalence of antibody to enterotoxigenic Escherichia coli in Ecuadorian and German children. J. Infect. Dis. 1990;162:974–977. doi: 10.1093/infdis/162.4.974. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty H., Moore J., Carlo W.A., Hartwell T.D., Wright L.L. A simulation based technique to estimate intracluster correlation for a binary variable. Contemp. Clin. Trials. 2009;30(1):71–80. doi: 10.1016/j.cct.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Clark M.L., Peel J.L. Perspectives in household air pollution research: who will benefit from interventions? Curr. Environ. Health Rep. 2014;1(3):250–257. [Google Scholar]
- 15.Clasen T., Roberts I., Rabie T., Schmidt W., Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst. Rev. 2006;3 doi: 10.1002/14651858.CD004794.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Crump J.A., Mendoza C.E., Priest J.W., Glass R.I., Monroe S.S., Dauphin L.A., Luby S.P. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am. J. Trop. Med. Hyg. 2007;77(1):136–141. [PubMed] [Google Scholar]
- 17.Dickinson K.L., Kanyomse E., Piedrahita R., Coffey E., Rivera I.J., Adoctor J., Wiedinmyer C. Research on emissions, air quality, climate, and cooking technologies in northern Ghana (REACCTING): study rationale and protocol. BMC Public Health. 2015;15(1):126. doi: 10.1186/s12889-015-1414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GBD 2013 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S.B., Bruce N.G., Grigg J., Hibberd P.L., Kurmi O.P., Lam K.B.H., Bar-Zeev N. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo M., Leon A.C. Statistical power and sample size requirements for three level hierarchical cluster randomized trials. Biometrics. 2008;64(4):1256–1262. doi: 10.1111/j.1541-0420.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayes R.J., Moulton L.H. Chapman & Hall; Boca Raton: 2009. Cluster Randomised Trials. [Google Scholar]
- 22.Hodge J.M., Clasen T.F. Carbon financing of household water treatment: background, operation and recommendations to improve potential for health gains. Environ. Sci. Technol. 2014;48(21):12509–12515. doi: 10.1021/es503155m. [DOI] [PubMed] [Google Scholar]
- 23.Khanna B., Cutler A., Israel N., Perry M., Lastovica A., Fields P., Gold B. Use caution with serologic testing for Helicobacter pylori infection in children. J. Infect. Dis. 1998;178:460–465. doi: 10.1086/515634. [DOI] [PubMed] [Google Scholar]
- 24.Lee A., Sanchez T.R., Shahriar M.H., Eunus M., Perzanowski M., Graziano J. A cross-sectional study of exhaled carbon monoxide as a biomarker of recent household air pollution exposure. Environ. Res. 2015;143:107–111. doi: 10.1016/j.envres.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindkvist P., Asrat D., Nilsson I., Tsega E., Olsson G.L., Wretlind B., Giesecke J. Age at acquisition of Helicobacter pylori infection: comparison of a high and a low prevalence country. Scand. J. Infect. Dis. 1996;28(2):181–184. doi: 10.3109/00365549609049072. [DOI] [PubMed] [Google Scholar]
- 26.Lipsitch M., Tchetgen E.T., Cohena T. Negative controls. Epidemiology. 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin W.J., Glass R.I., Araj H., Balbus J., Collins F.S., Curtis S., Bruce N.G. Household air pollution in low- and middle-income countries: health risks and research priorities. PLoS Med. 2013;10(6):e1001455. doi: 10.1371/journal.pmed.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masera O.R., Saatkamp B.D., Kammen D.M. From linear fuel switching to multiple cooking strategies: a critique and alternative to the energy ladder model. World Dev. 2000;28(12):2083–2103. [Google Scholar]
- 30.NISR . National Institute of Statistics of Rwanda; Kigali: 2014. Fourth Population and Housing Census, Rwanda, 2012. Main Indicators Report. [Google Scholar]
- 31.Pope D., Diaz E., Smith-Sivertsen T., Lie R.T., Bakke P., Balmes J.R., Bruce N.G. Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environ. Health Perspect. 2015;123(4):285. doi: 10.1289/ehp.1408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prüss-Ustün A., Bartram J., Clasen T., Colford J.M., Cumming O., Curtis V., Freeman M.C. Burden of disease from inadequate water, sanitation and hygiene in low-and middle-income settings: a retrospective analysis of data from 145 countries. Trop. Med. Int. Health. 2014;19(8):894–905. doi: 10.1111/tmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudan I., O’brien K.L., Nair H., Liu L., Theodoratou E., Qazi S., Child Health Epidemiology Reference Group Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health. 2013;3(1) doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz-Mercado I., Masera O., Zamora H., Smith K.R. Adoption and sustained use of improved cookstoves. Energy Policy. 2011;39(12):7557–7566. [Google Scholar]
- 35.Rylance J., Gordon S.B., Naeher L.P., Patel A., Balmes J.R., Adetona O., Martin W.J. Household air pollution: a call for studies into biomarkers of exposure and predictors of respiratory disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;304(9):L571–L578. doi: 10.1152/ajplung.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rwanda Ministry of Health . 2014. Trainer's Guide “Integrated Community Case Management of Childhood Illness.http://www.moh.gov.rw/index.php?id=134 English Version. January 2014. Available from. [Google Scholar]
- 37.Skogstrand K., Ekelund C.K., Thorsen P., Vogel I., Jacobsson B., Nørgaard-Pedersen B., Hougaard D.M. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J. Immunol. Methods. 2008;336(1):78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Smith K.R., McCracken J.P., Weber M.W., Hubbard A., Jenny A., Thompson L.M., Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378(9804):1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith K.R., Bruce N., Balakrishnan K., Adair-Rohani H., Balmes J., Chafe Z., Rehfuess E. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu. Rev. Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg E.B., Mendoza C.E., Glass R., Arana B., Lopez M.B., Mejia M., Bern C. Prevalence of infection with waterborne pathogens: a seroepidemiologic study in children 6-36 months old in San Juan Sacatepequez, Guatemala. Am. J. Trop. Med. Hyg. 2004;70(1):83. [PubMed] [Google Scholar]
- 41.Thomas E.A., Barstow C.K., Rosa G., Majorin F., Clasen T. Use of remotely reporting electronic sensors for assessing use of water filters and cookstoves in Rwanda. Environ. Sci. Technol. 2013;47(23):13602–13610. doi: 10.1021/es403412x. [DOI] [PubMed] [Google Scholar]
- 42.Tielsch J.M., Katz J., Zeger S.L., Khatry S.K., Shrestha L., Breysse P., LeClerq S.C. Designs of two randomized, community-based trials to assess the impact of alternative cookstove installation on respiratory illness among young children and reproductive outcomes in rural Nepal. BMC Public Health. 2014;14(1):1271. doi: 10.1186/1471-2458-14-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres-Dosal A., Pérez-Maldonado I.N., Jasso-Pineda Y., Salinas R.I.M., Alegría-Torres J.A., Díaz-Barriga F. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci. Total Environ. 2008;390(2):362–368. doi: 10.1016/j.scitotenv.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 44.Ungar B.L., Gilman R.H., Lanata C.F., Perez-Schael I. Seroepidemiology of Cryptosporidium infection in two Latin American populations. J. Infect. Dis. 1988;157(3):551–556. doi: 10.1093/infdis/157.3.551. [DOI] [PubMed] [Google Scholar]
- 45.UN DESA . Designing Household Survey Samples: Practical Guidelines. United Nations Publications; New York: 2008. Statistical division. [Google Scholar]
- 46.UNICEF . United Nations Children’s Fund; New York: 2012. Committing to Child Survival: a Promise Renewed. Progress Report 2012. [Google Scholar]
- 47.Vitral C.L., Yoshida C.F., Lemos E.R., Teixeira C.S., Gaspar A. Age-specific prevalence of antibodies to hepatitis A in children and adolescents from Rio de Janeiro, Brazil, 1978 and 1995: relationship of prevalence to environmental factors. Memórias do Inst. Oswaldo Cruz. 1998;93(1):1–5. doi: 10.1590/s0074-02761998000100001. [DOI] [PubMed] [Google Scholar]
- 48.Walker C.L.F., Perin J., Aryee M.J., Boschi-Pinto C., Black R.E. Diarrhea incidence in low-and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12(1):220. doi: 10.1186/1471-2458-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A., Black R.E. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO . World Health Organization; Geneva: 2010. Guidelines for Drinking Water Quality. 4th Ed. [Google Scholar]
- 51.WHO . World Health Organization; Geneva: 2014. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion. [PubMed] [Google Scholar]
- 52.WHO . World Health Organization; Geneva: 2014. Integrated Management of Childhood Illness: Chart Booklet.http://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/ Available at. [Google Scholar]
- 53.Zwane A.P., Zinman J., Van Dusen E., Pariente W., Null C., Miguel E., Duflo E. Being surveyed can change later behavior and related parameter estimates. Proc. Natl. Acad. Sci. 2011;108(5):1821–1826. doi: 10.1073/pnas.1000776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark M.L., Peel J.L., Balakrishnan K., Breysse P.N., Chillrud S.N., Naeher L.P. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ. Health Perspect. (Online) 2013;121(10):1120. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith K.R., Sagar A. Making the clean available: escaping India's chulha trap. Energy Policy. 2014;75:410–414. [Google Scholar]
- 56.Rosa G., Majorin F., Boisson S., Barstow C., Johnson M., Kirby M. Assessing the impact of water filters and improved cook stoves on drinking water quality and household air pollution: a randomised controlled trial in rwanda. PLoS One. 2014;9(3):e91011. doi: 10.1371/journal.pone.0091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makaka A., Breen S., Binagwaho A. Universal health coverage in Rwanda: a report of innovations to increase enrolment in community-based health insurance. Lancet. 2012;380:S7. [Google Scholar]
- 58.Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J., Hill M.N. Recommendations for blood pressure measurement in humans and experimental animals part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 59.Lammie P.J., Moss D.M., Goodhew E.B., Hamlin K., Krolewiecki A., West S.K., Priest J.W. Development of a new platform for neglected tropical disease surveillance. Int. J. Parasitol. 2012;42(9):797–800. doi: 10.1016/j.ijpara.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Clasen T.F., Alexander K.T., Sinclair D., Boisson S., Peletz R., Chang H.H. Interventions to improve water quality for preventing diarrhoea. Cochrane Library. 2015 doi: 10.1002/14651858.CD004794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dherani M., Pope D., Mascarenhas M., Smith K.R., Weber M., Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull. World Health Org. 2008;86(5):390C–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clasen T. Household water treatment and safe storage to prevent diarrheal disease in developing countries. Current Environ. Health Rep. 2015;2(1):69–74. doi: 10.1007/s40572-014-0033-9. [DOI] [PubMed] [Google Scholar]
- 63.Johnson M.A., Chiang R.A. Quantitative guidance for stove usage and performance to achieve health and environmental targets. Environ. Health Perspect. (Online) 2015;123(8):820. doi: 10.1289/ehp.1408681. [DOI] [PMC free article] [PubMed] [Google Scholar]