Abstract
Background
Babesia microti is an emerging tick-borne pathogen and the causative agent of human babesiosis. Mathematical modeling of the reproductive rate of B. microti indicates that it cannot persist in nature by horizontal tick-host transmission alone. We hypothesized that transplacental transmission in the reservoir population contributes to B. microti persistence and emergence in North American rodent populations.
Methods
Peromyscus leucopus were collected from Connecticut and Block Island, Rhode Island and analyzed using a highly specific quantitative PCR (qPCR) assay for infection with B. microti.
Results
In April, 100% (n = 103) of mice were infected with B. microti. Females exhibited significantly higher parasitemia than their offspring (P < 0.0001) and transplacental transmission was observed in 74.2% of embryos (n = 89). Transplacental transmission of B. microti is thus a viable and potentially important infectious pathway in naturally infected rodent species and should be considered in future theoretical and empirical studies.
Conclusions
To our knowledge, this study is the first to report transplacental transmission of B. microti occurring in its natural reservoir host, P. leucopus, in the United States and the only study that provides a quantitative estimate of parasitemia. This vector-independent pathway could contribute to the increased geographic range of B. microti or increase its abundance in endemic areas.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2875-8) contains supplementary material, which is available to authorized users.
Keywords: Ixodes scapularis, Rodent, Babesiosis, Emerging disease, Congenital transmission
Background
Babesia microti (Apicoplexa: Sporozoea) is a zoonotic intraerythrocytic apicomplexan parasite and is responsible for almost all cases of human babesiosis in the United States [1]. Human babesiosis is an emerging tick-borne disease that shares the same vector, the blacklegged tick (Ixodes scapularis), and dominant reservoir host, the white-footed mouse (Peromyscus leucopus), as the causative agent of Lyme disease, the spirochete Borrelia burgdorferi [2]. During the last 20 years, human babesiosis has spread in the United States [3, 4] following a similar trajectory to that of Lyme disease, although with a temporal lag [5–7]. Factors accounting for the delayed spread of babesiosis compared to Lyme disease include lower fitness in the enzootic cycle because of lower transmission from infected host to tick and lower trans-stadial transmission, greater asymptomatic infection in humans, insufficient physician awareness, and under-diagnosis in non- or newly-endemic areas [1, 5, 6].
An integrated measure of B. microti fitness (the basic reproductive number, R0) was estimated to be lower than the threshold for pathogen persistence (R0 < 1) under ecological conditions identified in long-term field studies [6, 8] implying that emergence of this pathogen should be unlikely in nature. However, despite the low predicted R0, B. microti has not only persisted in multiple locations with high infection prevalence in ticks regionally [5, 9–11], but it is also geographically expanding in the Northeast and upper Midwest regions of the USA [6]. This paradoxical finding suggests additional mechanism(s) enhancing B. microti transmission and persistence in the enzootic cycle may be occurring. Previous field studies reported higher average infection prevalences of B. burgdorferi (22.37%) compared to B. microti (9.53%) in nymphal I. scapularis ticks throughout the New England area [6]. However, very little is known about the B. microti infection status of P. leucopus in nature.
The aims of this research were to (i) determine if transplacental transmission of B. microti occurs in naturally infected, wild reservoir P. leucopus mice and (ii) quantify the level of transplacental transmission of host populations in B. microti-endemic coastal New England. As a comparison for early season infection prevalence we also screened for the presence of B. burgdorferi in the same host population; B. burgdorferi is not known to be transmitted transplacentally [12, 13].
Methods
Study site and animals
Adult Peromyscus leucopus were collected from two locations in Connecticut [Lake Gaillard (LG) 41°22'25.3"N, 72°46'43"W and Old Lyme (OL) 41°22'21.5"N, 72°20'37.6"W] and two locations on Block Island, Rhode Island [North Island (NI) 41°12'36.4"N, 71°34'18.8"W and Rodman’s Hollow (RH) 41°09'25.2"N, 71°35'22.9"W] for two trapping sessions from April 26 - May 2 (hereafter referred to as April) and July 23–31, 2016. In each trapping session, animals were trapped for two consecutive nights using Sherman live traps (7.62 × 8.89 × 22.86 cm; H.B. Sherman Traps, Inc. Tallahassee, FL) baited with peanut butter, oats, and sunflower seeds. Traps were arranged in nine 200 m transects with one trap placed every 10 m for a total of 180 traps at each location, except for NI where 100 m transects were used for a total of 90 traps.
Animals were removed from traps, morphological characteristics (age, sex, weight, body measurements, etc.) were collected, and attached larval and nymphal ticks were counted and removed from the ears and body. All animals were euthanized with an overdose of isoflurane and necropsied immediately in the field. All organs and tissues were preserved in liquid nitrogen, blood samples were dried on Whatman FTA cards (Fisher Scientific, Pittsburg, PA, USA), and the carcasses were wrapped in Whirl-Pak bags (Whirl-Pak®, Nasco, Fort Atkinson, WI, USA) and frozen in liquid nitrogen; embryos of pregnant females remained within the carcass. All samples were transferred to -80 °C for long term storage upon return to the laboratory. All animal procedures were in accordance with guidelines approved by the Columbia University Institutional Animal Care and Use Committee (IACUC no. AC-AAAL3656).
Embryo necropsy
To ensure no cross contamination of maternal blood occurred during the necropsy of embryos from the female’s body cavity, a new pair of autoclaved dissecting utensils were used for each adult female. Utensils were soaked in a 10% bleach solution and then in 70% ethanol for at least 2 min between sampling embryos from the same adult female. All embryos were removed from the body cavity of the female, washed with a saline solution, photographed, immediately cut into sections around each embryo, and then transferred to a separate sterile Petri dish. Embryos were found in various stages of development. Because the gestation time of P. leucopus is 22–28 days, embryos were separated into three developmental age categories (Week 1, Week 2, Week 3; Fig. 1) based on the size and the presence or absence of various developmental characteristics for each embryo (i.e. eye spot, placenta, limbs and tail, etc.). Week 1 embryos were very small with little to no distinguishing characteristics. Week 2 embryos were larger with a small placenta, could be removed from the uterus, and a distinctive eyespot was observed. Week 3 embryos were large with almost fully developed characteristics. A 25 mg sample of the following tissue types was collected: the uterine arteries and veins, the uterus surrounding each individual embryo, the placenta if present, and the embryonic sac surrounding each embryo if available. Utensils were cleaned with bleach and ethanol between each type of tissue collection. If the embryo was early in development the whole embryo was used for DNA extraction or the embryo was cut in half to equal 25 mg. If the embryo was in a later developmental stage the heart of the embryo was removed and used in the extraction process. All embryo samples were screened for the presence of B. microti using the quantitative PCR protocols described in the following section.
Fig. 1.
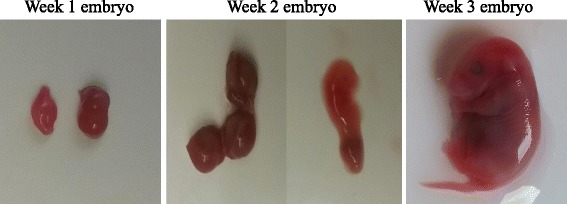
Pictures of embryos in the three stages of development, categorization was based on size and developmental characteristics of each embryo. Week 1 embryos were very small with little to no distinguishing characteristics. Week 2 embryos were larger and an embryo could be removed from its location in the uterus, a distinctive eye-spot was also observed. Week 3 embryos were large with almost fully developed characteristics
DNA extraction and PCRs
For all embryos and embryonic tissues, a 25 mg sample was collected as described in the previous section, DNA was extracted by hand using the Qiagen DNeasy Blood and Tissue kit following the manufacturer’s protocol (Qiagen, Valencia, CA, USA), and DNA concentration was analyzed using a spectrophotometer (Denovix Inc, New Castle County, Delaware, USA).
For adult samples, a 3 mm ear punch biopsy was collected from each mouse and DNA was extracted using the QIAcube HT DNA extraction system (Qiagen). DNA concentration was measured for each sample using a spectrophotometer (Denovix Inc). Each ear punch biopsy sample collected from adults was then tested in duplicate for the presence of B. burgdorferi using a quantitative PCR (qPCR) specific for a unique 69 bp segment of the 16S rRNA gene: forward primer (5'-GGC GGC ACA CTT AAC ACG TTA G-3'), reverse primer (5'-GCT GTA AAC GAT GCA CAC TTG GT-3'), probe (6FAM-TTC GGT ACT AAC TTT TAG TTA A-MGBNFQ) [14]. Samples were run on a 7500 real-time PCR system (Applied Biosystems®, ThermoFisher Scientific, Waltham, WA, USA) using TaqMan Fast Advanced chemistry (ThermoFisher Scientific, Waltham, WA) and cycling conditions consisted of: 95 °C for 20 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. Because B. burgdorferi is an extracellular bacterium and primarily observed in tissues, we restricted our screening of B. burgdorferi to only adult ear punch biopsy samples.
For adult individuals, DNA from dried blood samples was extracted from FTA cards using the QIAcube HT DNA extraction system or by hand using the Qiagen DNeasy Blood and Tissue kit following the manufacturer’s protocol (Qiagen). DNA concentration was analyzed using a spectrophotometer (Denovix Inc). Embryos, embryonic tissues, and adult blood samples, were screened in duplicate for the presence of B. microti using a qPCR designed specifically for detecting a 104 bp section of the 18S rRNA gene of B. microti: forward primer (5'-AAC AGG CAT TCG CCT TGA AT-3'), reverse primer (5'-CCA ACT GCT CCT ATT AAC CAT TAC TCT-3'), probe (6FAM-CTA CAG CAT GGA ATA ATG A-MGBNFQ) [15]. Babesia microti is primarily an intracellular erythrocytic protozoan and therefore we analyzed embryos, embryonic tissues, and adult dried blood samples for the presence of B. microti. Because blood samples could not be obtained from embryos, a 25 mg sample of heart tissue was also collected and analyzed from a subset of adult mice for direct comparison to embryos and embryonic tissues.
Average cycle threshold (CT) and quantity values were collected from each run and mean infection prevalence (number of infected individuals/total number of individuals) was calculated for each location. qPCR standards were constructed by separately cloning the aforementioned targeted regions of B. burgdorferi and B. microti into pUC57-Kan plasmids (GENEWIZ, Inc., South Plainfield, NJ, USA). A dilution series (106–1 copy number dilutions) for each pathogen was developed by combining a single uninfected I. scapularis nymph (courtesy of the CDC) and a known amount of plasmid DNA followed by DNA extraction [14, 15]. Quantification and normalization of each sample were completed as previously described [14, 16, 17]; results are expressed as gene copies per picogram (pg) of total DNA.
Babesia microti positive samples, determined via qPCR, were then subjected to a standard PCR using a specific primer set to amplify 437 bp of the B. microti 18S rRNA gene (forward PIRO-A: 5'-AAT ACC CAA TCC TGA CAC AGG G-3' and reverse PIRO-B: 5'-TTA AAT ACG AAT GCC CCC AAC-3') [18]. Amplification was performed using Platinum Superfi 2× PCR Master Mix (Invitrogen, ThermoFisher Scientific, Waltham, WA, USA) under the following conditions: 98 °C for 30 s, followed by 40 cycles of 98 °C for 10 s, 60 °C for 10 s, and 72 °C for 1 min, with a final elongation step of 72 °C for 5 min. PCR products were subjected to gel electrophoresis on a 1% agarose gel stained with ethidium bromide. Amplicons that produced bands of the correct size (~450 bp) were submitted for Sanger sequencing (Eurofins, Louisville, KY, USA) in both the forward and reverse directions. Consensus sequences were constructed and aligned with other orthologous B. microti sequences deposited in the GenBank database and previously described as human-infecting Clade 1 and nonhuman-associated Clade 2 and Clade 3 pathogens (AY144696, AY144701, AY144690; respectively) [19]. Alignments were completed by hand using BioEdit Sequencing Alignment Editor [20] and using the online Multiple Sequence Comparison by Log-Expectation Alignment (MUSCLE) program. Sequence diversity and a pairwise distance matrix were constructed using the maximum composite likelihood model in MEGA version 7.0 software [21].
Statistical analyses
Infection prevalence was calculated for sex of adults, embryonic tissue type, and embryonic stage of development. Significant differences between the proportion of individuals infected and not-infected with B. burgdorferi were evaluated using a Fisher’s exact test. A Student’s t-test was used to compare the number of nymphs collected from April mice, individuals infected with B. burgdorferi and/or B. microti in April mice between males and females, between CT and BI, and to compare female infection to embryo infection overall and between trapping sessions. A nonparametric Kruskal-Wallis test was performed to assess differences in B. microti infection in the stages of embryonic development and the average number of embryos in each developmental stage. A pairwise Wilcoxon test was used to determine the variation between each stage of development. Finally, an analysis of variance (ANOVA) was completed to determine differences in parasitemia between the three stages of embryonic development. Geometric means of duplicate samples were used in all calculations.
Results
All mice collected in April and only pregnant females (n = 6) and their embryos (n = 26) collected in July were necropsied and analyzed for the presence of B. burgdorferi and B. microti using qPCR. In the April sampling session, a total of 63 P. leucopus mice were collected from the Connecticut (CT) locations and 40 from the Block Island (BI) locations (Table 1). Of these, the combined proportion of mice with attached I. scapularis nymphs was 57.89% (n = 32 CT mice, n = 26 BI mice). Overall, 12.62% (CT = 9.53%; BI = 17.5%) of mice were infected with B. burgdorferi and 100% with B. microti. The average B. burgdorferi log mean copy number per pg total DNA (MCN) did not vary significantly between CT and BI (Table 1) or between infected males (nCT = 4; nBI = 5) and females (nCT = 2; nBI = 2). No significant differences in B. microti MCN were observed between CT and BI (Table 1) or between infected males (n = 55; MCN ± standard error = 5.25 ± 0.09) and females (n = 48; MCN = 5.20 ± 0.10).
Table 1.
Log mean copy number per pg total DNA (MCN) calculations and standard errors (SE) for Borrelia burgdorferi and Babesia microti from Connecticut locations: Lake Gaillard (LG) and Old Lyme (OL) and Block Island locations: North Island (NI) and Rodman’s Hollow (RH) for all Peromyscus leucopus collected in April
| B. burgdorferi | B. microti | ||||
|---|---|---|---|---|---|
| n | MCN | SE | MCN | SE | |
| Connecticut | 63 | 4.29 | 0.25 | 5.27 | 0.10 |
| LG | 27 | 3.95 | 0.29 | 5.43 | 0.18 |
| OL | 36 | 4.46 | 0.34 | 5.14 | 0.10 |
| Block Island | 40 | 4.35 | 0.20 | 5.17 | 0.10 |
| NI | 15 | 3.75 | 0.09 | 5.01 | 0.18 |
| RH | 25 | 4.59 | 0.17 | 5.27 | 0.11 |
Of the total small mammals sampled in 2016, 20 P. leucopus females were pregnant (nCT = 12; nBI = 8), including three and five mice collected on BI in April and July, respectively. All 12 pregnant females from CT were collected in April. One pregnant meadow vole (Microtus pennsylvanicus) was collected on BI in July and was included in some of the analyses. Four pregnant mice and the vole tested positive for B. burgdorferi (25.0%). All blood samples collected from adult pregnant females tested positive for the presence of B. microti via qPCR (100%) and no significant differences were observed between CT and BI females. The number of embryos per female (mouse only) ranged from 2–6 with an average of 4.5 embryos per female (Table 2; Additional file 1: Table S1). A significant difference among the average number of embryos was observed (Kruskal-Wallis χ2 = 8.13, df = 2, P = 0.0172). The average number of embryos in Week 1 (n = 3.4) was not significantly different from the number of embryos in Week 2 (n = 4.8; Wilcoxon test P = 0.0660), however there were significantly more Week 3 embryos compared to Week 1 (n = 5.1; Wilcoxon test P = 0.0360), but not compared to Week 2 (Wilcoxon test P = 0.4340; Fig. 2a). There were no significant differences observed in the average number of embryos between trapping sessions.
Table 2.
The mean number of embryos at each embryonic stage of development, the total number of embryos in each stage, the number of those embryos infected with Babesia microti, and the infection prevalence of mouse embryos for each stage of embryonic development is presented
| Mean no. embryos | No. embryos | No. infected | % infected | MCN | |
|---|---|---|---|---|---|
| Week 1 | 3.4 | 24 | 22 | 91.67 | 2.55 |
| Week 2 | 4.8 | 24 | 21 | 87.50 | 2.50 |
| Week 3 | 5.1 | 41 | 23 | 56.10 | 1.64 |
| Total | 4.5 | 89 | 66 | 74.16 | 2.22 |
See Additional file 1: Table S1 for detailed information of each individual female
Fig. 2.
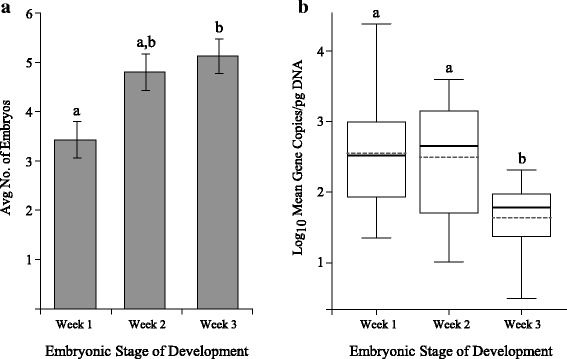
The average number of embryos in pregnant Peromyscus leucopus (a) and a box plot of the log mean copy number/pg DNA (MCN) of Babesia microti in embryos (b) in different stage of embryo development. Significantly fewer embryos were collected in Week 1 than in Week 3 (P = 0.0360). Parasitemia was significantly lower in Week 3 embryos compared to Week 1 and 2 (P < 0.0003 and P = 0.0022, respectively). Solid lines denote median values while dashed lines denote the means, different letters denote significant differences
A total of 89 mouse embryos were collected and tested for the presence of B. microti. Week 1 embryos showed the highest infection prevalence (91.67%), whereas Week 3 embryo infection was lowest (56.10%); the average infection prevalence among all embryos was 74.16% (Table 2). The average B. microti MCN was significantly different among the three stages of embryonic development (Kruskal-Wallis χ2 = 16.04, df = 2, P = 0.0003). Embryos from Week 1 (MCN = 2.55 ± 0.17) and Week 2 (MCN = 2.49 ± 0.18) displayed significantly higher parasitemia than embryos from the Week 3 stage of development (MCN = 1.64 ± 0.11; Wilcoxon test P = 0.0003 and P = 0.0022, respectively; Fig. 2b), but not between each other (Wilcoxon test P = 0.8948). Other reproductive tissue types (uterus, placenta, embryonic sac) also tested positive for B. microti (Additional file 2: Table S2), but due to the differences in embryo developmental stage not all tissue types could be collected at each of the three stages.
Females displayed significantly higher B. microti parasitemia (n = 21; MCN = 4.40 ± 0.21) than their embryos (n = 71; MCN = 2.29 ± 0.10; P < 0.0001; Fig. 3). April females (n = 15; MCN = 4.67 ± 0.24) showed significantly higher parasitemia than pregnant females from July (n = 6 including the M. pennsylvanicus female; MCN = 3.73 ± 0.35; P = 0.0502). Conversely, July embryos (n = 20; MCN = 2.66 ± 0.22) exhibited a significantly higher MCN than embryos from April (n = 51; MCN = 2.15 ± 0.10; P = 0.0477). To assess differences in MCN among different sample types, we also compared heart tissue (n = 11, MCN 4.61 ± 0.24) and blood samples (n = 11, MCN = 5.00 ± 0.26) collected from a subset of adult mice and found that they did not differ significantly (P = 0.2757).
Fig. 3.
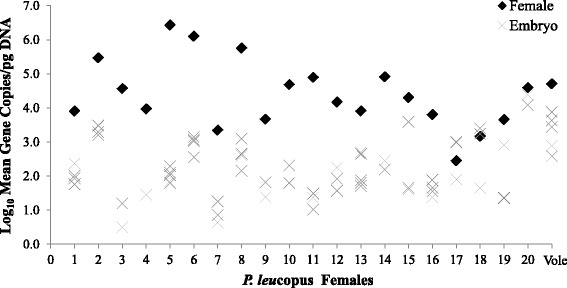
Babesia microti log mean copy number per pg of total DNA (MCN) comparing females to each of her infected embryos. Positions 1–20 are Peromyscus leucopus pregnant females; 1–16 were samples collected in April; 17–20 were collected in July, and the last position is of the single Microtus pennsylvanicus female (Vole) collected in July. Females (black diamonds) overall exhibited significantly higher parasitemia (MCN ± standard error, 4.405 ± 0.21) than their offspring (gray X; 2.291 ± 0.10; P < 0.0001)
Sequences in the forward and reverse directions of the 18S rRNA gene of B. microti were obtained for six individuals including each embryo, adult female uterine arteries and veins, uterus, placenta, and embryonic sac (total sequences = 76; Additional file 3: Figure S1). Consensus sequences were constructed and BLAST analysis (NCBI) confirmed all sequences to be strains of B. microti (XR_002459986 or AF028343). Most sampled sequences aligned nearly identically to the reference sequence for Clade 1 (AY144696), except for one sample of adult female arteries (2033-Arteries). The overall pairwise mean distance and sequence diversity was 0.005 for all embryonic samples (0.001 when 2033-Arteries was excluded) including the reference sequence for Clade 1 (AY144696) and 0.014 (0.011 when 2033-Arteries was excluded) when reference sequences from Clade 2 (AY144701) and Clade 3 (AY144690) were added. Unique B. microti sequences generated from this study have been deposited in GenBank (accession nos. MH221125, MH221126).
Discussion
Our study demonstrates for the first time that transplacental transmission of B. microti occurs in wild, naturally-infected populations of P. leucopus and M. pennsylvanicus in Connecticut and on Block Island, RI, USA, with 74% transmission efficiency from mother to fetuses. Although studies have previously reported transplacental transmission of Babesia canis canis in canines [22, 23], B. microti in BALB/c laboratory mice [24], and humans [25, 26] only one other study has reported vertical transmission of B. microti occurring in naturally infected rodent populations [27]. Tolkacz and colleagues [27] reported vertical transmission of B. microti in two species of vole (Microtus arvalis and M. oeconomus) in Poland, finding 81% infection occurring in embryos and 90% in pups. Here, we report transplacental transmission in P. leucopus and a third species of vole, M. pennsylvanicus.
All individuals were infected with B. microti in April, in contrast to only 12.6% of individuals infected with B. burgdorferi. These mice could have been infected by nymphal I. scapularis in the spring or could have survived and maintained the infection through the winter months (chronic infection). Because nymphal I. scapularis were found on 50.8% of mice collected in CT and 65.0% of mice collected on BI, it is possible these mice all became infected by questing nymphs once they emerged from their winter burrows. However, because a higher proportion of nymphal I. scapularis are typically infected with B. burgdorferi (22.37% average infection prevalence) than B. microti (9.53% average infection prevalence) in the New England area (comparing 10 studies and 9335 nymphal I. scapularis samples) [6], the higher B. microti infection prevalence in mice requires an alternative explanation. A combination of vertical transmission and chronic infection with B. microti through the winter months would amplify B. microti even before most ticks emerged from diapause, typically in mid to late May [6], providing a transmission advantage compared to B. burgdorferi. The duration of B. microti infection in naturally infected (via tick vector) P. leucopus is unclear; however, chronic infection has been shown to persist in laboratory P. leucopus for seven months and possibly longer [9, 10, 28, 29]. Chronic infection of B. microti was also observed for approximately five months in naturally infected voles from Russia [30]. In these ways mice infected over the transmission season (April-July) could remain infected until the following spring, when transplacental transmission could further increase early-season transmission. Resistance to re-infection is unknown in Peromyscus; consequently, mice could either become re-infected or B. microti could become reactivated every transmission season resulting in chronic infection and an increase of the pathogen in the population via vertical transmission. Reactivation of certain pathogens has been shown to occur during stressful life events (i.e. migration in birds) [31, 32]. Seasonal physiological stress (i.e. cold temperatures during winter months) may allow for reactivation of B. microti and provide an additional explanation for prolonged infection in wild P. leucopus.
The relative timing of insemination and infection in the mother may be important determinants of the survivorship and infection outcome of fetuses [24, 33]. The breeding season of P. leucopus in our study region starts around March, while nymphal tick activity typically starts in April or May, although both events are highly dependent on seasonal variation in temperature [34]. Furthermore, peak B. microti parasitemia in P. leucopus occurs 14 days after infection via tick bite [35]. Given the differences in mouse and tick life cycles, females captured in April were more likely inseminated before B. microti infection via ticks. The lower average number of Week 1 compared to Week 3 embryos may be explained by the higher likelihood of reabsorption of some or all embryos when the female is inseminated simultaneously or before infection. Reabsorption of embryos was suggested in a study using BALB/c laboratory mice which found that females inseminated in the acute phase of B. microti infection (0–12 days) did not produce any offspring [24]. Reabsorption of fetuses when mice are infected early in the gestation period has also been observed in other pathogens, such as Neospora caninum in BALB/c laboratory mice [33]. A significantly higher number of Week 3 embryos with significantly lower parasitemia compared to embryos in earlier stages of development (Week 1 and 2) was observed. This may also indicate that females became infected with B. microti after insemination or during later stages of gestation and the pathogen had not fully disseminated to all embryos when the female was euthanized. Experimental manipulation of the timing of infection and insemination is required to fully characterize the affect B. microti may have on developing fetuses and pathogen persistence.
Females exhibited significantly higher parasitemia compared to their offspring. Although the different tissues investigated could partially account for this difference, we found no significant differences in MCN in a subset of samples of adult heart tissue and blood. Decreased parasitemia in embryos may thus be due to limited placenta permeability. Although the placenta is known to function as a barrier to potentially harmful microbes and pathogens [36], multiple parasites and pathogens have developed ways of evading the placental barrier to infect a fetus, including another apicomplexan, Plasmodium spp. [37], as well as Toxoplasma gondii [38], Trypanosoma spp. [39], and several nematodes [40, 41]. Similar to Plasmodium spp., B. microti sporozoites invade host red blood cells and are able to breach the placental barrier to infect a female’s offspring [42]; exactly how these pathogens are able to diffuse through the placenta is unknown.
Tolkacz and colleagues [27] found different genotypes of B. microti in female voles and their offspring, although the mechanisms for these differences were not explained. This was not the case in our study, with sequences from P. leucopus and M. pennsylvanicus revealing high similarity between females and fetuses and overall. Low genome-wide sequence diversity was also observed in other studies [43, 44]. Some studies suggest that B. microti represents a rich species complex consisting of three distinct clades in the United States using the 18S rRNA gene; however genetic diversity within each clade is relatively invariant [19]. Strains in Clade 1 are found associated with human-biting Ixodes species, locations known to be endemic for human babesiosis, and exhibit the least amount of genetic diversity. Whereas strains in Clade 2 were isolated from carnivores and those in Clade 3 were found to infect rodents; neither Clade 2 nor 3 have been found to infect humans. Sequences from this study most closely aligned with the reference sequence from Clade 1 and these sequences showed a low sequence diversity of 0.001. One of our sequences (2033-Arteries) exhibited higher diversity than predicted by previous studies; this might have occurred due to multiple infections or interference from other pathogens during Sanger sequencing (resulting in a noisy chromatograph) and most likely does not represent a unique B. microti species.
Our field-based results indicate that transplacental transmission of B. microti is a potentially important pathway for infection in wild rodent species, P. leucopus and M. pennsylvanicus, which may partially explain B. microti emergence and geographic expansion. In endemic areas, transplacental transmission may enhance early season amplification of B. microti, contributing to higher infection prevalences in both hosts and vectors. Additional enhancement mechanisms have been described, including increased transmission of B. microti to ticks feeding on hosts coinfected with B. burgdorferi based in field and laboratory studies [6, 11, 35] and amplification driven by tick aggregation, as shown in an empirically-informed model [45]. Transplacental transmission in natural populations may also facilitate B. microti maintenance and spread in small rodent populations in areas with limited I. scapularis occurrence. For instance, in areas of Alaska, California, Colorado, Florida, Maine, and Montana that lack I. scapularis, small rodent populations (voles, shrews, cotton rats, other Peromyscus spp.) were infected with both human-infecting and non-human-associated strains of B. microti [46–50]. Some evidence suggests that nidicolous ticks (e.g. I. angustus, I. muris, I. spinipalpis) or other exophilic ticks (I. pacificus) may contribute moderate enzootic persistence in certain areas [48] in a ‘cryptic cycle’, but vertical transmission may also play a role. Irrespective of the maintenance mechanism in the enzootic cycle, transmission to humans and emergence would only occur when I. scapularis (a ‘bridge’ vector to humans) invades a region where B. microti had been enzootically maintained, as has been shown for B. burgdorferi [49, 51].
To confirm the relevance of transplacental and vertical transmission in B. microti, studies in a laboratory setting are required to specifically quantify the relative efficiencies of B. microti transmission routes, including tick-to-host transmission, vertical transmission in relation to insemination and infection timing, chronic infection, and xenodiagnoses and transmission efficiency of infected offspring. More extensive field studies quantifying the role of vertical transmission and chronic infection of B. microti should then be conducted to assess how these transmission pathways influence this pathogen’s persistence and transmission, in comparison to B. burgdorferi. As winter temperatures in these areas continue to increase each year [52], overwinter survival of mice may increase [53, 54], further enhancing the early season advantage of B. microti through chronic infection and vertical transmission.
Conclusions
We demonstrate that transplacental transmission of B. microti occurs with high efficiency in wild rodent populations in New England. This non-vector mediated transmission mode may result in significant pathogen amplification, in particular if combined with chronic infection in the host and increased overwintering survival in warming climates.
Additional files
Table S1. Information for each pregnant female and infection status of her embryos collected in April and July, 2016. Site: LG, Lake Gaillard; OL, Old Lyme; RH, Rodman’s Hollow; NI, North Island. Age of embryos: W1, Week 1; W2, Week 2; W3, Week 3. (DOCX 19 kb)
Table S2. Babesia microti infection prevalence in reproductive tissues (ES, embryonic sac). (DOCX 14 kb)
Figure S1. Babesia microti sequence alignment from embryos and tissues of the 18S rRNA gene for six females (labeled by a four digit identification number) including three reference sequences representing the three Babesia clades (Clade-1, AY144696; Clade-2, AY144701; Clade-3, AY144690). (DOCX 136 kb)
Acknowledgments
The authors would like to thank Sarah Dube, Elsbeth Kane, Christina Olbrantz and Evelyn Rynkiewicz for assistance collecting mice in the field and Maria Fernandez for statistical assistance.
Funding
This research was made possible by a grant from the National Institutes of Health, Ecology and Evolution of Infectious Disease Program to MDW (R01 GM105246).
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Unique B. microti sequences have been deposited in the GenBank database under the accession numbers MH221125 and MH221126. The raw data are available from the corresponding author upon reasonable request.
Abbreviations
- BI
Block Island, Rhode Island
- CT
Connecticut
- LG
Lake Gaillard
- MCN
log mean copy number/pg of DNA
- NI
North Island
- OL
Old Lyme
- qPCR
Quantitative polymerase chain reaction
- RH
Rodman’s Hollow
Authors’ contributions
DMT and MDW designed the study. DMT collected the data from the field and analyzed each individual in the laboratory. DMT performed all statistical analyses. DMT and MDW wrote the manuscript. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were in accordance with guidelines approved by the Columbia University Institutional Animal Care and Use Committee (IACUC no. AC-AAAL3656).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2875-8) contains supplementary material, which is available to authorized users.
Contributor Information
Danielle M. Tufts, Email: dt2503@columbia.edu
Maria A. Diuk-Wasser, Email: mad2256@columbia.edu
References
- 1.Vannier EG, Diuk-Wasser MA, Mamoun CB, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann NY Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- 3.Piesman J, Mather TN, Telford SR, III, Spielman A. Concurrent Borrelia burgdorferi and Babesia microti infection in nymphal Ixodes dammini. J Clin Microbiol. 1986;24:446–447. doi: 10.1128/jcm.24.3.446-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piesman J, Mather TN, Dammin GJ, Telford SR, III, Lastavica CC, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–1189. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- 5.Diuk-Wasser MA, Liu Y, Steeves T, Folsom-O’Keefe C, Dardick KR, Lepore T, et al. Monitoring human babesiosis emergence through vector surveillance, New England, USA. Emerg Infect Dis. 2014;20:225–231. doi: 10.3201/eid2002.130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter KS, Pepin KM, Webb CT, Gaff HD, Krause PJ, Pitzer VE, Diuk-Wasser MA. Invasion of two tick-borne diseases across New England: harnessing human surveillance data to capture underlying ecological invasion processes. Proc Biol Sci. 2016;283:S301–S327. doi: 10.1098/rspb.2016.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis S, Bent SJ. Loop analysis for pathogens: Niche partitioning in the transmission graph for pathogens of the North American tick Ixodes scapularis. J Theor Biol. 2011;269:96–103. doi: 10.1016/j.jtbi.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Mather TN, Telford SR, III, Moore SI, Spielman A. Borrelia burgdorferi and Babesia microti: Efficiency of transmission from reservoirs to vector ticks (Ixodes dammini) Exp Parasitol. 1990;70:55–61. doi: 10.1016/0014-4894(90)90085-Q. [DOI] [PubMed] [Google Scholar]
- 10.Telford SR, III, Spielman A. Reservoir competence of white-footed mice for Babesia microti. J Med Entomol. 1993;30:223–227. doi: 10.1093/jmedent/30.1.223. [DOI] [PubMed] [Google Scholar]
- 11.Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, et al. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One. 2014;9:9–13. doi: 10.1371/journal.pone.0099348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber MA, Zalneraitis EL. Childhood neurologic disorders and Lyme disease during pregnancy. Pediatr Neurol. 1994;11:41–43. doi: 10.1016/0887-8994(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro ED, Gerber MA. Lyme disease. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 6. Philadelphia: Elsevier Saunders; 2006. pp. 485–497. [Google Scholar]
- 14.Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollend L, Bent SJ, Krause PJ, Usmani-Brown S, Steeves TK, States SL, et al. Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector-Borne Zoonot. 2013;13:784–790. doi: 10.1089/vbz.2011.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3:e00434–e00412. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbour AG, Bunikis J, Fish D, Hanincova K. Association between body size and reservoir competence of mammals bearing Borrelia burgdorferi at an endemic site in the northeastern United States. Parasit Vectors. 2015;8:299. doi: 10.1186/s13071-015-0903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong PM, Katavolos P, Caporale DA, Smith RP, Spielman A, Telford SA. Diversity of Babesia infecting deer ticks (Ixodes dammini) Am J Trop Med Hyg. 1998;58:739–742. doi: 10.4269/ajtmh.1998.58.739. [DOI] [PubMed] [Google Scholar]
- 19.Goethert HK, Telford SR., III What is Babesia microti? Parasitol. 2003;127:301–309. doi: 10.1017/S0031182003003822. [DOI] [PubMed] [Google Scholar]
- 20.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 21.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Bio Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mierzejewska EJ, Welc-Faleciak R, Bednarska M, Rodo A, Bajer A. The first evidence for the vertical transmission of Babesia canis in a litter of Central Asian Shepherd dogs. Ann Agric Environ Med. 2014;21:500–503. doi: 10.5604/12321966.1120590. [DOI] [PubMed] [Google Scholar]
- 23.Adaszek L, Obara-Galek J, Piech T, Winiarczyk M, Kalinowski M, Winiarczyk S. Possible vertical transmission of Babesia canis canis from a bitch to her puppies: a case report. Vet Med-Czech. 2016;61:263–266. doi: 10.17221/8881-VETMED. [DOI] [Google Scholar]
- 24.Bednarska M, Bajer A, Drozdowska A, Mierzejewska EJ, Tolkacz K, Welc-Faleciak R. Vertical transmission of Babesia microti in BALB/c mice: Preliminary report. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0137731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.New DL, Quinn JB, Qureshi MZ, Sigler SJ. Vertically transmitted babesiosis. J Pediatr. 1997;131:163–164. doi: 10.1016/S0022-3476(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 26.Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18:1318–1321. doi: 10.3201/eid1808.110988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolkacz K, Bednarska M, Alsarraf M, Dwuznik D, Grzybek M, Welc-Faleciak R, et al. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae) Parasit Vectors. 2017;10:66. doi: 10.1186/s13071-017-2007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spielman A, Etkind P, Piesman J, Ruebush TK, II, Juranek DD, Jacobs MS. Reservoir hosts of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30:560–565. doi: 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- 29.Piesman J. Intensity and duration of Borrelia burgdorferi and Babesia microti infectivity in rodent hosts. Int J Parasitol. 1988;18:687–689. doi: 10.1016/0020-7519(88)90105-1. [DOI] [PubMed] [Google Scholar]
- 30.Rar V, Yakimenko V, Makenov M, Tikunov A, Epikhina T, Tancev A. High prevalence of Babesia microti ‘Munich’ type in small mammals from an Ixodes persulcatus/Ixodes trianguliceps sympatric area in the Omsk region, Russia. Parasitol Res. 2016;115:3619–3629. doi: 10.1007/s00436-016-5128-9. [DOI] [PubMed] [Google Scholar]
- 31.Gylfe A, Bergstrom S, Lundstrom J, Olsen B. Reactivation of Borrelia infection in birds. Nature. 2000;403:724–725. doi: 10.1038/35001663. [DOI] [PubMed] [Google Scholar]
- 32.Gern L, Humair PF. Ecology of Borrelia burgdorferi sensu lato in Europe. In: Gray J, Kahl O, Lane RS, Stanek G, editors. Lyme Borreliosis: Biology, epidemiology, and control. Wallinford, UK: CAB International Publishing; 2002. p. 149–174.
- 33.Quinn HE, Ellis JT, Smith NC. Neospora caninum: a cause of immune-mediated failure of pregnancy? Trends Parasitol. 2002;18:391–394. doi: 10.1016/S1471-4922(02)02324-3. [DOI] [PubMed] [Google Scholar]
- 34.Levi T, Keesing F, Oggenfuss K, Ostfeld RS. Accelerated phenology of blacklegged ticks under climate warming. Phil Trans R Soc B. 2015;370:20130556. doi: 10.1098/rstb.2013.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn JM, Krause PJ, Davis S, Vannier EG, Fitzpatrick MC, Rollend L, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the Northeastern United States. PLoS One. 2014;9:e115494. doi: 10.1371/journal.pone.0115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright C, Sibley CP. Placental transfer in health and disease. In: Kay HH, Nelson DM, Wang Y, editors. The placenta: From development to disease. West Sussex, UK: John Wiley and Sons; 2011. p. 66-74.
- 37.Poovassery J, Moore JM. Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta. Infect Immun. 2006;74:2839–2848. doi: 10.1128/IAI.74.5.2839-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhry SA, Gad N, Koren G. Toxoplasmosis and pregnancy. Can Fam Physician. 2014;60:334–336. [PMC free article] [PubMed] [Google Scholar]
- 39.Carlier Y, Truyens C, Deloron P, Peyron F. Congenital parasitic infections: a review. Acta Trop. 2012;121:55–70. doi: 10.1016/j.actatropica.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 40.da Costa-Macedo LM, Rey L. Ascaris lumbricoides in neonate: evidence of congenital transmission of intestinal nematodes. Rev Inst Med Trop Sao Paulo. 1990;32:351–354. doi: 10.1590/S0036-46651990000500007. [DOI] [PubMed] [Google Scholar]
- 41.Shoop WL, Michael BF, Eary CH, Haines HW. Transmammary transmission of Strongyloides stercoralis in dogs. J Parasitol. 2002;88:536–539. doi: 10.1645/0022-3395(2002)088[0536:TTOSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL. Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J Infect Dis. 2006;194:176–183. doi: 10.1086/505150. [DOI] [PubMed] [Google Scholar]
- 43.Goethert HK, Telford SR., III Not “out of Nantucket”: Babesia microti in southern New England comprises at least two major populations. Parasit Vectors. 2014;7:546. doi: 10.1186/s13071-014-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capri G, Walter KS, Mamoun CB, Krause PJ, Kitchen A, Lepore TJ, et al. Babesia microti from humans and ticks hold a genomic signature of strong population structure in the United States. BMC Genomics. 2016;17:888. doi: 10.1186/s12864-016-3225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randolph SE. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus) Parasitol. 1995;110:287–295. doi: 10.1017/S0031182000080872. [DOI] [PubMed] [Google Scholar]
- 46.Fay FG, Rausch RL. Parasitic organisms in the blood of arvicoline rodents in Alaska. J Parasitol. 1969;55:1258–1265. doi: 10.2307/3277271. [DOI] [Google Scholar]
- 47.Burkot TR, Schneider BS, Pieniazek NJ, Happ CM, Rutherford JS, Slemenda SB. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among pine voles Microtus ochrogaster in Colorado. Parasitology. 2000;121:595–599. [PubMed] [Google Scholar]
- 48.Goethert HK, Lubelcyzk C, LaCombe E, Holman M, Rand P, Smith RP, Telford SR., III Enzootic Babesia microti in Maine. J Parasitol. 2003;89:1069–1071. doi: 10.1645/GE-3149RN. [DOI] [PubMed] [Google Scholar]
- 49.Hamer SA, Hickling GJ, Sidge JL, Rosen ME, Walker ED, Tsao JI. Diverse Borrelia burgdorferi strains in a bird-tick cryptic cycle. Appl Environ Microbiol. 2011;77:1999–2007. doi: 10.1128/AEM.02479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark K, Savick K, Butler J. Babesia microti in rodents and raccoons from northeast Florida. J Parasitol. 2012;98:1117–1121. doi: 10.1645/GE-3083.1. [DOI] [PubMed] [Google Scholar]
- 51.Tsao JI. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet Res. 2009;40:36. doi: 10.1051/vetres/2009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes L. Biological consequences of global warming: is the signal already apparent? TREE. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- 53.Merritt JF, Zegers DA. Maximizing survivorship in cold: thermogenic profiles of non-hibernating mammals. Acta Theol. 2002;47:221–234. doi: 10.1007/BF03192489. [DOI] [Google Scholar]
- 54.Roy-Defresne E, Logan T, Simon JA, Chmura GL, Millien V. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: implications for the spread of Lyme disease. Plos One. 2013;8:e80724. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Information for each pregnant female and infection status of her embryos collected in April and July, 2016. Site: LG, Lake Gaillard; OL, Old Lyme; RH, Rodman’s Hollow; NI, North Island. Age of embryos: W1, Week 1; W2, Week 2; W3, Week 3. (DOCX 19 kb)
Table S2. Babesia microti infection prevalence in reproductive tissues (ES, embryonic sac). (DOCX 14 kb)
Figure S1. Babesia microti sequence alignment from embryos and tissues of the 18S rRNA gene for six females (labeled by a four digit identification number) including three reference sequences representing the three Babesia clades (Clade-1, AY144696; Clade-2, AY144701; Clade-3, AY144690). (DOCX 136 kb)
Data Availability Statement
The data supporting the conclusions of this article are included within the article. Unique B. microti sequences have been deposited in the GenBank database under the accession numbers MH221125 and MH221126. The raw data are available from the corresponding author upon reasonable request.


