Abstract
The classical view of heterotrimeric G protein signaling places G proteins at the cytoplasmic surface of the cell’s plasma membrane where they are activated by an appropriate G protein-coupled receptor. Once activated, the GTP-bound Gα and the free Gβγ are able to regulate plasma membrane-localized effectors, such as adenylyl cyclase, phospholipase C-β, RhoGEFs and ion channels. Hydrolysis of GTP by the Gα subunit returns the G protein to the inactive Gαβγ heterotrimer. Although all of these events in the G protein cycle can be restricted to the cytoplasmic surface of the plasma membrane, G protein localization is dynamic. Thus, it has become increasingly clear that G proteins are able to move to diverse subcellular locations where they perform non-canonical signaling functions. This chapter will highlight our current understanding of trafficking pathways that target newly synthesized G proteins to the plasma membrane, activation-induced and reversible translocation of G proteins from the plasma membrane to intracellular locations, and constitutive trafficking of G proteins.
Keywords: Assembly, G protein, Isoprenylation, Miristoylation, Palmitoylation, Trafficking
11.1 Introduction
A major role of heterotrimeric G proteins is to couple activated G protein-coupled receptors at the cell surface to appropriate intracellular signaling pathways (Preininger and Hamm 2004; Dorsam and Gutkind 2007). As the name implies, heterotrimeric G proteins are composed of three subunits, Gα, Gβ and Gγ, encoded by 16, 5 and 12 genes, respectively, in mammals. Based on sequence identity and functional similarity, Gα are typically grouped into four sub-families, termed Gαs, Gαi, Gαq and Gα12/13. Gβ and Gγ form an irreversible Gβγ dimer, with the exception of the Gβ5 subunit (Sondek and Siderovski 2001), and functional differences between different Gβγ combinations are not well-defined.
G proteins function as molecular switches by cycling between guanine-nucleotide bound states. The G protein cycle is characterized by Gα binding to either GDP or GTP and by dissociation and association of Gα and Gβγ. In the inactive state, the G protein exists as a heterotrimer in which Gα binds GDP. When a cell-surface GPCR is activated by binding an extracellular agonist, it interacts with its cognate heterotrimeric G protein at the cytoplasmic surface of the plasma membrane and catalyzes the release of GDP from Gα. Next, GTP replaces GDP by binding to Gα. GTP binding to Gα induces a conformational change that decreases its affinity for Gβγ and increases its affinity for effector proteins. Consequently, Gα and Gβγ dissociate, at least partially (Lambert 2008), and both GTP-bound Gα and free Gβγ are able to interact with and regulate numerous effector proteins, such as ion channels, adenylyl cyclases and phospholipases, to name a few classical examples. The intrinsic GTP hydrolysis activity of Gα, which is often greatly accelerated through binding to RGS (regulator of G protein signaling) proteins, returns Gα to the GDP-bound state and allows it to interact again with Gβγ, thereby reforming an inactive heterotrimer.
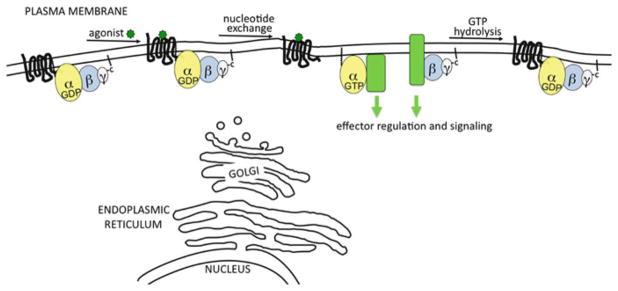
The classical view of heterotrimeric G protein signaling focuses on G proteins being associated with the cytoplasmic surface of the plasma membrane (Fig. 11.1). In this view, G proteins remain tethered to the PM while traversing through the G protein cycle and carrying out their cellular signaling functions. However, it has become clear that G proteins are not always localized at the cytoplasmic surface of the PM. Instead, G proteins dynamically localize to diverse subcellular locations and transit specific trafficking pathways. Thus, superimposed upon the classical view of G protein function, we must consider how trafficking of G proteins provides novel mechanisms for regulating G protein signaling. This chapter will discuss our current knowledge of G protein trafficking to the PM after synthesis, activation-dependent and constitutive internalization and recycling of G proteins, and how trafficking to unique subcellular locations provides novel G protein signaling functions.
Fig. 11.1. Classical view of G protein signaling.
In this view, heterotrimeric G proteins are restricted to the cytoplasmic surface of the PM. However, as discussed in this chapter, G proteins are found at a variety of subcellular locations and they can reversibly move between the PM and intracellular locations
11.2 Mechanisms of Membrane Binding by G Proteins
Localization to cellular membranes is required for many, though maybe not all, functions carried out by G proteins. Of major importance, several different lipids are covalently attached to G protein subunits, thereby serving as hydrophobic membrane anchors (Table 11.1). In addition, both Gα and Gβγ subunits appear to contain poly-basic motifs that provide electrostatic interactions with negatively charged regions of membranes. The interplay of these different membrane binding mechanisms is critical for controlling the trafficking of G proteins.
Table 11.1.
Sites of G protein lipid modifications
| Gα subunits | N-termini of α subunits | Lipid modification |
|---|---|---|
| αi1 | MGCTLSAEDKAAVERSKMID- | Myristoylation, Palmitoylation |
| αo1 | MGCTLSAEERAALERSKAIE- | Myristoylation, Palmitoylation |
| αZ | MGCRQSSEEKEAARRSRRID- | Myristoylation, Palmitoylation |
| αt | MGAGASAEEKHSREL- | Myristoylation |
| αs | MGCLGNSKTEDQRNEEDAQR- | Palmitoylation |
| αq | MTLESIMACCLSEEAKEARR- | Palmitoylation |
| α14 | MAGCCCLSAEEKESQRISAE- | Palmitoylation |
| α16 | MARSLRWRCCPWCLTEDEKA- | Palmitoylation |
| α12 | MSGVVRTLSRCLLPAEAGAR- | Palmitoylation |
| α13 | MADFLPSRSVLSVCFPGCVL- | Palmitoylation |
|
| ||
| Gγ subunits | C-termini of γ subunits | Lipid modification |
|
| ||
| γ1 | -KGIPEDKNPFKELKGGC ↓VIS | Farnesylation |
| γ2 | -TPVPASENPFREKKFFC ↓AIL | Geranylgeranylation |
The N-terminal sequences of several Gα and the C-terminal sequences of two Gγ are shown. Myristate links through an amide bond to an N-terminal glycine after removal of the initiating methionine as indicated by G. Palmitate attaches via a thioester bond to cysteine (in bold italics) residues near the N-terminus of Gα. γ1 and γ2 are isoprenylated through a thioether bond to a cysteine, indicated by C. After isoprenylation the C-terminal three amino acids are removed (↓), and the new C-terminus is carboxyl methylated. This is a representative listing of G protein subunits. In humans, 16 genes encode Gα (plus additional splice variants), 5 genes encode Gβ (Gβ proteins are not known to be lipid-modified), and 12 genes encode Gγ. Reprinted with permission from Marrari et al. (2007). Copyright 2007 American Chemical Society
When considering how membrane binding affects trafficking, it is important to consider that often two or more membrane binding mechanisms function together in a protein or protein complex. Whereas a single membrane binding signal, be it an attached lipid, polybasic sequence or other motif, may have weak affinity for a membrane, two or more signals can synergize to provide a high affinity multivalent membrane interaction. This is often referred to as the two-signal hypothesis or kinetic trapping (Dunphy and Linder 1998; Hancock et al. 1990; McLaughlin and Aderem 1995; Morales et al. 1998; Resh 1999). Another key point is that one of the membrane binding signals may direct the protein to a specific membrane or membrane domain. Lastly, changes in one of the membrane binding signals can allow regulation of membrane binding. As will be extensively discussed below, multiple membrane binding signals are found in heterotrimeric G proteins, and thus the combination of signals and the ability of different signals to be regulated can have profound effects on membrane binding by G proteins.
11.2.1 Gα Subunits Are N-Terminally Myristoylated and/or Palmitoylated
All Gα of the Gαi family are covalently modified by the 14-carbon saturated fatty acid myristate at an extreme N-terminal glycine (Table 11.1). After the initiating methione is removed by a methionyl amino peptidase, a prerequisite for myristoylation to occur, a glycine at position 2 becomes the new N-terminal amino acid. The attachment of myristate through an amide bond to the free amino group of the glycine is catalyzed by an N-myristoyl transferase (NMT), of which there are two isoforms in mammals, NMT1 and NMT2. NMTs recognize a consensus sequence of G2-X3-X4-X5-(S/T/C)6. The N-terminal glycine is essential; myristate is not attached to any other amino acid. Serine, threonine or cysteine is preferred at position 6, but other amino acids can be allowed. Moreover, other amino acids at positions 3–7 can influence the ability of an N-terminal sequence to be recognized and myristoylated by a NMT. Gα of the other families, Gαs, Gαq and Gα12, are not myristoylated simply because they lack a glycine at position 2 or fail to conform to the N-terminal consensus sequence even though they have a glycine at position, as in the case of Gαs.
Myristoylation typically occurs co-translationally as the nascent Gαi subunit is emerging from the ribosome. Although long thought to exclusively occur co-translationally, more recently it has become clear that myristoylation can occur post-translationally as well, usually when an internal glycine is exposed and becomes an N-terminal amino acid after cleavage by the apoptotic caspases (Martin et al. 2011). So far, there is no evidence to indicate that Gαi can be post-translationally myristoylated.
Myristate is not an abundant fatty acid in the cell, and thus the myristoylation process is very selective for myristate as opposed to other more abundant fatty acids (reviewed recently in Martin et al. 2011). However, the retinal-specific Gαt, as well as several other retinal proteins, has been found to be heterogeneously acylated at the N-terminal glycine (Kokame et al. 1992; Johnson et al. 1994; Neubert et al. 1992). Instead of only having myristate (C14:0, in which C14 indicates the carbon chain length, and 0 indicates the number of unsaturated bonds) attached, Gαt also exists in isoforms with attached 5-cis-tetradecenoic acid (C14:1), 5-cis, 8-cis- tetradecadienoic acid (C14:2) and laurate (C12). This heterogeneous N-acylation appears to occur only in the retinal photoreceptor cells due to unique characteristics of lipid metabolism in the retina compared to other cells in the body (Bereta and Palczewski 2011). The function of the differently modified isoforms of Gαt remains incompletely understood. Studies have indicated that laurate modified forms bind with lower affinity to Gβγ and have lower steady-state GTPase activity (Hashimoto et al. 2004; Kokame et al. 1992), suggesting that the lipid moiety can influence protein-protein interactions or intramolecular interactions. Alternatively, the functional differences may simply reflect that Gαt isoforms with less hydrophobic fatty acids attached have lower affinity for membranes and thus decreased function. Regardless, the existence of differently modified forms of Gαt provides the opportunity for important functional differences, and it remains possible that different isoforms of Gαt with different affinities for membranes could exhibit differences in trafficking. This speculation remains to be examined.
Lastly, myristoylation appears to be an irreversible modification. Since myristate is added co-translationally and mechanisms for its removal do not seem to exist, myristate appears to be attached to Gαi subunits throughout their lifetime; thus, myristoylation does not fit the concept of a regulatory modification. However, the relatively weak affinity of myristate for membranes, allows other factors to regulate the reversible membrane binding of myristoylated proteins (Resh 2006). For example, the covalent attachment of another lipid, such as palmitate (discussed below) can synergize with myristate to provide very strong membrane binding, while phosphorylation of a myristoylated protein can shift the protein off of the membrane by providing electrostatic repulsion from acidic membrane lipid headgroups. In this way covalently attached myristate, although irreversible, can affect membrane localization and trafficking of proteins, such as Gαi subunits.
In contrast to myristoylation, palmitoylation occurs post-translationally, is readily reversible, lacks a clear consensus motif and functions as a stronger membrane anchor due to its greater hydrophobicity. With the exception of Gαt and Gαgust, all Gα subunits can be palmitoylated at one or more cysteines located within the first 20 N-terminal amino acids (Table 11.1). For members of the Gαi family, except for Gαt and Gαgust which simply do not have any N-terminal cysteines, palmitoylation occurs at a cysteine at position 3 immediately adjacent to the glycine site of myristoylation. Because palmitoylation is a post-translational modification, co-translational myristoylation obviously happens before palmitoylation, and in fact myristoylation is a prerequisite for palmitoylation of Gαi subunits. Numerous studies have shown that glycine to alanine mutants at position 2 of Gαi subunits not only fail to be myristoylated but also lack palmitoylation (Wilson and Bourne 1995; Mumby et al. 1990; Jones et al. 1990; Hallak et al. 1994; Galbiati et al. 1994). Subunits of the Gαs, Gαq and Gα12 families all undergo palmitoylation in the absence of myristoylation. No clear sequence motif or position within the N-terminus is obvious for the sites of palmitoylation in the non-myristoylated Gα. In general sites of palmitoylation have not been easy to predict, although recently there have been advances in the computational prediction of palmitoylation sites (Ren et al. 2008; Hu et al. 2011; Li et al. 2011). For Gα subunits the key prerequisite for palmitoylation appears to be the existence of additional membrane targeting signals. These signals can be myristoylation, in the case of Gαi subunits, binding to other proteins, such as the Gβγ subunits, and polybasic membrane targeting motifs. This concept of multiple membrane targeting signals working to fine-tune membrane binding and trafficking is discussed further below.
In palmitoylation, the 16-carbon fatty acid palmitate is linked to a cysteine amino acid through a thioester bond. For many years the cellular machinery of palmitoylation remained elusive. That all changed with the identification of a family of proteins with palmitoyl acyltransferase activity (Lobo et al. 2002; Roth et al. 2002). This family of more than 20 proteins has been termed DHHC proteins for the presence of a conserved catalytic aspartate-histidine-histidine-cysteine motif. A great deal of current research is directed towards trying to understand the specificity of DHHC proteins. Several different DHHC proteins, such as DHHC3 and DHHC7, appear to be capable of mediating the palmitoylation of Gα subunits. Importantly, it was shown that siRNA-mediated depletion of DHHC3 and DHHC7 resulted in decreased palmitoylation of Gαq and a loss of its PM localization (Tsutsumi et al. 2009 ). Together with numerous studies in which cysteine sites of palmitoylation in various Gα were mutated to non-palmitoylatable serines or alanines (Bhattacharyya and Wedegaertner 2000; Hughes et al. 2001; Pedone and Hepler 2007 ; Wedegaertner et al. 1993, 1996; Wise et al. 1997), the DHHC knockdown approach establishes palmitoylation as an essential requirement for membrane localization of Gα.
Palmitoylation is a reversible modification, and indeed it has been shown using pulse-chase experimental approaches that the half-life of palmitate attached to Gα is relatively short, on the order of 1 h, compared to the much longer half-life of the Gα protein. More interestingly, the turnover of the palmitate attached to Gα, i.e., depalmitoylation, can be accelerated upon activation (Wedegaertner and Bourne 1994; Chen and Manning 2000; Bhamre et al. 1998; Loisel et al. 1999), suggesting that the palmitoylation status of Gα can be subject to regulation to affect localization and function. The cellular machinery that regulates depalmitoylation of Gα and other signaling proteins is being actively investigated (Zeidman et al. 2009), and acyl protein thioesterase 1 (APT1) is one key enzyme that can depalmitoylate Gα. APT1 in the yeast Saccharomyces cerevisiae was shown to be responsible for much of the depalmitoylating activity in yeast, including the depalmitoylation of the yeast Gα, Gpa1 (Duncan and Gilman 2002). Moreover, mammalian APT1 was over-expressed together with Gαs in cultured cells resulting in a more rapid turnover of palmitate attached to Gαs (Duncan and Gilman 1998). A more recent study showed that siRNA-mediated depletion of APT1 in cultured cells resulted in an increase in the fraction of Gα13 associated with membranes compared to cytoplasm; this result was consistent with the proposal that inhibition of Gα13 depalmitoylation leads to more palmitoylated Gα13 and thus more membrane bound Gα13. This study went on to suggest that APT1 regulation of the palmitoylation status of Gα13 is important for dendritic spine morphogenesis in neurons (Siegel et al. 2009). The reversibility of palmitoylation provides an important point of regulation for G protein signaling, and, as will be discussed below, cycles of palmitoylation and depalmitoylation can have important consequences for regulating G protein trafficking.
11.2.2 Gγ Subunits Are C-Terminally Isoprenylated
A variety of cellular peripheral membrane proteins, including all Gγ subunits, are post-translationally modified at their C-termini by an isoprenyl group, either a 15-carbon farnesyl or a 20-carbon geranylgeranyl (Table 11.1) (Lane and Beese 2006; Wright and Philips 2006). Farnesyl and geranylgeranyl transferases have been well characterized. These cytoplasmic enzymes specifically recognize a C-terminal CaaX motif on substrate proteins, in which a cysteine (C) is followed by two aliphatic (a) amino acids and the ultimate C-terminal amino acid (X) can be one of several possibilities. The last amino acid determines whether the substrate protein is farnesylated or geranylgeranylated. Gγ1, Gγ9 and Gγ11 are farnesylated due to having a serine in the C-terminal X position, while the other nine Gγ have a leucine in the X position and are geranylgeranylated. In either case, the isoprenyl group is attached via a thioether bond to the cysteine of the CaaX motif. One critical difference between the two isoprenyl modifications is that geranylgeranyl is more hydrophobic than farnesyl, and thus provides stronger membrane binding. Such differences in isoprenylation may contribute to functional differences of Gβγ isoforms. For a discussion of unique functions of Gβγ composed of select Gβ and Gγ subunits, see Chap. 10 in this book. Most proteins that are isoprenylated at the cysteine of the CaaX then undergo further processing consisting of proteolytic removal of the last three –aaX amino acids, thus providing an isoprenyl cysteine as the new C-terminal amino, followed by methylation of the C-terminal carboxyl group. The role of these subsequent modifications is not altogether clear. As discussed below, the –aaX proteolysis and carboxymethylation may be essential to direct the initial trafficking of nascent G proteins. Additionally, carboxymethylation contributes to an increased hydrophobicity of the Gγ C-terminus; studies suggest that this carboxymethylation is important for stronger membrane binding by farnesylated Gβγ, but not the more hydrophobic geranylgeranylated Gβγ (Michaelson et al. 2002). Our current knowledge is consistent with the following temporal model for Gβγ assembly and lipid modification: (1) Newly synthesized Gβ and Gγ are assembled in the cytoplasm; (2) Gγ, in the context of a Gβγ dimer, is either farnesylated or geranylgeranylated; (3) the C-terminal three (-aaX) amino acids are removed; and (4) the new C-terminus is carboxymethylated (Marrari et al. 2007). Importantly, several chaperone proteins have been identified that facilitate the formation of the Gβγ subunit after synthesis. See Chaps. 8 and 9 in this book for a detailed analysis of these chaperone proteins, and these are also briefly discussed later herein. In summary, the irreversible post-translational isoprenyl modification of Gγ provides an essential membrane anchor for Gβγ, and cooperates with myristate and/or palmitate modifications of Gα to affect the localization, function and trafficking, as discussed further below, of the G protein heterotrimer.
11.2.3 Polybasic Motifs in Gα and Gβγ
In addition to lipid modifications of the G protein subunits, regions of positive charge on the proteins have been shown to contribute to membrane localization. Structural studies of Gβ1γ1 originally showed that Gβ1 has a surface of positive charge that is predicted to facilitate Gβγ binding to membranes by ionic interactions with acidic membrane phospholipids (Sondek et al. 1996). The positively charged amino acids that comprise the basic region in Gβ1 are conserved in Gβ2, Gβ3 and Gβ4, consistent with an important role for this protein surface (Matsuda et al. 1994; Seitz et al. 1999; Murray et al. 2001). However, the importance of the basic region in Gβ in influencing membrane binding remains to be better defined. Many Gα have a large number of basic amino acids within the N-terminal helical region. For example Gαq contains 10 basic residues, both arginines and lysines, within amino acids 16–38. Most importantly, helical predictions indicate that most of the positively charged N-terminal amino acids align on one face of the helix, grouped together to form basic patches in position to interact with negatively charged surfaces of cellular membranes (Kosloff et al. 2002; Crouthamel et al. 2008, 2010; Pedone and Hepler 2007). Indeed, replacement of select N-terminal basic amino acids in Gαs and Gαq with non-charged alanines or glutamines results in decreased membrane localization of the mutant Gαs and Gαq compared to wild type (Crouthamel et al. 2008). Interestingly, these N-terminal polybasic patches are only found in the non-myristoylated Gα, i.e., the Gαs, Gαq and Gα12/13 families; Gα of the myristoylated Gαi family do not contain pronounced N-terminal polybasic regions (Kosloff et al. 2002). Thus, we can consider that the N-terminal polybasic regions take the place of myristoylation to function, in conjunction with binding to Gβγ, as initial membrane targeting signals required for promotion of palmitoylation and PM localization.
11.3 G Protein Trafficking to the Plasma Membrane After Biosynthesis
Work over the last decade has revealed complex and specific pathways that allow G proteins to move to the plasma membrane after synthesis. G proteins do not simply diffuse from the cytoplasm to the inner surface of the PM after synthesis. Instead, several chaperone proteins mediate proper folding and assembly of the subunits, and distinct trafficking pathways are followed that involve localization at the cytoplasmic surface of intracellular organelles (Fig. 11.2). The assembly of the Gβγ dimer has been well-reviewed recently (Willardson and Howlett 2007; Marrari et al. 2007; Dupre et al. 2009) and is also covered by other chapters in this collection; thus, here will be provided only an overview of the Gβγ assembly process, together with describing recent developments on understanding the assembly of the G protein heterotrimer and the organelles involved in trafficking to the PM.
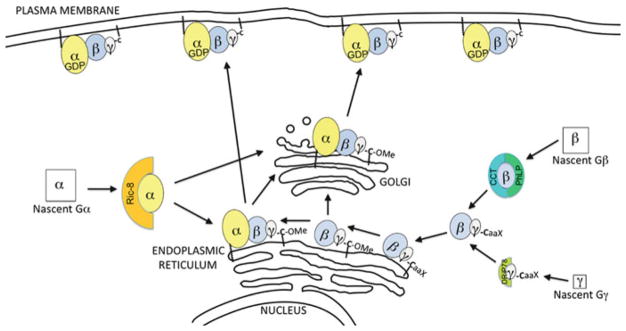
Fig. 11.2. Model of heterotrimeric G protein trafficking to the PM after synthesis.
Discussed in detail in the text, the following key steps are known to be involved in trafficking newly synthesized G proteins to the PM. Chaperone proteins have been identified for each G protein subunit, and these chaperones are required for proper folding and/or initial delivery of the subunit to a membrane. After formation of the Gβγ in the cytoplasm, the C-terminal tail (CaaX motif) of Gγ is modified with a farnesyl or geranylgeranyl group, and then Gβγ is targeted to the ER. At the ER, C-terminal proteolysis and carboxymethylation of Gγ occurs. Gα initially binds to Gβγ at either the ER or Golgi and undergoes palmitoylation. The fully lipid modified G protein heterotrimer then trafficks to the PM via the Golgi-dependent secretory pathway or through an uncharacterized Golgi-independent process
Our current knowledge indicates that assembly of the Gβγ dimer requires the following steps. Newly synthesized Gβ binds to the chaperonin containing tailless complex polypeptide 1 (CCT) which serves as a chaperone to prevent aggregation of unfolded Gβ (Kubota et al. 2006; Wells et al. 2006). Next, phosducin-like protein 1 (PhLP1) binds to the CCT- Gβ complex to further facilitate proper folding of Gβ (Lukov et al. 2005; Knol et al. 2005). Then phosphorylation of PhLP1 by casein kinase 2 (CK2) releases a complex of PhLP1 and properly folded Gβ from CCT (Lukov et al. 2006; Humrich et al. 2005). This phosphorylation event is particularly intriguing, as it suggests a regulatory mechanism for a cell to rapidly regulate levels of Gβγ (Willardson and Howlett 2007), and indeed different tissues have been shown to contain substantially different amounts of phosphorylated versus non-phosphorylated PhLP1 (Humrich et al. 2003). The upstream cellular signals and pathways that regulate PhLP1 phosphorylation by CK2 remain to be investigated. While nascent Gβ is bound by CCT and PhLP1 to promote its proper folding, one report has demonstrated that a similar chaperone function for nascent Gγ is performed by an ER-localized protein termed dopamine receptor-interacting protein 78 (DRiP78) (Dupre et al. 2007). Then lastly in the assembly process, Gβ and Gγ are released from their chaperones, PhLP1 and DRiP78, respectively, to bind each other and thereby form the properly folded Gβγ dimer. After completion of the Gβγ assembly process, Gγ is modified, as described above, by isoprenylation, C-terminal proteolysis and C-terminal methylation. Although assembled Gβγ has been shown to be cytoplasmic before undergoing lipid modification, the ER localization of DRiP78 suggests that the assembly of Gβγ may actually occur at the cytoplasmic surface of the ER. After isoprenylation of Gβγ, possibly near the ER, the subsequent proteolysis and methylation clearly occurs at the cytoplasmic surface of the ER. Thus, a fully assembled and modified Gβγ initially localizes at the ER.
Recent evidence suggests that specific chaperone proteins may also exist for Gα. In particular, a protein termed Ric-8 stabilizes newly synthesized Gα (Gabay et al. 2011). Ric-8, named for resistance to inhibitors of cholinesterase, was originally identified in a screen in C. elegans for activators of neurotransmission. Subsequent studies showed that Ric-8 functioned upstream of Gα and could bind to Gα. Next, it was shown that Ric-8, of which mammals have two forms, Ric-8A and Ric-8B, promoted GDP release from Gα in vitro (Tall et al. 2003). These results were exciting because they identified Ric-8 as an intracellular guanine-nucleotide exchange factor (GEF) for Gα; thus, G protein activation can occur in the absence of a cell-surface localized GPCR. However, studies on the depletion of Ric-8 in organisms or cultured cells revealed the surprising findings that Ric-8 was needed for proper localization and expression of Gα, suggesting that Ric-8 served a role beyond its function as a GEF. In Drosophila cells, loss of Ric-8 resulted in diminished amounts of Gαi and Gαo and in a failure of the Gα to reside at the PM (David et al. 2005; Hampoelz et al. 2005; Wang et al. 2005). Interestingly, Gβ levels were also reduced and mislocalized. Ric-8 does not directly interact with Gβγ; instead, diminished Gβγ is most likely a consequence of the lack of Gα. As will be discussed below, nascent Gβγ needs to interact with Gα to transit from the ER to the PM. Similar to the observations in Drosophila, in cultured mammalian cells depletion of Ric-8B caused a reduction in Gαs protein. Moreover, consistent with a chaperone function for Ric-8, overexpression of Ric-8A or Ric-8B promoted much higher levels of Gα from all four subfamilies, Gαi, Gαs, Gαq and Gα12/13. Ric-8A binds Gαi, Gαq and Gα12/13, while Ric-8B preferentially interacts with Gαs, suggesting that Ric-8 proteins might have a universal role as a chaperone for all Gα. To further support a role for Ric-8 as a Gα chaperone, a recent report showed in vitro that binding to Ric-8A increased the stability of Gαi (Thomas et al. 2011). A chaperone role for Ric-8 was more firmly established in another recent report. This studied showed that in cultured cells lacking either Ric-8A or Ric-8B the rate of degradation of select Gα was increased (Gabay et al. 2011). In cells lacking Ric-8A, Gαi and Gαq were efficiently translated; however, there seemed to be a specific defect in the Gα’s ability to initialize localize to membranes (Gabay et al. 2011), thus suggesting that Ric-8 is required for nascent Gα to arrive at a cellular membrane. In summary, accumulated evidence indicates that Ric-8 serves a chaperone function for newly synthesized Gα. Moreover, Ric-8 may help to deliver Gα to the ER for interaction with Gβγ. Further studies should shed even more light on the mechanistic details of how Ric-8 functions as a Gα chaperone.
As alluded to above, a great deal of evidence indicates that G protein heterotrimer formation is crucial for PM localization of Gα and Gβγ. In other words, Gα does not efficiently traffic to the PM unless it binds to Gβγ, while Gβγ does not efficiently traffic to the PM unless it binds to Gα. Our current understanding is that nascent Gα and Gβγ interact to form the heterotrimer at an intracellular location, likely the ER or Golgi, after interaction with the appropriate chaperones and processing of the C-terminus of Gγ as described above. The reciprocal requirement of Gα and Gβγ interaction for efficient PM targeting has been demonstrated in several ways (Marrari et al. 2007). Overexpression studies in cultured cells showed that overexpressed Gβγ localizes to predominantly intracellular membranes, but when various Gα are also overexpressed along with Gβγ both Gα and Gβγ display strong localization at the PM (Takida and Wedegaertner 2003; Michaelson et al. 2002; Evanko et al. 2001). Reciprocally, a number of studies have shown that overexpressed Gα displays both PM and cytoplasmic localization, but co-expression with Gβγ results in strong PM localization of Gα (Hepler et al. 1993). Another line of evidence supporting the importance of heterotrimer formation for PM localization of Gα and Gβγ is the use of mutants deficient in partner interaction. Heterotrimer crystal structures have defined protein surfaces that mediate the association of Gα and Gβγ. Thus, mutation of key Gβγ-contacting amino acids in the N-termini of several Gα results in decreased PM localization to the Gα (Fishburn et al. 2000; Evanko et al. 2000). Likewise, mutation of select Gα-interacting amino acids in Gβγ prevents its PM localization (Takida and Wedegaertner 2003). Such Gα-binding defective Gβγ localize primarily to ER membranes, where –CaaX processing of the Gγ takes place. Similarly, Gβγ-binding defective Gα localize to intracellular membranes, apparently ER (Fishburn et al. 2000), and cytoplasm (Evanko et al. 2000); intracellular membrane localization is observed for Gβγ-binding defective Gαz, which is co-translationally myristoylated, while cytoplasm localization is the predominant localization for Gβγ-binding defective Gαs and Gαq, both of which are non-myristoylated subunits (Fishburn et al. 2000; Evanko et al. 2000). Lastly, genetic deletions of specific G protein subunits provide further support for the reciprocal requirement of Gα and Gβγ interaction for PM localization. Because typical mammalian cells contain a number of different Gα and Gβγ subunits, deletion of one or two select subunits may not substantially affect overall Gα/Gβγ stoichiometry and thus may not reveal localization changes. However, model organisms or systems with fewer Gα and Gβγ have proved useful here. Yeast S. cerevisiae genetically lacking the Gα Gpa-1, and C. elegans embryos depleted of two critical Gα, GOA-1 and GPR-16, using RNAi, show a redistribution of Gβγ from the PM to intracellular sites (Song et al. 1996; Gotta and Ahringer 2001). Conversely, in Drosophila photoreceptor cells a loss of the eye-specific Gβ subunits results in a shift of Gαq, the key Gα in photoreceptor signaling, from membrane to cytosolic fractions (Kosloff et al. 2003; Elia et al. 2005). Taken together, a variety of studies indicate that formation of the heterotrimer is an important step in stable localization of both Gα and Gβγ at the PM.
A logical hypothesis to explain the above described reciprocal requirement is that the G protein heterotrimer is formed at an intracellular site before or enroute to trafficking of the heterotrimer to the PM (Marrari et al. 2007). Current evidence supports a model in which heterotrimer formation occurs at the cytoplasmic surface of the ER or Golgi. Moreover, evidence exists for trafficking of nascent G proteins to the PM via either a Golgi-dependent or –independent pathway. Consistent with assembly of the heterotrimer at the ER, mutant Gβγ that fails to bind Gα and wild type Gβγ that is expressed in the absence of Gα localize predominantly at the ER (Michaelson et al. 2002; Takida and Wedegaertner 2003). Because expression and interaction with Gα is required to shift Gβγ from the ER to the PM, the simplest explanation is that Gα must interact with Gβγ at the ER. Moreover, neither treatment of cells with the Golgi disruptor Brefeldin A nor expression of a dominant negative Sar1, which blocks ER to Golgi transport, prevented expressed Gα and Gβγ from localizing at the PM (Takida and Wedegaertner 2004; Fishburn et al. 1999; Gonzalo and Linder 1998). These studies suggested that not only would the heterotrimer form before trafficking to the Golgi, i.e., at the ER, but, in addition, trafficking to the PM would not require the Golgi. On the other hand, some studies have argued that Gα and Gβγ first interact at the Golgi and/or that the Golgi is required for movement of G proteins to the plasma membrane. One study observed apparent Golgi localization of co-expressed Gβγ and a palmitoylation site mutant of Gαi, and thus suggested that heterotrimer interaction and Gα palmitoylation takes place at the Golgi (Michaelson et al. 2002). A recent study showed that DHHC-3 and DHHC-7 are required for palmitoylation of Gαi, Gαs and Gαq, and that these palmitoyl acyltransferases are localized to the Golgi (Tsutsumi et al. 2009). Moreover, others have argued that the Golgi is the main subcellular site for palmitoylation of all peripheral membrane proteins (Rocks et al. 2010). Although the above studies suggest a functional Golgi may be required for palmitoylation and trafficking to the PM, they do not address whether or not the heterotrimer would first form at the ER before moving to the Golgi. Some light was shed on the question of the involvement of the Golgi in G protein trafficking to the PM by a report showing that the trafficking pathway depends on the protein complexes being formed. A functional Golgi was not required for PM localization of overexpressed Gα and Gβγ, as shown previously (Takida and Wedegaertner 2004); however, when a GPCR was also overexpressed, the Golgi was indeed required for PM localization of Gα, Gβγ and GPCR, suggesting that a heterotrimer in a complex with a GPCR takes a different pathway than a heterotrimer by itself (Dupre et al. 2006). Virtually all studies of G protein trafficking rely on overexpressed proteins; in the future it will be important to try to follow the route of G proteins that are endogenous or expressed at physiological levels.
In summary, heterotrimeric G proteins follow distinct trafficking pathways to reach the PM after synthesis (Fig. 11.2). Some of the key steps in trafficking routes include (1) localization of Gβγ at the cytoplasmic surface of the ER to undergo C-terminal modifications of Gγ; (2) Gα and Gβγ heterotrimer formation and Gα palmitoylation at an endomembrane site, either the cytoplasmic surface of the ER or Golgi; and (3) trafficking of the heterotrimer to the PM, either in a poorly defined Golgi-independent pathway from the ER to PM or in a Golgi-dependent pathway attached to the cytoplasmic surface of PM-destined vesicles. The different trafficking routes may reflect the intracellular formation of distinct protein complexes. Importantly, all of these novel trafficking steps provide opportunity for G proteins to regulate novel signaling pathways as well as targets for therapeutic intervention.
11.4 Activation-Dependent Internalization and Recycling of G Proteins
Once nascent G proteins reach the PM through specific trafficking pathways, they are able to carry out many of their classical signaling functions through interactions with GPCRs and effector proteins. However, G protein localization continues to be dynamic; they can rapidly and reversibly move from the PM to endomembrane locations. Over the last decade or more, evidence has emerged to support the notion that G proteins move throughout the cell in both constitutive and activation-dependent pathways (Figs. 11.3 and 11.4).
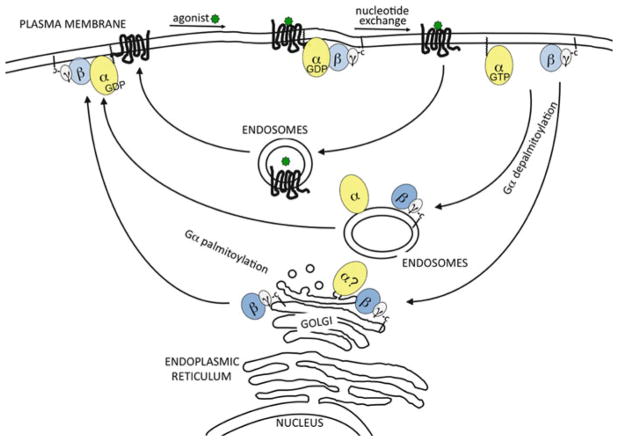
Fig. 11.3. Model of activation-induced G protein trafficking.
Upon GPCR-mediated activation of an appropriate heterotrimeric G protein at the cytoplasmic surface of the PM, different, but not necessarily mutually exclusive, pathways of reversible translocation have been described. One series of studies has shown that after GPCR activation select Gβγ can undergo rapid (within seconds) movement from the PM to the Golgi. Then, Gβγ can return from the Golgi to the PM with similar rapidity. This cycle of Gβγ movement has been described as diffusion-mediated. Interestingly, the rapid activation-induced Gβγ PM-Golgi cycle depends upon palmitoylation, and likely by extension depalmitoylation. However, Gβγ appears to undergo this cycle while Gα remains on the PM; thus, Gα at the Golgi during activation-induced rapid translocation is indicated by the question mark. In another pathway, GPCR-mediated activation promotes depalmitoylation of Gα allowing Gα to translocate off of the PM. This pathway is slower (minutes) than the PM-Golgi pathway. Gβγ has also been observed to show such slower movement off of PM along with Gα. Gα may simply be released into the cytoplasm by virtue of its depalmitoylation, or it may follow a vesicle-mediated pathway involving recycling endosomes together with Gβγ. In this slower pathway, Gα and Gβγ would return to the PM even slower (0.5–1 h). The figure also indicates that Gα and Gβγ traffic independently of GPCR internalization. The models in this figure focus on translocation of G proteins in non-visual systems. See the text and accompanying references for more details regarding light-activated translocation of visual G proteins
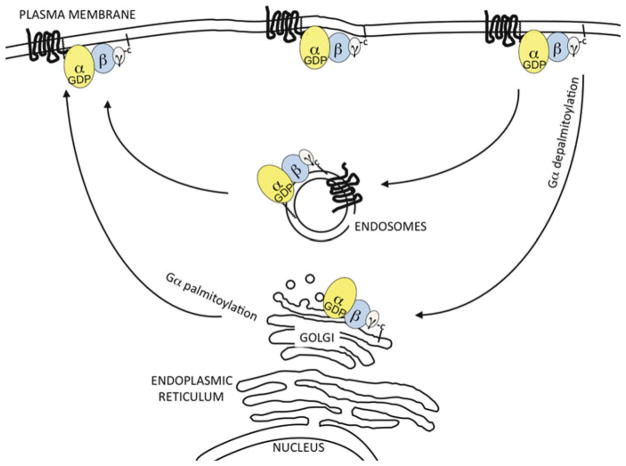
Fig. 11.4. Model of constitutive trafficking of G proteins.
Two pathways of constitutive, i.e., in the absence of GPCR-mediated activation, trafficking of heterotrimeric G proteins have been described. In one pathway, the G protein heterotrimer is constantly moving between the PM and Golgi. This pathway utilizes the palmitoylation cycle, as described for other palmitoylated peripheral membrane proteins, such as H-Ras. In this pathway, Gα in the context of the inactive heterotrimer undergoes continual depalmitoylation at the PM. Next, the depalmitoylated heterotrimer will move by diffusion to the Golgi where palmitoyl acyltransferases can be located. After re-palmitoylation of Gα, the heterotrimer returns by diffusion to the PM. In another described constitutive pathway, inactive Gα internalizes along with a non-activated GPCR. It is likely that Gβγ also follows this pathway, since it would be expected to be bound to inactive Gα. Thus, a G protein heterotrimer would traffic together with a constitutively internalizing GPCR. It is not known whether cycles of Gα depalmitoylation and palmitoylation would regulate this slower GPCR constitutive internalization-dependent pathway. See the text for details of these pathways of constitutive G protein movement
A variety of cell systems and experimental techniques have been used to establish that many G protein subunits translocate from the PM to intracellular locations in response to activation by an appropriate GPCR. One experimental system that has revealed a wealth of information about activation-induced G protein translocation is the mammalian visual system. A large number of studies have shown that activation of the GPCR rhodopsin by light in mammalian retinal photoreceptor cells results in a massive relocation of both the Gα and Gβγ subunits of transducin from the rod outer segment membranes to other subcellular locations (reviewed in Slepak and Hurley 2008; Artemyev 2008; Calvert et al. 2006). This G protein signaling system is somewhat unique due to the high concentration of rhodopsin and transducin and due to the fact that transducin has a weaker hydrophobic lipid attachment to membranes compared to other G proteins. Gαt is not palmitoylated. It is heterogeneously N-acylated by myristate (C14:0) and the less hydrophobic C14:1, C14:2 and C12 fatty acids. The transducin Gβ1γ1 is attached to the membrane by farnesylation of Gγ1; recall that farnesylation, like myristoylation, provides a relatively weak membrane anchor. The translocation of transducin off of the rod outer segment membrane is driven by activation-induced dissociation of the subunits. The αtβ1γ1 heterotrimer is attached tightly to the membrane due to the presence of two lipids, myristate and farnesyl. However, when light-activated rhodopsin promotes the loss of GDP and binding of GTP by Gαt, Gαt and Gβ1γ1 dissociate and now each subunit is able to translocate off the membrane due the weaker attachment by one lipid. Indeed, it has been demonstrated that more than 80% of Gαt and Gβ1γ1 are released from the membrane within minutes of light activation, and this dramatic translocation has the critical physiological role of allowing adaptation of the photoresponse to very bright conditions (Sokolov et al. 2002). After translocation off of rod outer segment membranes, current studies indicate that the transducin subunits spread to the inner segment of the photoreceptor cell, and then during dark adaptation transducin reforms the heterotrimer and returns to the outer segment membranes.
Further mechanistic details of the translocation process have been uncovered recently, and this continues to be a very active area of research. For Gβ1γ1, phosducin binds to it and thereby further decreases the membrane affinity of Gβ1γ1, at least partly by neutralizing electrostatic interactions of Gβ1γ1 with the membrane (Sokolov et al. 2004; Murray et al. 2001). In this way, phosducin promotes the translocation of Gβ1γ1. For Gαt, a computational study of its electrostatic potential using solved crystal structures indicated that negatively charged surface regions of Gαt would actually act as electrostatic repulsion to decrease the affinity of Gαt with a negatively charged membrane (Kosloff et al. 2008). Accordingly, once Gαt is dissociated from Gβ1γ1, the electrostatic repulsion would promote Gαt translocation. Additionally, the protein UNC119 was recently identified as a binding partner, with the exciting finding that UNC119 binds to the acylated N-terminus of Gαt in a complex in which the lipid is buried in a hydrophobic cavity of UNC119 (Zhang et al. 2011). The interaction of UNC119 with Gαt appears to promote the return of Gαt to outer segments (Zhang et al. 2011; Gopalakrishna et al. 2011). Another mechanistic detail that has been extensively addressed is the question of whether transducin translocation is driven by active transport or solely by diffusion. This has been a question of much debate and controversy in the field. Although some reports indicate that cytoskeletal elements are involved in transducin translocation and would thus serve as tracks for motor-driven active transport (Peterson et al. 2005), a number of researchers argue for diffusion-mediated transport, based a great deal on theoretical considerations (see these reviews for a in-depth discussion of mechanisms of transducin translocation, including diffusion versus active transport (Slepak and Hurley 2008; Artemyev 2008; Calvert et al. 2006)). Although some of the underlying mechanisms still need to be resolved, light-activated and reversible translocation of transducin clearly provides our most detailed and robust example of activation-induced trafficking of G proteins.
The Drosophila visual system has also been used as a model system to study G protein trafficking. Of particular interest, the visual system in flies uses palmitoylated Gαq to signal responses to light rather than the non-palmitoylated Gαt as is used in vertebrate visual signaling. Nonetheless, Gαq has been shown to translocate from membranes to the cytoplasm in response to light activation of photoreceptor cells (Kosloff et al. 2003; Cronin et al. 2004; Frechter et al. 2007). Similar to transducin in vertebrate visual signaling, Gαq translocation occurred in a matter of minutes after light activation. On the other hand, in Drosophila visual signaling it is not clear whether the relevant Gβγ traffics from the membrane after light activation, although studies using flies with reduced levels of Gβγ in the eye showed that Gαq needs Gβγ to return to the membrane. Furthermore, the recycling of Gαq required the photoreceptor-specific myosin III NINAC (Cronin et al. 2004). Although this study suggests that active transport would thus drive Gαq back to the membrane after light activation, more studies are needed to address the mechanism. Lastly, and again similar to vertebrate signaling, changes in the membrane content of Gαq allow adaptation to varying levels of light (Frechter et al. 2007). Thus, light-dependent translocation of G proteins appears to be a conserved mechanism for light adaptation.
Activation-induced G protein translocation has also been clearly identified to occur in non-visual signaling. Studies on Gαs provide the most information regarding activation-induced trafficking of G proteins in non-visual systems. A number of reports have demonstrated that Gαs can translocate from the PM to intracellular locations upon activation by a GPCR, such as the β2-adrenergic receptor (β2-AR), by cholera toxin or by introduction of a GTPase-inhibiting mutation (Allen et al. 2005; Ransnäs et al. 1989; Levis and Bourne 1992; Thiyagarajan et al. 2002; Wedegaertner and Bourne 1994; Wedegaertner et al. 1996; Yu and Rasenick 2002; Hynes et al. 2004). Immunofluorescence microscopy and biochemical fractionation of over-expressed and endogenous Gαs, as well as live cell imaging of Gαs fused to fluorescent proteins, have been used to document activation-induced translocation of Gαs. Agonist stimulation of β2-AR in cultured cells promotes Gαs translocation in a matter of 1–5 min, and indeed this represents a similar timecourse as observed for internalization of β2-AR (Hynes et al. 2004; Allen et al. 2005; Yu and Rasenick 2002 ; Wedegaertner et al. 1996). Some studies using immunofluorescence microscopy of fixed cells have observed β2-AR-activated Gαs to be homogeneously distributed throughout the cytoplasm, suggesting a simple diffusion of activated Gαs from the PM into the cytoplasm (Thiyagarajan et al. 2002; Wedegaertner et al. 1996), while other studies using live cell imaging have observed more punctate cytoplasmic localization of β2-AR-activated Gαs, suggesting vesicle-mediated trafficking of Gαs (Hynes et al. 2004; Allen et al. 2005; Yu and Rasenick 2002). The nature of the trafficking pathway utilized by activated Gαs for translocation off of the PM remains to be better understood. As described above for Gαt, more studies are needed to understand whether activation-induced translocation of Gαs, and other G proteins, is driven by diffusion or mediated by active transport on vesicles.
Although substantial evidence exists for activation-induced trafficking of Gαs, similar translocation has been much less documented for other non-visual system Gα. GPCR-induced redistribution of Gαq/11 has been demonstrated in mammalian cell culture; this is consistent with demonstrations of Gαq translocation in response to light in Drosophila photoreceptors, as described above. Accordingly, Gαq/11 translocation off of the PM was demonstrated in response to agonist activation of two Gq-coupled GPCRs, angiotensin II receptor and thyrotropin-releasing hormone receptor I (Drmota et al. 1998, 1999; Miserey-Lenkei et al. 2001). The temporal and spatial aspects of observed GPCR-induced Gαq/11 have differed. Gαq/11 appeared to translocate diffusely into the cytoplasm in 5–20 min in response to angiotensin II activation (Miserey-Lenkei et al. 2001); on the other hand, it took 2 h for Gαq/11 to translocate off of the PM after stimulation of the thyrotropin-releasing hormone receptor I, and Gαq/11 was observed in intracellular puncta, possibly vesicles (Drmota et al. 1998). As with Gαs, a great deal more studies with activation-induced trafficking of Gαq/11 are needed. Somewhat surprisingly, Gαt, Gαs and Gαq/11, both in Drosophila vision and non-visual cell culture, are the only Gα for which strong evidence has shown activation-induced trafficking. One interesting feature of these Gα is that they are either solely myristoylated (Gαt) or solely palmitoylated (Gαs and Gαq/11) but not both myristoylated and palmitoylated as is found in most members of the Gαi family. The inability of certain Gα, such as the myristoylated plus palmitoylated Gαi family members, to redistribute upon activation may be due to lipid modification differences, such as differences in rates or extents of depalmitoylation, as discussed below, or, alternatively, possibly the necessary imaging techniques have not yet been employed to observe such trafficking of Gαi.
Although much remains to be elucidated regarding the mechanistic details of activation-induced G protein trafficking, it is clear that one key step involves depalmitoylation of Gα (Marrari et al. 2007). Depalmitoylation dramatically decreases the membrane binding affinity of Gα, and thus promotes release from the PM. Gαt is the only Gα that does not undergo palmitoylation, and it was discussed above how activation decreases its membrane affinity and allow translocation. For other Gα, removal of attached palmitate is necessary to allow translocation into the cytoplasm. The strongest evidence for activation-induced depalmitoylation has been obtained for Gαs, maybe not surprisingly, since Gαs also provides the best non-visual system example of activation-induced translocation. Indeed, a strong correlation has been demonstrated for activation, translocation and depalmitoylation for Gαs. As observed for translocation of Gαs, mutational activation of Gαs or β2-AR-mediated activation Gαs promotes more rapid turnover of attached palmitate as revealed by palmitate labeling studies (Wedegaertner and Bourne 1994; Degtyarev et al. 1993; Mumby et al. 1994). Activation of appropriate GPCRs has also been shown to increase palmitate turnover on other Gα (Chen and Manning 2000; Stanislaus et al. 1997, 1998; Gurdal et al. 1997; Bhamre et al. 1998; Loisel et al. 1999; Stevens et al. 2001), including Gαq and Gαi, thus indicating that activation-induced depalmitoylation may occur in most Gα.
How does activation of a Gα promote its depalmitoylation? Recent results argue that in general depalmitoylation, through the acyl protein thioesterases APT1 and APT2, is a constitutive process, and intrinsic properties of a palmitoylated protein, such as membrane affinity and accessibility of the palmitoyl group to APT1/2, may regulate depalmitoylation (Rocks et al. 2010). Consistent with this idea, several studies have shown that downstream signaling does not affect the palmitate turnover or translocation of Gα (Kosloff et al. 2003; Cronin et al. 2004; Degtyarev et al. 1993). Also in line with a model of constitutive depalmitoylation, experiments with purified proteins or cell extracts show that Gβγ decreases the depalmitoylation of Gαs, suggesting that association with Gβγ inhibits accessibility of the depalmitoylating enzyme to the palmitoyl group attached to Gαs (Iiri et al. 1996; Wedegaertner and Bourne 1994). Because Gαs has a single attached palmitate, it may be more readily depalmitoylated compared to other Gα, such as Gαi, that are dually myristoylated and palmitoylated, simply because the Gαs palmitate is more accessible to the depalmitoylating enzyme. Taken together, current evidence indicates that a key step in activation-induced trafficking of palmitoylated Gα is an increased rate of depalmitoylation resulting from activation and dissociation from Gβγ.
Additional insight into activation-induced trafficking of G proteins has been obtained through comparisons of GPCR and G protein internalization. Gαs translocation off of the PM after activation does not require coincident internalization of a GPCR since Gαs translocation can be promoted by mutation or cholera toxin treatment, conditions under which a GPCR such as β2-AR remained localized at the PM (Levis and Bourne 1992; Wedegaertner et al. 1996). Moreover, Gαs and Gαq undergo GPCR activation-induced translocation even when GPCR internalization is blocked by mutation of the GPCR, expression of a dynamin dominant-negative mutant or treatment of cells with hypertonic sucrose (Wedegaertner et al. 1996; Hynes et al. 2004; Cronin et al. 2004; Miserey-Lenkei et al. 2001). However, another report showed that dominant negative dynamin blocked β2-AR-promoted translocation of Gαs in C6 glioma cells (Allen et al. 2005). It is possible that unique mechanisms of G protein internalization are used in different cells or that multiple pathways exist and alternative pathways can be utilized when one pathway is blocked. Dynamin can function in both clathrin-coated pit-mediated internalization as well lipid raft-mediated internalization of membrane proteins. Thus Gαs and other Gα may internalize in a lipid raft-dependent manner, at least in some situations, and this has been supported by results showing that Gαs and Gαq are enriched in lipid-raft fractions after activation, translocation of Gαs is inhibited by cholesterol depletion and internalized Gαs co-localizes with a lipid-raft marker (Allen et al. 2005; Hynes et al. 2004; Pesanova et al. 1999). In summary, results from visual and non-visual systems provide strong evidence for a trafficking model whereby activated Gα translocate independently of mechanisms responsible for GPCR internalization (Fig. 11.3). Nonetheless, the exact trafficking pathway for G proteins after movement off of the PM remains obscure.
Much of the above discussion has focused on activation-induced translocation of non-visual Gα subunits, particularly Gαs; however, there is evidence that non-visual Gβγ subunits also redistribute from the PM to intracellular locations, as occurs for Gβ1γ1 in vertebrate photoreceptors. One study used the technique of bimolecular fluorescence complementation (BiFC) to observe the localization of Gβ1γ7 during live cell imaging of transfected cells. It was found that upon agonist-stimulation of β2-AR Gβ1γ7 translocated from the PM to apparent vesicles, co-localizing with a fluorescent protein fusion of Gαs, and both Gαs and Gβ1γ7 displayed a similar time-course of redistribution, on the order of a few minutes (Hynes et al. 2004). This result suggested that activated Gαs and Gβ1γ7 could translocate together upon GPCR activation. Another report showed a translocation of expressed Gβ1γ2 from the PM to large endosomes after LPA stimulation; Gα translocation was not addressed (Garcia-Regalado et al. 2008). On the other hand, a series of live cell imaging studies suggested a very different picture (Akgoz et al. 2004, 2006; Azpiazu et al. 2006; Saini et al. 2007, 2009). Different combinations of Gβγ in which either the Gβ or Gγ subunit was fused to a fluorescent protein were expressed in cultured cells, along with various GPCRs and Gα. It was found that only certain combinations of Gβγ translocated upon GPCR activation, and the determining factor was the identity of the Gγ subunit. Gβγ translocation was not determined by whether the Gγ was geranylgeranylated or farnesylated, but rather depends on Gγ interactions with a GPCR. In this model, Gβγ combinations that contain a translocation competent Gγ associate with a particular GPCR via Gγ-mediated interactions, but upon activation the Gγ has a lowered affinity for the GPCR and thus the Gβγ is released from the PM (Saini et al. 2009). Remarkably, this can be a very fast process, with some Gβγ translocating off of the PM and localizing to the Golgi or ER in less that 10 s. Another surprising result of this series of studies is that no activation-induced translocation of Gα was observed, even though several different Gα, including Gαs, were tested, and rapid translocation of Gβγ was dependent upon palmitoylation. Thus, studies from this group have suggested a model whereby Gβγ translocates rapidly via diffusion to the Golgi or ER but Gα remains on the PM after activation of an appropriate GPCR (Akgoz et al. 2004, 2006; Azpiazu et al. 2006; Saini et al. 2007, 2009). Clearly, additional studies will be needed to reconcile different models of activation-induced G protein trafficking. Key questions to resolve include (1) Do both Gα and Gβγ translocate?; (2) If so, what are the rates of translocation, and are they vastly different for Gα and Gβγ?; and (3) Is translocation mediated by diffusion or vesicle-mediate active transport?
Lastly, in terms of activation-induced translocation of G proteins, most studies agree that it is a reversible process. As described above, there has been a substantial amount of research and debate as to whether G proteins that mediate visual signaling recycle to the PM via diffusion or active transport. However, very few studies have addressed the recycling of non-visual G proteins. Studies with Gαs have shown that after activation-induced translocation off of the PM, it returns to the PM over the course of approximately 1 h (Wedegaertner et al. 1996). This is slower than the initial translocation, which occurs within 10–15 min, yet Gαq in Drosophila photoreceptors recycles with a similar timecourse. Interesting, although Gαs and β2-AR do not appear to utilize the same mechanisms to translocate off of the PM upon activation, both proteins recycle to the PM at the same time when activation is terminated by the addition of an antagonist (Wedegaertner et al. 1996). Both β2-AR activation-induced translocation of Gαs and Gβ1γ7 and LPA receptor activation-induced translocation of Gβ1γ7 resulted in localization of the internalized subunits to Rab11 containing vesicles or structures; since Rab11 is associated with recycling endosomes, this colocalization suggests that internalized Gα and Gβγ may recycle via such a endosome-mediated pathway (Garcia-Regalado et al. 2008; Hynes et al. 2004). On the other hand, the series of studies that showed very rapid (<20 s) activation-dependent translocation of select Gβγ, but not Gα, showed a similar fast time course (seconds) for the recycling of Gβγ to the PM (Akgoz et al. 2004). Clearly, much work needs to be done to understand the recycling of G proteins to the PM once they have moved to intracellular locations upon activation.
11.5 Constitutive Trafficking of G Proteins
In addition to the evidence described above for activation-induced trafficking of G proteins, increasing evidence supports the idea that heterotrimeric G proteins, and indeed many peripheral membrane proteins in general, can traffic or shuttle between different membranes within the cell in the absence of an activation input (Fig. 11.4). One recently described constitutive trafficking pathway for peripheral membrane palmitoylated proteins invokes constitutive cycles of palmitoylation and depalmitoylation to direct movement between the PM and Golgi (Rocks et al. 2005, 2010). This model requires several key points, including (1) constitutive depalmitoylation of proteins is constantly occurring at all subcellular locations; (2) once depalmitoylated, a protein can then diffuse throughout the cell and sample any cellular membrane (PM, Golgi, ER, etc.); (3) the DHHC palmitoyl acyltransferases are concentrated at the Golgi, so that even though proteins are rapidly sampling various membranes, palmitoylation only occurs at the Golgi; (4) once a protein is palmitoylated at the Golgi, it is kinetically trapped there through strong hydrophobic anchoring to the Golgi membrane; and (5) specific transport, probably vesicle mediated, moves palmitoylated proteins from the Golgi to the PM. Most Gα are palmitoylated, and thus this model has relevance for defining and understanding the constitutive movement of G proteins.
Accordingly, recent data has supported rapid G protein shuttling between the PM and Golgi in a palmitoylation-dependent manner. Studies have used imaging techniques such as fluorescence recovery after photobleaching (FRAP), fluorescence loss in photobleaching (FLIP) and photoactivation to analyze the movement of G protein subunits fused to fluorescent proteins. Overexpression of all three subunits of a heterotrimer, in this case Gαo, Gβ1 and several different Gγ, including Gγ2, Gγ3, Gγ9 and Gγ11, showed substantial localization of the G protein to both the PM and an intracellular location, likely Golgi (Chisari et al. 2007). However, it should be noted that a number of studies overexpressing all three G protein subunits have observed predominant PM localization with little or no intracellular staining. Nonetheless, the above study used various live cell imaging techniques to demonstrate that there was a rapid and constitutive movement of all expressed G subunits between the Golgi and PM in both the anterograde and retrograde direction (Chisari et al. 2007). Based on the identical profile of movement of all three subunits, it appears that the inactive heterotrimer is constitutively trafficking, rather than Gα and Gβγ moving separately. Interestingly, the PM-Golgi shuttling was blocked by treatment with the palmitoylation inhibitor 2-bromopalmitate (2-BP) consistent with the model that a palmitoylation and depalmitoylation cycle mechanistically underlies this constitutive G protein trafficking. Both the retrograde and anterograde movement occurred within seconds and inhibitors of vesicle trafficking failed to block either direction of trafficking, suggesting that constitutive G protein trafficking occurs via diffusion (Chisari et al. 2007; Saini et al. 2009). Another recent study supported the model that G proteins can constitutively move between the PM and Golgi (Tsutsumi et al. 2009). Photoactivation and FRAP were used to follow the trafficking of Gαq when co-expressed with Gβ1γ2, and indeed movement between the PM and Golgi in both anterograde and retrograde directions was observed. Similar to the studies with the Gαo-containing heterotrimers (Chisari et al. 2007), the constitutive shuttling of Gαq-containing heterotrimers was blocked by 2-BP treatment (Tsutsumi et al. 2009). Moreover, the Gαq study implicated DHHC-3 and DHHC-7 as Golgi-localized palmitoyl acyltransferases responsible for palmitoylation of Gαq. The movement of Gαq between the PM and Golgi occurred in approximately 10 min, which was substantially slower than the shuttling of Gαo-containing heterotrimers which happened in a matter of seconds. The reason for this temporal difference awaits further experimentation.
Based on the above described studies and on extensive experiments with model palmitoylated proteins, such as H- and N-Ras (Rocks et al. 2005, 2010; Goodwin et al. 2005), a model for constitutive movement of heterotrimeric G proteins has been described (Saini et al. 2009). As with Ras and other palmitoylated peripheral membrane proteins, the model states that an inactive heterotrimer is constitutively depalmitoylated at the PM and then moves rapidly by diffusion to the Golgi, where it is re-palmitoylated and returns by rapid diffusion to the PM. This model should provide the basis for much future investigation to verify and mechanistically expand our knowledge of constitutive G protein trafficking. A number of questions need to be further addressed. First, it is difficult to understand how a heterotrimeric G protein can move from the Golgi to PM by simple diffusion. Once palmitoylation occurs at the Golgi, in this model, the heterotrimer is expected to be bound tightly to the membrane via multiple membrane binding motifs, including palmitate attached to Gα, geranylgeranylation of Gγ, and myristoylation or polybasic N-termini of Gα. Thus, it seems very unlikely that a fully lipid modified G protein heterotrimer could be simply released from a Golgi membrane to diffuse through the cytoplasm to the PM; it would likely require additional proteins that could extract the heterotrimer from the Golgi membrane and sequester the hydrophobic lipids for the trip through the cytoplasm. Likewise, similar questions exist regarding diffusion of the heterotrimer from the PM to the Golgi; however in this case the strength of membrane attachment is decreased by depalmitoylation. Nonetheless, a depalmitoylated heterotrimer could still contain more than one membrane binding motif (e.g., myristoylation of Gα together with prenylation of Gγ). Likely, additional proteins would also be needed to facilitate the diffusion of a depalmitoylated heterotrimer. An important and exciting goal for research in this area will be to identify proteins that bind the heterotrimer and sequester the attached lipids to facilitate diffusion between the cytoplasmic surfaces of different membranes. Another important point to address is that currently it is difficult to reconcile a model in which inactive heterotrimers rapidly and constitutively shuttle between the PM and Golgi and a model which states that in response to GPCR activation select Gβγ but not Gα move rapidly and reversibly from the PM to the Golgi or other intracellular locations (Saini et al. 2009 ). Future studies should help understand mechanistic differences between constitutive and activation-dependent trafficking of G proteins in a palmitoylation cycle-dependent manner.
No doubt future studies will reveal multiple levels of regulation that promote retention or release of a G protein on the PM or membrane of a particular organelle. In support of this, LRP6, a transmembrane PM-localized protein, was shown to promote PM localization of Gαs and Gβγ (Wan et al. 2011). In this study, siRNA depletion of LRP6 in cultured cells resulted in a partial redistribution of expressed Gαs and Gβγ from the PM into poorly defined cytoplasmic locations. Moreover, LRP6 binds inactive Gαs complexed with Gβγ. Thus, it was proposed the LRP6 enhances PM targeting of a Gαs-containing heterotrimer by helping to tether it to the PM. Obviously, such mechanisms are important to consider when pursuing models of G protein trafficking.
G proteins may additionally be able to constitutively traffic from the PM to intracellular locations in a manner independent of the above PM-Golgi palmitoylation cycle, although such pathways and mechanisms remain obscure. Interestingly, a number of studies have localized G protein subunits to endosomes, suggesting that G proteins may utilize endosome-mediated pathways for constitutive trafficking (Balbis et al. 2007; Scarselli and Donaldson 2009; Van Dyke 2004; Zheng et al. 2004). In most cases, it is uncertain whether endosome localization of G proteins should be interpreted as evidence for constitutive or activation-induced trafficking. As noted in the previous section, increased endosomal location of some G proteins has been detected upon GPCR activation. Nonetheless, a recent study provided evidence for a novel constitutive endosomal pathway for G proteins (Scarselli and Donaldson 2009). This study examined two GPCRs, β2-AR and the M3 muscarinic acetylcholine receptor (M3R), and found that they internalized in the absence of agonist, i.e., constitutively, and in a clathrin-independent manner. Surprisingly, Gαs and Gαq co-localized with β2-AR and M3R, respectively, on tubular recycling endosomes, suggesting that the cognate G protein constitutively traffics along with a GPCR following an endosomal pathway. It was not determined whether Gβγ also constitutively trafficked with the GPCRs; one would predict that indeed the heterotrimer would be trafficking under non-activating conditions. This endosomal pathway provides an intriguing way to constitutively deliver G proteins and GPCRs to endosomes, and possibly other locations. It will be exciting to determine whether the Golgi-mediated constitutive G protein shuttling pathway, described above, is mutually exclusive with this endosomal pathway, and what are the mechanisms that distinguish different pathways of constitutive trafficking.
11.6 Functional Implications of G Protein Trafficking
In conclusion, there is strong evidence that G proteins move within the cell both constitutively and in response to GPCR activation. A major challenge as research moves forward is to better define the underlying mechanisms that control G protein trafficking. Moreover, imaging studies will further resolve the subcellular locations through with G proteins traffic and will determine how G protein trafficking itineraries differ for unique heterotrimers and cell contexts.
The knowledge that G proteins indeed traffic within the cell begs the question of what is the role of such trafficking. One role for activation-induced translocation of G protein subunits is to turn off or decrease signaling by removing the G protein from a PM site of action. Clearly, light-induced translocation of G proteins in both vertebrate and invertebrate visual signaling plays such a role by allowing adaptation to bright light. A similar role has not been well-established for non-visual G protein signaling, yet there are some suggestive results. For example, a mutationally activated Gαs distributes between the PM and cytoplasm, due to more rapid palmitate turnover compared to wild type Gαs, but mutants that replace the single N-terminal site of palmitoylation with other signals, such as two lipid modifications, show enhanced PM localization and enhanced activation of its effector adenylyl cyclase (Thiyagarajan et al. 2002). Another study showed a heightened signaling response when a GPCR activated a heterotrimer containing a Gβγ that did not translocate compared to activation of a heterotrimer containing a translocating Gβγ (Chisari et al. 2009).
In contrast to regulating signals emanating from the PM, another important role for G protein trafficking is to deliver G proteins to diverse subcellular locations. It has become increasing clear that G proteins have novel signaling functions at a variety of intracellular locations (reviewed in Hewavitharana and Wedegaertner 2012). Select G protein subunits, Gα or Gβγ or both, have been localized to and shown to have functions at endosomes, Golgi, ER, mitochondria and even nuclei. Accordingly, intracellular trafficking pathways can target G proteins to these subcellular localizations to allow G protein regulation of non-canonical signaling, and moreover, regulation of activation-induced or constitutive trafficking pathways will influence intracellular signaling functions of G proteins. As one example, clear evidence has emerged that Gβγ subunits can localize to the cytoplasmic surface of Golgi membranes, where they regulate a signaling pathway involving a number of Golgi-localized signaling proteins, including PLCβ, PKD, PKCη and PI4-kinase, that ultimately controls the fission at the trans Golgi network of PM-destined vesicles (Diaz Anel and Malhotra 2005; Jamora et al. 1997, 1999; Diaz Anel 2007; Irannejad and Wedegaertner 2010; Saini et al. 2010). Gβγ must arrive at the Golgi through one or more of the trafficking pathways discussed in this review – trafficking of the G protein following biosynthesis, constitutive cycling between the PM and Golgi or activation-induced trafficking from the PM to Golgi. Indeed, one report correlated translocation of select Gβγ isoforms from the PM to Golgi after M1 muscarinic acetylcholine receptor activation with stimulation of insulin secretion (Saini et al. 2010). In the future, it is reasonable to think that a more detailed understanding of G protein trafficking will identify novel therapeutic targets to allow the inhibition or activation of a specific intracellular G protein function without affecting its other signaling functions.
References
- Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279(49):51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction. Cell Signal. 2006;18(10):1758–1768. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67(5):1493–1504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- Artemyev NO. Light-dependent compartmentalization of transducin in rod photoreceptors. Mol Neurobiol. 2008;37(1):44–51. doi: 10.1007/s12035-008-8015-2. [DOI] [PubMed] [Google Scholar]
- Azpiazu I, Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell Signal. 2006;18(8):1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbis A, Parmar A, Wang Y, Baquiran G, Posner BI. Compartmentalization of signaling-competent epidermal growth factor receptors in endosomes. Endocrinology. 2007;148(6):2944–2954. doi: 10.1210/en.2006-1674. pii:en.2006-1674. [DOI] [PubMed] [Google Scholar]
- Bereta G, Palczewski K. Heterogeneous N-terminal acylation of retinal proteins results from the retina’s unusual lipid metabolism. Biochemistry. 2011;50(18):3764–3776. doi: 10.1021/bi200245t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamre S, Wang HY, Friedman E. Serotonin-mediated palmitoylation and depalmitoylation of G alpha proteins in rat brain cortical membranes. J Pharmacol Exp Ther. 1998;286(3):1482–1489. [PubMed] [Google Scholar]
- Bhattacharyya R, Wedegaertner PB. Galpha 13 requires palmitoylation for plasma membrane localization, Rho-dependent signaling, and promotion of p115-RhoGEF membrane binding. J Biol Chem. 2000;275(20):14992–14999. doi: 10.1074/jbc.M000415200. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16(11):560–568. doi: 10.1016/j.tcb.2006.09.001. pii:S0962-8924(06)00237-6. [DOI] [PubMed] [Google Scholar]
- Chen CA, Manning DR. Regulation of galpha i palmitoylation by activation of the 5-hydroxytryptamine-1A receptor. J Biol Chem. 2000;275(31):23516–23522. doi: 10.1074/jbc.M003439200. [DOI] [PubMed] [Google Scholar]
- Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282(33):24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Saini DK, Cho JH, Kalyanaraman V, Gautam N. G protein subunit dissociation and translocation regulate cellular response to receptor stimulation. PLoS One. 2009;4(11):e7797. doi: 10.1371/journal.pone.0007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin MA, Diao F, Tsunoda S. Light-dependent subcellular translocation of Gqalpha in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J Cell Sci. 2004;117(Pt 20):4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- Crouthamel M, Thiyagarajan MM, Evanko DS, Wedegaertner PB. N-terminal polybasic motifs are required for plasma membrane localization of Galpha(s) and Galpha(q) Cell Signal. 2008;20(10):1900–1910. doi: 10.1016/j.cellsig.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouthamel M, Abankwa D, Zhang L, DiLizio C, Manning DR, Hancock JF, Wedegaertner PB. An N-terminal polybasic motif of Galphaq is required for signaling and influences membrane nanodomain distribution. Mol Pharmacol. 2010;78(4):767–777. doi: 10.1124/mol.110.066340. pii:mol.110.066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Martin CA, Segalen M, Rosenfeld F, Schweisguth F, Bellaiche Y. Drosophila Ric-8 regulates Galphai cortical localization to promote Galphai-dependent planar orientation of the mitotic spindle during asymmetric cell division. Nat Cell Biol. 2005;7(11):1083–1090. doi: 10.1038/ncb1319. pii:ncb1319. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TL. Increased palmitoylation of the Gs protein alpha subunit after activation by the beta-adrenergic receptor or cholera toxin. J Biol Chem. 1993;268(32):23769–23772. [PubMed] [Google Scholar]
- Diaz Anel AM. Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/ PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem J. 2007;406(1):157–165. doi: 10.1042/BJ20070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Anel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169(1):83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. pii:nrc2069. [DOI] [PubMed] [Google Scholar]
- Drmota T, Novotny J, Kim GD, Eidne KA, Milligan G, Svoboda P. Agonist-induced internalization of the G protein G11alpha and thyrotropin-releasing hormone receptors proceed on different time scales. J Biol Chem. 1998;273(34):21699–21707. doi: 10.1074/jbc.273.34.21699. [DOI] [PubMed] [Google Scholar]
- Drmota T, Novotny J, Gould GW, Svoboda P, Milligan G. Visualization of distinct patterns of subcellular redistribution of the thyrotropin-releasing hormone receptor-1 and gqalpha / G11alpha induced by agonist stimulation. Biochem J. 1999;340(Pt 2):529–538. [PMC free article] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273(25):15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein alpha subunit deacylation in vivo. J Biol Chem. 2002;277(35):31740–31752. doi: 10.1074/jbc.M202505200. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Linder ME. Signalling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436(1–2):245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signalling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281(45):34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282(18):13703–13715. doi: 10.1074/jbc.M608846200. pii:M608846200. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia N, Frechter S, Gedi Y, Minke B, Selinger Z. Excess of Gbetae over Gqalphae in vivo prevents dark, spontaneous activity of Drosophila photoreceptors. J Cell Biol. 2005;171(3):517–526. doi: 10.1083/jcb.200506082. pii:jcb.200506082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko DS, Thiyagarajan MM, Wedegaertner PB. Interaction with Gbetagamma is required for membrane targeting and palmitoylation of Galpha(s) and Galpha(q) J Biol Chem. 2000;275(2):1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gbeta gamma isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Galphas and Galphaq. J Biol Chem. 2001;276(26):23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Herzmark P, Morales J, Bourne HR. Gbetagamma and palmitate target newly synthesized Galphaz to the plasma membrane. J Biol Chem. 1999;274(26):18793–18800. doi: 10.1074/jbc.274.26.18793. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Pollitt SK, Bourne HR. Localization of a peripheral membrane protein: G beta gamma targets G alpha(z) Proc Natl Acad Sci USA. 2000;97(3):1085–1090. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechter S, Elia N, Tzarfaty V, Selinger Z, Minke B. Translocation of Gq alpha mediates long-term adaptation in Drosophila photoreceptors. J Neurosci. 2007;27(21):5571–5583. doi: 10.1523/JNEUROSCI.0310-07.2007. pii:27/21/5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay M, Pinter ME, Wright FA, Chan P, Murphy AJ, Valenzuela DM, Yancopoulos GD, Tall GG. Ric-8 proteins are molecular chaperones that direct nascent G protein alpha subunit membrane association. Sci Signal. 2011;4(200):ra79. doi: 10.1126/scisignal.2002223. pii:4/200/ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Guzzi F, Magee AI, Milligan G, Parenti M. N-terminal fatty acylation of the alpha-subunit of the G-protein Gi1: only the myristoylated protein is a substrate for palmitoylation. Biochem J. 1994;303(Pt 3):697–700. doi: 10.1042/bj3030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Regalado A, Guzman-Hernandez ML, Ramirez-Rangel I, Robles-Molina E, Balla T, Vazquez-Prado J, Reyes-Cruz G. G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction. Mol Biol Cell. 2008;19(10):4188–4200. doi: 10.1091/mbc.E07-10-1089. pii:E07-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9(3):585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Drake KR, Rogers C, Wright L, Lippincott-Schwartz J, Philips MR, Kenworthy AK. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170(2):261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna KN, Doddapuneni K, Boyd KK, Masuho I, Martemyanov KA, Artemyev NO. Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J Biol Chem. 2011;286(33):28954–28962. doi: 10.1074/jbc.M111.268821. pii:M111.268821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M, Ahringer J. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol. 2001;3(3):297–300. doi: 10.1038/35060092. [DOI] [PubMed] [Google Scholar]
- Gurdal H, Seasholtz TM, Wang HY, Brown RD, Johnson MD, Friedman E. Role of G alpha q or G alpha o proteins in alpha 1-adrenoceptor subtype-mediated responses in Fischer 344 rat aorta. Mol Pharmacol. 1997;52(6):1064–1070. doi: 10.1124/mol.52.6.1064. [DOI] [PubMed] [Google Scholar]
- Hallak H, Brass LF, Manning DR. Failure to myristoylate the alpha subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein kinase C. J Biol Chem. 1994;269(6):4571–4576. [PubMed] [Google Scholar]
- Hampoelz B, Hoeller O, Bowman SK, Dunican D, Knoblich JA. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nat Cell Biol. 2005;7(11):1099–1105. doi: 10.1038/ncb1318. pii:ncb1318. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Matsuda T, Matsuura Y, Haga T, Fukada Y. Production of N-lauroylated G protein alpha-subunit in Sf9 insect cells: the type of N-acyl group of Galpha influences G protein-mediated signal transduction. J Biochem. 2004;135(3):319–329. doi: 10.1093/jb/mvh039. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Kozasa T, Smrcka AV, Simon MI, Rhee SG, Sternweis PC, Gilman AG. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha. Activation of purified phospholipase C isozymes by G alpha subunits. J Biol Chem. 1993;268(19):14367–14375. [PubMed] [Google Scholar]
- Hewavitharana T, Wedegaertner PB. Non-canonical signaling and localizations of heterotrimeric G proteins. Cell Signal. 2012;24(1):25–34. doi: 10.1016/j.cellsig.2011.08.014. pii:S0898-6568(11)00260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LL, Wan SB, Niu S, Shi XH, Li HP, Cai YD, Chou KC. Prediction and analysis of protein palmitoylation sites. Biochimie. 2011;93(3):489–496. doi: 10.1016/j.biochi.2010.10.022. pii:S0300-9084(10)00383-4. [DOI] [PubMed] [Google Scholar]
- Hughes TE, Zhang H, Logothetis DE, Berlot CH. Visualization of a functional Galpha q-green fluorescent protein fusion in living cells. Association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not be activation mediated by receptors or AlF4. J Biol Chem. 2001;276(6):4227–4235. doi: 10.1074/jbc.M007608200. [DOI] [PubMed] [Google Scholar]
- Humrich J, Bermel C, Grubel T, Quitterer U, Lohse MJ. Regulation of phosducin-like protein by casein kinase 2 and N-terminal splicing. J Biol Chem. 2003;278(7):4474–4481. doi: 10.1074/jbc.M206347200. pii:M206347200. [DOI] [PubMed] [Google Scholar]
- Humrich J, Bermel C, Bunemann M, Harmark L, Frost R, Quitterer U, Lohse MJ. Phosducin-like protein regulates G-protein betagamma folding by interaction with tailless complex poly-peptide-1alpha: dephosphorylation or splicing of PhLP turns the switch toward regulation of Gbetagamma folding. J Biol Chem. 2005;280(20):20042–20050. doi: 10.1074/jbc.M409233200. [DOI] [PubMed] [Google Scholar]
- Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J Biol Chem. 2004;279(42):44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jones TL, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gs alpha by palmitate and the beta gamma subunit. Proc Natl Acad Sci USA. 1996;93(25):14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Wedegaertner PB. Regulation of constitutive cargo transport from the trans-Golgi network to plasma membrane by Golgi-localized G protein betagamma subunits. J Biol Chem. 2010;285(42):32393–32404. doi: 10.1074/jbc.M110.154963. pii:M110.154963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Takizawa PA, Zaarour RF, Denesvre C, Faulkner DJ, Malhotra V. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91(5):617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98(1):59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Ohguro H, Palczewski K, Hurley JB, Walsh KA, Neubert TA. Heterogeneous N-acylation is a tissue- and species-specific posttranslational modification. J Biol Chem. 1994;269:21067–21071. [PubMed] [Google Scholar]
- Jones TL, Simonds WF, Merendino JJ, Brann MR, Spiegel AM. Myristoylation of an inhibitory GTP-binding protein alpha subunit is essential for its membrane attachment. Proc Natl Acad Sci USA. 1990;87(2):568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol JC, Engel R, Blaauw M, Visser AJ, van Haastert PJ. The phosducin-like protein PhLP1 is essential for G{beta}{gamma} dimer formation in Dictyostelium discoideum. Mol Cell Biol. 2005;25(18):8393–8400. doi: 10.1128/MCB.25.18.8393-8400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein α-subunit. Nature. 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Selinger Z. Structural homology discloses a bifunctional structural motif at the N-termini of G alpha proteins. Biochemistry. 2002;41(49):14518–14523. doi: 10.1021/bi026729x. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z. Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22(3):459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosloff M, Alexov E, Arshavsky VY, Honig B. Electrostatic and lipid anchor contributions to the interaction of transducin with membranes: mechanistic implications for activation and translocation. J Biol Chem. 2008;283(45):31197–31207. doi: 10.1074/jbc.M803799200. pii:M803799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Kubota H, Nagata K. Cytosolic chaperonin protects folding intermediates of Gbeta from aggregation by recognizing hydrophobic beta-strands. Proc Natl Acad Sci USA. 2006;103(22):8360–8365. doi: 10.1073/pnas.0600195103. pii:0600195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA. Dissociation of heterotrimeric g proteins in cells. Sci Signal. 2008;1(25):re5. doi: 10.1126/scisignal.125re5. pii:scisignal.125re5. [DOI] [PubMed] [Google Scholar]
- Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47(4):681–699. doi: 10.1194/jlr.R600002-JLR200. pii:R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Levis MJ, Bourne HR. Activation of the α subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Shao YH, Deng NY. Improved prediction of palmitoylation sites using PWMs and SVM. Protein Pept Lett. 2011;18(2):186–193. doi: 10.2174/092986611794475084. pii:BSP/ PPL/ E pub/0239. [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277(43):41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Loisel TP, Ansanay H, Adam L, Marullo S, Seifert R, Lagace M, Bouvier M. Activation of the beta(2)-adrenergic receptor-Galpha(s) complex leads to rapid depalmitoylation and inhibition of repalmitoylation of both the receptor and Galpha(s) J Biol Chem. 1999;274(43):31014–31019. doi: 10.1074/jbc.274.43.31014. [DOI] [PubMed] [Google Scholar]
- Lukov GL, Hu T, McLaughlin JN, Hamm HE, Willardson BM. Phosducin-like protein acts as a molecular chaperone for G protein betagamma dimer assembly. EMBO J. 2005;24(11):1965–1975. doi: 10.1038/sj.emboj.7600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukov GL, Baker CM, Ludtke PJ, Hu T, Carter MD, Hackett RA, Thulin CD, Willardson BM. Mechanism of assembly of G protein betagamma subunits by protein kinase CK2-phosphorylated phosducin-like protein and the cytosolic chaperonin complex. J Biol Chem. 2006;281(31):22261–22274. doi: 10.1074/jbc.M601590200. pii:M601590200. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46(26):7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DD, Beauchamp E, Berthiaume LG. Post-translational myristoylation: fat matters in cellular life and death. Biochimie. 2011;93(1):18–31. doi: 10.1016/j.biochi.2010.10.018. pii:S0300-9084(10)00378-0. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Takao T, Shimonishi Y, Murata M, Asano T, Yoshizawa T, Fukada Y. Characterization of interactions between transducin α/βγ-subunits and lipid membranes. J Biol Chem. 1994;269(48):30358–30363. [PubMed] [Google Scholar]
- McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–280. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Ahearn I, Bergo M, Young S, Philips M. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell. 2002;13(9):3294–3302. doi: 10.1091/mbc.E02-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Lenkei Z, Parnot C, Corvol P, Clauser E. A functional enhanced green fluorescent protein (EGFP)-tagged angiotensin II at(1a) receptor recruits the endogenous Galphaq/11 protein to the membrane and induces its specific internalization independently of receptor-g protein coupling in HEK-293 cells. Mol Endocrinol. 2001;15(2):294–307. doi: 10.1210/mend.15.2.0600. [DOI] [PubMed] [Google Scholar]
- Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of G alpha z requires two signals. Mol Biol Cell. 1998;9(1):1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby SM, Heukeroth RO, Gordon JI, Gilman AG. G-protein α-subunit expression, myristoylation, and membrane association in COS cells. Proc Natl Acad Sci USA. 1990;87(2):728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91(7):2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D, McLaughlin S, Honig B. The role of electrostatic interactions in the regulation of the membrane association of G protein beta gamma heterodimers. J Biol Chem. 2001;276(48):45153–45159. doi: 10.1074/jbc.M101784200. [DOI] [PubMed] [Google Scholar]
- Neubert TA, Johnson RS, Hurley JB, Walsh KA. The rod transducin α subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267:18274–18277. [PubMed] [Google Scholar]
- Pedone KH, Hepler JR. The importance of N-terminal polycysteine and polybasic sequences for G14alpha and G16alpha palmitoylation, plasma membrane localization, and signaling function. J Biol Chem. 2007;282(35):25199–25212. doi: 10.1074/jbc.M610297200. [DOI] [PubMed] [Google Scholar]
- Pesanova Z, Novotny J, Cerny J, Milligan G, Svoboda P. Thyrotropin-releasing hormone-induced depletion of G(q)alpha/G(11)alpha proteins from detergent-insensitive membrane domains. FEBS Lett. 1999;464(1–2):35–40. doi: 10.1016/s0014-5793(99)01666-x. pii:S001457939901666X. [DOI] [PubMed] [Google Scholar]
- Peterson JJ, Orisme W, Fellows J, McDowell JH, Shelamer CL, Dugger DR, Smith WC. A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci. 2005;46(11):3988–3998. doi: 10.1167/iovs.05-0567. pii:46/11/3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preininger AM, Hamm HE. G protein signaling: insights from new structures. Sci STKE. 2004;2004(218):re3. doi: 10.1126/stke.2182004re3. pii:stke.2182004re3. [DOI] [PubMed] [Google Scholar]
- Ransnäs LA, Svoboda P, Jasper JR, Insel PA. Stimulation of β-adrenergic receptors of S49 lymphoma cells redistributes the α subunit of the stimulatory G protein between cytosol and membranes. Proc Natl Acad Sci USA. 1989;86:7900–7903. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21(11):639–644. doi: 10.1093/protein/gzn039. pii:gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451(1):1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2(11):584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307(5716):1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- Rocks O, Gerauer M, Vartak N, Koch S, Huang ZP, Pechlivanis M, Kuhlmann J, Brunsveld L, Chandra A, Ellinger B, Waldmann H, Bastiaens PI. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141(3):458–471. doi: 10.1016/j.cell.2010.04.007. pii:S0092-8674(10)00381-8. [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159(1):23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282(33):24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Chisari M, Gautam N. Shuttling and translocation of heterotrimeric G proteins and Ras. Trends Pharmacol Sci. 2009;30(6):278–286. doi: 10.1016/j.tips.2009.04.001. pii:S0165-6147(09)00056-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V, Gautam N. Regulation of Golgi structure and secretion by receptor-induced G protein betagamma complex translocation. Proc Natl Acad Sci USA. 2010;107(25):11417–11422. doi: 10.1073/pnas.1003042107. pii:1003042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarselli M, Donaldson JG. Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J Biol Chem. 2009;284(6):3577–3585. doi: 10.1074/jbc.M806819200. pii:M806819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz HR, Heck M, Hofmann KP, Alt T, Pellaud J, Seelig A. Molecular determinants of the reversible membrane anchorage of the G-protein transducin. Biochemistry. 1999;38(25):7950–7960. doi: 10.1021/bi990298+. pii:bi990298+ [DOI] [PubMed] [Google Scholar]
- Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, Hubel K, Dekker F, Hedberg C, Rengarajan B, Drepper C, Waldmann H, Kauppinen S, Greenberg ME, Draguhn A, Rehmsmeier M, Martinez J, Schratt GM. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11(6):705–716. doi: 10.1038/ncb1876. pii:ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepak VZ, Hurley JB. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life. 2008;60(1):2–9. doi: 10.1002/iub.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34(1):95–106. doi: 10.1016/s0896-6273(02)00636-0. pii:S0896627302006360. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem. 2004;279(18):19149–19156. doi: 10.1074/jbc.M311058200. pii:M311058200. [DOI] [PubMed] [Google Scholar]
- Sondek J, Siderovski DP. Ggamma-like (GGL) domains: new frontiers in G-protein signaling and beta-propeller scaffolding. Biochem Pharmacol. 2001;61(11):1329–1337. doi: 10.1016/s0006-2952(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379(6563):369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- Song J, Hirschman J, Gunn K, Dohlman HG. Regulation of membrane and subunit interactions by N-myristoylation of a G protein α subunit in yeast. J Biol Chem. 1996;271(34):20273–20283. doi: 10.1074/jbc.271.34.20273. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol Endocrinol. 1997;11(6):738–746. doi: 10.1210/mend.11.6.0005. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Ponder S, Ji TH, Conn PM. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: evidence from palmitoylation and overexpression of G proteins. Biol Reprod. 1998;59(3):579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- Stevens PA, Pediani J, Carrillo JJ, Milligan G. Coordinated agonist regulation of receptor and G protein palmitoylation and functional rescue of palmitoylation-deficient mutants of the G protein G11alpha following fusion to the alpha1b-adrenoreceptor: palmitoylation of G11alpha is not required for interaction with beta*gamma complex. J Biol Chem. 2001;276(38):35883–35890. doi: 10.1074/jbc.M103816200. [DOI] [PubMed] [Google Scholar]
- Takida S, Wedegaertner PB. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gbeta gamma. J Biol Chem. 2003;278(19):17284–17290. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- Takida S, Wedegaertner PB. Exocytic pathway-independent plasma membrane targeting of heterotrimeric G proteins. FEBS Lett. 2004;567(2–3):209–213. doi: 10.1016/j.febslet.2004.04.062. [DOI] [PubMed] [Google Scholar]
- Tall GG, Krumins AM, Gilman AG. Mammalian Ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J Biol Chem. 2003;278(10):8356–8362. doi: 10.1074/jbc.M211862200. pii:M211862200. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan MM, Bigras E, Van Tol HH, Hebert TE, Evanko DS, Wedegaertner PB. Activation-induced subcellular redistribution of G alpha(s) is dependent upon its unique N-terminus. Biochemistry. 2002;41(30):9470–9484. doi: 10.1021/bi025533u. [DOI] [PubMed] [Google Scholar]
- Thomas CJ, Briknarova K, Hilmer JK, Movahed N, Bothner B, Sumida JP, Tall GG, Sprang SR. The nucleotide exchange factor Ric-8A is a chaperone for the conformationally dynamic nucleotide-free state of Galphai1. PLoS One. 2011;6(8):e23197. doi: 10.1371/journal.pone.0023197. pii:PONE-D-11-08669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29(2):435–447. doi: 10.1128/MCB.01144-08. pii:MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke RW. Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiol. 2004;4:1. doi: 10.1186/1472-6793-4-1. pii:1472-6793-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Li J, Herbst K, Zhang J, Yu B, Wu X, Qiu T, Lei W, Lindvall C, Williams BO, Ma H, Zhang F, Cao X. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s) Sci Signal. 2011;4(164):ra15. doi: 10.1126/scisignal.2001464. pii:4/164/ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ng KH, Qian H, Siderovski DP, Chia W, Yu F. Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat Cell Biol. 2005;7(11):1091–1098. doi: 10.1038/ncb1317. pii:ncb1317. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gs alpha. Cell. 1994;77(7):1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J Biol Chem. 1993;268(33):25001–25008. [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR, von Zastrow M. Activation-induced subcellular redistribution of Gsa. Mol Biol Cell. 1996;7(8):1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, Dingus J, Hildebrandt JD. Role of the chaperonin CCT/TRiC complex in G protein betagamma-dimer assembly. J Biol Chem. 2006;281(29):20221–20232. doi: 10.1074/jbc.M602409200. pii:M602409200. [DOI] [PubMed] [Google Scholar]
- Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell Signal. 2007;19(12):2417–2427. doi: 10.1016/j.cell-sig.2007.06.013. pii:S0898-6568(07)00190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PT, Bourne HR. Fatty acylation of alpha z. Effects of palmitoylation and myristoylation on alpha z signaling. J Biol Chem. 1995;270(16):9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- Wise A, Parenti M, Milligan G. Interaction of the G-protein G11alpha with receptors and phosphoinositidase C: the contribution of G-protein palmitoylation and membrane association. FEBS Lett. 1997;407(3):257–260. doi: 10.1016/s0014-5793(97)00300-1. [DOI] [PubMed] [Google Scholar]
- Wright LP, Philips MR. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res. 2006;47(5):883–891. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Rasenick MM. Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Mol Pharmacol. 2002;61(2):352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Jackson CS, Magee AI. Protein acyl thioesterases (review) Mol Membr Biol. 2009;26(1):32–41. doi: 10.1080/09687680802629329. pii:907244142. [DOI] [PubMed] [Google Scholar]
- Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, Inana G, Whitby FG, Jorgensen EM, Hill CP, Tong L, Baehr W. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci. 2011;14(7):874–880. doi: 10.1038/nn.2835. pii:nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Lavoie C, Tang TD, Ma P, Meerloo T, Beas A, Farquhar MG. Regulation of epidermal growth factor receptor degradation by heterotrimeric Galphas protein. Mol Biol Cell. 2004;15(12):5538–5550. doi: 10.1091/mbc.E04-06-0446. pii:E04-06-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]