Abstract
Trifluoroacetic acid (TFA) is often used as a mobile phase modifier to enhance reversed phase chromatographic performance. TFA adjusts solution pH and is an ion-pairing agent, but it is not typically suitable for electrospray ionization-mass spectrometry (ESI-MS) and liquid chromatography/MS (LC/MS) because of its significant signal suppression. Supercharging agents elevate peptide and protein charge states in ESI, increasing tandem MS (MS/MS) efficiency. Here, LC/MS protein supercharging was effected by adding agents to LC mobile phase solvents. Significantly, the ionization suppression generally observed with TFA was, for the most part, rescued by supercharging agents, with improved separation efficiency (higher number of theoretical plates) and lowered detection limits.
Keywords: liquid chromatography, electrospray ionization mechanism, supercharging, ion suppression
Graphical Abstract
Several supercharging agents were tested, including novel agents N,N,N′N′-tetraethylsulfamide (TES) and 3-methyl-2-oxazolidone (MOZ). This use of supercharging reagents could apply broadly to LC-MS mobile phases containing TFA, and especially in protein analysis and peptide mapping. The success of two new agents predicted to supercharge based on pKBH+ < −1.7 confirms that the supercharging mechanism for increased positive ion charging requires low volatility, Brønsted bases weaker than H2O.
A mechanism for the suppression rescue is described which exploits the agents’ capacity to reduce solution phase ionization, hence decreasing trifluoroacetate anion concentrations in the droplet. The mechanism also addresses why signal improvement is not uniform across analytes.
1. Introduction
Over the past few decades, electrospray ionization mass spectrometry (ESI-MS) has become the premier analytical platform for the MS analysis of proteins and peptides. The hallmark of ESI-MS for proteins and other large biomolecules, multiple charging, extends the effective mass range of the analyzer in direct proportion to the number of charges per ion. Furthermore, the detection of multiply charged molecules at lower mass-to-charge (m/z) ratios, is more efficient than detecting equal-weight, singly charged ions. Compared to their lower-charged counterparts, higher charged proteins and peptides dissociate more efficiently, providing higher sequence coverage in tandem MS (MS/MS).
The term supercharging was first introduced by Williams and colleagues to describe the increased charging observed in the presence of m-nitrobenzyl alcohol (m-NBA), glycerol, ethylene glycol, and methoxyethanol. Of the four liquids, m-NBA was by far most notable for the extent by which it increased charge when added at levels below 5% [1]. Over time, the supercharging definition has become cloudy; in some articles it appears to apply to any observable increase in charge state, while in other articles it is reserved for charge-increases associated with addition of particular liquids and/or solids to the spray solvent. Inconsistencies exist even with the latter, narrower definition, because several additives are considered to be supercharging by some investigators, but not by others [2]. The disarray complicates attempts to discuss mechanisms. The best we can hope is to be clear about our meaning. We apply “supercharging” to describe analytes’ increased positive or negative ion charging when associated with limited addition of certain agents to native or denaturing solutions, but, for native ESI, only when that increase is not accompanied by classic signatures of unfolding (e.g., evidence of bimodal charge state distributions). Often the best evidence that a reagent increases charge states by a mechanism other than protein denaturation (unfolding) is its behavior with non-protein analytes (e.g., short peptides, oligosaccharides, or oligonucleotides).
After introducing the term, Williams and colleagues added m-chlorophenol, formamide, and dimethyl sulfoxide (DMSO) to their list of agents increasing charge [3]. We expanded the list with m-nitroacetophenone, m-nitrobenzonitrile, m-nitrophenethyl alcohol, sulfolane, m-trifluoromethyl-benzyl alcohol and others [2,4]. Investigating supercharging agents in native MS, we found that adding m-NBA or sulfolane to aqueous solutions can elevate charging, without dissociating noncovalent protein complexes [2,4,5]. Many other reports have detailed the application of ESI supercharging to studying noncovalent complex structure and protein conformation or have described new supercharging agents [6-17]. The benefits of supercharging for tandem MS of peptides and proteins, especially with electron capture dissociation (ECD) and electron transfer dissociation (ETD), have been demonstrated [8,18-21].
Coupling liquid chromatography with MS, LC/MS provides rapid, high-resolution separation and identification of analyte molecules from complex mixtures. Reversed-phase liquid chromatography (RPLC), relying largely upon the hydrophobic characters of sorbent, analyte, and organic solvent content, as well as the presence of various mobile phase additives, has become the standard platform for peptide analysis and bottom-up LC/MS proteomics.
Although the selection of buffer and pH can benefit separation efficiency by influencing the charge on polypeptide side chains, it may adversely affect the extent of adsorption to residual silanols (Si-OH) of the stationary phase. At pH ≥ 4, Si-OH is deprotonated and attracts cationic species passing through the column. This binding causes the solute plug containing polypeptide(s) of interest to broaden, resulting in wide peaks and poor selectivity. In such cases, additives such as trifluoroacetic acid (TFA) are added to the mobile phase to act as ion-pairing agents, shielding cationic species from interaction with negatively charged silanols to maintain ‘pseudoneutrality’ [22,23]. Ion pairing minimizes solute band broadening to narrow chromatographic peaks, i.e., substantially increasing theoretical plates and providing greater selectivity of analytes in complex mixtures.
Since the earliest applications of LC/MS to ESI, difficulty spraying aqueous TFA solutions has been recognized [24]. Spray instability and reduced analyte signal in the presence of TFA have generally been attributed to its high conductivity and surface tension. In pure, unassisted electrospray mode both high conductivity and high surface tension require voltages for spray onset that are close to those for corona discharge, resulting in either an unstable spray or reduced signal [25,26]. Furthermore, TFA anions can also pair with basic amino acid side chains, suppressing their ionization in the positive mode [26,27].
Attempts to overcome TFA signal suppression include employing post-column additives [24,26], reducing spray flow-rate or voltage [28,29], electrophoretically reducing the trifluoroacetate anion concentration post-column [30], and pneumatically- or ultrasonically-assisting the electrospray [22,31]. However, many of these modifications can compromise analytical performance, offsetting gains in sensitivity. Formic acid (FA) remains the preferred additive for LC/MS analysis, despite inferior chromatographic performance as compared to TFA. Its pKa is lower than acetic acid (3.75 vs. 4.67), such that at 0.1% (v/v) it yields a lower pH and a greater degree of protonation in solution. Formic acid is sometimes preferred over acetic acid simply because its odor is less objectionable.
For ESI-LC-MS/MS of tryptic peptides and phosphopeptides, benefit was found in adding the supercharging agent m-NBA to formic acid-containing mobile phases; e.g., 0.5% (v/v) m-NBA increased the average peptide charge state from +2.0 to +2.89 and the ETD-MS/MS fragmentation efficiency from 29% to 90% [19]. Li, et al. [21] demonstrated that 0.1% m-NBA improved both ETD-MS/MS and quantitation, while with acidic and high mass glycopeptides, Lin, et al. [32] observed improved sensitivity, charging, and chromatographic resolution from 0.5% m-NBA. Other labs noted m-NBA-increased ion signals, too [33]. MS/MS analyses of negatively charged glycosaminoglycans separated by hydrophilic interaction chromatography (HILIC) were improved by sulfolane addition, post-column [34]. Sharp’s laboratory [21] reported that the quality of peptide reversed phase LC separations was maintained with 0.1% m-NBA addition, but Meyer and Komives [33] found it to broaden peaks.
DMSO inclusion (5%) within the 0.5% FA mobile phase was reported to provide higher peak capacity for intact protein LC-MS [35]. LC-MS/MS benefits were also observed when 5% DMSO supplemented 0.2% FA mobile phase, increasing the number of peptides in a five-protein digest (trypsin, pepsin, or elastase) that were identified with an LTQ ion trap by 10-20%. Komives and colleagues attributed that increase to signals from higher charge states “coalescing” with that from the 2+; i.e., charge reductions that concentrate ion signals into a narrower range of charge states [33]. Substantially increased peptide signals in 5% DMSO were also observed by Kuster and colleagues [36], seen with 3% DMSO [37] and also with 1% [38]. Low-abundance peptides benefited more from the addition of DMSO than high-abundance peptides [36,38]. The performance enhancement also improved identification of phosphoryl- and acetyl-modifications [36]. Hahne et al. determined that coalescence (narrowed charge state distributions) explained only 10%–20% of the sensitivity gained with DMSO, attributing the rest to the higher boiling point and lower surface tension of DMSO, which would accelerate droplet fission compared to H2O. Sequestering peptides more rapidly into charged droplets was proposed to increase ionization efficiency [36]. The observed charge reduction was attributed to the high gas-phase basicity of DMSO (charge stripping). This explanation is reasonable, given that the proton affinity of DMSO is higher than that of H2O, but two points meriting consideration are that: (1) water’s proton affinity increases markedly with increasing cluster size, such that DMSO’s proton affinity is approximately equal to that of (H2O)3; larger water clusters have higher affinities [39]. (2) The extent of proton transfer within the ion source is controlled by kinetics, thus it depends on the density of gas phase DMSO. Whether the low volatility liquid’s evaporation rate is sufficiently fast to maintain the densities needed to account for all of the charge state reduction is an open question, and an alternative to ponder is that changes in solvent composition simply cause charges to be allocated differently when transitioning from droplets to gas phase ions [2].
In collaboration with the Chen group, the effects of supercharging agents in liquid sample desorption electrospray ionization (L-DESI) coupled with LC-MS were tested [40]. Proteins eluting in from the chromatographic column in TFA-containing mobile phase were ionized via an L-DESI spray probe incorporating solutions supplemented with m-NBA or sulfolane. Compared to standard L-DESI analyses, the addition of 50 mM m-NBA to the L-DESI spray shifted the maximum charge state of 14 kDa lysozyme from +12 to +17, with ~50-fold increased intensity [40]. Interestingly, supercharging combined with L-DESI revealed improved tolerance towards the TFA ion-pairing agent. Xu, et al. also found benefit by delivering m-NBA to TFA-containing mobile phases, in their case for monoclonal antibody analyses performed by size exclusion and reversed phase chromatography [41]. Delivering m-NBA post-column facilitated their characterization of low-level size variants. MS signals of antibody light and heavy chains were improved 7- and 2-fold, respectively.
The present study continues our investigations, examining the effects of supercharging agents on intact protein LC/MS with mobile phases containing either 0.1% TFA or 0.1% FA. In addition, two new agents are tested for their abilities to increase ESI charge states, to rescue ion signals from TFA-induced ion suppression, and to maintain superior chromatographic peak shapes and plate numbers. Finally, we explore the mechanism by which signal suppression is reduced, why the reduction is not uniform across analytes.
2. Materials and methods
2.1 Materials
Proteins bovine serum albumin (BSA; 66.3 kDa), lysozyme (14.3 kDa), myoglobin (16.9 kDa for apo-form), ubiquitin (8.6 kDa), ribonuclease A (RNase A; 13.7 kDa), and carbonic anhydrase (29 kDa), as well as supercharging agents m-nitrobenzyl alcohol (m-NBA, CAS 619-25-0), sulfolane (CAS 126-33-0), 3-nitrophenethyl alcohol (NPEA, CAS 52022-77-2), N,N,N′N′-tetraethylsulfamide (TES, CAS 2832-49-7), 3-methyl-2-oxazolidone (MOZ, CAS 19836-78-3), propylene carbonate (PC, CAS 108-32-7), and dimethyl sulfoxide (DMSO, CAS 67-68-5) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Protein mixtures were prepared in deionized water.
2.2 LC/MS of proteins
10 μL of the protein mixtures were injected onto an Agilent Zorbax C3 column (2.1 mm × 150 mm, 300 Å). LC/MS analysis was performed using an Agilent 1200 HPLC connected on-line with an ESI time-of-flight (TOF) mass spectrometer (Agilent 6220 ESI-TOF, Santa Clara, CA). A resolving power of 10,000 at m/z 1000 is routinely achieved on this instrument and the m/z range can be extended to 3200. All spectra were acquired in positive (+ESI) mode.
Solvent A was either 0.1% TFA or 0.1% FA in water. Solvent B was 90% acetonitrile (ACN) containing either 0.1% TFA or 0.1% FA and water. The flow was set to 200 μl/min. For intact protein analysis the following gradient was used: 5% B for 3 min, 5-25% B in 1 min, held at 25% for 6 min, 25-30% in 5 min, 30-45% in 19 min, 45-80% in 6 min, held at 80% for 5 min, returned to 5% B in 1 min, and equilibrated at 5% B for 9 min prior to next run (Figure S6).
For the analysis of a BSA tryptic digest, an Agilent Zorbax C18 column (2.1 mm × 150 mm, 300 Å) was used. The gradient employed was: 5% B for 3 min, 5-25% B in 1 min, held at 25% for 6 min, 25-30% in 5 min, from 30% to 80% in 3 min, held at 80% for 2 min, returned to 5% B in 1 min and equilibrated at 5% B for 9 min prior to next run. Supercharging agents m-NBA (0.1%, v/v), 3-NPEA (0.1%, v/v), TES, (0.1% v/v), 3-MOZ (0.1%, v/v), PC (0.1%, v/v), and DMSO (5%, v/v) were added to both aqueous and organic components of the mobile phase containing either 0.1% TFA or 0.1% FA.
An important consideration when spraying non-volatile liquids is their ability to foul the mass spectrometer. Investigators have elevated ion source temperatures to 150-275°C to reduce build-up of DMSO [33,37,42]. Additives have also been delivered post-column [41], and released only when needed for specific eluates [34].
3. Results and discussion
3.1 Supercharging agents’ effects on LC/MS signal intensities
It has been proposed that effective positive ion supercharging reagents must (1) be soluble, (2) interact with analytes, (3) be very weak Brønsted bases (pKBH+w ≤ −1.7) and (4) be similarly or less volatile than the bulk solvent to enable their concentration within the evaporating electrospray droplet [2]. For non-denaturing solutions, the reagent must shift protein charge state distributions when present at concentrations below those inducing conformational changes (in contrast to others [43-45], we distinguish charge increases driven by protein denaturation from those induced by a unique supercharging mechanism). Similarly, we predicted that very weak Brønsted acids (pKa > 15.7) would be effective negative ion supercharging reagents. These criteria enable reagents capable of supercharging to be predicted, and provide a means to test this theory [2].
Note that the mechanism we propose is described incorrectly by some publications [16,44] as gas phase “proton transfer between the protein and the reagents.” In our mechanism, the presence of a weakly basic (solution phase) reagent causes the ESI process to deposit less excess charge onto solvent, thus leaving more for analyte. This mechanism relies heavily on solution phase properties. For involatile supercharging agents, gas phase proton transfer effects are expected to be less important, simply because of the reagents’ low gas phase density.
The aprotic solvent sulfolane [4], one example of a dual-polarity supercharger, is consistent with the criteria above, being both a weak Brønsted base (pKBH+w = −12.9) and a weak Brønsted acid (pKa > 31). Molecules with demonstrated positive ion supercharging capacity: m-NBA [1], non-ionic saccharide detergents (i.e., octyl glucoside) [46], o-NBA, p-NBA, 3-nitrophenethyl alcohol, 3-nitroacetophenone, 3-nitrobenzonitrile, 3-trifluoromethylbenzene methanol [4], benzyl alcohol [4,15], 3-chlorothiete-1,1-dioxide, sulfolene [15], dimethyl sulfone, o-nitroanisole, p-nitroanisole [17], nitrobenzene [2,17,47], propylene carbonate [2,17,47], ethylene carbonate, butylene carbonate, 4-vinyl-1,3-dioxalan-2-one (vinyl-ethylene carbonate), 1,4-butanesultone and 1,3-propanesultone [17], are all weaker bases than H2O [2].
Supercharging agents at equal volume percentages were added to both solvent A and solvent B of the mobile phase. Agents reported to enhance protein charging (m-NBA, sulfolane, NPEA, or PC), were added, or one of two novel reagents selected for very weak Brønsted basicity and low volatility: N,N,N′N′-tetraethylsulfamide (TES) or 3-methyl-2-oxazolidone (MOZ). MOZ is an aprotic solvent, miscible with water and possessing a dielectric constant of 77.5 [48]. It is expected to be a weak base, given that carbamate basicities are intermediate between esters and amides [49]. TES, with dielectric constant = 29, is also expected to be less basic than water, because sulfonamides are even weaker bases than sulfones [50].
DMSO, a reagent widely reported [33,36-38,51-53] to reduce charging in positive ion mode (sub-charge) when added to peptide solutions and when added at low levels to protein solutions, is confusingly sometimes called supercharging [3,35,44], because larger additions to certain native-like protein solutions increased charging by denaturation. We also examined addition of DMSO to TFA-containing mobile phases, because it has consistently shown improved peptide LC-MS/MS performance for FA-containing mobile phases [33,36-38].
We observed modest increases in the average charge borne by small proteins eluting from 100 mM sulfolane mobile phases; e.g., increasing from +9.6 to +14.4 for RNase A (data not shown). However, larger proteins such as BSA displayed overabundant protein-sulfolane adducts that persisted even with lower sulfolane concentrations (down to 20 mM). In addition, chromatographic performance was compromised greatly, yielding irreproducible retention times and apparently permanent changes to the column surface matrix. Therefore, sulfolane was not tested further.
Consistent with our previous L-DESI-LC/MS study [40], adding other supercharging agents to TFA-containing HPLC mobile phases was seen to increase protein ion signals while maintaining chromatographic performance. A mixture containing intact proteins RNase A, ubiquitin, lysozyme, myoglobin, BSA, and carbonic anhydrase was analyzed. Upon addition of 0.1% m-NBA or 0.1% 3-NPEA to the mobile phase containing 0.1% TFA, the total ion current increased from 5- to 53-fold for BSA and RNase A, and 2- and 19-fold for BSA and lysozyme, respectively, relative to no addition of supercharging agents (Figure 1a, Table 1). Similar signal enhancements were observed in the presence of 0.1% TES and 0.1% MOZ (Figure 1b, Table 1). Addition of PC also increased signal (Table 1). Overall, the strongest signal enhancement from TFA mobile phases was observed with 0.1% m-NBA, although RNase A signals were increased more with TES or MOZ. Under our conditions, addition of 5% DMSO suppressed the protein signals (data not shown).
Figure 1.
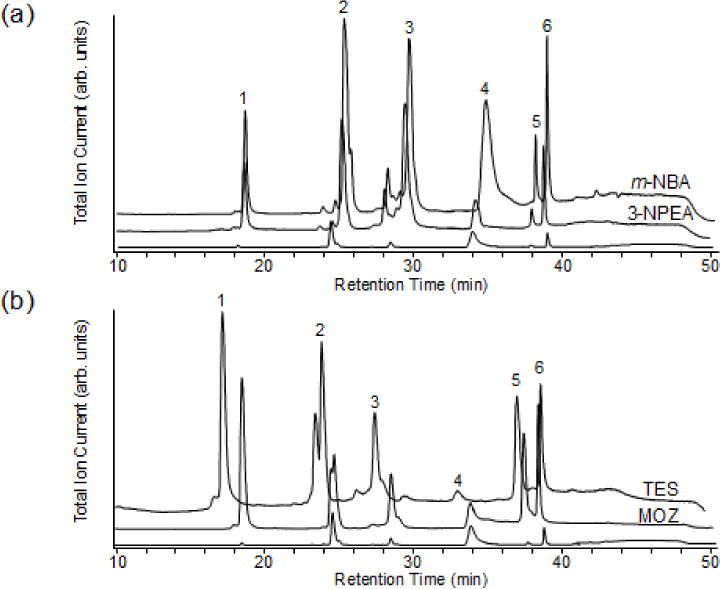
LC/MS of protein mixture containing (1) 17 μM RNase A, (2) 13 μM ubiquitin, (3) 7.7 μM lysozyme, (4) 15 μM BSA, (5) 6.5 μM myoglobin, and (6) 7.6 μM carbonic anhydrase with 0.1% TFA and (a) 0.1% m-NBA and 0.1% 3-NPEA, and (b) 0.1% MOZ and 0.1% TES. No supercharging agent was added to the measurements represented by the bottom trace in both (a) and (b).
Table 1.
Signal enhancement (fold increase) with addition of supercharging agents to 0.1% TFA ion pairing agent
| Protein | + m-NBA | + 3-NPEA | + TES | + MOZ | + PC |
|---|---|---|---|---|---|
| RNase A | 53 | 13 | 71 | 65 | 19 |
| Ubiquitin | 14 | 5 | 6 | 3 | 3 |
| Lysozyme | 39 | 19 | 17 | 11 | 5 |
| BSA | 5 | 2 | 2 | 2 | 4 |
| Myoglobin | 38 | 7 | 32 | 34 | 26 |
| Carbonic Anhydrase | 15 | 4 | 8 | 10 | 9 |
For mobile phases employing formic acid to ion pair with analytes, adding 0.1% m-NBA or 0.1% 3-NPEA was seen to increase the total ion current by 2 to 6-fold for smaller proteins (RNase A, ubiquitin, lysozyme) and 1.5 to 2-fold for BSA and carbonic anhydrase (Figures 2, S7). Addition of TES and MOZ increased ion intensities weakly; e.g., ubiquitin and lysozyme signals were approximately 1.3 times as intense in the presence of MOZ (Supplemental Figures S1, S7). PC, however, increased ubiquitin and insulin signals about 3- and 8-fold, respectively (Supplemental Figures S2, S7). The addition of 5% DMSO to the FA mobile phase did not suppress signals, counter to what we observed with TFA (data not shown).
Figure 2.
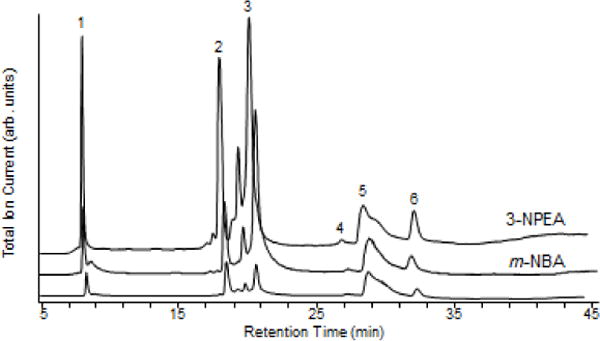
LC/MS of protein mixture containing (1) 17 μM RNase A, (2) 13 μM ubiquitin, (3) 7.7 μM lysozyme, (4) 15 μM BSA, (5) 6.5 μM myoglobin, and (6) 7.6 μM carbonic anhydrase with 0.1% formic acid and 0.1% m-NBA and 0.1% 3-NPEA. No supercharging agent was added to the measurements represented by the bottom trace.
To summarize, the 5 supercharging additives increased ion signals strongly from 0.1% TFA-containing mobile phases and less so (or not at all) from 0.1% FA phases. The smaller improvement seen with FA ion pairing mobile phases is perhaps not surprising, given that ion suppression is so severe in TFA solutions. DMSO was seen to decrease intact protein signals from TFA-containing mobile phases, while improving signals from FA mobile phases.
3.2 Supercharging agents’ effects on protein charge state distributions
In addition to increasing ion intensities, supercharging agents also increased average charge states of proteins separated by LC/MS. For example, upon addition of 0.1% m-NBA to the mobile phase containing TFA, there was a considerable increase in the charge state of lysozyme compared to the control (Figure 3, Table 2); the weighted average charge state increased from +6.1 to +11.4. Similar trends with respect to charge state distribution were observed for the other proteins in the mixture, e.g., the average charge states for RNase A and ubiquitin increased from +6.2 to +11.6 and +7.1 to +8.3 in the presence of m-NBA (Table 2). Enhanced protein charging was observed for the other supercharging agents investigated and also in the presence of FA modifier in the mobile phase (Figure 4, Table 2). The newly identified supercharging agents, TES and MOZ, performed about as well as the other agents. For instance, in the presence of TES, the average charge state of lysozyme increased from +6.1 to +10.6 (Figure 3), while the average charge state of RNase A increased from +6.2 to +9.7. Similarly, in the presence of MOZ, the average charge state of lysozyme increased from +6.1 to +10.3 (Table 2). Some caution is warranted when comparing the charge increases delivered by different supercharging agents under LC/MS conditions, because solvent compositions differ and retention times shift. In principle, composition changes can alter protein conformer populations. Nevertheless, the data consistently indicate that all four reagents deliver higher charge states.
Figure 3.
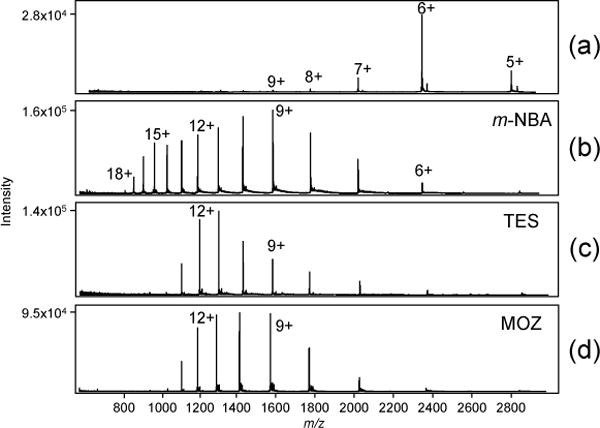
ESI-MS spectra of 7.7 µM lysozyme in 0.1% TFA (a) no supercharging agent, (b) with 0.1% m-NBA, (c) with 0.1% TES, and (d) 0.1% MOZ.
Table 2.
Average protein charge state with 0.1% TFA or 0.1% FA ion-pairing agent. n/a=not available.
| Protein | TFA | + m-NBA | + 3-NPEA | + TES | + MOZ |
|---|---|---|---|---|---|
| Lysozyme | 6.1 | 11.4 | 13.5 | 10.6 | 10.3 |
| RNase A | 6.2 | 11.6 | n/a | 9.7 | n/a |
| Protein | FA | + m-NBA | + 3-NPEA | + TES | + MOZ |
| Lysozyme | 9.7 | 12.9 | 12.5 | 12.0 | 12.6 |
| RNase A | 11.0 | 13.7 | 12.7 | n/a | 12.7 |
Figure 4.
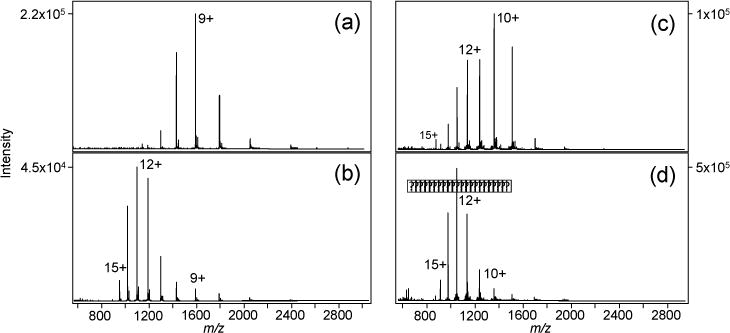
ESI-MS spectra of 7.7 µM lysozyme in 0.1% formic acid and (a) no MOZ, and (b) with 0.1% MOZ. ESI-MS spectra of 17 µM RNase A in 0.1% formic acid and (c) no MOZ, and (d) with 0.1% MOZ.
3.3 Supercharging agents’ effects on efficiency and limit of detection (LOD)
Most additives changed protein retention times slightly. For 0.1% TFA, retention times increased by about 0.5 and 0.25 min in the presence of m-NBA and 3-NPEA, respectively, while they decreased by about 0.5 and 0.5 – 1 min with TES and PC, respectively. MOZ did not change retention times significantly.
In 0.1% FA, retention times were only affected significantly by PC and then only the later eluting proteins myoglobin, BSA and carbonic anhydrase (Supplemental Figures S2 and S5).
In general, the supercharging agents tested had positive effects on chromatographic efficiency or plate number (N). Plate number (N) was calculated using the retention time (tR) and peak width at half height (W½) according to:
| (1) |
For TFA, efficiency improved for 3 - 4 of the 6 proteins in the mixture, depending on the supercharging additive. With 0.1% m-NBA, plate numbers more than doubled for RNase A and myoglobin, while 0.1% 3-NPEA addition improved myoglobin plate numbers almost 4-fold (Figure 5).
Figure 5.
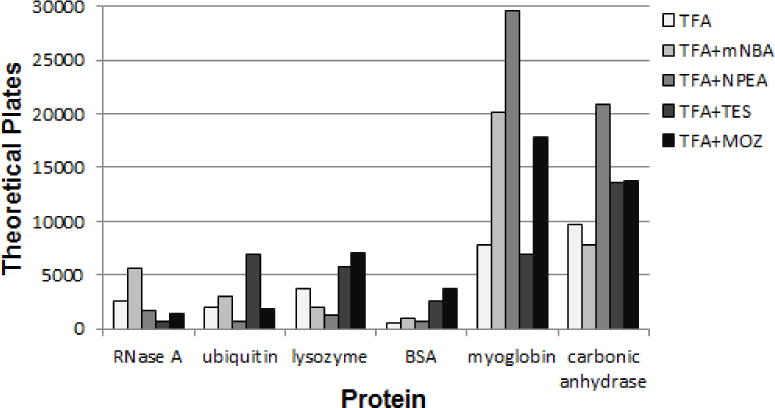
Number of theoretical plates for LC/MS of proteins with 0.1% TFA and supercharging agents.
With formic acid and 5% DMSO, the plate number for RNase A increased 4.5-fold, while that for carbonic anhydrase increased approximately 3.3-fold under the same conditions (Supplemental Figure S3).
With respect to TFA signal suppression and its impact on limits of detection (LOD), we observed that 0.1% m-NBA significantly rescued signals for 3 of the 6 proteins in the mixture (Table 3). LOD was determined from the lowest concentration that delivered a signal-to-noise ratio (S/N) ratio of 3:1. For example, RNase A showed a 5-fold decrease in LOD, while that for ubiquitin and lysozyme was decreased 3.3-fold. Therefore, by adding m-NBA to TFA-containing mobile phases, lower concentrations were detected for approximately half of the proteins in the mixture, while LODs for the other proteins were unchanged.
Table 3.
Limit of detection (M) with 0.1% TFA ion-pairing agent with and without m-NBA
| Protein | TFA | TFA + m-NBA | Fold Increase w/m-NBA |
|---|---|---|---|
| RNase A | 8.4 × 10−7 | 1.7 × 10−7 | 5.0 |
| Ubiquitin | 4.3 × 10−6 | 1.3 × 10−6 | 3.3 |
| Lysozyme | 2.6 × 10−6 | 7.7 × 10−7 | 3.3 |
| Myoglobin | 6.5 × 10−7 | 6.5 × 10−7 | No change |
| BSA | 1.5 × 10−5 | 1.5 × 10−5 | No change |
| Carbonic anhydrase | 2.5 × 10−7 | 2.5 × 10−7 | No change |
These effects in TFA contrasted with those in FA-containing mobile phases. Protein sensitivity was reduced by adding m-NBA to 0.1% FA eluent. Moreover, while 5% DMSO suppressed protein signal in 0.1% TFA, it significantly increased signals in 0.1% FA for lysozyme and carbonic anhydrase (data not shown).
3.4 LC-MS of a tryptic peptide mixture with supercharging agents
Peptide mapping is an important analytical tool used to test recombinant therapeutic protein lots prior to release for clinical trials [54,55]. It establishes protein identity by confirming primary structure or amino acid sequence lot-to-lot. Peptide mapping has also been used to establish the genetic stability of product-producing organisms throughout product life cycle [56]. Peptide mapping supports the monitoring of protein oxidation or deamidation, which may affect therapeutic function [57]. Consequently, methods to improve MS sensitivity for high resolution LC separations of peptides can be valuable.
As with intact protein mixtures, increased ion current was observed for the LC/MS analysis of BSA tryptic peptides using TFA as the ion pairing agent when either 0.1% m-NBA (Supplemental Figure S4) or 0.1% 3-NPEA (data not shown) was present. Relative intensities of the higher charge states also increased with either agent. For example, the average charge state of peptide TVMENFVAFVDK increased from +1.5 to +2.3 with m-NBA and to +2.5 with 3-NPEA. Increasing peptide charging aids MS/MS fragmentation for bottom-up proteomics strategies, especially with ETD sequencing methods [19].
3.5 Mechanism by which supercharging agents reverse TFA ion suppression
We, and others, have reported that m-NBA or sulfolane addition [32,40,41] can increase analyte ion signals in LC/MS with TFA. We attributed that increase to a related property of supercharging additives, and aprotic solvents in general to suppress ionization in solution [2]. By reducing the extent to which TFA ionizes within ESI droplets [60], these additives decrease trifluoroacetate anion concentrations and their accompanying analyte ion suppression. The agents concentrate within the droplet due to their low volatility, yet impact separation quality minimally, due to their almost negligible concentration in the bulk eluent. The success of strategies reducing anion concentrations by other means supports our mechanism [30,61].
The extent of improvement provided by supercharging agents is protein-dependent, generally following the order from most improved to least improved as RNase A, myoglobin, lysozyme, carbonic anhydrase, ubiquitin, and bovine albumin. The protein isoelectric points are 9.6, 6.97, 11.35, 5.9, 6.79, and 4.7, respectively. Clearly, basic proteins benefit more than acidic proteins. This behavior may reflect that TFA suppresses the signals of basic proteins (capable of much more trifluoroacetate anion binding) more strongly than acidic proteins. Reducing trifluoroacetate anion concentrations would then appear more beneficial to basic proteins.
Conclusion
Signal suppression caused by ion pairing agent TFA can be circumvented in LC-ESI-MS by including a modest amount of supercharging agent in the mobile phase. For the proteins investigated, ESI-MS signal intensities increased by up to 70-fold with 0.1% m-NBA, 3-NPEA, TES, MOZ, or PC, delivering lower detection limits. Moreover, the chromatographic plate numbers for some proteins increased significantly when 0.1% m-NBA supplemented the TFA-containing mobile phases. Signals were also increased from FA-containing mobile phases, albeit not as dramatically as from TFA. The average charge states of peptides and proteins increased in the presence of these additives, consistent with the known behavior of m-NBA, 3-NPEA, and PC. In addition, additives TES and MOZ also increased peptide and protein charge states, confirming that molecular candidates that supercharge peptides and proteins can be predicted based on their low volatility and very weak Brønsted basicity.
This strategy to overcome TFA-induced ion suppression succeeds because during chromatography the low supercharging agent concentrations do not seriously perturb the separations. During ESI, however, aconcentrate within the droplet because of their ultra-low evaporation rate. By suppressing TFA dissociation, the weak bases reduce trifluoroacetate anions and the signal suppression they cause. In principal, other solvents or solvent additives that reduce TFA dissociation may rescue suppressed ion signals, even if they do not increase charging in positive ion mode.
Supercharging reduces m/z requirements for the mass analyzer, improves mass resolution and mass accuracy, and for MS/MS, enhances fragmentation and sequence coverage. For reversed phase HPLC, TFA is a standard ion pairing agent for achieving high quality separations, yet it suppresses ESI-MS signals when coupled for mass detection. Supercharging agents can rescue that TFA-based ion suppression, thereby offering higher resolution LC/MS for protein measurements. Adding supercharging agents to the LC methods for separating intact proteins destined for interrogation by multiple reaction monitoring (MRM) is one area poised to benefit from increased sensitivity, especially when the intact protein approach is compared to traditional bottom-up methods [62,63]. Supercharging agent addition may provide an alternative to mixing small amounts of TFA and FA in balancing improved chromatographic efficiency with MS sensitivity [62].
Supplementary Material
Highlights.
New supercharging agents, tetraethylsulfamide and 2-methyl-2-oxazoline, identified
Using supercharging agents rescues LC/MS TFA-induced ion suppression
LC/MS protein sensitivity and LC resolution is improved with supercharging agents
Acknowledgments
Support from the US National Institutes of Health (R01GM103479 to J.A.L.) and the US Department of Energy (UCLA Institute of Genomics and Proteomics; DE-FC03-02ER63421) are acknowledged. We thank visiting student Daphney Sihwa (Spelman College, Atlanta, GA) for help with the peptide mapping studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iavarone AT, Jurchen JC, Williams ER. Supercharged Protein and Peptide Ions Formed by Electrospray Ionization. Anal Chem. 2001;73:1455–1460. doi: 10.1021/ac001251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogorzalek Loo RR, Lakshmanan R, Loo JA. What Protein Charging (and Supercharging) Reveal about the Mechanism of Electrospray Ionization. J Am Soc Mass Spectrom. 2014;25:1675–1693. doi: 10.1007/s13361-014-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iavarone AT, Williams ER. Mechanism of Charging and Supercharging Molecules in Electrospray Ionization. J Am Chem Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomeli SH, Peng IX, Yin S, Ogorzalek Loo RR, Loo JA. New Reagents for Increasing ESI Multiple Charging of Proteins and Protein Complexes. J Am Soc Mass Spectrom. 2010;21:127–131. doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA. Increasing Charge While Preserving Noncovalent Protein Complexes for ESI-MS. J Am Soc Mass Spectrom. 2009;20:593–596. doi: 10.1016/j.jasms.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Duijn E. Current Limitations in Native Mass Spectrometry Based Structural Biology. J Am Soc Mass Spectrom. 2010;21:971–978. doi: 10.1016/j.jasms.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Enyenihi AA, Yang H, Ytterberg AJ, Lyutvinskiy Y, Zubarev RA. Heme Binding in Gas-Phase Holo-Myoglobin Cations: Distal Becomes Proximal? J Am Soc Mass Spectrom. 2011;22:1763–1770. doi: 10.1007/s13361-011-0182-0. [DOI] [PubMed] [Google Scholar]
- 8.Yin S, Loo JA. Top-Down Mass Spectrometry of Supercharged Native Protein–Ligand Complexes. Int J Mass Spectrom. 2011;300:118–122. doi: 10.1016/j.ijms.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson CN, Benchaar SA, Miao Z, Loo JA, Chen H. Direct Ionization of Large Proteins and Protein Complexes by Desorption Electrospray Ionization-Mass Spectrometry. Analytical Chemistry. 2011;83:6468–6473. doi: 10.1021/ac201390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. Charge-State Dependent Compaction and Dissociation of Protein Complexes: Insights from Ion Mobility and Molecular Dynamics. J Am Chem Soc. 2012;34:3429–3438. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M, Dagana S, Wysocki VH. Impact of Charge State on Gas-Phase Behaviors of Noncovalent Protein Complexes in Collision Induced Dissociation and Surface Induced Dissociation. Analyst. 2013;138:1353–1362. doi: 10.1039/c2an36525a. [DOI] [PubMed] [Google Scholar]
- 12.Loo JA, Benchaar SA, Zhang J. Integrating Native Mass Spectrometry and Top-Down MS for Defining Protein Interactions Important in Biology and Medicine. Mass Spectrom. 2013;2:S0013. doi: 10.5702/massspectrometry.S0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lermyte F, Konijnenberg A, Williams JP, Brown JM, Valkenborg D, Sobott F. ETD allows for native surface mapping of a 150 kDa noncovalent complex on a commercial Q-TWIMS-TOF instrument. J Am Soc Mass Spectrom. 2014;25:343–350. doi: 10.1007/s13361-013-0798-3. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Richards MR, Kitova EN, Klassen JS. Influence of Sulfolane on ESI-MS Measurements of Protein-Ligand Affinities. J Am Soc Mass Spectrom. 2016;27:498–506. doi: 10.1007/s13361-015-1312-x. [DOI] [PubMed] [Google Scholar]
- 15.Douglass KA, Venter AR. Investigating the Role of Adducts in Protein Supercharging with Sulfolane. J Am Soc Mass Spectrom. 2012;23:489–497. doi: 10.1007/s13361-011-0319-1. [DOI] [PubMed] [Google Scholar]
- 16.Going CC, Xia A, Williams ER. New Supercharging Reagents Produce Highly Charged Protein Ions in Native Mass Spectrometry. Analyst. 2015;140:7184–7194. doi: 10.1039/c5an01710f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo AC, Donald WA. Solution Additives for Supercharging Proteins beyond the Theoretical Maximum Proton-Transfer Limit in Electrospray Ionization Mass Spectrometry. Anal Chem. 2014;85:4455–4462. doi: 10.1021/ac500304r. [DOI] [PubMed] [Google Scholar]
- 18.Iavarone AT, Paech K, Williams ER. Effects of Charge State and Cationizing Agent on the Electron Capture Dissociation of a Peptide. Anal Chem. 2004;76:2231–2238. doi: 10.1021/ac035431p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen F, Giessing AMB, Ingrell CR, Jensen ON. Peptide Sequencing and Characterization of Post-Translational Modifications by Enhanced Ion-Charging and Liquid Chromatography Electron-Transfer Dissociation Tandem Mass Spectrometry. Anal Chem. 2007;79:9243–9252. doi: 10.1021/ac701700g. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Ogorzalek Loo RR, Loo JA. Increasing Fragmentation of Disulfide-Bonded Proteins for Top-Down Mass Spectrometry by Supercharging. Int J Mass spectrom. 2015;377:546–556. doi: 10.1016/j.ijms.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Li Z, X B, Sharp JS. Supercharging by m-NBA Improves ETD-Based quantification of Hydroxyl Radical Protein Footprinting. J Am Soc Mass Spectrom. 2015;26:1424–1427. doi: 10.1007/s13361-015-1129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhlmann FE, Apffel A, Fischer SM, Goldberg G, Goodley PC. Signal Enhancement for Gradient Reverse-Phase High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry Analysis with Trifluoroacetic and other Strong Acid Modifiers by Postcolumn Addition of Propionic Acid and Isopropanol. J Am Soc Mass Spectrom. 1995;6:1221–1225. doi: 10.1016/1044-0305(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 23.Snyder LR, Kirkland JJ, Glajch JL. Practical HPLC Method Development. 2. John Wiley & Sons; Hoboken, NJ: 1997. [Google Scholar]
- 24.Eshraghi J, Chowdhury SK. Factors Affecting Electrospray Ionization of Effluents Containing Trifluoroacetic Acid for High-Performance Liquid Chromatography/Mass Spectrometry. Analytical Chemistry. 1993;65(23):3528–3533. doi: 10.1021/ac00071a035. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury SK, Chait BT. Method for the Electrospray Ionization of Highly Conductive Aqueous Solutions. Anal Chem. 1991;63:1660–1664. doi: 10.1021/ac00015a030. [DOI] [PubMed] [Google Scholar]
- 26.Apffel A, Fischer S, Goldberg G, Goodley PC, Kuhlmann FE. Enhanced Sensitivity for Peptide Mapping with Electrospray Liquid Chromatography-Mass Spectrometry in the Presence of Signal Suppression Due to Trifluoroacetic AcidCcontaining Mobile Phases. J Chromatography A. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- 27.Mirza UA, Chait BT. Effects of anions on the positive ion electrospray ionization mass spectra of peptides and proteins. Anal Chem. 1994;66(18):2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- 28.Griffin PR, Coffman JA, Hood LE, Yates JR., III Structural Analysis of Proteins by Capillary HPLC Electrospray Tandem Mass Spectrometry. International Journal of Mass Spectrometry and Ion Processes. 1991;111:131–149. [Google Scholar]
- 29.Huang EC, Henion JD. Packed-Capillary Liquid Chromatography/Ion-spray Tandem Mass Spectrometry Determination of Biomolecules. Anal Chem. 1991;63:732–739. [Google Scholar]
- 30.Wang NH, Lee WL, Her GR. Signal Enhancement for Peptide Analysis in Liquid Chromatography–Electrospray Ionization Mass Spectrometry with Trifluoroacetic Acid Containing Mobile Phase by Postcolumn Electrophoretic Mobility Control. Anal Chem. 2011;83:6163–6168. doi: 10.1021/ac2003714. [DOI] [PubMed] [Google Scholar]
- 31.Banks JF, Shen S, Whitehouse CM, Fenn JB. Ultrasonically Assisted Electrospray Ionization for LC/MS Determination of Nucleosides from a Transfer RNA Digest. Analytical Chemistry. 1994;66(3):406–414. doi: 10.1021/ac00075a015. [DOI] [PubMed] [Google Scholar]
- 32.Lin C-W, Haeuptle MA, Aebi M. Supercharging Reagent for Enhanced Liquid Chromatographic Separation and Charging of Sialylated and High-Molecular-Weight Glycopeptides for NanoHPLC–ESI-MS/MS Analysis. Anal Chem. 2016;88:8484–8494. doi: 10.1021/acs.analchem.6b00938. [DOI] [PubMed] [Google Scholar]
- 33.Meyer JG, Komives EA. Charge State Consolidation During Electrospray Ionization Improves Peptide Identification by Tandem Mass Spectrometry. J Am Soc Mass Spectrom. 2012;23:1390–1399. doi: 10.1007/s13361-012-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Shi X, Yu X, Leymarie N, Staples GO, Yin H, Killeen K, Zaia J. Improved Liquid Chromatography-MS/MS of Heparan Sulfate Oligosaccharides via Chip-Based Pulsed Makeup Flow. Anal Chem. 2011;83:8222–8229. doi: 10.1021/ac201964n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valeja SG, Tipton JD, Emmett MR, Marshall AG. New Reagents for Enhanced Liquid Chromatographic Separation and Charging of Intact Protein Ions for Electrospray Ionization Mass Spectrometry. Anal Chem. 2010;82:7515–7519. doi: 10.1021/ac1016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahne H, Pachi F, Ruprecht B, Maier SK, Klaeger S, Helm D, Médard G, Wilm M, Lemeer S, Kuster B. DMSO Enhances Electrospray Response, Boosting Sensitivity of Proteomic Experiments. Nature Methods. 2013 doi: 10.1038/nmeth.2610. [DOI] [PubMed] [Google Scholar]
- 37.Strzelecka D, Holman SW, Eyers CE. Evaluation of Dimethyl Sulfoxide (DMSO) as a Mobile Phase Additive During Top 3 Label-Free Quantitative Proteomics. Int J Mass Spectrom. 2015;391:157–160. doi: 10.1016/j.ijms.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judák P, Grainger J, Goebel C, Van Eenoo P, Deventer K. DMSO Assisted Electrospray Ionization for the Detection of Small Peptide Hormones in Urine by Dilute-and-Shoot-Liquid-Chromatography-High Resolution Mass Spectrometry. J Am Soc Mass Spectrom. 2017;28:1657–1665. doi: 10.1007/s13361-017-1670-7. [DOI] [PubMed] [Google Scholar]
- 39.Kawai Y, Yamaguchi S, Okada Y, Takeuchi K, Yamauchi Y, Ozawa S, Nakai H. Reactions of Protonated Water Clusters H+(H2O)n (n = 1–6) with Dimethylsulfoxide in a Guided Ion Beam Apparatus. Chem Phys Lett. 2003;377:69–73. [Google Scholar]
- 40.Liu Y, Miao Z, Lakshmanan R, Ogorzalek Loo RR, Loo JA, Chen H. Signal and Charge Enhancement for Protein Analysis by Liquid Chromatography-Mass Spectrometry with Desorption Electrospray Ionization. Int J Mass Spectrom. 2012;325–327:161–166. doi: 10.1016/j.ijms.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu CF, Zang L, Weiskopf A. Size-Exclusion Chromatography-Mass Spectrometry with m-Nitrobenzyl Alcohol as Post-Column Additive for Direct Characterization of Size Variants of Monoclonal Antibodies. J Chromatogr B. 2014;960:230–238. doi: 10.1016/j.jchromb.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Miladinovic SM, Fornelli L, Lu Y, Piech KM, Girault HH, Tsybin YO. In-Spray Supercharging of Peptides and Proteins in Electrospray Ionization Mass Spectrometry. Anal Chem. 2012;84:4647–4651. doi: 10.1021/ac300845n. [DOI] [PubMed] [Google Scholar]
- 43.Sterling HJ, Kintzer AF, Feld GK, Cassou CA, Krantz BK, Williams ER. Supercharging Protein Complexes from Aqueous Solution Disrupts their Native Conformations. J Am Soc Mass Spectrom. 2011;23:191–200. doi: 10.1007/s13361-011-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterling HJ, Prell JS, Cassou CA, Williams ER. Protein Conformation and Supercharging with DMSO from Aqueous Solution. J Am Soc Mass Spectrom. 2011;22:1178–1186. doi: 10.1007/s13361-011-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterling HJ, Williams ER. Origin of Supercharging in Electrospray Ionization of Non-Covalent Complexes from Aqueous Solution. J Am Soc Mass Spectrom. 2009;20:1933–1943. doi: 10.1016/j.jasms.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogorzalek Loo RR, Dales N, Andrews PC. The Effect of Detergents on Proteins Analyzed by Electrospray Ionization. In: Chapman JR, editor. Methods in Molecular Biology: Protein and Peptide Analysis by Mass Spectrometry. Vol. 61. Humana Press; Totowa, NJ: 1996. pp. 141–160. [DOI] [PubMed] [Google Scholar]
- 47.Loo JA, Lomeli SH, Ogorzalek-Loo RR. Factors that Promote ESI Multiple Charging for Proteins; Paper presented at the 58th ASMS Conference on Mass Spectrometry and Allied Topics; Salt Lake City, Utah. May 23–27, 2010. [Google Scholar]
- 48.Kelley MJ, Heineman WR. Electrochemical Studies in 3-Methyl-2-Oxazolidone. J Electroanal Chem. 1988;248:441–446. [Google Scholar]
- 49.Kurouchi H, Kawamoto K, Sugimoto H, Nakamura S, Otani Y, Ohwada T. Activation of Electrophilicity of Stable Y- Delocalized Carbamate Cations in Intramolecular Aromatic Substitution Reaction: Evidence for Formation of Diprotonated Carbamates Leading to Generation of Isocyanates. J Org Chem. 2012;77:9313–9328. doi: 10.1021/jo3020566. [DOI] [PubMed] [Google Scholar]
- 50.Chardin A, Laurence C, Berthelot M, Morris DG, Hydrogen-Bond Basicity of the Sulfonyl Group The Case of Strongly Basic Sulfonamidates RSO2NNMe3. J Chem Soc Perkin Trans. 1996;2:1047–1051. [Google Scholar]
- 51.Tjernberg A, Markova N, Griffiths WJ, Hallen D. DMSO-Related Effects in Protein Characterization. J Biomol Screening. 2006;11(2):131–137. doi: 10.1177/1087057105284218. [DOI] [PubMed] [Google Scholar]
- 52.Landreh M, Alvelius G, Johansson J, Jörnvall H. Protective Effects of Dimethyl Sulfoxide on Labile Protein Interactions during Electrospray Ionization. Anal Chem. 2014;85:4135–4139. doi: 10.1021/ac500879c. [DOI] [PubMed] [Google Scholar]
- 53.Chan DSH, Matak-Vinković D, Coyne AG, Abell C. Insight into Protein Conformation and Subcharging by DMSO from Native Ion Mobility Mass Spectrometry. Chem Select. 2016;1(18):5686–5690. doi: 10.1002/slct.201601402. [DOI] [Google Scholar]
- 54.Dick LWJ, Mahon D, Qiu D, Cheng KC. Peptide Mapping of Therapeutic Monoclonal Antibodies: Improvements for Increased Speed and Fewer Artifacts. J Chromatogr B. 2009;877:230–236. doi: 10.1016/j.jchromb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Bongers J, Cummings JM, Ebert MB, Federici L, Gledhill L, Gulati D, Hilliard GM, Jones BH, Lee KR, Mozdzanowski J, Naimoli M, Burman S. Validation of a Peptide Mapping Method for a Therapeutic Monoclonal Antibody: What Could We Possibly Learn About a Method We Have Run 100 Times? J Pharm Biomed Anal. 2000;21:1099–1128. doi: 10.1016/S0731-7085(99)00181-8. [DOI] [PubMed] [Google Scholar]
- 56.Chirino AJ, Mire-Sluis A. Characterizing Biological Products and Assessing Comparability Following Manufacturing Changes. Nature Biotech. 2004;22:1383–1391. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 57.Jenkins N, Murphy L, Tyther R. Post-Translational Modifications of Recombinant Proteins: Significance for Biopharmaceuticals. Mol Biotechnol. 2008;39:113–118. doi: 10.1007/s12033-008-9049-4.. [DOI] [PubMed] [Google Scholar]
- 58.Horvath C, Melander W, Molnar I, Molnar P. Enhancement of Retention by Ion-Pair Formation in Liquid hromatography with Nonpolar Stationary Phases. Anal Chem. 1977;14:12295–12305. doi: 10.1021/ac50022a048. [DOI] [Google Scholar]
- 59.Guo D, Mant CT, Hodges RS. Effects of Ion-Pairing Reagents on the Prediction of Peptide Retention in Reversed-Phase High-Resolution Liquid Chromatography. J Chromatogr A. 1987;386:205–222. doi: 10.1016/S0021-9673(01)94598-4. [DOI] [PubMed] [Google Scholar]
- 60.Streuli CA. Titrations in Nonaqueous Solvents. Anal Chem. 1964;36:363R–369R. [Google Scholar]
- 61.Chen J, Liu Z, Wang F, Mao J, Zhou Y, Liu MJ, Zou H, Zhang Y. Enhancing the Performance of LC-MS for Intact Protein Analysis by Counteracting the Signal Suppression Effects of Trifluoroacetic Acid During Electrospray. Chem Commun. 2015;51:14758–14760. doi: 10.1039/C5CC06072A. [DOI] [PubMed] [Google Scholar]
- 62.Wang EH, Combe PC, Schug KA. Multiple Reaction Monitoring for Direct Quantitation of Intact Proteins Using a Triple quadrupole Mass Spectrometer. J Am Soc Mass Spectrom. 2016;27:886–896. doi: 10.1007/s13361-016-1368-2. [DOI] [PubMed] [Google Scholar]
- 63.Wang EH, Appulage DK, McAllister EA, Schug KA. Ivestigation of Ion Transmission Effects on Intact Protein Quantification in a Triple Quadrupole Mass Spectrometer. J Am Soc Mass Spectrom. 2017;28:1977–1986. doi: 10.1007/s13361-017-1696-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


