Abstract
In root hairs of alfalfa (Medicago sativa), the requirement of Ca2+ for Nod factor signaling has been investigated by means of ion-selective microelectrodes. Measured 50 to 100 μm behind the growing tip, 0.1 μm NodRm-IV(C16:2,S) increased the cytosolic free [Ca2+] by about 0.2 pCa, while the same concentration of chitotetraose, the nonactive glucosamine backbone, had no effect. We demonstrate that NodRm-IV(C16:2,S) still depolarized the plasma membrane at external Ca2+ concentrations below cytosolic values if the free EGTA concentration remained low (≤0.01 mm). Externally added Sr2+ was able to replace Ca2+, and to some extent even enhanced the Nod-factor-induced depolarization, whereas with Mg2+ it was decreased. This suggests that the Nod factor response is triggered by Ca2+ from external stores. The addition of the endomembrane Ca2+-ATPase inhibitor 2,5-di(t-butyl)-1,4-benzohydroquinone, which presumably mobilizes Ca2+ from Ins(1,4,5)P3-sensitive stores, mimicked the Nod factor response, i.e. increased the cytosolic free [Ca2+], triggered Cl−-efflux, depolarized the plasma membrane, and alkalized the root hair space. In all cases a refractory state toward Nod factor perception was produced, indicating a shortcut of Nod factor signal transduction by releasing Ca2+ from internal stores. These latter results strongly support the idea that an elevation of cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal, which is consistent with the role of Ca2+ as a second messenger.
In legumes, nitrogen fixation takes place in highly specialized root organs (nodules) that result from symbiotic interaction between the host plant and soil bacteria known as rhizobia. During early stages of this association, molecular signals are synthesized by the rhizobia that are essential for initiating morphogenetic responses in the host plant. These signals, Nod factors, are lipochitooligosaccharides that are highly host specific due to their distinct structural modifications in different rhizobial species (Lerouge et al., 1990; Felle et al., 1995; Long, 1996; Schultze and Kondorosi, 1998).
Early events of Nod factor signaling are cytosolic pH changes (Felle et al., 1996) and delayed depolarization of the plasma membrane (Ehrhardt et al., 1992; Felle et al., 1995; Kurkdjian, 1995). Using ion-selective microelectrodes, we have demonstrated recently that the most rapid response to Nod factors is a Ca2+ influx from the root hair space into the cell. This triggers a number of events such as the activation of anion channels through which the cells lose Cl− rapidly, causing depolarization followed by K+ efflux (Felle et al., 1998). The Ca2+ channel antagonist nifedipine inhibited the Nod-factor-induced ion fluxes, and the Ca2+ ionophore A23187 mimicked the Nod factor responses, suggesting a role of Ca2+ in Nod signal transduction (Felle et al., 1998). A role of Ca2+ in Nod factor signaling has also been suggested by the absence of Ca2+ spiking in a nodulation-defective alfalfa mutant (Ehrhardt et al., 1996). Gehring et al. (1997) and DeRuijter et al. (1998) have demonstrated Nod-factor-induced elevation of the cytosolic free [Ca2+] in root hair tips. However, since these changes would primarily be connected with tip growth, whereas the experiments in this study were carried out well behind the tip, we did not consider their observations to be relevant for our investigations.
Testing the cytosolic free [Ca2+] within the root hairs of alfalfa provided evidence that the [Ca2+] increased in response to Nod factors; however, due to technical problems, this increase could not be brought into a clear temporal relationship with the Ca2+ influx, the Cl− efflux, or the depolarization. Taking this difficulty into account, we argued that the changes in cytosolic [Ca2+] need not necessarily spread across the entire cell, but could occur essentially in pockets, e.g. close to either vacuolar or endoplasmatic Ca2+ pools. Still, it left the question open as to what extent the concentration of cytosolic free Ca2+ is part of Nod factor signal transduction. To test this, we manipulated the cytosolic free [Ca2+] using an inhibitor of endomembrane Ca2+ ATPases, 2,5-di(t-butyl)-1,4-benzohydroquinone (BHQ), for its effect on the transduction of the Nod signal. Here we demonstrate that BHQ at submicromolar concentrations increases the cytosolic free [Ca2+] and strongly inhibits the transduction of the Nod signal, thus contributing to the idea that cytosolic Ca2+ is an important element in Nod factor signaling.
MATERIALS AND METHODS
General Assay Conditions
Seeds of alfalfa (Medicago sativa subsp. sativa cv Sitel) were surface-sterilized and prepared for treatment as described previously (Felle et al., 1995). Intact 2-d-old seedlings were fixed with wax on the bottom of a chamber that was constantly perfused with a solution containing 10 mm MES/Tris (mixed to pH 7.3), 0.1 mm KCl, 0.1 mm NaCl, and CaCl2 at the concentrations indicated in the text or figure legends. NodRm-IV(C16:2,S) from Sinorhizobium meliloti (Felle et al., 1995; Schultze et al., 1992) was prepared from an aqueous stock solution of 1 mm. Micromolar concentrations of BHQ were prepared from a 20 mm ethanolic stock solution, yielding a final maximal ethanol concentration of 0.05%. Within the measuring times (30 min) no side effects of these ethanol concentrations were detected.
Ion-Selective Microelectrodes
The electrical setup for the impalement of root hairs, the fabrication and application of ion-selective microelectrodes, and their intracellular or extracellular application has been described previously (Felle, 1987, 1988, 1994; Felle and Bertl, 1986). After internal silanization of the glass pipettes with tributylsilane (dissolved to 0.2% in chloroform), the respective sensor cocktail (Fluka) was mixed with polyvinylchloride:tetrahydrofuran (40 mg/mL) at a ratio of 30:70 (v/v) and backfilled into the tip using a long glass pipette. After letting the tetrahydrofuran evaporate, the undiluted sensor cocktail was added from the rear, followed by the respective reference solution. To prevent the cell turgor from pushing the sensor cocktail into the shank upon impalement, a constant pressure (5–10 bars) was applied from the rear of the electrode using a home-built pressure device. Ion-selective electrode and voltage reference were connected to a high-impedance amplifier (model FD 223, WP Instruments, Sarasota, FL), which simultaneously measured and subtracted the signals coming from the two electrodes. Signals were recorded on a chart recorder (model L-2200, Linseis, Germany). Since the two electrodes differ considerably in their response times to fast voltage changes, the differential signal (the net ion concentration in pX units) may show artifactual swings. Before measurements the ion-selective microelectrodes were precalibrated. Only electrodes that gave slopes of at least 25 mV/pCa or 55 mV/pH (or pCl, pK) were used. Calibrations that refer to the absolute pX values given were always carried out after the respective measurements.
RESULTS
Influence of External [Ca2+] on Nod-Factor-Induced Depolarization
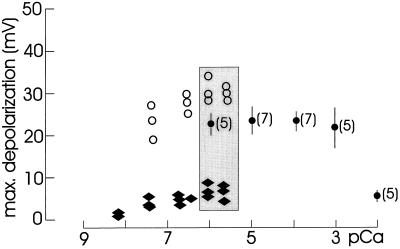
Recently, we demonstrated that the earliest response to Nod factors was a loss of Ca2+ from the root hair space and that this was necessary and sufficient to trigger downstream responses to Nod signals (Felle et al., 1998). To test the importance of extracellular Ca2+, we measured the response to Nod factors in the presence of different external Ca2+ concentrations. Figure 1 shows the maximal depolarizations recorded after adding 0.1 μm NodRm-IV(C16:2,S) to solutions with different Ca2+ concentrations. There was no significant response difference measured in the presence of Ca2+ solutions between 1 mm and solutions with no Ca2+ added (1–2 μm Ca2+, as measured with the Ca2+-selective microelectrode). However, the depolarizations were clearly less pronounced in the presence of 10 mm Ca2+.
Figure 1.
Maximal depolarizations of alfalfa root hairs in response to 0.1 μm NodRm-IV(C16:2,S), measured in different Ca2+ solutions (pCa) with and without EGTA, as indicated. Points are from single experiments (○, ♦) or mean values (•, ±se); numbers in brackets refer to the number of experiments. Shaded area compares the effect of NodRm-IV(C16:2,S) on membrane depolarization in the presence of 0 (•), 0.1 (♦), and 0.01 (○) mm EGTA at equal free [Ca2+].
To decrease free Ca2+ concentrations to below 1 μm, it was necessary to add the Ca2+ chelator EGTA. EGTA/Ca2+ solutions were calculated and the final [Ca2+] checked in the root hair space with a Ca2+-selective microelectrode. As shown in Figure 1, the free EGTA in these solutions played a critical role in the response of the root hairs to the Nod factor. Whereas in the presence of 0.1 mm free EGTA the depolarizations were clearly lower than in solutions without EGTA, the response was equal or even slightly better in the presence of 0.01 mm free EGTA. Solutions completely free of Ca2+ (excess EGTA) were not used because the recordings became unstable, probably due to an unspecific increase in membrane conductivity.
Sr2+, acting in a manner similar to Ca2+ (Bauer et al., 1998), can replace Ca2+. When 0.1 mm Sr2+ was added to the solution in which only a small response to 0.1 μm NodRm-IV(C16:2,S) was recorded (pCa 6.75, 0.1 mm free EGTA), the Nod factor substantially depolarized the root hairs (Fig. 2a). Decreasing the free EGTA to 0.01 mm stimulated the response to the Nod factor (Fig. 2a, compare tracks 1 and 4). In the presence of 0.1 mm Sr2+ the response was even stronger than the control without Sr2+ (track 3), whereas Mg2+ inhibited the response (track 5). In solutions with no EGTA added, Sr2+ generally induced stronger Nod factor responses than Ca2+ did at comparable concentrations (Fig. 2b).
Figure 2.
Membrane potential (Em) response of alfalfa root hairs to NodRm-IV(C16:2,S). a, Root hairs were preincubated in the indicated pCa solutions in the presence of 0.1 mm (traces 1 and 2) or 0.01 mm free EGTA (traces 3, 4, and 5). After the addition of 0.1 mm Sr2+ or Mg2+, respectively, 0.1 μm NodRm-IV(C16:2,S) (NF) was tested. At “//” drawings of curves were interrupted for 2 to 3 min to allow for normalization of Nod factor addition. b, Depolarization response to 0.01 μm NodRm-IV(C16:2,S) either in the presence of Sr2+ (1) or Ca2+ (2) at the indicated concentrations. Kinetics are representative examples of three to five recordings each carried out under equivalent conditions.
The Nod Factor Response in the Presence of High and Low External [Ca2+]
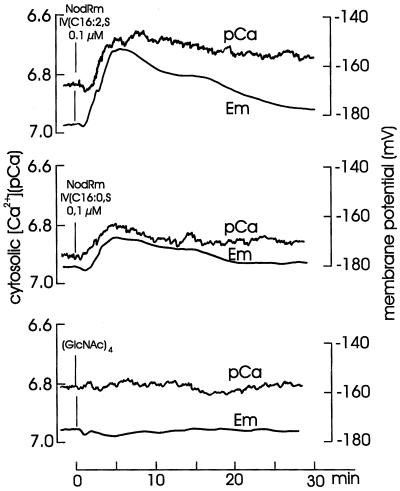
At standard external [Ca2+] of 0.1 mm, growing root hairs of alfalfa displayed a substantial tip-to-base [Ca2+] gradient, i.e. 604 to 967 nm in the tip compared with 95 to 235 nm in the middle or basal region (Felle et al., 1999). In a previous study we observed that the earliest response to Nod factors was a rapid loss of Ca2+ from the root hair space measured near the base of the root hairs (Felle et al., 1998). Therefore, the following measurements were carried out 50 to 100 μm behind the tip. As shown in Figure 3, the root hairs responded to 0.1 μm of the most effective Nod factor (NodRm-IV[C16:2,S]) with a marked increase in cytosolic free [Ca2+] of about 0.2 pCa, which in the presence of the Nod factor slightly recovered. The same concentration of NodRm-IV(C16:0,S) carrying a saturated lipid chain was less effective on both cytosolic [Ca2+] and depolarization. The glucosamine backbone chitotetraose had no significant effect at all.
Figure 3.
Effect of 0.1 μm NodRm-IV(C16:2,S), NodRm-IV(C16:0,S), and chitotetraose ([GlcNAc]4) on cytosolic free [Ca2+] (pCa) and membrane potential (Em) of alfalfa root hairs. Kinetics are representative examples of 21 (NodRm-IV[C16:2,S]), three (NodRm-IV[C16:0,S]), and five ([GlcNAc]4) carried out under equivalent conditions.
As shown in Figure 4a, the addition of 20 mm Ca2+ to the standard solution (0.1 mm Ca2+) increased the cytosolic [Ca2+] by 0.2 to 0.3 pCa, whereas 0.1 μm NodRm-IV(C16:2,S) added thereafter only evoked a minor increase in cytosolic [Ca2+] and a small depolarization. Changing the external [Ca2+] from 10 mm to a solution with no extra Ca2+ added (1–2 μm free Ca2+) decreased the cytosolic [Ca2+] just slightly below the level measured in the presence of 0.1 mm Ca2+. Lowering the external [Ca2+] to pCa 7.44 (36 nm Ca2+; free EGTA 0.01 mm) did not change the cytosolic free [Ca2+] any further. Subsequent addition of 0.1 μm NodRm-IV(C16:2,S), however, caused a rapid increase in cytosolic free [Ca2+] in the presence of 0.01 mm EGTA.
Figure 4.
Effect of external [Ca2+] on cytosolic free [Ca2+] (pCaC) and membrane potential (Em) of alfalfa root hairs before and after the addition of 0.1 μm NodRm-IV(C16:2,S). Shown is the Nod factor response in the presence of high extracellular [Ca2+] (a) and in the presence of low extracellular [Ca2+] (b). “Ca2+-free” refers to the addition of a solution with no extra Ca2+ added (i.e. 1–2 μm free Ca2+, as measured with a Ca2+-selective microelectrode within the root hair space). pCa 7.44 refers to a solution with 0.01 mm free EGTA. The initial transients on the Ca2+ traces arise from the different response times of the two electrodes. Kinetics are representative examples of four recordings each carried out under equivalent conditions.
BHQ Mimicks Nod Factor Action But Induces a Refractory State to Nod Factor Perception
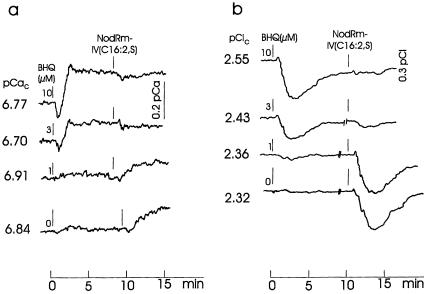
BHQ is known to inhibit Ca2+-ATPase in the ER of mammalian cells and to mobilize Ca2+, presumably from Ins(1,4,5)P3-sensitive stores (LLopis et al., 1991; Nakamura et al., 1992). Figure 5a shows that BHQ rapidly increases the cytosolic [Ca2+] in alfalfa root hairs at concentrations above 1 μm. NodRm-IV(C16:2,S) (0.1 μm) added in the presence of BHQ failed to cause a response at 3 and 10 μm BHQ, which previously had shown increased cytosolic [Ca2+]. However, in the presence of 1 μm or lower BHQ concentrations (which had no effect on cytosolic [Ca2+]), NodRm-IV(C16:2,S) clearly increased cytosolic [Ca2+]. A similar effect was observed for cytosolic [Cl−]. As Figure 5b shows, 10 μm BHQ transiently decreased cytosolic [Cl−] and prevented such a response by NodRm-IV(C16:2,S); 1 μm BHQ did not considerably change cytosolic [Cl−], but the subsequently added Nod factor did.
Figure 5.
Effect of BHQ at the indicated concentrations and subsequently added 0.1 μm NodRm-IV(C16:2,S) on cytosolic free [Ca2+] (pCaC) (a) and cytosolic [Cl−] (pClC) of M. sativa root hairs (b). At “//” drawings of curves are interrupted for 2 to 3 min to allow for normalization of Nod factor addition. The initial transients on the Ca2+ or Cl− traces arise from the different response times of the two electrodes. External [Ca2+] was 0.1 mm. Kinetics are representative examples of four recordings each carried out under equivalent conditions.
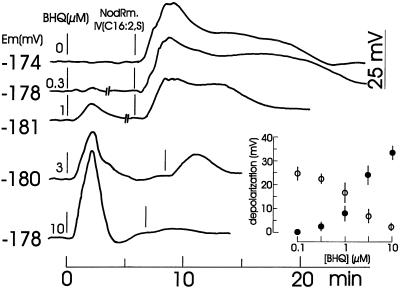
As shown in Figure 6, BHQ at concentrations above 1 μm transiently depolarized the root hairs, whereas submicromolar concentrations did not. After a pre-incubation with BHQ, the subsequent addition of 0.1 μm NodRm-IV(C16:2,S) in the presence of BHQ resulted in a concentration-dependent reduced depolarization.
Figure 6.
Effect of BHQ at the indicated concentrations on the membrane potential (Em) of alfalfa root hairs before and after the addition of 0.1 μm NodRm-IV(C16:2,S). At “//” drawings of curves were interrupted for 2 to 3 min to allow for normalization of Nod factor addition. External [Ca2+] was 0.1 mm. Kinetics are representative examples of three to five recordings carried out under equivalent conditions. Inset, Dose-response relationship of maximal Nod factor- (○) and BHQ-induced depolarizations (•) are plotted against the BHQ concentration during the experiment.
External alkalinization is a common phenomenon observed after the application of Nod factors and elicitors. Although its exact nature has not yet been elucidated, alkalinization is a good parameter with which to test the action of both symbiotic and defense agents (Felix et al., 1993; Nürnberger et al., 1994; Felle et al., 1998). As shown in Figure 7, 10 μm BHQ caused a transient alkalinization in the root hair space. Compared with the Nod-factor-induced alkalinization, it reached the peak faster and turned into an acidification at the end; 0.1 μm NodRm-IV(C16:2,S) added subsequently in the presence of BHQ only slightly alkalized the root hair space at that time. In contrast, when these agents were added in inverse order, BHQ alkalized the root hair space in the regular manner, indicating that the mechanisms leading to alkalinization came from different sources.
Figure 7.
Effect of 10 μm BHQ and 0.1 μm NodRm-IV(C16:2,S) on the pH of the alfalfa root hair space. External [Ca2+] was 0.1 mm. Results are representative of five equivalent tests each.
DISCUSSION
In this paper we demonstrate that external and cytosolic Ca2+ are important factors in Nod-factor-induced depolarization. It was interesting, however, to observe that substantial responses to NodRm-IV(C16:2,S) occurred even at external [Ca2+] far below the cytosolic value if EGTA did not hamper the response. According to Equation 1, this is not surprising. The electrochemical driving force for Ca2+ (ΔμCa/F) into the cell is given by:
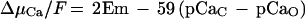 |
1 |
where Em is the membrane potential and pCaC and pCaO are the negative logarithms of the free [Ca2+] of the cytosol (c) and the outside (o), respectively. Inserting the membrane potential and the transmembrane [Ca2+] gradient, Equation 1 shows that even if the external [Ca2+] is less than the cytosolic [Ca2+], the inwardly directed driving force for Ca2+ is still sufficiently negative to drive Ca2+ into the cell. Unfortunately, because of the remaining free EGTA, it was not possible to exactly pinpoint the minimal external Ca2+ concentration required for a response to Nod factors. In the presence of 0.1 mm free EGTA, the cells ceased to respond around 10 nm (pCa 8.0). Extrapolating from the measuring points obtained in the presence of 0.01 mm EGTA, even lower [Ca2+] would be sufficient to support responsiveness.
It is intriguing that the Nod factor response is apparently insensitive over a wide range of [Ca2+]. This is because the measuring parameter, depolarization, is a secondary response apparently induced by elevated cytosolic [Ca2+]. It is not yet clear how much Ca2+ is actually needed to trigger the response, but it appears sufficient even at concentrations below 100 nm (Fig. 1). As demonstrated in Figure 2, Sr2+ could replace Ca2+ in triggering the Nod factor response, whereas Mg2+ could not, supporting the notion that external Ca2+ and its influx into the cytosol is essential for the Nod-factor-induced depolarization. This is in line with our previous observation that nifedipine, presumably through blocking much of the Ca2+ influx, prevent downstream Nod factor effects (Felle et al., 1998) such as Cl− efflux and subsequent depolarization (data not shown). The inhibitory effect of Mg2+ on the Nod factor response could be explained by obstructing Ca2+ from freely entering the respective channels.
We also present evidence that cytosolic [Ca2+] is an element of the Nod factor signal chain. BHQ obviously increased cytosolic free [Ca2+], transiently decreased cytosolic [Cl−] (Fig. 5), alkalized the root hair space (Fig. 7), transiently depolarized the plasma membrane, and inhibited the Nod signal (Fig. 6). BHQ mimicks the Nod factor response presumably just by increasing cytosolic [Ca2+], which was observed using the Ca2+ ionophore A23187 (Felle et al., 1998). Although both BHQ and Nod factors obviously trigger similar events through increasing cytosolic free [Ca2+], the data in Figure 6 clearly show that it matters which mechanism leads to the elevation of cytosolic [Ca2+]. With Nod factors, Ca2+ channels at the plasma membrane are activated, which may require the involvement of G-proteins (Pingret et al., 1998), whereas with BHQ, such a step was not necessary.
Whereas BHQ is poorly characterized in plant cells, it is known from mammalian cells that BHQ is a potent inhibitor of endomembrane Ca2+ ATPases of the E1E2-type, and it has been proposed that it mobilizes Ca2+ from Ins(1,4,5)P3-sensitive stores. Although we could clearly demonstrate that BHQ induced an increase in cytosolic [Ca2+], we had no way to tell whether this came from Ins(1,4,5)P3-sensitive or other stores. However, for the questions raised here such considerations would not be important, because we used BHQ only as a convenient tool with which to manipulate cytosolic free [Ca2+] and to test the effect this change would have on the Nod factor response.
The question of whether Nod factors drain intracellular Ca2+ stores has been addressed by Pingret et al. (1998) by applying the ryanodine receptor antagonist ruthenium red. Originally characterized in animal studies (see Galione, 1994), there is convincing evidence that a ryanodine receptor also exists in the endomembranes of plants (Knight et al., 1992; Allen et al., 1995). Pingret et al. (1998) observed that ruthenium red blocked the epidermal Nod factor response in alfalfa, and inferred that the transduction of the Nod factor signal required the mobilization of intracellular Ca2+ stores. Although they did not directly show that Nod factors increased cytosolic [Ca2+] and that ruthenium red prevented it, we demonstrate in this paper that Nod factors do cause an increase of the cytosolic [Ca2+] of alfalfa (Figs. 3–5), and this increase is indispensable for the activation of downstream events such as the activation of anion channels (Fig. 5b). On the other hand, using EGTA (to reduce external [Ca2+]) and La3+ (to block Ca2+-influx), Pingret et al. (1998) demonstrated that the investigated Nod factor response was also dependenton external Ca2+, an observation that corresponds with our observations.
The finding that BHQ at micromolar concentrations inhibits the Nod-factor-induced depolarization (and external alkalinization) is in agreement with the idea that events that require elevated cytosolic [Ca2+] are refractory to another Nod factor stimulus when triggered previously through increased cytosolic [Ca2+]. Since we have good indications now that the main cause for the Nod-factor-induced depolarization is a Ca2+-triggered stimulation of the Cl− efflux (Felle et al., 1998), previously elevated cytosolic [Ca2+] at the site of action would largely prevent such a stimulation, because BHQ, through increasing cytosolic Ca2+, would have already activated the anion channels. This idea is supported by the finding that BHQ itself caused Cl− efflux (Fig. 5b) and transient depolarization (Fig. 6), as well as by the observation that in the presence of 10 or 20 mm external Ca2+, the Nod factor response was strongly decreased, probably also due to the increased cytosolic [Ca2+] under these conditions (Figs. 1 and 4a). In this context it is interesting that BHQ induced a refractory state for Nod factor action, but not vice versa (Fig. 7). The reason for this could be that the Nod-factor-induced increase in cytosolic [Ca2+] is slightly transient or, more likely, that the suppression of the Nod factor response through increased cytosolic Ca2+ does not occur at the anion channel but at a more upstream part of the respective transduction chain.
Regardless of these considerations, an elevation of cytosolic Ca2+ appears to be a prerequisite for the initiation of a cascade of events at the plasma membrane of alfalfa root hairs, leading to rapid Cl− efflux and depolarization. Since the experiments with Sr2+ and nifedipine strongly indicate that this Ca2+ comes from the outside, these responses must be regarded as different from the Ca2+ spikes observed by Erhardt et al. (1996). Their delayed Nod-factor-induced Ca2+ signals very likely originate from intracellular stores around the nucleus. Both responses obviously target different stages within the framework of a complex Nod factor action. Whereas the spikes, by propagating along the root hair, may carry information from the nucleus to other parts of the cell, the more stationary Ca2+ changes presented in this paper, by triggering channel activities at the plasma membrane, may pave the way for the rhizobia to enter the cells. This could be initiated by localized alterations in the ion concentrations or transmembrane electrochemical ion gradients, including pH changes at the rhizobial docking site of the root hair. Both responses, however, underline the role of Ca2+ as a second messenger in Nod factor signaling.
Footnotes
This work was supported by the Commission of the European Community (TMR contract no. ERBFMRX–CT98–0243).
LITERATURE CITED
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Bethmann B, Popescu O, Hansen U-P, Simonis W, Schönknecht G. Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol. 1998;117:545–557. doi: 10.1104/pp.117.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JM, Sanders D. Inositol trisphosphate-mediated Ca2+-release in beet microsomes is inhibited by heparin. FEBS Lett. 1990;260:70–72. [Google Scholar]
- DeRujter NCA, Rook MB, Bisseling T, Emons AMC. Lipochitooligosaacharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 1998;13:341–350. [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:7–20. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Felle H. Proton transport and pH control in Sinapis alba root hairs: a study carried out with double-barreled pH-microelectrodes. J Exp Bot. 1987;38:340–354. [Google Scholar]
- Felle H. Cytosolic free calcium in Riccia fluitans and Zea mays: interaction of Ca2+ and pH? Planta. 1988;176:248–255. doi: 10.1007/BF00392452. [DOI] [PubMed] [Google Scholar]
- Felle HH. The H+/Cl−-symporter in root hair cells of Sinapis alba: an electrophysiological study using ion-selective microelectrodes. Plant Physiol. 1994;106:1131–1137. doi: 10.1104/pp.106.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H, Bertl A. The fabrication of H+-selective liquid membrane microelectrodes for use in plant cells. J Exp Bot. 1986;37:1416–1428. [Google Scholar]
- Felle HH, Kondorosi É, Kondorosi Á, Schultze M. Nod signal-induced plasma membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipochitooligosaccharide. Plant J. 1995;7:939–947. [Google Scholar]
- Felle HH, Kondorosi É, Kondorosi Á, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- Felle HH, Kondorosi É, Kondorosi Á, Schultze M. Plant J. 1998;13:455–463. [Google Scholar]
- Felle HH, Kondorosi É, Kondorosi Á, Schultze M (1999) Nod factors modulate the cytosolic free [Ca2+] differently in growing and non-growing root hairs of Medicago sativa. Planta (in press) [DOI] [PubMed]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Galione A. Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signaling. Mol Cell Endocrinol. 1994;98:125–131. doi: 10.1016/0303-7207(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Kabbara AA, Parish RW, Boukli NM, Broughton WJ. Rapid, plateau-like increases in intracellular free calcium are associated with Nod-factor-induced root hair deformation. Mol Plant-Microbe Interact. 1997;10:791–802. [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian AC. Plant Physiol. 1995;107:79–81. doi: 10.1104/pp.107.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulfated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- LLopis J, Chow SB, Kass GEN, Gahm A, Orrenius S. Comparison between the effects of the microsomal Ca2+-translocase inhibitors thapsigargin and 2,5-di(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991;277:553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SR. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Nakasaki Y, Matsuda N, Shihekawa M. Inhibition of sarcoplasmic reticulum Ca2+-ATPase by 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Biochem. 1992;112:750–755. doi: 10.1093/oxfordjournals.jbchem.a123970. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasmamembranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Pingret JL, Journet E-P, Barker DG. Rhizobium Nod factorsignaling: evidence for a G protein-mediated transduction mechanism. Plant Cell. 1998;10:659–671. doi: 10.1105/tpc.10.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Kondorosi Á. Regulation of symbiotic root nodule development. Annu Rev Genet. 1998;32:33–57. doi: 10.1146/annurev.genet.32.1.33. [DOI] [PubMed] [Google Scholar]
- Schultze M, Quiclet-Sire B, Kondorosi É, Virelizier H, Gluska JN, Endre G, Géro SD, Kondorosi Á. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci USA. 1992;89:192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]