Summary
A novel immune-modulatory therapy utilizing targeted delivery of cytokines to draining lymph nodes effectively reprograms Th2 allergic responses towards a Th1 and tolerogenic profile, resulting in protection from peanut antigen-induced anaphylaxis.
Keywords: allergy immunotherapy, anaphylaxis, peanut allergy, IL-12, Th1, Th2, lymph node targeting, nanoparticles
To the editor
Allergen immunotherapy is used to treat hypersensitivities to environmental or food allergens. It involves administering repeated and increasing doses of a soluble allergen until a maintenance dose is reached where symptoms are improved upon allergen exposure1. Although widely used, several limitations exist, including the risk of severe adverse events (e.g. fatal anaphylaxis), high costs of antigens and clinical monitoring, and requirement of many years of treatment2. Many patients remain refractory to treatment and for peanut (PN) allergy this strategy has a high risk of adverse reactions3. Thus, current immunotherapy protocols have distinct limitations and there is a major need for improved approaches, particularly for food allergies.
When effective, the mechanisms of allergen immunotherapy are not well understood, although it is thought to involve a combination of antigen-induced desensitization of effector immune cells and the development of tolerance. Skewing the Th1/Th2 profile towards a Th1 direction could markedly reduce Th2-mediated pathological responses to allergens. Thus, attempts have been made to block the production of Th2 cytokines, to directly inhibit their action, or to favor Th1 immunity by administering exogenous Th1-type cytokines, such as IL-12. For asthma treatment, exogenous IL-12 delivery was not effective, which was attributed to the difficulty of administering soluble cytokine to the lungs in sufficient amounts to improve outcomes4.
Recently, we showed that a significant and long-lasting effect can be achieved if immunomodulatory cytokines are targeted to draining lymph nodes (DLNs), the command structures for the development and refinement of immunity5. Cytokines were successfully targeted to DLNs by loading them into stable biodegradable nanoparticles (comprised of the carbohydrates heparin and chitosan)5. Injected at peripheral sites, these particles traffic via the lymphatics and, protected by the nanoparticle matrix, avoid degradation and dilution to reach DLNs in functionally relevant quantities. By subcutaneously injecting a vaccine antigen along with nanoparticles containing TNF, rapid germinal center development and protective immunity were evoked5. Furthermore, replacing TNF with IL-12 created particles that promoted IFN-production by T cells in DLNs5.
Allergic immune responses are characterized by a Th2 profile, promoted by cytokines such as IL-4 and IL-10, and by certain subclasses of antibodies, such as IgE and IgG16. In contrast, Th1-polarized immunity involves IL-12 production by dendritic cells (DCs) that, in turn, promotes IFN-γ production by T cells7. To test whether an immunotherapy involving the delivery of particulate IL-12 (pIL-12) along with PN-antigen can skew immunity towards a Th1-profile and protect from anaphylaxis, we used an “active sensitization” model of PN allergy (Fig. 1a). PN antigens were derived from crude PN extracts and contained known allergens, including Ara h 1–8 (Table S1 in this article’s Online Repository). Mice were subsequently given one of several experimental desensitization therapies (or control treatments, Fig. 1a), including pIL-12 versus soluble IL-12 (sIL-12) and particulate versus soluble IL-10 (pIL-10, sIL-10), which were administered with soluble PN-antigen in 4 treatments (Fig. 1a). While IL-12 was chosen due to its role in Th1-skewing, IL-10 was selected based on its contributions to establishing tolerance. At week 12, mice were challenged with sufficient PN-antigen to induce anaphylaxis. Severity of anaphylaxis was determined by quantifying the drop in temperature experienced after challenge, a standard measure of anaphylaxis in mice8. Naïve mice do not anaphylax when given a PN challenge; however, sensitized mice do (Fig. 1b). Neither the control treatments of PN alone or empty particles+PN significantly influenced anaphylaxis in PN-sensitized mice (Fig. 1b). In contrast, PN-sensitized mice given desensitization therapy of PN+pIL-12 did not anaphylax, while sIL-12 had no therapeutic effect (Fig. 1c). The Th2-promoting cytokine, IL-10, surprisingly, had the opposite effect in particulate form, resulting in more severe anaphylaxis when co-administered with PN during desensitization (Fig. 1d), likely due to its role in DLNs as a Th2 cytokine. In contrast, sIL-10 slightly improved the symptoms (Fig. 1d), potentially due to the immunosuppressive effect of IL-10 in peripheral tissue9. These results demonstrate the plasticity of allergic responses and that they can be reprogrammed differentially through choice and delivery of cytokines.
Figure 1. Nanoparticle-based IL-12 desensitization therapy protects from PN-induced anaphylaxis.
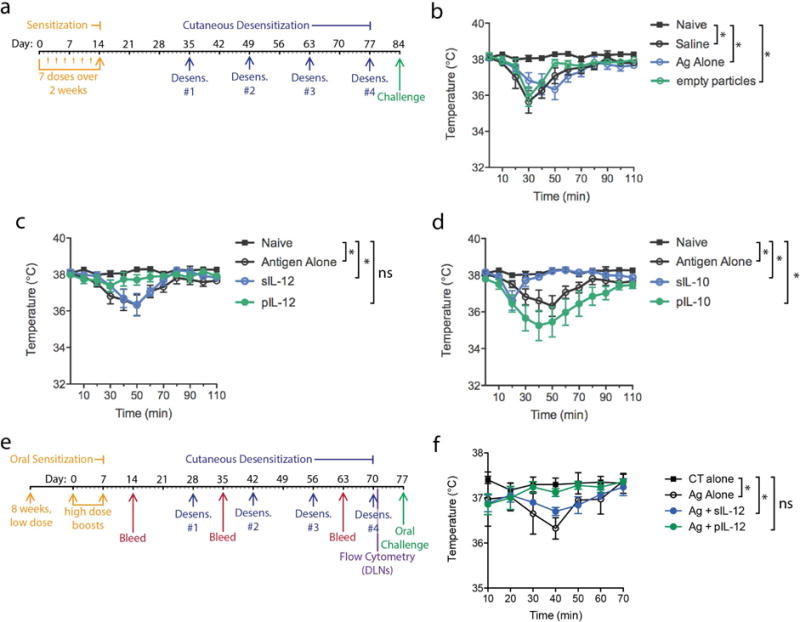
(a) Experimental time course for mouse sensitization to PN, desensitization and PN challenge. For sensitizations, mice were given 7 repeated low dose i.p. injections of PN antigen. Desensitization therapy involved co-administration of 1μg of PN antigen with and without 1ng of soluble or particulate cytokines (IL-12 or IL-10), or treatment with PN antigen alone without any cytokine-containing particles, or treatment with saline alone as control. Mice were challenged by i.p. injection with PN antigen to induce anaphylaxis. Temperatures are shown after challenge for controls and mice desensitized with (b) empty particles, (c) soluble versus particulate IL-12, or (d) soluble versus particulate IL-10. For b–c, Naïve indicates mice that were not PN-sensitized and all other groups were sensitized. pIL-12 and to a lesser extent sIL-10 effectively prevented PN-induced anaphylaxis. * indicates temperatures differ significantly between groups at >1 time point, determined by 2-way ANOVA. (e) Time course of oral sensitization, cutaneous desensitization and oral challenge is presented. For 8 weeks, 50μg of PN antigen was administered by oral gavage weekly. Subsequently, 2 high-dose oral gavage boosts of 1mg PN antigen containing cholera toxin (CT) were given. For some mice, tissues were harvested for immune profiling while others were challenged with 500μg of PN antigen, orally, and temperature was monitored. Control mice were given sensitization doses consisting of CT alone by oral gavage. (f) Anaphylaxis was monitored after oral PN challenge. PN-sensitized mice showed a significant drop in temperature compared to control un-sensitized mice that had received oral CT alone.
Subsequently, to test this strategy in a model that is more analogous to the natural route of PN exposure in humans, animals were sensitized and subsequently challenged orally (Fig. 1e). Desensitization therapy involved co-administration of PN-antigen with and without sIL-12 or pIL-12. Control mice maintained their body temperatures after a PN challenge, while PN-sensitized mice that were given mock-desensitization injections with only PN-antigen showed a modest but significant drop in temperature after PN challenge (Fig. 1f). Desensitization therapy with PN+sIL-12 also did not prevent anaphylaxis upon PN challenge (Fig. 1f). Mice given the desensitization therapy of PN+pIL-12 did not anaphylax and maintained their temperatures after challenge, similar to the naive control group (Fig. 1f). Thus, treatment of PN-sensitized animals with small amounts of PN combined with pIL-12 is an effective desensitization therapy to prevent oral PN-induced anaphylaxis.
To verify PN desensitization was dependent on the effects of pIL-12 in skewing immunity towards a Th1-profile, subclasses of PN-specific antibodies were monitored throughout the protocol (Fig. 2a–b). Animals produced PN-specific Th2-associated IgG1 (Fig. 2a) but not Th1-associated IgG2a (Fig. 2b) after oral sensitization. Consistent with Th1 skewing, serum IgG2a levels increased significantly after 3 doses of desensitization therapy in mice given PN+pIL-12 compared to mice given PN-antigen alone or PN+sIL-12 (Fig. 2b). Levels of IgG1 did not differ amongst groups after desensitization (Fig. 2a). Thus, pIL-12 desensitization increases the relative abundance of Th1-associated IgG2a in the serum (Fig. 2b). Since strong Th2 responses and atopic conditions are associated with elevated IgE levels and IgE also contributes to mast cell-mediated anaphylaxis, we expected IgE levels could be reduced by therapeutic Th1-reprogramming. Indeed, after desensitization, total serum IgE levels were decreased in animals receiving both sIL-12 and pIL-12 desensitization therapies, compared to those receiving control therapy of PN-antigen alone (Day-77, Fig. 2c). PN-specific IgE levels were also significantly reduced by sIL-12 or pIL-12 desensitization therapies (Fig. 2d).
Figure 2. Desensitization is characterized by skewing of the Th2 immune response towards a Th1 immune response.
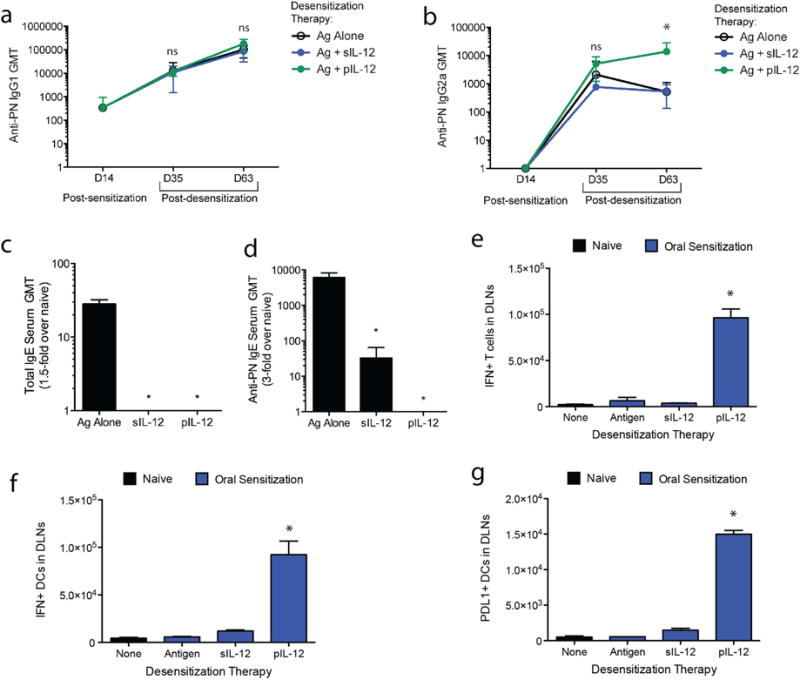
PN-specific (a) IgG1 and (b) IgG2a antibody subclass titers were measured at the pre- and post-desensitization therapy (days 14, 35 and 63) and are represented as the geometric mean titer (GMT) of at least 2-fold over naïve controls. (c) Total IgE and (d) PN-specific IgG levels on day 63 of the experimental protocol (post-desensitization) are represented as geometric mean titer (GMT) of at least 1.5-fold over naïve controls. Cells isolated from the lymph nodes of the skin 24h following the final desensitization challenge (day 70) were stained for IFN-γ production by (e) T cells and (f) DCs, and (g) PDL1 expression on DCs. For all panels, * indicates p<0.05 and “ns”=not significant.
Moreover, a Th1-associated activation profile is characterized by an abundance of IFNγ-producing T cells and DCs. To investigate whether Th1 immunity was enhanced by desensitization therapy, flow cytometry was performed 24h after administration of the final desensitization dose to determine the total number of IFNγ+ cells in the LNs draining the subcutaneous injection site of the desensitization therapy. The DLNs of mice given PN+pIL-12 showed significantly elevated numbers of INFγ+ T cells and DCs compared to controls, while those given PN-antigen alone or PN+sIL-12 did not (Fig. 2e–f). Mice given PN+pIL12 also had significantly increased numbers of DCs expressing PDL1 (Fig. 2g), which is associated with the development of tolerogenic immune responses.
Altogether we demonstrated that by targeting selected immunomodulatory cytokines to DLNs while administering antigens at peripheral sites, it is possible to achieve rapid reprogramming of DLNs, shifting the profile of antigen-specific immunity away from a harmful allergy-associated Th2 profile. Although effectiveness in humans has not yet been evaluated, compared to the existing analogous protocols for allergen immunotherapy in animal models, this is a dramatic reduction in time required for desensitization and can be instructive to the immune system with smaller quantities of antigen without gradual ramping of the allergen desensitization dose. These studies demonstrate the effectiveness of a novel desensitization strategy with advantages over traditional allergen immunotherapy.
In conclusion, we show that by utilizing our novel approach to deliver the Th1 cytokine, IL-12, to the DLNs, combined with soluble PN-antigen, we can effectively reprogram a Th2-associated allergic response towards a Th1-response and protect from PN-induced anaphylaxis.
Materials and Methods
Materials and methods in detail are described in this article’s online repository.
Online Methods
Animal Sources
Six-week-old female C57BL/6 mice were purchased from the National Cancer Institute. All animals were housed at the Duke University Vivarium and all experimental protocols were approved by the Duke University Institutional Animal Care and Use Committee.
Construction of nanoparticles
Heparin-chitosan nanoparticles were constructed based on a previously-described protocol1. In brief, this involved gradually combining of 0.1% heparin (Calbiochem) and 0.1% chitosan (Vanson), both in dH2O at approximately pH 4.5. The chitosan had been purified to drug grade, as previously described. One volume of 0.1% chitosan was added to five volumes of 0.1% heparin and vortexed for 30s. Addition of chitosan was repeated until a final 1:1 ratio of 0.1% chitosan to 0.1% heparin was achieved. After 10 min at room temperature, the pH was then adjusted to neutrality to prevent further aggregation. To load particles with cytokines, either recombinant IL-12 or IL-10 (both from R&D Systems) was vortexed for 10 min in 0.1% heparin before the addition of chitosan. Particles were centrifuged at 14,000 × g for 10 min at 4 °C, washed with water once, and then resuspended in PBS for injections. Each injection contained 100μg of heparin, 100μg of chitosan and 1ng of cytokine in particle form.
PN antigens
PN antigens, previously characterized in detail2, 3 were provided by Dr. Wesley Burkes.
Mass-spectrometry identification of major allergens present in the complex PN antigens
Quantitative proteomic analysis, LC-MS/MS was performed on soluble PN antigen at the Duke University’s Proteomics and Metabolomics facility. Acquired data was searched against the TrEMBL A. hypogaea database using a ‘Mascot’ search engine. Scaffold (version Scaffold_4.8.4, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications at greater than 99.0% probability and protein identifications at greater than 99.0% probability were used to achieve an FDR of less than 1.0% and contained at least 2 identified peptides. The data presented in Table S1 list the major proteins that were identified in our complex antigen sample, based on their total exclusive spectral counts.
Sensitization to peanut allergens and intraperitoneal challenges
For active sensitization to PN, mice were given 7 doses of PN antigen every 48h. Beginning week 5, desensitization therapy was initiated, which involved subcutaneous injection in the right rear footpad of soluble PN antigen (1μg) combined with nanoparticle-encapsulated cytokines (or appropriate controls) in a 20uL volume of PBS. Injections were repeated every two weeks for a total of 4 desensitization treatments. At week 12, animals were challenged with 0.5mg of PN antigen by i.p. injection. Anaphylaxis was monitored by measuring the animals’ temperatures at regular intervals by using rectal thermometer probe.
Sensitization to peanut allergens and oral challenges
For oral sensitization, mice were sensitized for 8 weeks with a low dose (50μg) of PN antigen by oral gavage, followed by two weeks of high dose oral gavage boosts (1mg) containing cholera toxin (CT), based on a published protocol4. Control mice received CT alone in PBS. The cutaneous desensitization protocol was initiated 3 weeks after beginning the high-dose sensitizing boosts and followed the same procedure, as described above, with 4 bi-weekly desensitization injections. Mice were challenged with 500μg of PN antigen in PBS by oral gavage and anaphylaxis was monitored, as above, by body temperature measurement.
Measurement of Th1/Th2 immune responses
For mice receiving oral PN sensitization and cutaneous desensitization therapy, blood was collected at the time points indicated in Fig. 1e and serum was isolated. For ELISAs, PN antigen was suspended in CBC buffer (15 mM Na2C03, 35 mM NaHCO3, pH 9.6) at a concentration of 2μg/mL, plated into 384-well microtiter plates and incubated overnight at 4°C. CBC buffer containing 3% nonfat dry milk was used to block, followed by washing four times with ELISA wash buffer (PBS, 0.05% Tween-20, 0.1% sodium azide). Serum samples were diluted 6-fold in serum diluent (PBS, 2.5% BSA, 2.5% nonfat dry milk, 5% goat serum, 0.1% sodium azide, 0.05% Tween-20) and added to ELISA plates that were incubated overnight at 4°C. After washing, alkaline phosphatase-conjugated anti-mouse IgG1, anti-mouse IgG2a, or anti-mouse IgE were added to the plates (Southern Biotechnology Associates), diluted 1:8000 in buffer containing PBS, 0.05% Tween-20, and 0.5% BSA. Plates were incubated at room temperature for 2h, washed four times with ELISA wash buffer and reacted with the alkaline phosphatase substrate Attophos (Roche Molecular Biochemicals). After incubation for 15 min, plates were read at 405 nm on a FluoroCount plate reader (Packard Instrument Company). Samples were considered positive for antigen-specific antibody when the relative light unit (RLU) value for the sample dilution was 2-fold higher than the RLU for a naive sample at the same dilution. To assess cellular responses after desensitization therapy, the popliteal LNs (which drains the footpad site of desensitization) were isolated from mice at the time point indicated in Fig. 1e, minced in RPMI containing 10% FBS and incubated for 30 min at 37°C with 100U/ml of Col lagenase A (Sigma). Single cell suspensions were produced by straining the disrupted LNs through a 70μM cell straining filter (BD Biosciences). After blocking with 1%BSA in PBS for 30min, cells were stained with the following antibodies: anti-CD3-FITC (Invitrogen), anti-CD11c-APC (BD Biosciences), and anti-PDL1-PE (ebioscience). After surface stains were completed, cells were fixed with 4% paraformaldehyde and treated with 1% saponin in PBS containing 1% BSA for intracellular staining using anti-IFN-PerCP-Cy5.5 (BD Biosciences). All data were acquired using a FACscaliber (BD Biosciences) and analyzed with CellQuest software.
Statistical analysis
Prism software was used for all statistical analyses. Flow cytometry and ELISA experiments were analyzed by 1-way ANOVA and temperature readings were analyzed by 2-way ANOVA, with Bonferroni’s multiple comparison test to determine p-values. Unless otherwise noted, error bars throughout the figures represent the SEM and n=5 animals per group. For all experiments, results were considered significant for p>0.05.
Supplementary Material
Acknowledgments
This work was funded by US National Institutes of Health grants R01 AI96305, R01 AI35678, 18 R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panel NI-SE, Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabar AI, Arroabarren E, Echechipia S, Garcia BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127:57–63. e1–3. doi: 10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–2. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan SA, O’Connor BJ, Matti S, Leckie MJ, Kanabar V, Khan J, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2149–53. doi: 10.1016/S0140-6736(00)03497-8. [DOI] [PubMed] [Google Scholar]
- 5.St John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–7. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helm RM, Burks AW. Mechanisms of food allergy. Curr Opin Immunol. 2000;12:647–53. doi: 10.1016/s0952-7915(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 7.Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy. 1999;29(Suppl 2):33–6. [PubMed] [Google Scholar]
- 8.Kind LS. Fall in rectal temperature as an indication of anaphylactic shock in the mouse. J Immunol. 1955;74:387–90. [PubMed] [Google Scholar]
- 9.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 1.St John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–7. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berglund JP, Szczepanski N, Penumarti A, Beavers A, Kesselring J, Orgel K, et al. Preparation and Analysis of Peanut Flour Used in Oral Immunotherapy Clinical Trials. J Allergy Clin Immunol Pract. 2017;5:1098–104. doi: 10.1016/j.jaip.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burks AW, Williams LW, Connaughton C, Cockrell G, O’Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90:962–9. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 4.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


