Abstract
Carotenoids are essential light-harvesting pigments in natural photosynthesis. They absorb in the blue–green region of the solar spectrum and transfer the absorbed energy to (bacterio-)chlorophylls, and thus expand the wavelength range of light that is able to drive photosynthesis. This process is an example of singlet–singlet excitation energy transfer, and carotenoids serve to enhance the overall efficiency of photosynthetic light reactions. The photochemistry and photophysics of carotenoids have often been interpreted by referring to those of simple polyene molecules that do not possess any functional groups. However, this may not always be wise because carotenoids usually have a number of functional groups that induce the variety of photochemical behaviours in them. These differences can also make the interpretation of the singlet excited states of carotenoids very complicated. In this article, we review the properties of the singlet excited states of carotenoids with the aim of producing as coherent a picture as possible of what is currently known and what needs to be learned.
Keywords: carotenoids, photosynthesis, singlet states
1. Introduction
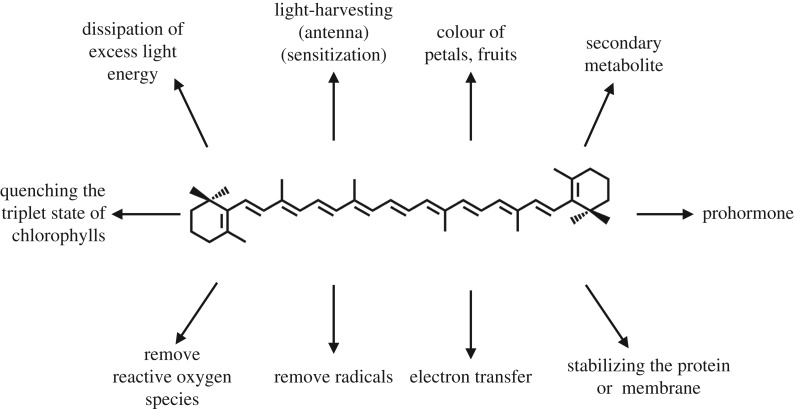
Carotenoids are a class of natural pigments. Over 750 species of carotenoid have been found in nature and have had their chemical structures determined [1,2]. Different carotenoids can have a wide variety of functions [3–8]. As illustrated in figure 1, they have light-harvesting and photoprotective functions in photosynthesis [9], they have pro-vitamin A activity in vision [10–12], they act as antioxidants in animals [13–16], they have an anti-ageing activity [17–19], they can stimulate the immune system [20,21] and they can exhibit an antitumour activity [22–24]. This extreme functional diversity means that what you see depends on which carotenoid you look at. There is a tendency for chemists to think that all carotenoids are the same as β-carotene. They are of course not, and this has led to considerable misunderstanding. Among the various functions of carotenoids listed above, those found in photosynthesis are the most well studied. However, especially in the case of the involvement of carotenoid excited singlet states, there are still a number of open questions, debates and indeed contradictions. In this review, we especially focus on the properties of these singlet excited states and their involvement in photosynthetic light harvesting. Trying to fully understand the molecular details of photosynthetic light harvesting so that they can be replicated in robust chemical systems is a major target of artificial photosynthesis [25].
Figure 1.
A schematic illustration that summarizes the functions of carotenoids in physiological systems. Chemical structure of β-carotene is shown as a representative of carotenoids.
Chlorophylls are the major light-absorbing pigments in photosynthesis. However, they cannot efficiently absorb light in the 450–550 nm region where the solar radiation profile (spectrum) at the surface of earth has its maximum intensity. This is precisely the region where carotenoids absorb light strongly. They are able to transfer this excitation energy to the chlorophylls by singlet–singlet excitation energy transfer, thereby making it available to power photosynthesis [26,27]. This energy-transfer reaction allows the carotenoids to function as accessory light-harvesting pigments, broadening the spectral range over which light can support photosynthesis. This role of carotenoids is particularly significant in the cases of purple photosynthetic bacteria, heterokontophyta (e.g. diatoms and brown algae) and dinoflagellates, all of which tend to occupy environmental niches where light intensity is usually limiting for growth. Especially in the case of dinoflagellates, for example, in their position in the water column most of the available solar energy is in the 450–550 nm region [26] and hence most of their photosynthesis is powered by light absorbed by carotenoids.
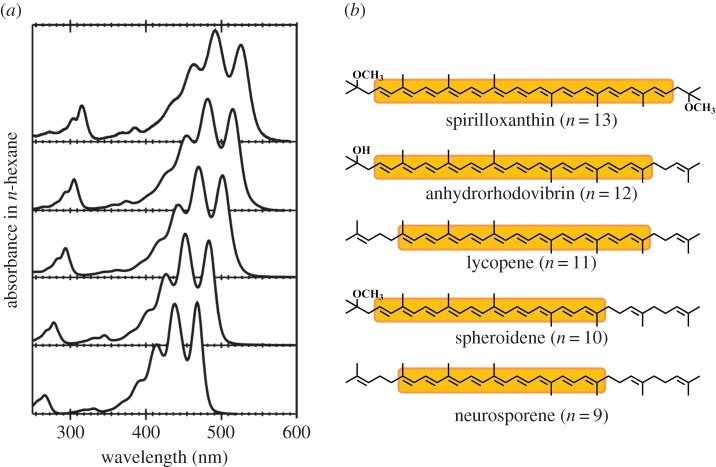
As illustrated in figure 2, carotenoids have strong absorption of visible light in the blue and green regions of the spectrum. This is why most of the carotenoids found in photosynthetic organisms have characteristic yellow, orange and red colours. The lowest excited singlet (S1) state in most pigment molecules represents the lowest energy, optically allowed one-photon transition from the ground state. The energy of this state then controls the colour of that pigment molecule. However, carotenoids have a non-standard pattern of excited states. The lowest energy, optically allowed excited singlet state is not the lowest energy singlet state. The lowest singlet excited energy state is formally a one-photon forbidden state. This unusual photophysical pattern is explained classically using symmetry rules that have been developed from studies of linear polyene molecules. In what follows below, we first describe the assignment of the S1 state from a historical point of view. Then we will expand this topic by considering other possible optically forbidden singlet excited states. Finally, time-resolved and coherent spectroscopy using the ultrafast laser facilities having time resolutions beyond 100 fs will be discussed because many of these states only exist on this ultrafast timescale. There are a number of good review articles that have already been published on the photophysics and photochemistry of carotenoids [9,25,28–34]. Therefore, we will concentrate here on the most recent studies as well as important milestones that have set the scene of our current understanding.
Figure 2.
(a) Absorption spectra in n-hexane solutions and (b) chemical structures of carotenoids bound to purple photosynthetic bacteria. Conjugated polyene backbones are highlighted with orange rectangles in (b).
2. The lowest excited singlet (S1) state of carotenoids
The photophysics and photochemistry of carotenoids are usually explained by referring to the results from the study of polyene molecules. Polyenes are linear conjugated chains of carbon atoms joined by alternating double and single bonds. According to the ‘classical’ textbook written by Hudson et al. [35], polyenes were (and continue to be) deservedly the objects of a good deal of experimental and theoretical attention. The historical importance of polyenes is due to their involvement in the development of molecular quantum theory and the understanding of fundamental molecular mechanisms of cis–trans photoisomerization. Before the pioneering work by Hudson & Kohler [36–38], polyenes were thought to be rather simple molecules, similar to that of other conjugated systems such as the polyacenes, and well described by approximate molecular orbital ideas. However, this turned out not to be the case. Referring to the exact words of Hudson et al. [35], ‘Polyene electronic structures are both more complicated and more interesting than was previously thought.’
The theoretical assignment of the electronic structures of unperturbed linear polyene molecules comes from the application of symmetry rules. Planarity and the C2 h point symmetry are the essential properties required to allow the precise designation of the singlet excited states of the polyene molecules to be described [38]. It is worth pointing out here that interpretations from simple linear polyenes can only be approximately applied to carotenoids because the presence of the methyl groups perturbs the planarity of the conjugated portion of the carotenoid molecules. It also should be pointed out here that the carotenoids in the light-harvesting (LH) complexes from phototrophs are distorted significantly so that the selection rules and the standard symmetry labels ought not to be relevant. In particular, the ‘dark’ Sx and S* states might not be properly understood in the C2 h framework. The recent paper by Fiedor et al. [39] makes this point explicitly, arguing in particular that the S1 state does not have oscillator strength not because of the selection rules but rather because of large distortions from planar conformations. This important issue will be touched upon again in a later section.
The presence of an excited singlet state, nearly forbidden in absorption, below the dipole-allowed state (11Bu state in C2 h symmetry) previously thought to be the lowest lying excited singlet, was first found for α,ω-dipenyl-1,3,5,7-octatetraene [36]. This finding was theoretically rationalized by Schulten & Karplus [40]. This one-photon forbidden lowest singlet excited state was characterized as a ‘doubly excited’ Ag state (21Ag state), which was only poorly described without extensive configuration interaction at that time. This ordering of electronic states, 21Ag below 11Bu, is now recognized as a general feature of polyene molecules whose number of conjugated double bonds (n) is greater than 4 [35]. The identification of the S1 (21Ag) state of short polyenes (n = 4–8) is due to measurements of fluorescence and fluorescence-excitation spectra [35,41,42]. It is noteworthy here, however, that a subtle reinterpretation of these observations may be required, because the S1 (21Ag) → S0 (1Ag) fluorescence experiments previously thought to have been carried out on pure all-trans isomers might actually be distorted due to the presence of cis isomers as impurities or formed as photochemical products from the S1 state [43]. Nevertheless, the overall state ordering has undoubtedly been correctly predicted by theoretical work on simple all-trans polyenes [44,45].
Tavan & Schulten [44,45] extended their theoretical work on linear polyenes. They described the excitations within Pariser–Parr–Pople (PPP) and Hubbard models by means of a multiple-reference double-excitation expansion. The PPP and Hubbard Hamiltonians give rise to another symmetry, the so-called ‘Pariser alternancy symmetry’ or ‘particle-hole symmetry’ [46]. This symmetry classifies ‘–’ and ‘+’ states, e.g. the ground state is designated as 11Ag– and the lowest optically allowed state as 11Bu+. The alternancy symmetry is useful for two reasons. Firstly, it allows the computational time to be reduced by taking account of the fact that the PPP and Hubbard many-electron Hamiltonians do not mix 1Ag–, 1Ag+, 1Bu– and 1Bu+ states. Secondly, the alternancy symmetry provides a very simple classification of ionic states that are ‘+’, and covalent states that are ‘–’. The singlet excited states of carotenoids are often designated in a similar manner by referring to this classification.
The lowest excited singlet (S1) state of carotenoids is frequently designated as the one photon forbidden 21Ag– state, assuming both the planarity and C2 h point symmetry of their polyene backbones. Hashimoto and Koyama were first to determine the Ag character of the S1 state of carotenoids using picosecond transient resonance Raman spectroscopy, which was then confirmed by Noguchi et al. [47–50]. These studies were further extended using a series of mono-cis isomers of both symmetric and asymmetric carotenoids [51,52]. The S1 species of carotenoids give rise to characteristic C = C stretching Raman lines at extraordinary high frequencies above 1750 cm−1. This unusual observation was explained based by the idea of vibronic coupling between the S0 (11Ag–) and S1 (21Ag–) states through ag-type C=C stretching symmetric vibration [53–55]. Therefore, the presence of the extraordinary high-frequency shifted C=C stretching mode can be taken as good evidence that the S1 electronic state of carotenoids has an Ag character that can induce vibronic coupling with the ground S0 state. The lowest singlet excited state, S1 has 21Ag− symmetry, and hence a one-photon-induced transition from the ground state is optically forbidden. The lowest optically allowed state is the 11Bu+ (S2) state. When the 11Bu+ state is induced by a short excitation pulse, it decays internally into the 21Ag− state within 100–300 fs. The S1 state typically then decays back to the ground state in a few picoseconds [56]. The exact rate constant of these processes depends upon factors such as the number of conjugated double bonds (n).
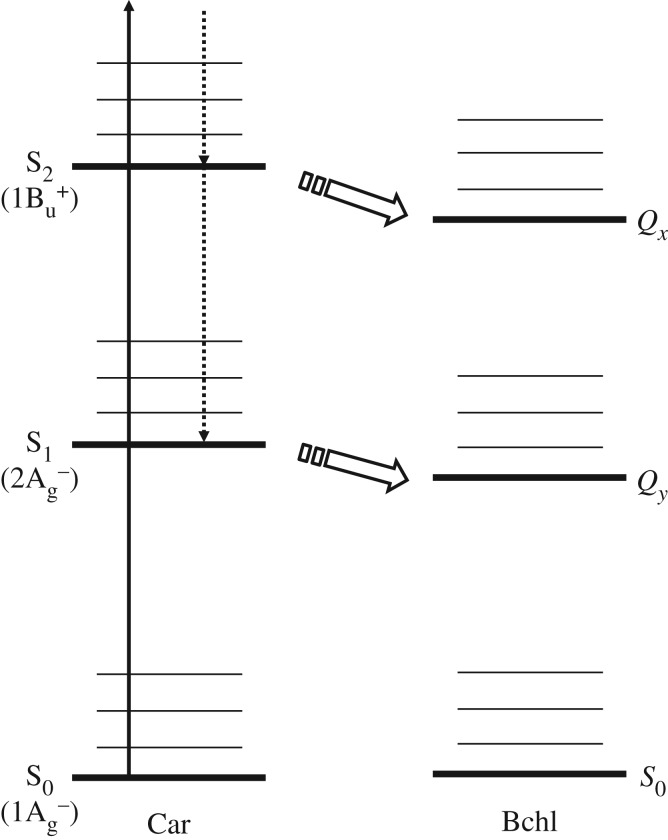
When the light-harvesting role of carotenoids in pigment–protein complexes from purple bacteria is considered, a simplified view is usually presented. This simplified picture is illustrated in figure 3. Probably the easiest way to begin this discussion is to describe the experiments of Macpherson et al. [57]. These authors compared the excited state kinetics of a carotenoid in organic solvent with the same carotenoid when bound within an LH2 complex. The idea was that any carotenoid singlet excited state that was capable of transferring energy to bacteriochlorophyll a in LH2 would be expected to have a shorter excited state lifetime in the antenna complex than in the organic solvent. Any energy transfer pathway would open another decay channel, thereby accelerating the overall rate of decay of that excited singlet state. Moreover, it was expected that the decay of the donor carotenoid excited singlet state should match the rate of the energy arriving at the acceptor bacteriochlorophyll a molecule. In the case of the carotenoid rhodopin glucoside the decay of the S2 state in the LH2 complex from Rbl. acidophilus was faster than that of the carotenoid in organic solvent (56 fs in LH2 and 133 fs in benzyl alcohol). The rise time of the arrival of the energy at both B800 and B850 matched the decay time of rhodopin glucoside's S2 state. The decay rate of the S1 state of rhodopin glucoside was the same in the LH2 complex as in organic solvent. In this case, the S1 state is not active in energy transfer to bacteriochlorophyll a. However, the LH2 complex from Rba. sphaeroides contains carotenoids with fewer conjugated double bonds and, in this case, the S1 state is able to transfer energy to the bacteriochlorophyll a molecules and its decay is accelerated in LH2 compared with organic solvent [58]. These findings show clearly how the S2 and S1 states are involved in determining the overall efficiency of excitation energy transfer from carotenoid to bacteriochlorophyll in the bacterial light-harvesting systems. The excited-state lifetimes of the S1 and S2 states of carotenoids are essential pieces of information when possible mechanisms of carotenoid-to-(bacterio)chlorophyll singlet–singlet energy transfer are being considered.
Figure 3.
Relative energy levels of the S1 and S2 excited singlet states of a typical carotenoid relative to bacteriochlorophyll (Bchl) a Qx and Qy transitions. These energy levels are valid for carotenoids containing up to 10 conjugated double bonds. Thick lines show the electronic states and thin lines show the vibrational states. When a carotenoid molecule is excited up to the S2 state (solid arrow), it shows vibrational relaxation and/or internal conversion to the S1 state (broken arrows). This process competes for the energy transfer (box arrows) to Bchl a.
The exact energies (0–0 origin) of the S2 and S1 states relative to those of the Qx and Qy states of (bacterio)chlorophyll are also important. The energy of the S2 state can be determined easily by ordinary absorption measurements because the transition from the ground (S0) to S2 states is optically allowed. On the contrary, the determination of the energy of the S1 state is not straightforward because of the optical forbiddenness. In the past, carotenoids had been thought to be non-fluorescent [59]. However, carotenoid fluorescence has clearly been demonstrated [60–66]. Although the fluorescence quantum yields from the S1 state are of the order of 10−5, the 0–0 origin of the S1 state has been suggested. Location of the S1 state has also been determined independently using the energy-gap law [67], by resonance Raman excitation profile measurements [68,69], and by near-IR (S1 → S2) transient absorption measurements [70,71]. Table 1 summarizes the singlet excited-state energies of various carotenoids reported so far. Depending on the methods that were used for determination, small but not negligible deviation of these values can be seen. Nevertheless, carotenoid to (bacterio)chlorophyll energy transfer has been discussed in most cases based on the very simple energy diagram as illustrated in figure 3 [77]. This, however, is probably an oversimplification as theoretical studies based primarily on symmetrical polyenes have predicted other possible excited singlet states such as 31Ag− and 11Bu− [44,45] (vide infra). The possibility of these multiple excited states, and indeed others, has made understanding carotenoid photophysics extremely complicated [75].
Table 1.
S0 (11Ag−) → S2 (11Bu+) and S0 (11Ag−) → S1(21Ag−) transition energies of various carotenoids. n, number of conjugated double bonds; THF, tetrahydrofuran; EPA, ether/isopentane/ethanol = 5/5/2(v/v/v); RT, room temperature.
| transition energy (cm−1) |
condition |
|||||
|---|---|---|---|---|---|---|
| n | carotenoid | S0(11Ag−) → S2(11Bu+) | S0(11Ag−) → S1(21Ag−) | solvent, temperature (K) | references | |
| 13 | all-trans-spirilloxanthin | 18 900(100) | 5% benzene/3-methyl pentane | 295,150 | [72,73] | |
| 18 083 | THF | RT | [74] | |||
| 12 | all-trans-anhydrorhodovibrin | 19 400 | n-hexane or 3-methyl pentane | 295 | [72] | |
| 19 100(100) | n-hexane | 295,150 | [72,73] | |||
| 11 | all-trans-β-carotene | 22 040 | n-hexane | 293 | [60] | |
| 21 390(100) | toluene, isopentane | 293,77 | [60] | |||
| 20 890(150) | quinoline | 293 | [60] | |||
| 20 300(100) | 13 200(300) | n-hexane | 170,RT | [63] | ||
| 19 700(100) | 13 100(300) | toluene | RT | [63] | ||
| 19 580 | isopentane | 4.2 | [60] | |||
| 19 380 | carbon disulfide | RT | [61] | |||
| 19 150 | n-hexane | 77 | [10] | |||
| 18 800(100) | 13 200(300) | carbon disulfide | RT | [75] | ||
| 18 400 | 14 500 | single crystal | 11 | [68] | ||
| β-apo-4'-carotene | 19 700(10) | EPA | 77 | [66] | ||
| all-trans-lycopene | 19 800(200) | n-hexane, acetone | 170,RT | [73] | ||
| 18 600 | n-hexane | 77 | [75] | |||
| 13 300 | 5% benzene/3-methyl pentane | RT | [76] | |||
| 10 | β-apo-6'-carotene | 20 450(15) | EPA | 77 | [66] | |
| rhodopin | 18 490 | carbon disulfide | RT | [61] | ||
| all-trans-spheroidene | 20 800 | methanol | RT | [64] | ||
| 20 250(150) | 14 200 | n-hexane | 200 | [65,72] | ||
| 19 600 | 14 200 | film, n-hexane | 77 | [69] | ||
| 18 138 | carbon disulfide | RT | [61] | |||
| 14 250(50) | n-hexane | 77, RT | [75,76] | |||
| 9 | all-trans-neurosporene | 21 100 | n-hexane | 190 | [65] | |
| 20 880(80) | n-hexane | 190, RT | [65] | |||
| 19 800 | n-hexane | RT | [61] | |||
| 15 300 | n-hexane | RT | [76] | |||
| β-apo-6'-carotenol | 20 490 | EPA | 77 | [62] | ||
| isozeaxanthin | 19 890 | EPA | 77 | [62] | ||
3. The 11Bu− and 31Ag− states
The efficiency of carotenoid-to-(bacterio)chlorophyll singlet energy transfer in light-harvesting complexes varies from 30 to nearly 100%, depending on the species of photosynthetic bacteria [29,30,31]. Until recently, it was thought that the mechanism of carotenoid-to-bacteriochlorophyll energy transfer could be fully explained based on the energy diagram illustrated in figure 3. The lifetimes of these singlet excited states depend on the extent of conjugation. In the case of β-carotene, for example, the lifetime of the S2 state is as short as 200 fs, while that of the S1 state is as long as 10 ps [56]. Time-resolved fluorescence spectroscopy with sub-picosecond time resolution shows clearly that energy transfer can take place from both the S2 and S1 states to bacteriochlorophyll [57]. The efficiency of carotenoid-to-bacteriochlorophyll energy transfer depends on how effectively the energy can be harvested from both these excited states.
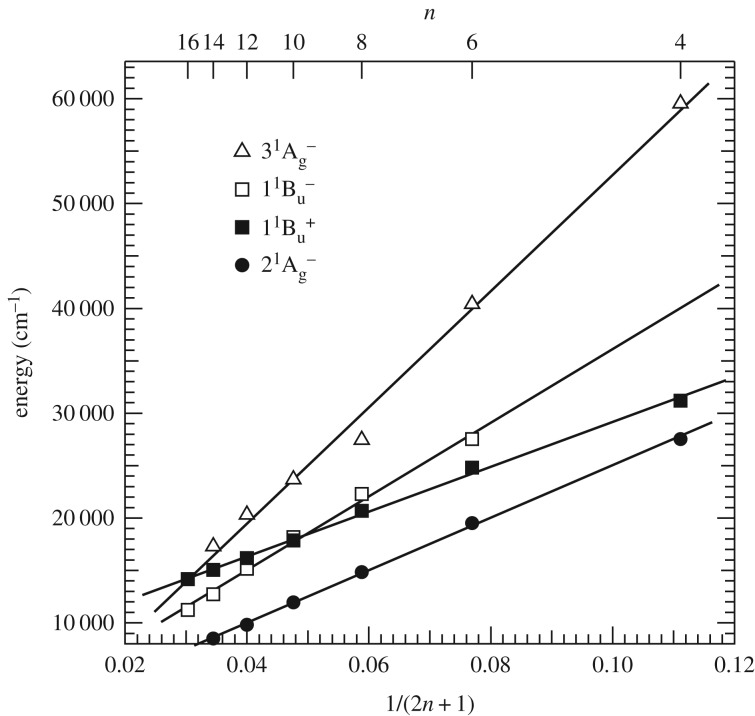
However, recent suggestions that other low-lying one-photon forbidden excited singlet states of carotenoids may also be involved in light harvesting have made the story more complicated. This is illustrated in figure 4, which shows the results of theoretical calculations by Tavan & Schulten [44,45]. They have predicted the presence of another one-photon forbidden singlet-excited state, namely the 11Bu– state, between S2 and S1 in the case of shorter polyene molecules with greater than four C=C double bonds. Indeed, extrapolation of their calculations suggests the presence of yet an additional 1Ag– (31Ag–) state between S2 and S1 for long polyene molecules with more than 10 C=C double bonds. Kurashige et al. [78] confirmed these predictions with more modern quantum chemical computations. They have applied multi-reference Møller–Plesset perturbation theory with complete active-space configuration interaction (CASCI-MRMP) to the study of the valence π → π* excited states of all-trans linear polyenes C2nH2n+2 (n = 3–14). This theory predicts that the 11Bu– state becomes lower than the 11Bu+ state at n ≥ 7 and that the 31Ag– state also becomes lower than the 11Bu+ state at n ≥ 11. This theoretical treatment has also been successfully expanded to the studies of all-trans α,ω-diphenyl polyenes and oligoacenes [79,80].
Figure 4.
Energy diagram calculated by Tavan & Schulten [44,45] using the PPPMRD-CI method for the low-lying singlet excited state of polyenes (n = 4–16).
These theoretical predictions were supported experimentally by the group of Koyama et al. using resonance Raman excitation profiles on solid crystalline carotenoids [75,81,82] and fluorescence, as well as steady-state absorption spectroscopies [72,73,83]. They further extended their studies using sub-picosecond time-resolved absorption and stimulated Raman spectroscopies [30,31,58,74,76,84–96]. They have interpreted all the observed excited-state dynamics of carotenoids following photoexcitation based on the ordering of the forbidden singlet excited states (31Ag–, 11Bu–, 21Ag–) presented by Tavan and Schulten. However, it should be noted that the spectral analyses of Koyama et al. probably depend too much on the application of a kinetic model that only considers the theory of Tavan and Schulten as a possibility. They also extended their analysis of their data beyond their actual detection limit of 100 fs time resolution. The conclusions of Koyama et al. now need to be tested with improved time resolution to see if they are still correct.
4. The other forbidden singlet excited states (S*, SX and X)
Another type of intermediate excited state, termed S*, has been found with carotenoids both free in solution and bound to light-harvesting complexes, revealing a further level of complication [97–101]. At the higher-energy side of the S1 → Sn transition, a new transient absorption band was detected by means of pump–probe time-resolved absorption spectroscopy and subsequent spectral analysis using SVD (singular value decomposition) and global fitting. This newly identified absorption band was assigned to the S* state. The lifetime of this particular state was determined to be between 5 and 12 ps, depending on both the species of carotenoid and whether it was present in the light-harvesting complex or in organic solvent. The S* state decayed into the triplet state when the carotenoid was bound to the LH1 or LH2 complex. However, when the carotenoid was free in organic solvent, the S* state decayed to the ground state without generating the triplet state. Applying a pump–dump and transient absorption technique for β-carotene, lycopene and zeaxanthin, Wohlleben et al. [100] re-examined the origin of the S* state with the carotenoid free in solution (S*sol). They suggested that the S*sol state is a vibrationally excited level of the electronic ground state (S*sol = hot S0), which is populated by a combination of impulsive Raman scattering of the pump pulse and S1 → S0 internal conversion. They also found the S* state of the protein-bound carotenoid and redesignated it as S*T. These ideas have recently been supported by Hashimoto et al. for spirilloxanthin both free in solution and bound to light-harvesting complexes [101]. However, the debate on the characterization of the S* state is still ongoing. Beck et al. [102] reinterpreted the radiationless decay of carotenoids after photoexcitation up to the S2 state by referring to a model derived from studies of polymethine cyanines [103]. They suggested that the S* state can be assigned to a low-lying S1 state structure with intramolecular charge transfer character and a pyramidal conformation (figure 5). On the other hand, quite recently, the group of Hauer et al. [104] have challenged this idea and presented a comprehensive and unified interpretation of S*-related features. They explained the features by vibronic transitions either from S1, from vibrationally excited levels on S0, or from both, depending on the chain length of the carotenoid investigated (figure 6). These discrepancies in the interpretation of the observed spectral data clearly demonstrate that while it is easy to measure spectral changes, it can be difficult to assign them. The problem is trying to determine from an absorption change whether it reflects different electronic states or different vibrational states. Involvement of vibrationally excited states in the relaxation process of carotenoids after photoexcitation was initially detected by time-resolved absorption spectroscopy [71,105–108], and has also been studied by time-resolved stimulated Raman spectroscopy [109–111].
Figure 5.
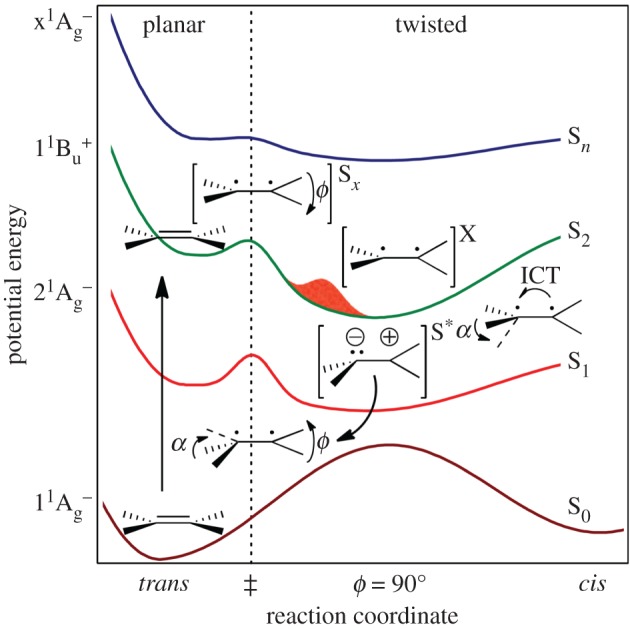
Proposed scheme for radiationless decay of carotenoids after optical preparation of the S2 state. The states that apply to planar structures are indicated by symmetry labels. Key points along the path back to the initial planar ground-state conformation are labelled with ethylenic structures, depicting the Sx, X and S* dark states as twisted structures near the S2 transition state and the S2 twisted minimum and pyramidal structures near the S1 state minimum, respectively. (Reproduced with permission from [102].)
Figure 6.
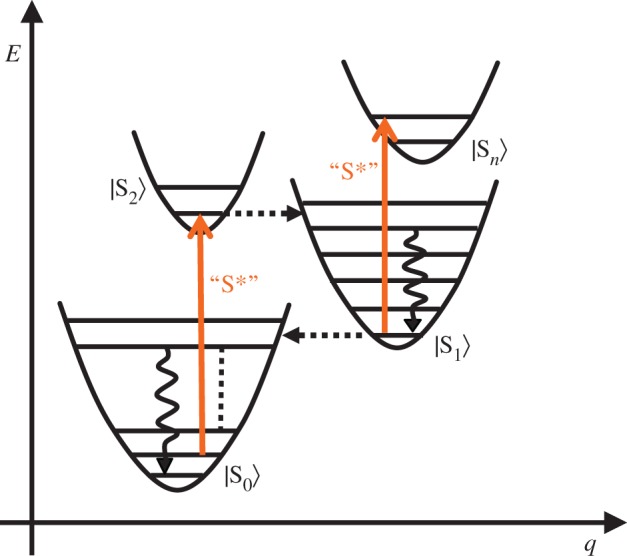
Energy-level scheme describing electronic (dashed horizontal arrows) and vibrational (wavy vertical arrows) energy relaxation in carotenoids along a reaction coordinate. Coloured vertical arrows indicate allowed electronic transitions. Electronic states are labelled in ket notation. (Reproduced with modification from [104] with permission.)
Carotenoids that contain carbonyl groups have the possibility of forming intramolecular charge-transfer states (SICT). These states have been well documented in the case of carotenoids such as peridinin and fucoxanthin (figure 7 for chemical structures of these molecules) [107,108,112–119]. The importance of this charge transfer state seems to be that it allows carotenoid to chlorophyll energy transfer to be highly efficient. Readers who are interested in more details about this state should consult the excellent review by Polívka & Sundström [28]. In our recent study, it was demonstrated that a large part of excitation energy captured by fucoxanthin bound to FCP (Mozuku FCP) is transferred to Chl a via the coupled S1/ICT state, resulting from a strong electronic dipole interaction between fucoxanthin and Chl a [117]. This strong dipole interaction was attributed to the ICT character of the excited state of fucoxanthin, enabling it to enhance the transition dipole moment of the S1/ICT state. Indeed, the enhancement of the excitation energy-transfer efficiency from carotenoid to bacteriochlorophyll is demonstrated by incorporating fucoxanthin into the LH1 complexes from a purple photosynthetic bacterium [120]. However, the nature and origin of the S1/ICT state of carbonyl carotenoids is yet to be fully understood. To try to get more information on this point, the ultrafast excited state dynamics of fucoxanthin and its homologues have been investigated [115].
Figure 7.
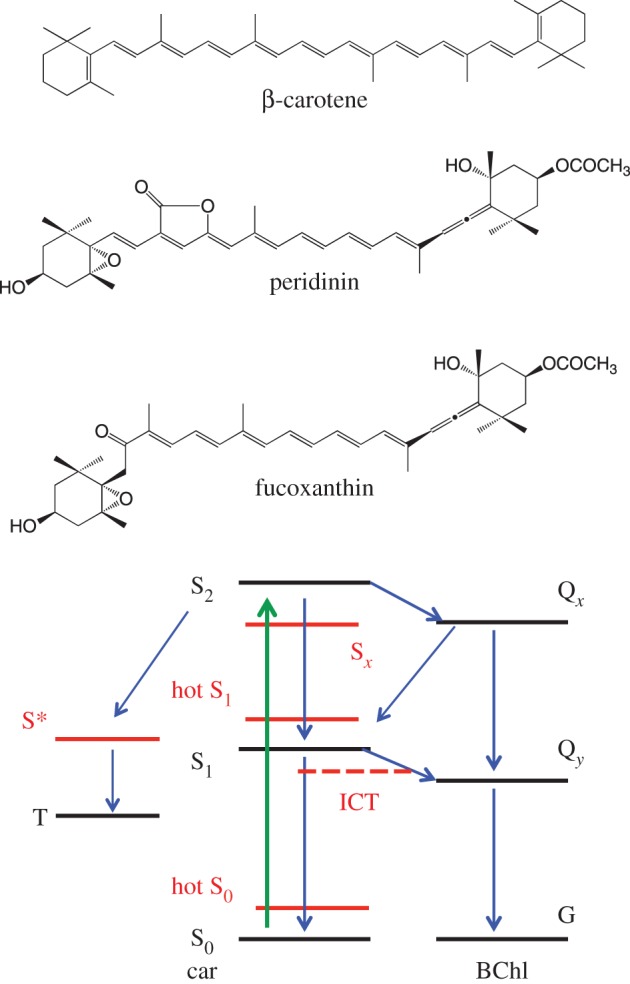
Chemical structures of β-carotene, peridinin and fucoxanthin, and a schematic description of energy diagrams together with relaxation and energy-transfer pathways of carotenoids following photoexcitation up to the S2 state.
The spectroscopic properties of fucoxanthin in polar (methanol) and non-polar (cyclohexane) solvents were studied [115]. Transient absorption associated with the optically forbidden S1 (21Ag−) and/or the ICT states were observed following one-photon excitation to the optically allowed S2 (11Bu+) state in methanol. The transient absorption measurements carried out in methanol showed that the ratio of the ICT-to-S1 state formation increased with decreasing excitation energy. The ICT character was clearly visible in the steady-state absorption in methanol based on a Franck–Condon analysis. The results suggest that two spectroscopic forms of fucoxanthin, blue and red, exist in a polar environment. The spectroscopic properties of fucoxanthin in methanol were further studied by femtosecond pump–probe measurements in the near-infrared region, where transient absorption associated with the optically allowed S2 (11Bu+) state and stimulated emission from the strongly coupled S1/ICT state were observed following one-photon excitation to the S2 state [118]. The results showed that the amplitude of the stimulated emission from the S1/ICT state increased with decreasing excitation energy, demonstrating that the red form of fucoxanthin exhibits a stronger ICT character. The magnitude of ICT character of carbonyl carotenoids has often been evaluated from (1) solvent polarity-dependent S1/ICT lifetimes, (2) amplitudes of the ICT transient absorption and stimulated emission bands and (3) the dipole moment of the ICT state. Femtosecond pump–probe spectroscopic measurements were performed on fucoxanthin homologues with varying numbers of conjugated double bonds (n = 4–8) [119,121]. The ICT properties of fucoxanthin homologues were characterized by the S1/ICT lifetimes and the transient absorption and stimulated emission bands due to the S1/ICT state.
Figure 7 shows a schematic illustration of the relative energies of the carotenoid excited singlet states discussed above together with the proposed relaxation pathways from the S2 state as well as the energy-transfer pathways between carotenoid and bacteriochlorophyll. As the relaxation from the S2 state is very fast, ultrafast vibrational spectroscopies are going to be important to try to clarify further the structure–function relationship of the above singlet excited states [34].
5. Time-resolved and coherent spectroscopies beyond 100 fs time resolution
Recently, it has become possible to use much shorter femtosecond pulses. When this was done by Cerullo et al. [122], the data claimed the presence of an intermediate state between S2 and S1. This state was formed as S2 decayed and gave rise to S1 as it decayed. However, with these extremely fast reactions it was not possible to be sure that this intermediate state was another pure excited singlet state such as 11Bu−. Therefore, this dark state was tentatively designated as SX. Since this time there have been many studies that have suggested that such an intermediate state is required to fully explain the experimental data. It has also been suggested that these results could be due the appearance of a nonlinear optical effect [123]. There have even been further studies that have not seen or required the presence of such an extra intermediate state to fit the data [29]. This has led to a lot of confusion. Most recently, broadband two-dimensional (2D) electronic spectroscopic measurements on light-harvesting proteins from purple bacteria and isolated carotenoids have been performed in order to characterize in more detail the excited-state manifold of carotenoids that channel energy to bacteriochlorophyll molecules. The data revealed a well-resolved signal (cross peak) consistent with a previously postulated carotenoid dark state, the presence of which was confirmed by global kinetic analysis. The most recent results, therefore, suggest that a carotenoid dark state does have a role in mediating energy flow from carotenoid to bacteriochlorophyll [124], and this state was designated as X. All these findings clearly suggest the presence of a dark state in between S2 and S1. However, it is worth pointing out that it is worth looking back at some older up-conversion experiments by the group of Gillbro et al. [57]. Carotenoids in solution show the decay time 150 fs for S2 emission. If S2 in a few fs goes to another state, then one has to assume that the other intermediate state emits. If that state emits, why doesn't it absorb? This old emission study should not be forgotten and strongly implies that there is more to be understood here.
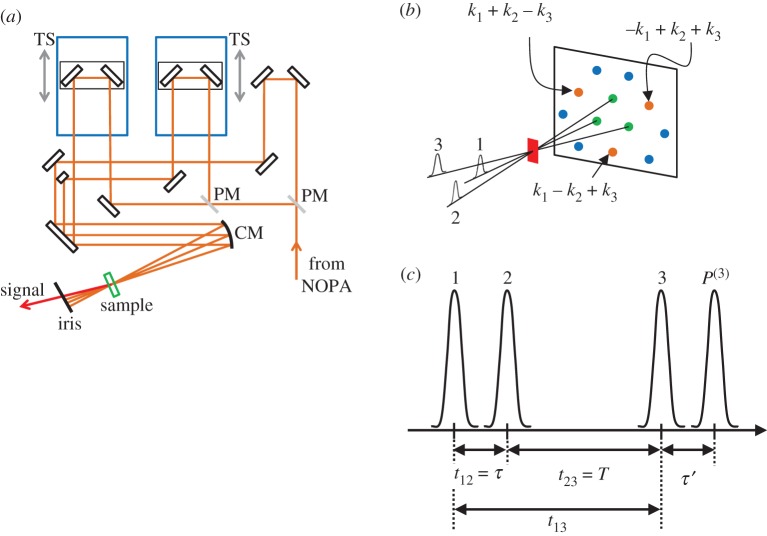
Another sophisticated ultrafast spectroscopic technique called four-wave mixing (FWM) or coherent spectroscopy has been employed in order to investigate the ultrafast photophysics of carotenoids. FWM measurement is performed with an optical configuration illustrated in figure 8a. Namely, laser light is split to three using a beam splitter, and two of them are independently guided towards translational stages in order to induce time delays among three laser pulses. If these three laser pulses are well focused onto a single spot in the sample, FWM signals can be observed. This type of optical configuration, where three laser pulses excite the sample from three distinct directions, is called the BOXCARS configuration.
Figure 8.
(a) Optical configuration of the interferometer used for the measurements of four-wave mixing (FWM) signals. The light from a non-collinearly phase-matched optical parametric amplifier (NOPA) is split into three using two pellicle mirrors (PM). Three laser pulses thus produced are then focused to excite the sample using a collimating mirror (CM). Time intervals between each of these three laser pulses were controlled using two translational stages (TS). The FWM signals are generated along the directions that satisfy the phase-match condition. One of these signals is selected using an iris diaphragm. (b) When three laser pulses are irradiated to the sample, the FWM signals can be generated along the directions that satisfy the phase-match conditions. (c) The relation between coherent time τ and population time T. t12 (t13) is the time interval between pulses 1 and 2 (1 and 3) when they arrive at the sample.
When such three laser pulses simultaneously reach to the sample (zero time delays), FWM signals are generated in the area surrounding the transmitted excitation laser light. As illustrated in figure 8b, if the wavevectors of excitation and signal lights are defined, respectively, as ki = (1, 2, 3) and ks, FWM signals appear at the direction that satisfies the relation of ks = ±k1 ± k2 ± k3 and ks = 2ki−kj(j = 1, 2, 3, and j ≠ i). It should be noted here that FWM signals are strong enough for carotenoids to be seen with the naked eye. This means that carotenoids are suitable molecules for investigation of their nonlinear optical responses. Information concerning the coherence can be obtained by investigating the time evolution of the FWM signals.
FWM signals in carotenoids have been reported for β-carotene and its homologues, lycopene, astaxanthin and spheroidene [125–137]. As an example, the results with β-carotene are given here. Figure 9a shows the time evolution of an FWM signal with β-carotene. In this example, the abscissa gives the time interval T between pulse 2 and pulse 3 (figure 8c). The time interval τ between pulse 1 and pulse 2 was set to be zero. The FWM signal that is measured under this condition is frequently called the transient grating (TG) signal. The intense signal that appears around the time origin in figure 9a is assigned as a coherent spike. Following this spike signal, a coherent vibration signal with a very fast period of oscillation of about 20–30 fs can be observed on top of the slowly decaying background that has a lifetime of about 5 ps.
Figure 9.
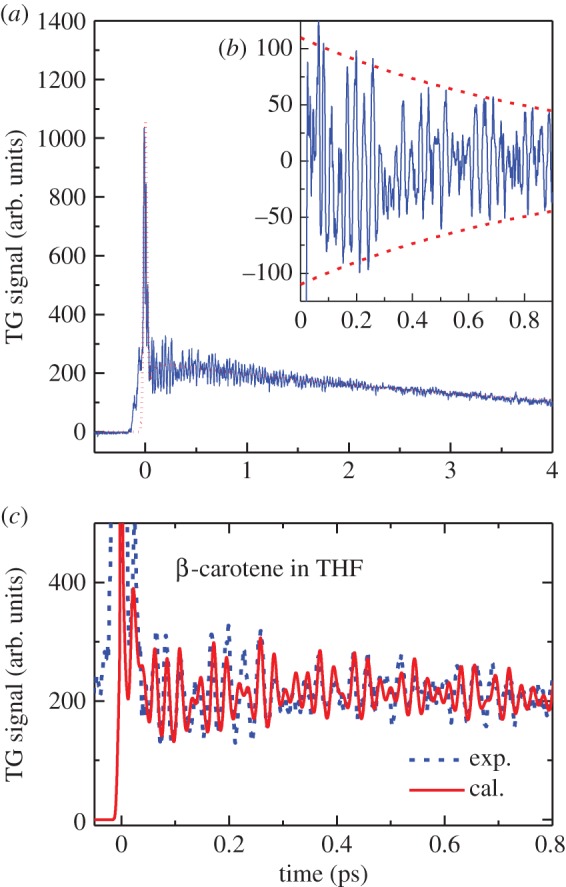
(a) Experimentally observed time evolution of the transient-grating (TG) signal. A slowly varying background shown with dotted lines reflects the lifetime of the electronic excited states. If the background is subtracted from the original TG signal, the coherent vibration component can be extracted as shown in (b). The decay time of coherent vibration is determined to be around 1 ps. (c) Comparison of the experimentally observed TG signal (broken line) and the result of theoretical calculation (solid line).
The origin of this coherent vibration can be clarified if the TG signal in figure 9b is Fourier transformed. It can be readily understood, based on the comparison with the Raman spectrum of β-carotene shown in figure 10b, that the peaks obtained by the Fourier transformation of the coherent vibration (figure 10a) show good coincidence with those of the ground-state Raman spectrum of β-carotene. Namely, the peaks that appeared at ν1 = 1522 cm−1 and ν2 = 1157 cm−1, respectively, are attributed to the totally symmetric vibration of C=C and C–C stretchings, and the peak that appeared at ν3 = 1007 cm−1 is attributed to the in-plane rocking vibration of methyl groups. These vibrational modes appeared because all the β-carotene molecules under inspection start to vibrate in phase, i.e. coherently, following the impulsive excitation with ultrashort laser pulses. The stretching vibrations of carotenoids usually appear in the 1000–1500 cm−1 frequency domain. These frequencies correspond to 30–20 fs in the time domain, if the frequency to time domain conversion is performed. Therefore, coherent vibration can be induced in carotenoids if we use the sub-20 fs ultrashort laser pulses for the FWM experiment.
Figure 10.
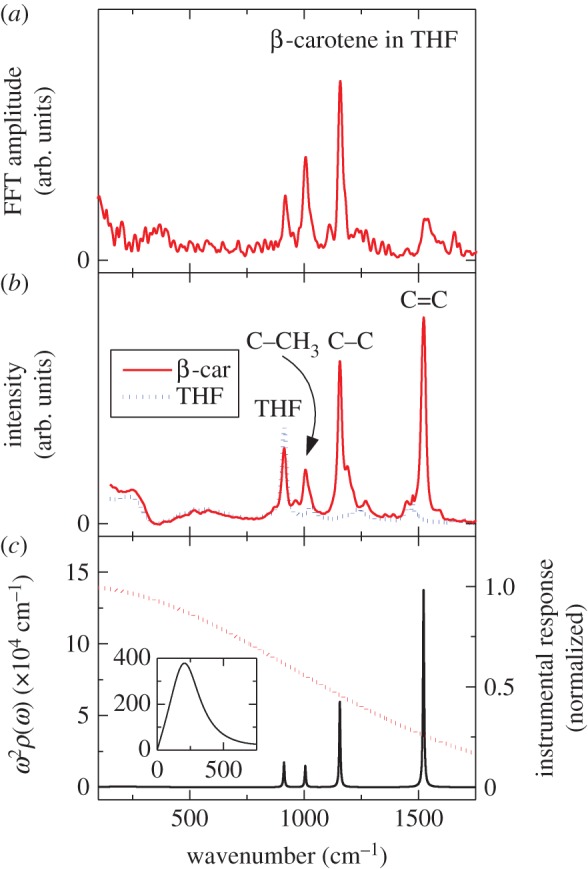
(a) Fourier-transformed spectrum of the coherent signal component shown in figure 9b. (b) Raman spectra of β-carotene and solvent THF (tetrahydrofuran). (c) Spectral density (solid line) and the response function of the detecting system (dotted line). The inset shows the spectral density that reflects the system–bath (β-carotene and solvent THF) interaction in the low-frequency regime.
The most important information that is obtained by the measurement of FWM signals is the coupling between carotenoids and their surrounding environment. This information is reflected in spectral density (figure 10c). It is known that there are couplings with slow vibrations of 100 fs (approx. 300 cm−1) or less in organic solvents [127,131,133]. Obtaining the spectral density, various optical responses including absorption and fluorescence spectra can be calculated and, therefore, the precise discussion on the experimental data based on theoretical models becomes feasible [138]. One of those examples, figure 9c, shows the results of the theoretical calculation for the FWM signal. The experimental result is in good agreement with the theoretical calculation. As shown here, spectral density includes meaningful information; however, there are a few reports on carotenoids bound to pigment–protein complexes [139]. On the other hand, many studies have already been performed on the coherent vibrations that directly reflect the effect of coupling with the surrounding environment in bacteriochlorophyll [140–147]. Revealing the correlation among these coherent vibrations as well as the role of coherent vibration in excitation energy transfer will be rewarding challenges for the future.
Coherent vibrations are also observed in the electronic excited state of carotenoids. The group of Motzkus et al. were able to observe the coherent vibration in the S1 state of β-carotene with 20 fs temporal and 10 cm−1 spectral resolutions [126,128,130,148–153]. They introduced a pre-pump pulse that excites β-carotene to its S2 state for FWM measurement (pump-FWM) to produce a populated S1 state via internal conversion from the S2 state. They concluded that the coherence of the molecular vibrations is not conserved during the process of S2 → S1 internal conversion [126]. They also claimed that the lifetime of coherent vibration in the S1 state is an order of magnitude smaller than that in the ground state for all the vibrational modes. Quite recently, they further extended their study to a series of open-chain carotenoids with different numbers of conjugated double bonds n = 9, 10, 11 and 13 (neurosporene, spheroidene, lycopene and spirilloxanthin, respectively), and a closed-chain carotenoid (lutein) [154]. They have interpreted their data on the relaxation from S2 to S1 based on the model including the forbidden singlet states (31Ag– and 11Bu–). They were successful to detect the frequency shift of the C=C stretching mode along the course of deactivation. They suggested that the vibrational dynamics directly after the initial excitation of carotenoids is dominated by two different vibronic couplings: (1) diabatic mixing between 1Bu states takes place only for shorter open-chain carotenoids (n = 9 and 10), where the vibrational levels of the 11Bu+ and 11Bu– states are energetically close (figure 11). The interaction between these states leads to a typical frequency downshift after the deactivation of the Franck–Condon region. (2) Adiabatic vibronic coupling between the 1Ag states is a well-known general feature of the Raman spectra of carotenoids (see §2), which is responsible for the generation of the typical S1 C=C stretching frequency at 1800 cm−1. Nevertheless, their results suggest that it does not lead to any modification of the vibrational dynamics during 11Bu+ deactivation, because adiabatic vibronic coupling does not take place between 11Bu+ and 21Ag– states. The evolution of the S1 C=C stretching frequency at 1800 cm−1 as well as of other modes for carotenoids without diabatic mixing follows a frequency upshift due to potential anharmonicity (figure 11). This is quite a new interpretation that warrants further study on both the experimental and theoretical sides.
Figure 11.
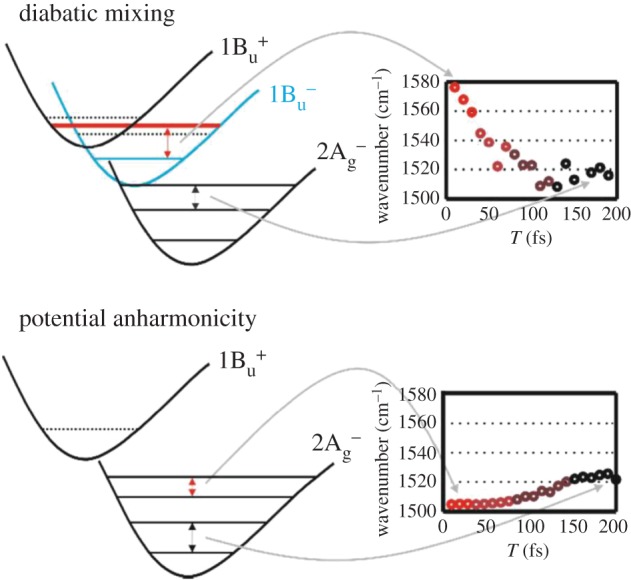
Scheme of vibrational frequencies shifts within the initial 200 fs dynamics. Diabatic mixing between 1Bu+ and 1Bu– leads to a frequency downshift during the evolution from the 1Bu+/1Bu– mixed potential to the 2Ag– potential. The red line in the 1Bu+/1Bu– mixed potential depicts the shifted vibrational level due to the diabatic mixing. Frequency upshifts due to the 2Ag– potential anharmonicity can be observed in the initial dynamics when diabatic mixing does not play a role. The intermediate states in the scheme for describing potential anharmonicity were omitted for clarity. (Reproduced with permission from [154].)
6. Conclusion
The properties of the singlet excited state of carotenoids, which have the major role in photosynthetic light harvesting, were reviewed extensively. The underlying photophysics to understand the forbidden singlet excited states is based on the historical work by Tavan & Schulten [44,45] which assumes both the planarity and C2 h point symmetry of the polyene backbone of the carotenoids. Quite recently, Fiedor et al. [39] have raised an objection to this idea. This is because structures of many naturally occurring carotenoids are asymmetric due to the side groups that are coupled with the polyene backbone as has been described in the textbook by Hudson et al. [35]. They proposed that the reason for inactivity of the S0 → S1 transition of carotenoids is not due to the symmetry, but it is due to a severe molecular deformation in the S1 state, which cannot be accessed by one-photon excitation from the ground state. This is quite an interesting new idea, but a more sophisticated experimental and computational effort is needed to better understand this issue and to see what the correct interpretation really is.
Carotenoids are indeed fascinating molecules. They have remarkable photophysical and photochemical properties [155]. Though a lot of details are known about the properties of the singlet-excited states of carotenoids, there are still a lot more to be unravelled. Now is an exciting time to be involved in carotenoids research. It has been particularly notable, as physical methods have evolved and have been applied to studying carotenoids, how the knowledge of the way in which carotenoids function in photosynthesis has advanced. We expect this trend to continue. One can highlight areas where we expect these developments to really help the understanding of the molecular mechanisms by which carotenoids discharge their photosynthetic functions. Examples are the further application of advanced two-dimensional coherent time-resolved spectroscopies [156,157] and time-resolved stimulated Raman spectroscopy [158–161]. These methods should be able to help resolve the ongoing problems of understanding the pattern of carotenoid excited singlet states and their involvement in light harvesting. They should be able to resolve the key issues of which absorption changes reflect discrete electronic states and which come from different vibrational ones. Sorting this out will hopefully remove many of the current controversies.
Data accessibility
This article has no additional data.
Authors' contribution
H.H. and R.J.C. conceived the themes of this manuscript. H.H., C.U., N.Y., A.T.G. and H.H. drafted the manuscript. All the authors gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
H.H. thanks JSPS KAKENHI, Grant-in-Aids for Basic Research (B) (no. 16H04181) and Scientific Research on Innovative Areas ‘Innovations for Light-Energy Conversion (I4LEC)’ (nos. 17H06433 and 17H0637) for financial support. R.J.C. and A.T.G. wish to gratefully thank the BBSRC and Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences under Award number DE-SC 0001035 for financial support.
References
- 1.Pfander H, Gerspacher M, Rychener M, Schwabe R. 1987. Key to carotenoids: 2nd and enlarged edition. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 2.Britton G, Liaaen-Jensen S, Pfander H. 2004. Carotenoids: handbook. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 3.Britton G, Liaaen-Jensen S, Pfander H. 1995. Carotenoids vol. 1a: isolation and analysis. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 4.Britton G, Liaaen-Jensen S, Pfander H. 1995. Carotenoids vol. 1b: spectroscopy. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 5.Britton G, Liaaen-Jensen S, Pfander H. 1996. Carotenoids vol. 2: synthesis. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 6.Britton G, Liaaen-Jensen S, Pfander H. 1998. Carotenoids vol. 3: biosynthesis and metabolism. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 7.Britton G, Liaaen-Jensen S, Pfander H. 2008. Carotenoids vol. 4: natural functions. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 8.Britton G, Liaaen-Jensen S, Pfander H. 2009. Carotenoids vol. 5: nutrition and health. Basel, Switzerland: Birkhäuser Verlag. [Google Scholar]
- 9.Hashimoto H, Uragami C, Cogdell RJ. 2016. Carotenoids and photosynthesis. Subcell. Biochem. 79, 111–139. ( 10.1007/978-3-319-39126-7_4) [DOI] [PubMed] [Google Scholar]
- 10.Schalch W. 1992. Carotenoids in the retina—a review of their possible role in preventing or limiting damage caused by light and oxygen. In Free radicals and aging (eds Emerit I, Chance B), pp. 280–298. Basel, Switzerland: Birkhäuser Verlag. [DOI] [PubMed] [Google Scholar]
- 11.von Lintig J, Kiser PD, Golczak M, Palczewski K. 2010. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends. Biochem. Sci. 35, 400–410. ( 10.1016/j.tibs.2010.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Lintig J. 2012. Provitamin A metabolism and functions in mammalian biology. Am. J. Clin. Nutr. 96, 1234S–1244S. ( 10.3945/ajcn.112.034629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. 1996. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384, 240–242. ( 10.1016/0014-5793(96)00323-7) [DOI] [PubMed] [Google Scholar]
- 14.Edge R, McGarvey DJ, Truscott TG. 1997. The carotenoids as anti-oxidants—a review. J. Photochem. Photobiol. B Biol. 41, 189–200. ( 10.1016/s1011-1344(97)00092-4) [DOI] [PubMed] [Google Scholar]
- 15.Kurihara H, Koda H, Asami S, Kiso Y, Tanaka T. 2002. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci. 70, 2509–2520. ( 10.1016/s0024-3205(02)01522-9) [DOI] [PubMed] [Google Scholar]
- 16.Fiedor J, Burda K. 2014. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 6, 466–488. ( 10.3390/nu6020466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krinsky NI, Mayane ST, Sies H. 2004. Carotenoids in health and disease. New York, NY: Marcel Dekker. [Google Scholar]
- 18.Cooper DA. 2004. Carotenoids in health and disease: recent scientific evaluations, research recommendations and the consumer. J. Nutr. 134, 221S–224S. ( 10.1093/jn/134.1.221S) [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Koo N, Min DB. 2004. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp. Rev. Food Sci. Food Safety 3, 21–33. ( 10.1111/j.1541-4337.2004.tb00058.x) [DOI] [PubMed] [Google Scholar]
- 20.Bendich A. 1989. Carotenoids and the immune system. In Carotenoids: chemistry and biology (eds Krinsky NI, Mathews-Roth MM, Taylor RF), pp. 323–335. Boston, MA: Springer. [Google Scholar]
- 21.Navara KJ. 2003. Dietary carotenoid pigments and immune function in a songbird with extensive carotenoid-based plumage coloration. Behav. Ecol. 14, 909–916. ( 10.1093/beheco/arg085) [DOI] [Google Scholar]
- 22.Mathews-Roth MM. 1982. Antitumor activity of β-carotene, canthaxanthin and phytoene. Oncology 39, 33–37. ( 10.1159/000225601) [DOI] [PubMed] [Google Scholar]
- 23.Jyonouchi H, Sun S, Iijima K, Gross MD. 2000. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 36, 59–65. ( 10.1207/S15327914NC3601_9) [DOI] [PubMed] [Google Scholar]
- 24.Soltani F, Ramezani M, Amel Farzad S, Mokhtarzadeh A, Hashemi M. 2017. Comparison study of the effect of alkyl-modified and unmodified PAMAM and PPI dendrimers on solubility and antitumor activity of crocetin. Artif. Cells Nanomed. Biotechnol. 45, 1356–1362. ( 10.1080/21691401.2016.1236805) [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto H, Uragami C. 2015. Artificial photosynthesis producing solar fuels: natural tactics of photosynthesis. In From molecules to materials: pathways to artificial photosynthesis (eds Rozhkova EA, Ariga K), pp. 57–70. Dordrecht, The Netherland: Springer International Publishing. [Google Scholar]
- 26.Green BR, Parson WW. 2003. Light-harvesting antennas in photosynthesis. Dordrecht, The Netherland: Kluwer Academic Publishers. [Google Scholar]
- 27.Fromme P. 2008. Photosynthetic protein complexes; a structural approach. Weinheim, Germany: Wiley-Blackwell. [Google Scholar]
- 28.Polívka T, Sundström V. 2004. Ultrafast dynamics of carotenoids excited states – from solution to natural and artificial systems. Chem. Rev. 104, 2021–2071. ( 10.1021/cr020674n) [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto H, Yanagi K, Yoshizawa M, Polli D, Cerullo G, Lanzani G, De Silvestri S, Gardiner AT, Cogdell RJ. 2004. The very early events following photoexcitation of carotenoids. Arch. Biochem. Biophys. 430, 61–69. ( 10.1016/j.abb.2004.04.022) [DOI] [PubMed] [Google Scholar]
- 30.Koyama Y, Kakitani Y. 2006. Mechanisms of carotenoid-to-bacteriochlorophyll energy transfer in the light harvesting antenna complexes 1 and 2: dependence on the conjugation length of carotenoids. In Chlorophylls and bacteriochlorophylls (eds Grimm B, Porra RJ, Rüdiger W, Scheer H), pp. 431–443. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 31.Koyama Y, Kakitani Y, Watanabe Y. 2008. Photophysical properties and light-harvesting and photoprotective functions of carotenloids in bacterial photosynthesis: structural selections. In Primary processes of photosynthesis - part 1: principles and apparatus (ed. Renger G.), pp. 151–201. Cambridge, MA: RSC Publishing. [Google Scholar]
- 32.Polívka T, Sundström V. 2009. Dark excited states of carotenoids: consensus and controversy. Chem. Phys. Lett. 477, 1–11. ( 10.1016/j.cplett.2009.06.011) [DOI] [Google Scholar]
- 33.Polívka T, Frank HA. 2010. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 43, 1125–1134. ( 10.1021/ar100030m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto H, Sugisaki M, Yoshizawa M. 2015. Ultrafast time-resolved vibrational spectroscopies of carotenoids in photosynthesis. Biochim. Biophys. Acta 1847, 69–78. ( 10.1016/j.bbabio.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 35.Hudson BS, Kohler BE, Schulten K. 1982. Linear polyene electronic structure and potential surfaces. In Excited states (ed. Lim ED.), pp. 1–95. New York, NY: Academic Press. [Google Scholar]
- 36.Hudson BS, Kohler BE. 1972. A low-lying weak transition in the polyene α,ω-diphenyloctatetraene. Chem. Phys. Lett. 14, 299–304. ( 10.1016/0009-2614(72)80119-2) [DOI] [Google Scholar]
- 37.Hudson BS, Kohler BE. 1973. Polyene spectroscopy: the lowest energy excited singlet state of diphenyloctatetraene and other linear polyenes. J. Chem. Phys. 59, 4984–5002. ( 10.1063/1.1680717) [DOI] [Google Scholar]
- 38.Hudson BS, Kohler BE. 1974. Linear polyene electronic structure and spectroscopy. Ann. Rev. Phys. Chem. 25, 437–460. ( 10.1146/annurev.pc.25.100174.002253) [DOI] [Google Scholar]
- 39.Fiedor L, Heriyanto FJ, Pilch M. 2016. Effects of molecular symmetry on the electronic transitions in carotenoids. J. Phys. Chem. Lett. 7, 1821–1829. ( 10.1021/acs.jpclett.6b00637) [DOI] [PubMed] [Google Scholar]
- 40.Schulten K, Karplus M. 1972. On the origin of a low-lying forbidden transition in polyenes and related molecules. Chem. Phys. Lett. 14, 305–309. ( 10.1016/0009-2614(72)80120-9) [DOI] [Google Scholar]
- 41.Simpson JH, Mclaughlin L, Smith DS, Christensen RL. 1987. Vibronic coupling in polyenes: high-resolution optical spectroscopy of all-trans-2,4,6,8,10,12,14-hexadecaheptaene. J. Chem. Phys. 87, 3360–3365. ( 10.1063/1.452978) [DOI] [Google Scholar]
- 42.Kohler BE, Spangler C, Westerfield C. 1988. The 21Ag state in the linear polyene 2,4,6,8,10,12,14,16-octadecaoctaene. J. Chem. Phys. 89, 5422–5428. ( 10.1063/1.455594) [DOI] [Google Scholar]
- 43.Christensen RL, Galinato MGI, Chu EF, Fujii R, Hashimoto H, Frank HA. 2007. Symmetry control of radiative decay in linear polyenes: low barriers for isomerization in the S1 state of hexadecaheptaene. J. Am. Chem. Soc. 129, 1769–1775. ( 10.1021/ja0609607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavan P, Schulten K. 1986. The low-lying electronic excitations in long polyenes: a PPP-MRD-CI study. J. Chem. Phys. 85, 6602–6609. ( 10.1063/1.451442) [DOI] [Google Scholar]
- 45.Tavan P, Schulten K. 1987. Electronic excitations in finite and infinite polyenes. Phys. Rev. B 36, 4337–4358. ( 10.1103/PhysRevB.36.4337) [DOI] [PubMed] [Google Scholar]
- 46.Pariser R. 1956. Theory of the electronic spectra and structure of the polyacenes and of alternant hydrocarbons. J. Chem. Phys. 24, 250–268. ( 10.1063/1.1742461) [DOI] [Google Scholar]
- 47.Hashimoto H, Koyama Y. 1989. The C=C stretching Raman lines of β-carotene isomers in the S1 state as detected by pump–probe resonance Raman spectroscopy. Chem. Phys. Lett. 154, 321–325. ( 10.1016/0009-2614(89)85363-1) [DOI] [Google Scholar]
- 48.Hashimoto H, Koyama Y. 1989. Raman spectra of all-trans-β-carotene in the S1 and T1 states produced by direct photoexcitation. Chem. Phys. Lett. 163, 251–256. ( 10.1016/0009-2614(89)80045-4) [DOI] [Google Scholar]
- 49.Hashimoto H, Koyama Y. 1989. Raman spectra of all-trans-β-apo-8'-carotenal in the S1 and T1 states: a picosecond pump-and-probe technique using ML-Qs pulse trains. Chem. Phys. Lett. 162, 523–527. ( 10.1016/0009-2614(89)87018-6) [DOI] [Google Scholar]
- 50.Noguchi T, Kolaczkowski S, Arbour C, Aramaki S, Atkinson GH, Hayashi H, Tasumi M. 1989. Resonance Raman spectrum of the excited 2Ag state of β-carotene. Photochem. Photobiol. 50, 603–609. ( 10.1111/j.1751-1097.1989.tb04315.x) [DOI] [Google Scholar]
- 51.Hashimoto H, Koyama Y, Hirata Y, Mataga N. 1991. S1 and T1 species of β-carotene generated by direct photoexcitation from the all-trans, 9-cis, 13-cis, and 15-cis isomers as revealed by picosecond transient absorption and transient Raman spectroscopies. J. Phys. Chem. 95, 3072–3076. ( 10.1021/j100161a022) [DOI] [Google Scholar]
- 52.Hashimoto H, Miki Y, Kuki M, Shimamura T, Utsumi H, Koyama Y. 1993. Isolation by high-pressure liquid chromatography of the cis-trans isomers of β-apo-8'-carotenal. Determination of their S0-state configurations by NMR spectroscopy and prediction of their S1- and T1-state configurations by transient Raman spectroscopy. J. Am. Chem. Soc. 115, 9216–9225. ( 10.1021/ja00073a042) [DOI] [Google Scholar]
- 53.Auerbach RA, Christensen RL, Granville MF, Kohler BE. 1981. Absorption and emission of 2,12-dimethyltridecahexaene. J. Chem. Phys. 74, 4–9. ( 10.1063/1.440857) [DOI] [Google Scholar]
- 54.Kasama A, Taya M, Kamisuki T, Adachi Y, Maeda S. 1987. Resonance CARS of diphenylpolyenes in the excited states: vibronic shift of a 21Ag state vibration. In Time-resolved vibrational spectroscopy (ed. Atkinson GH.), pp. 304–319. New York, NY: Gordon and Breach. [Google Scholar]
- 55.Lasaga AC, Aerni RJ, Karplus M. 1980. Photodynamics of polyenes: the effect of electron correlation on potential surfaces. J. Chem. Phys. 73, 5230–5243. ( 10.1063/1.439951) [DOI] [Google Scholar]
- 56.Frank HA. 2001. Spectroscopic studies of the low-lying singlet excited electronic states and photochemical properties of carotenoids. Arch. Biochem. Biophys. 385, 53–60. ( 10.1006/abbi.2000.2091) [DOI] [PubMed] [Google Scholar]
- 57.Macpherson AN, Arellano JB, Fraser NJ, Cogdell RJ, Gillbro T. 2001. Efficient energy transfer from the carotenoid S2 state in a photosynthetic light-harvesting complex. Biophys. J. 80, 923–930. ( 10.1016/s0006-3495(01)76071-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rondonuwu FS, Yokoyama K, Fujii R, Koyama Y, Cogdell RJ, Watanabe Y. 2004. The role of the 11Bu− state in carotenoid-to-bacteriochlorophyll singlet-energy transfer in the LH2 antenna complexes from Rhodobacter sphaeroides G1C, Rhodobacter sphaeroides 2.4.1, Rhodospirillum molischianum and Rhodopseudomonas acidophila. Chem. Phys. Lett. 390, 314–322. ( 10.1016/j.cplett.2004.03.089) [DOI] [Google Scholar]
- 59.Cogdell RJ, Frank HA. 1987. How carotenoids function in photosynthetic bacteria. Biochim. Biophys. Acta 895, 63–79. ( 10.1016/S0304-4173(87)80008-3) [DOI] [PubMed] [Google Scholar]
- 60.Bondarev SL, Bachilo SM, Dvornikov SS, Tikhomirov SA. 1989. S2→ S0 fluorescence and transient Sn ← S1 absorption of all-trans-β-carotene in solid and liquid solutions. J. Photochem. Photobiol. A Chem. 46, 315–322. ( 10.1016/1010-6030(89)87048-0) [DOI] [Google Scholar]
- 61.Gillbro T, Cogdell RJ. 1989. Carotenoid fluorescence. Chem. Phys. Lett. 158, 312–316. ( 10.1016/0009-2614(89)87342-7) [DOI] [Google Scholar]
- 62.Cosgrove SA, Guite MA, Burnell TB, Christensen RL. 1990. Electronic relaxation in long polyenes. J. Phys. Chem. 94, 8118–8124. ( 10.1021/j100384a026) [DOI] [Google Scholar]
- 63.Bondarev SL, Knyukshto VN. 1994. Fluorescence from the S1(21Ag) state of all-trans-β-carotene. Chem. Phys. Lett. 225, 346–350. ( 10.1016/0009-2614(94)87092-6) [DOI] [Google Scholar]
- 64.DeCoster B, Christensen RL, Gebhard R, Lugtenburg J, Farhoosh R, Frank HA. 1992. Low-lying electronic states of carotenoids. Biochim. Biophys. Acta 1102, 107–114. ( 10.1016/0005-2728(92)90070-I) [DOI] [PubMed] [Google Scholar]
- 65.Fujii R, Onaka K, Kuki M, Koyama Y, Watanabe Y. 1998. The 2Ag− energies of all-trans-neurosporene and spheroidene as determined by fluorescence spectroscopy. Chem. Phys. Lett. 288, 847–853. ( 10.1016/S0009-2614(98)00376-5) [DOI] [Google Scholar]
- 66.Christensen RL, Goyette M, Gallagher L, Duncan J, DeCoster B, Lugtenburg J, Jansen FJ, van der Hoef I. 1999. S1 and S2 states of apo- and diapocarotenes. J. Phys. Chem. A 103, 2399–2407. ( 10.1021/jp983946s) [DOI] [Google Scholar]
- 67.Frank HA, Desamero RZB, Chynwat V, Gebhard R, van der Hoef I, Jansen FJ, Lugtenburg J, Gosztola D, Wasielewski MR. 1997. Spectroscopic properties of spheroidene analogs having different extents of π-electron conjugation. J. Phys. Chem. A 101, 149–157. ( 10.1021/jp962373l) [DOI] [Google Scholar]
- 68.Hashimoto H, Koyama Y, Mori Y. 1997. Mechanism activating the 21Ag state in all-trans-β-carotene crystal to resonance Raman scattering. Jpn. J. Appl. Phys. 36, L916–L918. ( 10.1143/JJAP.36.L916) [DOI] [Google Scholar]
- 69.Sashima T, Shiba M, Hashimoto H, Nagae H, Koyama Y. 1998. The 2Ag− energy of crystalline all-trans-spheroidene as determined by resonance-Raman excitation profiles. Chem. Phys. Lett. 290, 36–42. ( 10.1016/s0009-2614(98)00481-3) [DOI] [Google Scholar]
- 70.Polívka T, Herek JL, Zigmantas D, Akerlund HE, Sundström V. 1999. Direct observation of the (forbidden) S1 state in carotenoids. Proc. Nat. Acad. Sci. USA 96, 4914–4917. ( 10.1073/pnas.96.9.4914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Polívka T, Zigmantas D, Frank HA, Bautista JA, Herek JL, Koyama Y, Fujii R, Sundström V. 2001. Near-infrared time-resolved study of the S1 state dynamics of the carotenoid spheroidene. J. Phys. Chem. B 105, 1072–1080. ( 10.1021/jp002206s) [DOI] [Google Scholar]
- 72.Fujii R, Ishikawa T, Koyama Y, Taguchi M, Isobe Y, Nagae H, Watanabe Y. 2001. Fluorescence spectroscopy of all-trans-anhydrorhodovibrin and spirilloxanthin: detection of the 1Bu− fluorescence. J. Phys. Chem. A 105, 5348–5355. ( 10.1021/jp010150b) [DOI] [Google Scholar]
- 73.Fujii R, Fujino T, Inaba T, Nagae H, Koyama Y. 2004. Internal conversion of 1Bu+ → 1Bu− → 2Ag− and fluorescence from the 1Bu− state in all-trans-neurosporene as probed by up-conversion spectroscopy. Chem. Phys. Lett. 384, 9–15. ( 10.1016/j.cplett.2003.11.074) [DOI] [Google Scholar]
- 74.Nishimura K, Rondonuwu FS, Fujii R, Akahane J, Koyama Y, Kobayashi T. 2004. Sequential singlet internal conversion of 1Bu+→3Ag−→1Bu−→2Ag−→(1Ag− ground) in all-trans-spirilloxanthin revealed by two-dimensional sub-5-fs spectroscopy. Chem. Phys. Lett. 392, 68–73. ( 10.1016/j.cplett.2004.04.109) [DOI] [Google Scholar]
- 75.Sashima T, Koyama Y, Yamada T, Hashimoto H. 2000. The 1Bu+, 1Bu−, and 2Ag− energies of crystalline lycopene, β-carotene, and mini-9-β-carotene as determined by resonance-Raman excitation profiles: dependence of the 1Bu− state energy on the conjugation length. J. Phys. Chem. B 104, 5011–5019. ( 10.1021/jp994185b) [DOI] [Google Scholar]
- 76.Rondonuwu FS, Watanabe Y, Fujii R, Koyama Y. 2003. A first detection of singlet to triplet conversion from the 11Bu− to the 13Ag state and triplet internal conversion from the 13Ag to the 13Bu state in carotenoids: dependence on the conjugation length. Chem. Phys. Lett. 376, 292–301. ( 10.1016/s0009-2614(03)00983-7) [DOI] [Google Scholar]
- 77.Zhang JP, Fujii R, Qian P, Inaba T, Mizoguchi T, Koyama Y, Onaka K, Watanabe Y, Nagae H. 2000. Mechanism of the carotenoid-to-bacteriochlorophyll energy transfer via the S1 state in the LH2 complexes from purple bacteria. J. Phys. Chem. B 104, 3683–3691. ( 10.1021/jp993970l) [DOI] [Google Scholar]
- 78.Kurashige Y, Nakano H, Nakao Y, Hirao K. 2004. The π→π* excited states of long linear polyenes studied by the CASCI-MRMP method. Chem. Phys. Lett. 400, 425–429. ( 10.1016/j.cplett.2004.10.141) [DOI] [Google Scholar]
- 79.Mizukami W, Kurashige Y, Ehara M, Yanai T, Itoh T. 2009. Ab initio study of the excited singlet states of all-trans α,ω-diphenylpolyenes with one to seven polyene double bonds: simulation of the spectral data within Franck–Condon approximation. J. Chem. Phys. 131, 174313 ( 10.1063/1.3261729) [DOI] [PubMed] [Google Scholar]
- 80.Kurashige Y, Yanai T. 2014. Theoretical study of the π→π* excited states of oligoacenes: a full π-valence DMRG-CASPT2 study. Bull. Chem. Soc. Jpn. 87, 1071–1073. ( 10.1246/bcsj.20140180) [DOI] [Google Scholar]
- 81.Sashima T, Nagae H, Kuki M, Koyama Y. 1999. A new singlet-excited state of all-trans-spheroidene as detected by resonance-Raman excitation profiles. Chem. Phys. Lett. 299, 187–194. ( 10.1016/S0009-2614(98)01278-0) [DOI] [Google Scholar]
- 82.Furuichi K, Sashima T, Koyama Y. 2002. The first detection of the 3Ag− state in carotenoids using resonance-Raman excitation profiles. Chem. Phys. Lett. 356, 547–555. ( 10.1016/S0009-2614(02)00412-8) [DOI] [Google Scholar]
- 83.Wang P, Nakamura R, Kanematsu Y, Koyama Y, Nagae H, Nishio T, Hashimoto H, Zhang JP. 2005. Low-lying singlet states of carotenoids having 8–13 conjugated double bonds as determined by electronic absorption spectroscopy. Chem. Phys. Lett. 410, 108–114. ( 10.1016/j.cplett.2005.05.037) [DOI] [Google Scholar]
- 84.Zhang JP, Inaba T, Watanabe Y, Koyama Y. 2000. Excited-state dynamics among the 1Bu+, 1Bu− and 2Ag− states of all-trans-neurosporene as revealed by near-infrared time-resolved absorption spectroscopy. Chem. Phys. Lett. 332, 351–358. ( 10.1016/s0009-2614(00)01275-6) [DOI] [Google Scholar]
- 85.Zhang JP, Inaba T, Watanabe Y, Koyama Y. 2000. Sub-picosecond time-resolved absorption spectroscopy of all-trans-neurosporene in solution and bound to the LH2 complex from Rhodobacter sphaeroides G1C. Chem. Phys. Lett. 331, 154–162. ( 10.1016/S0009-2614(00)01165-9) [DOI] [Google Scholar]
- 86.Zhang JP, Inaba T, Koyama Y. 2001. The role of the newly-found 1Bu− state of carotenoid in mediating the 1Bu+-to-2Ag− internal conversion and the excited-state dynamics of carotenoid and bacteriochlorophyll in a bacterial antenna complex. J. Mol. Struct. 598, 65–78. ( 10.1016/s0022-2860(01)00806-7) [DOI] [Google Scholar]
- 87.Zhang JP, Fujii R, Koyama Y, Rondonuwu FS, Watanabe Y, Mortensen A, Skibsted LH. 2001. The 1Bu-type singlet state of β-carotene as a precursor of the radical cation found in chloroform solution by sub-picosecond time-resolved absorption spectroscopy. Chem. Phys. Lett. 348, 235–241. ( 10.1016/S0009-2614(01)01157-5) [DOI] [Google Scholar]
- 88.Zhang JP, Inaba T, Watanabe Y, Koyama Y. 2001. Partition of carotenoid-to-bacteriochlorophyll singlet-energy transfer through two channels in the LH2 complex from Rhodobacter sphaeroides G1C. Chem. Phys. Lett. 340, 484–492. ( 10.1016/S0009-2614(01)00451-1) [DOI] [Google Scholar]
- 89.Zhang JP, Skibsted LH, Fujii R, Koyama Y. 2001. Transient absorption from the 1Bu+ state of all-trans-β-carotene newly identified in the near-infrared region. Photochem. Photobiol. 73, 219–222. ( 10.1562/0031-8655(2001)073%3C0219:Taftus%3E2.0.Co;2) [DOI] [PubMed] [Google Scholar]
- 90.Rondonuwu FS, Watanabe Y, Zhang JP, Furuichi K, Koyama Y. 2002. Internal-conversion and radiative-transition processes among the 1Bu+, 1Bu− and 2Ag− states of all-trans-neurosporene as revealed by subpicosecond time-resolved Raman spectroscopy. Chem. Phys. Lett. 357, 376–384. ( 10.1016/S0009-2614(02)00491-8) [DOI] [Google Scholar]
- 91.Fujii R, Inaba T, Watanabe Y, Koyama Y, Zhang JP. 2003. Two different pathways of internal conversion in carotenoids depending on the length of the conjugated chain. Chem. Phys. Lett. 369, 165–172. ( 10.1016/S0009-2614(02)01999-1) [DOI] [Google Scholar]
- 92.Akahane J, Rondonuwu FS, Fiedor L, Watanabe Y, Koyama Y. 2004. Dependence of singlet-energy transfer on the conjugation length of carotenoids reconstituted into the LH1 complex from Rhodospirillum rubrum G9. Chem. Phys. Lett. 393, 184–191. ( 10.1016/j.cplett.2004.06.021) [DOI] [Google Scholar]
- 93.Koyama Y, Rondonuwu FS, Fujii R, Watanabe Y. 2004. Light-harvesting function of carotenoids in photo-synthesis: the roles of the newly found 11Bu− state. Biopolymers 74, 2–18. ( 10.1002/bip.20034) [DOI] [PubMed] [Google Scholar]
- 94.Ikuta M, Yabushita A, Rondonuwu FS, Akahane J, Koyama Y, Kobayashi T. 2006. The 1Bu+ → 3Ag− → 1Bu− → 2Ag− internal conversion in carotenoids following the energy-gap law identified by 5 fs spectroscopy. Chem. Phys. Lett. 422, 95–99. ( 10.1016/j.cplett.2006.02.042) [DOI] [Google Scholar]
- 95.Rondonuwu FS, Kakitani Y, Tamura H, Koyama Y. 2006. Singlet internal conversion processes in the order of 1Bu+ → 3Ag− → 1Bu− → 2Ag− → 1Ag− in all-trans-spheroidene and lycopene as revealed by subpicosecond time-resolved Raman spectroscopy. Chem. Phys. Lett. 429, 234–238. ( 10.1016/j.cplett.2006.07.061) [DOI] [Google Scholar]
- 96.Sutresno A, Kakitani Y, Zuo P, Li C, Koyama Y, Nagae H. 2007. Presence and absence of electronic mixing in shorter-chain and longer-chain carotenoids: assignment of the symmetries of 1Bu− and 3Ag− states located just below the 1Bu+ state. Chem. Phys. Lett. 447, 127–133. ( 10.1016/j.cplett.2007.08.081) [DOI] [Google Scholar]
- 97.Gradinaru CC, Kennis J, Papagiannakis E, van Stokkum IH, Cogdell RJ, Fleming GR, Niederman RA, van Grondelle R. 2001. An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc. Natl Acad. Sci. USA 98, 2364–2369. ( 10.1073/pnas.051501298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papagiannakis E, Das SK, Gall A, van Stokkum IHM, Robert B, van Grondelle R, Frank HA, Kennis JTM. 2003. Light harvesting by carotenoids incorporated into the B850 light-harvesting complex from Rhodobacter sphaeroides R-26.1: excited-state relaxation, ultrafast triplet formation, and energy transfer to bacteriochlorophyll. J. Phys. Chem. B 107, 5642–5649. ( 10.1021/jp027174i) [DOI] [Google Scholar]
- 99.Papagiannakis E, van Stokkum IHM, van Grondelle R, Niederman RA, Zigmantas D, Sundström V, Polívka T. 2003. A near-infrared transient absorption study of the excited-state dynamics of the carotenoid spirilloxanthin in solution and in the LH1 complex of Rhodospirillum rubrum. J. Phys. Chem. B 107, 11 216–11 223. ( 10.1021/jp034931j) [DOI] [Google Scholar]
- 100.Wohlleben W, Buckup T, Hashimoto H, Cogdell RJ, Herek JL, Motzkus M. 2004. Pump-deplete-probe spectroscopy and the puzzle of carotenoid dark states. J. Phys. Chem. B 108, 3320–3325. ( 10.1021/jp036145k) [DOI] [Google Scholar]
- 101.Kosumi D, Maruta S, Horibe T, Nagaoka Y, Fujii R, Sugisaki M, Cogdell RJ, Hashimoto H. 2012. Ultrafast excited state dynamics of spirilloxanthin in solution and bound to core antenna complexes: identification of the S* and T1 states. J. Chem. Phys. 137, 064505 ( 10.1063/1.4737129) [DOI] [PubMed] [Google Scholar]
- 102.Beck WF, Bishop MM, Roscioli JD, Ghosh S, Frank HA. 2015. Excited state conformational dynamics in carotenoids: dark intermediates and excitation energy transfer. Arch. Biochem. Biophys. 572, 175–183. ( 10.1016/j.abb.2015.02.016) [DOI] [PubMed] [Google Scholar]
- 103.Sanchez-Galvez A, Hunt P, Robb MA, Olivucci M, Vreven T, Schlegel HB. 2000. Ultrafast radiationless deactivation of organic dyes: evidence for a two-state two-mode pathway in polymethine cyanines. J. Am. Chem. Soc. 122, 2911–2924. ( 10.1021/ja993985x) [DOI] [Google Scholar]
- 104.Balevicius V Jr, Abramavicius D, Polivka T, Galestian Pour A, Hauer J. 2016. A unified picture of S* in carotenoids. J. Phys. Chem. Lett. 7, 3347–3352. ( 10.1021/acs.jpclett.6b01455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andersson PO, Gillbro T. 1995. Photophysics and dynamics of the lowest excited singlet-state in long substituted polyenes with implications to the very long-chain limit. J. Chem. Phys. 103, 2509–2519. ( 10.1063/1.469672) [DOI] [Google Scholar]
- 106.Hörvin BH, Zigmantas D, Sundström V, Polívka T. 2002. Dynamics of vibrational relaxation in the S1 state of carotenoids having 11 conjugated C=C bonds. Chem. Phys. Lett. 355, 465–470. ( 10.1016/s0009-2614(02)00268-3) [DOI] [Google Scholar]
- 107.Bautista JA, Connors RE, Raju BB, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR, Frank HA. 1999. Excited state properties of peridinin: observation of a solvent dependence of the lowest excited singlet state lifetime and spectral behavior unique among carotenoids. J. Phys. Chem. B 103, 8751–8758. ( 10.1021/jp9916135) [DOI] [Google Scholar]
- 108.Frank HA, Bautista JA, Josue J, Pendon Z, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR. 2000. Effect of the solvent environment on the spectroscopic properties and dynamics of the lowest excited states of carotenoids. J. Phys. Chem. B 104, 4569–4577. ( 10.1021/jp000079u) [DOI] [Google Scholar]
- 109.Yoshizawa M, Aoki H, Hashimoto H. 2001. Vibrational relaxation of the 2Ag− excited state in all-trans-β-carotene obtained by femtosecond time-resolved Raman spectroscopy. Phys. Rev. B 63, 180301 ( 10.1103/PhysRevB.63.180301) [DOI] [Google Scholar]
- 110.Yoshizawa M, Aoki H, Hashimoto H. 2002. Femtosecond time-resolved Raman signals on ultrafast dynamics in all-trans-β-carotene. Bull. Chem. Soc. Jpn. 75, 949–955. ( 10.1246/bcsj.75.949) [DOI] [Google Scholar]
- 111.McCamant DW, Kim JE, Mathies RA. 2002. Vibrational relaxation in β-carotene probed by picosecond stokes and anti-stokes resonance Raman spectroscopy. J. Phys. Chem. A 106, 6030–6038. ( 10.1021/jp0203595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zigmantas D, Polivka T, Hiller RG, Yartsev A, Sundstrom V. 2001. Spectroscopic and dynamic properties of the peridinin lowest singlet excited states. J. Phys. Chem. A 105, 10 296–10 306. ( 10.1021/jp010022n) [DOI] [Google Scholar]
- 113.Zigmantas D, Hiller RG, Sundström V, Polívka T. 2002. Carotenoid to chlorophyll energy transfer in the peridinin–chlorophyll-a–protein complex involves an intramolecular charge transfer state. Proc. Natl. Acad. Sci. USA 99, 16 760–16 765. ( 10.1073/pnas.262537599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kosumi D, et al. 2009. One- and two-photon pump-probe optical spectroscopic measurements reveal the S1 and intramolecular charge transfer states are distinct in fucoxanthin. Chem. Phys. Lett. 483, 95–100. ( 10.1016/j.cplett.2009.10.077) [DOI] [Google Scholar]
- 115.Kosumi D, et al. 2011. Ultrafast excited state dynamics of fucoxanthin: excitation energy dependent intramolecular charge transfer dynamics. Phys. Chem. Chem. Phys. 13, 10 762–10 770. ( 10.1039/c0cp02568b) [DOI] [PubMed] [Google Scholar]
- 116.Kosumi D, et al. 2011. Ultrafast S1 and ICT state dynamics of a marine carotenoid probed by femtosecond one- and two-photon pump-probe spectroscopy. J. Lumin. 131, 515–518. ( 10.1016/j.jlumin.2010.09.018) [DOI] [Google Scholar]
- 117.Kosumi D, Kita M, Fujii R, Sugisaki M, Oka N, Takaesu Y, Taira T, Iha M, Hashimoto H. 2012. Excitation energy-transfer dynamics of brown algal photosynthetic antennas. J. Phys. Chem. Lett. 3, 2659–2664. ( 10.1021/Jz300612c) [DOI] [PubMed] [Google Scholar]
- 118.Kosumi D, Fujii R, Sugisaki M, Oka N, Iha M, Hashimoto H. 2014. Characterization of the intramolecular transfer state of marine carotenoid fucoxanthin by femtosecond pump-probe spectroscopy. Photosynth. Res. 121, 61–68. ( 10.1007/s11120-014-9995-6) [DOI] [PubMed] [Google Scholar]
- 119.Kosumi D, Kajikawa T, Okumura S, Sugisaki M, Sakaguchi K, Katsumura S, Hashimoto H. 2014. Elucidation and control of an intramolecular charge transfer property of fucoxanthin by a modification of its polyene chain length. J. Phys. Chem. Lett. 5, 792–797. ( 10.1021/jz5000287) [DOI] [PubMed] [Google Scholar]
- 120.Yukihira N, et al. 2017. Strategies to enhance the excitation energy-transfer efficiency in a light-harvesting system using the intra-molecular charge transfer character of carotenoids. Faraday Discuss. 198, 59–71. ( 10.1039/c6fd00211k) [DOI] [PubMed] [Google Scholar]
- 121.Kosumi D, Kajikawa T, Yano K, Okumura S, Sugisaki M, Sakaguchi K, Katsumura S, Hashimoto H. 2014. Roles of allene-group in an intramolecular charge transfer character of a short fucoxanthin homolog as revealed by femtosecond pump–probe spectroscopy. Chem. Phys. Lett. 602, 75–79. ( 10.1016/j.cplett.2014.04.022) [DOI] [Google Scholar]
- 122.Cerullo G, Polli D, Lanzani G, De Silvestri S, Hashimoto H, Cogdell RJ. 2002. Photosynthetic light harvesting by carotenoids: detection of an intermediate excited state. Science 298, 2395–2398. ( 10.1126/science.1074685) [DOI] [PubMed] [Google Scholar]
- 123.Kosumi D, Komukai M, Hashimoto H, Yoshizawa M. 2005. Ultrafast dynamics of all-trans-β-carotene explored by resonant and nonresonant photoexcitations. Phys. Rev. Lett. 95, 213 601–213 604. ( 10.1103/PhysRevLett.95.213601) [DOI] [PubMed] [Google Scholar]
- 124.Ostroumov EE, Mulvaney RM, Cogdell RJ, Scholes GD. 2013. Broadband 2D electronic spectroscopy reveals a carotenoid dark state in purple bacteria. Science 340, 52–56. ( 10.1126/science.1230106) [DOI] [PubMed] [Google Scholar]
- 125.Siebert T, Engel V, Materny A, Kiefer W, Schmitt M. 2003. Probing the kinetics of a nonadiabatic transition initiating out of vibrationally excited as well as ground state modes with femtosecond time-resolved transient gratings. J. Phys. Chem. A 107, 8355–8362. ( 10.1021/jp022650q) [DOI] [Google Scholar]
- 126.Hornung T, Skenderović H, Motzkus M. 2005. Observation of all-trans-β-carotene wavepacket motion on the electronic ground and excited dark state using degenerate four-wave mixing (DFWM) and pump-DFWM. Chem. Phys. Lett. 402, 283–288. ( 10.1016/j.cplett.2004.11.135) [DOI] [Google Scholar]
- 127.Sugisaki M, Yanagi K, Cogdell RJ, Hashimoto H. 2007. Unified explanation for linear and nonlinear optical responses in β-carotene: a sub-20-fs degenerate four-wave mixing spectroscopic study. Phys. Rev. B 75, 155110 ( 10.1103/PhysRevB.75.155110) [DOI] [Google Scholar]
- 128.Hauer J, Buckup T, Motzkus M. 2007. Pump-degenerate four wave mixing as a technique for analyzing structural and electronic evolution: multidimensional time-resolved dynamics near a conical intersection. J. Phys. Chem. A 111, 10 517–10 529. ( 10.1021/jp073727j) [DOI] [PubMed] [Google Scholar]
- 129.Sugisaki M, Fujiwara M, Yanagi K, Cogdell RJ, Hashimoto H. 2008. Four-wave mixing signals from β-carotene and its n=15 homologue. Photosyn. Res. 95, 299–308. ( 10.1007/s11120-007-9265-y) [DOI] [PubMed] [Google Scholar]
- 130.Buckup T, Hauer J, Mohring J, Motzkus M. 2009. Multidimensional spectroscopy of β-carotene: vibrational cooling in the excited state. Arch. Biochem. Biophys. 483, 219–223. ( 10.1016/j.abb.2008.10.031) [DOI] [PubMed] [Google Scholar]
- 131.Fujiwara M, Yamauchi K, Sugisaki M, Gall A, Robert B, Cogdell R J, Hashimoto H. 2008. Energy dissipation in the ground-state vibrational manifolds of β-carotene homologues: a sub-20-fs time-resolved transient grating spectroscopic study. Phys. Rev. B 77, 205118 ( 10.1103/PhysRevB.77.205118) [DOI] [Google Scholar]
- 132.Sugisaki M, Fujiwara M, Nair SV, Ruda HE, Cogdell RJ, Hashimoto H. 2009. Excitation-energy dependence of transient grating spectroscopy in β-carotene. Phys. Rev. B 80, 035118 ( 10.1103/PhysRevB.80.035118) [DOI] [Google Scholar]
- 133.Christensson N, Polivka T, Yartsev A, Pullerits T. 2009. Photon echo spectroscopy reveals structure–dynamics relationships in carotenoids. Phys. Rev. B 79, 245118 ( 10.1103/PhysRevB.79.245118) [DOI] [Google Scholar]
- 134.Fujiwara M, Sugisaki M, Gall A, Robert B, Cogdell RJ, Hashimoto H. 2009. Ultrafast optical responses of β-carotene and lycopene probed by sub-20-fs time-resolved coherent spectroscopy. J. Lumin. 129, 1808–1812. ( 10.1016/j.jlumin.2009.04.098) [DOI] [Google Scholar]
- 135.Sugisaki M, Fujiwara M, Kosumi D, Fujii R, Nango M, Cogdell RJ, Hashimoto H. 2010. Comparison of transient grating signals from spheroidene in an organic solvent and in pigment–protein complexes from Rhodobacter sphaeroides 2.4.1. Phys. Rev. B 81, 245112 ( 10.1103/PhysRevB.81.245112) [DOI] [Google Scholar]
- 136.Sugisaki M, Kosumi D, Saito K, Cogdell RJ, Hashimoto H. 2011. Control of coherent vibronic oscillations in β-carotene by ultrashort laser pulses. Phys. Status Solidi C 8, 151–154. ( 10.1002/pssc.201000679) [DOI] [Google Scholar]
- 137.Sugisaki M, Kosumi D, Saito K, Cogdell RJ, Hashimoto H. 2011. Strong coherent coupling of vibronic oscillations in spheroidene. Phys. Proc. 13, 74–77. ( 10.1016/j.phpro.2011.02.018) [DOI] [Google Scholar]
- 138.Mukamel S. 1995. Principles of nonlinear optical spectroscopy. Oxford, UK: Oxford University Press. [Google Scholar]
- 139.Sugisaki M, Fujiwara M, Fujii R, Nakagawa K, Nango M, Cogdell RJ, Hashimoto H. 2009. Transient grating spectroscopy in photosynthetic purple bacteria Rhodobacter sphaeroides 2.4.1. J. Lumin. 129, 1908–1911. ( 10.1016/j.jlumin.2009.01.027) [DOI] [Google Scholar]
- 140.Vos MH, Lambry JC, Robles SJ, Youvan DC, Breton J, Martin JL. 1991. Direct observation of vibrational coherence in bacterial reaction centers using femtosecond absorption spectroscopy. Proc. Natl Acad. Sci. USA 88, 8885–8889. ( 10.1073/pnas.88.20.8885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vos MH, Rappaport F, Lambry JC, Breton J, Martin JL. 1993. Visualization of coherent nuclear motion in a membrane protein by femtosecond spectroscopy. Nature 363, 320–325. ( 10.1038/363320a0) [DOI] [Google Scholar]
- 142.Vos MH, Jones MR, Hunter CN, Breton J, Martin JL. 1994. Coherent nuclear dynamics at room temperature in bacterial reactioncenters. Proc. Natl Acad. Sci. USA 91, 12 701–12 705. ( 10.1073/pnas.91.26.12701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jimenez R, van Mourik F, Yu JY, Fleming GR. 1997. Three-pulse photon echo measurements on LH1 and LH2 complexes of Rhodobacter sphaeroides: a nonlinear spectroscopic probe of energy transfer. J. Phys. Chem. B 101, 7350–7359. ( 10.1021/jp970299g) [DOI] [Google Scholar]
- 144.Groot ML, Yu JY, Agarwal R, Norris JR, Fleming GR. 1998. Three-pulse photon echo measurements on the accessory pigments in the reaction center of Rhodobacter sphaeroides. J. Phys. Chem. B 102, 5923–5931. ( 10.1021/jp9808680) [DOI] [Google Scholar]
- 145.Agarwal R, Yang M, Xu QH, Fleming GR. 2001. Three pulse photon echo peak shift study of the B800 band of the LH2 complex of Rps. acidophila at room temperature: a coupled master equation and nonlinear optical response function approach. J. Phys. Chem. B 105, 1887–1894. ( 10.1021/jp0031146) [DOI] [Google Scholar]
- 146.Shelly KR, Carson EA, Beck WF. 2003. Vibrational coherence from the dipyridine complex of bacteriochlorophyll a: intramolecular modes in the 10–220-cm−1 regime, intermolecular solvent modes, and relevance to photosynthesis. J. Am. Chem. Soc. 125, 11 810–11 811. ( 10.1021/ja0366890) [DOI] [PubMed] [Google Scholar]
- 147.Shelly KR, Golovich EC, Beck WF. 2006. Intermolecular vibrational coherence in bacteriochlorophyll a with clustered polar solvent molecules. J. Phys. Chem. B 110, 20 586–20 595. ( 10.1021/jp062909v) [DOI] [PubMed] [Google Scholar]
- 148.Buckup T, Hauer J, Voll J, Vivie-Riedle R, Motzkus M. 2011. A general control mechanism of energy flow in the excited state of polyenic biochromophores. Faraday Discuss. 153, 213–225. ( 10.1039/c1fd00037c) [DOI] [PubMed] [Google Scholar]
- 149.Marek MS, Buckup T, Motzkus M. 2011. Direct observation of a dark state in lycopene using pump-DFWM. J. Phys. Chem. B 115, 8328–8337. ( 10.1021/jp202753j) [DOI] [PubMed] [Google Scholar]
- 150.Philip KJ, Motzkus M, Buckup T. 2011. Selective nonlinear response preparation using femtosecond spectrally resolved four-wave-mixing. J. Chem. Phys. 135, 224 505 ( 10.1063/1.3666846) [DOI] [PubMed] [Google Scholar]
- 151.Kraack JP, Wand A, Buckup T, Motzkus M, Ruhman S. 2013. Mapping multidimensional excited state dynamics using pump-impulsive-vibrational-spectroscopy and pump-degenerate-four-wave-mixing. Phys. Chem. Chem. Phys. 15, 14 487–14 501. ( 10.1039/c3cp50871d) [DOI] [PubMed] [Google Scholar]
- 152.Marek MS, Buckup T, Southall J, Cogdell RJ, Motzkus M. 2013. Highlighting short-lived excited electronic states with pump-degenerate-four-wave-mixing. J. Chem. Phys. 139, 074202 ( 10.1063/1.4818164) [DOI] [PubMed] [Google Scholar]
- 153.Buckup T, Motzkus M. 2014. Multidimensional time-resolved spectroscopy of vibrational coherence in biopolyenes. Annu. Rev. Phys. Chem. 65, 39–57. ( 10.1146/annurev-physchem-040513-103619) [DOI] [PubMed] [Google Scholar]
- 154.Miki T, Buckup T, Krause MS, Southall J, Cogdell RJ, Motzkus M. 2016. Vibronic coupling in the excited-states of carotenoids. Phys. Chem. Chem. Phys. 18, 11 443–11 453. ( 10.1039/c5cp07542d) [DOI] [PubMed] [Google Scholar]
- 155.Frank HA, Young AJ, Britton G, Cogdell RJ. 1999. The photochemistry of carotenoids. In Advances in photosynthesis and respiration, vol. 8 (ed. Govindjee), pp. 235–244. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 156.Cho M. 2008. Coherent two-dimensional optical spectroscopy. Chem. Rev. 108, 1331–1418. ( 10.1021/cr078377b) [DOI] [PubMed] [Google Scholar]
- 157.Cho M. 2009. Two-dimensional optical spectroscopy. Boca Raton, FL: CRC Press. [Google Scholar]
- 158.Yoshizawa M, Kurosawa M. 1999. Femtosecond time-resolved Raman spectroscopy using stimulated Raman scattering. Phys. Rev. A 61, 013808 ( 10.1103/PhysRevA.61.013808) [DOI] [Google Scholar]
- 159.Kukura P, McCamant DW, Mathies RA. 2007. Femtosecond stimulated Raman spectroscopy. Annu. Rev. Phys. Chem. 58, 461–488. ( 10.1146/annurev.physchem.58.032806.104456) [DOI] [PubMed] [Google Scholar]
- 160.Frontiera RR, Mathies RA. 2011. Femtosecond stimulated Raman spectroscopy. Laser Photon. Rev. 5, 102–113. ( 10.1002/lpor.200900048) [DOI] [Google Scholar]
- 161.Challa JR, Du Y, McCamant DW. 2012. Femtosecond stimulated Raman spectroscopy using a scanning multichannel technique. Appl. Spectrosc. 66, 227–232. ( 10.1366/11-06457) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.