Abstract
The chloroplast signal recognition particle (cpSRP) is a protein complex consisting of 54- and 43-kD subunits encoded by the fifty-four chloroplast, which encodes cpSRP54 (ffc), and chaos (cao) loci, respectively. Two new null alleles in the ffc locus have been identified. ffc1-1 is caused by a stop codon in exon 10, while ffc1-2 has a large DNA insertion in intron 8. ffc mutants have yellow first true leaves that subsequently become green. The reaction center proteins D1, D2, and psaA/B, as well as seven different light-harvesting chlorophyll proteins (LHCPs), were found at reduced levels in the young ffc leaves but at wild-type levels in the older leaves. The abundance of the two types of LHCP was unaffected by the mutation, while two others were increased in the absence of cpSRP54. Null mutants in the cao locus contain reduced levels of the same subset of LHCP proteins as ffc mutants, but are distinguishable in four ways: young leaves are greener, the chlorophyll a/b ratio is elevated, levels of reaction center proteins are normal, and there is no recovery in the level of LHCPs in the adult plant. The data suggest that cpSRP54 and cpSRP43 have some nonoverlapping roles and that alternative transport pathways can compensate for the absence of a functional cpSRP.
Chloroplasts contain a minimum of four pathways for targeting proteins to the thylakoid membrane (for reviews, see Cline and Henry, 1996; Schnell, 1998). Luminal proteins use either the chloroplast Sec (cpSec) pathway or the ΔpH pathway; integral membrane proteins use the chloroplast signal recognition particle (cpSRP) pathway or insert by an apparently spontaneous mechanism where no soluble or membrane factors have been found to be required. Only the cpSec and cpSRP pathways have soluble factor requirements. For the Sec pathway, these factors include cpSecA (Yuan et al., 1994; Nohara et al., 1995; Voelker et al., 1997) and ATP (Kirwin et al., 1988; Hulford et al., 1994; Karnauchov et al., 1994; Yuan and Cline, 1994). For the cpSRP pathway the factors include cpSRP54 (Franklin and Hoffman, 1993; Li et al., 1995; Schuenemann et al., 1998; Klimyuk et al., 1999), GTP (Hoffman and Franklin, 1994), and at least one additional soluble factor (Payan and Cline, 1991; Schuenemann et al., 1998). A cpSecY/E complex acts as the translocase for the cpSec pathway (Laidler et al., 1995; Schuenemann et al., 1999); the translocase for the cpSRP is unknown. A trans-thylakoid pH gradient is not essential but stimulates transport for both pathways (Cline et al., 1992, 1993). The ΔpH pathway is distinguished by an absolute requirement for a ΔpH (Mould and Robinson, 1991; Cline et al., 1992; Klosgen et al., 1992; Brock et al., 1995) and the integral membrane protein Hcf 106 (Voelker and Barkan, 1995b; Settles et al., 1997); cpSecY is not required (Schuenemann et al., 1999).
One striking outcome from in vitro studies was the observation that protein substrates exhibit a strict dependence for a particular pathway. For example, the 33-kD subunit of the oxygen-evolving complex (OE33), plastocyanin, and PSI-F use the Sec pathway (Hulford et al., 1994; Karnauchov et al., 1994; Yuan and Cline, 1994), OE23, OE17, PSII-T, and PSI-N use the ΔpH pathway (Cline et al., 1993; Mant et al., 1994; Kapazoglou et al., 1995), light-harvesting chlorophyll (Chl) protein b1 (Lhcb1) uses the cpSRP pathway (Li et al., 1995; Schuenemann et al., 1998), and PSII-W, PSII-X, and coupling factor II proteins insert spontaneously (Michl et al., 1994; Kim et al., 1996, 1998). Cyt F may be an exception; although it uses the Sec pathway (Voelker and Barkan, 1995; Nohara et al., 1996; Mould et al., 1997), it is capable of interacting with cpSRP54 in vitro (High et al., 1997).
More recently, in vitro studies have been supplemented by in vivo analysis. Null mutations in Hcf106 (Voelker and Barkan, 1995b), SecA (tha1) (Voelker and Barkan, 1995b; Voelker et al., 1997), and SecY (csy1) (Roy and Barkan, 1998) have been isolated in maize and all are lethal in the homozygous state. Hcf106 and tha1 mutants were selectively defective, but not completely deficient, in the transport of ΔpH and cpSec pathway proteins, respectively. These data suggested that proteins have pathway preferences but these preferences are not absolute. The SecY mutant had a phenotype that was more severe than the hcf106/tha1 double mutant, implying that SecY is used in the cpSRP pathway and/or that additional SecY-utilizing targeting pathways remain to be elucidated (Roy and Barkan, 1998).
Mutants in the cpSRP pathway have also been isolated in Arabidopsis, and the phenotypes are much milder than those of the maize mutants described above (Pilgrim et al., 1998; Klimyuk et al., 1999). A null mutant in cpSRP43, chaos (cao), was found to be chlorotic, had an elevated Chl a/b ratio, was selectively deficient in light-harvesting Chl proteins (LHCPs) relative to other thylakoid proteins, and was viable (Klimyuk et al., 1999). Mutants deficient in cpSRP54, presumably due to cosuppression, were isolated from Arabidopsis transformed with mutant cpSRP54 constructs (Pilgrim et al., 1998). These mutants were also viable and, surprisingly, had a distinct phenotype from the chaos mutant. The transgenic mutants produced yellow first true leaves that became green 3 to 4 d later. Chl a/b ratios were unaffected in both yellow and green leaves. Unlike the chaos mutant, many chloroplast proteins were reduced in the first true leaves, and the affected proteins were found at normal levels in older plants.
Earlier biochemical studies established that cpSRP43 and cpSRP54 form a complex and work together to promote the biogenesis of the major LHCP, Lhcb1 (Schuenemann et al., 1998; Klimyuk et al., 1999). For example, only the complex, not the individual cpSRP subunits, can bind to Lhcb1 and keep it soluble in aqueous solution. Likewise, both subunits are required for LHCP integration into thylakoid membranes. Given the requirement of both substrates for activity in LHCP biogenesis, it was expected that mutant alleles in fifty-four chloroplasts, which encode cpSRP54, would have the same phenotype as cao mutant alleles. That different phenotypes are observed suggest that cpSRP54 and cpSRP43 might have some nonoverlapping roles. Alternatively, a true null allele in the ffc locus might have a different phenotype from the co-suppressor line, more closely resembling the chaos phenotype. To further examine this possibility, we isolated and characterized true null alleles in the ffc locus. Our results clearly indicate that ffc and chaos mutants have distinct phenotypes, and therefore cpSRP54 and cpSRP43 do not always function in concert.
MATERIALS AND METHODS
Plant Growth Conditions and Transformation
George Redei deposited a collection of mutants in the Arabidopsis Biological Resource Center that are referred to as yellow heart (19 lines) or yellow green (32 lines) that resemble transgenic mutants deficient in cpSRP54 levels (Pilgrim et al., 1998). Seeds were obtained from this collection and grown on plates as described previously (Pilgrim et al., 1998).
Extraction of Proteins and Immunoblot Analysis
For assaying cpSRP54 in fresh leaves, extracts were made by harvesting tissue directly into liquid N2 and grinding in ice-cold buffer (1 m Na2HPO4, 1 mm PMSF, 1 mm benzamidine, 5 mm ε-amino-n-caproic acid, 10 μg/mL leupeptin, 10 μg/mL antipain, and 1 mm p-hydroxymercuribenzoate) with a mortar and pestle. Homogenate was centrifuged for 2 min at 13,000g in a microfuge at 4°C to pellet insoluble material, and soluble extracts were analyzed directly as described previously (Pilgrim et al., 1998). For assaying Chl, the pellet was resuspended in 1 mL of extraction buffer, and 200 μL of sample was mixed with 800 μL of acetone, centrifuged, and assayed (Arnon, 1949). The remaining sample was pelleted and solubilized in 8 m urea and 0.1% (w/v) SDS. Protein concentration was determined using the bicinchoninic acid assay (Smith et al., 1985). Proteins were resolved by SDS-PAGE and either stained with Coomassie Blue or transferred to nitrocellulose membranes, followed by the detection of proteins using enhanced chemiluminescence, as described previously (Pilgrim et al., 1998).
Antibodies
Antisera against Arabidopsis cpSRP54 (CIW 24) was raised in rabbits (Cocalico Biologicals, Reamstown, PA) against a protein expressed in Escherichia coli containing the first 33 amino acids of the mature protein fused to residues 317 to 488 followed by GSHHHHHH. The construct used to express the antigen was made as an in-frame deletion of pNH4 (Pilgrim et al., 1998). pNH4 was digested with EcoRV and BglII, the recessed ends were filled in with Klenow subunit, and the plasmid was religated. An IgG fraction was prepared from crude serum by ammonium sulfate precipitation and chromatography on DEAE-Sephadex columns (Harlow and Lane, 1988).
Antisera against broad bean Lhca1 (Hoffman et al., 1987), cpSRP43 (Klimyuk et al., 1999), OE33 (Pilgrim et al., 1998), and SecY (Schuenemann et al., 1999) were prepared as described previously. Antisera against Lhca2, Lhcb1, Lhcb2, Lhcb3, Lhcb5, and Lhcb6 were generously provided by Andrew Staehelin (Sigrist and Staehelin, 1992, 1994); Lhca3 and Lhca4 by Stefan Jansson (Krol et al., 1995); barley Lhcb4 (monoclonal 14-6D1c11) by David Simpson (Knoetzel and Simpson, 1991), spinach psbS and D1 (D1HuSa2) by Bertil Andersson and Klaas-Jan van Wijk (Stockholm University), spinach PSI core by Roberto Barbato (Universita del Piemonte Orientale, Alessandria, Italy), D2 (2 mKan1) by Eva Aro (University of Turku, Finland), pea OE23 by Ken Cline (University of Florida, Gainesville), maize CytF by Alice Barkan (Barkan et al., 1986), and pea ClpC by John Shanklin (Shanklin et al., 1995).
Extraction of RNA, DNA, and Hybridization Analysis
RNA extractions were performed using a plant total RNA kit (RNeasy, Qiagen, Valencia, CA) as recommended by the manufacturer. Northern analysis was conducted exactly as described previously (Pilgrim et al., 1998). Arabidopsis genomic DNA was isolated as described by Murray and Thompson (1980). For Southern blots, 10 μg of DNA was digested overnight in the appropriate enzyme in a volume of 200 μL. DNA was precipitated in alcohol, fractionated on 0.7% (w/v) agarose gels, and blotted to nitrocellulose membranes in 20× SSC as described previously (Sambrook et al., 1989). DNA was fixed to the membrane by UV cross-linking with a Stratalinker (Stratagene), prehybridized in hybridization buffer (6× SSC, 1× Denhardt's solution, 0.5% [w/v] SDS, 20 μg/mL yeast tRNA, and 0.05% [w/v] sodium PPi) for 4 h at 65°C, and hybridized to probes (2 × 106 dpm/mL) for 16 h. Filters were washed in 0.1× SSC/0.1% SDS for 5 min at room temperature, followed by 60-min and 5-min washes at 65°C.
PCR and Sequencing
PCR was performed in a thermal cycler (MJ Research, Waterstown, MA). Generally, 50 ng of genomic DNA was amplified in a 25-μL reaction containing 0.2 mm of each deoxynucleotide, 1.5 mm MgCl2, 0.5 unit of Taq polymerase, and 10× buffer supplied by the manufacturer (MBI Fermentas, Amherst, NY). For labeling probes by PCR, 1 ng of plasmid DNA and 0.5 μm of each primer were combined with 1 nmol of dTTP, 1 nmol of dATP, 1 nmol of dGTP, and 50 pmol of dCTP in a total volume of 7 μL. Three microliters (10 pmol) of [α-32P]dCTP (3,000 μCi/mmol, NEN) and 15 μL of Chill Out (MJ Research) were added, and the sample was placed on ice.
Each reaction received 20 μL of dilute Taq polymerase (1 unit) in 1.5× Taq PCR buffer. A three-step program was used: 94°C for 2 min (1 cycle), 30 cycles of 92°C for 30 s, annealing for 30 s, extension at 72°C for 1 min/kb, and a final extension for 72°C for 5 min. Long PCR was performed with the Expand PCR kit from Boehringer Mannheim according to the manufacturer's directions. PCR products were purified on quick-spin columns (QiaQuick, Qiagen), sequenced directly using a dye deoxy terminator cycle-sequencing kit (Prism Ready Reaction, Applied Biosystems), and analyzed on a genetic analyzer (model Prism 310, Applied Biosystems). Genomic clones were sequenced similarly using primers that hybridize along the length of the gene.
Gene Isolation
Texas A & M University (College Station) and Institut fur Genbiologische Forschung, Berlin bacteria artificial chromosome (BAC) filters were generously provided by Joe Ecker (University of Pennsylvania, Philadelphia). Filters were hybridized against a 5′ end probe made from digesting ffc cDNA with SacI and BglII. The probe was labeled using a random oligonucleotide-labeling kit according to the manufacturer's directions (Pharmacia). BAC DNA preparations were made from the strains containing the hybridizing BAC clones. BAC DNA was restricted with EcoRI and analyzed on Southern blots probed with the 5′ SacI-BglII fragment and a 3′ end BglII-HindIII (the HindIII site originating from the polylinker) fragment. Clones F11D5, F8C6, F2F2, and F22J14 yielded the appropriately sized restriction fragments. The Ffc gene was subcloned from F22J14 in three pieces: a 5-kb EcoRI-SacI fragment containing the promoter and the 5′ end of the coding region was subcloned into the same sites of Puc19; a 1.9-kb XhoI-BglII fragment was subcloned into the XhoI-BamHI sites of Bluescript SK+ (Stratagene); and a 1.4-kb BglII-EcoRI fragment extending downstream from the coding region was subcloned into the BamHI and EcoRI sites of Bluescript SK+. The cloning strategy left a 434-bp gap between the two BglII sites. The missing sequence was amplified by PCR and the PCR product was sequenced directly.
RESULTS
CS 3149 and CS 3153 Are Allelic, Single-Gene Mutations in ffc
To determine whether any of the George Redei mutants might lack cpSRP54, we acquired all 51 lines and analyzed the level of cpSRP54 in leaf tissue by immunoblot analysis. Two pigment mutants created by x-ray mutagenesis of wild-type Arabidopsis ecotype Columbia, CS 3149 (originally scored as yellow heart), and CS 3153 (originally scored as yellow-green) (Fig. 1A, e and f) contained no detectable cpSRP54 in 50 μg of total protein (data not shown).
Figure 1.
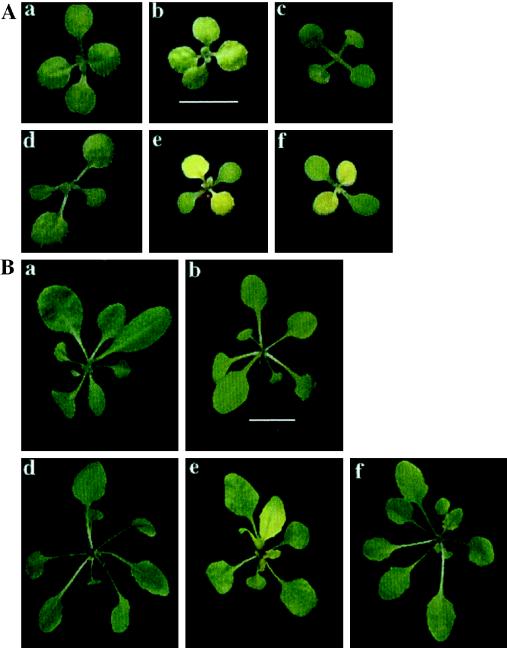
Visible appearance of Arabidopsis wild type and cpSRP mutants. Seeds were grown on plates lacking Suc under constant illumination. Plants were photographed under identical conditions at 10 d (A) and 28 d (B). a, Landsberg wild type; b, chaos (cpSRP43 mutant); c, ffc1-2::54His (cpSRP54 mutant transformed with cpSRP54 His); d, Columbia wild type; e, ffc1-1 (cpSRP54 mutant); and f, ffc1-2 (cpSRP54 mutant). The scales in panel b of both A and B correspond to 0.5 cm.
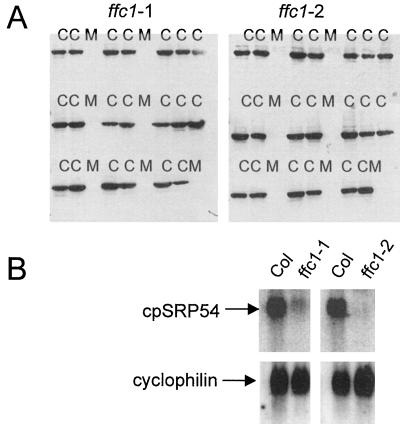
When CS 3149 and CS 3153 were each crossed to wild-type Columbia, all progeny in the F1 generation had a wild-type phenotype, indicating that both mutations are recessive (data not shown). Both mutations segregated 3:1 in the F2 generation, indicating that single genes were causing the phenotype. To confirm that the mutant phenotype segregates with the null allele, 18 wild-type and nine mutant seedlings were randomly chosen from the F2 progeny, scored for phenotype, and analyzed for cpSRP54. All seedlings with the mutant phenotype lacked cpSRP54, while those having the wild-type phenotype did contain the protein (Fig. 2A). In three crosses made between the two mutants, all progeny in the F1 generation had the mutant phenotype, indicating that CS 3149 and CS 3153 are allelic. Both mutants produced wild-type kanamycin-resistant transformants when transformed with a wild-type construct encoding a His-tagged version of cpSRP54 (a 3153 transformant is shown in Fig. 1A, c). These results indicated that the two mutants contained null alleles of ffc; we designated CS 3149 as ffc1-1 and CS 3153 as ffc1-2.
Figure 2.
Identification of ffc null alleles. A, Cosegregation of the mutant phenotype and the null ffc allele. F2 progeny from backcrosses between ffc1-1 and ffc1-2 to ecotype Columbia were grown under constant illumination on plates lacking both Suc and the selectable marker. From each backcross, seven mutant (M) and 20 wild-type plants (C) were sampled at random, and protein was extracted from the leaf tissue and analyzed by immunoblot analysis using antisera raised against cpSRP54. Cross-reacting proteins were detected by enhanced chemiluminescence. B, ffc transcripts are low or undetectable in ffc mutants. Each lane contained 5 μg of total RNA. Blots were hybridized against a SacI-BglII probe from pNH10 and an EcoRI-BamHI probe from cyclophilin to monitor for equal loading.
Northern-Blot Analysis
To determine if ffc1-1 and ffc1-2 lacked ffc transcript, RNA was isolated from both lines and the level of ffc transcript was measured by northern-blot analysis (Fig. 2B). Samples blotted against a cyclophilin probe revealed that loading differences were minimal. ffc1-1 contained full-length ffc transcript, but the level was reduced compared with the wild type. In contrast, full-length transcript was not detected in the ffc1-2 mutant. These data suggested that the ffc1-1 may contain a point mutation, whereas ffc1-2 may contain a deletion, insertion, or DNA rearrangement.
Isolation of the Ffc Gene
To facilitate the mutational analysis of the ffc mutants, we isolated the Ffc gene by screening BAC library filters and sequenced the clone (accession no. AF092168). A map depicting the 13 exons, pertinent restriction sites, and primers is shown in Figure 3A. From PCR amplification of the yUP YAC library, we tentatively mapped ffc to chromosome 5 between the markers M447 and g4090.
Figure 3.
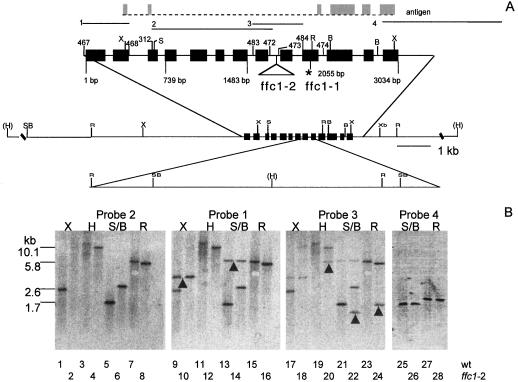
Molecular basis of the ffc alleles. A, Restriction map of ffc. Exons used to make recombinant antigen are marked in gray. Probes used for hybridization are numbered 1 to 4. The position of the exons in the ffc gene is indicated by the solid black boxes. Numbers above the gene are arbitrary designations of primers used in the study. Numbers below the gene correspond to the nucleotide sequence. Restriction sites are indicated as follows: X, XhoI; S, SacI; R, EcoRI; B, BglII; H, HindIII; Xb, XbaI; and SB, SacI or BglII site. HindIII sites marked in parentheses have not been mapped relative to the internal restriction sites. The DNA insertion in ffc1-2 in intron 8 is indicated by the triangle and is drawn to scale in the lower restriction map. The asterisk indicates the stop codon in exon 10. B, Southern-blot analysis of the ffc1-2 allele. Each lane contained 10 μg of genomic DNA from either ecotype Columbia (odd lanes) or the ffc1-2 mutant line (even lanes). The first three panels represent the same blot hybridized successively to probes 2, 1, and 3. Blots were not stripped of probes prior to rehybridization. Arrows indicate bands that were detected only upon rehybridization. A new sample was hybridized to probe 4 in the fourth panel.
Identification of the Mutation in ffc1-1
Southern blotting revealed no anomalies in the ffc1-1 allele (data not shown), further suggesting that ffc1-1 had a point mutation. To test this idea, PCR products spanning the entire ffc1-1 allele were generated and directly sequenced. A single mutation was detected at position 2,055 that changed the codon for R288, CGA, into the stop codon TGA (Fig. 3A). Conceivably, a truncated protein with a predicted molecular mass of 31 kD is expressed; however, no such protein was detected. The antibody used (CIW24) was raised against a fusion protein containing the N-terminal 33 amino acids of cpSRP54 fused to the C terminus (amino acids 317−488) (Fig. 3A). Either the truncated product is unstable or the antiserum is not effective at recognizing the N-terminal 33 amino acids.
Identification of the Mutation in ffc1-2
Differences between the wild-type and ffc1-2 alleles were evident from the results of Southern-blot analysis (Fig. 3B). In the first three panels of Figure 3B, lanes 1 to 24, a single blot was successively hybridized to three different probes without stripping. Newly appearing bands are marked on the blots with an arrow. Probe 2, which was generated by PCR using the primers 312 and 472, hybridized to smaller XhoI (2.6 kb versus a barely visible 12 kb) and SacI/BglII fragments (1.7 versus 2.6 kb) and larger HindIII (12 versus 10 kb) and EcoRI fragments (6.0 versus 5.9 kb) in the wild type compared with ffc1-2. This result indicated that the ffc1-2 allele is altered between the SacI-BglII sites (642−2,232 bp). A simple deletion can be ruled out because hybridizing fragments are both smaller and larger in the mutant. However, the data could be explained by a large (10 kb) DNA insertion within this region, as depicted in Figure 3A.
To test this idea further, the blot was hybridized to probe 1. This probe, generated by PCR using primers 467 and 468, hybridizes to the 5′ end of the gene. Two bands that were not detected by probe 2, a 3.7-kb XhoI fragment (Fig. 3B, lanes 9 and 10) and a 6.0-kb SacI-BglII fragment (lanes 13 and 14), were detected by probe 1 in both the wild-type and ffc1-2 alleles. This indicated that there were no major differences in the 5′ end of the gene (up to 642 bp). A second blot (Fig. 3B, panel 4) hybridized against probe 4, made by random hexamer labeling of a BglII-XbaI fragment corresponding to the 3′ end of the gene, also did not reveal differences between the two alleles digested with SacI-BglII and EcoRI. These data corroborated the previous finding and narrowed the location of the mutation to within the SacI-EcoRI sites (642−2,068 bp).
With the exception of intron 8 (1,670−1,780 bp), all regions from 642 to 2,068 bp were amplified by PCR and determined to be free of mutations by sequence analysis. Attempts at amplifying a large DNA insert in intron 8 were unsuccessful. However, the insert could be detected by hybridization to probe 3, which spans intron 8. Additional fragments (1.6-kb EcoRI, 1.4-kb SacI/BglII, and 6-kb HindIII) were detected for the ffc1-2 allele but not the wild type. These data can all be reconciled with the insertion model depicted in Figure 3A.
Null ffc Mutants Have a Yellow Heart/Virescent Phenotype; chaos Mutants Are Chlorotic
Having confirmed that we isolated true null mutants in the ffc gene, we compared the ffc and chaos mutants grown under identical conditions on agar plates lacking Suc. As was seen for the transgenic cosuppression lines (Pilgrim et al., 1998), both ffc mutants produced yellow first true leaves with green cotyledons. These leaves became green within 1 week and all subsequent leaves were green (Fig. 1A, e and f versus Fig. 1B, e and f). Leaves in the ffc1-1 mutant plants exhibited a premature leaf yellowing beginning in the 4th week of growth. As this phenotype was not observed in ffc1-2 or in cosuppressor lines, it could not have been due exclusively to the absence of cpSRP54.
Chaos plants did not produce yellow first true leaves; all leaves were pale green (Fig. 1A, b). Thus, there is a noticeable developmental effect for the ffc mutants that is not observed in chaos mutants. In the older plants, we could not distinguish between chaos and ffc mutants based on color. Both were paler than the respective wild-type plants (Columbia for ffc and Landsberg for chaos). The mutant plants grew considerably slower than wild-type plants on soil under a 16-h photoperiod.
Elevated Chl a/b Ratios Are Observed in chaos But Not in ffc Mutants
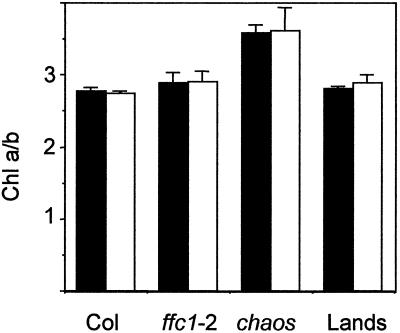
All Chl b is associated with LHCP, whereas Chl a is associated with both LHCP and reaction center proteins (Thornber and Highkin, 1974). Therefore, a change in the Chl a/b ratio reflects a change in the relative abundance of LHCP to other Chl-containing proteins. Klimyuk et al. (1999) had previously reported that Chl a/b ratios were elevated in chaos plants grown under a number of different light conditions, which is consistent with the observation that these plants had reduced levels of LHCP relative to other Chl a-containing proteins. In contrast, cosuppressor ffc lines had wild-type Chl a/b ratios in both the yellow first true leaves, where Chl content was reduced by over 75%, and in the older leaves, where Chl was reduced by about 30% (Pilgrim et al., 1998). To determine whether Chl a/b ratios were altered in ffc null mutants, measurements were made in 10- and 24-d-old leaves of ffc1-2, chaos, Columbia, and Landsberg. Elevated Chl a/b ratios were observed in chaos in both the young and old leaves, but were normal in the ffc1-2 leaves (Fig. 4). These data indicated that the composition of pigment proteins is different in the two mutant lines.
Figure 4.
Chl a/b ratios in wild-type and mutant tissue. Leaves were extracted from ecotype Columbia (Col), ffc1-2, chaos, and ecotype Landsberg (Lands) at 10 and 24 d, and Chl a and b were measured as described in the text.
Immunoblot Analysis of LHCP in chaos and ffc1-2
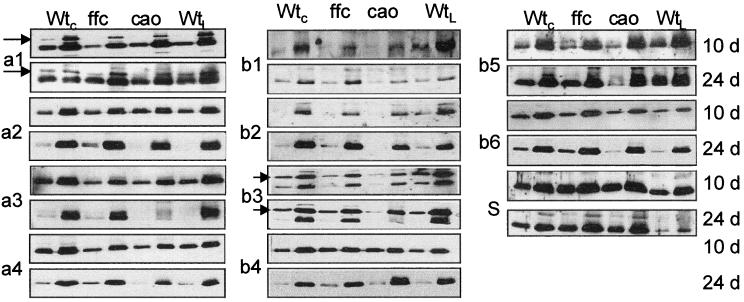
The most abundant proteins of the thylakoid membrane are members of the LHCP family. LHCP proteins are grouped into 12 distinct gene families (Jansson, 1994). Four gene families encode proteins associated with PSI (Lhca1−4), seven gene families encode proteins associated with PSII (Lhcb1−6 and psbS), and the 12th family is comprised of early light-inducible proteins that accumulate under periods of stress (Jansson, 1994). Together, these proteins represent about 50% of the total Chl and a significant fraction of the membrane protein (Green and Salter, 1996). To determine whether the levels of individual LHCP are affected by a mutation in cpSRP, we performed immunoblot analysis with antibodies specific for all the LHCP families except early light-inducible proteins. Analysis was performed on the first true leaves harvested from 10-d-old plants and the shoots of 24-d-old plants. At least two levels of protein were loaded for each blot to ensure that the signal was in the linear response range, and blots were repeated at least three times. Results from representative experiments are shown in Figure 5. Three types of responses were observed. In the first type, exemplified by psbS and Lhcb4, elevated levels of protein were observed at both the 10- and 24-d time points for both mutants. In the second, exemplified by Lhca2 and Lhcb6, wild-type levels of proteins were found at both time points for both mutants. For the third response, LHCP were reduced in chaos plants at both 10 and 24 d but were reduced in ffc plants only at the 10-d time point. It is noteworthy that the same subset of proteins, Lhca1, Lhca3, Lhca4, Lhcb1, Lhcb2, Lhcb3, and Lhcb5, exhibited this response. These results suggest that at least seven of the LHCPs are dependent on the cpSRP pathway for targeting, while four proteins are either less dependent or independent of cpSRP.
Figure 5.
Immunoblot analysis of LHCP in mutant and wild-type tissue. Proteins were extracted as described in the text. Samples were loaded at low and high concentrations to ensure linearity of the immuno response. Each blot was repeated at least three times. WtC, Wild-type Columbia; ffc, ffc1-2; cao, chaos; WtL, wild-type Landsberg. The antibody dilution used (in parentheses) and the amount of membrane protein loaded in each lane were as follows: Lhca 1 (1:1,000) 10 d, 0.5/2.5 μg, 24 d, 1/3 μg; Lhca2 (1:200) 10 and 24 d, 1/5 μg; Lhca3 (1:1,000) 10 d, 0.5/1.5 μg, 24 d, 0.5/2.5 μg; Lhca4 (1:1,000) 10 d, 7.5/15 μg, 24 d, 10/40 μg; Lhcb1 (1:50) 10 and 24 d, 10/20 μg; Lhcb2 (1:50) 10 and 24 d, 0.5/1.5 μg; Lhcb3 (1:30) 10 and 24 d, 1/3 μg; Lhcb4 (1:100) 10 and 24 d, 2.5/7.5 μg; Lhcb5 (1:100) 10 d, 0.5/1.5 μg, 24 d, 1/3 μg; Lhcb6 (1:100) 10 d, 2/4 μg, 24 d, 2/6 μg; psbS (1:1,000) 10 d, 7.5/15 μg, 24 d, 10/20 μg.
Reaction Center Proteins Are Affected in ffc But Not in chaos
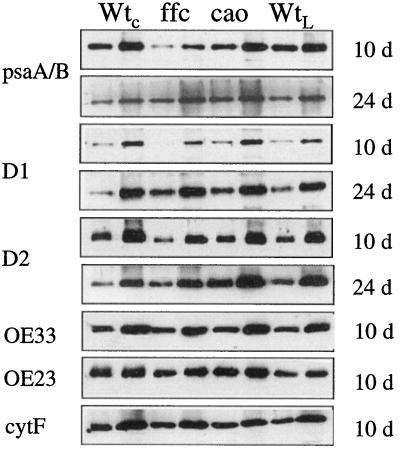
The reaction center of PSII is comprised of the D1 and D2 polypeptides. Likewise, the reaction center for PSI is comprised of psaA and psaB proteins. All four polypeptides are hydrophobic Chl proteins encoded by the chloroplast genome. In a previous investigation, reaction center proteins were not found to be affected by the chaos mutation (Klimyuk et al., 1999), whereas levels of these proteins were reduced in transgenic plants deficient in cpSRP54 (Pilgrim et al., 1998). Levels of these proteins were re-examined in both null mutants, and the results shown in Figure 6 confirm the previous findings. Levels of reaction center proteins were comparable or greater than wild type for the chaos mutant. Levels of reaction center proteins were substantially reduced in the 10-d-old ffc mutant, but were normal or even elevated in the 24-d-old plants. Thus, reaction center proteins are adversely affected in the 10-d-old ffc mutant and not in chaos. These results suggest the involvement of cpSRP54, not cpSRP43, in the biogenesis of chloroplast-encoded proteins.
Figure 6.
Immunoblot analysis of the reaction center polypeptides OE33, OE23, and CytF in mutant and wild-type tissue. Proteins were extracted and analyzed as described in the legend to Figure 5. The antibody dilution used (in parentheses) and the amount of membrane protein loaded in each lane were as follows: psaA/B (1:1,000) 10 d, 3/6 μg, 24 d, 2.5/5 μg; D1 (1:250) 10 d, 3/6 μg, 24 d, 2.5/7.5 μg; D2 (1:1,000) 10 and 24 d, 2.5/7.5 μg; OE33 (1:10,000) 10 and 24 d, 0.1/0.3 μg; OE23 (1:10,000) 10 and 24 d, 0.5/1.5 μg; CytF (1:500) 10 and 24 d, 2.5/7.5 μg.
Another chloroplast-encoded protein, CytF, which is diminished in maize mutants lacking SecA (Voelker and Barkan, 1995b), was unaffected by mutation of either cpSRP subunit (Fig. 6). This observation demonstrates that not all chloroplast proteins are affected by the loss of cpSRP54. Furthermore, it demonstrates that the biogenesis of CytF is not especially cpSRP dependent. Another SecA-dependent protein, OE33, was also unaffected by the mutations (Fig. 6). Similarly, the ΔpH-dependent protein OE23 (Fig. 6) and the soluble protein rbcS (data not shown) were also unaffected. Clearly, mutations in cpSRP have specific effects on a subset of proteins.
Effects on Protein Transport Machinery
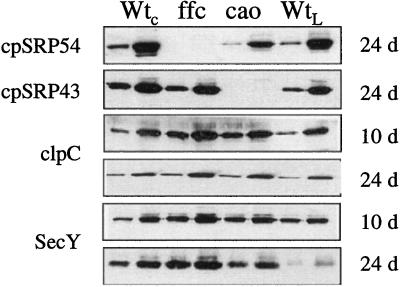
As was previously observed, cpSRP54 and cpSRP43 were not detected in the ffc and chaos mutants, respectively (Fig. 7). In the absence of cpSRP43, levels of cpSRP54 were somewhat reduced, suggesting that the complex stabilizes cpSRP54 or that expression of cpSRP54 is influenced by the abundance of cpSRP43. In contrast, no reduction in cpSRP43 was observed in the absence of cpSRP54. Chaperones are often found at elevated levels in tissues subjected to stress. We previously observed that both Hsp70 and ClpC increased in the ffc cosuppressor lines (Pilgrim et al., 1998). Hsp70 facilitates protein folding and plays a role in protein translocation across membranes (Craig et al., 1990). ClpC is a protein related to Hsp90 and is found associated with the envelope translocon (Nielsen et al., 1997). In both null mutants the levels of ClpC were elevated at both stages of development (Fig. 7); Hsp70 did not appear to be significantly altered (data not shown). Another protein found at elevated levels in the mutants was cpSecY, which forms a protein translocation channel in the thylakoid membrane (Laidler et al., 1995; Schuenemann et al., 1999). In both mutants, the level of SecY was elevated at both developmental stages (Fig. 7). The increases in ClpC and SecY may comprise a compensation mechanism to alleviate the defect of functional cpSRP.
Figure 7.
Immunoblot analysis of cpSRP, clpC, and SecY in mutant and wild-type tissue. Proteins were extracted and analyzed as described in the legend to Figure 5. The antibody dilution used (in parentheses) and the amount of membrane protein loaded in each lane were as follows: cpSRP54 (1:5,000) 10 and 24 d, 5/25 μg; cpSRP43 (1:2,000) 10 and 24 d, 15/40 μg; clpC (1:5,000) 10 and 24 d, 1/5 μg; SecY (1:2,000) 10 d, 2/5 μg, 24 d, 7.5/15 μg.
DISCUSSION
Reconstitution studies have demonstrated that cpSRP43 and cpSRP54 form a protein complex, cpSRP, that is required for the biogenesis of Lhcb1 in vitro (Schuenemann et al., 1998). The chaos and ffc mutants provide genetic confirmation for these studies. Of the 11 members of the LHCP protein family that we tested, the same seven were adversely affected by a mutation in the ffc or cao alleles. As loss of either protein affected the same subset of proteins, this specificity is consistent with the idea that the complex is the active form with the loss of either protein leading to inactivity.
In at least four noteworthy respects, chaos and ffc mutants have distinctive phenotypes. First, the two mutants can be visually distinguished at the young seedling stage, where the first true leaves of chaos mutants are greener. Second, the Chl a/b ratio of chaos plants is significantly higher than that of ffc mutants, resembling wild-type levels. Third, the ffc mutation but not the cao mutation adversely affects reaction center proteins. Fourth, the phenotype of the ffc mutant becomes considerably less severe as the plant matures. This recovery is manifested by a greening of the leaves and a relative increase in the amount of all of the thylakoid proteins that were adversely affected in the 10-d-old plants. Thus, the distinct phenotypes observed clearly support the idea that cpSRP43 and cpSRP54 have nonoverlapping roles.
A previous study showed that all cpSRP43 was complexed to cpSRP54, while a second pool of cpSRP54 was associated with 70S ribosomes free of cpSRP43 (Schuenemann et al., 1998). The existence of two forms of cpSRP54 but just one form of cpSRP43 suggests the reason for the different phenotypes. When cpSRP54 is associated with cpSRP43, the resulting complex has the ability to promote the post-translational targeting of LHCP, and, indeed, the same set of LHCP proteins are affected by a mutation in either subunit of cpSRP. When cpSRP54 is not associated with cpSRP43, it is primarily associated with 70S ribosomes (Franklin and Hoffman, 1993). Conceivably, the ribosome-bound cpSRP54 plays a role analogous to cytoplasmic SRP54 in mediating the cotranslational targeting of hydrophobic proteins to the membrane.
Biochemical support for this idea has recently been obtained by Nilsson et al. (1999), who observed that ribosome nascent chains containing the D1 protein were efficiently cross-linked to endogenous cpSRP54 in a chloroplast translation extract. The observation that reaction center proteins are affected in the ffc but not the chaos mutants directly supports the notion that cpSRP54 plays a role in the biogenesis of some of the chloroplast-encoded proteins. Because cytoplasmic SRP54 is always associated with an RNA (Walter and Johnson, 1994), it will be of interest to determine the nature of the association of cpSRP54 with the 70S ribosome. If cpSRP54 has maintained its ancient role (analogous to cytosolic SRP54) and has simultaneously adapted a new, more specialized role through binding to cpSRP43, an ffc/chaos double mutant will be expected to have the same phenotype as the ffc mutant. This investigation is currently under way.
One interesting outcome from the analysis of the cpSRP mutants is the fact that LHCP proteins insert into the membrane in the absence of functional cpSRP. Although it cannot be excluded that the individual subunits are partially active in vivo, this possibility seems unlikely given that the subunits are inactive individually in vitro (Schuenemann et al., 1998). More likely, an alternative transport pathway normally targets a fraction of the LHCP molecules. In support of this idea, it has been argued that the cpSRP evolved more recently than the LHCPs, as the chromo domain motif found in cpSRP43 has never been observed in prokaryotic genomes, whereas prokaryotic proteins related to LHCP have been described (Dolganov et al., 1995; Klimyuk et al., 1999). Thus, prior to cpSRP, there was likely a pre-existing mechanism for the targeting of LHCPs, and such a system may still be operational but has yet to be described. One possibility is that LHCP is also targeted via vesicles emanating from the envelope membrane. Such a pathway might escape detection, as it is unlikely that a vesicular-targeting pathway would be reconstituted using the assays currently applied to study the cpSRP pathway. Vesicles emanating from the envelope membranes are evident during certain stages of plastid development (Hoober et al., 1991; Hugueney et al., 1995). Furthermore, the dynamin protein family, some members of which are known to be involved in vesicular trafficking (Schmid et al., 1998), contains chloroplast homologs that are required for normal chloroplast development (Kang et al., 1998; Park et al., 1998).
A second interesting outcome is the discovery that four LHCP proteins, Lhca2, Lhcb4, Lhcb6, and psbS, were not adversely affected by a mutation in either cpSRP subunit. The expression of these proteins might be up-regulated in the mutants, thereby masking the targeting defect, or the targeting of these proteins might be less dependent or independent of cpSRP. In vitro studies with Lhca2 indicated that this protein requires GTP and stroma for integration into the thylakoid, suggesting that it does utilize the cpSRP pathway (Hoffman and Franklin, 1994). However, the psbS protein can insert into thylakoid membranes without any additional soluble or membrane factors (Kim et al., 1999). The requirement for cpSRP may be more definitively addressed by in vitro reconstitution experiments with the individual proteins.
Perhaps the most puzzling result was the finding that the ffc mutants recover, while the chaos mutants do not. The recovery is seen for both LHCP and reaction center proteins. The fact that the chaos mutant does not show a developmental effect indicates that the requirement for cpSRP is not reduced as the plants age. Rather, it appears that a compensation mechanism is induced in ffc but not in chaos. One possibility, given the differential effects on reaction center proteins in the two mutants, is that the recovery system is induced in response to a limitation in reaction center proteins. Whatever mechanism facilitates the targeting of the reaction center proteins may also promote the insertion of LHCP. Thus, these studies suggest that one to two transport mechanisms, in addition to the four already described, remain to be discovered.
ACKNOWLEDGMENTS
We thank Joe Ecker for providing the Arabidopsis genomic library on BAC filters, Chris Somerville for the yUP YAC library, Luc Adam for the genomic DNA isolation procedure, Andrew Staehlin, Stefan Jansson, David Simpson, Bertil Andersson, Klaas Jan van Wijk, Roberto Barbato, Ken Cline, Alice Barkan, and John Shanklin for antibodies, Courtney Riggle for excellent technical assistance, and Danja Schuenemann for critically reading the manuscript.
Footnotes
This work was supported by grant no. GM42609–02 from the U.S. Department of Agriculture to N.E.H. This is Carnegie Institution of Washington publication no. 1,411.
LITERATURE CITED
- Arnon D. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Miles D, Taylor W. Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J. 1986;5:1421–1427. doi: 10.1002/j.1460-2075.1986.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock IW, Mills JD, Robinson D, Robinson C. The delta pH-driven, ATP-independent protein translocation mechanism in the chloroplast thylakoid membrane: kinetics and energetics. J Biol Chem. 1995;270:1657–1662. doi: 10.1074/jbc.270.4.1657. [DOI] [PubMed] [Google Scholar]
- Cline K, Ettinger WF, Theg SM. Protein-specific energy requirements for protein transport across or into thylakoid membranes: two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992;267:2688–2696. [PubMed] [Google Scholar]
- Cline K, Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Dev Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- Cline K, Henry R, Li CJ, Yuan JG. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 1993;12:4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E, Kang PJ, Boorstein W. A review of the role of 70 kDa heat shock proteins in protein translocation across membranes. Antonie Leeuwenhoek. 1990;58:137–146. doi: 10.1007/BF00548924. [DOI] [PubMed] [Google Scholar]
- Dolganov NAM, Bhaya D, Grossman AR. Cyanobacterial protein with similarity to the chlorophyll a/b-binding proteins of higher plants: evolution and regulation. Proc Natl Acad Sci. 1995;92:636–640. doi: 10.1073/pnas.92.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin AE, Hoffman NE. Characterization of a chloroplast homologue of the 54-kda subunit of the signal recognition particle. J Biol Chem. 1993;268:22175–22180. [PubMed] [Google Scholar]
- Green BR, Salter AH. Light regulation of nuclear-encoded thylakoid proteins. In: Andersson B, Salter AH, Barber J, editors. Molecular Genetics of Photosynthesis. Oxford: IRL Press; 1996. pp. 75–103. [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- High S, Henry R, Mould RM, Valent Q, Meacock S, Cline K, Gray JC, Luirink J. Chloroplast SRP54 interacts with a specific subset of thylakoid precursor proteins. J Biol Chem. 1997;272:11622–11628. doi: 10.1074/jbc.272.17.11622. [DOI] [PubMed] [Google Scholar]
- Hoffman NE, Franklin AE. Evidence for a stromal GTP requirement for the integration of a chlorophyll a/b-binding polypeptide into thylakoid membranes. Plant Physiol. 1994;105:295–304. doi: 10.1104/pp.105.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NE, Pichersky E, Malik VS, Castresana C, Ko K, Darr SC, Cashmore AR. A cDNA clone encoding a photosystem I protein with homology to photosystem II chlorophyll a/b-binding polypeptides. Proc Natl Acad Sci USA. 1987;84:8844–8848. doi: 10.1073/pnas.84.24.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober JK, Boyd CO, Paavola LG. Origin of thylakoid membranes in Chlamydomonas reinhardtii y-1 at 38°C. Plant Physiol. 1991;96:1321–1328. doi: 10.1104/pp.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Dharlingue A, Kuntz M, Camara B. Identification of a plastid protein involved in vesicle fusion and/or membrane protein translocation. Proc Natl Acad Sci USA. 1995;92:5630–5634. doi: 10.1073/pnas.92.12.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulford A, Hazell L, Mould RM, Robinson C. Two distinct mechanisms for the translocation of proteins across the thylakoid membrane, one requiring the presence of a stromal protein factor and nucleotide triphosphates. J Biol Chem. 1994;269:3251–3256. [PubMed] [Google Scholar]
- Jansson S. The light-harvesting chlorophyll a/b binding proteins. Biochim Biophys Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Kang SG, Jin JB, Piao HL, Pih KT, Jang HJ, Lim JH, Hwang I. Molecular cloning of an Arabidopsis cDNA encoding a dynamin-like protein that is localized to plastids. Plant Mol Biol. 1998;38:437–447. doi: 10.1023/a:1006099718761. [DOI] [PubMed] [Google Scholar]
- Kapazoglou A, Sagliocco F, Dure L. PSII-T, a new nuclear encoded lumenal protein from photosystem II: targeting and processing in isolated chloroplasts. J Biol Chem. 1995;270:12197–12202. doi: 10.1074/jbc.270.20.12197. [DOI] [PubMed] [Google Scholar]
- Karnauchov I, Cai DG, Schmidt I, Herrmann RG, Klosgen RB. The thylakoid translocation of subunit 3 of photosystem I, the psaF gene product, depends on a bipartite transit peptide and proceeds along an azide-sensitive pathway. J Biol Chem. 1994;269:32871–32878. [PubMed] [Google Scholar]
- Kim SJ, Jansson S, Hoffman NE, Robinson C, Mant A. Distinct 'assisted' and 'spontaneous' mechanisms for the insertion of polytopic chlorophyll-binding proteins into the thylakoid membrane. J Biol Chem. 1999;274:4715–4721. doi: 10.1074/jbc.274.8.4715. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Robinson C, Mant A. Sec-SRP-independent insertion of two thylakoid membrane proteins bearing cleavable signal peptides. FEBS Lett. 1998;424:105–108. doi: 10.1016/s0014-5793(98)00148-3. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Robinson D, Robinson C. An Arabidopsis thaliana cDNA encoding PS II-X, a 41 kDa component of photosystem II: a bipartite presequence mediates SecA/Delta pH-independent targeting into thylakoids. FEBS Lett. 1996;390:175–178. doi: 10.1016/0014-5793(96)00658-8. [DOI] [PubMed] [Google Scholar]
- Kirwin P, Elderfield PD, Williams RS, Robinson C. Transport of proteins into chloroplasts: organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988;263:18128–18132. [PubMed] [Google Scholar]
- Klimyuk VI, Persello-Cartieaux F, Havaux M, Contard P, Schuenemann D, Meiherhoff K, Gouet P, Jones JDG, Hoffman NE, Nussaume L. Molecular identification of CHAOS a plant-specific component of the chloroplast signal recognition particle. Plant Cell. 1999;11:87–99. doi: 10.1105/tpc.11.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosgen RB, Brock IW, Herrmann RG, Robinson C. Proton gradient-driven import of the 16 kda oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992;18:1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Knoetzel J, Simpson D. Expression and organisation of antenna proteins in the light-sensitive and temperature-sensitive barley mutant Chlorina-104. Planta. 1991;185:111–123. doi: 10.1007/BF00194522. [DOI] [PubMed] [Google Scholar]
- Krol M, Spangfort MD, Huner NPA, Oquist G, Gustafsson P, Jansson S. Chlorophyll a/b-binding proteins, pigment conversions, and early light-induced proteins in a chlorophyll b-less barley mutant. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidler V, Chaddock AM, Knott TG, Walker D, Robinson C. A SecY homolog in Arabidopsis thaliana: sequence of a full-length cDNA clone and import of the precursor protein into chloroplasts. J Biol Chem. 1995;270:17664–17667. doi: 10.1074/jbc.270.30.17664. [DOI] [PubMed] [Google Scholar]
- Li XX, Henry R, Yuan JG, Cline K, Hoffman NE. A chloroplast homologue of the signal recognition particle subunit SRP54 is involved in the posttranslational integration of a protein into thylakoid membranes. Proc Natl Acad Sci USA. 1995;92:3789–3793. doi: 10.1073/pnas.92.9.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant A, Nielsen VS, Knott TG, Moller BL, Robinson C. Multiple mechanisms for the targeting of photosystem I subunits F, H, K, L, and N into and across the thylakoid membrane. J Biol Chem. 1994;269:27303–27309. [PubMed] [Google Scholar]
- Michl D, Robinson C, Shackleton JB, Herrmann RG, Klosgen RB. Targeting of proteins to the thylakoids by bipartite presequences: cFoll is imported by a novel, third pathway. EMBO J. 1994;13:1310–1317. doi: 10.1002/j.1460-2075.1994.tb06383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould RM, Knight JS, Bogsch E, Gray JC. Azide-sensitive thylakoid membrane insertion of chimeric cytochrome f polypeptides imported by isolated pea chloroplasts. Plant J. 1997;11:1051–1058. doi: 10.1046/j.1365-313x.1997.11051051.x. [DOI] [PubMed] [Google Scholar]
- Mould RM, Robinson C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991;266:12189–12193. [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp 100 molecular chaperone. EMBO J. 1997;16:935–946. doi: 10.1093/emboj/16.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RJ, Brunner J, Hoffman NE, van Wijk KJ. Targeting and interactions of ribosome nascent chain complexes of the chloroplast-encoded D1 thylakoid membrane protein. EMBO J. 1999;18:733–742. doi: 10.1093/emboj/18.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara T, Asai T, Nakai M, Sugiura M, Endo T. Cytochrome f encoded by the chloroplast genome is imported into thylakoids via the SecA-dependent pathway. Biochem Biophys Res Commun. 1996;224:474–478. doi: 10.1006/bbrc.1996.1051. [DOI] [PubMed] [Google Scholar]
- Nohara T, Nakai M, Goto A, Endo T. Isolation and characterization of the cDNA for pea chloroplast SecA evolutionary conservation of the bacterial-type SecA-dependent protein transport within chloroplasts. FEBS Lett. 1995;364:305–308. doi: 10.1016/0014-5793(95)00415-6. [DOI] [PubMed] [Google Scholar]
- Park JM, Cho JH, Kang SG, Jang HJ, Pih KT, Piao HL, Cho MJ, Hwang I. A dynamin-like protein in Arabidopsis thaliana is involved in biogenesis of thylakoid membranes. EMBO J. 1998;17:859–867. doi: 10.1093/emboj/17.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan LA, Cline K. A stromal protein factor maintains the solubility and insertion competence of an imported thylakoid membrane protein. J Cell Biol. 1991;112:603–613. doi: 10.1083/jcb.112.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim ML, van Wijk KJ, Parry DH, Sy DAC, Hoffman NE. Expression of a dominant negative form of cpSRP54 inhibits chloroplast biogenesis in Arabidopsis. Plant J. 1998;13:177–186. doi: 10.1046/j.1365-313x.1998.00021.x. [DOI] [PubMed] [Google Scholar]
- Roy LM, Barkan A. A SecY homologue is required for the elaboration of the chloroplast thylakoid membrane and for normal chloroplast gene expression. J Cell Biol. 1998;141:385–395. doi: 10.1083/jcb.141.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmid SL, McNiven MA, DeCamilli P. Dynamin and its partners: a progress report. Curr Opin Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Schnell DJ. Protein targeting to the thylakoid membrane. Annu Rev Plant Physiol. 1998;49:97–126. doi: 10.1146/annurev.arplant.49.1.97. [DOI] [PubMed] [Google Scholar]
- Schuenemann D, Amin P, Hartmann E, Hoffman NE. Chloroplast SecY is complexed to SecE and involved in the translocation of the 33 kD but not the 23 kD subunit of the oxygen-evolving complex. J Biol Chem. 1999;274:12177–12182. doi: 10.1074/jbc.274.17.12177. [DOI] [PubMed] [Google Scholar]
- Schuenemann D, Gupta S, Persello-Cartieaux F, Klimyuk VI, Jones JDG, Nussaume L, Hoffman NE. A novel signal recognition particle targets light harvesting proteins to the thylakoid membranes. Proc Natl Acad Sci USA. 1998;95:10312–10316. doi: 10.1073/pnas.95.17.10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles AM, Yonetani A, Baron A, Bush DR, Cline K, Martienssen R. Sec-independent protein translocation by the maize Hcf106 protein. Science. 1997;278:1467–1470. doi: 10.1126/science.278.5342.1467. [DOI] [PubMed] [Google Scholar]
- Shanklin J, Dewitt ND, Flanagan JM. The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: an archetypal two-component ATP-dependent protease. Plant Cell. 1995;7:1713–1722. doi: 10.1105/tpc.7.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist M, Staehelin LA. Identification of type-1 and type-2 light-harvesting chlorophyll-a/b-binding proteins using monospecific antibodies. Biochim Biophys Acta. 1992;1098:191–200. doi: 10.1016/s0005-2728(05)80336-6. [DOI] [PubMed] [Google Scholar]
- Sigrist M, Staehelin LA. Appearance of type 1, 2, and 3 light-harvesting complex II and light-harvesting complex I proteins during light-induced greening of barley (Hordeum vulgare) etioplasts. Plant Physiol. 1994;104:135–145. doi: 10.1104/pp.104.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thornber JP, Highkin HR. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974;41:109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Voelker R, Barkan A. Nuclear genes required for post-translational steps in the biogenesis of the chloroplast cytochrome b(6)f complex in maize. Mol Gen Genet. 1995a;249:507–514. doi: 10.1007/BF00290576. [DOI] [PubMed] [Google Scholar]
- Voelker R, Barkan A. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 1995b;14:3905–3914. doi: 10.1002/j.1460-2075.1995.tb00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R, Mendel-Hartvig J, Barkan A. Transposon-disruption of a maize nuclear gene, tha1, encoding a chloroplast SecA homologue: in vivo role of cp-SecA in thylakoid protein targeting. Genetics. 1997;145:467–478. doi: 10.1093/genetics/145.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Yuan JG, Cline K. Plastocyanin and the 33-kDa subunit of the oxygen-evolving complex are transported into thylakoids with similar requirements as predicted from pathway specificity. J Biol Chem. 1994;269:18463–18467. [PubMed] [Google Scholar]
- Yuan JG, Henry R, McCaffery M, Cline K. SecA homolog in protein transport within chloroplasts: evidence for endo- symbiont-derived sorting. Science. 1994;266:796–798. doi: 10.1126/science.7973633. [DOI] [PubMed] [Google Scholar]