Abstract
Introduction:
Converging clinical and biological evidence suggest sex is an important factor when selecting a pharmacological intervention for smoking cessation. The current investigation used network meta-analyses to estimate sex differences in the comparative efficacy of transdermal nicotine (TN), varenicline, and sustained release (SR) bupropion for smoking cessation.
Methods:
Systematically searched previously published reviews and databases (Medline, PsycINFO, Embase) of randomized, double-blind, placebo-controlled trials of bupropion-SR, TN, and varenicline for cigarette smoking cessation in primary care/general community samples were included.
Results:
Thirty-two studies met all criteria and 28 (88%) were included in the final analyses, representing 14 389 smokers (51% female). Results of the full sample (women and men combined) mirrored those from a Cochrane Tobacco Addiction Group network meta-analysis of smoking cessation pharmacotherapy, showing VAR>TN=BUP. All medications improved quit rates over placebo for both women and men. Relative to placebo, varenicline efficacy was similar for women and men. Significant sex differences were evident when comparing varenicline versus TN and varenicline versus bupropion. For women, varenicline was more efficacious than TN (RR = 1.41; 95% CI = 1.12,1.76) and bupropion (RR = 1.38; 95% CI = 1.08,1.77). For men, outcomes for those treated with TN and bupropion were similar to those treated with varenicline. There were no differences in efficacy when comparing bupropion versus TN.
Conclusions:
The advantage of varenicline over bupropion SR and TN is greater for women than men. Clinicians should strongly consider varenicline as the first option treatment for women. Among men, the advantage of varenicline over TN or bupropion is less clear.
Implications:
This study provides information for the sex-informed treatment of nicotine addiction among cigarette smokers. Relative to placebo, women and men achieved similar outcomes when treated with varenicline; however the advantages of varenicline over transdermal patch and bupropion were greater for women compared to men.
Introduction
Despite continuing declines in cigarette smoking, approximately one out of every six adults in the United States continues to smoke,1 and smoking remains the leading cause of preventable death in the United States, killing approximately 556 000 Americans per year.2,3 Pharmacotherapy can help smokers quit,4,5 yet the likelihood of successfully quitting remains very low.6
There are currently three types of FDA approved pharmacotherapies for smoking cessation: five variants of nicotine replacement therapy (NRT; transdermal nicotine [TN], gum, lozenge, nasal spray, oral inhaler), varenicline, and sustained release (SR) bupropion. A recent network meta-analysis and overview conducted by the Cochrane Tobacco Addiction Group which was not sex-specific concluded that each medication improved the likelihood of cessation relative to placebo (NRT: odds ratio [OR] = 1.84; varenicline: OR = 2.88; bupropion: OR = 1.82).5 In head-to-head comparisons, varenicline was more efficacious than NRT (OR = 1.57) and bupropion (OR = 1.59), with no difference in efficacy between NRT and bupropion.5
Empirical evidence identifies meaningful sex differences in smoking cessation medication efficacy, with hypothesized mechanisms related to sex differences in smoking and metabolism of smoking cessation pharmacotherapy. In a meta-analysis, Perkins and Scott7 found that TN was 40% more efficacious for men compared to women at 6-months post quit attempt. McKee and colleagues8 recently published a meta-analysis of varenicline clinical trials, finding greater efficacy for women compared to men at the end of treatment and at 6-months (46% more efficacious at end of treatment; 34% more efficacious at 6-months). In their meta-analysis of bupropion trials, Scharf and Shiffman9 found no difference in the efficacy by sex at end of treatment, although rates of quitting were lower overall among women. These previous meta-analyses were limited to examining sex differences for individual medications. While findings provided evidence for clinically meaningful sex differences, none included sex-specific head-to-head comparisons of medication efficacy. Consequently, existing literature provides limited information for tailoring clinical decisions based on the cigarette smoker’s sex. If important sex differences exist, the ability to tailor decisions to these differences will improve smoking cessation rates for both women and men.
We conducted a sex-specific network meta-analysis to compare the efficacy of TN, bupropion SR, and varenicline for smoking cessation in primary care and community volunteer clinical trials. We included only randomized, double-blind, placebo controlled trials. Our outcome of interest was biochemically verified abstinence at 6-months post quit attempt. Based on the above noted empirical evidence, we hypothesized that varenicline and bupropion would be more efficacious than TN for women, and that varenicline would be more efficacious than both TN and bupropion for men.
Methods
Search Procedures, Inclusion Criterion, and Data Extraction
For each medication, we initially identified studies for inclusion from previously published meta-analyses of sex differences in medication efficacy known to the authors.7–9 Using AMSTAR criteria10 we determined the search strategies of the original reviews were of sufficient quality to not need repeating for the time-period covered in each review. We then updated our sample of trials by conducting systematic literature searches (in MEDLINE, Embase, and PyscINFO) for the time period since the publication of prior reviews. Searches were initially conducted between January and November 2015, and verified for completeness in December 2015.
For TN we searched titles, abstracts, and key words for ((transdermal nicotine patch OR nicotine patch) AND smoking), limiting the search to trials published from 2008 to 2015 (ie, the time period since Perkins and Scott7 published their meta-analysis of sex differences in TN efficacy). For bupropion we used the search terms: ((bupropion SR OR sustained-release bupropion OR Zyban OR Wellbutrin) AND smoking), limiting the search to 2004–2015. For varenicline we used the search terms ((varenicline OR Chantix OR Champix) AND smoking), limiting the search to 2014–2015.
Our inclusion criteria were designed to maximize the internal validity of comparisons by generating a sample of studies generally consistent in methods and sample. The inclusion criteria were as follows: (1) randomized, double-blind, placebo-controlled trial of one of the three medications of interest for cigarette smoking cessation (ie, not other forms of tobacco/nicotine); (2) primary care or general community participants; (3) treatment with only a medication of interest and a matched placebo condition of only placebo, with the exception that treatment could include behavioral counseling if both treatment and placebo participants received the same counseling; (4) the medication was offered as the first-line treatment (eg, we excluded studies where non-responders to an initial medication were then randomized to the medication of interest, and re-treatment investigations); (5) biochemically confirmed abstinence at 6-months post quit date (point-prevalence abstinence when available, continuous when not; see following paragraph); (6) inclusion of men and women; (7) all analytic cells had sample sizes > 0; (8) exclusion of those with serious acute mental health, physical health, or substance use disorders; and (9) adults 18 and older.
We elected to use point prevalence abstinence as our primary outcome, then used continuous abstinence if point prevalence was not available. Although continuous measures of abstinence have been recommended as the standard for smoking cessation clinical trials,11 these recommendations have also acknowledged that many smokers “slip-up” during an abstinence attempt and that requiring smokers to remain completely abstinent for a continuous abstinence outcome may be overly strict. Several of the clinical trials included in this review employed an overly strict definition of continuous abstinence, leading to our decision to use point prevalence. However, it is important to note that empirical data suggest there is little practical difference between analyzing point prevalence versus continuous abstinence data. A recent review of smoking cessation clinical trials found that relative risk and OR estimates comparing active versus control conditions were identical when using point prevalence versus continuous abstinence.12
For each medication’s literature review, two independent reviewers examined all abstracts and identified publications for potential inclusion, based on study inclusion criteria. We included any abstract identified by either reviewer in a full publication review to make final decisions on manuscript inclusion. Two reviewers than separately reviewed each full publication and independently determined whether each publication met our inclusion criteria. We computed kappa agreement statistics, and then disagreements were resolved between the two reviewers.
Selected studies were added to those identified through previous meta-analyses, and the following data were extracted into a standardized form: country, study design, participants, proportion female, inclusion/exclusion criteria, randomization procedure, allocation concealment procedure, blinding procedure, treatment regimen, outcome measurement, attrition details, counseling regimen, and results. When results were not presented by sex in a manuscript, authors were contacted in an attempt to acquire additional data. Pharmaceutical industry representatives were also contacted for additional data.
Analyses
We utilized network meta-analysis to generate sex-specific indirect head-to-head comparisons of treatments. Network meta-analysis deviates from traditional meta-analysis by using indirect rather than direct comparisons between therapies. For example, if there are three treatments in a head-to-head comparison (A, B, and C), a traditional meta-analysis would only include multi-armed investigations in which these three treatments were directly compared (A vs. B vs. C). In network meta-analyses, comparisons between A versus C are estimated from trials comparing A versus B and trials comparing B versus C.13 For the current investigation, placebo treatment groups served as an anchor from which indirect comparisons between varenicline, bupropion, and TN were inferred. Although previous meta-analyses did not examine sex differences, network meta-analysis has been successfully used to compare the relative efficacy of NRT, bupropion, and varenicline by the Cochrane Tobacco Addiction Group.5
Network meta-analyses assume that the indirect comparisons equal the direct comparisons. In practice, this “consistency assumption” is unlikely to be strictly met, resulting in the development of methods to analytically account for inconsistency. For the current investigation, we followed methods published by Salanti et al.,13 utilizing an arms-based method of comparison and accounting for inconsistency by specifying random effects in the multivariate meta-analytic model, nesting multi-arm comparisons within study. We were unable to conduct direct comparison meta-analyses due to the small number of original multi-arm trials with sex-specific abstinence rates meeting inclusion criteria (n = 4).
All analyses were conducted using the Metafor program in R.14 Treatment results were coded as the natural logarithm of the probability of abstinence in each treatment group, using the logarithmic risk ratio for comparisons. Effect sizes were weighted using the inverse variance method.15 We tested sex differences in comparisons by including sex × comparison interactions in an arms-based network meta-analysis model, specifying random effects for treatment arm. We then computed separate models of treatment comparisons for women only, men only, and women and men combined.
We included behavioral counseling as a control variable in all models, due to prior evidence of smoking cessation effect size variance by sex and treatment with or without counseling.7 Studies in which behavioral counseling was a formal component of the treatment regimen, beyond brief advice or counseling (usually multiple sessions lasting at least 20 minutes), were coded as 1, others were coded as 0.
In order to validate our sample of studies, we first conducted analyses for men and women combined, and compared our results to a previously published Cochrane network meta-analysis for smoking cessation pharmacotherapy. For these analyses, we alternatively used the OR as our effect size to be consistent with the Cochrane review.
Given that we were unable to obtain sex-specific results from a proportion of eligible bupropion studies, we also conducted analyses to validate that our bupropion sample was representative of all eligible studies. We compared results for men and women combined for the studies included in our meta-analysis to results for men and women combined from all eligible studies. We then conducted our primary analyses, examining sex-specific comparisons of medication efficacy.
Results
Search Results
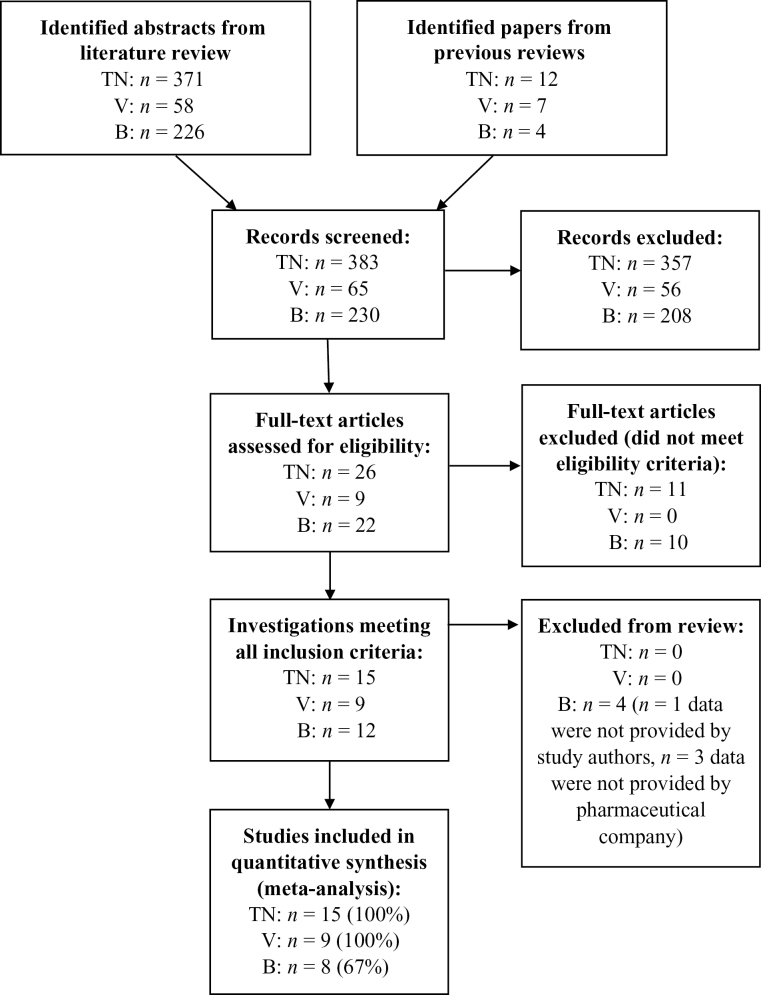
A flow diagram of search results is depicted in Figure 1. Our review included 28 of 32 eligible investigations identified for inclusion (88%). Four of these investigations included multiple active treatment arms, and therefore in some cases search results presented below overlap for different medications. For TN, 12 investigations from previous meta-analyses met all study inclusion criteria, all of which were included in the current investigation.16–27 Our search provided an additional 371 abstracts for review, 14 of which were considered for further review. Three of these met all inclusion criteria28–30 (κ = 1.0). Study authors provided summary data for each trial. In sum our review included data from 15 active TN arms (100% inclusion) with a combined sample size of n = 3882 (women: n = 2021; men: n = 1861).
Figure 1.
Flow chart for study inclusion. TN = Transdermal nicotine trials; V = Varenicline trials; B = Bupropion SR trials. Note: Results for different medications overlap for 4 investigations that included multiple treatment arms. This review included a total of 28 investigations of 32 eligible studies, for an overall inclusion rate of 88%.
For varenicline, seven investigations from a previous meta-analysis met inclusion criteria.31–37 Summary data for each trial were provided by Pfizer, Inc.8 Our literature search generated 58 abstracts, two of which met all inclusion criterion30,38 (κ = 1.0). Study authors provided data for both additional investigations. In sum our review included nine varenicline treatment arms (100% inclusion) with a sample size of n = 2530 (women: n = 1087; men: n = 1443).
For bupropion SR, four investigations from a previous meta-analysis met all study inclusion criterion.39–42 We were unable to obtain sex-specific results from one trial.41 For a second trial,22 we imputed sex-specific data from the 52-week outcome, based on the observation of little change in abstinence between 26–52 weeks (see Table 1 for method of imputation). We computed models both with and without this trial (see “Sensitivity Analyses”). Our literature review identified 226 additional abstracts, 18 of which were included in a full manuscript review. Eight of these met all inclusion criteria (κ = 1.0).29,32,33,38,43–46 Three trials were not able to be included because we were unable to obtain data from pharmaceutical companies.32,33,44 Authors provided summary data for the remaining five trials. In total, we included nine bupropion SR active treatment arms from eight investigations out of a possible 12 (67% inclusion) for a combined sample size of n = 1971 (women: n = 1138; men: n = 833 men). See “Sensitivity Analysis” for an evaluation of the representativeness of our bupropion SR sample.
Table 1.
Sex Differences in Comparative Pharmacotherapy Efficacy
| Sex difference interaction effect size (women vs. men [reference]) [risk ratio (95% CI)] | p | |
|---|---|---|
| Head-to-head comparisons | ||
| Varenicline vs. TN | 1.19 (1.01, 1.40) | .04 |
| Varenicline vs. bupropion SR | 1.22 (1.02, 1.47) | .03 |
| Bupropion SR vs. TN | 0.97 (0.79, 1.19) | .77 |
| Comparisons to placebo | ||
| Varenicline vs. Placebo | 1.01 (0.86, 1.19) | .89 |
| TN vs. Placebo | 0.85 (0.71, 1.02) | .08 |
| Bupropion SR vs. Placebo | 0.83 (0.68, 1.01) | .06 |
CI = confidence interval; SR = sustained release; TN = Transdermal nicotine. p-values < .05 were considered statistically significant.
In addition to the treatment arms noted above, the included investigations generated a combined placebo treatment sample of n = 6006 (women: n = 3136; men: n = 2870) from 28 investigations. See “Sensitivity Analysis” for an evaluation of the effect of combining placebo treatment arms from trials of different medications.
Study Characteristics and Bias Assessment
Studies included in this review are summarized in Supplementary Table S1, alongside judgments for risk of bias. For risk assessment, we evaluated studies on four criteria: randomization procedure, allocation concealment, blinding procedure, and attrition.5,47 All included trials were randomized, double-blind, placebo controlled trials; however, consistent with methods published by the Cochrane Tobacco Group5,47 studies were deemed to have unclear risk of bias if authors did not describe methods for randomization, allocation concealment, or blinding in detail.5,47 Studies were also considered to have unclear risk of bias if attrition was not fully reported. When possible we deferred to previously published Cochrane meta-analyses for risk of bias assessment.5,47 Unclear risk of bias, due to insufficiently described methods, was present in 10 (of 28) trials for randomization, 13 trials for allocation concealment, 15 trials for blinding, and four trials for attrition.
Sample Validation
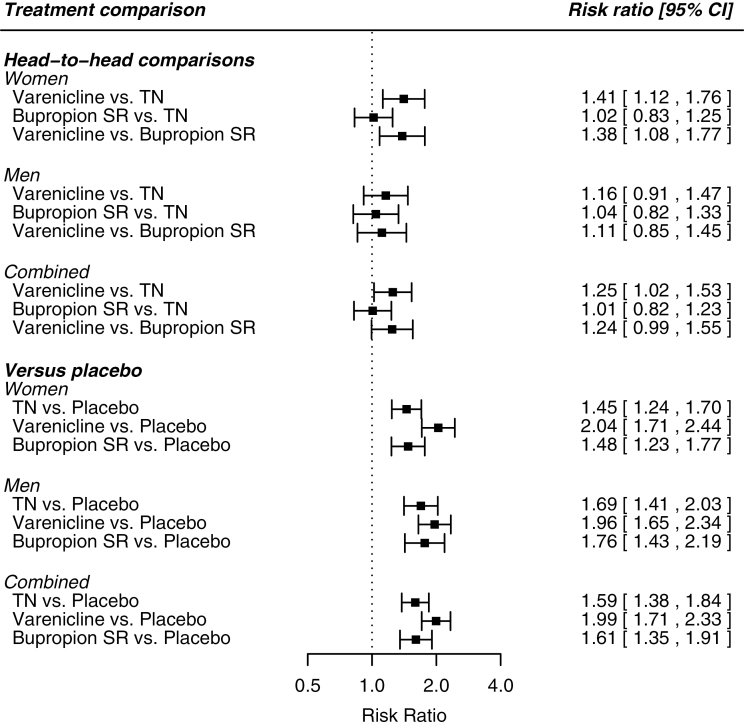
To validate our sample, we compared our results for women and men combined (Figure 2) to a previously published Cochrane network meta-analysis of smoking cessation pharmacotherapy.5 Comparisons were made using ORs rather than relative risk (RR) because ORs were reported in the Cochrane review. In the current investigation, in comparison to placebo, the ORs and 95% confidence intervals (CI) for varenicline, TN, and bupropion were: 2.64 (2.14, 3.26), 1.81 (1.50, 2.18), and 1.81 (1.44, 2.26). The corresponding ORs from the Cochrane meta-analysis were: 2.89 (2.40, 3.48), 1.91 (1.71, 2.14), and 1.85 (1.63, 2.10). For head-to-head comparisons in the current investigation, results were as follows: varenicline versus TN—1.67 (1.24, 2.27); varenicline versus bupropion—1.65 (1.19, 2.30); TN versus bupropion: 1.01 (0.77, 1.34). The corresponding figures from the Cochrane meta-analysis were: varenicline versus TN—1.51 (1.22, 1.87); varenicline versus bupropion—1.56 (1.26, 1.93); bupropion versus TN—0.97 (0.83, 1.13). In summary, all differences between this meta-analytic sample and the previous Cochrane sample were small or negligible, suggesting our sample was representative of varenicline, bupropion SR, and TN clinical trials. The small differences between this study and the Cochrane sample were likely the result of our more strict inclusion criteria, as well as differences in the timeframe of the outcome measure (6 months vs. 6+ months) and primary use of point prevalence versus continuous abstinence.
Figure 2.
Network analysis head-to-head comparisons for women, men, and both sexes combined.
In order to evaluate the representativeness of our bupropion SR trials, we acquired sex-combined outcome data from the four studies for which we were not able to retrieve sex-specific findings. We then compared sex-combined results from our eight included trials to those from all 12 identified studies. The results comparing bupropion to placebo from our included sample of trials were: RR = 1.54 (95% CI = 1.24, 1.93). The results from all identified studies were: RR = 1.62 (95% CI = 1.38, 1.91). The consistency of results between our included investigations and the full sample of identified studies supports the representativeness of our bupropion sample.
Network Analyses
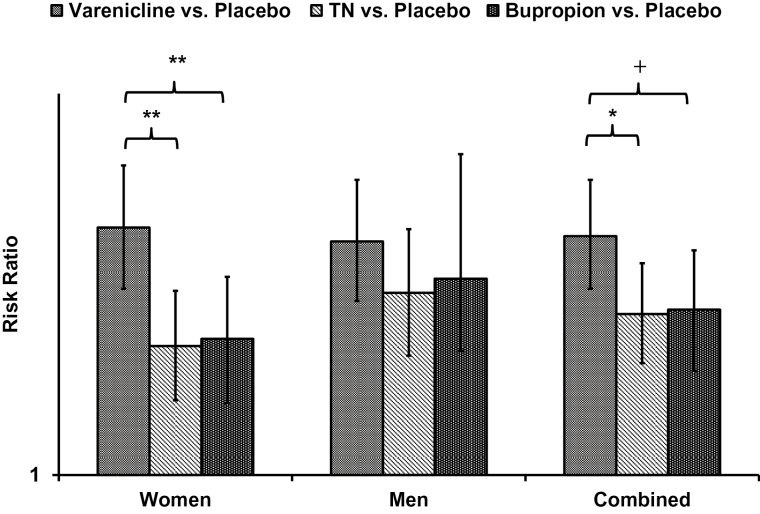
Table 1 shows risk ratios and 95% CI for sex by treatment comparison interactions. There were significant (p < .05) sex differences for the comparisons between varenicline versus TN (RR=1.19, 95% CI = 1.01, 1.40) and varenicline versus bupropion (RR = 1.22, 95% CI = 1.02, 1.47). Sex-specific meta-analytic RRs and 95% CIs for head-to-head comparisons are illustrated using a forest plot in Figure 2. The results are alternatively presented in Figure 3, showing each medication’s comparison to placebo, and the significance of relative differences in comparison to placebo, by sex.
Figure 3.
Network meta-analysis placebo comparison effect sizes (95% confidence interval [CI]), with differences in effect size by medication. +p < .10, *p < .05, **p < .01. TN = Transdermal nicotine.
Women
In head-to-head comparisons, varenicline significantly outperformed both TN and bupropion (Figure 2). The difference between bupropion and TN was negligible and non-significant. The meta-analytic residual heterogeneity among effect sizes was 12% (Q30 = 34.03). Relative to placebo, all three medications significantly increased the odds of 6-month abstinence.
Men
All head-to-head comparison differences were small and were statistically non-significant (Figure 2). The meta-analytic residual heterogeneity among effect sizes was 32% (Q30 = 43.93). Relative to placebo, all 3 medications significantly increased the odds of 6-month abstinence.
Sensitivity Analyses
Meta-analytic results were not substantively affected by the following study sample manipulations: (1) removing six TN trials with dosages less than 21mg over 24 hours for at least some participants,16,18–20,23,28 (2) removing one bupropion treatment arm for which we imputed results,22 (3) removing two investigations in which samples were selected based on cigarettes per day (one bupropion study of light smokers,43 one TN study of heavy smokers25), and (4) removing seven TN trials that measured sustained abstinence rather than point-prevalence abstinence.16,18,20,21,23–25 We also conducted analyses without controlling for counseling, which did not have a meaningful effect on study findings.
In order to examine the impact of combining different placebo types into a single placebo control arm, we evaluated separate models for each medication, comparing each active treatment group to only placebo groups from that treatment’s corresponding studies (eg, TN abstinence rates vs. placebo patch abstinence rates). Results were highly consistent with those from the fully combined network meta-analysis models for men, women, and combined samples, providing evidence that sample inconsistency did not have a large impact on study findings.
Discussion
Compared to placebo, women and men treated with varenicline achieved similar cessation outcomes at 6 months; however, the benefits of varenicline over TN and bupropion SR were greater for women compared to men. Women treated with varenicline were 41% more likely to achieve 6-month abstinence compared to women treated with TN, and were 38% more likely to achieve 6-month abstinence than women treated with bupropion. For men, the benefit of varenicline over TN (16%) and bupropion (11%) were smaller and were not statistically significant. There was no sex difference in the relative efficacy of TN in comparison to bupropion; for both women and men, differences between the two medications were negligible. Based on placebo comparisons, the pattern of findings for sex differences were the result of roughly equivalent efficacy of varenicline for women and men, combined with lower efficacy of TN and bupropion for women compared to men.
Results from our full sample, with women and men combined, closely followed those from a previously published network meta-analysis published by the Cochrane Tobacco Addiction Group, validating the representativeness of our sample of studies. The Cochrane review used continuous abstinence and our review used point prevalence, supporting the interchangeability of outcome selection. Our results suggest that combining men and women for analyses had the effect of underestimating the relative benefit of varenicline over bupropion and TN for women, while overestimating differences in efficacy for men.
Examination of the residual effect-size heterogeneity in meta-analytic models (I2) from the full model compared to separate models for women and men further demonstrated the importance of examining women and men separately. Residual heterogeneity was reduced from 49% when women and men were combined to 12% for women and 32% for men when considered separately. These results suggest sex contributes to variation in effect size between investigations, and that findings are more consistent between studies when women and men are considered separately.
Our findings support the use of varenicline as the first option treatment for women who are trying to quit smoking. The advantage of varenicline over both bupropion and TN may be the result of sex differences in metabolism of smoking cessation pharmacotherapy. Both nicotine and bupropion48 undergo substantial hepatic metabolism via cytochrome P450 CYP2B6 enzymes, and prior studies have demonstrated greater CYP2B6 function in women compared to men.49,50 Regarding nicotine, empirical investigations have shown women metabolize nicotine at a faster rate than men,51,52 and that faster metabolizers have poorer smoking cessation outcomes with TN.30,53 Despite the potential for sex differences in bupropion metabolism, these differences are less well studied. Contrarily, varenicline does not undergo significant CYP enzyme metabolism,54 and has demonstrated similar pharmacokinetic properties for women and men.
In addition to potential metabolism-related mechanisms, women are more influenced by non-pharmacologic drivers of cigarette smoking (eg, negative affect, smoking-related cues), while men are more influenced by the pharmacological effects of nicotine.55 Varenicline has demonstrated efficacy for reducing cue reactivity56 and is minimally metabolized,30,54 making it a potentially potent medication for women attempting to quit. Further, women versus men smokers have lower availability of beta2 nicotinic acetylcholine receptors (nAChRs) compared to sex-matched nonsmokers.57 That is, it appears that female tobacco smokers do not show the nicotine-induced up-regulation of beta2-nAChRs that has been widely reported in the preclinical and postmortem literature.58 It is hypothesized that nicotine replacement may be less effective in women as there are fewer available beta2 receptors for NRT to occupy. While preclinical studies have shown that both nicotine and varenicline have similar effects on nAChR function,59 these studies were restricted to male mice. It is unknown whether varenicline produces sexually dimorphic effects on nicotinic receptor function.
We had anticipated greater efficacy for bupropion compared to TN for women, based on evidence that bupropion reduces negative-affect related withdrawal.38 However, negative affect reduction may only account for a small proportion of bupropion’s efficacy as a smoking cessation aid.60 The mechanisms through which bupropion SR aids in smoking cessation are not fully understood. Bupropion acts both as a dopamine re-uptake inhibitor as well as a non-competitive antagonist of nicotinic acetylcholine receptors.61 A review of bupropion for smoking cessation concluded the medication may work by mimicking nicotine, thus reducing withdrawal and simulating its reinforcing effects.61 It is hypothesized that men’s smoking is more dependent on nicotine-based reinforcement, whereas women’s smoking is more strongly tied to affect regulation.62,63 Results from the current investigation, showing no difference between bupropion and TN for men or women, are consistent with such a mechanism.
Among men, based on placebo comparisons, varenicline appeared to be most efficacious, with a risk ratio of 1.96, compared to 1.69 for TN and 1.76 for bupropion. However, this difference in risk ratios may be misleading, given the exponential scale of the measure. Head-to-head comparisons demonstrated the advantages of varenicline over TN and bupropion were small (16% and 11%, respectively) and did not reach statistical significance in our analyses. Consequently the clinical implications of these meta-analytic findings for men are less clear. With less of a difference between medications compared to women, treatment decisions may be more strongly influenced by other factors such as potential contraindications, patient experience and preference, and medication availability.
Clinicians and smokers may be concerned about reports of neuropsychiatric adverse events associated with the use of varenicline. The medication carries a black box warning label from the FDA stemming from early post-marketing reports of adverse psychiatric events (eg, suicide, aggression) among users.64 Although investigation of adverse effects associated with varenicline use for smoking cessation is ongoing, a number of large scale studies have not substantiated the presence of increased risk for neuropsychiatric adverse events.65–79 For example, recently reported results from a study of smokers with (n = 4074) and without (n = 3984) psychiatric disorders demonstrated no evidence of neuropsychiatric adverse events included suicidal ideation and behaviors.80 Although clinicians should be aware of the potential for important rare affect-related risks associated with varenicline use, greater transparency of scientific information will aid both clinicians and consumers when making treatment decisions.75
There are limitations of this investigation to consider. Four of 12 eligible bupropion trials were not included due to an inability to acquire sex-specific results, highlighting the importance of including sex-specific results in original publications. However, we provided evidence that our sample of bupropion studies was representative of all that met inclusion criteria. Further, our sex-combined results were highly consistent with those from a previous network meta-analysis of smoking cessation medications by the Cochrane Tobacco Group.5 For example, relative to placebo, this previous meta-analysis found ORs of 1.80 for NRT, 1.82 for bupropion, and 2.88 for varenicline, compared to corresponding figures of 1.81, 1.80, and 2.64, respectively, from the current investigation.
We did not include forms of NRT other than TN in our review. This decision was based on the availability of previously published meta-analytic data on sex-specific abstinence rates for TN trials dating back to the 1990s which were unavailable for the other formulations of NRT. The Cochrane Tobacco Group previously found little difference in efficacy between various formulations of NRT,5 bolstering confidence that TN is an adequate marker for other NRT. TN is also the most commonly used form of NRT.4 While combined NRT81 is becoming the standard of care option for NRT, we made an a priori decision to not include combination NRT. This decision was the result of an insufficient number of eligible trials for adequately powered sex-specific analyses. A PubMed review identified only one trial meeting all eligibility criteria.29 Sex-specific analyses of combination NRT will become more feasible and valid as more trials are conducted.
Our study was a network analysis by design and therefore all comparisons between medications were indirect. We were unable to conduct direct comparison meta-analyses due to the small number of original multi-arm trials with sex-specific abstinence rates meeting inclusion criteria (n = 4). To account for potential inconsistencies between study samples, we employed previously validated analytic techniques. However, the results should be treated as retrospective and observational,13 and consequently we cannot rule out the possibility of residual confounding bias impacting the study results.
The sample was restricted to trials involving primary care and general community participants, limiting the ability to generalize findings to specific groups of cigarette smokers, such as those with specific mental or physical health concerns. None of the studies in the meta-analysis accounted for or examined differences by menstrual cycle, hormone levels, or pre versus post-menopausal status among women, which may affect treatment outcomes.82
Conclusion
This investigation highlights the broad importance of the inclusion and reporting of sex-specific results in clinical trials, even when statistically significant differences are not found. Such practices will aid in future attempts to synthesize evidence of sex differences across investigations and improve treatment for both men and women. This study’s results provide novel guidance for the sex-specific treatment of smoking. While women and men achieved similar cessation outcomes relative to placebo when treated with varenicline, varenicline significantly outperformed bupropion and TN among women but not men. These findings identify varenicline as a particularly potent first option treatment for women. Among men, the advantage of varenicline over bupropion or TN is less clear.
Funding
Funding was provided by the Grace J. Fippinger Foundation (PIs: CMM and PHS), grant Number P50 DA033945 from NIDA, and the Office of Research on Women’s Health (ORWH), Office of the Director, NIH (PI: SAM), and by the Yale BIRCWH Scholar Program on Women’s Health and Addictive Behaviors (ORWH, NIDA, NIAAA; K12 DA031050; PI: CMM).
Declaration of Interests
PHS and SAM have investigator-initiated research funding from Pfizer, Inc. The current investigation was not funded by Pfizer, nor did Pfizer have a role in conducting or reporting of the current investigation. The other authors have no conflicts to disclose.
Supplementary Material
Acknowledgments
We are grateful to the following for their effort in providing data for the investigation: Dr Kenneth Ward, Dr Caryn Lerman, Dr Paul Wileyto, Dr Megan Piper, Dr Paul Cinciripini, Dr Jason Robinson, Dr Danielle McCarthy, Dr Sharon Hall, Dr Lisa Cox, and Pfizer, Inc.
References
- 1. Jamal A, Homa D, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR. 2015;64(44):1233–1259. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/ Accessed March 17, 2016. [Google Scholar]
- 3. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality—beyond established causes. N Engl J Med. 2015;372(7):631–640. doi:10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 4. Kasza KA, Hyland AJ, Borland R, et al. Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2013;108(1):193–202. doi:10.1111/j.1360-0443.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis (Review). Cochrane Db Syst Rev. 2013;5:1–52. doi:10.1002/14651858.CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borland R, Partos TR, Yong H-H, Cummings KM, Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction. 2012;107(3):673–682 doi:10.1111/j.1360-0443.2011.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–1250. doi:10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 8. McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH. Sex differences in varenicline efficacy for smoking cessation: a meta-analysis [published online ahead of print October 6, 2015]. Nicotine Tob Res. doi:10.1093/ntr/ntv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–1469. doi:10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 10. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):1–7. doi:10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3): 299–303. doi:10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 12. Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res. 2010;12(7):756–762. doi:10.1093/ntr/ntq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salanti G, Higgins JPT, Ades AE, Ioannidis JPA. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. doi:10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- 14. Wolfgang V. Conducting meta-analyses in R with the Metafor package. J Stat Soft. 2010;36(3):1–48. http://brieger.esalq.usp.br/CRAN/web/packages/metafor/vignettes/metafor.pdf Accessed March 17, 2016. [Google Scholar]
- 15. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 16. Abelin T, Müller P, Buehler A, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet. 1989;333(8628):7–10. doi:10.1016/S0140-6736(89)91671-1. [DOI] [PubMed] [Google Scholar]
- 17. Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self-help video for cigarette smoking cessation. J Consult Clin Psychol. 1997;65(4):663–672. doi:10.1037/0022-006x.65.4.663. [DOI] [PubMed] [Google Scholar]
- 18. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Resp J. 1996;9(4):643–651. http://erj.ersjournals.com/erj/9/4/643.full.pdf Accessed March 17, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Sachs DL, Säwe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Arch Intern Med. 1993;153(16):1881–1890. doi:10.1001/archinte.1993.00410160041003. [PubMed] [Google Scholar]
- 20. Tonnesen P, Norregaard J, Simonsen K, Säwe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med. 1991;325(5):311–315. doi:10.1056/NEJM199108013250503. [DOI] [PubMed] [Google Scholar]
- 21. Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. Brit J Gen Pract. 1996;46(404):145–148. http://bjgp.org/bjgp/46/404/145.full.pdf Accessed March 17, 2016. [PMC free article] [PubMed] [Google Scholar]
- 22. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340(9):685–691. doi:10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 23. Shiffman S, Sweeney CT, Dresler CM. Nicotine patch and lozenge are effective for women. Nicotine Tob Res. 2005;7(1):119–127. doi:10.1080/14622200412331328439. [DOI] [PubMed] [Google Scholar]
- 24. Ehrsam RE, Buhler A, Muller P, et al. Weaning of young smokers using a transdermal nicotine patch. Swiss Medical Forum. 1991;80(7):145–150. http://europepmc.org/abstract/med/2008547 Accessed March 17, 2016. [PubMed] [Google Scholar]
- 25. Hughes JR, Lesmes GR, Hatsukami DK, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tob Res. 1999;1(2):169–174. doi:10.1080/14622299050011281. [DOI] [PubMed] [Google Scholar]
- 26. Richmond RL, Harris K, de Almeida Neto A. The transdermal nicotine patch: results of a randomised placebo-controlled trial. Med J Aust. 1994;161(2):130–135. http://europepmc.org/abstract/med/8028537 Accessed March 17, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–562. doi:10.1037/0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 28. Ward KD, Asfar T, Al Ali R, et al. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction. 2013;108(2):394–403. doi:10.1111/j.1360-0443.2012.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi:10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lerman C, Schnoll RA, Hawk LW, Jr, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. doi:10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niaura R, Hays JT, Jorenby DE, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin. 2008;24(7):1931–1941. doi:10.1185/03007990802177523. [DOI] [PubMed] [Google Scholar]
- 32. Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 33. Jorenby DE, Hays J, Rigotti NA, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29(6):1040–1056. doi:10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 35. Bolliger CT, Issa JS, Posadas-Valay R, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2011;33(4):465–477. doi:10.1016/j.clinthera.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 36. Rennard S, Hughes J, Cinciripini PM, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012;14(3):343–350. doi:10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. doi:10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 38. Cinciripini PM, Robinson JD, Karam-Hage M, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70(5):522–533. doi:10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi:10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- 40. Collins BN, Wileyto EP, Patterson F, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine Tob Res. 2004;6(1):27–37. doi:10.1080/14622200310001656830. [DOI] [PubMed] [Google Scholar]
- 41. Dale LC, Glover ED, Sachs DPL, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest. 2001;119(5):1357. doi:10.1378/chest.119.5.1357. [DOI] [PubMed] [Google Scholar]
- 42. Smith SS, Jorenby DE, Leischow SJ, et al. Targeting smokers at increased risk for relapse: treating women and those with a history of depression. Nicotine Tob Res. 2003;5(1):99–109. doi:10.1080/1462220021000060437. [DOI] [PubMed] [Google Scholar]
- 43. Cox LS, Nollen NL, Mayo MS, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. 2012;104(4):290–298. doi:10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction. 2004;99(9):1206–1218. doi:10.1111/j.1360-0443.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 45. Lerman C, Shields PG, Wileyto EP, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22(5):541–548. doi:10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- 46. McCarthy DE, Piasecki TM, Lawrence DL, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob. Res. 2008;10(4):717–729. doi:10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 47. Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation (Review). Cochrane Db Syst Rev. 2008;3: 1–160. doi:doi/10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 48. Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther. 2005;27(11):1685–1695. doi:10.1016/j.clinthera.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 49. Lamba V, Lamba J, Yasuda K, et al. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther. 2003;307(3):906–922. doi:10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- 50. Ilic K, Hawke RL, Thirumaran RK, et al. The influence of sex, ethnicity, and CYP2B6 genotype on bupropion metabolism as an Index of hepatic CYP2B6 activity in humans. Drug Metab Dispos. 2013;41(3):575–581. doi:10.1124/dmd.112.048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chenoweth MJ, Novalen M, Hawk LW, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomark Prev. 2014;23(9): 1773–1782. doi:10.1158/1055–9965.epi-14–0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80(4):319–330. doi:10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 53. Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. doi:10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 54. Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49(12): 799–816. doi:10.2165/11537850. [DOI] [PubMed] [Google Scholar]
- 55. Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. doi:10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Franklin T, Wang Z, Suh JJ, et al. Effects of varenicline on smoking cue–triggered neural and craving responses. Arch Gen Psychiatry. 2011;68(5):516–526. doi:10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cosgrove KP, Esterlis I, McKee SA, et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69(4):418–427. doi:10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- 59. Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13(1):41–46. doi:10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67(2):219–223. doi:10.1016/S0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 61. Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10(3):219–231. doi:10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- 62. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi:10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 63. Verplaetse TL, Weinberger AH, Smith PH, et al. Targeting the Noradrenergic System for Gender-Sensitive Medication Development for Tobacco Dependence. Nicotine Tob Res. 2015;17(4):486–495. doi:10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. Plos One. 2011;6(11):e27016–e27016. doi:10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kotz D, Viechtbauer W, Simpson C, van Schayck OCP, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med. 2015;3(10):761–768. doi:10.1016/S2213-2600(15)00320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Evins A, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145–154. doi:10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thomas KH, Martin RM, Davies NM, Metcalfe C, Windmeijer F, Gunnell D. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347 doi:10.1136/bmj.f5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cinciripini PM, Robinson JD, Karam-Hage M, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70(5):522–533. doi:10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Anthenelli RM, Morris C, Ramey TS, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med. 2013;159(6):390–400. doi:10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- 70. Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry. 2013;170(12):1460–1467. doi:10.1176/appi.ajp.2013.12121599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pasternak B, Svanström H, Hviid A. Use of varenicline versus bupropion and risk of psychiatric adverse events. Addiction. 2013;108(7):1336–1343; 1338p. doi:10.1111/add.12165. [DOI] [PubMed] [Google Scholar]
- 72. Meyer TE, Taylor LG, Xie S, et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction. 2013;108(1):203–210; 208p. doi:10.1111/j.1360-0443.2012.04024.x. [DOI] [PubMed] [Google Scholar]
- 73. Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline in smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(5):654–660. doi:10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 74. Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33(4):289–301. doi:10.2165/11319180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 75. Hughes JR. Varenicline as a cause of suicidal outcomes. Nicotine Tob Res. 2015;18(1):2–9. doi:10.1093/ntr/ntu275. [DOI] [PubMed] [Google Scholar]
- 76. Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ. 2009;339:b3805. doi:10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre–post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146–154. doi:10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 78. FDA. FDA Drug Safety Communication: Safety review update of Chantix (varenicline) and risk of neuropsychiatric events 2015. www.fda.gov/Drugs/DrugSafety/ucm276737.htm Accessed January 16, 2016.
- 79. Garza D, Murphy M, Tseng L-J, Riordan HJ, Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol Psychiatry. 2011;69(11):1075–1082. doi:10.1016/j.biopsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 80. Anthenelli RM, Prochaska JJ, Benowitz NL, West R. Evaluating adverse events in a global smoking cessation study (EAGLES): A randomized, controlled trial comparing the safety and efficacy of the first-line smoking cessation aids in smokers with and without psychiatric disorders. Paper presented at: Annual meeting of the Society for Research on Nicotine and Tobacco; March 2–5, 2016; Chicago, IL http://c.ymcdn.com/sites/www.srnt.org/resource/resmgr/Conferences/2016_Annual_Meeting/Program/FINAL_SRNT_Abstract_WEB02171.pdf Accessed March 17, 2016. [Google Scholar]
- 81. Fiore M, Jaen CR, Baker T, et al. Treating tobacco use and dependence: 2008 update: U.S. Department of Health and Human Services, Public Health Service. 2008. [Google Scholar]
- 82. Weinberger AH, Smith PH, Allen SS, et al. Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17(4):407–421. doi:10.1093/ntr/ntu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.