ABSTRACT
Respiratory syncytial virus (RSV) infection is a leading cause of hospitalization and mortality in young children. Protective therapy options are limited. Currently, palivizumab, a monoclonal IgG1 antibody, is the only licensed drug for RSV prophylaxis, although other IgG antibody candidates are being evaluated. However, at the respiratory mucosa, IgA antibodies are most abundant and act as the first line of defense against invading pathogens. Therefore, it would be logical to explore the potential of recombinant human IgA antibodies to protect against viral respiratory infection, but very little research on the topic has been published. Moreover, it is unknown whether human antibodies of the IgA isotype are better suited than those of the IgG isotype as antiviral drugs to combat respiratory infections. To address this, we generated various human IgA antibody formats of palivizumab and motavizumab, two well-characterized human IgG1 anti-RSV antibodies. We evaluated their efficacy to prevent RSV infection in vitro and in vivo and found similar, but somewhat decreased efficacy for different IgA subclasses and formats. Thus, reformatting palivizumab or motavizumab into IgA reduces the antiviral potency of either antibody. Moreover, our results indicate that the efficacy of intranasal IgA prophylaxis against RSV infection in human FcαRI transgenic mice is independent of Fc receptor expression.
KEYWORDS: antibody glycosylation, dIgA, Fc receptor, fusion protein, IgG, mIgA, motavizumab, neutralizing antibodies, palivizumab, RSV, sIgA
Introduction
Immunoglobulins of the IgA isotype play an important role in defense against invading pathogens at mucosal surfaces.1 In humans there are two IgA subclasses, IgA1 and IgA2, which mainly differ in the length of their hinge regions and the abundance of post-translational modifications such as glycosylation.2 IgA is produced in different molecular forms: as a monomeric molecule (mIgA) by B cells in the blood and bone marrow, and as a dimeric molecule (dIgA) by B cells in the lamina propria. dIgA is transported by the polymeric immunoglobulin receptor (pIgR) from the basolateral side of the mucosal epithelium to the luminal side, where it is released together with the secretory component (SC, remnant of the pIgR) as secretory IgA (sIgA).3 dIgA and sIgA are mostly found at the mucosa, where they can bind and neutralize pathogens intra- or extracellularly.4 In contrast, mIgA is mainly present in serum and can efficiently engage FcαRI-expressing myeloid cells to induce cellular effector functions.5 Therefore, IgA monoclonal antibodies (mAbs) are investigated as therapeutics to target tumor cells via neutrophils and macrophages in a process referred to as antibody-dependent cell-mediated cytotoxicity (ADCC).6 ADCC may also be an important mechanism in the elimination of virus-infected cells, as shown by Ravetch and colleagues using IgG antibodies against influenza virus.7
Respiratory syncytial virus (RSV) is a common pathogen that can cause severe acute lower respiratory tract infection with potentially fatal outcome in high-risk individuals, e.g., preterm infants, infants with congenital heart disease, immunocompromised patients and the elderly. Currently, the high-risk infants are prophylactically treated in the clinic with the monoclonal IgG1 antibody palivizumab (Synagis®), which targets the RSV fusion (F) protein. The efficacy of this antibody has been shown in two large clinical trials resulting in 55% and 45% reduction in RSV-associated hospitalization.8,9 To date, palivizumab is the only US Food and Drug Administration (FDA)-approved mAb directed against an infectious agent, and it is administered via intramuscular injections solely to infants at high-risk for severe RSV infection. Since the drug is only partly effective, substantial efforts have been undertaken to develop new anti-RSV antibodies with improved affinity and better neutralizing capacity. Motavizumab, the affinity-matured variant of palivizumab, reduced RSV titers in the lungs of RSV-infected cotton rats by 100-fold compared to palivizumab,10 but did not receive FDA approval due to higher number of allergic skin reactions in motavizumab- compared to palivizumab-treated patients.11
The presence of RSV neutralizing IgG antibodies in serum has been correlated with protection against RSV infection.12 Recently, mucosal IgA RSV neutralizing antibodies (nAbs) were shown to better correlate with RSV protection than serum IgG RSV nAbs.13 Experimental infection of adults with RSV induces memory IgG B cells, but does not induce memory IgA B cells.13 The lack of IgA memory B cell formation may explain why individuals can become repeatedly infected with the same RSV strain.13,14 RSV vaccines that manage to induce high levels of mucosal IgA instead of IgG only may, therefore, provide better protection against infection. Whether localization of the antibody (i.e., serum vs. mucosa) or, in addition, the antibody target and isotype (e.g., IgG vs. IgA) affect the effectiveness of antiviral antibodies with the same antigen specificity remains incompletely understood. In vitro comparison of an anti-hemagglutinin (HA) monoclonal IgA and IgG derived from the same parental hybridoma (thus assumed to have the same epitope specificity), suggested that IgA has increased potential to prevent influenza virus infection compared to IgG.15 However, intranasal (i.n.) delivery of a mouse monoclonal IgA (HNK20) against the RSV F protein was less effective against RSV infection in mice than a different mouse IgG antibody (133-1H) that binds to the same antigenic site.16 Recently, we showed that i.n. administration of antibodies derived from human amniotic fluid protected mice against RSV infection and that protection by IgG lasted at least one week.17
While the potential of related mouse IgA and IgG antibodies against RSV infection has been previously investigated, no study has ever compared the efficacy between (human) IgA and IgG anti-RSV antibodies with identical Fab sequence. Also, the difference in antiviral activity between human IgA subclasses (i.e., IgA1 vs IgA2) and formats (i.e., monomeric, dimeric and secretory IgA) of the same antigen specificity is unknown. Since IgA is the predominant antibody isotype at mucosal surfaces and the first line of defense against viral respiratory infection, we aimed to compare the anti-RSV efficacy of all the different human IgA formats with IgG. We generated recombinant human IgA and IgG antibodies with identical Fab sequence as palivizumab and motavizumab and compared their efficacy in vitro and in vivo against RSV infection.
Results
Recombinant antibody production and characterization
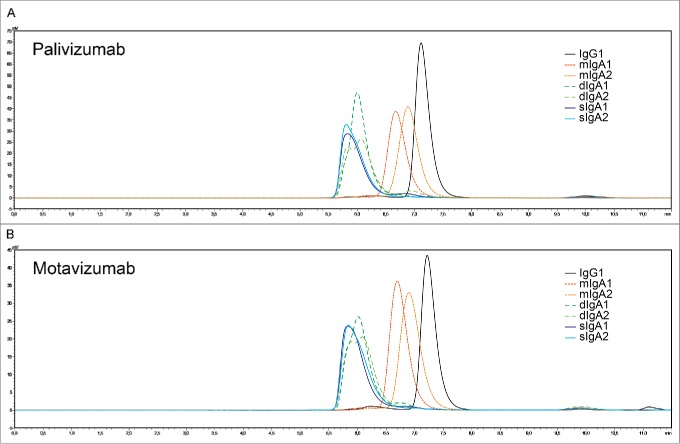
The palivizumab and motavizumab antibody panels consisted of monomeric IgG1, and IgA1 and IgA2 antibodies of the mIgA, dIgA and sIgA format. The production and purification of recombinant human mIgA and dIgA have been previously described.18,19 We have set up a new production method for human sIgA, adapted from Moldt and colleagues.20 This new method yielded almost 100% sIgA formation, compared to 30% sIgA formation by Moldt and colleagues. All antibodies were analyzed by high-performance size-exclusion chromatography (HPSEC) to confirm their purity and monomeric, dimeric or secretory nature (Fig. 1). All antibody formats, except dIgA2, showed a main single peak. The dIgA2 format of both palivizumab and motavizumab showed a double peak, which is known to be characteristic for human dIgA2.21 The purity of all tested antibodies was above 95%.
Figure 1.
(A) Palivizumab and (B) motavizumab recombinant antibodies were analyzed by HPSEC. All antibody formats, except dIgA2, showed a main single peak. dIgA2 showed a characteristic double peak. The purity of all tested antibodies was >95%.
In addition, antibody glycan profiles were analyzed to investigate differences in glycosylation between the recombinant IgG and the various IgA subclasses and formats (Supplementary Fig. 1 and 2, and supplementary methods). Differences in IgG glycosylation are known to influence FcγR-dependent activities,22 such as ADCC, but little is known about the effect of IgA glycosylation. In general, the antibody N-glycosylation characteristics were highly similar between palivizumab and motavizumab, but several differences could be found between the IgG and IgA variants. The commercially available palivizumab and the recombinant IgGs mainly showed three fucosylated diantennary glycans, namely with zero, one or two galactoses. IgA1 and IgA2 in addition displayed various degrees of sialylation, high mannose glycans, and low levels of tri- and tetraantennary species. Sialylation proved to be present in both α2,3- and α2,6-linkage (for an overview of the major N-glycosylation characteristics, see Supplementary Fig. 1 and 2). To characterize the binding of the recombinant antibodies, their ability to bind to RSV-infected HEp-2 cells was determined by flow cytometry. For each antibody panel, the mIgA, dIgA and sIgA antibodies within the IgA1 or IgA2 subclass bound very similar to the RSV-infected cells, although for some of the concentrations in the dilution series a statistically significant difference in antibody binding was observed (Fig. 2). Overall, these results indicate that dimerization and secretory antibody formation did not influence their target binding capacity.
Figure 2.
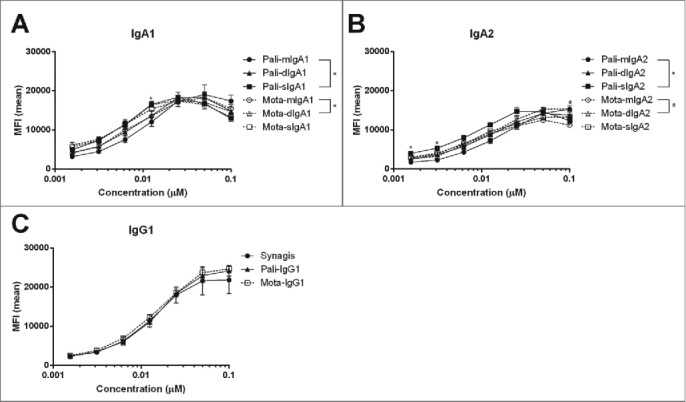
Binding of RSV-specific human IgA and IgG to RSV-infected HEp-2 cells. RSV-infected HEp-2 cells were stained with a dilution series of IgG and IgA variants of palivizumab (pali) and motavizumab (mota) and then analyzed by flow cytometry. (A) IgA1, (B) IgA2, and (C) IgG1 recombinant antibodies. Synagis: commercially available palivizumab. Data represent the mean ± SEM of duplicate measurements. Kruskal-Wallis test, #/*P < 0.05.
RSV neutralization
Next, we compared the RSV neutralization capacity of the palivizumab and motavizumab antibodies by equimolar concentrations of IgA and IgG among the different antibody subclasses. For IgA1 and IgA2, the monomeric, dimeric and secretory forms of each mAb showed a comparable capacity to neutralize RSV (Fig. 3A and B), except for palivizumab IgA2, where sIgA2 was less effective than mIgA2 and dIgA2. All motavizumab antibodies were more effective than their palivizumab counterparts. Moreover, antibodies of the IgG1 subclass (Fig. 3C) neutralized RSV slightly better than those of the IgA1 or IgA2 subclasses, although only the differences between palivizumab IgG1 and sIgA2 were statistically significant (Table 1).
Figure 3.
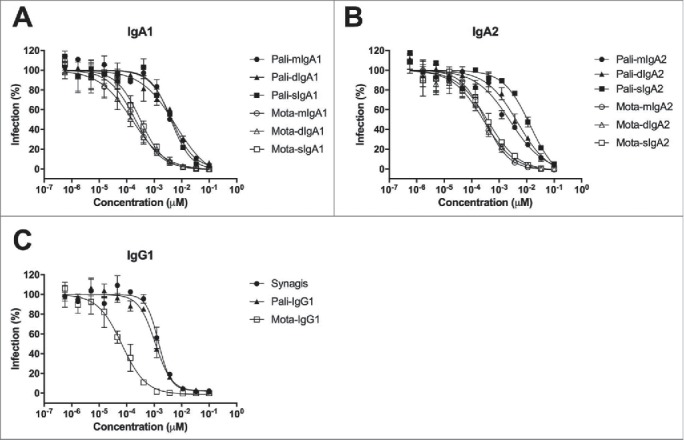
RSV neutralizing activity of human IgG and IgA variants of palivizumab and motavizumab. RSV-GFP was preincubated with a dilution series of recombinant IgG and IgA variants of palivizumab (pali) and motavizumab (mota) before infection of HEp-2 cells. GFP fluorescence of the infected cells was analyzed by flow cytometry. Graphs show the neutralizing activity of (A) IgA1, (B) IgA2, and (C) IgG1 recombinant antibodies. Synagis: commercially available palivizumab. Data represent the mean ± SEM of triplicate measurements.
Table 1.
In vitro RSV neutralizing activity of human IgG and IgA.
| Palivizumab EC50 (nM) | Motavizumab EC50 (nM) | |
|---|---|---|
| Synagis | 1.41400 | |
| IgG1 | 0.95850 | 0.07823 |
| mIgA1 | 3.77700 | 0.19860 |
| mIgA2 | 2.97200 | 0.29760 |
| dIgA1 | 5.53250 | 0.16985 |
| dIgA2 | 4.35550 | 0.50825 |
| sIgA1 | 3.30550 | 0.40325 |
| sIgA2 | 14.23000* | 0.56330 |
Synagis: commercially available palivizumab. Kruskal-Wallis test, *P < 0.05 compared to IgG1.
ADCC of RSV-infected HEp-2 cells
In pediatric patients with RSV-induced bronchiolitis, neutrophils constitute approximately 80% of the cellular infiltrate in the airways.23 To determine if Fc receptors (FcRs, i.e., FcγRs for IgG and FcαRI for IgA) on human neutrophils can play a role in the clearance of RSV-infected cells by the recombinant palivizumab and motavizumab antibodies, we performed in vitro ADCC assays using isolated human blood neutrophils as effector cells and RSV-infected HEp-2 cells as target cells. Lysis of the infected cells was observed with all antibodies in the presence of neutrophils (Fig. 4). The neutrophils showed a trend towards more lysis with monomeric IgA1 and IgA2 than with IgG, which had comparable efficacy to dimeric and secretory IgA. However, these differences did not reach statistical significance. The same results were obtained for both the palivizumab (Fig. 4A) and motavizumab (Fig. 4B) antibody panels.
Figure 4.
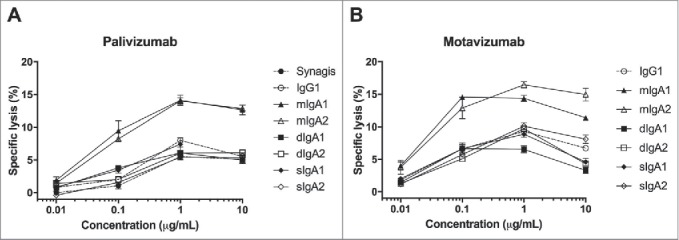
Neutrophils mediate ADCC of RSV-infected HEp-2 cells with RSV-specific IgA. Purified human blood neutrophils were incubated with 51Cr-labeled RSV-infected HEp-2 cells in the presence of IgA (solid lines) and IgG (dashed lines) variants of (A) palivizumab and (B) motavizumab. Radioactivity in the cell supernatant was measured after 4 hours to determine the percentage of cell lysis. Synagis: commercially available palivizumab. Data represent the mean ± SEM of triplicate measurements from one representative donor out of three. Kruskal-Wallis test, results were not significant.
Anti-RSV IgA prophylaxis in mice
Finally, we investigated the prophylactic efficacy of the recombinant palivizumab and motavizumab antibodies in BALB/c mice. The mice were treated i.n. with 0.05 mg/kg antibody or phosphate-buffered saline (PBS) one day before RSV infection, and five days later bronchoalveolar lavage (BAL) was performed to determine the virus load. Recombinant palivizumab IgG and IgA prophylaxis in wild-type mice significantly reduced the RSV load compared to PBS treatment (Fig. 5A). Similar to the in vitro RSV neutralization results, prophylaxis with IgG was more effective than with IgA. Palivizumab IgG1-treated mice demonstrated a statistically significant decrease in RSV load in the BAL fluid compared to palivizumab mIgA1-, mIgA2- and sIgA2-treated mice, but was equally effective as dIgA1, dIgA2 and sIgA1 treatment.
Figure 5.
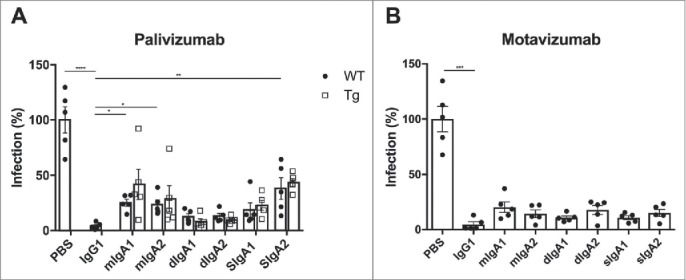
In vivo protection against RSV infection by intranasally administered RSV-specific IgA and IgG. RSV load was determined in bronchoalveolar fluid from wild-type (WT; closed circles) and human FcαRI transgenic (Tg; open squares) BALB/c mice prophylactically treated with recombinant IgA and IgG variants of (A) palivizumab and (B) motavizumab. Graphs show the percentage of RSV infection; the mean RSV load of the PBS-treated group was set to 100% infection. N=5 mice per group. Data represent the mean ± SEM. Kruskal-Wallis test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Since FcRs may also be involved in antibody-mediated elimination of virus-infected cells in vivo, as previously shown by Ravetch and colleagues for FcγRs and IgG targeting influenza HA,7 we aimed to investigate the involvement of FcαRI in IgA in vivo protection against RSV infection. However, as mice lack a homologue of human FcαRI,24 we used human FcαRI transgenic mice, which express this receptor on their myeloid cells.25 Palivizumab IgA prophylaxis in FcαRI transgenic mice did not result in better protection against RSV infection compared to wild-type mice (Fig. 5A). Consequently, the recombinant motavizumab antibodies were tested only in wild-type mice. As observed with palivizumab, motavizumab IgG seemed to be slightly more effective than the motavizumab IgA antibodies, but none of the differences in RSV load between motavizumab IgG and IgA treatment were statistically significant (Fig. 5B).
Discussion
Immunoprophylaxis for RSV infection has been proven effective by the successful clinical use of the monoclonal IgG1 antibody palivizumab, although not all infants benefit from this treatment. While several other IgG antibody candidates are being evaluated, one would think that the natural isotype for mucosal surfaces, i.e., IgA, is a far better candidate for (prophylactic) treatment of lung viruses. Here, we investigated the prophylactic efficacy of recombinant human IgA formats of palivizumab and motavizumab, both well-characterized human IgG1 antibodies against RSV, and compared their anti-viral efficacy in vitro and in vivo by i.n. administration in BALB/c mice. We found that most of these human IgA formats had similar, but slightly reduced protective capacity compared to IgG against RSV infection in mice and that the in vitro RSV neutralizing capacity of these antibodies correlated with their prophylactic efficacy upon i.n. administration.
Strikingly, mucosal IgA RSV nAbs were recently shown to better correlate with RSV protection than serum IgG RSV nAbs.13 Also, most vaccines are designed to induce high levels of mucosal IgA for better protection against respiratory infections. However, the effectiveness of antibodies in preventing respiratory infection seems to rely mostly on their abundance and localization, with only minor influence of the isotype. Mucosal antibodies, whether IgA or IgG, are closer to the site of infection than serum antibodies, and this may therefore better reflect the protective capacity against respiratory infections. Moreover, the longer half-life of IgG compared to IgA antibodies in serum benefits the effectiveness of IgG therapeutics when administered intramuscularly or intravenously. The serum half-life of human IgA1 and IgA2 antibodies is lower than human IgG in mice (28.6–32.2 hours for IgA1 and 16.5–20.4 hours for IgA2 vs 9 days for IgG).18,26 This half-life has been extended by glycoengineering26,27 and integration of an albumin binding domain (ABD) to enable recycling via the neonatal Fc receptor (FcRn),18 but, despite these modifications, the IgA half-life remained lower than the half-live of IgG. Our data suggest that the half-life of IgG and IgA at the mucosa may be very similar when administered i.n., since both antibody formats demonstrated comparable protective efficacy against RSV infection in vivo. Several differences in antibody N-glycosylation were found between the IgG and IgA variants of both palivizumab and motavizumab, but these differences did not appear to affect RSV neutralization, as this was similar between the various antibody formats.
The production and purification of IgA antibodies has been a challenge for many years. Unlike IgG antibodies, which were easily purified using protein A and G that bind to the Fc region of IgG (but not IgA), no good method existed for the purification of IgA. Jacalin, a lectin that binds to O-linked glycoproteins, has been previously used to purify IgA1 from mixtures containing IgA2 and IgG. More recently, the purification of recombinant antibodies containing human IgA constant region by kappa affinity purification has been described,28 which overcomes this problem. While antibody yields in a single mammalian/eukaryote cell-line are usually high for IgG and mIgA, the yields of sIgA, and to a lesser extent dIgA, are extremely low.29,30 Alternatively, recombinant sIgA production in plants is very promising, although the antibody glycosylation patterns differ from those in mammalian cells.31 Recently, Moldt and colleagues have described a method that improves sIgA production and purification by allowing free SC to bind to dIgA bound on a Protein L column.20 However still, their average sIgA yield was 30%. We have adapted this method by allowing free SC to bind to purified dIgA in solution. This yielded almost 100% sIgA formation. Therefore, our method to produce sIgA apparently is among the most efficient methods described to date.
Another factor hampering the detailed study of human IgA antibodies in mice is the fact that mice do not express a homologue of human FcαRI.24 We previously generated human FcαRI transgenic mice and showed that IgA anti-tumor therapy is enhanced in these transgenic mice compared to wild-type mice.25 Recently, the requirement of FcγRs for the therapeutic activity of broadly neutralizing anti-influenza IgG antibodies was shown.7 However, in the present study, the presence of human FcαRI on mouse myeloid cells in the transgenic mice did not improve the prophylactic efficacy of recombinant human IgA anti-RSV. A possible explanation for this may be the therapeutic use of the anti-influenza antibodies in the previous study compared to the prophylactic use of the anti-RSV antibodies in the present study. Therefore, our prophylactic in vivo setting may have favored immune exclusion (i.e., RSV neutralization at the extracellular lumen) above FcR-dependent cellular effector functions (usually directed at infected target cells). Moreover, unlike human IgA, human IgG can induce cellular effector functions in wild-type mice by binding to mouse FcγRs.32 These mouse FcγRs have similar affinity as human FcγRs for human IgG.33,34 Therefore, we cannot exclude FcγR enhancement of the anti-viral activity of the tested human IgG anti-RSV antibodies in wild-type mice. To determine this, Fcerg1−/− mice, which lack activating FcγRs, would be required. However, since FcαRI in the transgenic mice did not enhance the anti-RSV activity of the IgA antibodies, we speculate that endogenous FcγRs in the wild-type mice also did not contribute to the anti-RSV activity of the IgG antibodies. Accordingly, the prophylactic efficacy of the RSV nAbs in vivo was found to correlate with their RSV neutralizing capacity in vitro.
The role of FcRs in mediating anti-viral antibody therapy is likely to depend on the epitope or target. Ravetch and colleagues have shown that anti-influenza broadly neutralizing IgG antibodies directed against the HA stalk region require FcγR binding for their in vivo activity, whereas IgG antibodies directed against the HA head domain do not depend on FcγR interaction for their in vivo activity.7 Whether RSV nAbs that bind to epitopes different than the palivizumab and motavizumab epitope (e.g., antigenic site zero) require FcR interaction for their in vivo activity remains to be investigated.
Strikingly, in vitro lysis of RSV-infected HEp-2 cells was observed with palivizumab and motavizumab IgA in the presence of human blood neutrophils, suggesting a role for FcαRI on human neutrophils. Nevertheless, this effect was not observed when human monocytes, which also express high levels of FcαRI, were used as effector cells (data not shown). This may be due to different killing mechanisms employed by these cells, i.e., phagocytosis by monocytes and poorly understood mechanisms by neutrophils. However, we and others have previously demonstrated that IgA can induce lysis of tumor cells in vitro with both human macrophages and neutrophils.18,25,35 Moreover, tumor cell lysis by these effector cells was higher with IgA than with IgG. Similarly, a recombinant IgA1 against HIV (F4251g8 IgA1 variant) performed better in antibody-dependent cell-mediated viral inhibition than the IgG variant.36 In the present study, we observed the highest lysis of RSV-infected HEp-2 cells with mIgA and lower lysis with IgG, dIgA and sIgA (independent of the antibody subclass), although the differences were not statistically significant. Based on these results, it is tempting to speculate that, in humans, FcαRI on neutrophils may still enhance IgA-mediated clearance of RSV-infected cells and benefit i.n. IgA treatment (i.e., therapeutic setting) over IgG.
In conclusion, we generated and examined a panel of recombinant human IgA variants of established IgG anti-RSV antibodies and found that reformatting IgG into IgA reduces the antibody protective efficacy against RSV infection in vitro and in mice. We found no role for FcαRI in IgA prophylaxis against RSV infection in human FcαRI transgenic mice, but did observe a trend towards increased lysis of RSV-infected HEp-2 cells by human blood neutrophils in vitro in the presence of IgA. Since lysis with monomeric IgA tended to be better than with IgG, future studies will be needed to determine whether RSV-specific IgA may be more beneficial than IgG in a therapeutic setting compared to a prophylactic setting.
Materials and methods
Cells and viruses
FreeStyle™ 293-F cells (Invitrogen) were maintained in FreeStyle™ 293 Expression Medium (Invitrogen) at 37°C, 8% CO2 in an orbital shaker (125 rpm). HEp-2 cells (ATCC) were maintained in IMDM (Gibco) supplemented with penicillin (100 Units/mL; Life Technologies), streptomycin (100 µg/mL; Life Technologies) and 10% fetal calf serum (FCS) at 37°C, 5% CO2. RSV-A2 and RSV-GFP were propagated in HEp-2 cells (70–80% confluent), purified by polyethylene glycol 6000 precipitation, resuspended in PBS supplemented with 10% sucrose and stored in liquid nitrogen.
Antibody production and purification
Variable heavy and light chain (HC, LC) sequences of the recombinant IgA and IgG antibodies are derived from palivizumab (Medi-493; Accession Number DB00110) and motavizumab (Medi-524)37 and were synthesized by ShineGene. Variable sequences were cloned into Lonza expression vectors: pEE14.4-kappaLC, pEE14.4-IgG1, pEE14.4-IgA1 and pEE14.4-IgA2(m1). mIgA and dIgA were co-produced by transient transfection of HEK 293-F cells with plasmids encoding the HC, LC and His-tagged joining chain (JC; kindly provided by Dr. Gestur Vidarsson) and pAdvantage (Accession Number U47294; Promega) using 293fectin™ Transfection Reagent (Invitrogen) according to the manufacturer's instructions. IgG1 was produced in the same way, except that the JC plasmid was excluded. sIgA was produced by incubating purified dIgA overnight (O/N) with supernatant of HEK 293-F cells transiently transfected with a plasmid encoding the SC (kindly provided by Dr. Jenny Woof, with courtesy of Dr. Charlotte Kaetzel) and pAdvantage. Antibody-SC-containing supernatants were harvested 4 days after transfection and diluted 1:1 in PBS before purification. IgG1 was purified using Hi-trap protein A columns (GE Healthcare), eluted with 0.1 M sodium acetate (Sigma Aldrich) pH 2.5 and directly neutralized with 1 M Tris-HCL (Roche Diagnostics) pH 8.8. Antibody-containing fractions were pooled and dialyzed to PBS. mIgA and dIgA were first co-purified by anti-kappa affinity chromatography (HiTrap KappaSelect, GE-Healthcare) and bound protein was eluted using 0.1 M glycine (VWR International) pH 2.5 and directly neutralized with 1 M Tris-HCl pH 8.8. Antibody-containing fractions were pooled and dialyzed in His-binding buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0). Then, dIgA (containing his-tagged JC) was separated from mIgA by nickel affinity chromatography (HisTrap HP, GE-Healthcare). mIgA remained in the flow-through and bound dIgA was eluted using His-elution buffer (His-binding buffer comprising 250 mM imidazole). Finally, both the mIgA and dIgA fractions were subjected to size-exclusion chromatography (SEC) (HiPrep 26/60 Sephacryl S-300 High Resolution, GE Healthcare) using PBS. sIgA was first purified by anti-kappa chromatography followed by SEC. Purified antibodies were analyzed on a Shimadzu Prominence Modular HPLC by HPSEC (Yarra™ 3u SEC-2000 column; Phenomenex) with 100 mM sodium phosphate, 150 mM NaCl pH 6.8 as mobile phase (flowrate of 0.35 mL/min and detection at 280 nm).
Quantification of antibody concentrations
Antibody concentrations were determined as follows: . Correction factor IgG: 1.36; IgA: 1.4. Alternatively, antibody concentrations were determined by enzyme-linked immunosorbent assay (ELISA). 96-well plates (NUNC Maxisorp) were coated O/N at 4°C with 50 ng goat-anti-hKappa antibody (Southern Biotech) in PBS. In between steps, plates were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBS-T). Plates were blocked for 1 h at 37°C with 1% bovine serum albumin (BSA; Roche) in PBS-T. Two-fold antibody dilutions were added and incubated for 1.5 h at room temperature (RT). Palivizumab (Synagis®; MedImmune) and purified human IgA (Bethyl) were used as calibration standards. Horseradish peroxidase (HRP)-labeled goat-anti-hIgG (0.5 μg/mL, Jackson, 109-035-088) or HRP-labeled goat-anti-hIgA (0.5 μg/mL, Southern Biotech, 2050–05) were used as detection antibody for 1 h at RT. Plates were developed with ABTS substrate (Roche) and the absorbance was measured at 415 nm with a Multiscan RC (Thermolab systems).
Fluorescent-activated cell sorting (FACS)-based antibody binding assay
HEp-2 cells (70-80% confluent in T175 flasks) were infected with 1 × 105 plaque-forming unit (PFU) RSV-A2 and incubated O/N at 37°C, 5% CO2. Cells were harvested by trypsinization, seeded at 1 × 105 cells/well in V-bottom 96-wells plates (Greiner bio-one), and incubated with a dilution series of recombinant palivizumab and motavizumab or PBS for 45 min at RT. After three washes with PBS, Fluorescein isothiocyanate (FITC)-labeled goat-anti-human kappa (0.5 µg/mL, Southern Biotech, 2060–02) was added for 45 min at RT. Finally, cells were washed with PBS, fixed with 1% formaldehyde and resuspended in PBS to analyze antibody binding by flow cytometry (BD Bioscience, Canto II and FACS Diva software).
FACS-based RSV neutralization assay
HEp-2 cells (5 × 104 cells/well) were seeded in flat bottom 96-well plates (Nunc Microwell) and infected the next day with RSV-GFP (1 × 105 PFU) that was pre-incubated for 1 h at 37°C with several dilutions of recombinant palivizumab, recombinant motavizumab or IMDM culture medium. After 20–24 h, cells were trypsinized, fixed with 1% formaldehyde and resuspended in PBS to analyze the GFP expression by flow cytometry (BD Bioscience, Canto II and FACS Diva software). To calculate the neutralization percentages, the mean fluorescence intensity (MFI) values of uninfected control cells and infected control cells were set to 100% and 0% neutralization, respectively.
Human neutrophil ADCC assay
ADCC assays with 51Cr-labeled target cells were performed as previously described.38,39 Briefly, polymorphonuclear neutrophils (PMN) were isolated from healthy individuals (MiniDonorDienst, UMC Utrecht) by Ficoll-Histopaque separation (GE Healthcare; Sigma-Aldrich) and then incubated with 51Cr-labeled RSV-infected HEp-2 cells (effector-to-target ratio = 40:1) and the recombinant antibodies. After 4 h incubation at 37°C, 5% CO2, the supernatant was harvested and radioactivity was measured with a liquid scintillation counter (MicroBeta; Perkin Elmer). Specific lysis was calculated as follows: . RSV-infected HEp-2 cells with PMN in culture medium or 5% Triton X-100 (Roche Diagnostics) were used to determine minimal- and maximum-release, respectively.
RSV challenge studies in mice
FcαRI transgenic BALB/c mice were housed and bred at our local animal facility and were aged 8–20 weeks at start of the experiment.25 Wild-type female littermates of the same age were used or additional wild-type female BALB/c mice aged 6–8 weeks were purchased from Janvier Labs. Anesthetized mice (4% isoflurane) were treated i.n. with 50 µL antibody (0.05 mg/kg) or PBS and were challenged i.n. 1 day later with 3 × 106 PFU RSV-A2 in 50 µL PBS. At day five post infection the mice were euthanized by intraperitoneal injection of sodium pentobarbital (200 µL of 60 mg/mL). Lungs were lavaged with 1 mL PBS and the bronchioalveolar lavage fluid was used to determine the viral load, as described previously.40 The RSV load (depicted as % infection) was calculated as follows: .
Study approval
All animal studies were approved by the local Committee for Animal Experimentation.
Statistics
Statistical analysis and non-linear regression analysis were performed with GraphPad Prism 6 software.
Chemicals, reagents and enzymes
High purity water (MQ) was generated using a Q-Gard 2 system (Millipore, Amsterdam, Netherlands), maintained at ≥ 18 MΩ. Ethanol, trifluoroacetic acid (TFA), sodium dodecyl sulfate (SDS), Na2HPO4 × 2H2O, KH2PO4 and NaCl were purchased from Merck (Darmstadt, Germany). Nonidet P-40 substitute (NP-40), 1-hydroxybenzotriazole (HOBt), super-DHB and 50% NaOH originated from Sigma-Aldrich (Steinheim, Germany), while 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) hydrochloride was bought from Fluorochem (Hadfield, U.K.), peptide-N-glycosidase F (PNGase F) from Roche Diagnostics (Mannheim, Germany), and HPLC SupraGradient acetonitrile (ACN) from Biosolve (Valkenswaard, Netherlands).
Glycan release and ethyl esterification
In triplicate for each sample, protein N-glycosylation was released by PNGase F as previously described.41 Briefly, 20 µL 2% SDS was added to 10 µL sample, followed by 10 min incubation at 60°C. After letting the samples cool to room temperature, 20 µL release mix was added containing 2% NP-40 and 2.5 mU PNGase F in 2.5x PBS (14.25 g/L Na2HPO4 × 2H2O, 1.25 g/L KH2PO4 and 21.25 g/L NaCl), followed by overnight incubation at 37°C.
Next, 5 µL of release mixtures was derivatized by adding 35 µL 0.25 M EDC + 0.25 M HOBt in ethanol and incubating for 1 h at 37°C, resulting in lactones for α2,3-linked sialic acids and ethyl esters for α2,6-linked sialic acids.42 40 µL ACN was subsequently added to prepare for glycan enrichment.
The glycans were recovered from the reaction mixture by hydrophilic interaction liquid chromatography (HILIC) solid phase extraction (SPE), using cotton as stationary phase.43 In short, 200 µL pipette tips were packed with 200 µg cotton, washed three times with 100 µL MQ, and three times with 100 µL 85% ACN. Next, the glycans were loaded by pipetting the samples up and down 30 times, followed by three washes with 100 µL 85% ACN 1% TFA, another three washed with 100 µL 85% ACN, and eluted in 10 µL MQ.
MALDI-TOF-MS measurement
One microliter of the eluted samples was spotted on a MTP AnchorChip 600/384 TF MALDI target (Bruker Daltonics, Bremen, Germany), and mixed on-plate with 1 µL 5 mg/mL super-DHB 1 mM NaOH in 50% ACN. Matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF)-mass spectrometry (MS) was carried out using reflectron positive ion mode of an UltraFlextreme system (Bruker Daltonics) controlled by Flexcontrol 3.4 Build 135 (Bruker Daltonics). The instrument was calibrated before measurement using a peptide calibration standard (Bruker Daltonics). Laser power was set as high as possible to still allowing baseline separation of isotopic peaks. Sample spectra were acquired by summing 10000 laser shots in a random walking pattern, at a frequency of 1000 Hz, and using a window from m/z 1000 to 5000 with suppression up to m/z 950.
Data analysis
FlexAnalysis 3.3 (build 65) was used to process the spectra, encompassing smoothing (Savitzky-Golay, width 0.2, cycles 1), baseline subtraction (Top-hat), peak picking (Snap, S/N ≥ 10), and internal calibration on affirmed glycan compositions. Peak lists were exported with S/N and area, and further analyzed in Excel. Based on the average extracted m/z values, the most likely N-glycan compositions were assigned (H = hexose; N = N-acetylhexosamine; F = deoxyhexose (fucose); L = α2,3-linked (lactonized) N-acetylneuraminic acid; E = α2,6-linked (ethyl esterified) N-acetylneuraminic acid), and these compositions were integrated from all spectra making use of MassyTools version 1.0.0.44 Extracted glycans areas within a spectrum were normalized to the total sum of areas, which was further used for repeatability and derived trait calculation. Figures were assigned with structures created in GlycoWorkbench 2.1 build 146, following CFG annotation.45
Supplementary Material
Abbreviations
- ABD
albumin binding domain
- ADCC
antibody-dependent cell-mediated cytotoxicity
- BAL
bronchoalveolar lavage
- BSA
bovine serum albumin
- dIgA
dimeric IgA
- ELISA
enzyme-linked immunosorbent assay
- F protein
fusion protein
- FACS
fluorescent-activated cell sorting
- FcRn
neonatal Fc receptor
- FcRs
Fc receptors
- FDA
US Food and Drug Administration
- FITC
Fluorescein isothiocyanate
- GFP
green fluorescent protein
- HA
hemagglutinin
- HC
heavy chain
- HPSEC
high-performance size-exclusion chromatography
- HRP
horseradish peroxidase
- i.n.
intranasal
- JC
joining chain
- LC
light chain
- mAbs
monoclonal antibodies
- MFI
mean fluorescence intensity
- mIgA
monomeric IgA
- nAbs
neutralizing antibodies
- O/N
overnight
- PBS-T
PBS Tween
- PBS
phosphate-buffered saline
- PFU
plaque-forming unit
- pIgR
polymeric immunoglobulin receptor
- PMN
polymorphonuclear neutrophils
- RSV
respiratory syncytial virus
- RT
room temperature
- SC
secretory component
- SEC
size-exclusion chromatography
- sIgA
secretory IgA
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Jenny Woof and Dr. Charlotte Kaetzel for providing the SC plasmid and Dr. Gestur Vidarsson for providing the His-tagged JC plasmid.
Funding
S.R.J. is funded by an ERC stimulation grant of the Utrecht University, and M.N. by the Dutch Technology Foundation [STW project 13017]. K.R.R. and M.W. were supported by the European Union (Seventh Framework Program HighGlycan project, grant number 278535).
References
- 1.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590–7. doi: 10.1038/mi.2011.39. PMID:21937984. [DOI] [PubMed] [Google Scholar]
- 2.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcalpha receptor interactions. J Biol Chem. 1998;273:2260–72. doi: 10.1074/jbc.273.4.2260. PMID:9442070. [DOI] [PubMed] [Google Scholar]
- 3.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598–602. doi: 10.1038/mi.2011.37. PMID:21956244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–67. doi: 10.1038/nrmicro2384. PMID:20694027. [DOI] [PubMed] [Google Scholar]
- 5.Brandsma AM, Jacobino SR, Meyer S, ten Broeke T, Leusen JH. Fc receptor inside-out signaling and possible impact on antibody therapy. Immunol Rev. 2015;268:74–87. doi: 10.1111/imr.12332. PMID:26497514. [DOI] [PubMed] [Google Scholar]
- 6.Leusen JH. IgA as therapeutic antibody. Mol Immunol. 2015;68:35–9. doi: 10.1016/j.molimm.2015.09.005. PMID:26597204. [DOI] [PubMed] [Google Scholar]
- 7.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–51. doi: 10.1038/nm.3443. PMID:24412922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, Connor EM, Sondheimer HM. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40. doi: 10.1067/S0022-3476(03)00454-2. PMID:14571236. [DOI] [PubMed] [Google Scholar]
- 9.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- 10.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, et al.. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–24. doi: 10.1086/514115. PMID:9359721. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell-Estrany X, Simoes EA, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics. 2010;125:e35–51. doi: 10.1542/peds.2008-1036. PMID:20008423. [DOI] [PubMed] [Google Scholar]
- 12.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: Establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–82. doi: 10.1016/S0264-410X(03)00355-4. PMID:12850364. [DOI] [PubMed] [Google Scholar]
- 13.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CA, Wrammert J, Openshaw PJ, Chiu C, The MOSAIC Investigators. Impaired Antibody-mediated Protection and Defective IgA B Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am J Respir Crit Care Med. 2015;191:1040–9 doi: 10.1164/rccm.201412-2256OC. PMID:25730467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. PMID:3706232.24465606 [DOI] [PubMed] [Google Scholar]
- 15.Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One. 2014;9:e85582. doi: 10.1371/journal.pone.0085582. PMID:24465606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RG, Crowe JE, Jr Johnson TR, Tang YW, Graham BS. Passive IgA monoclonal antibody is no more effective than IgG at protecting mice from mucosal challenge with respiratory syncytial virus. J Infect Dis. 1999;180:1324–7. doi: 10.1086/315037. PMID:10479165. [DOI] [PubMed] [Google Scholar]
- 17.Jacobino SR, Nederend M, Hennus M, Houben ML, Ngwuta JO, Viveen M, Coenjaerts FE, Hack CE, van Neerven RJ, Graham BS, et al.. Human amniotic fluid antibodies protect the neonate against respiratory syncytial virus infection. J Allergy Clin Immunol. 2016;138:1477–1480.e5. doi: 10.1016/j.jaci.2016.06.001. PMID:27448445. [DOI] [PubMed] [Google Scholar]
- 18.Meyer S, Nederend M, Jansen JH, Reiding KR, Jacobino SR, Meeldijk J, Bovenschen N, Wuhrer M, Valerius T, Ubink R, et al.. Improved in vivo anti-tumor effects of IgA-Her2 antibodies through half-life extension and serum exposure enhancement by FcRn targeting. MAbs. 2016;8:87–98. doi: 10.1080/19420862.2015.1106658. PMID:26466856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohse S, Derer S, Beyer T, Klausz K, Peipp M, Leusen JH, van de Winkel JG, Dechant M, Valerius T. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186:3770–8. doi: 10.4049/jimmunol.1003082. PMID:21317397. [DOI] [PubMed] [Google Scholar]
- 20.Moldt B, Saye-Francisco K, Schultz N, Burton DR, Hessell AJ. Simplifying the synthesis of SIgA: combination of dIgA and rhSC using affinity chromatography. Methods. 2014;65:127–32. doi: 10.1016/j.ymeth.2013.06.022. PMID:23811333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohse S, Brunke C, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, van der Winkel JG, Leusen JH, et al.. Characterization of a mutated IgA2 antibody of the m(1) allotype against the epidermal growth factor receptor for the recruitment of monocytes and macrophages. J Biol Chem. 2012;287:25139–50. doi: 10.1074/jbc.M112.353060. PMID:22679018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, Mok JY, et al.. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front Immunol. 2017;8:877. doi: 10.3389/fimmu.2017.00877. PMID:28824618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geerdink RJ, Pillay J, Meyaard L, Bont L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J Allergy Clin Immunol. 2015;136:838–47. doi: 10.1016/j.jaci.2015.06.034. PMID:26277597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abi-Rached L, Dorighi K, Norman PJ, Yawata M, Parham P. Episodes of natural selection shaped the interactions of IgA-Fc with FcalphaRI and bacterial decoy proteins. J Immunol. 2007;178:7943–54. doi: 10.4049/jimmunol.178.12.7943. PMID:17548632. [DOI] [PubMed] [Google Scholar]
- 25.Boross P, Lohse S, Nederend M, Jansen JH, van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et al.. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med. 2013;5:1213–26. doi: 10.1002/emmm.201201929. PMID:23918228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouwendal GJ, van der Lee MM, Meyer S, Reiding KR, Schouten J, de Roo G, Egging DF, Leusen JH, Boross P, Wuhrer M, et al.. A comparison of anti-HER2 IgA and IgG1 in vivo efficacy is facilitated by high N-glycan sialylation of the IgA. MAbs. 2016;8:74–86. doi: 10.1080/19420862.2015.1102812. PMID:26440530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohse S, Meyer S, Meulenbroek LA, Jansen JH, Nederend M, Kretschmer A, Klausz K, Moginger U, Derer S, Rosner T, et al.. An Anti-EGFR IgA That Displays Improved Pharmacokinetics and Myeloid Effector Cell Engagement In Vivo. Cancer Res. 2016;76:403–17. doi: 10.1158/0008-5472.CAN-15-1232. PMID:26634925. [DOI] [PubMed] [Google Scholar]
- 28.Beyer T, Lohse S, Berger S, Peipp M, Valerius T, Dechant M. Serum-free production and purification of chimeric IgA antibodies. J Immunol Methods. 2009;346:26–37. doi: 10.1016/j.jim.2009.05.002. PMID:19427867. [DOI] [PubMed] [Google Scholar]
- 29.Berdoz J, Blanc CT, Reinhardt M, Kraehenbuhl JP, Corthesy B. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc Natl Acad Sci U S A. 1999;96:3029–34. doi: 10.1073/pnas.96.6.3029. PMID:10077631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chintalacharuvu KR, Morrison SL. Production of secretory immunoglobulin A by a single mammalian cell. Proc Natl Acad Sci U S A. 1997;94:6364–8. doi: 10.1073/pnas.94.12.6364. PMID:9177223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul M, Reljic R, Klein K, Drake PM, van Dolleweerd C, Pabst M, Windwarder M, Arcalis E, Stoger E, Altmann F, et al.. Characterization of a plant-produced recombinant human secretory IgA with broad neutralizing activity against HIV. MAbs. 2014;6:1585–97. doi: 10.4161/mabs.36336. PMID:25484063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51. doi: 10.1111/imr.12350. PMID:26497511. [DOI] [PubMed] [Google Scholar]
- 33.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189:3430–8. doi: 10.4049/jimmunol.1200356. PMID:22956577. [DOI] [PubMed] [Google Scholar]
- 34.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi: 10.1182/blood-2008-09-179754. PMID:19018092. [DOI] [PubMed] [Google Scholar]
- 35.Brandsma AM, Ten Broeke T, Nederend M, Meulenbroek LA, van Tetering G, Meyer S, Jansen JH, Beltran Buitrago MA, Nagelkerke SQ, Nemeth I, et al.. Simultaneous Targeting of FcgammaRs and FcalphaRI Enhances Tumor Cell Killing. Cancer Immunol Res. 2015;3:1316–24. doi: 10.1158/2326-6066.CIR-15-0099-T. PMID:26407589. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Duval M, Lewis C, Gawron MA, Wang R, Posner MR, Cavacini LA. Impact of IgA constant domain on HIV-1 neutralizing function of monoclonal antibody F425A1g8. J Immunol. 2013;190:205–10. doi: 10.4049/jimmunol.1201469. PMID:23183895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Pfarr DS, Johnson S, Brewah YA, Woods RM, Patel NK, White WI, Young JF, Kiener PA. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–65. doi: 10.1016/j.jmb.2007.02.024. PMID:17362988. [DOI] [PubMed] [Google Scholar]
- 38.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–37. doi: 10.1161/CIRCULATIONAHA.108.771048. PMID:18519846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould JM, Weiser JN. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J Infect Dis. 2002;186:361–71. doi: 10.1086/341658. PMID:12134232. [DOI] [PubMed] [Google Scholar]
- 40.van de Pol AC, Wolfs TF, van Loon AM, Tacke CE, Viveen MC, Jansen NJ, Kimpen JL, Rossen JW, Coenjaerts FE. Molecular quantification of respiratory syncytial virus in respiratory samples: reliable detection during the initial phase of infection. J Clin Microbiol. 2010;48:3569–74. doi: 10.1128/JCM.00097-10. PMID:20660210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhaak LR, Huhn C, Waterreus WJ, de Boer AR, Neususs C, Hokke CH, Deelder AM, Wuhrer M.. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins.. Anal Chem 2008;80:6119–26 [DOI] [PubMed] [Google Scholar]
- 42.Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M.. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem 2014;86:5784–93 [DOI] [PubMed] [Google Scholar]
- 43.Selman MH, Hemayatkar M, Deelder AM, Wuhrer AM. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem 2011;83:2492–9 [DOI] [PubMed] [Google Scholar]
- 44.Jansen BC, Reiding KR, Bondt A, Ederveen AL Hipgrave, Palmblad M, Falck D, Wuhrer M,. MassyTools: A High-Throughput Targeted Data Processing Tool for Relative Quantitation and Quality Control Developed for Glycomic and Glycoproteomic MALDI-MS. J Proteome Res 2015;14:5088–98 [DOI] [PubMed] [Google Scholar]
- 45.Ceroni A, Maass K, Geyer H, Geyer R Dell A Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans.. J Proteome Res 2008;7:1650–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.