Abstract
The Yes-associated protein 1 (YAP1), a major downstream effector of the Hippo pathway, functions as a transcriptional regulator and plays an important role in cellular control of organ size and tumor growth. Elevated oncogenic activity of YAP1 has been clarified in different types of human cancers, which contributes to cancer cell survival and chemoresistance. However, the molecular mechanism of YAP1 overexpression in cancers is still not clear. Here we demonstrate that the deubiquitination enzyme USP9X deubiquitinates and stabilizes YAP1, thereby promoting cancer cell survival. Increased USP9X expression correlates with increased YAP1 protein in human breast cancer lines and patient samples. Moreover, depletion of USP9X increases YAP1 poly-ubiquitination, which in turn elevates YAP1 turnover and cell sensitivity to chemotherapy. Overall, our study establishes the USP9X-YAP1 axis as an important regulatory mechanism of breast cancer and provides a rationale for potential therapeutic interventions in the treatment of breast cancer.
Introduction
The Hippo/YAP1 signaling pathway is one of the most important players in organ size regulation and tissue homeostasis. The misregulation of Hippo/YAP1 pathway is involved in cancer development 1–3. As a transcriptional co-activator, YAP1 does not contain any DNA-binding domains, which elicits its transcription activation via interaction with other transcription factors including TEAD transcription factor family members, SMAD2, β-catenin, and Runt-related transcription factor 2 (RUNX2) to promote proliferation and tumor growth 2, 4–7.
The activity of YAP1 is tightly regulated at physiological conditions, where elevated YAP1 activity and/or overexpression have been observed in different kinds of cancer types 3. In humans, the kinase cascade MST1/2-Lats1/2 functions to inactivate YAP1 by directly phosphorylating YAP1 at Ser 127, which consequentially results in cytoplasmic retention of phosphorylated YAP1 via binding to 14-3-3 8, 9. Conversely, dephosphorylated YAP1 localizes to the nucleus, which in turn induce gene expression that promotes cell proliferation and organ growth 10, 11. Recently, several labs have shown that cellular energy stress induces LKB1/AMPK-dependent activation of Hippo pathway kinase cascades, which subsequently phosphorylates and inactivates YAP1 activity 12–15. Moreover, AMPK directly phosphorylates YAP1 and abolishes the YAP1-TEAD interaction 12, 13. c-Abl also phosphorylates YAP1 at Y357 site following DNA damage, which in turn enhanced YAP1 bind to p73 and activates p73-regulated pro-apoptotic target genes expression 16. In addition, the tyrosine kinase YES1, phosphorylated YAP1 and triggered the localization of the YAP1-TBX5-β-catenin complex to the promoters of anti-apoptotic genes 4.
In addition to the regulatory mechanisms controlling its phosphorylation and localization, YAP1 can be regulated by other post-translational modification. YAP1 is coordinately phosphorylated by Lats and CK1, and this phosphorylation regulates YAP1 ubiquitination and degradation through β-TRCP E3 ubiquitin ligase 17. A recent study reported that Fbxw7 regulates YAP1 stability through ubiquitin-proteasomal degradation in hepatocellular carcinoma 18. However, the mechanisms governing YAP1 protein stability in human cancers remain largely unknown. Thus, the identification of the signaling pathway controlling YAP1 stabilization will be important to demostrate YAP1 biology funciton and can be exploited for potential therapeutic interventions.
Here, we report that USP9X regulates breast cancer cell proliferation and cancer cell response to therapeutic drugs through the YAP1 pathway. Mechanistically, USP9X deubiquitinates and stabilizes YAP1. In addition, depletion of USP9X decreases breast cancer proliferation, tumorigenesis, and chemoresistance in a YAP1 dependent manner. Furthermore, USP9X overexpression is observed in breast cancers, which is correlated with the high expression of YAP1, suggesting that the USP9X-YAP1 axis may play a role in the pathogenesis of breast cancers.
Results
USP9X is a bona fide DUB targeting YAP1 protein for deubiquitination and stabilization
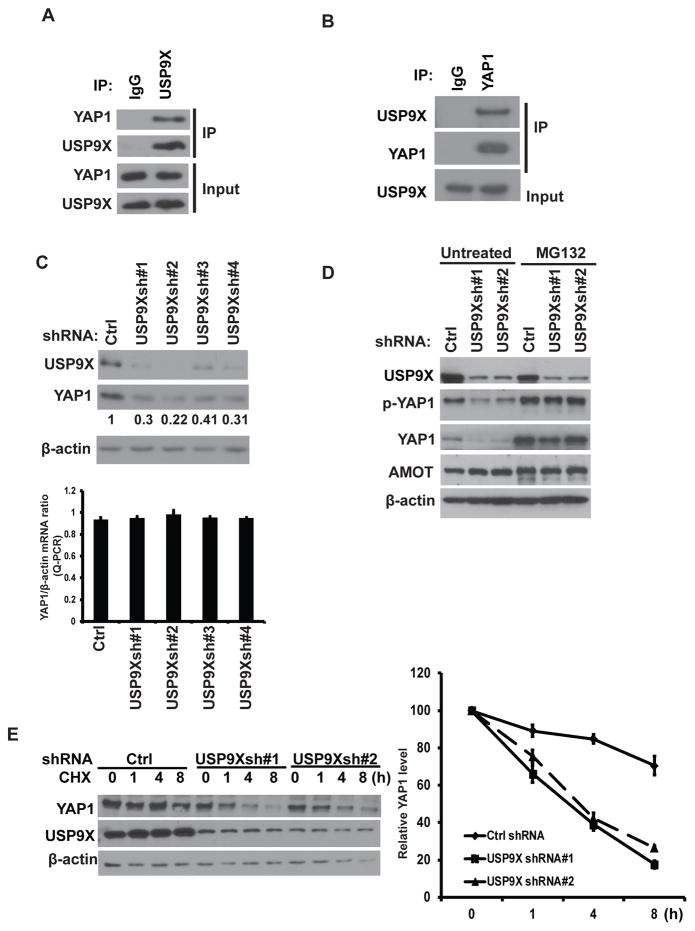
Previous studies showed that YAP1 ubiquitination and degradation were mediated by several E3 ligases, such as β-TRCP and Fbxw7. However, the process of YAP1 deubiquitination is still unclear. Multiple proteomic databases showed USP9X in YAP1 purification complex. (https://www.ncbi.nlm.nih.gov/gene/10413) and (http://www.genecards.org/cgi-bin/carddisp.pl?gene=YAP1&keywords=yap1). Therefore, we first tested the interaction between USP9X and YAP1. We found that endogenous USP9X coimmunoprecipitated with endogenous YAP1 in the co-immunoprecipitation (Co-IP) experiment (Figure 1A–B). The interaction of USP9X and YAP1 led us to test a potential role for the deubiquitination enzyme USP9X in the regulation of YAP1 turnover and function. We found overexpression of wild type (WT) USP9X but not the catalytic inactive mutant (CS mutant) in MDA-MB-231 cells dramatically increased YAP1 protein level (Supplementary Figure 1A). Concersely, depletion of USP9X in MDA-MB-231 cells significantly decreased YAP1 protein level but did not affect YAP1 mRNA level (Figure 1C). Depletion of USP9X in ovarian cancer cell line OVCAR8 also downregulated YAP1 protein levels (Supplementary Figure 1B). In addition, treating cells with the proteasome inhibitor, MG132, could rescue the decreased YAP1 protein level in cells depleted of USP9X (Figure 1D). Previous studies showed that USP9X deubiquitinates MCL1 and promotes cancer cell survival in human follicular lymphomas and diffuse large B cell lymphomas 19. We did observed a moderate decrease of MCL1 protein level in USP9X-depleted cells (Supplementary Figure 1D). Moreover, USP9X was reported to target Angiomotin (AMOT) and Angiomotin-like 2 (AMOTL2), which in turn regulates YAP1 activation 20, 21. However, in our study, depletion of USP9X did not affect AMOT levels (Figure 1D and Supplementary Figure 1B–C). We then hypothesized that USP9X may regulates YAP1 stability. Indeed, YAP1 protein was less stable after USP9X knockdown (Figure 1E). Moreover, overexpression of USP9X, dramatically increased YAP1 stabilization (Supplementary Figure 1E), while overexpression of the CS mutant decreased YAP1 stability. Taken together, these results suggest that USP9X directly regulates YAP1 stability.
Figure 1. USP9X binds and stabilizes YAP1.
(A–B) HEK293T cell were lysed and IPed with indicated antibodies. The immunocomplexes were subjected to Western blot. (C) MDA-MB-231 Cells stably expressed ctrl or USP9X shRNAs were subjected to western blot and qRT-PCR to examine the indicated protein and mRNA level. Lower panel: YAP1 mRNA level relative to β-actin was quantified (mean ± s.d. (n=6)). (D) Cells as in (C) were left untreated or treated with MG-132 and Western blot was performed to examine the indicated protein levels. (E) CHX pulse-chase assay was performed in cells as in (C). Right panel: the protein levels of YAP1 relative to β-actin (mean ± s.e.m. of three independent experiments)
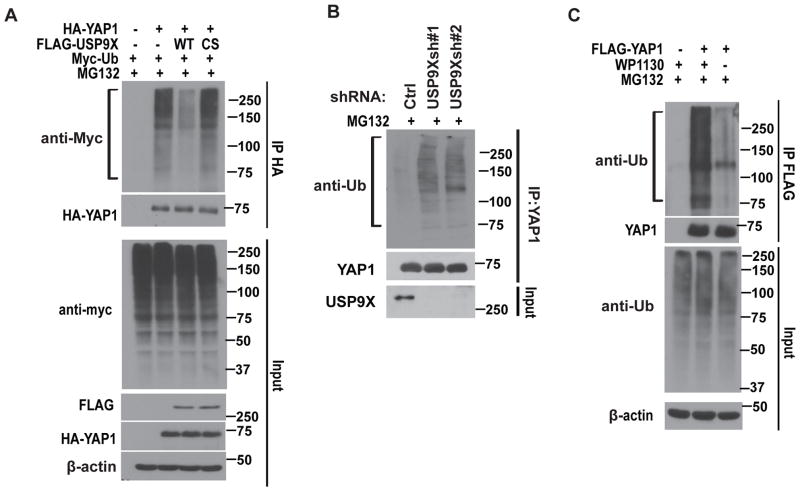
To further investigate whether USP9X functions as a DUB for YAP1 protein, we performed a deubiquitination assay by co-transfecting cells with WT USP9X or the CS mutant in the presence of MG132. A significant decrease in polyubiquitylated YAP1 protein was observed in cells transfected with WT USP9X, whereas the expression of CS mutant was not able to decrease YAP1 ubiquitination (Figure 2A). On the other hand, depletion of USP9X significantly increased YAP1 ubiquitination (Figure 2B). In addition, the USP9X inhibitor, WP1130 22 significantly enhanced YAP1 ubiquitination (Figure 2C). Furthermore, USP9X removed K48-linked polyubiquitination of YAP1 (Supplementary Figure 2A). Taken together, these results suggest that USP9X is a DUB that regulates YAP1 deubiquitination and stability.
Figure 2. USP9X deubiquitinates YAP1.
(A) Cells were transfected with HA-YAP1, FLAG-USP9X and Myc-Ub as indicated. The polyubiquitylated YAP1 protein was detected by anti-Myc antibody. (B) Cells stably expressed control or USP9X shRNAs were subjected to deubiquitination assay and the polyubiquitylated YAP1 protein was detected by the anti-Ub antibody. (C) Cells transfected with FLAG-YAP1 were treated with or without WP1130. The polyubiquitylated YAP1 protein was examined as in (B).
USP9X regulates cell proliferation through YAP1
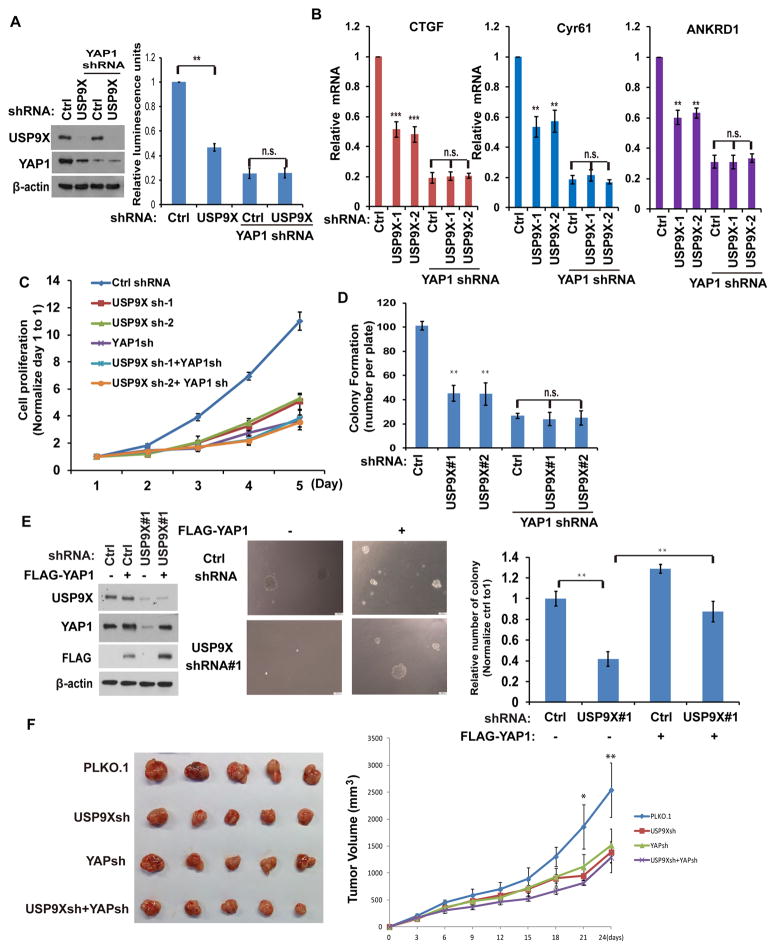
To assess the effect of USP9X on YAP1 transactivation, we utilized a YAP1 dependent luciferase reporter system which consists of a 5xUAS- luciferase reporter and a Gal4 DNA-binding domain fused to TEAD4 (Gal4-TEAD4) 9. The reporter activity was strongly suppressed in cells stably expressing USP9X or YAP1 shRNA (Figure 3A). Depletion of USP9X in cells depleted of YAP1 had no further inhibitory effect on reporter activity, indicating that USP9X suppresses the reporter activity mainly through YAP1 (Figure 3A). In addition, we examined the YAP1-regulated gene transcripts (CTGF, CYR61 and ANKRD1) and found depletion of USP9X dramatically decreased the transcripts of CTGF, CYR61 and ANKRD1 in control cells but not YAP1 knockdown cells (Figure 3B). These results suggested that USP9X regulates YAP1 dependent transcription. As a key effector of the Hippo signaling pathway, YAP1 regulates organ size and tumor growth by modulating cell proliferation. Next, we investigated potential functions of USP9X in cancer cell proliferation and anchorage-independent cancer cell growth. Depletion of USP9X led to decreased cell proliferation (Figure 3C), while depletion of both USP9X and YAP1 did not cause a further decrease in cell proliferation compared with cells depleted of YAP1 alone. Similar effects were observed in colony formation assays (Figure 3D). taken together, all these results suggest that USP9X regulates the proliferation of cancer cells in a YAP1 dependent manner. Next, we tested whether YAP1 is able to rescue the proliferation defect caused by USP9X depletion. As shown in Figure 3E, depletion of USP9X caused a dramatic loss of colony forming ability, while the reconstitution of YAP1 was able to efficiently rescue this defect. On the other hand, overexpression of AMOT could not rescue cancer cell growth in USP9X depleted cells (Supplementary Figure 3A). Xenograft experiments further showed that depletion of USP9X or YAP1 similarly inhibited tumor growth, while the combined knockdown of USP9X and YAP1 did not further reduce tumor growth (Figure 3F). This is consistent with in vitro results using cancer cell lines and indicates that USP9X affects tumor cell proliferation by regulating YAP1.
Figure 3. USP9X regulates cell proliferation and anchorage-independent growth through YAP1.
(A) MDA-MB-231 cells were transfected with indicated constructs with the luciferase reporter constructs. The YAP1 activity were analyzed by the luciferase reporter assay (mean ± s.d. (n=6)). (B) YAP-regulated gene transcripts were detected by qRT-PCR in MDA-MB-231 cells stably expressing indicated shRNA. The data were normalized with the β-actin mRNA (mean ± s.d. (n=3)). (C) The cells generated as in B were measured cell proliferation by MTS assay (mean ± s.e.m. of three independent experiments). (D) The cells were generated as in B and colony-formation assays were performed (mean ± s.e.m. of three independent experiments). (E) Cells stably expressed Ctrl or USP9X shRNAs together with or without FLAG-YAP1. Cells were cultured in soft agar and cell growth was measured (mean ± s.e.m. of three independent experiments). (F) Cells generated as in (A) were injected into the immunodeficient mice as described in methods. Tumor growth was measured every 3 days (mean ± s.d. of 5 mice). All of the statistical analyses were performed with the ANOVA. *, p<0.05; **, p<0.01. ***, p<0.001
USP9X is positively correlated to YAP1 in clinical breast cancer samples
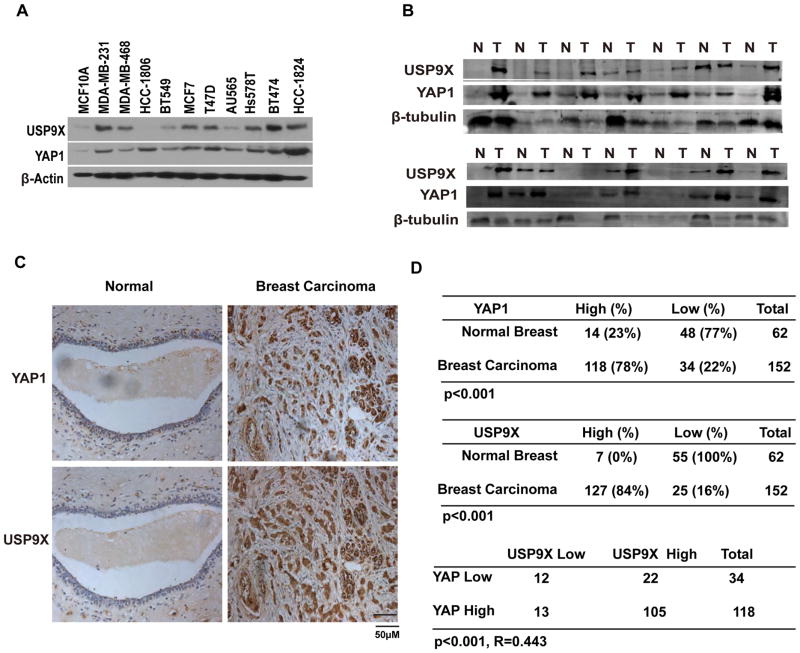
Since YAP1 plays a key role in human cancer development, it is possible that USP9X promotes deubiquitination and stabilization of YAP1 in human cancers. We then examined the expression of USP9X and YAP1 in breast cancer cell lines and cancer tissue samples. As shown in Figure 4A, USP9X is overexpressed in most of the breast cancer cell lines. Furthermore, high USP9X protein levels correlate with increased YAP1 in most of the breast cancer samples (Figure 4B). To further confirm the results, we further performed immunohistochemical staining of USP9X and YAP1 in breast cancer tissue microarrays. As shown in Figure 4C–D, both USP9X and YAP1 are overexpressed in breast carcinoma compared to normal breast tissues. Furthermore, USP9X positively correlated with YAP1 expression level but not with MCL1 or AMOT level in breast carcinoma (Figure 4D and Supplementary Figure 4A–B). Taken together, these results indicate that USP9X is upregulated in breast carcinoma, positively correlating with YAP1 expression.
Figure 4. USP9X expression positively correlates with YAP1 expression in clinical breast cancer samples.
(A) Expression of USP9X and YAP1 in the mammary epithelial cell line and breast cancer cell lines as indicated. (B) A subset of the breast tumor and normal tissues were subjected to westernblot to examine the YAP1 and USP9X protein levels. (C) Representative staining of USP9X and YAP1 in breast carcinoma and peritumoral breast tissues. (D) Quantification of USP9X and YAP1 protein levels in normal and breast carcinoma and the correlation study of USP9X and YAP1 expression level in breast carcinoma. Statistical analyses were performed with the χ2 test. R: The Pearson correlation coefficient.
USP9X regulates response of breast cancer cells to chemotherapy through YAP1
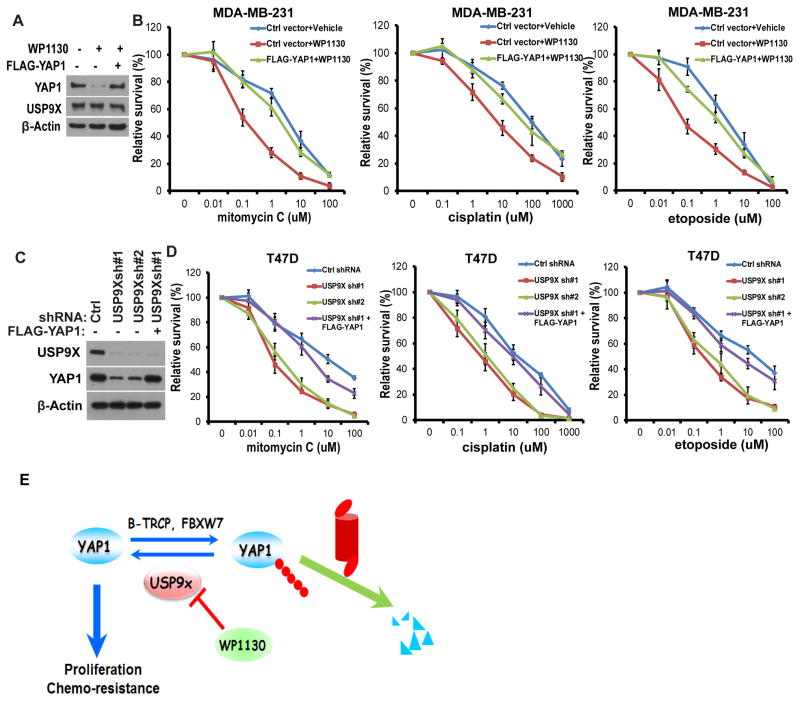
The activation of transcriptional coactivator YAP1 has been shown to play a role in chemoresistance in different malignancies like ovarian cancer, melanoma, and hepatocellular carcinoma. Considering that YAP1 is stabilized by USP9X, we tested whether inhibition of USP9X activity by its pharmacological inhibitor or specific shRNA affects cell response to chemotherapy. First, cells were treated with vehicle or USP9X inhibitor WP1130, and the responses to mitomycin C (MMC), cisplatin and etoposide were subsequently measured. We found that the USP9X inhibitor WP1130 promoted YAP1 degradation (Figure 5A). Additionally, WP1130 sensitized cells to chemotherapy drugs treatment (Figure 5B), while cells reconstituted with FLAG-YAP1 were significantly resistant to chemotherapy drugs treatment even in the presence of WP1130 treatment. Similarly, depletion of USP9X in cells using two specific shRNAs significantly decreased YAP1 protein levels (Figure 5C) and increased cellular sensitivity to MMC, cisplatin and etoposide (Figure 5D), while reconstituting USP9X depleted cells with FLAG-YAP1 reversed chemotherapy drugs hypersensitivity (Figure 5D). These results establish an important role of USP9X in regulating chemotherapeutic response, at least in part through YAP1.
Figure 5. USP9X regulates the breast cancer cells response to chemotherapy.
(A) MDA-MB-231 cells were stably expressed with the indicated constructs and treated with either vehicle or USP9X inhibitor WP1130 for 12h. The cells were subjected to westernblot to examine the indicated protein levels. (B) Cells as in (A) were treated with MMC, cisplatin and etoposide and cell survival was determined (mean ± s.d. (n=6)). (C) T47D cells were stably expressed ctrl or USP9X shRNA together with or without FLAG-YAP1. The cells were subjected to westernblot to examine the indicated protein levels. (D) Cells as in (C) were treated with MMC, cisplatin and etoposide and cell survival was determined (mean ± s.d. (n=6)). (E) The experimental model of how USP9X regulates YAP1.
In summary, we demonstrate that USP9X interacts with YAP1 and promotes its deubiquitination, ultimately leading to YAP1 stabilization (Figure 5E). We reveal USP9X as a positive regulator of the YAP1 in breast cancer cell proliferation and a potential biomarker for predicting chemoresistance.
Discussion
YAP1, a major factor of the Hippo tumor suppressor pathway, is known to play significant roles in multiple cellular processes, such as cell proliferation, differentiation, stemness as well as apoptosis 2, 23–29. YAP1 has been reported both as a tumor suppressor and an oncogene candidate 8, 30. Previous study reported that low YAP1 levels prevent nuclear ABL1-mediated apoptosis in hematologic malignancies, while restored YAP1 levels by genetic inactivation of STK4 triggers cell death in vitro and in vivo 31. In addition, YAP1 can be directly phosphorylated by c-Abl following DNA damage, while in turn facilitates YAP1 bind to p73 and co-activates p73 dependent pro-apoptotic target genes 16. In contrast, YAP1 was also reported to function as a potent oncogene and is associated with the prognosis of many human cancers including prostate, breast, ovarian, and hepatocellular cancers 3, 8, 15, 32. Several recent studies have elucidated that two different E3 ubiquitin-ligases (SCFβ-TrCP and SCFFbw7) control YAP1 ubiquitination and degradation. A DUB protein, DUB3 controls the protein stability of multiple components of Hippo pathway, including the E3 ligase ITCH, the LATS kinases and the AMOT family proteins, which in turn induces YAP1 turnover 33. Moreover, previous studies showed that USP9X targets Angiomotin (AMOT) and Angiomotin-like 2 (AMOTL2), which in turn regulates YAP1 activation 20, 21. However, in our study, depletion of USP9X did not affect AMOT levels in both breast and ovarian cancer cell lines (Figure 1D and Supplementary Figure 1B–C). Although we could not completely exclude the possibility that USP9X regulates Amot in other cancer models, such as the regulation of Amot by USP9X in ccRCC model 20. However, deubiquitinases that directly mediate and oppose YAP1 ubiquitination has not been identified yet.
The deubiquitination enzymes are proteases that remove Ub chains from diverse substrates and regulate multiple cellular processes, such as cell survival, cell differentiation, chromosome remodeling as well as apoptosis 19, 34–37. Similar to YAP1, the biological effects of USP9X in human cancers are complex, which could be regulated by different proteins targeted by USP9X in different cellular context. In pancreatic ductal adenocarcinoma (PDA), USP9X was reported to be a tumor suppressor, in which USP9X is the most commonly mutated gene and was inactivated in more than half of the tumors 38. USP9X has also been reported to regulate cellular sensitivity to chemo and radiotherapies. USP9X deubiquitinates and stabilizes MCL1 in distinct human cancers including human follicular lymphomas, diffuse large B cell lymphomas, glioblastoma, colon and lung cancers 19. The USP9X inhibitor WP1130 or depletion of USP9X by its specific shRNAs induces MCL-1 degradation in cells and increases tumor cell sensitivity to chemo and radiotherapies 39. On the other hand, a recent study showed that USP9X regulates cell survival and radio-sensitivity without affecting MCL-1 protein level in Jurkat cells and several glioblastoma cell lines, indicating MCL-1-independent mechanisms 40. Thus, identifying novel substrates other than MCL-1 is essential for understanding USP9X biology and its implication in tumorigenesis and drug resistance.
Here, we reveal a connection between USP9X and the Hippo-YAP pathway in breast cancer. In this study, we demonstrate that USP9X may target YAP1 for deubiquitination and stabilization, thereby promoting breast cancer growth and tumor progression. Anti-cancer effects of USP9X inhibition could be partially reversed by restoring YAP1 expression in vitro and in vivo. Interestingly, USP9X was positively correlated to YAP1 protein expression in breast cancers. Our study provides evidence of USP9X-YAP1 as a potential biomarker for predicting chemoresistance in breast cancers. In addition, targeting the USP9X-YAP1 axis could be used to sensitize cancer cells to radio and chemotherapy, although clinical effect of USP9X/YAP1 inhibitors needs to be further tested. Furthermore, although our study mostly focused on Paclitaxel and breast cancers, our finding that USP9X-YAP1 axis affects the response to chemotherapeutic agents would have broader implications for the treatment of other cancers and should be examined in the future.
Materials and Methods
Cell culture, constructs, and antibodies
We purchased mammary epithelial cell line MCF10A and breast cancer cell lines MDA-MB-231, HCC-1806, MDA-MB-468, BT549, MCF7, T47D, AU565, Hs578T, BT474, HCC-1824 from ATCC. All the cell lines have tested and confirmed by the Mayo Clinic medical genome facility Center. USP9X WT and its inactive mutants were purchased from MRC PPU (University of Dundee). The luciferase reporter constructs were kindly provided by Kunliang Guan 9. YAP1 cDNA was kindly provided by Junjie Chen 11 and we subcloned it to pCMV-HA and pLV.3-FLAG vectors.
Anti-USP9X (Catalog No: 55054-1-AP) antibody was purchased from Proteintech. Anti-p-YAP1 (4911S) antibody was purchased from cell signaling. Anti-YAP1 (SC15407) and anti-Ub (sc-8017) antibodies were purchased from Santa Cruz. Antibodies against HA (H9658), FLAG (F1804), β-actin (A1978) and β-Tubulin (T8328) were purchased from sigma.
RNA interference
USP9X shRNAs were kindly provided by Drs. William G. Kaelin and Qing Zhang 36: USP9xsh#1, GGTCGTTACAGCTAGTATTTA; USP9xsh#2, CGCCTGATTCTTCCAATGAAA; USP9xsh#3, CGACCCTAAACGTAGACATTA; USP9xsh#4, GAGAGTTTATTCACTGTCTTA. YAP1 shRNAs were purchased from Sigma (NM_006106.3-1354s21c1). We generated the lentiviruses for USP9X and YAP1 shRNAs according to the standard protocol.
Deubiquitination assay in vivo
HEK293T cells transfected with HA-YAP1, FLAG-USP9X and Myc-Ub as indicated were incubated with 25 μM MG132. After 4 h incubation, the cells were carefully washed with pre-chilled PBS for 2 times and harvested. Then the cells were lysed in 120 μl buffer with 10% glycerol, 2% SDS, pH 6.8 Tris-HCl (62.5 mM), 1 mM iodoacetamide and 20 mM NEM. Then the cell lysates were boiled for 15 min and then diluted with NTEN lysis buffer containing protease inhibitors and deubiquitination inhibitors in 1:10 ratio. After 20 min, the cell lysates were IPed with indicated antibodies and protein A/G beads for 4h (4°C). After washing the beads, the immunocomplexes were subjected to western blot.
Cell survival assay
The breast cancer cell lines stably expressed with the indicated constructs were treated with mitomycin C, cisplatin or etoposide at indicated concentrations. After 72 h, the plates were read in an Epoch2 microplate reader (BioTek Instruments). The cell survival ratio was examined by MTS assay (Promega).
Soft agar colony-formation assays
The breast cancer cells (MDA-MB-231) stably expressed Ctrl or USP9X shRNA together with FLAG-YAP1 were plated in 0.3% (w/v) agarose and the base layer is a 0.6% (w/v) agarose. Both of the layers contained the complete medium. After 2 weeks, colonies were counted by using a light microscope with 4X NA 0.10 objective lens (ECLIPSE 80i; Nikon).
Tumor growth study in xenograft
MDA-MB-231 cells stably expressed the control, USP9X, YAP1 or USP9X together with YAP1 shRNAs were injected subcutaneously into female athymic nu/nu mice (NCI) mammary fat pad (106 cells/mouse) by randomization. Every 3 days, the tumor volumes were measured following standard protocol. Data were analyzed using ANOVA test. By following the blinding procedures, two persons as a study group performed all the mice experiments. Dr. Lei Li injected the cells into the mice and Dr. Yunhui Li, who is totally blinded to the experiment group, measure the tumor and analyzed the data. The experiment protocol was approved by the IACUC at Mayo Clinic.
Mice were subjected to euthanasia when following situations were happen: displaying pain or distress, such as weight loss (> 10% of body weight), not eating or drinking, lethargy, lying down, or difficulty in breathing.
Immunohistochemical staining
The tissue microarray was purchased from Biomax (BR1101, BR1002 and BC08022). The immunohistochemical staining and quantification were described previously 41. The immunostaining was blindly scored by pathologists. The IHC score was calculated as described previously 42. The χ2 test and the Pearson correlation coefficient were used for statistical analysis of the correlation between USP9X and YAP1.
Statistical analysis
Data are represented as the mean ± s.e.m. of 3 independent experiments for cell proliferation and soft agar assays. Data are represented as the mean ± s.d. (n=6) for cell survival assay. Data are represented as the mean ± s.d. of 5 mice for the tumor growth study in xenograft. The Student’s t-test, ANOVA and χ2 test were utilized for the statistical analyses (*, p<0.05; **, p<0.01; ***, p<0.001).
Supplementary Material
Acknowledgments
The authors thank Drs William G. Kaelin, Qing Zhang, Kunliang Guan and Junjie Chen for kindly providing constructs. This study was supported by NSFC (81773758, 81572770, 31371367), National Institutes of Health grant (CA203971, CA203561, CA189666, CA130996), Ovarian Cancer SPORE in Mayo Clinic (P50CA136393), and the Pilot Project Award Program of Mayo Clinic Fraternal Order of Eagles Cancer Research Fund.
Footnotes
Conflict of Interests
There is no conflict of interests with the contents of this article for all the authors.
References
- 1.Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- 2.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S, et al. TGF-beta Targets the Hippo Pathway Scaffold RASSF1A to Facilitate YAP/SMAD2 Nuclear Translocation. Mol Cell. 2016;63:156–166. doi: 10.1016/j.molcel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Vitolo MI, Anglin IE, Mahoney WM, Jr, Renoud KJ, Gartenhaus RB, Bachman KE, et al. The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biol Ther. 2007;6:856–863. doi: 10.4161/cbt.6.6.4241. [DOI] [PubMed] [Google Scholar]
- 8.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Eyss B, Jaenicke LA, Kortlever RM, Royla N, Wiese KE, Letschert S, et al. A MYC-Driven Change in Mitochondrial Dynamics Limits YAP/TAZ Function in Mammary Epithelial Cells and Breast Cancer. Cancer Cell. 2015;28:743–757. doi: 10.1016/j.ccell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13:110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 20.Thanh Nguyen H, Andrejeva D, Gupta R, Choudhary C, Hong X, Eichhorn PJ, et al. Deubiquitylating enzyme USP9x regulates hippo pathway activity by controlling angiomotin protein turnover. Cell Discov. 2016;2:16001. doi: 10.1038/celldisc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Kim M, Park SJ, Lee C, Lim DS. Role of Angiomotin-like 2 mono-ubiquitination on YAP inhibition. EMBO Rep. 2016;17:64–78. doi: 10.15252/embr.201540809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 23.Piersma B, de Rond S, Werker PM, Boo S, Hinz B, van Beuge MM, et al. YAP1 Is a Driver of Myofibroblast Differentiation in Normal and Diseased Fibroblasts. Am J Pathol. 2015;185:3326–3337. doi: 10.1016/j.ajpath.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Zhi X, Zhao D, Zhou Z, Liu R, Chen C. YAP promotes breast cell proliferation and survival partially through stabilizing the KLF5 transcription factor. Am J Pathol. 2012;180:2452–2461. doi: 10.1016/j.ajpath.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, et al. YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung KH, McCarthy RL, Zhou C, Uprety N, Barton MC, Beretta L. MicroRNA Regulates Hepatocytic Differentiation of Progenitor Cells by Targeting YAP1. Stem Cells. 2016;34:1284–1296. doi: 10.1002/stem.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 2014;26:273–287. doi: 10.1016/j.ccr.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Svalina MN, Keller C. YAPping about differentiation therapy in muscle cancer. Cancer Cell. 2014;26:154–155. doi: 10.1016/j.ccr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strano S, Blandino G. YAP1 meets tumor suppression. Mol Cell. 2007;27:863–864. doi: 10.1016/j.molcel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HT, Kugler JM, Cohen SM. DUB3 Deubiquitylating Enzymes Regulate Hippo Pathway Activity by Regulating the Stability of ITCH, LATS and AMOT Proteins. PLoS One. 2017;12:e0169587. doi: 10.1371/journal.pone.0169587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 35.Nagai H, Noguchi T, Homma K, Katagiri K, Takeda K, Matsuzawa A, et al. Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol Cell. 2009;36:805–818. doi: 10.1016/j.molcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X, Zhai B, Koivunen P, Shin SJ, Lu G, Liu J, et al. Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev. 2014;28:1429–1444. doi: 10.1101/gad.242131.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savio MG, Wollscheid N, Cavallaro E, Algisi V, Di Fiore PP, Sigismund S, et al. USP9X Controls EGFR Fate by Deubiquitinating the Endocytic Adaptor Eps15. Curr Biol. 2016;26:173–183. doi: 10.1016/j.cub.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson LF, Sun H, Liu Y, Potu H, Kandarpa M, Ermann M, et al. Targeting deubiquitinase activity with a novel small-molecule inhibitor as therapy for B-cell malignancies. Blood. 2015;125:3588–3597. doi: 10.1182/blood-2014-10-605584. [DOI] [PubMed] [Google Scholar]
- 40.Wolfsperger F, Hogh-Binder SA, Schittenhelm J, Psaras T, Ritter V, Bornes L, et al. Deubiquitylating enzyme USP9x regulates radiosensitivity in glioblastoma cells by Mcl-1-dependent and -independent mechanisms. Cell Death Dis. 2016;7:e2039. doi: 10.1038/cddis.2015.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Fang Y, Zhang H, Deng M, Gao B, Niu N, et al. HEATR1 Negatively Regulates Akt to Help Sensitize Pancreatic Cancer Cells to Chemotherapy. Cancer Res. 2016;76:572–581. doi: 10.1158/0008-5472.CAN-15-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo K, Li Y, Yin Y, Li L, Wu C, Chen Y, et al. USP49 negatively regulates tumorigenesis and chemoresistance through FKBP51-AKT signaling. Embo J. 2017;36:1434–1446. doi: 10.15252/embj.201695669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.