Abstract
Study Objectives:
The aim of the study was to investigate the cognitive function of patients with primary restless legs syndrome/Willis-Ekbom disease (RLS/ WED) in a Chinese population.
Methods:
A total of 40 patients with RLS/WED who were drug naïve and 40 controls, matched by age, sex, and educational level, were evaluated by cognitive function assessments, including the Chinese version of the Mini-Mental State Examination (MMSE-C), clock drawing test (CDT), Auditory Verbal Learning Test (AVLT), Rey-Osterrieth Complex Figure Test (CFT), and Stroop Color Word Test (SCWT).
Results:
Patients with RLS/WED showed worse performance on the SCWT (Stroop Card C time: 102.36 ± 17.12 versus 87.08 ± 7.73 seconds, P = .033; Interference Index: 3.39 ± 0.38 versus 2.90 ± 0.15, P < .0001), CFT (24.05 ± 9.28 versus 33.74 ± 1.59, P = .008), and CDT than controls (16-score method: 10.13 ± 3.94 versus 13.98 ± 1.79, P = .0002) after adjusting for Hamilton Anxiety Scale score, Hamilton Depression Scale score, Epworth Sleepiness Scale score, and Pittsburgh Sleep Quality Index total score to eliminate the confounders of concomitant sleep disturbances, anxiety, and depression.
Conclusions:
Our study suggested that cognitive functions involving executive and visuospatial domains might be disturbed in Chinese patients with primary RLS/WED.
Citation:
Li G, Tang H, Chen J, Qi X, Chen S, Ma J. Executive and visuospatial dysfunction in patients with primary restless legs syndrome/Willis-Ekbom disease: study of a Chinese population. J Clin Sleep Med. 2018;14(5):785–790.
Keywords: executive dysfunction, restless legs syndrome/Willis-Ekbom disease, visuospatial dysfunction
BRIEF SUMMARY
Current Knowledge/Study Rationale: The relationship between restless legs syndrome/Willis-Ekbom disease (RLS/WED) and cognitive dysfunction is unclear; some studies have reported cognitive deficits in patients with RLS/WED and others have not.
Study Impact: This is the first study to investigate cognitive dysfunction in patients with primary RLS/WED in a Chinese population. In this 1:1 matched case-control study, we demonstrated that executive and visuospatial functions were disturbed in Chinese patients with primary RLS/WED.
INTRODUCTION
Restless legs syndrome/Willis-Ekbom disease (RLS/WED), is a common sleep disorder characterized by an urge to move legs, generally accompanied by various kinds of discomfort sensations at night or rest, which can be relieved partially or totally by movement.1 It is not uncommon for patients with RLS/WED to report cognitive impairment.2 Some studies have shown that patients with RLS/WED had cognitive deficits whereas others did not.2–7 The cognitive deficits reported in patients with RLS/WED usually involve executive function and is assessed by the following tests: semantic memory test,2,4,6 Stroop test,2,4 D2 Cancellation Task,4 Wisconsin Card Sorting
Test (number of errors and number of nonperseverative errors)5 and Trail Making Test-B.6 However, most of these studies were performed in patients with mild or intermittent RLS/WED. Cognitive deficits in patients with severe or chronic-persistent RLS are less studied. It is important to investigate whether other cognitive function might also be affected in RLS/WED. Therefore, we conducted a case-control study to investigate the relationship between cognitive function and RLS/WED in a Chinese population.
METHODS
Study Procedure
Patients with RLS/WED were enrolled from outpatient clinics in Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, from January 2015 to November 2016. The diagnosis of RLS/WED was made by two RLS/ WED specialists based on revised International RLS/WED Study Group diagnostic criteria.8 Diseases that mimic RLS/ WED were excluded, such as hypnic jerks, sleep-related leg cramps, positional discomfort, or neuropathy. Extensive physical examination was performed by two neurologists (Gen Li and Jianfang Ma). Ferritin level was tested in all patients with RLS. Exclusion criteria included secondary RLS/ WED, sleep-related breathing disorder (SRBD), or primary insomnia. All patients with RLS/WED were drug naïve and symptom free when we made the assessment. Control subjects were recruited healthy community volunteers and evaluated by RLS/WED specialists for exclusion of RLS/WED. Other neurological diseases, other sleep disorders, or psychiatric diseases were also excluded in the RLS/WED and control groups. Control subjects were strictly matched for age (within 1 year), sex, and education levels without SRBD or insomnia. History of hypertension and diabetes were also taken. Neuropsychological panel adopted in our study to evaluate the cognitive function included the Chinese version of the Mini-Mental State Examination (MMSE-C) for comprehensive cognitive fields, clock drawing test (CDT) for executive function and visuospatial function, Auditory Verbal Learning Test (AVLT) for memory, Rey-Osterrieth Complex Figure Test (CFT) for visuospatial function, and Stroop Color Word Test (SCWT) for executive function. Stroop interference index was calculated as Stroop Card C time divided by Stroop Card A time. Hamilton Depression Rating Scale (HAMD) and Hamilton Anxiety Rating Scale (HAMA) were used to assess the degree of depression and anxiety. International RLS/WED Study Group (IRLSSG) rating scale was used to assess the severity of RLS/WED. The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep condition. Epworth Sleepiness Scale (ESS) was used to assess daytime sleepiness. These scales have been evaluated in Chinese patients in various studies.9–13 Normal or borderline values of these scales are shown in Table S1 in the supplemental material.14 All cognitive tests were done between 9:00 am – 4:00 pm at an outpatient clinic.
All participants signed consent forms and this study was approved by the ethic committee of Ruijin Hospital, affiliated with the Shanghai Jiao Tong University School of Medicine.
Assessment of CDT
We assessed CDT by two well-trained raters. Several evaluation methods were used, such as 3-score method, 5-score method, and 16-score method. We resolved discrepancies by discussion with a senior investigator.
Statistical Analysis
Statistical analysis was performed using SAS software package (version 9.4 TS1M2; SAS Institute Inc., Cary, North Carolina, United States). Chi-square test and Student t test were performed to compare demographic features and confounding factors between RLS/WED and non-RLS/WED participants. Analysis of covariance was used. Logistic regression analysis was performed with odds ratios (OR) and 95% confidence intervals (CI) calculated. A level of P < .05 was regarded as statistically significant.
RESULTS
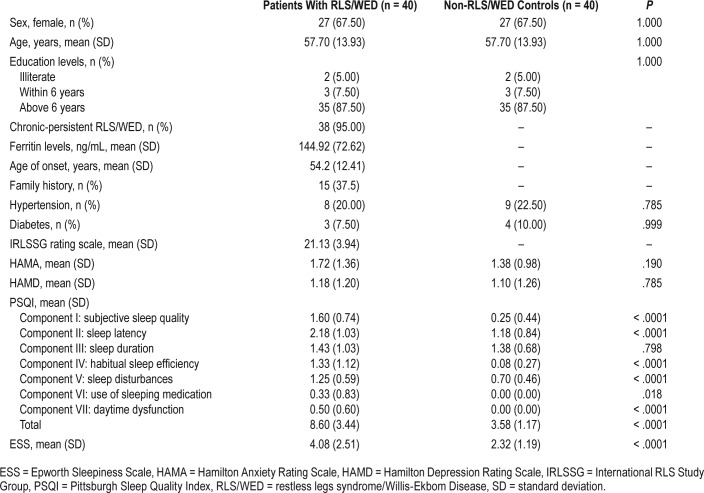
In total, 40 patients with RLS/WED and 40 non-RLS/WED normal controls were enrolled in our study, matched by age, sex, and education level. Of the patients with RLS/WED, 15 had a family history of the syndrome. The average age of RLS/WED onset was 54.2 years. The average ferritin level in those with RLS/WED was 144.92 ng/mL. Eight or 20% of the patients with RLS/WED had hypertension and 3 (7.5%) had diabetes. Nine or 22.5% of control group subjects had hyper-tension and 4 (10%) had diabetes. There were no statistical differences between the RLS/WED group and control group on hypertension and diabetes (hypertension: P = .785; diabetes: P = .999). The average IRLSSG rating scale score for the RLS group was 21.13 ± 3.94. Approximately 38 of the patients with RLS/WED (95%) experienced chronic-persistent RLS/ WED and 27 (67.5%) were female. There were no significant differences of anxiety scores (HAMA) and depression scores (HAMD) between patients with RLS/WED and controls. However, PSQI scores (except for component III) and ESS scores were significantly higher in patients with RLS/WED than controls (Table 1).
Table 1.
Demographic information and sleep assessment of cases and controls.
The patients with RLS/WED described their symptoms as uncomfortable sensations that were alleviated by movement or massage at night. Some could describe their symptoms vividly, like feelings of “ants in the legs,” “burning” or “itching.” Others could not describe the sensation, reporting that they were just uncomfortable.
The cognitive tests were compared between the RLS/WED and control groups after adjusting for PSQI, ESS, HAMA, and HAMD in order to prevent the interfering of sleep condition, anxiety, and depression comorbidities in RLS/WED.
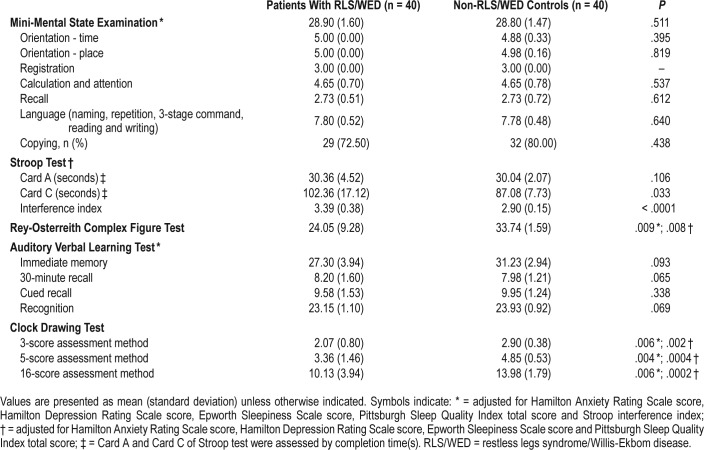
For SCWT, we found that time completing Card C and Stroop interference index was much longer in the patients with RLS/WED than controls. Card C time for RLS/WED and controls was 102.36 ± 17.12 and 87.08 ± 7.73 seconds, respectively; P = .033. The interference index for RLS/WED and controls was 3.39 ± 0.38 and 2.90 ± 0.15, respectively; P < .0001. These results indicate executive dysfunction in patients with RLS/ WED (Table 2).
Table 2.
Cognitive assessments in patients with RLS/WED and controls.
In addition, we used the CFT to investigate the visual-spatial ability of patients with RLS/WED. We found significantly lower scores in patients with RLS/WED than controls (RLS/ WED: 24.05 ± 9.28, controls: 33.74 ± 1.59, P = .008). As executive dysfunction may interfere with CFT results, we performed the statistical analysis of the CFT scores after adjusting for Stroop interference index and the results were similar (RLS/ WED: 24.05 ± 9.28, controls: 33.74 ± 1.59, P = .009), suggesting a visual-spatial disability in patients with RLS/WED that was independent of executive dysfunction (Table 2).
Regarding CDT, patients with RLS/WED performed worse than controls (3-score: 2.07 ± 0.80 versus 2.90 ± 0.38, P = .002; 5-score: 3.36 ± 1.46 versus 4.85 ± 0.53, P = .0004; and 16-score: 10.13 ± 3.94 versus 13.98 ± 1.79, P = .0002 for RLS/WED versus controls, respectively). After further adjusting for Stroop interference index scores for potential confounding effects of executive dysfunction, CDT test results remained worse for the RLS/WED group (3-score: P = .006, 5-score: P = .004, and 16-score: P = .006) (Table 2). These results also indicated a visuospatial disability in patients with RLS/WED that was independent of executive dysfunction.
There were no statistical differences of AVLT and MMSE scores between the RLS/WED and control groups, suggesting that memory ability and overall cognitive function were not disturbed in patients with RLS/WED (Table 2).
DISCUSSION
Our study found that cognitive domains disturbed in patients with RLS/WED include executive and visual-spatial function; however, memory and overall cognitive function were mostly preserved. To our knowledge, this is the first study to demonstrate a visuospatial dysfunction in patients with RLS/WED.
Executive dysfunction has already been reported in patients with RLS/WED.2,4,5 Frontal lobe dysfunction was thought to contribute to the executive dysfunction in those with RLS. Cranial magnetic resonance imaging (MRI) detected gray matter or adjacent white matter changes in middle orbitofrontal gyrus, inferior frontal gyrus and anterior cingulate cortex of patients with RLS/WED by voxel-based morphometry and diffusion tensor imaging techniques.15–17 Event-related potentials (ERPs) revealed much lower P300 amplitudes in frontal and central locations of the brain in patients with RLS/WED.18 In addition, decreased gamma-band phase synchrony was found in patients with RLS/WED, especially in the frontal region. These findings from MRI and ERP studies show frontal or pre-frontal cortical dysfunction that can account for the executive dysfunction in patients with RLS/WED.19,20
Visuospatial dysfunction of patients with RLS/WED seems to be an independent finding from our study, supported by CFT and CDT results after adjusting for executive dysfunction by Stroop interference index. It is difficult to understand the mechanism underlying the visuospatial disability in RLS/ WED. Several explanations were proposed. First, thalamus abnormality (anterior thalamic nucleus) might cause the visuospatial disability, especially spatial location learning.21 Reduced iron content, dysregulated dopamine, and glutamate transmission in thalamus were also detected in patients with RLS/WED by various brain image studies.17,22–29 It is possible that thalamus dysregulation contributes to the visuospatial dys-function. Second, comorbidities such as sleep problems might contribute to the visuospatial disability in patients with RLS/ WED.6,30 More studies are needed to confirm and reveal the underlying mechanism of visuospatial disability in RLS/WED.
The strengths of our study are that the patients with RLS/ WED enrolled in our study were from outpatient clinics and nearly 95% had chronic persistent RLS/WED. The diagnosis was based on revised RLS/WED diagnostic criteria by two experienced RLS/WED specialists. All possible RLS/WED mimics and secondary causes of RLS/WED were excluded.
This study has some weaknesses and limitations. First, we did not perform a complete neuropsychological assessment. Some cognitive domains, such as verbal fluency and naming functions, were not assessed in our study. It was reported that patients with RLS/WED had verbal fluency in both initials and categories that also reflected prefrontal lobe functions.4,6 So it is possible that other cognitive domains are disturbed in RLS/WED, and this disturbance may affect the executive and visual-spatial dysfunctions indirectly. Second, we did not study other forms of RLS/WED like mild intermittent RLS/ WED. It is uncertain whether the cognitive dysfunction found in our study is the same in other types of RLS/WED. Third, cranial images or neurophysiological examinations, such as ERPs and MRI, were not performed in the current study. The neuropsychological assessments adopted in our study can be confounded by many factors such as the assessment errors of raters and performances of patients with RLS/WED. Fourth, we excluded peripheral neuropathy via physical examination and history rather than electromyography. Considering that our sample size is small, it is possible we have false-positive findings. Finally, it is possible that other factors such as sleep disruption might contribute to the cognitive dysfunction in patients with RLS,4,31 although we adjusted for PSQI and ESS scores in our logistic regression analysis. For example, Fulda et al. performed a study in which they enrolled fewer participants (RLS = 23 versus control = 23), used questionnaires to assess sleep disturbances, and analyzed by correlation and t test. Fulda et al. found that impaired sleep quality affected the category fluency task in patients with RLS.4 However, they performed another study 1 year later that included more participants (RLS = 41 versus control = 133), used similar questionnaires, and used a different analysis model (regression model). This study showed that the cognitive dysfunction in patients with frequent RLS was independent from their concomitant sleep problems.5 Interestingly, Gamaldo et al. found that patients with RLS were even better than sleep-restricted control patients in verbal fluency tests; this was explained by a probable sleep loss adaption mechanism. However, the sample size of this study was small (RLS = 16 versus sleep restricted controls = 13).31 Methodological difference might account for opposing results, including study design, sleep assessment, sample size, and statistical analysis. Therefore, both comprehensive subjective and objective assessments should be performed to study cognitive function in a larger cohort of patients with RLS/WED in future studies.
In conclusion, our study found disturbed cognitive function involving the executive and visuospatial domains in Chinese patients with primary RLS/WED. These results call for more investigation to reveal possible mechanisms underlying this phenomenon.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interests. This study was supported by grants from National Natural Science Fund (81200979 and 81571103), Natural Science Foundation of Science and Technology of Shanghai (15ZR1426700), and The National Key R&D Program of China (2016YFC1306000 and 2016YFC1305804).
ACKNOWLEDGMENTS
Author contributions: Dr. Gen Li and Dr. Huidong Tang performed the cognitive assessment of RLS/WED and control, statistical analysis and drafted the manuscript. Dr. Xuemei Qi and Dr. Jie Chen performed the cognitive assessment of RLS/WED and controls. Dr. Huidong Tang made the diagnosis of RLS/WED and reevaluated the cognitive tests. Dr. Jianfang Ma designed the study, double-checked the diagnosis of RLS/WED, statistical analysis and revised the manuscript. Prof. Shengdi Chen supervised the study and revised the manuscript.
ABBREVIATIONS
- CDT
clock drawing test
- CFT
Rey-Osterrieth Complex Figure Test
- CI
confidence interval
- ERP
event-related potential
- ESS
Epworth Sleepiness Scale
- HAMA
Hamilton Anxiety Rating Scale
- HAMD
Hamilton Depression Rating Scale
- IRLSSG
International RLS/WED Study Group
- MMSE
Mini-Mental State Examination
- MMSE-C
Mini-Mental State Examination-Chinese version
- MRI
magnetic resonance imaging
- OR
odds ratio
- PSQI
Pittsburgh Sleep Quality Index
- RLS/WED
restless legs syndrome/Willis-Ekbom disease
- SCWT
Stroop Color Word Test
- SD
standard deviation
- SRBD
sleep-related breathing disorder
REFERENCES
- 1.Allen RP. Restless legs syndrome/Willis Ekbom disease: evaluation and treatment. Int Rev Psychiatry. 2014;26(2):248–262. doi: 10.3109/09540261.2014.904279. [DOI] [PubMed] [Google Scholar]
- 2.Celle S, Roche F, Kerleroux J, et al. Prevalence and clinical correlates of restless legs syndrome in an elderly French population: the synapse study. J Gerontol A Biol Sci Med Sci. 2010;65(2):167–173. doi: 10.1093/gerona/glp161. [DOI] [PubMed] [Google Scholar]
- 3.Driver-Dunckley E, Connor D, Hentz J, et al. No evidence for cognitive dysfunction or depression in patients with mild restless legs syndrome. Mov Disord. 2009;24(12):1840–1842. doi: 10.1002/mds.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulda S, Beitinger ME, Reppermund S, Winkelmann J, Wetter TC. Short-term attention and verbal fluency is decreased in restless legs syndrome patients. Mov Disord. 2010;25(15):2641–2648. doi: 10.1002/mds.23353. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S, Szesny N, Ising M, et al. Further evidence for executive dysfunction in subjects with RLS from a non-clinical sample. Sleep Med. 2011;12(10):1003–1007. doi: 10.1016/j.sleep.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7(1):25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Rist PM, Elbaz A, Dufouil C, Tzourio C, Kurth T. Restless legs syndrome and cognitive function: a population-based cross-sectional study. Am J Med. 2015;128(9):1023.e33–1023.e39. doi: 10.1016/j.amjmed.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Wan Y, Cheng Q, et al. Malnutrition and associated factors in Chinese patients with Parkinson's disease: results from a pilot investigation. Parkinsonism Relat Disord. 2010;16(2):119–123. doi: 10.1016/j.parkreldis.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Zhao Q, Teramukai S, et al. Executive function predicts survival in Alzheimer disease: a study in Shanghai. J Alzheimers Dis. 2010;22(2):673–682. doi: 10.3233/JAD-2010-100318. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang JP, Wang G, Cheng Q, et al. Cognitive impairment and the associated risk factors among the elderly in the Shanghai urban area: a pilot study from China. Transl Neurodegener. 2012;1(1):22. doi: 10.1186/2047-9158-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009;23(3):253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 13.Lin YN, Zhou LN, Zhang XJ, Li QY, Wang Q, Xu HJ. Combined effect of obstructive sleep apnea and chronic smoking on cognitive impairment. Sleep Breath. 2016;20(1):51–59. doi: 10.1007/s11325-015-1183-1. [DOI] [PubMed] [Google Scholar]
- 14.Qi Hao G, Bian HZ. Neuropsychological Assessment. 2nd ed. Shanghai, China: Shanghai Science and Technology Press; 2016. [Google Scholar]
- 15.Hornyak M, Ahrendts JC, Spiegelhalder K, et al. Voxel-based morphometry in unmedicated patients with restless legs syndrome. Sleep Med. 2007;9(1):22–26. doi: 10.1016/j.sleep.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo G, Manners D, Vetrugno R, et al. Combined brain voxel-based morphometry and diffusion tensor imaging study in idiopathic restless legs syndrome patients. Eur J Neurol. 2012;19(7):1045–1049. doi: 10.1111/j.1468-1331.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 17.Unrath A, Müller HP, Ludolph AC, Riecker A, Kassubek J. Cerebral white matter alterations in idiopathic restless legs syndrome, as measured by diffusion tensor imaging. Mov Disord. 2008;23(9):1250–1255. doi: 10.1002/mds.22074. [DOI] [PubMed] [Google Scholar]
- 18.Jung KY, Koo YS, Kim BJ, et al. Electrophysiologic disturbances during daytime in patients with restless legs syndrome: further evidence of cognitive dysfunction? Sleep Med. 2011;12(4):416–421. doi: 10.1016/j.sleep.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Choi JW, Ko D, Lee GT, Jung KY, Kim KH. Reduced neural synchrony in patients with restless legs syndrome during a visual oddball task. PLoS One. 2012;7(7):e42312. doi: 10.1371/journal.pone.0042312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tippin J, Aksan N, Dawson J, Anderson SW, Rizzo M. Sleep remains disturbed in patients with obstructive sleep apnea treated with positive airway pressure: a three-month cohort study using continuous actigraphy. Sleep Med. 2016;24:24–31. doi: 10.1016/j.sleep.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau PH, Tsenkina Y, Lecourtier L, et al. Lesions of the anterior thalamic nuclei and intralaminar thalamic nuclei: place and visual discrimination learning in the water maze. Brain Struct Funct. 2013;218(3):657–667. doi: 10.1007/s00429-012-0419-0. [DOI] [PubMed] [Google Scholar]
- 22.Allen RP, Barker PB, Horská A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome increased and related to disturbed sleep. Neurology. 2013;80(22):2028–2034. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astrakas L, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou M. T2 relaxometry and fMRI of the brain in late-onset restless legs syndrome. Neurology. 2008;71(12):911–916. doi: 10.1212/01.wnl.0000325914.50764.a2. [DOI] [PubMed] [Google Scholar]
- 24.Červenka S, Pålhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129(Pt 8):2017–2028. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 25.Etgen T, Draganski B, Ilg C, et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. NeuroImage. 2005;24(4):1242–1247. doi: 10.1016/j.neuroimage.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Ku J, Cho YW, Lee YS, et al. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014;15(3):289–294. doi: 10.1016/j.sleep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Allen RP, Earley CJ, et al. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82. doi: 10.1016/j.sleep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo G, Manners D, Testa C, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Disord. 2013;28(13):1886–1890. doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 29.Winkelman JW, Schoerning L, Platt S, Jensen JE. Restless legs syndrome and central nervous system gamma-aminobutyric acid: preliminary associations with periodic limb movements in sleep and restless leg syndrome symptom severity. Sleep Med. 2014;15(10):1225–1230. doi: 10.1016/j.sleep.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Johar H, Kawan R, Emeny RT, Ladwig KH. Impaired sleep predicts cognitive decline in old people: findings from the prospective KORA Age Study. Sleep. 2016;39(1):217–226. doi: 10.5665/sleep.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamaldo CE, Benbrook AR, Allen RP, Ognutimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9(5):500–505. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.