Abstract
Study Objectives:
Longitudinal studies support the usage of positive airway pressure (PAP) therapy in treating obstructive sleep apnea (OSA) to improve cardiovascular disease. However, the anticipated benefit is not ubiquitous. In this study, we elucidate whether PAP therapy leads to immediate improvements on endothelial function, a subclinical marker of cardiovascular status, by examining the effect of circulating exosomes, isolated from patients before and after PAP therapy, on naive endothelial cells.
Methods:
We isolated plasma-derived circulating exosomes from 12 patients with severe OSA and obesity hypoventilation syndrome (OHS) before and after 6 weeks of PAP therapy, and examined their effect on cultured endothelial cells using several in vitro reporter assays.
Results:
We found that circulating exosomes contributed to the induction and propagation of OSA/OHS-related endothelial dysfunction (ie, increased permeability and disruption of tight junctions along with increased adhesion molecule expression, and reduced endothelial nitric oxide synthase expression), and promoted increased monocyte adherence. Further, when comparing exosomes isolated before and after PAP therapy, the disturbances in endothelial cell function were attenuated with treatment, including an overall cumulative decrease in endothelial permeability in all 12 subjects by 10.8% (P = .035), as well as detection of a subset of 4 differentially expressed exosomal miRNAs, even in the absence of parallel changes in systemic blood pressure or metabolic function.
Conclusions:
Circulating exosomes facilitate important intercellular signals that modify endothelial phenotype, and thus emerge as potential fundamental contributors in the context of OSA/OHS-related endothelial dysfunction. Exosomes may not only provide candidate biomarkers, but are also a likely and plausible mechanism toward OSA/OHS-induced cardiovascular disease.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Title: AVAPS-AE Efficacy Study, URL: https://clinicaltrials.gov/ct2/show/NCT01368614, Identifier: NCT01368614
Citation:
Bhattacharjee R, Khalyfa A, Khalyfa AA, Mokhlesi B, Kheirandish-Gozal L, Almendros I, Peris E, Malhotra A, Gozal D. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: a proof of concept study. J Clin Sleep Med. 2018;14(5):797–807.
Keywords: cardiovascular disease, endothelial function, exosomes, obstructive sleep apnea, positive airway pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent studies show mixed results on the therapeutic benefits of positive airway pressure (PAP) therapy for improving cardiovascular function in adults with sleep-disordered breathing. Few such studies have addressed the early subclinical improvements in cardiovascular function, including endothelial function, which may shed insight into the mechanisms accounting for how PAP therapy can improve cardiovascular disease.
Study Impact: Here, we show that circulating exosomes isolated from patients with obesity hypoventilation syndrome adversely affect naïve endothelial integrity in vitro and such deleterious effects are improved following just 6 weeks of PAP therapy.
INTRODUCTION
Obstructive sleep apnea (OSA) is highly prevalent, affecting 22% of men and 17% of women.1 The prevalence of OSA appears to be overall increasing,2 likely due to increased patient and physician awareness and enhanced referral patterns, along with improvements in the diagnostic approaches of OSA, but is also likely to be attributable to the epidemic of obesity, the latter constituting a major and significant risk factor for development of OSA.3 Obesity hypoventilation syndrome (OHS) affects nearly 10% to 20% of obese patients with OSA.4 OHS is defined by daytime hypercapnia and hypoxemia (PaCO2 ≥ 45 mmHg and PaO2 < 70 mmHg at sea level) in an obese patient (body mass index [BMI] ≥ 30 kg/m2) with sleep-disordered breathing in the absence of any other detectable cause of hypoventilation.5
Recent large-scale longitudinal population-based studies have revealed that the risk of cardiovascular disease in adults is independently associated with OSA.6 Both the Busselton Health Study and the Wisconsin Sleep Cohort have reported that severe OSA in adults is associated with a threefold increase in the risk of all-cause mortality, and a higher cardiovascular mortality at 18-year follow-up, respectively.7,8 Furthermore, the presence of OHS also adds to the risk of cardiovascular morbidity and mortality.4,9 Among the relevant cardiovascular outcomes, OSA is strongly associated with hypertension,10 myocardial ischemia,11 arrhythmias,12 ischemic stroke,13,14 such that the cumulative effect of OSA on the cardiovascular system is likely accountable for the reported increases in both fatal and nonfatal cardiovascular events.15,16
Therapy of OSA/OHS through application of positive airway pressure (PAP) treatment prevents episodic airway collapse by maintaining airway patency during sleep and is clearly beneficial in treating OSA/OHS, even if its ability to reverse underlying cardiovascular disease and reduce fatal and nonfatal cardiovascular events remains unclear.17–20 However, PAP therapy decreases systemic blood pressure and surges in blood pressure during sleep.21,22 Indeed, in a multicenter randomized controlled trial, Martinez-Garcia and colleagues demonstrated that 12 weeks of CPAP therapy resulted in significant decreases in 24-hour mean blood pressure (3.1 mmHg, P = .02) and 24-hour diastolic blood pressure (3.2 mmHg, P = .005) in patients with resistant hypertension, and the beneficial effect was significantly associated with the duration of nocturnal CPAP therapy.23
The putative mechanisms underlying the adverse cardiovascular outcomes of OSA have been intensely explored24,25 and among them, evidence of increased sympathetic nervous system outflow and endothelial dysfunction are particularly prominent. In this study, we hypothesized that circulating exosomes, which contain cellular bioactive cargo including microRNAs, are not only biomarkers of end-organ morbidity, but are also important contributors to modifying the cardiovascular phenotype in severe OSA/OHS, by inducing physiological alterations of vascular endothelial cells and by disrupting endothelial function. As a corollary to such hypothesis, we would anticipate that subclinical improvements from PAP therapy can become readily detectable in exosomal biological properties, and therefore be identifiable much earlier than the possible PAP-changes in large vessels, such as those traditionally assessed for monitoring blood pressure.
Exosomes consist of 30–100 nm vesicular structures generated within the endosomal network that begin with inward budding of the cell membrane to form early endosomes, followed by second inward budding of the endosomal membrane to form the various intraluminal vesicles. These endovesicles will then fuse with the cell membrane, and are released to the extracellular space in an exocytotic fashion. Once in the circulation or in any other intercellular space, exosomes can then specifically bind, integrate, and selectively affect target cells.26,27 Thus, exosomal cargo exchanges represent potential and intriguing pathways of intercellular communication between target and recipient cells through delivery of proteins, lipids, RNAs, nontranscribed RNAs, microRNAs (miRNAs), and cell-free DNA, which in concert can modify the phenotype of the target cells.28
Here, we isolated exosomes from severe OSA/OHS patients at diagnosis and following 6 weeks of PAP therapy to evaluate whether plasma exosomes can elicit in vitro functional alterations in naïve endothelial cells, and can therefore be contemplated as not only biomarkers, but also as effectors of anticipated improvements in endothelial function following PAP therapy. Accordingly, the effects of circulating exosomes on endothelial cell monolayer impedance, expression of tight-junction function proteins, endothelial nitric oxide synthase (eNOS), and adhesion molecules, and monocyte attachment to endothelial cells were assessed as reporter assays of the OSA-induced phenotypic changes in endothelial function.
METHODS
Subjects
This was a substudy of a two-center randomized controlled trial evaluating the efficacy of three different modalities of PAP therapy in patients with OHS (clinical trial registration NCT01368614). These PAP modalities included average volume assured pressure support with auto-expiratory positive airway pressure (AVAPS-AE), continuous positive airway pressure (CPAP), and bilevel PAP. The primary study was designed to test the hypothesis that in patients with OHS, the AVAPS-AE mode provides a benefit in daytime gas exchange at 6 weeks that is equivalent or no worse than bilevel PAP and CPAP therapy. Six weeks was chosen as the duration of follow-up, because it typically takes 2 to 4 weeks for daytime hyper-capnia to improve in patients with OHS who are adherent to PAP therapy.4
Our inclusion criteria were age 18 year or older and age 75 years or younger, diagnosis of OHS in the past 3 months but no initiation of PAP therapy, body mass index (BMI) ≥ 30 kg/m2, daytime partial pressure of arterial CO2 (PaCO2) ≥ 45 mmHg, daytime pH > 7.35 from an arterial blood gas, presence of OSA with an apnea-hypopnea index (AHI) ≥ 5 events/h of sleep, and forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) > 70% (ie, no evidence of chronic obstructive pulmonary disease or obstructive airways disease). We excluded acutely ill and unstable patients, a hospitalization for respiratory exacerbation fewer than 6 weeks prior to screening visit, and evidence of alkalosis (pH > 7.45) on the arterial blood gas measurement.
Between November 2011 and February 2014, 51 patients evaluated in the Sleep Disorders Clinic were referred to the University of Chicago research site for suspicion of OHS and 46 patients attended the research screening visit. Two patients declined consent to the substudy whereas the remaining 44 provided written informed consent. Due to lack of hypercapnia on the screening baseline arterial blood gas (PaCO2 < 45 mmHg), 19 subjects were excluded. Two participants were excluded because they were undergoing active PAP therapy, one was excluded due to obstructive airways disease on spirometry (FEV1/FVC < 70%), and one had a BMI of less than 30 kg/m2. An additional four participants did not show up for the first appointment after the initial screening. Thus, the final analytic cohort for the substudy consisted of 17 patients with OHS. Of these 17 participants, pre-treatment and posttreatment blood samples were available in 12 participants for the purposes of the current analysis. Therefore, the final analytic cohort consisted of 12 patients with OSA/ OHS (5 male and 7 female) who underwent two venipunctures 6 weeks apart after being prescribed PAP therapy. The 5 excluded patients without pretreatment and posttreatment blood samples were similar to the 12 patients included in this analysis based on age, sex, race, BMI, and AHI. The main protocol and the substudy were approved by the University of Chicago Institutional Review Board (protocol # 10-702-A-CR004), and all participants provided written informed consent.
Following informed consent, obese adult patients with OSA/OHS underwent diagnostic polysomnography (PSG). Patients were then randomized to receive different modalities of positive airway pressure (PAP) and underwent a full night of PAP titration in the sleep laboratory. All subjects returned for a third in-laboratory PSG 6 weeks after home PAP therapy. The PSG at 6 weeks was performed with the patients using their home PAP devices without any additional titration (see supplemental material for further details). All patients had PAP adherence appraised using remote data device adherence monitoring. Further details regarding PSG procedures, PAP titration, PAP adherence monitoring, questionnaires, and blood pressure measurements are provided in the supplemental material.
Exosome Isolation, Labeling, and Characterization
Exosomes were isolated from plasma using a commercially available kit (Life Technologies, Carlsbad, California, United States). Briefly, plasma was centrifuged at 2000 xg for 20 minutes to remove cell/debris. The supernatants were collected and 0.2 volume of the Total Exosome Isolation Reagent was added. The mixtures were incubated at 4°C for 30 minutes followed by centrifugation at 10,000 xg for 10 minutes, and pel-lets were solubilized in 1x phosphate buffered saline (PBS). Isolated exosomal fractions were then carefully characterized and confirmed using electron microscopy (Figure S1 in the supplemental material) and western blot analysis (Figure S2 in the supplemental material) in a subset of patients, as recommended by the recent Consensus Guidelines.29 Exosome quantification was performed in all subjects both before and after PAP therapy using ExoQuant (Life Technologies, Carlsbad, California, United States). There were no observed differences in exosome quantification before and after therapy (data not shown). Equivalent amounts of plasma exosomes isolated from each subject before and after receiving PAP therapy were then applied to naïve endothelial cells in vitro.
Endothelial Cell Culture
Brain Endothelioma 3 cell lines (always used before passage 4–6) were purchased from Lonza (catalog #CC- 2543; Allendale, New Jersey, United States) and grown in Dulbecco modified Eagle medium (DMEM) supplemented and incubated at 37°C and 5% CO2.
Electric Cell-Substrate Impedance Sensing and Immunofluorescence
To examine the effect of exosomes on endothelial cell mono-layer barrier, endothelial cells were grown to confluence into electric cell-substrate impedance sensing (ECIS) arrays as a single confluent monolayer. As previously described30 exosomes were added in duplicate wells and changes in impedance across the monolayer were continuously monitored in the ECIS instrument (Applied Biophysics Inc., Troy, New York, United States) for up to 24 hours.31,32
For immunofluorescence staining, confluent endothelial cell monolayers were grown on 12 coverslips for 24 hours in DMEM media containing 10% fetal bovine serum (FBS), and then washed with DMEM media containing 2% FBS. Isolated exosomes from subjects were added individually to coverslips for 24 hours. Cells were fixed with 4% (w/v) paraformaldehyde in PBS for 20 minutes at room temperature (RT) and then washed again with PBS. The cell membranes were permeabilized by incubation with 0.25% (v/v) Triton-X-100 in PBS for 10 minutes at RT. After washing with PBS the samples were blocked with 3% (w/v) bovine serum albumin in PBS for 45 minutes at RT to block unspecific binding sites, and followed by overnight incubation at 4°C with ZO-1, 1:400; vascular endothelial (VE)-cadherin, 1:400 (Life Technologies, Grand Island, New York, United States), intercellular adhesion molecule-1 (ICAM-1),1:250; and vascular cellular adhesion molecule-1 (VCAM-1), 1:100 (Santa Cruz Biotechnology, Inc., Dallas, Texas, United States). Alexa 488 or Alexa-594 were used as secondary antibodies (1:400, 2 mg/mL; Life Technologies, Grand Island, New York, United States) and nuclear staining with DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride); 1:1000; Life Technologies, Grand Island, New York, United States) were performed. Appropriate controls and preadsorption experiments were performed to ascertain the specificity of the staining. Images were captured with a Leica SP5 Tandem Scanner Spectral 2-photon confocal microscope (Leica Microsystems, Inc., Buffalo Grove, Illinois, United States) with a 63× oil-immersion lens.
Western Blot Analysis
Lysates of exosomes were separated and isolated exosomes were homogenized in radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCL [pH 7.4], 1 mmol/L EDTA, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% Triton, complete pro-tease inhibitor cocktail tablet [Roche, Basel, Switzerland]). Proteins were separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes using an electrotransfer apparatus (Bio-Rad, Hercules, California, United States). The membranes were blocked with 3% non-fat dry milk (1 hour) and probed with primary polyclonal antibody (CD63 antisera (System Biosciences), which detects a tetraspanin that is a ubiquitous exosome protein (1:1,000) overnight at 4°C. The next day, membranes were washed and incubated with secondary antibody (horseradish peroxidase conjugated, 1:10,000) for 60 minutes at RT, and developed with enhanced chemiluminescence detection system (GE Healthcare, Piscataway, New Jersey, United States). The images were recorded in chemipro-gram of the gel documentation system (Bio-Rad) and band intensity was normalized with respective glyceraldehyde 3-phosphate dehydrogenase intensity calculated through Image Lab densitometry software (Bio-Rad).
miRNA Isolation and Microarray Hybridization
Total RNA including miRNA was extracted from isolated exosomes using miRNeasy Mini Kit-column-based system following the manufacturer's instructions (Qiagen, Turnberry Lane, Valencia, California, United States). Exosomes were isolated as indicated above and the pellet were solubilized in 700 μL of Qiazol Column. The columns were dried for 5 minutes before elution. Total RNA was eluted by adding 14 μL of DNAse-RNase-free water to the membrane of the spin column and incubating for 1 minute before centrifugation at 13,000 xg for 1 minute at RT.31,33 The RNA quality and integrity was determined using the Eukaryote Total RNA Nano 6000 LabChip assay (Agilent Technologies, Santa Clara, California, United States) on the Agilent 2100 Bioanalyzer. The quality of miRNA was determined using Agilent Small RNA Kit according to the manufacturer's protocol. Both total RNA and miRNA samples were quantified on a Nanodrop 2000 (Ambion, Austin, Texas, United States).
Each sample was prepared according to Agilent's miRNA recommended approach using the one-color technique, and profiled on the Agilent human miRNA microarray (Agilent Technologies). Each array consisted of 60-mer DNA probes synthesized in situ that represent 2,006 human miRNA. Total RNA including enriched miRNA was dephosphorylated with calf intestine alkaline phosphatase (GE Healthcare Europe GmbH), denatured with dimethyl sulfoxide, and labeled with pCp-Cy3 using T4 RNA ligase (GE Healthcare Europe GmbH). The labeled RNAs were hybridized to custom human miRNAs microarrays 8x60K (Agilent, Santa Clara, California, United States). Following hybridization and washing, the arrays were scanned with an Agilent microarray scanner using high dynamic-range settings as specified by the manufacturer. Microarray results were extracted using Agilent Feature Extraction software (v12.0) to quantify signal intensities.31,33 The quality control for each miRNA was evaluated based on their Agilent Feature Extraction quality control report. MiRNA microarray data were log-base 2 transformed and quantile normalized. The normalized data were expressed as the difference of log of g processed signal (Agilent Feature Extraction). Undetected probes were excluded from further analysis. Background-subtracted intensities were normalized for detected miRNA probes using the quantile method across all miRNA microarray experiments as described previously.31,33
Quantitative Reverse Transcription Polymerase Chain Reaction Assays
Quantitative polymerase chain reaction analysis was performed for selected messenger RNAs (mRNAs) (ICAM, VCAM, eNOS, and β integrin) using ABI PRISM 7500 System (Applied Biosystems, Foster City, California, United States). For RNA, cDNA was synthesized using 250 ng of total RNA using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, California, United States). Human TATA-box binding protein (TBP gene) was used as a reference gene to normalize the expression ratios. The cycle number (Ct) values were averaged and the difference between the TBP Ct and the gene of interest Ct were calculated to calculate the relative expression of the gene of interest, and the data were normalized to corresponding pretreatment.
Monocyte Attachment Assays
Monocyte attachment to endothelial cells was assessed 24 hours after plasma exosome administration in a subset of patients, as previously described.34 For additional information, please see the supplemental material.
Data Analyses
Results are presented as means ± standard deviation, unless stated otherwise. All numerical data were subjected to statistical analysis using paired t tests or analysis of variance followed by post hoc tests (Tukey) as appropriate. Chi-square analysis was performed on categorical data concerning demographic characteristics of the various groups. Finally, canonical correlation analyses were performed to explore the relationships between sets of variables. Statistical analyses were performed using SPSS version 21.0 (SPPS Inc., Chicago, Illinois, United States). For all comparisons, a two-tailed value of P < .05 was considered to define statistical significance.
miRNA Microarray Analysis
The false discovery rate was controlled at 5% to correct for multiple comparisons. miRNA target predictions for differentially expressed miRNAs were initially computationally predicted using established miRNA target-prediction programs including DIANA-mirPath v3.0 (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index), and miRWalk2.0: a comprehensive atlas of microRNA-target interaction (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/generetsys-self.html). In order to improve the reliability of the miRNA targets, only target genes predicted by at least three of the programs were selected. The predicted genes of individual miRNA were uploaded to the online DAVID program (http://david.abcc.ncifcrf.gov/) for their functional annotation and clustering analysis. The software performs an enrichment analysis of multiple miRNA target genes by comparing each set of miRNA targets to all known KEGG pathways (Kyoto Encyclopedia of Genes and Genomes). The pathways exhibiting a false discovery rate adjusted value of P < .05 were considered significantly enriched between the compared classes.
RESULTS
Subject Characteristics
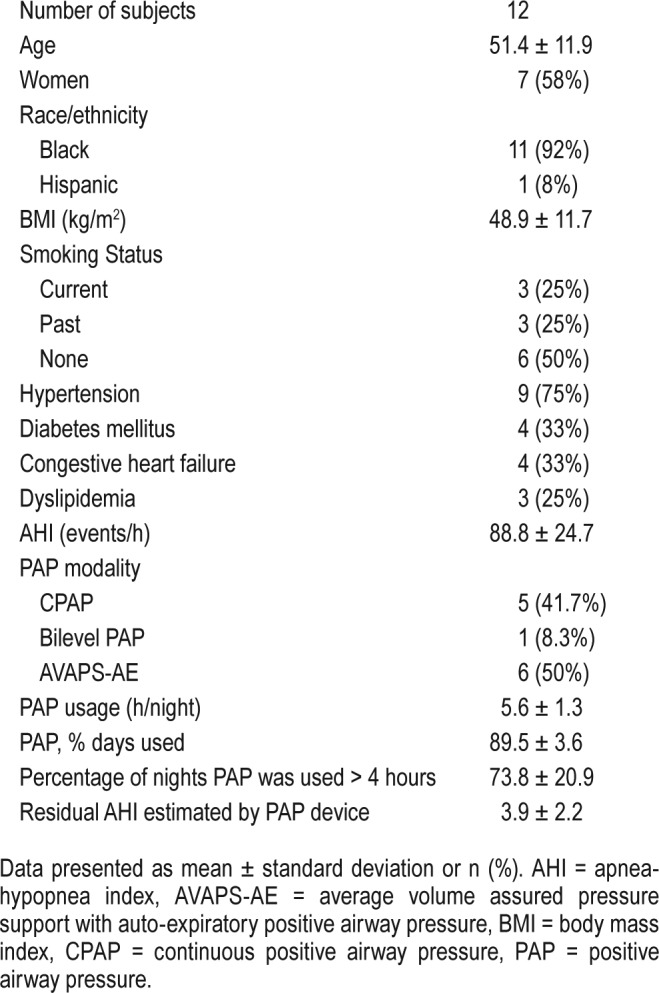
A total of 12 severe OSA/OHS subjects were included in this substudy and all patients returned for follow-up in 6 weeks. Individual and group demographic data are presented in Table 1 and Table S1 in the supplemental material. Women represented 58% of the cohort. Mean age was 51.4 ± 11.9 years and mean BMI was 48.9 ± 11.7 kg/m2. Nearly all subjects were African American (92%). The mean baseline AHI was 88.8 ± 24.7 events/h, which improved significantly after 6 weeks of PAP therapy (residual AHI estimated by PAP devices 3.9 ± 2.2 events/h, P < .001). Mean PAP therapy usage per night was 5.6 ± 1.3 hours, mean percentage of nights of PAP usage was 89.5 ± 3.6%, and mean percentage of nights where PAP was used for more than 4 hours was 73.8 ± 20.9%.
Table 1.
Demographic summary of subjects.
PAP therapy led to significant improvement in daytime sleepiness, quality of life, and daytime hypoventilation. Moreover, significant improvements were observed in polysomno-graphic measures of sleep quality, sleep-disordered breathing, and gas exchange during sleep (Table 2). Despite these clinically significant improvements, there were no significant improvements in systolic and diastolic blood pressure or in metabolic and inflammatory markers after 6 weeks of PAP therapy (Table 2).
Table 2.
Clinical Summary of 12 subjects before and after PAP therapy.
Exosomal Effects on Endothelial Cell Monolayers
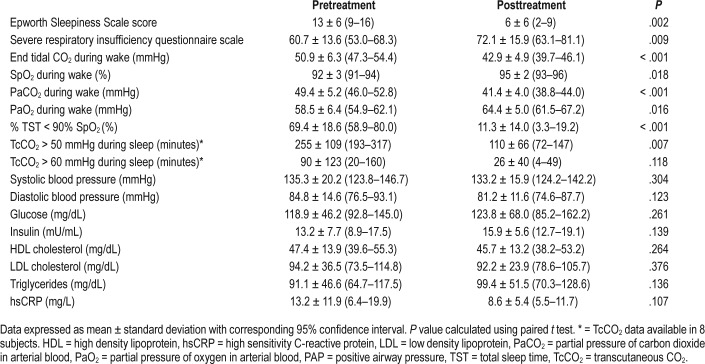
Exosomes from subjects isolated before and after 6 weeks of PAP therapy were added to brain endothelioma 3 (BEnd3) endothelial cells. ECIS normalized resistance values were monitored continuously, and substantial decrements in mono-layer resistance were observed following administration of exosomes isolated from all subjects with severe OSA/OHS compared to control (Figure 1A). Following 6 weeks of PAP therapy, although decrements in monolayer impedance were clearly apparent, their effect was significantly attenuated when compared to pre-PAP (Figure 1A; P < .001) denoting that the degree of endothelial barrier integrity was improved. Figure 1B shows the individual changes in ECIS resistance values illustrating that most but not all patients had measurable improvement, further supporting the large variability in patient responses following 6 weeks of PAP therapy. The overall cumulative improvement in endothelial resistance in all 12 subjects was +10.8% (P = .035; of note, exclusion of one outlier subject, #10 resulted in markedly improved statistical significance: P = .0049).
Figure 1. Exosome-mediated in vitro effects on endothelial cell monolayer resistance and membrane tight junction proteins.
(A) Ensemble-averaged curves of ECIS measured endothelial cell barrier resistance changes over time after administration of exosomes from adult patients with severe OSA/OHS before (Pre-PAP; red line) and after (Post-PAP; green line) positive airway pressure therapy compared to endothelial cells incubated with plasma free media and empty exosomes (control; black line). (The y axis refers to a ratio of ECIS values to baseline ECIS values at start of experiment). (B) Evaluation of ECIS measured endothelial cell barrier resistance changes of all 12 individual patients (pt) comparing the effects on endothelial cells 24 hours after exosome administration. ECIS changes were evaluated before (Pre-PAP) and after 6 weeks positive airway pressure therapy (Post-PAP). The overall cumulative improvement in endothelial cell monolayer resistance for all 12 subjects was +10.8% (P = .035). (C) Effect of plasma exosomes on tight junction and membrane structure in naive endothelial cells. Representative images of at least six separate experiments illustrate exosome-induced changes in expression of VE-cadherin and zonula occludens (ZO)-1 expression patterns. The scale bars for all the representative images are 25 μm. Left figures represent before and right figures represent after PAP therapy. ECIS = electric cell-substrate impedance sensing, OHS = obesity hypoventilation syndrome, OSA = obstructive sleep apnea, PAP = positive airway pressure.
In addition to ECIS assays, immunohistochemistry assessments confirmed that exosomes incubated with BEnd3 endothelial cells induced disruption of cell membrane integrity, as illustrated by the discontinuity of VE-cadherin along the membrane, and the altered topographic distribution of the tight junction protein ZO-1 (Figure 1C). When exosomes isolated from the same patients after PAP therapy were added to BEnd3 endothelial cells, there was a marked increase in circumferential membrane restricted staining of VE-cadherin and ZO-1, implying improved endothelial barrier integrity, thereby corroborating the improvements in membrane barrier resistance observed in the ECIS assays. Control subjects not shown as patients were compared before and after PAP therapy.
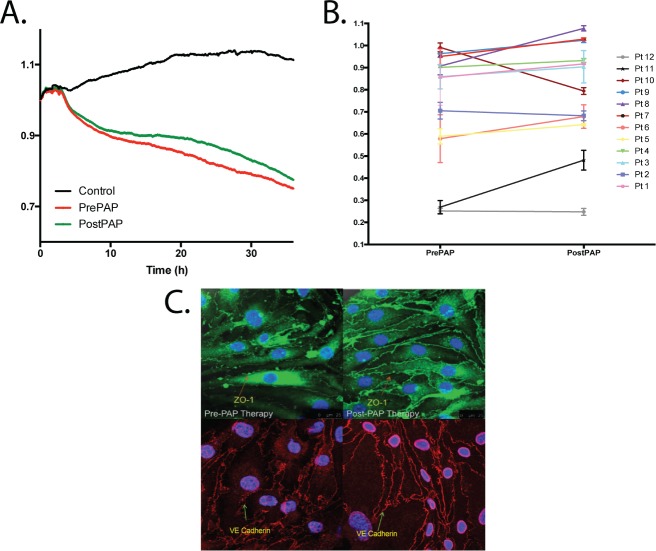
Exosomal Effects on Endothelial Cellular Adhesion
The distribution and abundance of the adhesion molecules ICAM-1 and VCAM-1 was examined using immunohistochemical approaches (Figure 2A). Quantification using immunohistochemistry imaging was not performed, rather, expression of adhesion molecules was evaluated. Exosomes isolated from patients prior to PAP therapy resulted in marked increases in the expression of both adhesion molecules throughout the surface of naïve BEnd3 cells. However, attenuated immunofluorescence reactivity emerged for both ICAM-1 and VCAM-1 when exosomes isolated from the same patients but following PAP therapy were applied.
Figure 2. Exosome-mediated effects on endothelial cell expression of adhesion molecules, monocyte attachment of endothelial cells and changes in mRNA expression.
(A) Effect of plasma exosomes on adhesion molecule expression in naive endothelial cells. Representative images of at least six separate experiments show exosome-induced changes in expression of vascular cellular adhesion molecule (VCAM) and intercellular-adhesion molecule 1 (ICAM) with visually apparent reduction in post-PAP. (B) Effect of plasma exosomes on attachment of red fluorescent protein tagged monocytes. Representative images are shown for at least six separate experiments in different subjects. The scale bars for all the representative images are 25 μm. Left figures represent before and right figures represent after PAP therapy. (C) Quantification of monocyte attachment was performed by measuring immunofluorescence intensity of endothelial cells inoculated with exosomes from six subjects before (Pre PAP) and after 6 weeks of positive airway pressure therapy (Post PAP), and compared to plasma free media with empty exosomes (control). The net reduction in monocyte attachment from to Pre PAP to Post PAP was statistically significant (P = .0423). (D) Changes in mRNA fold changes of ICAM-1, VCAM-1, β-integrin, and eNOS expression in endothelial cells treated with either pre-PAP or post-PAP exosomes. PAP therapy was associated with significant reductions in the expression of both ICAM-1 and VCAM-1, along with significant increases in eNOS mRNA expression (P < .05 for all comparisons). eNOS = endothelial nitric oxide synthase, PAP = positive airway pressure.
As a proof of concept that the enhanced expression of the adhesion molecules is indicative of increased propensity for intercellular attachment, we next evaluated the degree of monocyte attachment using red fluorescent protein (RFP) tagged bone marrow monocytes to BEnd3 endothelial cells previously treated with exosomes from four subjects before and after 6 weeks of PAP therapy (Figure 2B). Monocyte attachment was markedly increased compared to vehicle controls in untreated subjects, and a significant reduction in monocyte attachment emerged in the same subjects following 6 weeks of PAP therapy (Figure 2C; P = .0423).
Further, ICAM-1, VCAM-1, and β-integrin mRNA expression were all markedly upregulated in naïve endothelial cells treated with exosomes from subjects at baseline, and reductions in expression occurred after PAP therapy for both ICAM-1 and VCAM-1 but not for β-integrin (Figure 2D). In addition, because endothelial function is highly dependent on eNOS, we also evaluated eNOS mRNA expression and observed an increase in eNOS mRNA expression in BEnd3 cells after PAP when compared to pre-PAP (Figure 2D; P < .05).
miRNA Array Analyses
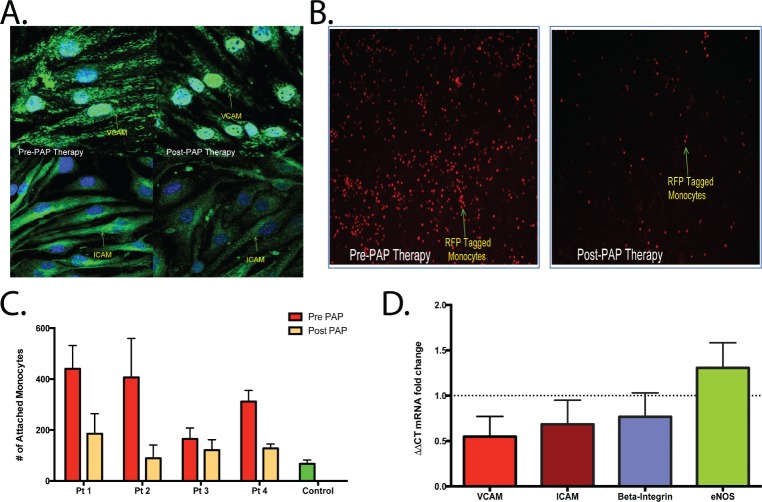
miRNA array analyses of exosomal cargo using techniques as previously described30 in eight pretreated and post-PAP subjects were performed to identify uniquely differentially expressed miRNAs, and revealed four differentially expressed miRNAs (Figure 3), which were validated using quantitative reverse transcription polymerase chain reaction. Further exploration of the potential gene pathways associated with these miRNAs was conducted in silico and are shown in Figure 3. The functional significance of these four differentially expressed miRNAs is not currently well established given their novelty and will need to be addressed with further studies.
Figure 3. Differentially expressed miRNAs in exosomal cargo in subjects pretreatment versus posttreatment (n = 8).
DISCUSSION
In the current study, we provide evidence that in patients with severe OSA/OHS, 6 weeks of PAP therapy leads to marked improvements in circulating exosomal cargo effects on the endothelium. This improvement was present despite the lack of detectable improvement in routinely measured clinical and laboratory surrogates of cardiovascular risk such as blood pressure, hs-CRP or dyslipidemia. Furthermore, we show plasma exosomes from adult subjects with severe OSA/OHS induce endothelial dysfunction as reflected by several reporter assays in naïve cells in vitro, and that such properties are attenuated after 6 weeks of PAP therapy.
The assumption that both obesity and severe OSA/OHS, known risk factors for cardiovascular disease, act initially to disturb endothelial homeostasis is based on an extensive body of literature.33,35–39 From this first site of vascular injury, multiple pathways operate to propagate and promote the appearance of specific cardiovascular morbidities such as hyper-tension or ischemic heart disease. Conversely, the likelihood of documenting any improvements in established cardiovascular disease is low even if adherence to PAP is optimal, as recently illustrated by experiments in a murine model of chronic OSA,40 and certainly even less likely when PAP adherence is poor.20 However, it is likely that removal of the injurious processes elicited by the conjoint effects of OSA and obesity will begin with changes in cellular function, and that such changes are then reflected by alterations in circulating exosomal cargo properties. Based on such assumptions, we examined subjects with severe OSA/OHS who were reevaluated following just 6 weeks of PAP therapy. Accordingly, we did not observe, nor did we expect to detect any improvements in systemic blood pressure or metabolic function given that these types of improvements are likely to emerge after more extended periods of therapy.23,41,42 Our study did not include measurements of brachial artery flow mediated dilatation, which would have been useful to assess changes in large-vessel endothelial function.
Notwithstanding, PAP therapy for such short duration led to reduced deleterious effects of circulating exosomes on endothelial function, as illustrated first by an in vitro assay that specifically evaluates endothelial barrier integrity. The observed variability in our findings measuring endothelial barrier integrity may be related to variability among patients, or variability related to the ECIS assay. Although the clinical significance of a small albeit significant improvement in endothelial monolayer impedance is not well defined, the premise of increased endothelial barrier integrity would imply improved endothelial function. These findings of improved endothelial function were further corroborated using in vitro assays evaluating endothelial cell-monocyte adhesion, as well as endothelial cell eNOS mRNA expression. Thus, whether we consider exosomal properties as biomarkers of improvement or as effectors of end-organ morbidity, it becomes apparent that even after short therapeutic interventions, such as 6 weeks of PAP, there are significant and detectable improvements. We therefore surmise that the reversibility of OSA-induced end-organ morbidity is the one that needs to be questioned, rather than the reversibility of the mechanisms that ultimately induce that end-organ morbidity, because such mechanisms emerge as rapidly responsive to treatment. We acknowledge that our findings diverge from the recently published SAVE trial,20 a large randomized control study in which CPAP therapy was not found to elicit a cardiovascular benefit. However, it is important to emphasize that our cohort was more adherent to PAP therapy; our study examined changes in vitro to naive nondiseased endothelial cells. A lack of cardiovascular improvement by CPAP therapy observed in the SAVE trial is possibly related to the premise that in many adults with OSA, the deleterious consequences of OSA to diseased blood vessels are longstanding and thereby irreversible which cannot be corrected with PAP therapy. Nonetheless, evidence suggests that CPAP therapy, even in patients with advanced cardiovascular disease, results in a statistically significant albeit small improvement in both systolic and diastolic blood pressure.43,44
Exosomes have been shown to transfer several bioactive elements in their cargo to target cells including miRNAs, thus participating in miRNA-based signaling,45,46 and dysregulation of miRNA content in the exosomes can in turn result in altered target cell function, and ultimately promote the development of a variety of diseases.47,48 Furthermore, it appears that exosomes modify the phenotype of target cells chiefly through their intravesicular miRNA content.49–51 In a previous study, we demonstrated that clinically identified endothelial dysfunction in a large set of children with either OSA or obesity was accompanied by parallel changes in the properties of circulating exosomes, when the latter was evaluated using some of the reporter assays used herein.30 Furthermore, we identified a candidate exosomal miRNA, namely miRNA-630, as being mechanistically involved in mediating endothelial function, as illustrated by the use of selective agomirs and antagomirs in some of the experiments. Further support for the biomarker capabilities of plasma miRNAs as indicators of cardiovascular susceptibility to OSA in children has also been reported.28 However, we should also remark that even if we assume that the endothelium is as vulnerable in children as it is in adults, the duration of OSA and obesity is much shorter, and as such the proportion of children manifesting cardiovascular morbidity is smaller, the duration and magnitude of the morbidity are likely reduced, and the reversibility of such morbidity with treatment is much more likely to be increased in children. Further, in our smaller cohort of obese adults, who are phenotypically quite different from young children, especially given the influence of numerous confounding factors and comorbidities, we observed a different profile of exosomal miRNA cargo. The observed differences in exosomal miRNA content following PAP therapy, while novel, needs further investigation to elucidate the functional implications and clinical significance of these changes.
Based on the aforementioned considerations, the fact that improvements in exosomal properties on endothelial targets are detectable in adult patients with severe obesity and severe OSA/OHS following just 6 weeks of therapy is notable by itself. However, even though no changes occurred in cardiovascular or metabolic function, current findings suggest that the subclinical improvements reflect reduced burden of disease even very early following initiation of PAP therapy, and further introduce the conceptual framework whereby plasma exosomes are central to the pathophysiology of cardiovascular morbidity in obesity and OSA/OHS, and that improvements in cardiovascular status are likely to become manifest as recovery of endothelial function early in the course of treatment. Unfortunately, due to the limited amounts of plasma available from the research subjects, we cannot infer as to the specific cellular sources of the exosomes that contributed to the in vitro endothelial functional abnormalities.
As with any study, we should acknowledge some of its limitations. First, the small sample size of only obese patients with OSA/OHS will undoubtedly require additional studies in expanded cohorts to enable translation of potential exosomal molecular miRNA targets into clinical practice. Second, not all patients had improvements with PAP, opening the opportunity to investigate exosomal cargo determinants of therapeutic responsiveness, important a priori knowledge that could facilitate precision therapy for OSA43,52,53 and OHS. Indeed, we have previously documented that circulating miRNAs may enable accurate prediction of favorable response to PAP in patients with resistant hypertension and OSA.54 Thus, future studies should explore whether the miRNA content of exosomes can reliably differentiate PAP responders from nonresponders. It is also unclear at this stage whether targeted exosomal miRNA therapy using agomirs/antagomirs may potentiate the recovery of endothelial function and improve the reversibility of the cardiovascular morbidity that might be already established at diagnosis. Finally, the restricted quantities of plasma that were available from the participants were constrained by the bio-ethical committee, and precluded exploration of differences in miRNA exosomal cargo in a cell-source specific manner. Our study restricted therapy to only 6 weeks of therapy, and therefore longitudinal assessments over more extended periods of PAP therapy are needed to assess the temporal trajectories of changes in exosomes and in cardiovascular function.
CONCLUSIONS
In summary, naive endothelial responses to circulating plasma exosomes reveal substantial deleterious alterations in patients with severe OSA/OHS that are collectively improved following short-term PAP therapy. These findings illustrate the presence of a dichotomous set of responses whereby early improvements in exosomal cargo effects on endothelium are present in the absence of concurrent amelioration in cardiovascular clinical metrics. Furthermore, we surmise that assessments of exosomal functional properties and cargo may potentially define cardiovascular responses to PAP therapy and shed insight into discriminating PAP therapy “responders” from “nonresponders,” thereby enabling precision approaches if such assumptions are confirmed in future studies.
DISCLOSURE STATEMENT
LKG and DG were supported by National Institutes of Health grant R01HL130984. RB is supported by Scientist Development Grant from the American Heart Association (3SDG14780079). BM is supported by National Institutes of Health grant R01HL119161. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: conception and design: RB, AK, LKG, DG; analysis and interpretation: RB, AK, AAK, IA, EP, BM, AM, DG; drafting the manuscript for important intellectual content: RB, LKG, BM, AM, DG.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- bEnd3
brain endothelialioma 3
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DAPI
4',6-Diamidino-2-Phenylindole, Dihydrochloride
- DMEM
Dulbecco modified Eagle medium
- DNA
deoxyribonucleic acid
- ECIS
electric cell-substrate impedance sensing
- EDTA
ethylenediaminetetraacetic acid
- eNOS
endothelial nitric oxide synthase
- FBS
fetal bovine serum
- ICAM-1
intercellular adhesion molecule-1
- miRNA
micro ribonucleic acid
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PaCO2
pressure of arterial carbon dioxide
- PaO2
pressure of arterial oxygen
- PAP
positive airway pressure
- PBS
phosphate buffered saline
- PSG
polysomnography
- RNA
ribonucleic acid
- RT
room temperature
- SD
standard deviation
- SpO2
oxyhemoglobin saturation
- TBP
TATA-box binding protein
- TcCO2
transcutaneous carbon dioxide
- TST
total sleep time
- VCAM-1
vascular cellular adhesion molecule-1
- VE-cadherein
vascular endothelial cadherin
- ZO-1
zonula occludens-1
REFERENCES
- 1.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 4.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55(10):1347–1362. discussion 1363-1365. [PubMed] [Google Scholar]
- 5.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5(2):218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47(4):1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(6):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 9.Castro-Anon O, Perez de Llano LA, De la Fuente Sanchez S, et al. Obesityhypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One. 2015;10(2):e0117808. doi: 10.1371/journal.pone.0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 11.Peled N, Abinader EG, Pillar G, Sharif D, Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol. 1999;34(6):1744–1749. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 12.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 14.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 16.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 17.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 18.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Somers VK. Obstructive sleep apnoea, metabolic syndrome, and cardiovascular outcomes. Eur Heart J. 2004;25(9):709–711. doi: 10.1016/j.ehj.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 20.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 21.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter JR, Fonkoue IT, Grimaldi D, et al. Positive airway pressure improves nocturnal beat-to-beat blood pressure surges in obesity hypoventilation syndrome with obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R602–R611. doi: 10.1152/ajpregu.00516.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Rai AJ, DeCastro GJ, et al. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods. 2015;87:26–30. doi: 10.1016/j.ymeth.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014;12:162. doi: 10.1186/1479-5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, et al. Circulating plasma extracellular microvesicle microrna cargo and endothelial dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(9):1116–1126. doi: 10.1164/rccm.201602-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keese CR, Bhawe K, Wegener J, Giaever I. Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques. 2002;33(4):842–824. 846, 848–850. doi: 10.2144/02334rr01. [DOI] [PubMed] [Google Scholar]
- 32.Rahim S, Uren A. A real-time electrical impedance based technique to measure invasion of endothelial cell monolayer by cancer cells. J Vis Exp. 2011;(50) doi: 10.3791/2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brant LC, Wang N, Ojeda FM, et al. Relations of metabolically healthy and unhealthy obesity to digital vascular function in three community-based cohorts: a meta-analysis. J Am Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalyfa A, Zhang C, Khalyfa AA, et al. Effect on intermittent hypoxia on plasma exosomal micro RNA signature and endothelial function in healthy adults. Sleep. 2016;39(12):2077–2090. doi: 10.5665/sleep.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selthofer-Relatic K, Bosnjak I, Kibel A. Obesity related coronary microvascular dysfunction: from basic to clinical practice. Cardiol Res Pract. 2016;2016:8173816. doi: 10.1155/2016/8173816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virdis A. Endothelial dysfunction in obesity: role of inflammation. High Blood Press Cardiovasc Prev. 2016;23(2):83–85. doi: 10.1007/s40292-016-0133-8. [DOI] [PubMed] [Google Scholar]
- 37.Bruyndonckx L, Hoymans VY, Lemmens K, Ramet J, Vrints CJ. Childhood obesity-related endothelial dysfunction: an update on pathophysiological mechanisms and diagnostic advancements. Pediatr Res. 2016;79(6):831–837. doi: 10.1038/pr.2016.22. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Yu W, Gao M, et al. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyos CM, Melehan KL, Liu PY, Grunstein RR, Phillips CL. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med Rev. 2015;20:15–26. doi: 10.1016/j.smrv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Cortese R, Gileles-Hillel A, Khalyfa A, et al. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: potential role of CD36. Sci Rep. 2017;7:43648. doi: 10.1038/srep43648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35(1 Pt 1):144–147. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 42.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21(2):241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 43.Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145(4):762–771. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 44.Castro-Grattoni AL, Torres G, Martinez-Alonso M, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00651-2017. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301(5631):336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 47.Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18(3):215–222. doi: 10.1097/PPO.0b013e318250c001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diehl P, Fricke A, Sander L, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93(4):633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114(2):345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 51.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82(4):412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 52.Kolanis S, Pilavakis M, Sofogianni A, Tziomalos K. Is there a role for continuous positive airway pressure treatment in the management of obstructive sleep apnea-related hypertension? Curr Hypertens Rev. 2017;13(2):89–92. doi: 10.2174/1573402113666170612094750. [DOI] [PubMed] [Google Scholar]
- 53.Bratton DJ, Stradling JR, Barbe F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69(12):1128–1135. doi: 10.1136/thoraxjnl-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66(9):1023–1032. doi: 10.1016/j.jacc.2015.06.1315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.