Abstract
Background
Intra-tumoral heterogeneity has been recently addressed in different types of cancer, including breast cancer. A concept describing the origin of intra-tumoral heterogeneity is the cancer stem-cell hypothesis, proposing the existence of cancer stem cells that can self-renew limitlessly and therefore lead to tumor progression. Clonal evolution in accumulated single cell genomic alterations is a further possible explanation in carcinogenesis. In this study, we addressed the question whether intra-tumoral heterogeneity can be reliably detected in tissue-micro-arrays in breast cancer by comparing expression levels of conventional predictive/prognostic tumor markers, tumor progression markers and stem cell markers between central and peripheral tumor areas.
Methods
We analyzed immunohistochemical expression and/or gene amplification status of conventional prognostic tumor markers (ER, PR, HER2, CK5/6), tumor progression markers (PTEN, PIK3CA, p53, Ki-67) and stem cell markers (mTOR, SOX2, SOX9, SOX10, SLUG, CD44, CD24, TWIST) in 372 tissue-micro-array samples from 72 breast cancer patients. Expression levels were compared between central and peripheral tumor tissue areas and were correlated to histopathological grading. 15 selected cases additionally underwent RNA sequencing for transcriptome analysis.
Results
No significant difference in any of the analyzed between central and peripheral tumor areas was seen with any of the analyzed methods/or results that showed difference. Except mTOR, PIK3CA and SOX9 (nuclear) protein expression, all markers correlated significantly (p < 0.05) with histopathological grading both in central and peripheral areas.
Conclusion
Our results suggest that intra-tumoral heterogeneity of stem-cell and tumor-progression markers cannot be reliably addressed in tissue-micro-array samples in breast cancer. However, most markers correlated strongly with histopathological grading confirming prognostic information as expression profiles were independent on the site of the biopsy was taken.
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1495-6) contains supplementary material, which is available to authorized users.
Keywords: Intratumoral heterogeneity, Breast cancer, Stem cells, Tumor progression, Tissue micro array
Background
Different types of cancer, including breast cancer, were found to present intra-tumoral heterogeneity, which can occur as genetic, phenotypic or functional diversity in spatial or temporal patterns [1]. The two most commonly accepted concepts describing the origin of tumor heterogeneity are the cancer stem cell (CSC) hypothesis and the Darwinian clonal evolution model [2, 3]. Both thesis consider gain of proliferative potential through single cells that acquired multiple molecular alterations. The Darwinian clonal evolution model proposes natural selection through varying degrees of genetic instability, leading to multiple subpopulations with different genetic aberrations. On the other hand, the cancer stem cell hypothesis posits the existence of a small population of tumorigenic cells within a tumor, that can self-renew limitlessly and therefore induces tumor growth, disease progression, propensity for metastasis and the generation of heterogeneity [3–5]. Moreover, cancer stem cells lead to therapy resistance [5–7]. In 2003, Al-Hajj et al. first described the presence of cancer stem cells in human breast cancer [6, 8].
When using core needle biopsy or fine-needle aspirations for tumor diagnostics only a small amount of tissue is examined. Therapeutic decisions are based on those single tumor samples. Accordingly, the findings of regional intra-tumoral heterogeneity of biomarkers query the significance of single biopsy interpretations with implications for accurate tumor classification [9–11]. Huebschman et al. previously examined adjacent cells in triple negative breast cancer at which a significant molecular heterogeneity, including as well markers of the “stem cell” type, was found [10]. Allott et al. revealed spatial heterogeneity to be a source of core-to-core discordance in status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) on tissue micro arrays (TMAs) when analyzed using automated digital image. They therefore suggested intra-tumoral heterogeneity to have possible implications for breast cancer classifications [11]. To optimize therapy, molecular biomarkers are required, that exactly predict clinical disease outcomes and can be targets of molecular therapies [12].
In our study, we addressed the question whether intra-tumoral heterogeneity of breast stem cell and tumor progression markers can be reliably detected in TMA based tumor samples by comparing protein and gene expression levels between central and peripheral tumor parts. For this purpose, a special TMA was constructed containing peripheral and central primary breast cancer and as well as available also metastatic samples. Furthermore, we proofed central and peripheral areas whether they bear the same prognostic information regarding stem cell and tumor progression markers on the base of histopathological grading.
Methods
Patient cohort
In this study, a special tissue micro array (TMA) was constructed using 372 formalin-fixed, paraffin embedded (FFPE) tissue samples from 72 breast cancer patients. The cohort contained 372 samples from the Institute of Pathology and Molecular Pathology, University Hospital of Zurich, collected between 1998 and 2011. Samples for the TMA were punched from central and peripheral primary tumor tissue areas in all n = 72 cases. The distance between central and peripheral areas was on average 1 cm. Samples from axillary lymph node or skin metastases were available in n = 11 cases. Clinico-pathological data such as histological grading, subtype and tumor/nodal stage and information about patient follow-up could be retrieved from the database of the Institute of Pathology and Molecular Pathology, University Hospital of Zurich. Additional data on patient survival was provided by the Breast Center Seefeld, Zurich.
Clinico-pathological parameters
In total, 57 of 72 (79%) cases were invasive ductal carcinomas (NST, non-special type) (G1: n = 16, G2: n = 16, G3: n = 25), n = 9 (13%) cases were invasive lobular carcinomas (G1: n = 0, G2: n = 8, G3: n = 1) and n = 5 (7%) tumors were classified as other histological subtypes. Information on histological subtype was missing in n = 1 (1%) case.
Altogether, pT stage was available in 67 of 72 cases (93%). The information was missing for n = 5 (7%) patients. N = 31 (43%) tumors were staged pT1 (G1: n = 12, G2: n = 8, G3: n = 11), n = 26 (36%) tumors were staged pT2 (G1: n = 6, G2: n = 10, G3: n = 10), n = 9 (13%) tumors were staged pT3 (G1: n = 0, G2: n = 5, G3: n = 4) and n = 1 (1%) tumor was classified pT4 (G1: n = 0, G2: n = 0, G3: n = 1).
Nodal stage was available in n = 63 cases. The information was missing in n = 9 (13%) patients. N = 28 (39%) cases were nodal negative (pN0, G1: n = 11, G2: n = 8, G3: n = 9). Of the nodal positive patients, n = 26 (36%) tumors were classified as pN1 (G1: n = 7, G2: n = 7, G3: n = 12), n = 5 (7%) tumors were classified as pN2 (G1: n = 1, G2: n = 3, G3: n = 1) and n = 4 (6%) tumors were staged as pN3 (G1: n = 0, G2: n = 2, G3: n = 3).
Clinico-pathological parameters are summarized in Table 1.
Table 1.
Clinico-pathological parameters
| n = 72s | Histological grading | |||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | Total | |||||
| n | % | n | % | n | % | n | % | |
| Type of tissue | ||||||||
| Tumor center | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| Tumor periphery | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| Normal tissue | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| Lymph node metastasis | 1 | 5 | 6 | 23 | 4 | 15 | 11 | 15 |
| Skin metastasis | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 1 |
| Total | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| Histological subtype | ||||||||
| Invasive ductal | 16 | 84 | 16 | 62 | 25 | 93 | 57 | 79 |
| Invasive lobular | 0 | 0 | 8 | 31 | 1 | 4 | 9 | 13 |
| Other | 3 | 16 | 2 | 8 | 0 | 0 | 5 | 7 |
| Unknown | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 1 |
| Total | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| pT stage | ||||||||
| pT1 | 12 | 63 | 8 | 31 | 11 | 41 | 31 | 43 |
| pT2 | 6 | 32 | 10 | 38 | 10 | 37 | 26 | 36 |
| pT3 | 0 | 0 | 5 | 19 | 4 | 15 | 9 | 13 |
| pT4 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 1 |
| Unknown | 1 | 5 | 3 | 12 | 1 | 4 | 5 | 7 |
| Total | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
| pN stage | ||||||||
| pN0 | 11 | 58 | 8 | 31 | 9 | 33 | 28 | 39 |
| pN1 | 7 | 37 | 7 | 27 | 12 | 44 | 26 | 36 |
| pN2 | 1 | 5 | 3 | 12 | 1 | 4 | 5 | 7 |
| pN3 | 0 | 0 | 2 | 8 | 2 | 7 | 4 | 6 |
| Unknown | 0 | 0 | 6 | 23 | 3 | 11 | 9 | 13 |
| Total | 19 | 100 | 26 | 100 | 27 | 100 | 72 | 100 |
Tissue micro array (TMA)
Formalin-fixed, paraffin embedded (FFPE) tissue samples from 72 breast cancer patients were arranged into one tissue micro array (TMA 208) using a method of construction previously described [13, 14]. From each patient (n = 72) two tissue cores of central tumor parts, as well as two tissue cores of peripheral tumor parts and at least one core of normal tissue were punched and added to the TMA. Areas to be selected for the TMA were determined on hematoxylin and eosin (HE) stained large tissue sections from routine diagnostic slides. Additionally, in 15% of cases (n = 11) two cores from lymph node metastasis and in 1% of cases (n = 1) two cores of skin metastasis were added to the TMA. Selection of tumor areas were processed for the TMA in the same way as with the primary tumors.
Tissue cores were grouped per patient and according to the histopathological grading [15]. Of 72 cases 19 were well differentiated (G1), n = 26 were moderately differentiated (G2) and n = 27 were poorly differentiated (G3).
Immunohistochemistry
All immunohistochemical stains were performed in the Immunohistochemistry Laboratory of the Institute of Pathology and Molecular Pathology, University Hospital Zurich, with following clones and pretreatment modalities using the automated Ventana Benchmark stain system (Additional file 1: Table S1).
Scoring of immunohistochemical stains
The expressions of E-cadherin, p63, CK5/6, CD24, mTOR and PIK3CA were scored with a two-tiered scoring system (negative vs. positive) being membranous and/or nuclear positive.
The expressions of SLUG, SOX2, SOX9, SOX10, PTEN, TWIST, p53 and CD44 were scored with a three-tiered scoring system (negative, ≤ 50% of the cells positive, > 50% of the cells positive), being cytoplasmic and/or nuclear positive.
The membranous expressions of HER2 and EGFR were scored with a four-tiered scoring system (negative, ≤ 10% positive, > 10% but incomplete positive, strong circular positivity in > 10% of the cells), according to current diagnostic guidelines as score 0, 1+, 2+, 3+ [16].
The nuclear expressions of ER, PR and Ki-67 were scored with a scoring system representing the estimated proportion of positive tumor cells (negative, 10% positive, 20% positive, 30% positive, 40% positive, 50% positive, 60% positive, 70% positive, 80% positive, 90% positive, 100% positive).
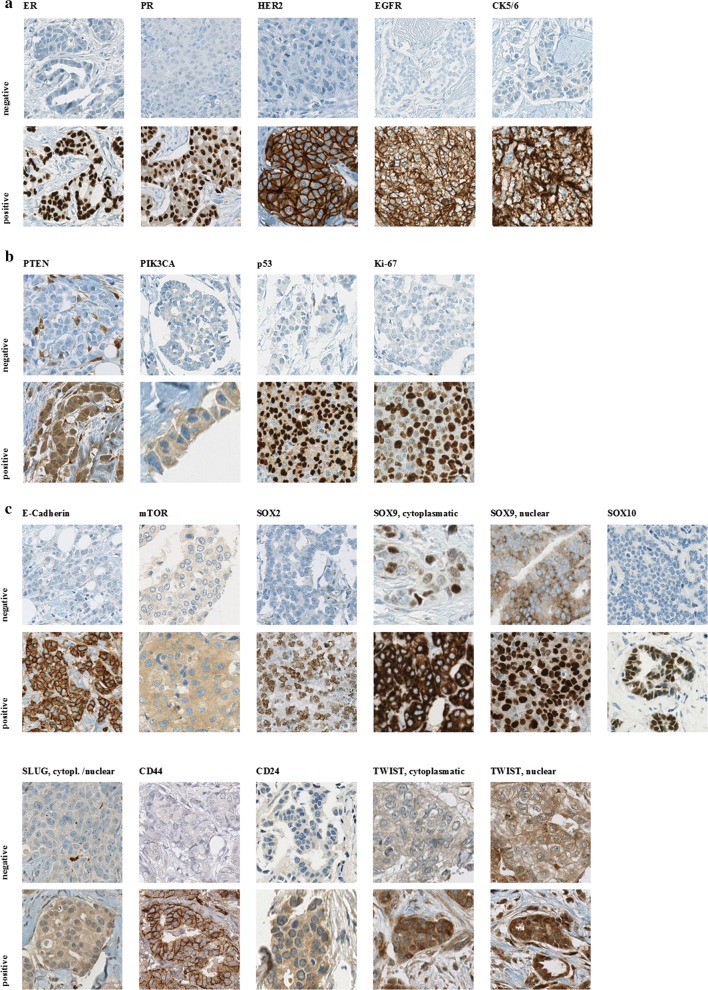
Representative stains from each stain are shown in Fig. 1.
Fig. 1.
Representative immunohistochemical stains according to biological features. a Conventional predictive/prognostic markers, b tumor progression markers, c stem cell markers
Fluorescent in situ hybridization (FISH) reaction
HER2
The HER2 gene was tested by using a dual fluorescence kit (PathoVysion, Vysis, Abbott AG, Diagnostic Division Baar, Switzerland) containing the HER2 gene (17q11.2-q12, directly labeled with fluorescent spectrum orange) and CEP17 (17p11.1-q11.1, labeled with fluorescent spectrum green). The whole procedure was carried out using the PathoVysion probes on the fully automated Leica Bond autostainer (Leica Biosystems, Nunningen, Switzerland).
PTEN
The PTEN gene was visualized by the Vysis PTEN probe (Vysis, Abbott AG, Diagnostic Division Baar, Switzerland). The kit contained the PTEN gene (10q23) labelled with orange and the CEP10 probe (10p11.1-q11.1) labeled with green. The procedure was carried out using the Leica Bond autostainer according to the manufacturer’s instructions.
PIK3CA
The Vysis PIK3CA dual probe (labelled as green, locus 3q26.32) together with the CEP3 probe (labelled as orange, locus 3p11.1-q11.1) were used (Vysis, Abbott AG, Diagnostic Division Baar, Switzerland). The procedure was done on the Leica Bond autostainer.
Scoring of fluorescent in situ hybridization (FISH) reactions
HER2
The 2013 ASCO-CAP guidelines were used for interpreting the signals in the FISH tests [16]. The number of signal copies and the ratios (HER2/CEP17) were calculated for each probe. Gene copies (> 6 in at least 10% of the tumor surface) or cluster formations (small clusters ~ 6 copies, larger clusters ~ 12 copies) were defined as amplified. Similarly, a ratio > 2 was defined as amplified (positive), a ratio < 2 was set as negative (Fig. 2).
Fig. 2.
Representative areas of FISH reactions with negative and amplified samples: HER2, PTEN, PIK3CA
PTEN, PIK3CA
Scoring was done in an analogous way as in HER2 FISH assays described above.
Illustrative areas of FISH reactions are shown in Fig. 2.
Transcriptome (mRNA) analysis
15 cases, five from each histological grading (5 cases G1, 5 cases G2, 5 cases G3) were selected for transcriptome analysis using next generation sequencing. The same central and peripheral areas of the paraffin blocks that were used for TMA construction were punched for reception of material for RNA extraction. HE slide controls were used for the area selection for the mRNA tissue preparation.
Paraffin blocks were punched both in the center and in the tumor periphery. RNA was isolated from these punches using the Maxwell FFPE RNA isolation kit (Promega) according to the manufacturer’s manual in the Institute of Pathology and Molecular Pathology University Hospital Zürich. The RNA amount was quantified by Qubit measurement. The fragment size of the RNA was determined by the DV200 analysis using the bioanalyzer. Transcriptome analysis was performed at the Functional Genomics Center Zurich, Switzerland.
The SMARTer Stranded Total RNA-Seq Kit-Pico Input Mammalian (Clontech Laboratories, Inc., A Takara Bio Company, California, USA) was used in the preparation of libraries for sequencing. No fragmentation was performed. Total RNA samples (10 ng) were reverse-transcribed using random priming into double-stranded cDNA in the presence of a template switch oligo (TSO). When the reverse transcriptase reaches the 5′ end of the RNA fragment, the enzyme’s terminal transferase activity adds non-templated nucleotides to the 3′ end of the cDNA. The TSO pairs with the added non-templated nucleotide, enabling the reverse transcriptase to continue replicating to the end of the oligonucleotide. This resulted in a cDNA fragment that contains sequences derived from the random priming oligo and the TSO. PCR amplification using primers binding to these sequences was performed. The PCR added full-length Illumina adapters, including the index for multiplexing. Ribosomal cDNA was cleaved by ZapR in the presence of the mammalian-specific R-Probes. Remaining fragments were enriched with a second round of PCR amplification using primers designed to match Illumina adapters.
The quality and quantity of the enriched libraries were validated using the Tapestation (Agilent, Waldbronn, Germany). The product is a smear with an average fragment size of approximately 360 bp. The libraries were normalized to 10 nM in Tris–Cl 10 mM, pH8.5 with 0.1% Tween 20. The libraries were sequenced single end 125 bp using the Illumina HiSeq 4000 (Illumina, Inc, California, USA).
The RNA-seq data analysis consisted of the following steps:
Read-alignment was done with STAR (version 2.5.3a). As reference, we used the Ensembl genome build GRCh38.p10. With the gene annotations of Ensembl release 91.
The STAR alignment options were “–outFilterType BySJout–outFilterMatchNmin 30–outFilterMismatchNmax 10–outFilterMismatchNoverLmax 0.05–alignSJDBoverhangMin 1–alignSJoverhangMin 8–alignIntronMax 1,000,000–alignMatesGapMax 1,000,000–outFilterMultimapNmax 50”.
Gene expression values were computed with the function featureCounts from the R package Rsubread.
Differential expression was computed using the generalized linear model implemented in the Bioconductor package EdgeR. In the statistical model, we considered as factors the tumor area and the patient [17–19].
Statistical analysis
IBM SPSS Statistics 23 was used for statistical analysis of the correlations of intratumoral heterogeneity and grading to stem cell characteristics and tumor progression markers. Frequency distribution was showed using crosstabs as a multivariate statistical method. Chi square and Spearman rank correlation coefficient were calculated to estimate the strength of the correlation as well as fisher’s exact test. p-value < 0.05 were interpreted as statistically significant. Correlation matrix graphics were constructed using the Correlation Matrix STHDA.com online software.
Results
Intra-tumoral heterogeneity
Expression levels of all markers were compared between tissue cores of central and peripheral tumor parts to assess intra-tumoral heterogeneity. Comparison with tissue samples of lymph node metastasis was limited due to small number of samples. All three groups of markers (1) conventional predictive markers (ER, PR, HER2, EGFR and CK5/6), (2) tumor progression markers (PTEN, PIK3CA, p53, Ki-67) and (3) stem cell markers (E-cadherin, mTOR, SOX2, SOX9, SOX10, SLUG, CD44, CD24 and TWIST) were analyzed. There was no significant difference in the expression level in any of the analyzed markers in terms of central and peripheral tumor areas. Central and peripheral spots showed a high correlation (as detailed in Additional file 2: Table S2). Additionally, there was a complete concordance between central tumor areas and metastatic lesions in all marker expression.
Correlation to histological grading
All previously described markers were correlated to histopathological grading. Conventional parameters (ER, PR, HER2 status, EGFR, CK5/6), as expected significantly correlated to histological grading (p < 0.05).
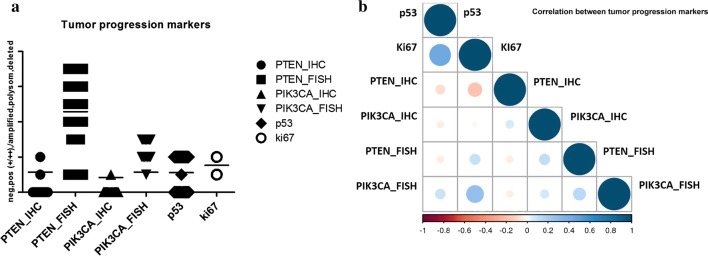
Tumor progression markers as PIK3CA FISH amplification, p53 and Ki-67 immunohistochemical expression showed a strong significant correlation between histological grading and expression level (p < 0.0001). PTEN both with immunohistochemistry and FISH correlated slightly with histological grading (p < 0.05). PIK3CA protein expression did not show significant correlation to grading.
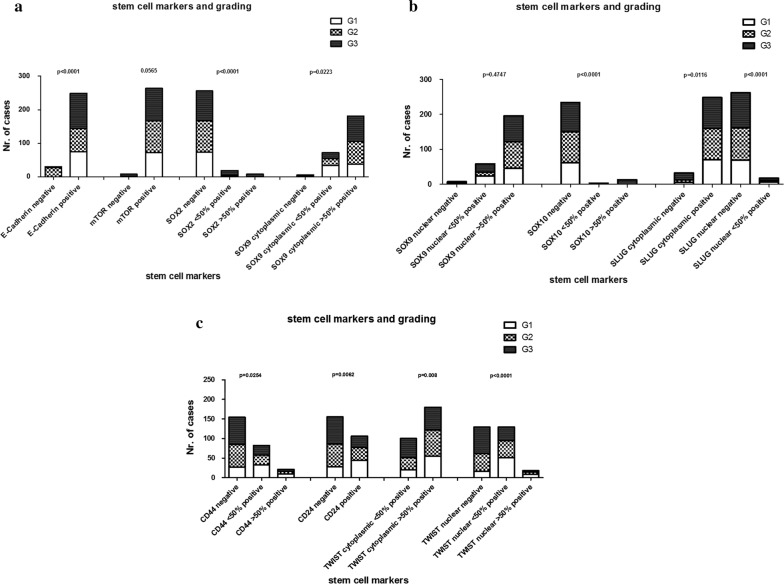
Among stem cell markers, a strong significant correlation between histological grading and expression level was found with E-cadherin, SOX2, SOX10, CD24 and TWIST. Furthermore, a slight significant correlation was seen with SOX9 (cytoplasmatic), SLUG (cytoplasmatic) and CD44. SOX9 (nuclear) and mTOR expression showed no correlation to histological grading.
Details of the individual stains are shown in Additional file 3: Table S3 and in Figs. 3a, b, 4a–c.
Fig. 3.
Graphical illustration of association between histological grading (a) and conventional prognostic/predictive markers (b) and tumor progression markers. p-values reflect Fisher’s exact test results
Fig. 4.
a–c Graphical illustration of association between histological grading and stem cell markers. p-values reflect Fisher’s exact test results
Correlation of conventional prognostic/predictive markers with stem cell and progression markers
Among the conventional predictive markers, a strong significant correlation was found between estrogen receptor and all the other conventional predictive markers, as well as between progesterone receptor and EGFR, HER2 IHC and CK5/6, between HER2 IHC and CK5/6 and HER2 FISH and between EGFR and CK5/6.
When correlated to tumor progression markers, a slight significant correlation was seen between estrogen receptor and PTEN (IHC and FISH), estrogen receptor and PIK3CA (IHC) as well as between progesterone receptor and PIK3CA (IHC). Comparison of ER and PR to Ki-67, HER2 IHC to PTEN FISH, p53 and Ki-67, as well as comparison of EGFR to p53 and Ki-67 and CK5/6 to PIK3CA, p53 and Ki-67 revealed a strong significant correlation.
The correlation of conventional predictive markers to stem cell markers showed numerous significant correlations. Especially the hormone receptors and EGFR revealed a strong significant correlation to stem cell markers.
Details of stain distribution and significant correlations are shown in Additional file 4: Table S4 and in Fig. 5a, b.
Fig. 5.
Graphical illustration of stain distribution (a) and correlation (b) among prognostic-predictive markers. Bars indicate mean values
Correlation of tumor progression markers to other markers
Correlation of the tumor progression markers PTEN, PIK3CA, p53 and Ki-67 to other tumor progression markers as well as to conventional predictive markers and stem cell markers was investigated.
Among the tumor progression markers, a significant correlation was only seen between PTEN and PIK3CA (IHC) and p53 and PIK3CA (IHC).
Tumor progression markers and stem cell markers revealed numerous strong significant correlations with each other. No significant correlations could be seen between tumor progression markers and SOX9 and CD44.
Comparison of tumor progression markers to conventional predictive markers is described above.
Details of stain distribution and significant correlations are shown in Additional file 5: Table S5 and in Fig. 6a, b.
Fig. 6.
Graphical illustration of stain distribution (a) and correlation (b) among tumor progression markers. Bars indicate mean values
Comparison of stem cell markers to other markers
Comparison of stem cell markers to conventional markers and tumor progression markers is described above.
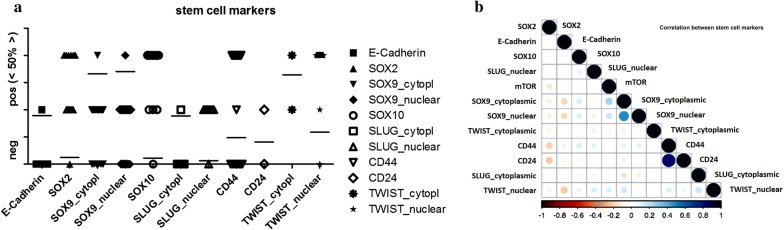
Among the stem cell markers numerous significant correlations were found. No significant correlation was seen between SOX10 and other stem cell markers.
Details of stain distribution and significant correlations are shown in Additional file 6: Table S6 and in Fig. 7a, b.
Fig. 7.
Graphical illustration of stain distribution (a) and correlation (b) among stem cell markers. Bars indicate mean values
Transcriptome (mRNA) analysis
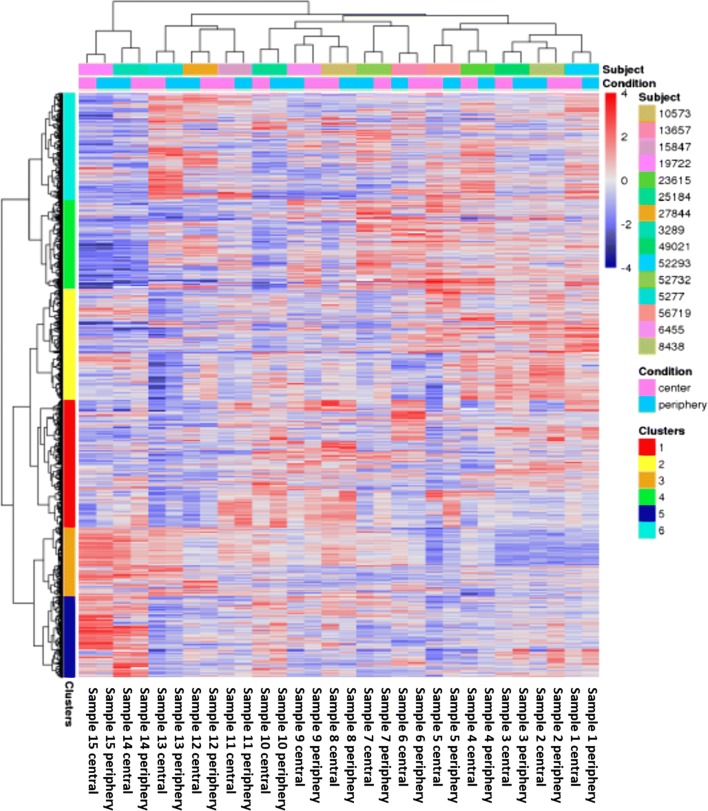
The sample clustering for the 2000 most variable genes in the experiment of the mRNA expression profiles shows that samples from the same patient are grouping together (Fig. 8). There is no indication for a differential expression signature between tumor areas based on the explorative cluster analysis. The sample to sample correlation illustrated in Fig. 9a confirms a strong positive correlation for all patient sample pairs included in this study. Focusing only on the 100 most variable genes provides very similar results (Fig. 9b).
Fig. 8.
Heatmap showing the most variable 2000 genes in the RNA-Seq data. Sample and gene clustering was applied. The color indicates the log2-foldchange in comparison to the overall samples mean of the corresponding gene
Fig. 9.
Heatmap showing the sample to sample correlation based on Kendall’s rank correlation coefficient for a all expressed genes (mean count > 20) and b 100 most variable genes across all samples
Furthermore, the expression profiles between central and peripheral tumor areas were directly compared considering the patient as an additional factor in the linear model to remove the influence of different genetic backgrounds. The resulting p value histogram for all genes is following a uniform distribution (Fig. 10). This suggests that there is no gene signature which significantly differs between both tumor areas. No candidate gene was detected by applying a fold-change cut off > 2 and p-value ≤ 0.01.
Fig. 10.

p-value histogram from the differential expression analysis. Absent and present genes in the data are separated by the color
Based on these results, there is no indication for any differentially expressed gene between periphery and center in this data.
Discussion
In our study, we addressed the question whether a tissue micro array based approach can sufficiently detect intra-tumoral heterogeneity in stem cell and tumor progression markers in breast cancer by comparing expression levels between central and peripheral tumor parts. Our results suggest, that based on protein and DNA expression levels in tissue micro array core sections, no reliable distinction could be determined.
We are not aware of any similar approach in the literature describing the value of TMA in the intra-tumoral heterogeneity of stem cell and tumor progression markers in breast cancer.
Our findings partially contradict previous studies revealing intra-tumoral heterogeneity in breast cancer in tissue section based in the expression level of prognostic markers [9]. The finding of intra-tumoral heterogeneity in Ki-67 expression levels was confirmed in a recent study by Focke et al. [20]. On the other hand, when comparing tissue array-based measures of ER and PR status with immunohistochemistry, intra-tumoral heterogeneity, although present, did not affect exact tumor classification [21]. Similarly, in a study of Yang et al. investigating spatial heterogeneity of breast cancer stem cell markers, few patients revealed intra-tumoral heterogeneity, but no association with prognosis was seen [22].
Contrariwise, our results are in accordance to the data of Alkatout et al. where as well no difference of the expression levels of SLUG and TWIST between tumor center and tumor periphery was seen [23].
Spatial intra-tumoral heterogeneity has been described in connection with epithelial-mesenchymal transition (EMT), as breast cancer genome has been shown to change dynamically during cancer development from primary to metastatic tumor [24]. EMT is a process during which epithelial cells lose their epithelial cell characteristics and acquire mesenchymal phenotype and EMT cells gain motility and become invasive [25–29]. Hence, EMT has been considered as an important mechanism in tumor cell progression and metastasis, and consistently, the expression of EMT markers has been shown to be associated with poor prognosis [25–29]. There is increasing evidence that EMT is associated with acquisition of stem-cell properties [29, 30]. In a study of well-differentiated colorectal cancers and their metastasis a dedifferentiation and loss of epithelial properties was seen at the invasive tumor margin, and Brabletz et al. therefore proposed occurrence of EMT preferably at peripheral tumor sites [23, 31]. However, this hypothesis could not be confirmed by Alkatout et al. No significant difference of expression levels of the EMT markers SLUG, TWIST, SNAIL and Zeb1 between tumor center and tumor periphery could be revealed [23]. On the other hand, Connor et al. found a higher proliferative activity (Ki-67 index) at the tumor periphery than in the tumor center [32]. As described above, our results are in accordance to the data of Alkatout et al. We could not find a difference of expression levels of SLUG and TWIST between tumor center and periphery either. A possible interpretation of these findings is that epithelial-mesenchymal transition is not only occurring at the tumor periphery, but at multiple sites within the tumor [23].
The second aim of the study was to proof prognostic value of the special TMA containing different tumor areas by comparing expression levels of conventional predictive tumor markers, tumor progression markers and stem cell markers with histopathological grading. In breast cancer diagnostics hormone receptors (ER, PR) as well as HER2 are established prognostic and predictive biomarkers [33–35]. Based on the current TMA, we could proof a strong negative correlation between hormone receptor expression levels and histopathological grading and a significant positive correlation between histopathological grading and HER2/EGFR/CK5/6 expression, corresponding with literature data and confirming prognostic value of the TMA independently of the site of the biopsy was taken.
Ki-67 and p53 are connected to cell proliferation and if increased or overexpressed are associated with higher grading, metastatic potential and shortened overall-survival [33, 35–37]. A shortened time to progression and decreased survival has furthermore been seen with PTEN deletion and PIK3CA activating mutations [38, 39]. In accordance with these findings we found a significant positive correlation between Ki-67, p53 and PIK3CA expression levels and histopathological grading, whereas a negative correlation was seen with PTEN expression.
Previous studies about the predictive value of CD44 and CD24 have showed controversial results. In 2003, a CD44+/CD24− phenotype was first identified as a highly tumorigenic subpopulation of tumor cells with stem-cell-like characteristics [5, 6, 8]. Tumors showing this phenotype were described to favor occurrence of distant metastasis, but no significant correlation with clinical outcome was seen [40–43]. In studies separately analyzing CD44 as a predictive marker an association between CD44 positivity and increased progression-free and disease-free survival was found [44, 45]. The role of CD24 as a predictive marker was as well discussed controversial. The data of Kristiansen et al. revealed an association to shortened disease-free-survival, while Jang et al. found no significant correlation to prognosis [46, 47]. In our study, a slight respectively strong negative correlation between CD44 and CD24 and histopathological grading was seen, being concordant with the results of Dan et al. and Diaz et al.
TWIST, SLUG and SOX9 have lately been described as cancer stems cell markers inducing EMT [48–51]. In consistence with our findings, previous studies found these markers to be associated with higher histopathological grading and shortened overall survival [48, 50–55].
Through loss of E-cadherin tumor cells gain the ability to migrate and E-cadherin negativity was found to correlate with higher tumor grade and stage and poor prognosis [56, 57]. However, in contrast to these results, our data revealed a positive correlation to histopathological grading.
SOX2 and SOX10 are often present in breast cancer, although their role is poorly understood yet. Our data further support recent suggestions that SOX2 and SOX10 correlate to higher histopathological grading and hence to reduced overall survival [51, 58].
mTOR is part of a pathway playing an important role in tumorigenesis. Our findings are in agreement with a recent study of Ding et al. where no significant correlation between mTOR expression and clinical outcome was seen [59].
Intratumoral heterogeneity of tumor infiltrating lymphocytes (TIL) was addressed in a previous study assessing different anatomical regions of breast cancer on large tissue sections and showing that TIL-s at the infiltrating margins are more numerous than in the intratumoral stromal tissue [60]. Interestingly, the same trend was found when metastatic lesions were compared with the primary tumor suggesting that the primary tumor most likely determines the TIL composition in the metastatic lesion which mirrors the immunological pattern of primary tumor [60].
Regarding the correlation between stem cell, tumor progression and conventional prognostic markers, we could show in our study, that within the current TMA cohort most markers displayed significant inter-marker correlation, independently from the anatomical site of the tumor sample taken. Most breast tumors are estrogen receptor and progesterone receptor positive and are associated with better overall survival. Further, HER2 negativity was found to correlate with ER and PR positivity and was as well described to be associated with favorably prognosis [33, 35, 38]. Ki-67, as a proliferation marker, as well as p53 are linked with poor prognosis. Their expression levels negatively correlate with ER and PR expression levels and positively correlate with HER2 status [33, 37, 61, 62]. All these findings are in complete agreement with the results of our study, where a strong significant correlation between the hormone receptors and a negative correlation between ER/PR and HER2, Ki-67 and p53 was seen. On the other hand, no correlation was found between p53 and Ki-67 expression levels.
Trastuzumab is a monoclonal antibody, used for treatment of metastatic HER2-positive breast cancer. Lately, it was suggested that mutations of the PI3 K/AKT pathway and loss of PTEN could interfere with trastuzumab treatment and lead to resistance. Recent studies therefore questioned whether PIK3CA and PTEN could be used as predictive markers of trastuzumab efficacy [38, 63]. We revealed a strong significant correlation between HER2 status and PTEN expression level and between PTEN and PIK3CA but not between HER2 status and PIK3CA expression level was seen. The results agreed with previous studies of Razis et al. and Lebok et al., where as well a correlation between HER2 and PTEN was found [38, 39]. So far, PTEN loss and PIK3CA mutations are proposed to be mutually exclusive, what seems to be inconsistent with our finding of a significant correlation between the two markers, even though mutation status of PIK3CA was not assessed in our study [38]. However in one recent study by Sueta et al., a combined analysis of PTEN loss and PIK3CA mutations was shown to potentially identify patients who are unlikely to respond to trastuzumab therapy in neoadjuvant setting [64]. It seems, that complete pathological response, pCR is lower if an activating PIKC3CA mutation and/or low level or PTEN expression is present [64]. PTEN is a negative regulator of the PIK3CA pathway, consequently, simultaneous PIK3CA mutations and low PTEN expression can stronger predict response to trastuzumab therapy then one marker alone, which can potentially identify patients who can benefit from PIK3 K targeted therapies [64]. The negative and positive correlation between PIKC3CA and PTEN protein expression and gene copy numbers in our study corroborate with these observations.
So far, only few studies investigated the association of CD24/CD44 to other markers. Collina et al. described a slight statistical link between CD44 and Ki-67, which could not be confirmed by our study [43]. Jang et al. previously found a negative, respectively positive correlation between CD44/CD24 and HER2 status [47]. Our data revealed as well an inverse correlation between HER2 and CD44, but inconsistent with the results of Jang et al. an inverse association to CD24 too.
The over-expression of most stem cell markers has been linked to poor prognosis and to hormone receptor negativity and HER2 positivity, which also could be confirmed in our data [48, 54, 56, 58, 65]. The numerous correlations among the stem cell markers, further providing a similar predictive value [48, 54, 56, 58, 65].
Conclusion
Based on the data in our study, a tissue micro array based approach failed to deliver sufficient information on intra-tumoral heterogeneity in breast cancer stem cell and tumor progression markers by comparing central and peripheral tumor areas. Further studies using single cell genomic alterations including further mRNA based assessments are needed to fully understand the role and interaction between stem cell marker and intra-tumoral heterogeneity also in terms of tumor progression in breast cancer. On the other hand, the anatomical site of the tumor samples did not influence prognostic information on these markers which was independent from the site of biopsy was taken.
Additional files
Additional file 1: Table S1. Immunohistochemical stains and laboratory details.
Additional file 2: Table S2. Analysis of intratumoral heterogeneity.
Additional file 3: Table S3. Correlation to histopathological grading.
Additional file 4: Table S4. Conventional predictive/prognostic markers—significant correlations.
Additional file 5: Table S5. Tumor progression markers—significant correlations.
Additional file 6: Table S6. Stem cell markers—significant correlations.
Authors’ contributions
PK conducted analyses, interpreted the results and drafted the manuscript. CG constructed the TMA. HJ and BB contributed to design and data interpretation. BP provided clinical information. SNF extracted RNA. CA and LO performed mRNA sequencing and statistical analysis and contributed to data interpretation. ZV designed the study, interpreted the results and finalized the text. All authors read and approved the final manuscript.
Acknowledgements
We thank Mr. André Fitsche and Mrs. Susanne Dettwiler for technical assistance in preparing slides and performing immunohistochemical and in situ hybridization assays.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All definitive data generated or analyzed during this study are included in this published article. Further raw data analyzed during the study are available from the corresponding author on request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was part of a larger breast cancer project of the Institute of Surgical Pathology, University Hospital Zurich, previously approved by the Ethical Committee of the Canton Zurich (KEK-ZH NR: 2012-0553). Informed consent was not necessary as the data were analyzed in an anonymous way.
Funding
No funding was necessary for this project.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
The original version of this article was revised: originally an incorrect Fig. 7b was published.
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1495-6) contains supplementary material, which is available to authorized users.
A correction to this article is available online at https://doi.org/10.1186/s12967-018-1553-0.
Change history
7/2/2018
Following publication of the original article [1], a typesetting mistake is reported. For Fig. 7b, a copy of Fig. 6b has been published. The correct Fig. 7b is given in this correction and the original article has been updated.
Contributor Information
Pascale Kündig, Email: pascale.kuendig@hotmail.com.
Charlotte Giesen, charlotte.giesen@googlemail.com.
Hartland Jackson, Email: hartland.jackson@uzh.ch.
Bernd Bodenmiller, Email: bernd.bodenmiller@imls.uzh.ch.
Bärbel Papassotirolopus, Email: b.papassotiropoulos@brust-zentrum.ch.
Sandra Nicole Freiberger, Email: Sandra.Freiberger@usz.ch.
Catharine Aquino, Email: catharine.aquino@fgcz.ethz.ch.
Lennart Opitz, Email: lennart.opitz@fgcz.ethz.ch.
Zsuzsanna Varga, Phone: +41 44 255 2449, Email: zsuzsanna.varga@usz.ch.
References
- 1.Renovanz M, Kim EL. Intratumoral heterogeneity, its contribution to therapy resistance and methodological caveats to assessment. Front Oncol. 2014;4:142. doi: 10.3389/fonc.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, Rosen JM. Developmental insights into breast cancer intratumoral heterogeneity. Trends Cancer. 2015;1(4):242–251. doi: 10.1016/j.trecan.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210. doi: 10.1186/bcr3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014;28(12):1101–1107. [PubMed] [Google Scholar]
- 5.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;42(Suppl 1):S3–S17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Angeloni V, Tiberio P, Appierto V, Daidone MG. Implications of stemness-related signaling pathways in breast cancer response to therapy. Semin Cancer Biol. 2015;31:43–51. doi: 10.1016/j.semcancer.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Shao J, Fan W, Ma B, Wu Y. Breast cancer stem cells expressing different stem cell markers exhibit distinct biological characteristics. Mol Med Rep. 2016;14(6):4991–4998. doi: 10.3892/mmr.2016.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis GA, Cohen C. Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray-based study. Appl Immunohistochem Mol Morphol. 2010;18(5):433–441. doi: 10.1097/PAI.0b013e3181dddb20. [DOI] [PubMed] [Google Scholar]
- 10.Huebschman ML, Lane NL, Liu H, Sarode VR, Devlin JL, Frenkel EP. Molecular heterogeneity in adjacent cells in triple-negative breast cancer. Breast Cancer (Dove Med Press). 2015;7:231–237. doi: 10.2147/BCTT.S87041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allott EH, Geradts J, Sun X, Cohen SM, Zirpoli GR, Khoury T, et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast Cancer Res. 2016;18(1):68. doi: 10.1186/s13058-016-0725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke HB. Predicting clinical outcomes using molecular biomarkers. Biomark Cancer. 2016;8:89–99. doi: 10.4137/BIC.S33380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195(1):72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- 14.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 15.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, de Vijver MJ. WHO classification of tumours of the breast. Lyon: IARC; 2012. [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 17.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y, Smyth GK, Shi W. The subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41(10):e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Focke CM, Decker T, van Diest PJ. Intratumoral heterogeneity of Ki67 expression in early breast cancers exceeds variability between individual tumours. Histopathology. 2016;69(5):849–861. doi: 10.1111/his.13007. [DOI] [PubMed] [Google Scholar]
- 21.Barry WT, Kernagis DN, Dressman HK, Griffis RJ, Hunter JD, Olson JA, et al. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J Clin Oncol. 2010;28(13):2198–2206. doi: 10.1200/JCO.2009.26.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Cao L, Sun Z, Jin J, Fang H, Zhang W, et al. Evaluation of breast cancer stem cells and intratumor stemness heterogeneity in triple-negative breast cancer as prognostic factors. Int J Biol Sci. 2016;12(12):1568–1577. doi: 10.7150/ijbs.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkatout I, Wiedermann M, Bauer M, Wenners A, Jonat W, Klapper W. Transcription factors associated with epithelial-mesenchymal transition and cancer stem cells in the tumor centre and margin of invasive breast cancer. Exp Mol Pathol. 2013;94(1):168–173. doi: 10.1016/j.yexmp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Sato F, Saji S, Toi M. Genomic tumor evolution of breast cancer. Breast Cancer. 2016;23(1):4–11. doi: 10.1007/s12282-015-0617-8. [DOI] [PubMed] [Google Scholar]
- 25.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11(6):213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z. The biological and clinical importance of epithelial–mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol. 2015;141(2):189–201. doi: 10.1007/s00432-014-1752-x. [DOI] [PubMed] [Google Scholar]
- 27.Mego M, Mani SA, Lee BN, Li C, Evans KW, Cohen EN, et al. Expression of epithelial–mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2012;130(4):808–816. doi: 10.1002/ijc.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi Y, Lee HJ, Jang MH, Gwak JM, Lee KS, Kim EJ, et al. Epithelial–mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum Pathol. 2013;44(11):2581–2589. doi: 10.1016/j.humpath.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Sarkissyan M, Vadgama JV. Epithelial–mesenchymal transition and breast cancer. J Clin Med. 2016 doi: 10.3390/jcm5020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anwar TE, Kleer CG. Tissue-based identification of stem cells and epithelial-to-mesenchymal transition in breast cancer. Hum Pathol. 2013;44(8):1457–1464. doi: 10.1016/j.humpath.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor AJM, Pinder SE, Elston CW, Bell JA, Wencyk P, Robertson JFR, et al. Intratumoural heterogeneity of proliferation in invasive breast carcinoma evaluated with MIBI antibody. Breast. 1997;6(4):171–176. doi: 10.1016/S0960-9776(97)90568-3. [DOI] [Google Scholar]
- 33.Patani N, Martin LA, Dowsett M. Biomarkers for the clinical management of breast cancer: international perspective. Int J Cancer. 2013;133(1):1–13. doi: 10.1002/ijc.27997. [DOI] [PubMed] [Google Scholar]
- 34.Aleskandarany MA, Soria D, Green AR, Nolan C, Diez-Rodriguez M, Ellis IO, et al. Markers of progression in early-stage invasive breast cancer: a predictive immunohistochemical panel algorithm for distant recurrence risk stratification. Breast Cancer Res Treat. 2015;151(2):325–333. doi: 10.1007/s10549-015-3406-3. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A, Jinno H, Ando T, Fujii T, Nakamura T, Saito J, et al. Biological markers of invasive breast cancer. Jpn J Clin Oncol. 2016;46(2):99–105. doi: 10.1093/jjco/hyv153. [DOI] [PubMed] [Google Scholar]
- 36.Cidado J, Wong HY, Rosen DM, Cimino-Mathews A, Garay JP, Fessler AG, et al. Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget. 2016;7(5):6281–6293. doi: 10.18632/oncotarget.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shokouh TZ, Ezatollah A, Barand P. Interrelationships between Ki67, HER2/neu, p53, ER, and PR status and their associations with tumor grade and lymph node involvement in breast carcinoma subtypes: retrospective-observational analytical study. Medicine (Baltimore). 2015;94(32):e1359. doi: 10.1097/MD.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128(2):447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 39.Lebok P, Kopperschmidt V, Kluth M, Hube-Magg C, Özden C, et al. Partial PTEN deletion is linked to poor prognosis in breast cancer. BMC Cancer. 2015;15:963. doi: 10.1186/s12885-015-1770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44 +/CD24 −/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11(3):1154–1159. [PubMed] [Google Scholar]
- 41.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, et al. The clinicopathologic and prognostic significance of CD44 +/CD24(−/low) and CD44 −/CD24 + tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39(7):1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44 +/CD24 − phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, et al. Prognostic value of cancer stem cells markers in triple-negative breast cancer. Biomed Res Int. 2015;2015:158682. doi: 10.1155/2015/158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dan T, Hewitt SM, Ohri N, Ly D, Soule BP, Smith SL, et al. CD44 is prognostic for overall survival in the NCI randomized trial on breast conservation with 25 year follow-up. Breast Cancer Res Treat. 2014;143(1):11–18. doi: 10.1007/s10549-013-2758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, et al. CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res. 2005;11(9):3309–3314. doi: 10.1158/1078-0432.CCR-04-2184. [DOI] [PubMed] [Google Scholar]
- 46.Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schlüns K, Denkert C, et al. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9(13):4906–4913. [PubMed] [Google Scholar]
- 47.Jang MH, Kang HJ, Jang KS, Paik SS, Kim WS. Clinicopathological analysis of CD44 and CD24 expression in invasive breast cancer. Oncol Lett. 2016;12(4):2728–2733. doi: 10.3892/ol.2016.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue K, Fry EA. Novel molecular markers for breast cancer. Biomark Cancer. 2016;8:25–42. doi: 10.4137/BIC.S38394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakravarty G, Moroz K, Makridakis NM, Lloyd SA, Galvez SE, Canavello PR, et al. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp Biol Med (Maywood). 2011;236(2):145–155. doi: 10.1258/ebm.2010.010086. [DOI] [PubMed] [Google Scholar]
- 50.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riemenschnitter C, Teleki I, Tischler V, Guo W, Varga Z. Stability and prognostic value of Slug, Sox9 and Sox10 expression in breast cancers treated with neoadjuvant chemotherapy. Springerplus. 2013;2:695. doi: 10.1186/2193-1801-2-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markiewicz A, Ahrends T, Wełnicka-Jaśkiewicz M, Seroczyńska B, Skokowski J, Jaśkiewicz J, et al. Expression of epithelial to mesenchymal transition-related markers in lymph node metastases as a surrogate for primary tumor metastatic potential in breast cancer. J Transl Med. 2012;10:226. doi: 10.1186/1479-5876-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren H, Du P, Ge Z, Jin Y, Ding D, Liu X, et al. TWIST1 and BMI1 in cancer metastasis and chemoresistance. J Cancer. 2016;7(9):1074–1080. doi: 10.7150/jca.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YQ, Wei XL, Liang YK, Chen WL, Zhang F, Bai JW, et al. Over-expressed twist associates with markers of epithelial mesenchymal transition and predicts poor prognosis in breast cancers via erk and Akt activation. PLoS ONE. 2015;10(8):e0135851. doi: 10.1371/journal.pone.0135851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pomp V, Leo C, Mauracher A, Korol D, Guo W, Varga Z. Differential expression of epithelial–mesenchymal transition and stem cell markers in intrinsic subtypes of breast cancer. Breast Cancer Res Treat. 2015;154(1):45–55. doi: 10.1007/s10549-015-3598-6. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Yin S, Zhang L, Liu W, Chen B. Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget. 2017 doi: 10.18632/oncotarget.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shamir ER, Ewald AJ. Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration. Curr Top Dev Biol. 2015;112:353–382. doi: 10.1016/bs.ctdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang YH, Luo MH, Ni YB, Tsang JY, Chan SK, Lui PC, et al. Increased SOX2 expression in less differentiated breast carcinomas and their lymph node metastases. Histopathology. 2014;64(4):494–503. doi: 10.1111/his.12257. [DOI] [PubMed] [Google Scholar]
- 59.Ding XF, Li LF, Zhou XL, Guo LN, Dou MM, Chi YY, et al. P-mTOR expression and implication in breast carcinoma: a systematic review and meta-analysis. PLoS ONE. 2017;12(1):e0170302. doi: 10.1371/journal.pone.0170302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016;5(6):e1153208. doi: 10.1080/2162402X.2016.1153208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Payandeh M, Shahriari-Ahmadi A, Sadeghi M, Sadeghi E. Correlations between HER2 expression and other prognostic factors in breast cancer: inverse relations with the Ki-67 index and P53 status. Asian Pac J Cancer Prev. 2016;17(3):1015–1018. doi: 10.7314/APJCP.2016.17.3.1015. [DOI] [PubMed] [Google Scholar]
- 62.Rosa FE, Caldeira JRF, Felipes J, Bertonha FB, Quevedo FC, Domingues MAC, et al. Evaluation of estrogen receptor α and β and progesterone receptor expression and correlation with clinicopathologic factors and proliferative marker Ki-67 in breast cancers. Hum Pathol. 2008;39(5):720–730. doi: 10.1016/j.humpath.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Adamczyk A, Niemiec J, Janecka A, Harazin-Lechowska A, Ambicka A, Grela-Wojewoda A, et al. Prognostic value of PIK3CA mutation status, PTEN and androgen receptor expression for metastasis-free survival in HER2-positive breast cancer patients treated with trastuzumab in adjuvant setting. Pol J Pathol. 2015;66(2):133–141. doi: 10.5114/pjp.2015.53009. [DOI] [PubMed] [Google Scholar]
- 64.Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, Hayashi M, Takeshita T, Yamamoto S, et al. An integrative analysis of PIK3CA mutation, PTEN, and INPP4B expression in terms of trastuzumab efficacy in HER2-positive breast cancer. PLoS ONE. 2014;9(12):e116054. doi: 10.1371/journal.pone.0116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Wu Y, Abbatiello TC, Wu WL, Kim JR, Sarkissyan M, et al. Slug contributes to cancer progression by direct regulation of ERα signaling pathway. Int J Oncol. 2015;46(4):1461–1472. doi: 10.3892/ijo.2015.2878. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Immunohistochemical stains and laboratory details.
Additional file 2: Table S2. Analysis of intratumoral heterogeneity.
Additional file 3: Table S3. Correlation to histopathological grading.
Additional file 4: Table S4. Conventional predictive/prognostic markers—significant correlations.
Additional file 5: Table S5. Tumor progression markers—significant correlations.
Additional file 6: Table S6. Stem cell markers—significant correlations.
Data Availability Statement
All definitive data generated or analyzed during this study are included in this published article. Further raw data analyzed during the study are available from the corresponding author on request.