Abstract
Drosophila melanogaster has been used as a very versatile and potent model in the past few years for studies in metabolism and metabolic disorders, including diabetes types 1 and 2. Drosophila insulin signaling, despite having seven insulin-like peptides with partially redundant functions, is very similar to the human insulin pathway and has served to study many different aspects of diabetes and the diabetic state. Yet, very few studies have addressed the chronic nature of diabetes, key for understanding the full-blown disease, which most studies normally explore. One of the advantages of having Drosophila mutant viable combinations at different levels of the insulin pathway, with significantly reduced insulin pathway signaling, is that the abnormal metabolic state can be studied from the onset of the life cycle and followed throughout. In this review, we look at the chronic nature of impaired insulin signaling. We also compare these results to the results gleaned from vertebrate model studies.
1. Introduction
Diabetes is a chronic metabolic malaise that affects and is forecast to affect many millions of people in the world [1]. It is a disease caused by insulin deficiency or loss of insulin action. In addition to genetic factors, certain lifestyles such as high dietary fat content and physical inactivity are risk factors for the development of diabetes [2]. It has outpaced many other diseases and is predicted to become one of the major health concerns in the future [3]. According to data cited by the World Health Organization, by 2014 incidence of diabetes had risen to 8.5% [3]. In Mexico, for example, 2017 figures show that over 15% of adults are diabetic, which is a very high incidence and concern [4]. As of now, diabetes is an incurable and incapacitating disease with a long and protracted progression. It is also a disease being diagnosed more often in younger patients [2].
In human diabetic patients where the condition has existed for some time, there are several comorbidities. It courses with macrovascular complications, leading to heart disease and stroke, and increased cardiovascular morbidity and mortality. In addition, microvascular complications lead to nephropathy, retinopathy, and neuropathy [1]. Little is known of the onset and early progression of the disease, except for familial cases, which are the minority, and the higher risk of diabetes type 2 for babies where mothers had hyperglycemia or diabetes [2, 5].
Diabetes mellitus is divided into basically two types: type 1 and type 2, a division that reflects the cause of the metabolic dysfunction. Diabetics type 1 have a reduction in insulin secretion, and as a consequence, blood glucose does not attain homeostatic levels after food ingestion and digestion. Physicians normally treat them by prescribing exogenous insulin injections on a regular basis. These diabetics represent around 10% of all diabetic patients, and in most cases, their condition is due to the death of pancreatic Langerhans islets ß-type cells, which normally secrete insulin to clear elevated glucose levels from the bloodstream, like after a meal [6]. It leads to elevated blood glucose levels, as expected, and to general body wasting.
Diabetes type 2 represents the majority of cases, ranging between 90 and 95% of all diabetic patients. It is characterized by a combination of insulin resistance and insulin secretion defects, resulting in relative insulin deficiency and hyperglycemia [6]. Diabetic type 2 patients normally represent patients that have had a long progression, initially suffering from metabolic syndrome, and/or being overweight, and/or being obese for several years. Environmental factors, like diet and level of physical exercise, also play an important role in the inception and progression of the disease, as noted above.
Finally, there is also a third type of diabetes: gestational diabetes. This form of diabetes occurs in pregnant women, leads to increased risk of diabetes for the offspring, and may lead to diabetes type 2 in the mothers after birth [2].
There are, in sum, many factors causing diabetes type 2, both genetic and environmental, and the composite picture is complex, as it may change depending on the actual combination present in populations and individual patients [2]. While all of the factors cited above are recognized contributing factors, it is not clear how they weigh in the initiation and early progression of the disease. Therefore, it is important to elucidate the precise molecular mechanisms underlying the development and progression of the disease.
In general, the diabetic state is multifactorial encompassing several origins and progressions. Studying its causes, effects, and consequences is paramount in the actual diabetes “epidemic,” but it is not easy or even possible to study many of these aspects using human patients as test subjects. Scientists have developed model systems where diabetes can be controlled to a higher extent, and in which experimental setups with a high degree of rigor and reproducibility can be used, with genetic uniformity, and highly controlled environments. Principles uncovered in these systems can then be applied in a more general fashion, as the insulin pathway and glucose control is a common, evolutionarily conserved mechanism in the animal kingdom (Figure 1).
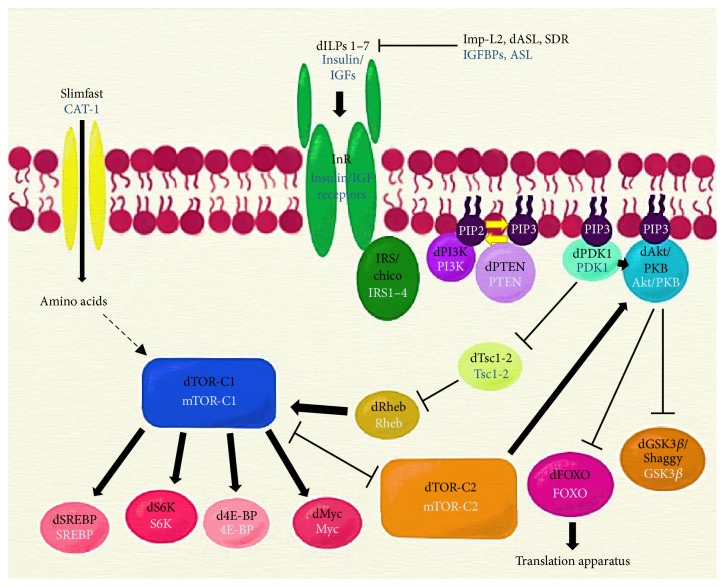
Figure 1.
The insulin signaling pathway. The binding of insulin to its receptor initiates a phosphorylation cascade that results in the regulation of metabolism through several effectors. Names for the vertebrate counterparts of the pathway appear below their Drosophila names. CAT-1: cationic amino acid transporter-1; Imp-L2: ecdysone-inducible gene L2; IGFBPs: insulin-like growth factor binding proteins; ASL: acid-labile subunit; SDR: secreted decoy of InR; dILPs 1–7: insulin-like ligands 1–7; IGFs: insulin-like growth factors; InR: insulin receptor; IRS/chico: insulin receptor substrate; PI3K: phosphatidylinositol 3-kinase (two subunits: Pi3K92E is the catalytic subunit, and Pi3K21B is the regulatory subunit); PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol 3,4,5-trisphosphate; PTEN: phosphatase and tensin homolog; dPDK1: 3-phosphoinositide dependent protein kinase-1; GSK3β: glycogen synthase kinase 3 beta; Tsc1-2: tuberous sclerosis proteins 1 and 2; Rheb: Ras homolog enriched in brain; TOR-C1: target of rapamycin complex 1 (the TOR-C1 complex consists primarily of TOR, regulatory associated protein of TOR (raptor), and lethal with Sec-13 protein 8 (LST8)); TOR-C2: target of rapamycin complex 2 (the TOR-C2 complex consists primarily of TOR, rapamycin-insensitive companion of TOR (Rictor), and stress-activated protein kinase-interacting protein 1 (Sin1)); Myc: Myc protein; SREBP: sterol regulatory element-binding protein; S6K: ribosomal protein S6 kinase beta-1; 4E-BP: eukaryotic translation initiation factor 4E-binding protein 1; FoxO: Forkhead box O transcription factor. Dashed lines indicate an indirect interaction; arrows and bar-headed lines indicate activation and inhibition, respectively.
1.1. The Insulin Pathway
Insulin is an anabolic hormone in glucose homeostasis in experimentally pancreatomized dogs [7] discovered by Banting and Best, who won the Nobel Prize for this discovery [8]. In general in vertebrates, insulin is secreted from pancreatic Langerhans islets ß-type cells in response to increased glucose levels. In some teleost fish, insulin is produced in Brockmann's bodies [9]. Secreted insulin in the bloodstream binds to membrane receptors, especially in muscle cells, and initiates a transduction cascade that leads to glucose internalization and an anabolic response. In invertebrates, the insulin molecule is slightly longer, has one more disulphide bridge, and is secreted from specialized neurons (insulin-producing cells, or IPC) and glia in the brain [10]. Recently, in a striking novel use, insulin-like peptides have been identified in the venom of certain Conus mollusks able to bind insulin receptor molecules and induce hypoglycemia in fish prey [11, 12].
Insulin is a small polypeptide constituted by two chains linked by disulphide bonds, synthesized from the same gene [13]. Whereas vertebrates have one insulin gene, the Drosophila genome codes for seven several insulin-like peptides, secreted from the insulin-producing cells (IPC) of the brain. A further eight Drosophila insulin-like peptide, DILP8, is really a relaxin homolog, binding to a different type of receptor, and controlling corporal symmetry [14–17].
The Drosophila insulin-like peptides (ILPs) also have nonredundant functions [18–20]. The ILP2 peptide has the highest homology to the vertebrate insulin gene and is synthesized together with ILP1, ILP3, and ILP5 in the IPCs of the brain, and their synthesis depends on ILP3. ILP3 expression also activates the insulin pathway in the fat body [21]. ILP4, ILP5, and ILP6 are expressed in the midgut, ILp7 is expressed in the ventral nerve chord, and ILp2 is also expressed in the salivary glands and imaginal discs [22]. The Drosophila IPCs are the equivalent of the mammalian Langerhans' islets ß pancreatic cells [23]. ILP6 is synthesized in the fat body and can partially substitute for ILP2 and ILP5. ILP2 loss-of-function mutations lead to an increase in lifespan, while loss in ILP6 causes reduced growth [23, 24].
An insulin monomer is around 50 amino acid residues in length, but dimers form in solution. Insulin is synthesized as a single polypeptide called preproinsulin, which is processed in the endoplasmic reticulum forming proinsulin, which then undergoes maturation through the action of peptidases releasing a fragment called the C-peptide and the A and B chains, linked by disulfide bonds. Mature insulin is exocytosed into the circulation by glucose stimulation and binds to plasma membrane receptors with tyrosine kinase activity.
Insulin is a potent anabolic hormone in vertebrates [25]. It also exerts a variety of actions in flies including effects on glucose, lipid, and protein metabolism. It directly promotes growth and proliferation in tissues, rather than differentiation [26, 27]. In vertebrates, insulin stimulates glucose uptake in skeletal muscle and fat, promotes glycogen synthesis in skeletal muscle, suppresses hepatic glucose production, and inhibits lipolysis in adipocytes [28]. Although vertebrate skeletal muscle, liver, and adipose tissue are considered the main target tissues of insulin action, there is evidence that insulin has important physiological functions in other tissues such as the brain, pancreas, heart, and endothelial cells [29, 30]. Pretty much the same is true for invertebrates in equivalent tissues, where insulin action has been shown to impinge on the physiology of many tissues, including the brain [31]. In vertebrates, there are insulin-growth factor binding proteins (IGFBPs) that conform to an evolutionarily conserved superfamily, regulating insulin-growth factor function; in Drosophila the homolog is ecdysone-inducible gene 2 (Imp-L2) [32].
In vertebrates, it is thought that insulin-like growth factor binding proteins, IGFBPs, and a third protein, ALS (acid-labile subunit), form ternary complexes with IGFs to regulate IGF function, separating insulin functions from IGFs functions [33]. In flies, ILPs have both vertebrate insulin and IGF functions. The Drosophila genome codes for a putative IGFBP-acid-labile subunit (IGFBP-ALS) homolog, convoluted, that has been shown to bind in vitro by ectopic expression to ILPs and Imp-L2 forming a ternary complex [34]. However, mutations (even null mutations) in convoluted have mutant phenotypes that differ from insulin pathway mutants. Convoluted mutants are larval lethal and affect tracheal morphogenesis and motor axon guidance. In addition, convoluted has a higher homology to extracellular matrix proteins like Chaoptin than to vertebrate ALS [35, 36]. Taken together, all of these facts cast doubt on whether a fly ALS homolog actually exists. It seems reasonable to postulate that since ILPs are both insulin and IGFs in flies, no separation in complexes is necessary.
Insulin or insulin-like peptides bind to the insulin receptor (IR), a heterotetrameric protein that consists of two extracellular α-subunits and two transmembrane β-subunits connected by disulfide bridges [37–40]. Insulin binding oligomerizes the receptors, allowing for cross-phosphorylation of the receptor molecules in tyrosine (tyr) residues in the IR domain of the intracellular part of the β-subunit. Despite some differences, vertebrate and invertebrate insulin receptors are equivalent [41], as chimeric fruit fly-vertebrate insulin receptors have been shown to be activated with a similar mechanism as vertebrate insulin receptors in mammalian cells [42]. In flies, there is also a secreted decoy of the insulin receptor, secreted decoy of InR (Sdr), that binds some dILPs in circulation in the hemolymph, necessary for the negative regulation of Dilp action [43].
Phosphorylation in InR tyr residues in the intracellular part of the β-subunit, and the carboxy-terminal extension in the fruit fly insulin receptor [44], leads to the generation of protein binding sites. This leads to the subsequent recruitment, binding, and tyr phosphorylation of members of the insulin receptor substrate (IRS) family proteins [37]. In Drosophila, besides the carboxy-terminus extension of the insulin receptor, the IRS homologs chico [45] and Lnk [46] act as IRS type molecules. Whereas chico is the sole IRS homolog in flies [45], Lnk is the fly homolog of vertebrate SH2B adaptor proteins [47]. Lnk acts as an adaptor molecule that favors Chico and InR membrane localization [46].
The phosphorylated tyrosine residues in both the activated receptors and the IRS proteins create further binding sites for other molecules, like the catalytic subunit of phosphatidylinositol 3′ kinase (Pi3K92E), which via the regulatory subunit, Pi3K21B, can now be brought in proximity to its substrate, phosphatidylinositol (4,5) bisphosphate [48].
The phosphorylated residues of vertebrate IRS1 (or Chico, in the fruit fly) mediate an association with the SH2 domains of the p85 regulatory subunit of phosphatidylinositol 3-kinase (Pi3K) (Pi3k21B in flies [49]) leading to activation of the p110 catalytic subunit, which then catalyzes the formation of phosphatidylinositol (3,4, 5) trisphosphate (PI 3, 4, 5-P3) from phosphatidylinositol (4,5) bisphosphate (PI 4, 5-P2) in the inner leaflet of the plasma membrane [50]. This then creates binding sites for proteins with pleckstrin homology domains (PH) [51], like the phosphoinositide dependent kinase (PDK1) [52] and protein kinase B (PKB, also known as Akt) [53]. Both proteins bind, via their PH domains, the phosphatidylinositol trisphosphate generated in the inner membrane leaflet of the plasma membrane via action of Pi3K92E [54, 55]. PDK1, a serine-threonine kinase, then phosphorylates and activates Akt [56, 57].
The phosphorylating activity of Pi3K92E is counteracted by PTEN (phosphatase and tensin homolog deleted in chromosome ten), a lipid and protein phosphatase and tumor suppressor gene in vertebrates and flies [58, 59]. The lipid phosphatase activity, of phosphatidylinositol (3,4, 5) trisphosphate to phosphatidylinositol (4,5) bisphosphate is thought to be the main catalytic activity. It is deregulated in many tumor types in humans and in neurodegenerative diseases, like Parkinson's disease [60]. In Drosophila, another negative regulator of Pi3K92E is Susi, binding to the p60 regulatory subunit of PI3K, the 60 Kd molecular weight subunit [61, 62].
Akt/PKB is considered a critical node in insulin signaling. Akt/PKB acts by phosphorylating many different proteins [40]. In so doing, Akt/PKB activates different outcomes: (1) Glut 4-mediated glucose transport in vertebrates, by activating the protein Akt substrate of 160 kDa (AS160), (2) glycogen synthesis through inhibition of glycogen synthase kinase 3ß (GSKß), and hence, favoring glycogen synthase (GS) activity, (3) protein synthesis through the mammalian target of rapamycin (TOR) pathway, (4) inhibition of the Forkhead transcription factor FoxO, a major positive catabolic regulator, and (5) others targets, such as the SIK2 (salt-inducible kinase 2) [22, 63–66]. In Drosophila, Melted interacts with both FoxO and the TOR kinase via the tuberous sclerosis complex 2 protein (TSC2) and acts as a bridge within the insulin pathway regulating the activities of these two proteins [67].
Besides the direct effect on glucose metabolism, GSK3β also regulates cellular metabolism through the inhibitory regulation of transcription factors that globally control specific metabolic programs, and many of them are also regulated by TOR complexes: cell survival or proliferation (including c-Myc), the sterol regulatory element-binding proteins (SREBP1c), hypoxia-inducible factor 1-alpha (HIF1a), and the nuclear factor- (erythroid-derived 2-) like 2 (Nrf2) [68]. Thus, Akt signaling can stabilize these proteins by inhibiting GSK3β and by indirectly activating TOR-C1 [27]. There is evidence that the insulin pathway control of Myc is evolutionarily conserved in Drosophila. In biochemical experiments in tissue culture cells and in ectopic expression studies, the Drosophila insulin pathway, via inhibition of shaggy, the Drosophila homolog of GSK3β, regulated Drosophila Myc protein stability. Drosophila myc is coded by the gene diminutive [69, 70].
Perhaps the best-documented cases of downstream components activated by Akt/PKB are the target of rapamycin (TOR) kinase and the FoxO transcription factor. The ser/thr kinase TOR interacts with different proteins to form the complexes TOR-C1 and TOR-C2 [62, 71]. This kinase positively regulates cell growth, proliferation, motility, and survival. TOR-C1 appears to play a role in acute feedback inhibition of Akt, negatively regulating insulin action. Activation of TOR is not direct from Akt/PKB: in the Drosophila ovaries, Akt/PKB represses the proline-rich Akt substrate 40 kDa (PRAS40). There is also a PRAS40 homolog in vertebrates [72]. In the fly ovaries, PRAS40 represses TOR, decoupling reproduction from growth in the cells of this organ [73]. In other tissues, Akt/PKB phosphorylates and might repress TSC1 (in flies) and TSC2, which are normally thought to repress the GTP-binding protein and GTPase Rheb that activates TOR-C1, yet it is unclear whether indeed this is the case [74]. TOR-C1 activation leads to longer S1 phase in cells [75, 76].
TOR is another central component downstream of insulin signaling. The TOR kinase in the TOR-C1 complex phosphorylates and regulates several proteins. TOR kinase in the TOR-C1 complex phosphorylates (1) S6 kinase, to promote translation (S6 is a component of the ribosomes) [77], (2) the translation regulatory factor 4E-BP, which also promotes protein synthesis [78], (3) the transcription factor Myc [69, 79, 80], (4) SREBP [81], and (5) autophagy proteins (phosphorylation of these autophagy proteins represses them) [82]. TOR also regulates endocytosis to promote growth and repress catabolism [83]. Besides regulation by the insulin pathway, TOR-C1 is also regulated via a nutrient sensing signaling pathway, specifically via the activity of the amino acid transporter Slimfast [84]. dS6K promotes ILP2 expression in the IPCs [21]. ILP secretion by IPCs is controlled by nutritional status, and this nutritional status is conveyed to IPCs by fat body cells, which secrete the Unpaired2 cytokine in fed conditions, which regulates GABAergic neurons in the brain, releasing the GABAergic tonic inhibition they exert on the IPCs, leading to ILP secretion [85, 86].
The other Akt/PKB well-studied target is FoxO. FoxO is a transcription factor (a family in mammals) that favors catabolism, counteracts anabolism, and is phosphorylated by Akt/PKB to repress its activity [22, 87]. Activation of insulin signaling leads to acute translocation of FoxO proteins out of the nucleus and attenuation of their transcriptional program [88, 89]. In vertebrates, the Forkhead box O (FoxO) family consists of FoxO1, FoxO3, FoxO4, and FoxO6 proteins; a distinct gene encodes each one. Drosophila has only one such gene [90, 91]. FoxO proteins bind to the insulin response element (IRE) to stimulate target gene expression on diverse pathways including cell metabolism, proliferation, differentiation, oxidative stress, cell survival, senescence, autophagy, and aging, counteracting insulin action [92].
FoxO repression via insulin signaling activity results in an attenuation of FoxO-dependent expression of genes like those coding for glucose 6-phosphatase or phosphoenolpyruvate carboxykinase [93, 94]. Among other genes, FoxO-regulated antioxidants include the Mn-dependent superoxide dismutase [95]. Signaling pathways that regulate stress and redox status also regulate FoxO proteins, thus, impinge on insulin signaling and the diabetic state: p38, AMP-activated protein kinase (AMPK), among others. The NAD-dependent protein deacetylase sirtuin-1 (Sirt1) directly modifies FoxO transcription factors and promotes their nuclear translocation and activation of target genes [96]. In addition, the acetylation state of histones and the FoxO coactivator PGC-1α (peroxisome proliferator-activated receptor γ-coactivator-1α) may modify the effect of a stimulus on FoxO-induced gene transcription [97]. In conclusion, the FoxO genes transcription is regulated by a variety of physiological cues and pathological stress stimuli frequently associated with increased oxidative stress.
1.2. Oxidative Stress and Insulin Signaling
Oxidative stress is considered a key factor in the development and progression of diabetes and its complications [98]. In vertebrates, Sestrins 1–3 (Sesns) form a family of conserved stress-responsive proteins [99]. The Sesns regulate the insulin pathway by regulating the AMP kinase and TOR [100, 101]. Sesn1 was identified as the product of a gene (PA26) activated by the transcription factor p53 in cells exposed to genotoxic stress. Later, it was isolated also as a FoxO responsive gene in growth factor stimulated cells [102]. Sesn 2 promotes the degradation of Kelch-like protein 1 (Keap1) leading to upregulation of Nrf2 signaling and the induction of genes for antioxidant enzymes. The adaptor protein p62 is required for the Sesn 2-dependent activation of Nrf2 [103]. Sesns block TOR-C1 activation and thereby reduce reactive oxygen species accumulation [104]. In Drosophila, a single Sestrin homolog has been isolated and characterized. It is activated by accumulation of reactive oxygen species and regulates insulin signaling. Mutant flies suffer from metabolic disarray, muscle wasting, and mitochondrial dysfunction [105]. The Drosophila Sestrin acts through two GATOR protein complexes. These GATOR complexes regulate the activity of the RagB GTPase, necessary for TOR-C1 activity. The Drosophila Sestrin binds to GATOR2. Bound to Sestrin, GATOR2 frees GATOR1. Free GATOR1 inhibits RagB function by activating its GTPase activity, thus, inhibiting TOR-C1 activation [106, 107]. In vertebrates, the GATOR complexes act in the same fashion; GATOR complexes are evolutionarily conserved in metazoans [108].
Reduction of energy levels in the cells causes the activation of the AMP-activated protein kinase, AMP kinase. This, in its turn, results in TSC2 phosphorylation and subsequent TOR-C1 inhibition. A hypoxic state also reduces TOR activity via the hypoxia-inducible factor-1 (Hif-1) that affects the hypoxia-induced response genes Redd1 [109] and Scylla [110]. Scylla forms a complex with charybdis, negatively regulating TOR-C1 acting downstream of Akt/PKB and upstream of TSC [110].
2. Experimental Animal Models: Vertebrates
Type 1 diabetes is characterized by progressive β-cell destruction. Insulin resistance in target tissues characterizes type 2 diabetes. The majority of obese individuals do not become diabetic, although over weight or obesity are clear risk factors for diabetes. In the United States, 87.5% of adults over 18 years old were overweight (including obese and morbidly obese individuals), and an estimated 12.2% of the population is diabetic in 2017 [111], suggesting that β-pancreatic cells failure is required to cause hyperglycemia [112]. Due to its overall evolutionary conservation, animal models are used to identify mechanisms, principles, and potential drug targets, besides elucidating general underpinnings of biological metabolic significance. Many animal models of diabetes are currently available for elucidating the pathophysiology of diabetes and testing novel therapies for complications. However, since diabetes etiology is multifactorial, no single animal model may exactly replicate the human situation. Several of these animal models can be used to study chronic diabetes phenotypes.
In principle, all of the models reviewed below could be used for chronic aspects of diabetes and the accruement and evolution of the diabetic state. In spite of this opportunity, in most cases experiments are begun when the diabetic model organisms have advanced to a frank diabetic state (for example, when the resting glucose level is above 250–300 mg/dl several days/weeks after streptozotocin (STZ) injection in rats; see below). It is desirable to study the initial states, starting when the STZ injection is given, and studying the acquirement of the diabetic state, as well as its ulterior evolution. The models reviewed here could well serve or have served this purpose.
2.1. Chemical Induction of Diabetes
Alloxan and STZ treatments are the most used diabetes models for diabetic complications in vertebrates. Both chemicals are toxic glucose analogues transported into the cells via the Glut 2 transporter [113]. Both treatments lead to necrosis, importantly of insulin-producing cells, but by different mechanisms. Alloxan generates toxic free radicals, leading to cell death via necrosis. STZ is cleaved, generating free methylnitrosourea that induces DNA fragmentation and necrotic cell decay [114]. Although STZ may also have toxic effects on other organs, its effectiveness and side effects depend mainly on tissue-specific Glut 2 expression, animal age, and nutritional status [114]. STZ administration to 0–2-day-old rats induces an inadequate beta cell mass used as a type 2 diabetes model [115].
A variety of mammals, rodents, rabbits, dogs, pigs, and nonhuman primates, have been used as models of STZ- and alloxan-induced diabetes. The small size of rodents and rabbits results advantageous for maintenance costs, especially in longitudinal studies, but somewhat limits sample material available per animal. In recent years, the pig has gained importance because of its size and close similarity to human physiology. Minipigs have clear advantages over domestic pigs, and genetic modifications leading to diabetic phenotypes have been developed [116].
2.2. Genetic Vertebrate Models of Diabetes
Yet to date, rodents represent the predominant vertebrate species used in biomedical research because of traditional use and accumulated knowledge, known animal husbandry, evolutionarily conserved metabolic pathways, the capacity to conduct experiments in organs and study physiology, and, more recently, genetic manipulation possibilities. Among them, several genetic mutant strains are extensively used, depending on the diabetic aspect under study.
The Akita mice have an Ins2+/C96Y mutation, a single nucleotide substitution in the insulin 2 gene (Ins2). This mutation causes reduced insulin secretion, resulting in the development of type 1 diabetes [117]. The db/db mouse is the most popular model of type 2 diabetes. They have a deletion mutation in the leptin receptor resulting in defective receptor function for the adipocyte-derived hormone leptin. This mutation leads to the developing of obesity, insulin resistance, and diabetes [118]. Other mutants with altered metabolism such as the agouti (Ay) mouse, a polygenic model of obesity-induced diabetes, and the ApoE −/− (apoliporotein E deficient) mouse, an atherosclerosis model, are available [119–122].
The Zucker fatty (ZF) rat ports a homozygous missense mutation (fatty, fa) in the leptin receptor gene and develops obesity without diabetes, although rats develop progressive insulin resistance and glucose intolerance [123]. The Wistar fatty (WF) rat is a congenic strain of the Wistar Kyoto rat that also has a fa/fa homozygous missense mutation in the leptin receptor gene. This strain develops obesity [124, 125]. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is a recognized model of type 2 diabetes. Rats show impaired glucose tolerance, observed from 8 weeks of age, hyperglycemia, and peripheral insulin resistance [126, 127]. The Goto-Kakizaki (GK) rat is a model of nonobese type 2 diabetes. This is a Wistar substrain that develops mild hyperglycemia, insulin resistance, and hiperinsulinemia [128–131]. The ZDF-Leprfa/Crl rat was originated in a colony of Zucker rats, expressing type 2 diabetes, among other models [132–134].
In addition, there are also a variety of different polygenic models of obesity that include the KK-AY mice [135], New Zealand obese (NZO) mice [136], the TALLYHO/Jng mice [137], and the OLETF rats [127], besides diet-induced models of obesity [30]. These models also lead to diabetic states.
3. Invertebrate Insulin Signaling
Besides vertebrate diabetes models, two main invertebrate models have been used in experiments. These two invertebrate models are the nematode Caenorhabditis elegans and the fruit fly Drosophila melanogaster.
3.1. Caenorhabditis elegans
In the nematode Caenorhabditis elegans some of the insulin pathway main components were first characterized, like the nematode homolog, age-1. In C. elegans, faulty insulin signaling leads to life extension, metabolism changes, disrupted growth, and stress resilience, reminiscent of some diabetic phenotypes [138, 139].
In this nematode the insulin pathway genes were discovered by virtue of their control of dauer larva formation and longevity, evidencing a relationship between aging/nutrition/lifespan [140]. Dauer larvae are formed between larval stages two and three and represent an alternative third larval stage that can survive harsh environmental conditions for up to four months. The pivotal genes in the C. elegans insulin pathway are evolutionarily conserved. The insulin receptor homolog is daf-2 (from abnormal dauer formation), the IRS homolog is ist-1 [141], the PI3K catalytic subunit is age-1 (from aging alteration), and the regulatory subunit is aap-1 [141], PTEN is daf-18, Akt is Akt-1 and Akt-2, and FoxO is daf-16 [142]. Similar to the case in Drosophila, the insulin pathway is unique and required for many functions including nutritional assessment and metabolism, growth, lifecycle, longevity, and behavior. The study of dauer larvae formation in C. elegans has already yielded insights into metabolic/nutritional control and longevity with relevance to humans [143]. There have also been studies regarding behavior modifications, degenerative diseases, and the roles played by FoxO transcription factors in the worm and humans [144]. Learning, memory, and organismal growth are also other chronic conditions where research in C. elegans insulin pathway has pinpointed general functions [142].
3.2. Drosophila and Diabetes
D. melanogaster insulin signaling has been evolutionarily conserved, and both types 1 and 2 diabetes can be modeled. Reducing or nearly abrogating expression of the insulin-like peptides (ILP) in the fly can achieve type 1 diabetes [23]. On the other hand, several manipulations can lead to diabetes type 2: mutations in the insulin pathway components downstream from the ILPs [27, 45], dietary manipulations leading to obesity, metabolic imbalance, and hyperglycemia [145–148], or studies in other Drosophila species with different lifestyles/diets [149, 150]. As these different experimental protocols, applied in genetically homogeneous fly populations converge, essentially, in a reproducibly diabetic state and faulty insulin signaling, they can all be used for longitudinal studies characterizing the accruement and evolution of compromised diabetic signaling, diabetic phenotypes, and their consequences.
Drosophila is uniquely poised to study the insulin pathway and diabetes chronic aspects: it has a very well-developed genetic toolkit simply not available, or not as easily amenable, and with higher genetic background homogeneity and rigor as other models, a very highly polished sequenced genome, a “simplified” insulin pathway, with components exhibiting far less redundancy than, for example, vertebrate models, and the availability of different species with similar sequenced genomes that represent “natural” experiments with different lifestyles and diets, among other advantages. It is particularly of note the capacity to generate different types of genetic mosaics in the whole organism, allowing study and analysis of the cell, tissue, and organismal consequences, and cell independence of mutations, and the localization of functional “foci.” Another related advantage is the possibility of generating space and time limited genetic mosaics that can be used to distinguish between developmental defects versus metabolic defects, for example.
3.2.1. Different Drosophila Species Lead to Different Lifestyles
Most of the well-known Drosophila species are saprophytic [151]. Inside this big genus (over 2,000 species described so far), there are both generalist and specialist ones, with omnivorous or restricted diets. The environmental conditions that each population faces, together with the availability of nutrients, altitude, latitude, temperature, and so on, can impinge on differences and adaptations that have effects, whether direct or indirect, on insulin signaling. It can affect the levels of activity and, in general, the lifestyle of populations. There are studies examining the effect of varying diets in different Drosophila species, whether or not they support life of the organisms in a long-term basis [149, 150, 152]. Some of these changes may or may not have to do with adaptations involving the insulin pathway [153, 154]. In any case, the fact that the genomes plus many other ecological and genomic variables are already known [155–157] implies a great advantage for insulin pathway studies of these ecologically diverse species [158]. This avenue of research represents a window of opportunity as there are more and more Drosophila species characterized that can be cultivated in the laboratory, with their genomes sequenced, available for study [159].
Other examples of studies with different Drosophila species include D. simulans, where metabolic rate, longevity, and resistance to stress have been studied [160, 161]. D. sechellia, found only in the Seychelles archipelago and requiring the fruit Morinda citrifolia as specialized and niche nutrition, toxic for other species, has been thoroughly researched [162, 163]. A recent adaptation of a population of D. yakuba to the same nutritional resource as D. sechellia in an island population (as opposed to conspecific populations in the continent), namely, Morinda fruit, is striking. This represents a particularly interesting case of a recent adaptation to a major diet shift [164]. D. mojavensis requires cacti as a feeding resource and has even specialized to different host cacti in different populations [165]. Together, they may allow dissection of the mechanisms behind the differences and preferences for specific nutrients, oviposition sites, and their tolerance and metabolism. For example, D. mojavensis shows a better resistance to the presence of alcohol, a product of the fermentation of cacti [166]. It will be interesting to study in these examples the changes, if any, in the insulin pathway due to their specialized and restricted nutritional resources, and how might a diabetic state alter their metabolism.
3.2.2. Drosophila melanogaster and Insulin Signaling
In D. melanogaster, the growth of the organism is regulated by insulin signaling and the interaction of this signal with the levels of juvenile hormone and ecdysone. Additionally, there is the role played by the kinase TOR of the insulin pathway, which, as stated above, couples growth with the amount of available nutrients [167], at least in part via the amino acid transporter Slimfast [84]. Since the fruit fly is a poikilothermic organism, it is affected by ambient temperature in a direct way, presenting a larger size at lower temperatures, and an increase in size with latitude and altitude [168]. The number of ovarioles in females is also susceptible to these factors, being lower in tropical populations and it has been shown that insulin signaling activity underlies these differences [169, 170].
Besides growth hormones, diet, and temperature, gut microbiota can modulate insulin pathway activity. Between populations and lines there are differences in the microbiome, and this influences insulin signaling [171, 172]. Strains infected with the Wolbachia endosymbiont exhibit increased insulin signaling, whereas lack of Wolbachia worsens insulin mutants phenotypes, particularly the decline in fecundity and adult weight [173]. Loss of ILPs in the brain, on the other hand, extends lifespan if Wolbachia is present [24]. Lactobacillus partially rescues growth in poorly fed larvae and Acetobacter pomorum also modulates insulin signaling [174, 175]. All of these factors have to be taken into consideration, ideally, when longitudinal studies are performed, since many of these factors may vary with age, independent of the status of the fly.
Longevity has also been tied at times with insulin signaling [176–178]. Experimental model organism lines that were selected because of their increased lifespan, often present changes in the insulin pathway. Hoffman et al. [179] performed WGS and GWAS studies on long-lived Drosophila strains and found that the metabolites that decline with age are associated with glycolysis and the metabolism of glycophospholipids. Changes were observed associated with age and sex in biogenic amines, and carnitines, required for the transfer of fatty acids in the mitochondria where they pass through beta-oxidation generating acetyl-coA required for the Krebs cycle.
3.2.3. Inducing the Diabetic State through Diet
Providing Drosophila diets with increased or decreased nutrients provokes the deregulation of its metabolism and of insulin signaling. High-sugar as well as high-protein diets increase insulin-like peptide expression (ILPs) [148, 180]; this initial increase in ILP expression is consistent with what is observed in vertebrates in the accruement of insulin resistance, where the organism initially tries to increase its insulin production to compensate for excess nutrient input. However, in vertebrates, the eventual deterioration of beta cells leads to ultimate failure of this initial compensation [181]. Similarly, in overfed flies, the fat body secondarily reduces its insulin response to increased circulating ILPs, and this diminution decreases significantly as flies age, rendering flies completely resistant at advanced ages [147]. These results support the observation that a diet rich in fat initially increases levels of different ILPs, rescuing at a first stage the overfed phenotype by means of hyperinsulinemia. This increase in insulin signaling, plus hyperglycemia, though, leads to an increase in free fatty acids by inappropriate lipolysis and the generation of insulin resistance, particularly in the fat body. High fat diets also contribute to heart dysfunction [145, 182].
Sugar, lipid, and protein variation in diets have led to effects in fertility, longevity, sugar and fat accumulation, weight changes, induction of insulin resistance, and aging [147, 183]. In general, the results are consistent with protein and carbohydrate balance determining lifespan. In some cases diets high in carbohydrates and low in proteins allow greater longevity, often accompanied by lower fecundity. In other studies, extra protein intake results in lean and longer lived flies, while carbohydrate intake leads to obese flies; finally, balanced, intermediate carbohydrate:protein ratios diets have also been found to lead to longer-lived flies [183–187].
Dietary restriction has been variously applied to flies, often leading to longer life-spans and insulin signaling involvement [188–190]. Dietary restriction effects are also seen in immunity via insulin signaling regulation [191]. The insulin signaling is clearly part of the equation in all these studies, although it may not be the sole determinant [192, 193]. One such type of dietary restriction is caloric restriction. Caloric restriction is defined as a reduction in caloric intake without malnutrition and has shown in several models a positive effect on calorie consumption, including yeast and C. elegans besides D. melanogaster [194–196]. Another variation of diet manipulation with nutritional and lifespan consequences is methionine availability in the diet [197]. Also, parental obesity leads to transgenerational effects [198].
Clearly, diet manipulation can be used to generate and evolve diabetic states akin to diabetes type 2 in flies, and by its very nature, it is easily amenable for longitudinal studies. Yet an important problem besieging all these studies, and one that may explain conflicting results, is that all these diet regimes are semidefined chemically, at best, so that studies that modify diets are very difficult to compare. They typically define “protein” as amount of yeast in the medium, or “carbohydrate” as unrefined sugar or molasses, for example, which are clearly broad generalizations, as yeast cells have carbohydrates, lipids, and other nutrients besides proteins, and unrefined sugar or molasses are not only composed of carbohydrates. In the future, diets should strive to be defined chemically, so that they can be comparable and used reproducibly by different laboratories. In addition, total consumption should be measured, since unconstrained flies have free access to their food source and are able to regulate their caloric intake, at least in the case of D. melanogaster [183]. Also, these studies are subject to environmental variations, the use of different sexes, different genetic backgrounds, different strains, different age of flies, and so on, all of which affect the outcome of the studies. And while these studies show that changes in diet have clear effects on the body and insulin signaling, lack of definition constitutes a limiting factor in these experimental approaches.
3.2.4. Diabetes in Flies by Virtue of Mutations in the Insulin Pathway
Flies homozygous mutant for genes in the insulin pathway are born diabetic. There are advantages to this approach: the nature of the defect is known, and the genetic background and environmental conditions can be controlled in a rigorous manner. The different stages of the lifecycle can also be exploited, with stages where feeding occurs (larvae, adults), and stages without food input (pupae). Faulty insulin signaling by virtue of mutations can both be used to model diabetes type 1 (ILPs loss-of-function mutations [23], and even explore genes regulating insulin secretion [199]), and also diabetes type 2, with loss-of-function mutations in the rest of the pathway, as the net effect would be insulin resistance [27, 31, 71]. Furthermore, both whole organisms can be mutant (in hypomorphic conditions, as null alleles are mostly lethal), or only in selected tissues and organs ([18, 45, 48, 77, 91, 200, 201], among other references cited throughout this review).
Drosophila has seven insulin-like peptides (ILPs). They are partially redundant, so knock-outs for a particular ILP usually have moderate effects, and it is necessary to have more than one ILP gene loss-of-function mutation to generate lethality [23, 24]. In contrast, mutations in InR, Dp110, and other components of the pathway are homozygous lethal, so heterozygous (as there are some dominant effects [45, 202]) or heteroallelic flies are often used. The latter have the benefit of allocating more robustly the defects observed to the studied mutation (for example, see [31]). chico (the insulin receptor substrate fly homolog) is a particular case. The homozygous mutant chico1 allele was originally described as viable, helping establish the typical mutant phenotypes of partial loss-of-function of insulin pathway mutants [45]. Later it was found out that, when devoid of the endosymbiont Wolbachia, chico loss-of-function conditions are homozygous lethal, underscoring the close association between gut microbiota and insulin signaling [173].
The most common phenotypes caused by mutants in the insulin pathway are a decrease in fertility, decreased size of organisms, changes in longevity (decrease in normal conditions, often an increase in longevity when there is caloric restriction), defects in fat body morphology, in heart, retina, and brain physiology, increased levels of triacylglycerides, and higher amounts of circulating sugars in the hemolymph [31, 45, 71, 145, 147, 203–205].
Insulin/TOR pathway function is critical in the regulation of growth, autophagy, cell and organism survival, and anabolism (regulating lipid and carbohydrate homeostasis) [22]. Lack of nutrients or ATP impedes its function, and overfeeding can lead to loss of balance: insulin participates in the accumulation of lipids and carbohydrates, so that an excessive intake of nutrients can lead to hyperactivation of the pathway, and lipid and glycogen accumulation. TOR kinase is regulated by insulin signaling and by amino acids, acting as a central point in metabolism regulation. It has also been implicated in aging [206–208].
Besides nutritional input, there are other conditions that regulate insulin signaling: as an example, one such state is the systemic response to stress (which, of course, is activated by lack of nutrition, among other stimuli). One such stress trigger is infection, and innate immunity activation. Activation of Toll in the fat body leads to the induction of immunity, redistribution of resources, and activation of JNK and NFK-β by inflammation. Here, attenuation of insulin signaling leads to FoxO activity, which regulates genes participating in stress response and metabolic control: blockade of gluconeogenesis, glycogenolysis, and the use of storage lipids for catabolism [209]. It may also increase longevity if FoxO is upregulated in adipose or intestinal tissue [210]. In addition, chronic intestinal activation of FoxO may lead to deregulation of lipid homeostasis [211].
3.2.5. Towards the Characterization of Chronic Diabetes in Flies
What can be studied in these fly diabetes models in a longitudinal study? Nearly every aspect of diabetes mellitus mentioned so far: from initial phenotypes, to its evolution and consequences at old age, up to death. We have discussed above metabolic imbalances and longevity as two of the most studied effects [212, 213]. Other mutant phenotypes include decreased fertility and altered physiology of various organs and systems (nervous system, heart, fat tissue, muscles, etc.), for example, showing involvement of nervous system function: electrical activity or octopamine neurotransmission [214, 215] or heart dysfunction [216].
Perturbations like chronic stress can be addressed, and the effects of external factors on the diabetic state, such as nutritional variation, light regime, and temperature, can also be addressed, for example, the effect of artificial sweeteners upon insulin pathway signaling [217] or the effect of xenobiotics on the insulin pathway [218]. Fundamentally, though, the inception and “normal” progression of the mutant condition or the diseased state can be closely followed, for example, the relationship between sleep and metabolism alterations via insulin signaling [219–221]. Unfortunately, to this day few studies have consciously addressed these chronic aspects of diabetes, although some do compare flies at different stages, like during oogenesis [222], or pupariation [223], or neurite remodeling [224], or adult stages [225].
Most of the studies to date that touch on longitudinal aspects address longevity, fertility [226], and its phenocritical period [23, 202, 227, 228], like the earlier appearance of locomotor defects [229], size and growth phenotypes [45, 200, 201, 230], and metabolism [71]. In summary, the Drosophila model represents a window of opportunity not only to study fundamental aspects of diabetes and the diabetic state, but also its complications, and the effect of various external stimuli and factors in the accruement and development of the disease. Future studies will, undoubtedly, target and address these issues to a much greater extent.
4. Conclusions
Notwithstanding the ubiquity and utility of vertebrate models of diabetes (especially rodent models), and when compared with other experimental models of diabetes, Drosophila has clear advantages. Despite sometimes ill-defined parameters in diet regimes and environment, the model strengths, namely, a robust, extensive, and highly developed genetic system, with a plethora of isolated and characterized insulin pathway and general metabolism genes, ease of manipulation and use, low cost, high fertility, and numbers, short generation time, high evolutionary conservation, and homogeneous genetic backgrounds, among other positive characteristics, make the fly a premier system for insulin pathway and metabolic studies.
Although many aspects of diabetes mellitus have been studied in the different fly models specially, there is still a dearth of longitudinal studies. In such studies, ideally exception should be taken of the differences that occur in metabolism and lifestyle normally as flies age; that is to say, these studies should always par appropriate control flies with experimental ones throughout the life cycle with the same genetic background, to effectively tease away differences due to the occurrence and evolution of the diabetic state from normal aging. This is especially true of a disease that touches many different aspects of the organisms' wellbeing, and one that by its very nature is very pleiotropic and polygenic.
Notwithstanding this, the fruit fly represents one of the more promising, rigorous, and thoroughly researched models of diabetes in which we carry out such research. Due to great evolutionary conservation, it allows for particularly detailed and controlled studies covering nearly all aspects of this chronic and fatal disease. Compared to other available models, that is, vertebrate studies, be it organismal or even cell tissue culture ones, the fly favorably compares, allowing for more holistic and encompassing approaches.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Narayan K. M. V., Zhang P., Kanaya A. M., et al. Diabetes: The Pandemic and Potential Solutions. In: Jamison D. T., Breman J. G., Measham A. R., editors. Disease Control Priorities in Developing Countries. 2nd. Washington, DC, USA: 2006. [Google Scholar]
- 2.Chen L., Magliano D. J., Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nature Reviews Endocrinology. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 3.Mathers C. D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3(11, article e442) doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OECD. Health at a Glance 2017: OECD Indicators. Paris, France: OECD Publishing; 2017. Diabetes prevalence. [Google Scholar]
- 5.Dodds S. The How-To for Type 2: An Overview of Diagnosis and Management of Type 2 Diabetes Mellitus. Nursing Clinics of North America. 2017;52(4):513–522. doi: 10.1016/j.cnur.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Kahn B. B., Flier J. S. Obesity and insulin resistance. The Journal of Clinical Investigation. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banting F. G., Best C. H., Collip J. B., Campbell W. R., Fletcher A. A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Canadian Medical Association Journal. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 8.NM AB. AB NM, Frederick G. Banting-Facts, 2014.
- 9.Fortin J. S., Santamaria-Bouvier A., Lair S., Dallaire A. D., Benoit-Biancamano M.-O. Research open access anatomic and molecular characterization of the endocrine pancreas of a teleostean fish: Atlantic wolffish (Anarhichas lupus) Zoological Studies. 2015;54 doi: 10.1186/s40555-014-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biology. 2002;12(15):1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 11.Menting J. G., Gajewiak J., MacRaild C. A., et al. A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nature Structural & Molecular Biology. 2016;23(10):916–920. doi: 10.1038/nsmb.3292. [DOI] [PubMed] [Google Scholar]
- 12.Safavi-Hemami H., Gajewiak J., Karanth S., et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proceedings of the National Acadamy of Sciences of the United States of America. 2015;112(6):1743–1748. doi: 10.1073/pnas.1423857112/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- 14.Vallejo D. M., Juarez-Carreño S., Bolivar J., Morante J., Dominguez M. A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science. 2015;350(6262, article no. 6767) doi: 10..1126/science.aac6767. [DOI] [PubMed] [Google Scholar]
- 15.Colombani J., Andersen D. S., Boulan L., et al. Drosophila Lgr3 Couples Organ Growth with Maturation and Ensures Developmental Stability. Current Biology. 2015;25(20):2723–2729. doi: 10.1016/j.cub.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Colombani J., Andersen D. S., Léopol P. Secreted peptide dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336(6081):582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 17.Garelli A., Gontijo A. M., Miguela V., Caparros E., Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336(6081):579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- 18.Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Current Biology. 2001;11(4):213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 19.Nässel D. R. Neuropeptides in the nervous system of Drosophila and other insects: Multiple roles as neuromodulators and neurohormones. Progress in Neurobiology. 2002;68(1):1–84. doi: 10.1016/S0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q., Brown M. R. Signaling and function of insulin-like peptides in insects. Annual Review of Entomology. 2006;51(1):1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 21.Wessells R., Fitzgerald E., Piazza N., et al. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8(5):542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teleman A. A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochemical Journal. 2010;425(1):13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 23.Broughton S. J., Piper M. D. W., Ikeya T., et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Acadamy of Sciences of the United States of America. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grönke S., Clarke D.-F., Broughton S., Andrews T. D., Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genetics. 2010;6(2) doi: 10.1371/journal.pgen.1000857.e1000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldfine I. D., Youngren J. F. Contributions of the American Journal of Physiology to the discovery of insulin. American Journal of Physiology-Endocrinology and Metabolism. 1998;274(2):E207–E209. doi: 10.1152/ajpendo.1998.274.2.E207. [DOI] [PubMed] [Google Scholar]
- 26.Murillo-Maldonado J. M., Bou Zeineddine F., Stock R., Thackeray J., Riesgo-Escovar J. R. Insulin receptor-mediated signaling via phospholipase C-γ regulates growth and differentiation in drosophila. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0028067.e28067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murillo-Maldonado J. M., Riesgo-Escovar J. R. Development and diabetes on the fly. Mechanisms of Development. 2017;144:150–155. doi: 10.1016/j.mod.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 28.LeRoith D., Accili D. Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. Nature Clinical Practice Endocrinology & Metabolism. 2008;4(3):164–172. doi: 10.1038/ncpendmet0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muniyappa R., Montagnani M., Koh K. K., Quon M. J. Cardiovascular actions of insulin. Endocrine Reviews. 2007;28(5):463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T., Kubota N., Kadowaki T. Imbalanced Insulin Actions in Obesity and Type 2 Diabetes: Key Mouse Models of Insulin Signaling Pathway. Cell Metabolism. 2017;25(4):797–810. doi: 10.1016/j.cmet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Murillo-Maldonado J. M., Sánchez-Chávez G., Salgado L. M., Salceda R., Riesgo-Escovar J. R. Drosophila insulin pathway mutants affect visual physiology and brain function besides growth, lipid, and carbohydrate metabolism. Diabetes. 2011;60(5):1632–1636. doi: 10.2337/db10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honegger B., Galic M., Köhler K., et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. Journal of Biology. 2008;7(3, article no. 10) doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan C., Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. General and Comparative Endocrinology. 2005;142(1-2):44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Arquier N., Géminard C., Bourouis M., et al. Drosophila ALS Regulates Growth and Metabolism through Functional Interaction with Insulin-Like Peptides. Cell Metabolism. 2008;7(4):333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Swanson L. E., Yu M., Nelson K. S., Laprise P., Tepass U., Beitel G. J. Drosophila convoluted/dALS is an essential gene required for tracheal tube morphogenesis and apical matrix organization. Genetics. 2009;181(4):1281–1290. doi: 10.1534/genetics.108.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consortium F. B. Flybase - the Drosophila database. Nucleic Acids Research. 1994;22(17):3456–3458. doi: 10.1093/nar/22.17.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi C. M., Emanuelli B., Kahn C. R. Critical nodes in signalling pathways: insights into insulin action. Nature Reviews Molecular Cell Biology. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 38.Cohen P., Alessi D. R., Cross D. A. E. PDK1, one of the missing links in insulin signal transduction? FEBS Letters. 1997;410(1):3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- 39.Draznin B. Molecular mechanisms of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of p85α: the two sides of a coin. Diabetes. 2006;55(8):2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- 40.Hajduch E., Litherland G. J., Hundal H. S. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Letters. 2001;492(3):199–203. doi: 10.1016/s0014-5793(01)02242-6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez R., Tabarini D., Azpiazu N., Frasch M., Schlessinger J. The Drosophila insulin receptor homolog: A gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO Journal. 1995;14(14):3373–3384. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi T., Fernandez R., Roth R. A. Comparison of the Signaling Abilities of the Drosophila and Human Insulin Receptors in Mammalian Cells. Biochemistry. 1995;34(15):4962–4968. doi: 10.1021/bi00015a007. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto N., Nakamori R., Murai T., Yamauchi Y., Masuda A., Nishimura T. A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in drosophila. Genes & Development. 2013;27(1):87–97. doi: 10.1101/gad.204479.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan Y., Chen C., Cao Y., Garofalo R. S. The Drosophila insulin receptor contains a novel carboxyl-terminal extension likely to play an important role in signal transduction. The Journal of Biological Chemistry. 1995;270(9):4236–4243. doi: 10.1074/jbc.270.9.4236. [DOI] [PubMed] [Google Scholar]
- 45.Böhni R., Riesgo-Escovar J., Oldham S., et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97(7):865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 46.Almudi I., Poernbacher I., Hafen E., Stocker H. The Lnk/SH2B adaptor provides a fail-safe mechanism to establish the Insulin receptor-Chico interaction. Cell Communication and Signaling. 2013;11(1, article no. 26) doi: 10.1186/1478-811X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werz C., Köhler K., Hafen E., Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genetics. 2009;5(8) doi: 10.1371/journal.pgen.1000596.e1000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leevers S. J., Weinkove D., MacDougall L. K., Hafen E., Waterfield M. D. The drosophila phosphoinositide 3-kinase Dp 110 promotes cell growth. EMBO Journal. 1996;15(23):6584–6594. [PMC free article] [PubMed] [Google Scholar]
- 49.Weinkove D., Leevers S. J., MacDougall L. K., Waterfield M. D. p60 is an adaptor for the Drosophila phosphoinositide 3-kinase, Dp110. The Journal of Biological Chemistry. 1997;272(23):14606–14610. doi: 10.1074/jbc.272.23.14606. [DOI] [PubMed] [Google Scholar]
- 50.Vanhaesebroeck B., Leevers S. J., Panayotou G., Waterfield M. D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends in Biochemical Sciences. 1997;22(7):267–272. doi: 10.1016/S0968-0004(97)01061-X. [DOI] [PubMed] [Google Scholar]
- 51.Haslam R. J., Koide H. B., Hemmings B. A. Pleckstrin domain homology. Nature. 1993;363(6427):309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- 52.Rintelen F., Stocker H., Thomas G., Hafen E. PDK1 regulates growth through Akt and S6K in Drosophila. Proceedings of the National Acadamy of Sciences of the United States of America. 2001;98(26):15020–15025. doi: 10.1073/pnas.011318098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franke T. F., Tartof K. D., Tsichlis P. N. The SH2-like Akt homology (AH) domain of c-akt is present in multiple copies in the genome of vertebrate and invertebrate eucaryotes. Cloning and characterization of the Drosophila melanogaster c-akt homolog Dakt1. Oncogene. 1994;9(1):141–148. [PubMed] [Google Scholar]
- 54.Powell D. J., Hajduch E., Kular G., Hundal H. S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Molecular and Cellular Biology. 2003;23(21):7794–7808. doi: 10.1128/mcb.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayascas J. R. Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle. 2008;7(19):2978–2982. doi: 10.4161/cc.7.19.6810. [DOI] [PubMed] [Google Scholar]
- 56.Bayascas J. R., Wullschleger S., Sakamoto K., et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Molecular and Cellular Biology. 2008;28(10):3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alessi D. R., Andjelkovic M., Caudwell B., et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO Journal. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 58.Goberdhan D. C. I., Paricio N., Goodman E. C., Mlodzik M., Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes & Development. 1999;13(24):3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang H., Potter C. J., Tao W., et al. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126(23):5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 60.Kim R. H., Mak T. W. Tumours and tremors: How PTEN regulation underlies both. British Journal of Cancer. 2006;94(5):620–624. doi: 10.1038/sj.bjc.6602994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wittwer F., Jaquenoud M., Brogiolo W., et al. Susi, a negative regulator of Drosophila PI3-Kinase. Developmental Cell. 2005;8(6):817–827. doi: 10.1016/j.devcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Manning B. D. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. The Journal of Cell Biology. 2004;167(3):399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hresko R. C., Mueckler M. mTOR·RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. The Journal of Biological Chemistry. 2005;280(49):40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence J. C., Jr., Roach P. J. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46(4):541–547. doi: 10.2337/diab.46.4.541. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence J. C., Jr., Skurat A. V., Roach P. J., Azpiazu I., Manchester J. Glycogen synthase: Activation by insulin and effect of transgenic overexpression in skeletal muscle. Biochemical Society Transactions. 1997;25(1):14–19. doi: 10.1042/bst0250014. [DOI] [PubMed] [Google Scholar]
- 66.Liu P., Cheng H., Roberts T. M., Zhao J. J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Reviews Drug Discovery. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teleman A. A., Chen Y.-W., Cohen S. M. Drosophila melted modulates FOXO and TOR activity. Developmental Cell. 2005;9(2):271–281. doi: 10.1016/j.devcel.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Kaidanovich-Beilin O., Woodgett J. R. GSK-3: Functional Insights from Cell Biology and Animal Models. Frontiers in Molecular Neuroscience. 2011;4, article 40 doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellosta P., Gallant P. Myc function in Drosophila. Genes & Cancer. 2010;1(6):542–546. doi: 10.1177/1947601910377490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parisi F., Riccardo S., Daniel M., et al. Drosophila insulin and target of rapamycin (TOR) pathways regulate GSK3 beta activity to control Myc stability and determine Myc expression in vivo. BMC Biology. 2011;9, article no. 65 doi: 10.1186/1741-7007-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teleman A., Ratzenböck I., Oldham S. Drosophila: A Model for Understanding Obesity and Diabetic Complications. Experimental and Clinical Endocrinology & Diabetes. 2012;120(4):184–185. doi: 10.1055/s-0032-1304566. [DOI] [PubMed] [Google Scholar]
- 72.Kovacina K. S., Park G. Y., Bae S. S. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. The Journal of Biological Chemistry. 2003;278(12):10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 73.Pallares-Cartes C., Cakan-Akdogan G., Teleman A. A. Tissue-Specific Coupling between Insulin/IGF and TORC1 Signaling via PRAS40 in Drosophila. Developmental Cell. 2012;22(1):172–182. doi: 10.1016/j.devcel.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 74.Schleich S., Teleman A. A. Akt phosphorylates both Tsc1 and Tsc2 in drosophila, but neither phosphorylation is required for normal animal growth. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006305.e6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel P. H., Thapar N., Guo L., et al. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. Journal of Cell Science. 2003;116(17):3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 76.Patel P. H., Tamanoi F. Using Drosophila and Yeast Genetics to Investigate a Role for the Rheb GTPase in Cell Growth. Methods in Enzymology. 2005;407:443–454. doi: 10.1016/S0076-6879(05)07036-9. [DOI] [PubMed] [Google Scholar]
- 77.Montagne J., Stewart M. J., Stocker H., Hafen E., Kozma S. C., Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285(5436):2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 78.Teleman A. A., Chen Y.-W., Cohen S. M. 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes & Development. 2005;19(16):1844–1848. doi: 10.1101/gad.341505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teleman A. A., Hietakangas V., Sayadian A. C., Cohen S. M. Nutritional Control of Protein Biosynthetic Capacity by Insulin via Myc in Drosophila. Cell Metabolism. 2008;7(1):21–32. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Kuo Y., Huang H., Cai T., Wang T. Target of rapamycin complex 2 regulates cell growth via Myc in drosophila. Scientific Reports. 2015;5 doi: 10.1038/srep10339.10339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porstmann T., Santos C. R., Griffiths B., et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metabolism. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tóth M. L., Sigmond T., Borsos É., et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4(3):330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 83.Hennig K. M., Colombani J., Neufeld T. P. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. The Journal of Cell Biology. 2006;173(6):963–974. doi: 10.1083/jcb.200511140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colombani J., Raisin S., Pantalacci S., Radimerski T., Montagne J., Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114(6):739–749. doi: 10.1016/S0092-8674(03)00713-X. [DOI] [PubMed] [Google Scholar]
- 85.Géminard C., Rulifson E. J., Léopold P. Remote Control of Insulin Secretion by Fat Cells in Drosophila. Cell Metabolism. 2009;10(3):199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Rajan A., Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee S., Dong H. H. FoxO integration of insulin signaling with glucose and lipid metabolism. Journal of Endocrinology. 2017;233(2):R67–R79. doi: 10.1530/JOE-17-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brunet A., Bonni A., Zigmond M. J., et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 89.Kops G. J. P. L., de Ruiter N. D., De Vries-Smits A. M. M., Powell D. R., Bos J. L., Burgering B. M. T. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398(6728):630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 90.Kramer J. M., Davidge J. T., Lockyer J. M., Staveley B. E. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Developmental Biology. 2003;3, article 5 doi: 10.1186/1471-213X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Junger M. A., Rintelen F., Stocker H., et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. Journal of Biological Chemistry. 2003;2(3, article 20) doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Accili D., Arden K. C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 93.Schmoll D., Walker K. S., Alessi D. R., et al. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the Forkhead transcription factor FKHR: Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. The Journal of Biological Chemistry. 2000;275(46):36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 94.Barthel A., Schmoll D., Unterman T. G. FoxO proteins in insulin action and metabolism. Trends in Endocrinology & Metabolism. 2005;16(4):183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Kops G. J. P. L., Dansen T. B., Polderman P. E., et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419(6904):316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 96.Webb A. E., Brunet A. FOXO transcription factors: Key regulators of cellular quality control. Trends in Biochemical Sciences. 2014;39(4):159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 98.Erol A. Insulin resistance is an evolutionarily conserved physiological mechanism at the cellular level for protection against increased oxidative stress. BioEssays. 2007;29(8):811–818. doi: 10.1002/bies.20618. [DOI] [PubMed] [Google Scholar]
- 99.Budanov A. V., Lee J. H., Karin M. Stressin' Sestrins take an aging fight. EMBO Molecular Medicine. 2010;2(10):388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J. H., Budanov A. V., Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metabolism. 2013;18(6):792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parmigiani A., Nourbakhsh A., Ding B., et al. Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Reports. 2014;9(4):1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nogueira V., Park Y., Chen C.-C., et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14(6):458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bae S. H., Sung S. H., Oh S. Y., et al. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of keap1 and prevent oxidative liver damage. Cell Metabolism. 2013;17(1):73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Budanov A. V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J. H., Budanov A. V., Park E. J., et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327(5970):1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J. S., Ro S. H., Kim M., et al. Corrigendum: Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific Reports. 2015;5, article 14029 doi: 10.1038/srep14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim J. S., Ro S. H., Kim M., et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific Reports. 2015;5, article 9502 doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peng M., Yin N., Li M. O. SZT2 dictates GATOR control of mTORC1 signalling. Nature. 2017;543(7645):433–437. doi: 10.1038/nature21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brugarolas J., Lei K., Hurley R. L., et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes & Development. 2004;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reiling J. H., Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes & Development. 2004;18(23):2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prevention CfDCa. National Diabetes Statistics Report. National Institute of Diabetes and Digestive and Kidney Diseases. 2017, https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- 112.Kahn S. E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 113.Bonner-Weir S. Regulation of pancreatic beta-cell mass in vivo. Recent Progress in Hormone Research. 1994;49:91–104. doi: 10.1016/b978-0-12-571149-4.50008-8. [DOI] [PubMed] [Google Scholar]
- 114.Larsen M. O., Wilken M., Gotfredsen C. F., Carr R. D., Svendsen O., Rolin B. Mild streptozotocin diabetes in the Göttingen minipig. A novel model of moderate insulin deficiency and diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2002;282(6):E1342–E1351. doi: 10.1152/ajpendo.00564.2001. [DOI] [PubMed] [Google Scholar]
- 115.Rolo A. P., Palmeira C. M. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Hattangady N. G., Rajadhyaksha M. S. A brief review of in vitro models of diabetic neuropathy. International Journal of Diabetes in Developing Countries. 2009;29(4):143–149. doi: 10.4103/0973-3930.57344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J., Takeuchi T., Tanaka S., et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. The Journal of Clinical Investigation. 1999;103(1):27–37. doi: 10.1172/jci4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hummel K. P., Dickie M. M., Coleman D. L. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 119.Martinez H. G., Quinones M. P., Jimenez F., et al. Critical role of chemokine (C–C motif) receptor 2 (CCR2) in the KKAy+Apoe–/– mouse model of the metabolic syndrome. Diabetologia. 2011;54(10):2660–2668. doi: 10.1007/s00125-011-2248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proceedings of the National Acadamy of Sciences of the United States of America. 1992;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meir K. S., Leitersdorf E. Atherosclerosis in the apolipoprotein E-deficient mouse: a decade of progress. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(6):1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 122.Matsuo T., Shino A. Induction of diabetic alterations by goldthioglucose-obesity in KK, ICR and C57BL mice. Diabetologia. 1972;8(6):391–397. doi: 10.1007/BF01212165. [DOI] [PubMed] [Google Scholar]
- 123.Regulation of food intake in fatty and lean growing Zucker rats. Nutrition Reviews. 1977;35(7):181–183. doi: 10.1111/j.1753-4887.1977.tb06586.x. [DOI] [PubMed] [Google Scholar]
- 124.Ikeda H., Shino A., Matsuo T., Iwatsuka H., Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981;30(12):1045–1050. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- 125.Kitada M., Takeda A., Nagai T., Ito H., Kanasaki K., Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of sirt1 in diabetic wistar fatty (fa/fa) rats: a model of type 2 diabetes. Journal of Diabetes Research. 2011;2011:11. doi: 10.1155/2011/908185.908185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katsuda Y., Sasase T., Tadaki H., et al. Contribution of hyperglycemia on diabetic complications in obese type 2 diabetic SDT fatty rats: Effects of SGLT inhibitor phlorizin. Journal of Experimental Animal Science. 2014;64(2):161–169. doi: 10.1538/expanim.14-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katsuda Y., Ohta T., Miyajima K., et al. Diabetic complications in obese type 2 diabetic rat models. Journal of Experimental Animal Science. 2014;63(2):121–132. doi: 10.1538/expanim.63.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Akash M. S. H., Rehman K., Chen S. Goto-kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Current Diabetes Reviews. 2013;9(5):387–396. doi: 10.2174/15733998113099990069. [DOI] [PubMed] [Google Scholar]
- 129.Östenson C.-G., Efendic S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes, Obesity and Metabolism. 2007;9(supplement 2):180–186. doi: 10.1111/j.1463-1326.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 130.Brøndum E., Nilsson H., Aalkjær C. Functional Abnormalities in Isolated Arteries from Goto-Kakizaki and Streptozotocin-treated Diabetic Rat Models. Hormone and Metabolic Research. 2005;37:56–60. doi: 10.1055/s-2005-861370. [DOI] [PubMed] [Google Scholar]
- 131.Janssen U., Vassiliadou A., Riley S. G., Phillips A. O., Floege J. The quest for a model of type II diabetes with nephropathy: The Goto Kakizaki rat. Journal of Nephrology. 2004;17(6):769–773. [PubMed] [Google Scholar]
- 132.Arndt T., Wedekind D., Jörns A., et al. A novel Dock8 gene mutation confers diabetogenic susceptibility in the LEW.1AR1/Ztm-iddm rat, an animal model of human type 1 diabetes. Diabetologia. 2015;58(12):2800–2809. doi: 10.1007/s00125-015-3757-7. [DOI] [PubMed] [Google Scholar]
- 133.Jörns A., Günther A., Hedrich H.-J., Wedekind D., Tiedge M., Lenzen S. Immune cell infiltration, cytokine expression, and β-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54(7):2041–2052. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- 134.Lenzen S., Tiedge M., Elsner M., et al. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44(9):1189–1196. doi: 10.1007/s001250100625. [DOI] [PubMed] [Google Scholar]
- 135.Chakraborty G., Thumpayil S., Lafontant D.-E., Woubneh W., Toney J. H. Age dependence of glucose tolerance in adult KK-A y mice, a model of non-insulin dependent diabetes mellitus. Lab Animal. 2009;38(11):364–368. doi: 10.1038/laban1109-364. [DOI] [PubMed] [Google Scholar]
- 136.Veroni M. C., Proietto J., Larkins R. G. Evolution of insulin resistance in New Zealand obese mice. Diabetes. 1991;40(11):1480–1487. doi: 10.2337/diab.40.11.1480. doi: 10.2337/diab.40.11.1480. [DOI] [PubMed] [Google Scholar]
- 137.Denvir J., Boskovic G., Fan J., Primerano D. A., Parkman J. K., Kim J. H. Whole genome sequence analysis of the TALLYHO/Jng mouse. BMC Genomics. 2016;17(1, article no. 907) doi: 10.1186/s12864-016-3245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bharill P., Ayyadevara S., Alla R., Shmookler Reis R. J. Extreme depletion of PIP3 accompanies the increased life span and stress tolerance of PI3K-null C. elegans mutants. Frontiers in Genetics. 2013;4, Article 34 doi: 10.3389/fgene.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gami M. S., Wolkow C. A. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell. 2006;5(1):31–37. doi: 10.1111/j.1474-9726.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Riddle D. L., Swanson M. M., Albert P. S. Interacting genes in nematode dauer larva formation. Nature. 1981;290(5808):668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 141.Wolkow C. A., Muñoz M. J., Riddle D. L., Ruvkun G. Insulin receptor substrate and p55 orthologous adaptor proteins function in the Caenorhabditis elegans daf-2/insulin-like signaling pathway. The Journal of Biological Chemistry. 2002;277(51):49591–49597. doi: 10.1074/jbc.M207866200. [DOI] [PubMed] [Google Scholar]
- 142.Murphy C. T., Hu P. J. Insulin/insulin-like growth factor signaling in C. elegans. WormBook. 2013:1–43. doi: 10.1895/wormbook.1.164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suh Y., Atzmon G., Cho M.-O., et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Acadamy of Sciences of the United States of America. 2008;105(9):3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hesp K., Smant G., Kammenga J. E. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Experimental Gerontology. 2015;72:1–7. doi: 10.1016/j.exger.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 145.Birse R. T., Choi J., Reardon K., et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metabolism. 2010;12(5):533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim J., Neufeld T. P. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nature Communications. 2015;6, article no. 7846 doi: 10.1038/ncomms7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morris S. N. S., Coogan C., Chamseddin K., et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochimica et Biophysica Acta. 2012;1822(8):1230–1237. doi: 10.1016/j.bbadis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Musselman L. P., Fink J. L., Narzinski K., et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Disease Models & Mechanisms. 2011;4(6):842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matzkin L. M., Johnson S., Paight C., Bozinovic G., Markow T. A. Dietary protein and sugar differentially affect development and metabolic pools in ecologically diverse Drosophila. Journal of Nutrition. 2011;141(6):1127–1133. doi: 10.3945/jn.111.138438. [DOI] [PubMed] [Google Scholar]
- 150.Matzkin L. M., Johnson S., Paight C., Markow T. A. Preadult Parental Diet Affects Offspring Development and Metabolism in Drosophila melanogaster. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059530.e59530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Markow T. A., O'Grady P. M., ebrary Inc. Drosophila a guide to species identification and use. 2006.
- 152.Matzkin L. M., Watts T. D., Bitler B. G., Machado C. A., Markow T. A. Functional genomics of cactus host shifts in Drosophila mojavensis. Molecular Ecology. 2006;15(14):4635–4643. doi: 10.1111/j.1365-294X.2006.03102.x. [DOI] [PubMed] [Google Scholar]
- 153.Matzkin L. M., Mutsaka K., Johnson S., Markow T. A. Metabolic pools differ among ecologically diverse Drosophila species. Journal of Insect Physiology. 2009;55(12):1145–1150. doi: 10.1016/j.jinsphys.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Markow T. A., Matzkin L. M., Watts T. D. Desiccation resistance in four Drosophila species: Sex and population effects. Flight Journal. 2007;1(5):268–273. doi: 10.4161/fly.5293. [DOI] [PubMed] [Google Scholar]
- 155.Sanchez-Flores A., Peñaloza F., Carpinteyro-Ponce J., et al. Genome evolution in three species of cactophilic drosophila. G3: Genes, Genomes, Genetics. 2016;6(10):3097–3105. doi: 10.1534/g3.116.033779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schaeffer S. W., Bhutkar A., McAllister B. F., et al. Polytene chromosomal maps of 11 drosophila species: The order of genomic scaffolds inferred from genetic and physical maps. Genetics. 2008;179(3):1601–1655. doi: 10.1534/genetics.107.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song X., Goicoechea J. L., Ammiraju J. S. S., et al. The 19 genomes of Drosophila: A BAC library resource for genus-wide and genome-scale comparative evolutionary research. Genetics. 2011;187(4):1023–1030. doi: 10.1534/genetics.111.126540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alvarez-Ponce D., Aguadé M., Rozas J. Network-level molecular evolutionary analysis of the insulin/TOR signal transduction pathway across 12 Drosophila genomes. Genome Research. 2009;19(2):234–242. doi: 10.1101/gr.084038.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cornell-University, Drosophila Stock Center, 2017, http://blogs.cornell.edu/drosophila/
- 160.Ballard J. W. O., Melvin R. G., Miller J. T., Katewa S. D. Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell. 2007;6(5):699–708. doi: 10.1111/j.1474-9726.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 161.Melvin R. G., Van Voorhies W. A., Ballard J. W. O. Working harder to stay alive: metabolic rate increases with age in Drosophila simulans but does not correlate with life span. Journal of Insect Physiology. 2007;53(12):1300–1306. doi: 10.1016/j.jinsphys.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 162.Jones C. D. The genetics of adaptation in Drosophila sechellia. Genetica. 2005;123(1-2):137–145. doi: 10.1007/s10709-004-2728-6. [DOI] [PubMed] [Google Scholar]
- 163.Amlou M., Moreteau B., David J. R. Genetic analysis of Drosophila sechellia specialization: Oviposition behavior toward the major aliphatic acids of its host plant. Behavior Genetics. 1998;28(6):455–464. doi: 10.1023/A:1021689312582. [DOI] [PubMed] [Google Scholar]
- 164.Yassin A., Debat V., Bastide H., Gidaszewski N., David J. R., Pool J. E. Recurrent specialization on a toxic fruit in an island Drosophila population. Proceedings of the National Acadamy of Sciences of the United States of America. 2016;113(17):4771–4776. doi: 10.1073/pnas.1522559113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matzkin L. M. Ecological genomics of host shifts in Drosophila mojavensis. Advances in Experimental Medicine and Biology. 2014;781:233–247. doi: 10.1007/978-94-007-7347-9_12. [DOI] [PubMed] [Google Scholar]
- 166.Starmer W. T., Heed W. B., Rockwood Sluss E. S. Extension of longevity in Drosophila mojavensis by environmental ethanol: differences between subraces. Proceedings of the National Acadamy of Sciences of the United States of America. 1977;74(1):387–391. doi: 10.1073/pnas.74.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mirth C. K., Shingleton A. W. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Frontiers in Endocrinology. 2012;3 doi: 10.3389/fendo.2012.00049.Article 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jalal M., Andersen T., Hessen D. O. Temperature and developmental responses of body and cell size in Drosophila; effects of polyploidy and genome configuration. Journal of Thermal Biology. 2015;51:1–14. doi: 10.1016/j.jtherbio.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 169.Paaby A. B., Schmidt P. S. Dissecting the genetics of longevity in drosophila melanogaster. Flight Journal. 2009;3(1):29–38. doi: 10.4161/fly.3.1.7771. [DOI] [PubMed] [Google Scholar]
- 170.Green D. A., II, Extavour C. G. Insulin signalling underlies both plasticity and divergence of a reproductive trait in Drosophila. Proceedings of the Royal Society B Biological Science. 2014;281(1779) doi: 10.1098/rspb.2013.2673.20132673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong A. C.-N., Dobson A. J., Douglas A. E. Gut microbiota dictates the metabolic response of Drosophila to diet. Journal of Experimental Biology. 2014;217(11):1894–1901. doi: 10.1242/jeb.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chaston J. M., Dobson A. J., Newell P. D., Douglas A. E. Host genetic control of the microbiota mediates the Drosophila nutritional phenotype. Applied and Environmental Microbiology. 2016;82(2):671–679. doi: 10.1128/AEM.03301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ikeya T., Broughton S., Alic N., Grandison R., Partridge L. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proceedings of the Royal Society B Biological Science. 2009;276(1674):3799–3807. doi: 10.1098/rspb.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shin S. C., Kim S.-H., You H., et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334(6056):670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 175.Newell P. D., Douglas A. E. Interspecies Interactions Determine the Impact of the Gut Microbiota on Nutrient Allocation in Drosophila melanogaster. Applied and Environmental Microbiology. 2014;80(2):788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Toivonen J. M., Partridge L. Endocrine regulation of aging and reproduction in Drosophila. Molecular and Cellular Endocrinology. 2009;299(1):39–50. doi: 10.1016/j.mce.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 177.Erdogan C. S., Hansen B. W., Vang O. Are invertebrates relevant models in ageing research? Focus on the effects of rapamycin on TOR. Mechanisms of Ageing and Development. 2016;153:22–29. doi: 10.1016/j.mad.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 178.Min K.-J., Yamamoto R., Buch S., Pankratz M., Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7(2):199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hoffman J. M., Soltow Q. A., Li S., Sidik A., Jones D. P., Promislow D. E. L. Effects of age, sex, and genotype on high-sensitivity metabolomic profiles in the fruit fly, Drosophila melanogaster. Aging Cell. 2014;13(4):596–604. doi: 10.1111/acel.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buch S., Melcher C., Bauer M., Katzenberger J., Pankratz M. J. Opposing Effects of Dietary Protein and Sugar Regulate a Transcriptional Target of Drosophila Insulin-like Peptide Signaling. Cell Metabolism. 2008;7(4):321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 181.Samuel V. T., Shulman G. I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. The Journal of Clinical Investigation. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heinrichsen E. T., Zhang H., Robinson J. E., et al. Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Molecular Metabolism. 2014;3(1):42–54. doi: 10.1016/j.molmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bruce K. D., Hoxha S., Carvalho G. B., et al. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Experimental Gerontology. 2013;48(10):1129–1135. doi: 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee K. P. Dietary protein: Carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: A test using a chemically defined diet. Journal of Insect Physiology. 2015;75:12–19. doi: 10.1016/j.jinsphys.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 185.Skorupa D. A., Dervisefendic A., Zwiener J., Pletcher S. D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7(4):478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pletcher S. D., Libert S., Skorupa D. Flies and their Golden Apples: The effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Research Reviews. 2005;4(4):451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 187.Tatar M., Post S., Yu K. Nutrient control of Drosophila longevity. Trends in Endocrinology & Metabolism. 2014;25(10):509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Piper M. D. W., Skorupa D., Partridge L. Diet, metabolism and lifespan in Drosophila. Experimental Gerontology. 2005;40(11):857–862. doi: 10.1016/j.exger.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 189.Partridge L., Piper M. D., Mair W. Dietary restriction in Drosophila. Mechanisms of Ageing and Development. 2005;126(9):938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 190.Mair W., Piper M. D. W., Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biology. 2005;3(7, article e223) doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee J.-E., Rayyan M., Liao A., Edery I., Pletcher S. D. Acute Dietary Restriction Acts via TOR, PP2A, and Myc Signaling to Boost Innate Immunity in Drosophila. Cell Reports. 2017;20(2):479–490. doi: 10.1016/j.celrep.2017.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lian T., Gaur U., Yang D., Li D., Li Y., Yang M. Epigenetic mechanisms of dietary restriction induced aging in Drosophila. Experimental Gerontology. 2015;72:38–44. doi: 10.1016/j.exger.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 193.Post S., Tatar M. Nutritional geometric profiles of insulin/IGF expression in Drosophila melanogaster. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155628.e0155628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lakowski B., Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proceedings of the National Acadamy of Sciences of the United States of America. 1998;95(22):13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin S.-J., Kaeberlein M., Andalis A. A., et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418(6895):344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 196.Mair W., Goymer P., Pletcher S. D., Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301(5640):1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- 197.Lee B. C., Kaya A., Ma S., et al. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature Communications. 2014;5, article 3592 doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Palu R. A. S., Praggastis S. A., Thummel C. S. Parental obesity leads to metabolic changes in the F2 generation in Drosophila. Molecular Metabolism. 2017;6(7):631–639. doi: 10.1016/j.molmet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park S., Alfa R. W., Topper S. M., Kim G. E. S., Kockel L., Kim S. K. A Genetic Strategy to Measure Circulating Drosophila Insulin Reveals Genes Regulating Insulin Production and Secretion. PLoS Genetics. 2014;10(8) doi: 10.1371/journal.pgen.1004555.e1004555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oldham S., Böhni R., Stocker H., Brogiolo W., Hafen E. Genetic control of size in Drosophila. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355(1399):945–952. doi: 10.1098/rstb.2000.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oldham S., Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends in Cell Biology. 2003;13(2):79–85. doi: 10.1016/S0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 202.Clancy D. J., Gems D., Harshman L. G., et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 203.Alfa R. W., Kim S. K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Disease Models & Mechanisms. 2016;9(4):365–376. doi: 10.1242/dmm.023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Garofalo R. S. Genetic analysis of insulin signaling in Drosophila. Trends in Endocrinology & Metabolism. 2002;13(4):156–162. doi: 10.1016/S1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- 205.Lehmann M. Endocrine and physiological regulation of neutral fat storage in Drosophila. Molecular and Cellular Endocrinology. 2018;461:165–177. doi: 10.1016/j.mce.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011;10(1):5–9. doi: 10.1111/j.1474-9726.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Partridge L., Alic N., Bjedov I., Piper M. D. W. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Experimental Gerontology. 2011;46(5):376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hwangbo D. S., Gersham B., Tu M., Palmer M., Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429(6991):562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 209.DiAngelo J. R., Bland M. L., Bambina S., Cherry S., Birnbaum M. J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proceedings of the National Acadamy of Sciences of the United States of America. 2009;106(49):20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Biteau B., Karpac J., Supoyo S., Degennaro M., Lehmann R., Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genetics. 2010;6(10):p. e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Karpac J., Biteau B., Jasper H. Misregulation of an Adaptive Metabolic Response Contributes to the Age-Related Disruption of Lipid Homeostasis in Drosophila. Cell Reports. 2013;4(6):1250–1261. doi: 10.1016/j.celrep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Song W., Cheng D., Hong S., et al. Midgut-Derived Activin Regulates Glucagon-like Action in the Fat Body and Glycemic Control. Cell Metabolism. 2017;25(2):386–399. doi: 10.1016/j.cmet.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Musselman L. P., Fink J. L., Baranski T. J. CoA protects against the deleterious effects of caloric overload in Drosophila1. Journal of Lipid Research. 2016;57(3):380–387. doi: 10.1194/jlr.M062976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Augustin H., McGourty K., Allen M. J., et al. Reduced insulin signaling maintains electrical transmission in a neural circuit in aging flies. PLoS Biology. 2017;15(9) doi: 10.1371/journal.pbio.2001655.e2001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li Y., Hoffmann J., Li Y., et al. Octopamine controls starvation resistance, life span and metabolic traits in Drosophila. Scientific Reports. 2016;6 doi: 10.1038/srep35359.35359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Na J., Musselman L. P., Pendse J., et al. A Drosophila Model of High Sugar Diet-Induced Cardiomyopathy. PLoS Genetics. 2013;9(1) doi: 10.1371/journal.pgen.1003175.e1003175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Q.-P., Lin Y. Q., Zhang L., et al. Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metabolism. 2016;24(1):75–90. doi: 10.1016/j.cmet.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 218.Williams M. J., Wiemerslage L., Gohel P., Kheder S., Kothegala L. V., Schiöth H. B. Dibutyl phthalate exposure disrupts evolutionarily conserved insulin and glucagon-like signaling in drosophila males. Endocrinology. 2016;157(6):2309–2321. doi: 10.1210/en.2015-2006. [DOI] [PubMed] [Google Scholar]
- 219.Yurgel M. E., Masek P., DiAngelo J., Keene A. C. Genetic dissection of sleep–metabolism interactions in the fruit fly. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2015;201(9):869–877. doi: 10.1007/s00359-014-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Robertson M., Keene A. C. Molecular mechanisms of age-related sleep loss in the fruit fly-a mini-review. Gerontology. 2013;59(4):334–339. doi: 10.1159/000348576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cong X., Wang H., Liu Z., He C., An C., Zhao Z. Regulation of sleep by insulin-like peptide system in drosophila melanogaster. SLEEP. 2015;38(7):1075–1083. doi: 10.5665/sleep.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mensah L. B., Goberdhan D. C. I., Wilson C. MTORC1 signalling mediates PI3K-dependent large lipid droplet accumulation in Drosophila ovarian nurse cells. Biology Open. 2017;6(5):563–570. doi: 10.1242/bio.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Y., Liao S., Veenstra J. A., Nässel D. R. Drosophila insulin-like peptide 1 (DILP1) is transiently expressed during non-feeding stages and reproductive dormancy. Scientific Reports. 2016;6 doi: 10.1038/srep26620.26620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gu T., Zhao T., Hewes R. S. Insulin signaling regulates neurite growth during metamorphic neuronal remodeling. Biology Open. 2014;3(1):81–93. doi: 10.1242/bio.20136437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haselton A. T., Fridell Y.-W. C. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. AGING. 2010;2(8):523–526. doi: 10.18632/aging.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hsu H.-J., Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proceedings of the National Acadamy of Sciences of the United States of America. 2009;106(4):1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clancy D. J., Gems D., Hafen E., Leevers S. J., Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296(5566):p. 319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 228.Giannakou M. E., Goss M., Jünger M. A., Hafen E., Leevers S. J., Partridge L. Long-lived Drosophila with over-expressed dFOXO in adult fat body. Science. 2004;305(5682):p. 361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 229.Jones M. A., Gargano J. W., Rhodenizer D., Martin I., Bhandari P., Grotewiel M. A forward genetic screen in Drosophila implicates insulin signaling in age-related locomotor impairment. Experimental Gerontology. 2009;44(8):532–540. doi: 10.1016/j.exger.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Glatter T., Schittenhelm R. B., Rinner O., et al. Modularity and hormone sensitivity of the Drosophila melanogaster insulin receptor/target of rapamycin interaction proteome. Molecular Systems Biology. 2011;7, article no. 547 doi: 10.1038/msb.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]