Abstract
Advances in medical care have led to an increase in the number of octogenarians and even older patients, forming an important and unique patient subgroup. It is clear that advancing age is an independent risk factor for the development of most arrhythmias, causing substantial morbidity and mortality. Patients ≥80 years of age have significant structural and electrical remodeling of cardiac tissue; accrue competing comorbidities; react differently to drug therapy; and may experience falls, frailty, and cognitive impairment, presenting significant therapeutic challenges. Unfortunately, very old patients are under-represented in clinical trials, leading to critical gaps in evidence to guide effective and safe treatment of arrhythmias. In this state-of-the-art review, we examine the pathophysiology of aging and arrhythmias and then present the available evidence on age-specific management of the most common arrhythmias, including drugs, catheter ablation, and cardiac implantable electronic devices.
Keywords: ablation, antiarrhythmic drugs, electrophysiology, geriatrics, octogenarians
The global population of people ≥80 years of age is currently 137 million and is expected to triple by 2050 (1). There is a high prevalence of cardiovascular disease in this age group, including arrhythmias. In the United States, estimated survival after age 80 years is 9.73 years in women and 8.28 years in men (2). Therefore, the prevalence of arrhythmias in individuals ≥80 years of age will likely continue to rise, presenting significant treatment challenges to clinicians and an economic burden to society. Unfortunately, octogenarians and even older patients are under-represented in clinical trials. Therefore, guideline recommendations based on younger populations may not be relevant in the very elderly. In this state-of-the-art review, we examine the available evidence on age-specific management of arrhythmias in “very old” patients who are 80 years and beyond (Central Illustration). In the absence of more specific data, we discuss the evidence from “younger” geriatric populations, with the caveat that it is uncertain whether the inferences thus reached can be generalized to octogenarians.
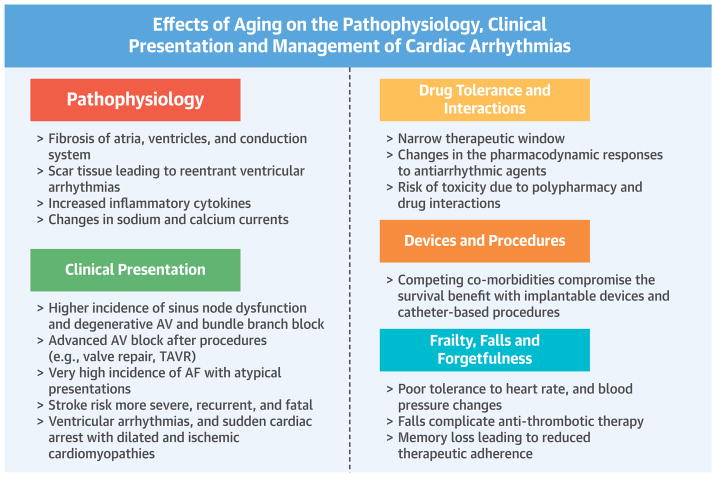
CENTRAL ILLUSTRATION. Effects of Aging on Cardiac Arrhythmias: Etiology, Clinical Presentation, and Management.
Aging leads to progressive degenerative changes of the contractile and conduction systems of the heart. Since the reparative process is slow, there is replacement fibrosis, which leads to structural and electrical conduction heterogeneity. Supraventricular and ventricular arrhythmias are then triggered. Pharmacotherapy can be challenging due to a narrow therapeutic window and risk of toxicity. Elderly patients often have concomitant structural heart disease requiring transcatheter or surgical procedures, which can lead to new arrhythmias requiring catheter ablation or implantable devices. AF = atrial fibrillation; AV = atrioventricular; TAVR = transcatheter aortic valvular replacement.
PATHOPHYSIOLOGY OF ARRHYTHMIAS IN THE ELDERLY
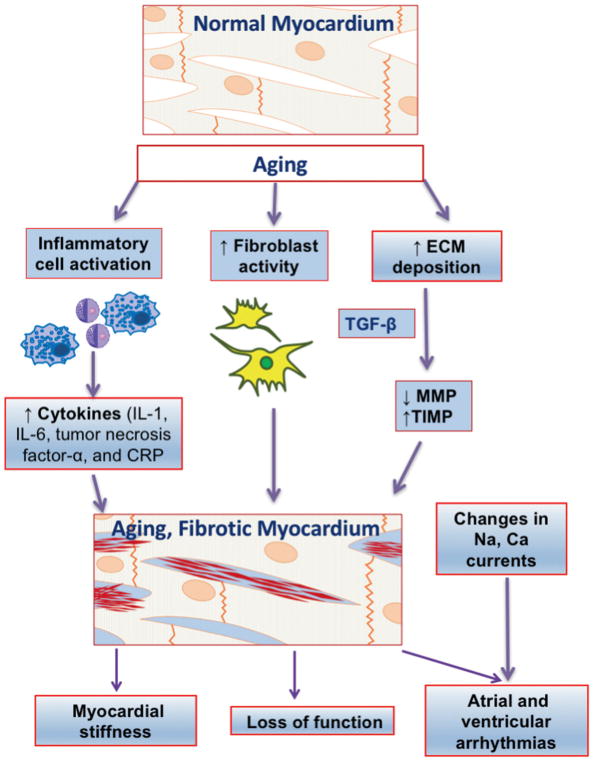
The heart undergoes complex changes during aging that affect cellular composition and the extracellular matrix. The increase in oxidative stress due to the increase in reactive oxygen species production with age results in an overall enhancement in the rate of cardiomyocyte death and increased fibrosis (3). In contrast to ischemia or an infiltrative process, which is characterized by acute or subacute cardiomyocyte damage and replacement fibrosis, aging-related cardiac fibrosis is accompanied by significant degenerative changes. Matrix metalloproteinases are a family of proteolytic enzymes that are highly expressed in the left ventricle and are involved in the degradation of the extracellular matrix. Tissue inhibitors of metalloproteinases are low-molecular-weight molecules that bind to matrix metalloproteinases forming complexes that exhibit inhibitory control on matrix metalloproteinases. Changes in the matrix metalloproteinase/tissue inhibitor ratio may be a potential mechanism for increased extracellular matrix volume in aging ventricles that correlates with left ventricular hypertrophy and fibrosis (Figure 1). Fibrotic changes in aged hearts reduce the safety margin for conduction by promoting conduction block and re-entry when the availability of the fast sodium current is reduced by premature electrical stimulation. Oxidative stress, which is believed to be an independent mediator of age-related arrhythmias, reduces repolarization reserve by enhancing late sodium current (INa-L), late calcium current (ICa-L), and the Na-Ca exchanger to favor the formation of early after depolarizations. The latter can initiate ventricular tachycardia (VT) or ventricular fibrillation in structurally remodeled hearts (4). The effects of aging on the heart and its relationship to arrhythmias are shown in Figure 2.
FIGURE 1. Pathophysiology of Arrhythmias in Elderly.
Aging hearts demonstrate a typical milieu of sterile inflammation, with enhanced proinflammatory (IL-1, IL-6, tumor necrosis factor-α, and CRP) and profibrotic (TGF-β) cytokine release. Proinflammatory activity leads to progressive loss of cardiomyocyte numbers. The reduction in the proteolytic activity of MPs with increased expression of the tissue inhibitor of MPs, in association with a pro-fibrotic cytokine TGF-β, promotes fibrosis. Ca = calcium; CRP = C-reactive protein; ECM = extracellular matrix; IL = interleukin; MMP = matrix metalloproteinase; Na = sodium; TIMP = tissue inhibitor of matrix metalloproteinase; TGF = transforming growth factor;.
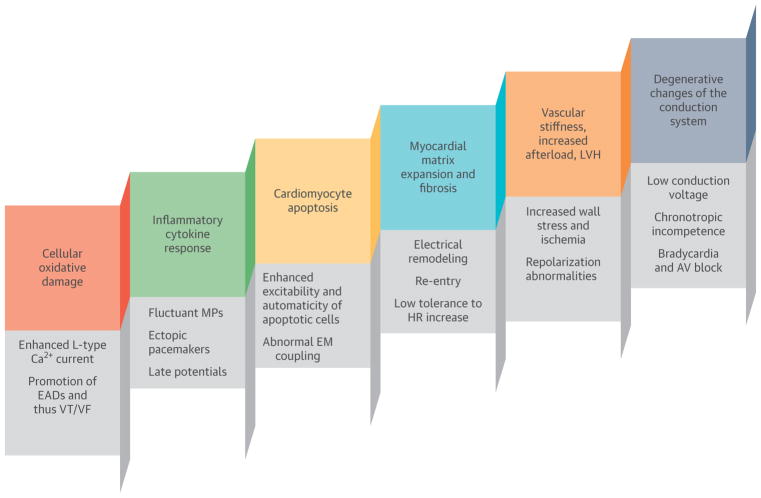
FIGURE 2. Age-Associated Pathological Changes and Their Effect on the Initiation, Manifestation, and Recurrence of Cardiac Arrhythmias.
The major culprit mechanisms are related to cellular oxidative damage, cardiomyocyte apoptosis, and increased extracellular matrix volume that can lead to abnormal automaticity, re-entry, and repolarization abnormalities. In addition, ischemic and inflammatory mechanisms are responsible for fluctuating membrane potentials, ectopic pacemakers, and ultimately, myocardial fibrosis. Overall, aging hearts show electrical signal heterogeneity, abnormal electromechanical coupling, atrial and ventricular remodeling, low conduction voltage, and an increased incidence of both atrial and ventricular arrhythmias. Fibrofatty changes of the conduction system lead to bradycardia, AV block, and chronotropic incompetence. AV = atrioventricular; Ca2+ = calcium; EADs = early afterdepolarizations; EM = electromechanical; HR = heart rate; MPs = membrane potentials; VF = ventricular fibrillation; VT = ventricular tachycardia.
ATRIAL REMODELING
Prior studies have shown remodeling of the left atrium in older patients with left ventricular diastolic dysfunction. Such effects are caused either by loss of mechanical atrial function or chronically elevated wall stress. Atrial structural heterogeneity due to fibrosis leads to a loss of synchrony, and stiffness leads to a loss of compliance. In search of the underlying atrial substrate for atrial fibrillation (AF) that often develops with aging, Kistler et al. (5) demonstrated age-associated electrical changes in the atria, including low voltages and conduction slowing. However, animal models have not shown significant age-related changes in sodium current density in either the right or left atrium. Similarly, basal calcium current reduction in aged cells was unaccompanied by changes in calcium channel availability or recovery from inactivation. Therefore, it is unlikely that conduction changes in aged atrial tissues are due to changes in intrinsic channel function, but rather due to the complex interplay between age-related fibrosis and a trigger mechanism for initiation and propagation of dysrhythmias (6).
REMODELING OF CONDUCTION SYSTEM
Studies demonstrate that aging is associated with deposition of fatty and fibrous tissue in the sinoatrial node and the conduction system and reduction in the density of autonomic innervation (7). In addition, pre-clinical studies in rabbits have shown an aging-related slowdown of electrical conduction velocity throughout the His-Purkinje network (8), which is manifested in humans by prolongation of the HV interval. Kuo et al. (9) showed that the atrioventricular (AV) nodal recovery curve remains unchanged once one reaches adulthood, whereas the AV nodal effective refractory period becomes slightly longer after age 60 years (9).
MYOCARDIAL REMODELING
Compared to non-senescent hearts, aging hearts demonstrate excess collagen deposition, reduced cardiomyocyte density, and an altered geometrical configuration of collagen fibers (10). Such structural disarray can trigger ventricular arrhythmias. With aging, terminally-differentiated cells like cardiomyocytes are associated with autophagy, apoptosis, and failure of cell repair. Cardiomyocyte apoptosis is associated with increased excitability and automaticity of the contractile elements, which can lead to the development of ventricular arrhythmias (11). Fibrotic and myocyte-depleted ventricles can also create the substrate for re-entrant ventricular arrhythmias.
Aging is associated with increased myocardial inflammation mediated by circulating pro-inflammatory cytokines. Although the mechanisms underlying inflammation are not clear, there is evidence that inflammatory cytokines can lead to calcium channel dysfunction, action potential prolongation, and initiation of triggered activity predisposing to cardiac arrhythmias (12). Inflammatory processes in cardiac myocytes and the interstitium can lead to fluctuations in membrane potential. Ongoing chronic tissue damage and the reparative process favor the development of ectopic pacemakers, late potentials, and re-entry as a result of inhomogeneous stimulus conduction (13).
BRADYARRHYTHMIAS
The intrinsic rate of the sinoatrial node, which is around 85 to 105 beats/min after complete autonomic blockade, declines with age. However, resting heart rate does not significantly change with aging due to the relative balance in the sympathetic and parasympathetic nervous systems. There is also an age-related decline in maximal heart rate response to exercise by 0.7 beats/min annually after early adulthood (7). In patients ≥80 years of age without cardiovascular disease, there is a nocturnal decline in heart rate by about 14 beats/min and sinus arrhythmia with pauses of 1.5 to 2 s in up to 12% of patients (14). The 24-h ambulatory electrocardiography of 1,372 participants in the Cardiovascular Health Study showed an increased incidence of bradycardia with advancing age, about 10% in men ≥80 years of age (15).
SINUS NODE DYSFUNCTION
Sinus node dysfunction (SND), also known as sick sinus syndrome, is most often diagnosed in the seventh or eighth decade of life. It is estimated that the incidence of SND in the United States will be around 172,000 annually by 2060, mainly due to an increased incidence in patients ≥75 years of age (16). In addition to dizziness or syncope, SND may cause fatigue and exercise intolerance due to “chronotropic incompetence,” an inability to mount the age-predicted maximal heart rate, which decreases with age, in response to exercise.
ATRIOVENTRICULAR BLOCK
The prevalence of first-degree AV block follows a J-shaped curve (17). A prolonged PR interval is common in patients younger than 40 years of age and athletes, likely due to high vagal tone. The prevalence of first-degree AV block starts to increase again in the fifth and sixth decades of life, continues to increase with advancing age, and peaks in the ninth decade in Caucasians and the tenth decade in African Americans (17,18). This rise in prevalence of first-degree AV block is thought to be due to age-related and/or pathological fibrosis of the conduction system (17). Although largely considered benign, a prolonged PR interval has been associated with increased AF, left ventricular dysfunction or heart failure, and mortality (19). In a study of patients ≥85 years of age, first-degree AV block as a single electrocardiographic finding was the strongest predictor of mortality (20). However, patients with first-degree AV block are generally asymptomatic and progression to more advanced heart block is infrequent.
There has been some debate as to whether type 1 second-degree AV block is benign in the elderly, as they are likely to have more fibrosis of the conduction system and structural heart disease (21). In a retrospective study of propensity-matched, elderly veterans with type 1 second-degree AV block, implantation of pacemakers or implantable cardioverter-defibrillators (ICDs) was associated with longer survival, even when ICDs were excluded from the analysis (22). These results should be interpreted with caution and cannot be generalized to support routine implantation of pacemakers in asymptomatic elderly patients with type 1 second-degree AV block. Symptomatic type I second-degree AV block, on the other hand, is best treated with permanent pacing, regardless of the age of the patient.
Although elderly patients have a higher prevalence of distal (His or infra-His) conduction disease leading to type 2 second- or third-degree AV block, the evaluation and indications for pacemakers do not differ from younger patients. Advanced AV block caused by cardiac amyloidosis, a condition that is increasingly diagnosed in the elderly, requires implantation of a permanent pacemaker due to its progressive course. Many elderly patients with symptomatic severe aortic stenosis who were previously deemed inoperable are undergoing transcatheter aortic valve replacement that may cause advanced AV block. The rate of pacemaker implantation may vary depending upon the type of valve and was reported to be around 12% in the 2016 Transcatheter Valve Therapy (TVT) Registry (23).
BUNDLE BRANCH BLOCK
The prevalence of bundle branch block increases with age. In 1 study (24), 855 men ≥50 years of age were followed for 30 years and were found to have an increasing prevalence of bundle branch block from 1% to 17%. At 80 years of age, the prevalence of right bundle branch block and left bundle branch block was 11.3% and 5.7%, respectively. After 18 years of follow-up, only 21% and 11% of patients with new right bundle and left bundle branch block, respectively, in the Framingham Heart Study cohort were free of cardiovascular disease (25). However, there is no specific evaluation recommended in asymptomatic elderly patients with bundle branch block.
Bifascicular block typically occurs in the elderly and is associated with a high risk of subsequent advanced AV block, syncope, and even sudden cardiac death (SCD), especially in the presence of alternating bundle branch block, type 2 or advanced second-degree AV block, or transient third-degree AV block (26). Current guidelines recommend pacemaker implantation in patients with bifascicular block and unexplained syncope, or in asymptomatic patients with an HV interval ≥100 ms or atrial pacing-induced nonphysiological block during electrophysiological study. However, in a study of elderly patients (mean age 77 years) with bifascicular block and unexplained syncope treated with pacemakers, 16% still had recurrent syncope (27). This study underscores the need for comprehensive evaluation of syncope prior to pacemaker placement.
PACEMAKERS
An analysis of Dutch registry data of 96,900 patients undergoing initial pacemaker implantation showed that 32.6% of patients were ≥80 years of age (28). The most common indications for pacemakers were SND (42.3%) and AV block (38.9%). In an analysis of 178,000 pacemakers implanted between 1997 and 2004 in the United States, patients ≥75 years of age accounted for 64% of all implantations (29).
Pacemakers implanted to treat SND have not been shown to improve survival. Dual-chamber pacing (DDDR) can improve quality of life and functional status in elderly patients (30). In patients with SND treated with atrial pacing, the median annual combined incidence of second- and third-degree AV block is 0.6% (0% to 4.5%), with a mean prevalence of 2.1% (0% to 11.9%) (31). Although the incidence of AV block is low, many elderly patients are likely to require AV nodal blocking agents for AF, heart failure, or coronary artery disease, putting them at risk of developing symptomatic AV block. In addition, atrial-based pacing is associated with a higher risk of paroxysmal AF (hazard ratio [HR]: 1.27; 95% confidence interval [CI]: 1.03 to 1.56) and reoperation (HR: 1.99; 95% CI: 1.53 to 2.59) (32). Therefore, dual-chamber pacing (DDDR) is usually preferred in elderly patients with SND.
In many studies that included octogenarians and nonagenarians, the rates of survival and procedural complications after pacemaker implantation have been comparable to age- and sex-matched control subjects (33). In a pooled meta-analysis (34) of randomized clinical trials, the rate of early complications was 5.1% in patients ≥75 years of age (mean age 81.6 years) compared with 3.4% in younger patients (p = 0.006), mainly driven by pneumothorax and lead dislodgement, whereas older patients had a lower risk of lead fracture. Atrial-based pacing was associated with more complications than ventricular pacemakers in both age groups (34). In a large population-based study (35) from the United States (n = 115,683 patients), the unadjusted mortality rates after pacemaker implantation in patients 70 to 79 years, 80 to 89 years, and ≥90 years of age were 0.60%, 0.99%, and 1.87%, respectively. On multivariate analysis, advancing age continued to predict mortality (odds ratio: 1.54 for age 80 to 89 years and 2.8 for age ≥90 years). However, the presence of comorbidity was shown to be a stronger predictor for mortality (odds ratio: 2.69 for moderate comorbidity and 5.00 for severe comorbidity), and there was a linear age-comorbidity interaction (Figure 3). The unadjusted rates of major complications for each age were 5.61%, 6.13%, and 6.31%, respectively. This age-related difference in the rates of procedural complications disappeared on multivariate analysis. Therefore, the incremental risk of age in octogenarians and nonagenarians is clinically modest and should not preclude referral for pacemaker implantation.
FIGURE 3. Role of Advanced Age and Existing Medical Comorbidities in Predicting Mortality Rate After Pacemaker Implantation.
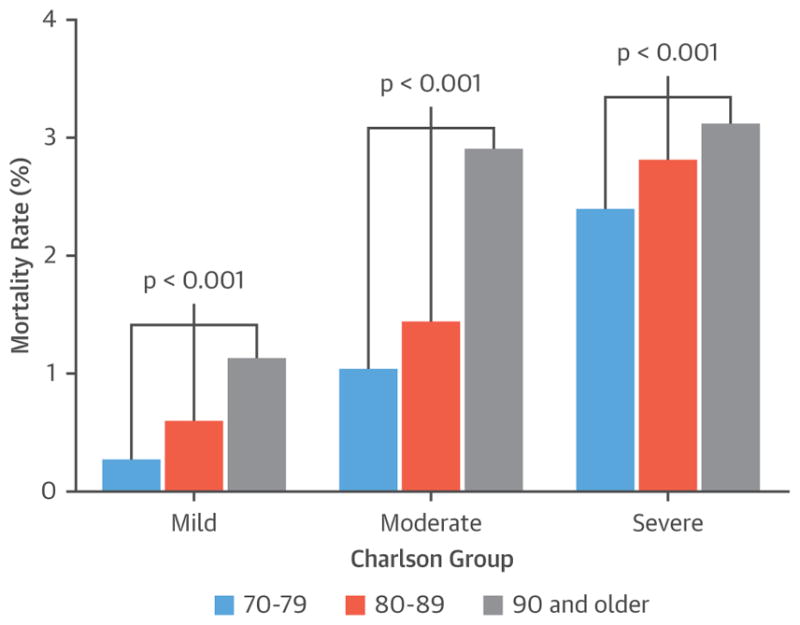
The bar diagrams represent mortality rates in patients with mild (group 1), moderate (group 2), and severe (group 3) Charlson comorbidity indexes. In each group, patients who are 90 years of age and older had significantly increased mortality rates after initial pacemaker implantation (age–comorbidity level interactions, p = 0.004). Study results are from a cross-sectional analysis of 2004 to 2008 hospital discharge information from the Healthcare Cost and Utilization Projection Nationwide Inpatient Sample administrative database. Reproduced with permission from Mandawat et al. (35).
Single-chamber leadless intracardiac pacemakers may be an attractive option for selected elderly patients, especially if they have persistent AF or are expected to need infrequent ventricular pacing. The studies of 2 different leadless pacemaker systems included relatively older patients (mean age 75.8 and 75.9 years) and showed lower complication rates (6.7% and 4%) compared with historical control subjects of transvenous pacemakers (36,37). There was a trend toward an increased risk of cardiac perforation or pericardial effusion (1.5% and 1.6%), particularly in older female patients.
ECTOPIC BEATS
Premature atrial and ventricular complexes are frequently present in the healthy elderly population. In the Cardiovascular Health Study (15), 24-h ambulatory monitoring in 60- to 85-year-old healthy individuals showed that 86% of patients had premature atrial complexes, with 26% having >36 premature atrial complexes/h. Premature ventricular complexes have been found in 82% of elderly subjects, including 3- to 5-beat runs of nonsustained VT. Dukes et al. (38) demonstrated in Cardiovascular Health Study participants that a high burden of premature ventricular complexes was associated with increased left ventricular systolic dysfunction, incident heart failure, and mortality. The specificity for predicting a 15-year risk of heart failure was >99% for a PVC burden of 10%.
TACHYARRHYTHMIAS
SUPRAVENTRICULAR TACHYCARDIA
AV node reentrant tachycardia and AV re-entrant (accessory pathway-mediated) tachycardia commonly present earlier in life because they originate from congenital conduction substrates. Late-onset presentations are likely due to an age-related increase in triggers for reentry, changes in the relative conduction characteristics of the re-entrant pathways, and/or intolerance to tachycardia.
Atrial tachycardia represents 3% to 17% of supra-ventricular tachycardias (SVTs) that present for ablation (39). One large review showed that the proportion of atrial tachycardia increased with age and accounted for 23% of SVTs above the age of 70 years. Animal models suggest an age-related decline in the ability to modulate atrial electrophysiological responses to neurohormonal stimuli, which may contribute to the increased incidence of atrial tachycardia in the elderly (40).
Most published data on the mechanisms and relative frequency of SVTs come from older studies before catheter ablation became widespread. With increasing age, the atrial effective refractory period increases and conduction in the slow AV nodal or accessory pathway decreases, leading to increased tachycardia cycle lengths (41,42). Elderly patients tend to have slower rates with SVT, but they may more often present with angina or heart failure (42).
In a comparison of younger versus older patients (oldest age 70 years) undergoing electrophysiological studies for pre-excitation syndromes, tachyarrhythmia rates in younger patients were higher, but there was no age-related difference in the ability to induce SVT. Anterograde conduction in the setting of AF was more rapid in younger patients (43).
Given that most patients with AV node re-entrant tachycardia or AV re-entrant tachycardia present at an earlier age and that catheter ablation is successfully pursued, few patients >80 years of age present for ablation of these arrhythmias today. Older patients with pre-excitation who have lived a lifetime without symptoms do not need routine electrophysiological studies for evaluation, especially because the conduction properties of the accessory pathway likely deteriorate with age. On the other hand, AF becomes more likely with advancing age. Although rapid conduction over the accessory pathway is not that likely, treatment of AF itself potentially creates some dilemmas, especially with respect to the use of AV nodal blocking drugs. Antiarrhythmic drugs (AADs) may be helpful in such cases, but most electrophysiologists would have a low threshold for accessory pathway ablation, perhaps in conjunction with pulmonary vein isolation. In a follow-up study after accessory pathway ablation, older age and longer duration of AF were found to predict AF recurrence (44).
Initial treatment for regular, narrow complex SVT usually involves beta-blockers or calcium-channel blockers. AADs may be an acceptable approach when AV nodal blockers alone are ineffective and frailty/limited life expectancy/comorbidities make catheter ablation an unacceptable option.
CATHETER ABLATION OF SVT
The major theoretical concern with AV node re-entrant tachycardia ablation in the elderly is the creation of iatrogenic complete heart block (45). In a study by Rostock et al. (46), despite a higher incidence of baseline first-degree AV block in patients age ≥75 years, there was no post-ablative complete heart block at an average follow up of 37 months. Other studies have also shown no significant differences in recurrence or post-operative complete heart block between elderly and younger patients.
A retrospective evaluation of 961 patients with AV re-entrant tachycardia, about one-half of whom underwent ablation, compared long-term outcomes in their elderly (age >65 years) cohort compared with their younger patients at an average follow-up of 5.6 years (47). The incidence of poorly tolerated tachycardia (4.2% vs. 0.6%; p = 0.001) and the risk of major complications (10% vs. 1.9%; p = 0.006) were more frequent in elderly patients.
In patients with atrial tachycardia, older age is a predictor of multiple atrial foci, which affects the risk of recurrence (48). There are no large series specifically on ablation for atrial tachycardia in octogenarians that would provide information on major complications and success rates.
ATRIAL FIBRILLATION
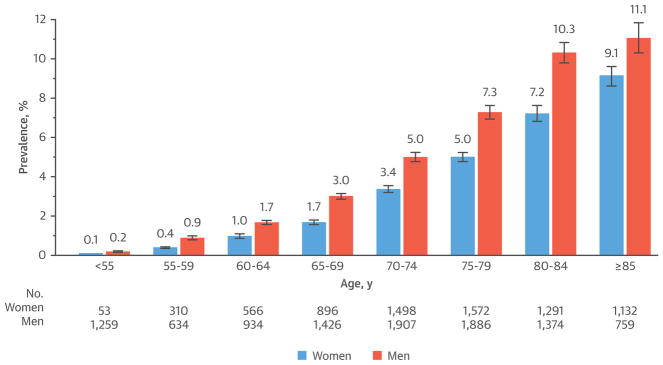
AF is the most common arrhythmia in the elderly and affects about 1 in 10 individuals over the age of 80 years (49) (Figure 4). It has been projected that the prevalence of AF will increase by 2.5-fold in the United States by 2050 and >50% of the patients will be ≥80 years of age (49). In addition to an increased risk of stroke and heart failure, AF in the elderly has been associated with a decline in physical performance and cognitive ability (50,51), shorter disability-free survival, and higher mortality (52,53). In elderly patients, AF is generally associated with comorbidities, and lone AF is less common compared with the younger population. The elderly with AF tend to have atypical presentations, with palpitations present in only 1 in 10 patients ≥80 years of age (54). In the STROKESTOP (Systematic ECG Screening for Atrial Fibrillation Among 75 Year Old Subjects in the Region of Stockholm and Halland, Sweden) study (55), population-based screening of 75- to 76-year-old patients increased the detection of AF by 33%, highlighting the preponderance of asymptomatic AF in the elderly.
FIGURE 4. Prevalence of Diagnosed Atrial Fibrillation Stratified by Age and Sex.
Reproduced with permission from Go et al. (49).
ANTICOAGULATION FOR STROKE PREVENTION
One-third of strokes in patients ≥80 years of age are attributable to AF. The cumulative effects of comorbidities such as hypertension and heart failure over many years, along with the known effects of aging on the heart, such as fibrosis, likely contribute to the increasing incidence of strokes in elderly AF patients. The CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke or TIA, Vascular disease, Age 65–74 years, and Female Sex) score for predicting the risk of stroke in AF highlights the importance of advanced age as a risk factor. The HAS-BLED (Hypertension, Abnormal liver or renal function, Stroke, Bleeding history or predisposition, Labile INR, Elderly [age ≥65 years], Drugs/alcohol) score, which is used to predict the risk of major bleeding, also recognizes advanced age as a risk factor. However, a study of a Danish cohort of AF patients (56) demonstrated a greater net clinical benefit of warfarin in patients with a higher bleeding risk defined by a HAS-BLED score ≥3. Given that these scoring systems highlight the increased risk of both thromboembolism and bleeding in AF patients ≥80 years of age, the decision to initiate oral anticoagulation should be individualized (Table 1).
TABLE 1.
Anticoagulation for Very Elderly AF Patients
| 1. Very elderly AF patients are at an increased risk of both stroke and bleeding, but the benefit of anticoagulation outweighs the risk of bleeding. |
| 2. Warfarin and DOACs are both good options. |
| 3. Renal function may dictate choice of anticoagulation. |
| 4. Although there are no head-to-head trials, dabigatran 150 mg twice daily compared with warfarin was associated with an increased risk of extracranial bleeding in octogenarians, whereas apixaban was superior to warfarin in both efficacy and safety, independent of age. |
| 5. Compliance is key, so patient comprehension and shared decision making are important. |
AF = atrial fibrillation; DOAC = direct oral anticoagulants.
With the introduction of dabigatran and the factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban), there has been a paradigm shift in the approach to anticoagulation in AF. Direct oral anticoagulants may be preferred over warfarin in elderly patients because of predictable pharmacokinetics, less interaction with other drugs and food, and no requirement for routine testing. Although the efficacy and safety of the direct oral anticoagulants have not been specifically studied in very elderly patients, they are fairly well represented in the 4 landmark anticoagulation trials (Table 2). Apixaban was shown to be superior to warfarin in reducing the risk of stroke or systemic embolism with a lower risk of intracranial or extracranial hemorrhage in all age groups including ≥80 years of age (57). In separate analyses of rivaroxaban (58) and edoxaban (59) compared with warfarin, there was no age-treatment interaction with respect to efficacy and safety. Dabigatran 150 mg twice daily compared with warfarin was associated with a higher risk of major extracranial bleeding in patients ≥80 years (60). The availability of a U.S. Food and Drug Administration–approved reversal agent, idarucizumab, may factor into the decision to choose dabigatran.
TABLE 2.
The Risks and Benefits of Direct Oral Anticoagulants With Warfarin in Elderly Patients With Nonvalvular Atrial Fibrillation
| Anticoagulants (Ref. #) | Parent Trial | Proportion of Very Elderly Patients | Efficacy | Risk |
|---|---|---|---|---|
| Dabigatran vs. warfarin (60) | RE-LY (n = 18,113) | 16.7% ≥80 yrs (n = 3,025) | 150 mg bid: Trend toward lower risk of stroke or systemic embolism with dabigatran in age ≥85 yrs Lower risk of stroke or systemic embolism in age <75 yrs 110 mg bid: Trend toward lower risk of stroke or systemic embolism with dabigatran in patients age <75 and ≥85 yrs |
Major extracranial bleeding 150 mg bid: Higher risk with dabigatran in ≥80 yrs of age* (HR 1.68) Lower risk with dabigatran in younger patients 110 mg bid: No difference in ≥80 yrs Lower with dabigatran in younger patients Major intracranial hemorrhage 150 or 110 mg bid: Trend toward lower risk with dabigatran in age ≥85 yrs Lower risk with dabigatran in age <75 yrs |
| Apixaban†‡ vs. warfarin (57) | ARISTOTLE (n = 18,201) | 13% ≥80 yrs (n = 2,436) | Lower risk of stroke or systemic embolism with apixaban in all age groups | Lower risk of major bleeding and intracranial bleeding with apixaban in all age groups |
| Rivaroxaban§ vs. warfarin (58) | ROCKET AF (n = 14,276) | 44% ≥75 yrs (n = 6,229) 4.6% ≥85 yrs (n = 663) |
No difference in the risk of stroke or systemic embolism in all age groups | No difference in major bleeding or hemorrhagic stroke in all age groups |
| Edoxaban vs. warfarin (59) | ENGAGE AF-TIMI 48 (n = 21,105) | 17% ≥80 yrs (n = 3,591) | No difference in the risk of stroke or systemic embolism in all age groups | Lower major bleeding or intracranial hemorrhage with edoxaban in all age groups |
p value significant for age-treatment interaction.
The 2.5 mg twice daily (bid) dose was used in the study if patients had at least 2 of the following factors: ≥80 years of age, weight ≥60 kg, and creatinine >1.5 mg/dl.
69% of patients age ≥80 years received 5 mg bid and 31% received 2.5 mg bid per pre-specified criteria.
Dose of rivaroxaban 20 mg daily, or 15 mg daily if glomerular filtration rate (GFR) <50 ml/min.
ARISTOTLE = Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; ENGAGE AF-TIMI = Effective aNticoaGulation with factor Xa next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction; RE-LY = Randomized Evaluation of Long-Term Anticoagulant Therapy; ROCKET AF = Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
Despite strong evidence of effectiveness, anticoagulation is still underutilized in AF, especially in patients ≥80 years of age (61,62). Hylek et al. (61) demonstrated that warfarin was discontinued in 26% of patients ≥80 years of age by the end of the first year, mostly due to concerns related to safety (Figure 5). However, randomized controlled trials have demonstrated that anticoagulation in the elderly can be managed safely and effectively. In the BAFTA (Birmingham Atrial Fibrillation Treatment of the Aged) study (63), dose-adjusted warfarin was associated with a 2% absolute risk reduction of fatal or disabling stroke, intracranial hemorrhage, or systemic embolism in patients ≥75 years of age (mean age 82 years), with a trend toward a lower rate of extracranial bleeding. In a subsequent study, the greatest net benefit of warfarin in AF was demonstrated in patients ≥85 years of age and those with a prior history of ischemic stroke (64).
FIGURE 5. Risk of Stopping Warfarin in the First Year on the Basis of Perceived Safety Concerns by Age.
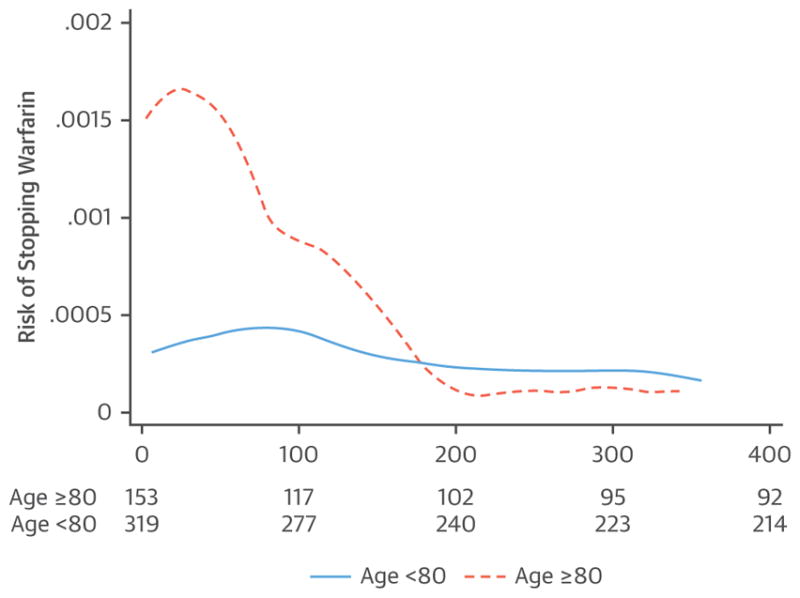
The y-axis represents the smoothed hazard estimates over time for the 2 age groups (<80 and ≥80 years). Numbers below the graph are the number of patients on warfarin at that time point (p < 0.001, log-rank test). The risk of stopping warfarin peaked early and then, beginning at 6 months, approximated that of younger patients. Reproduced with permission from Hylek et al. (61).
The higher bleeding risk in the elderly is often attributed to frailty and the propensity to falls, leading to withholding of anticoagulation in octogenarians. However, 1 analysis concluded that an individual would have to fall >295 times before the risk of subdural hematoma related to falls would outweigh the benefit of anticoagulation (65).
In elderly AF patients, the presence of dementia could pose a bleeding risk due to underdosing or overdosing of oral anticoagulants. However, in a recent study of veterans (mean age 80 years) with AF, continuation of warfarin after a diagnosis of dementia was associated with a reduction of stroke and mortality, without an increase in major bleeding (66).
There is an increased prevalence of cerebral microbleeds and cerebral amyloid angiopathy in the elderly, leading to an increased risk of intracranial hemorrhage (67). Evidence to support routine imaging of the brain for the screening of cerebral amyloid angiopathy before initiating anticoagulation is lacking, but it would be prudent to review a brain magnetic resonance image if previously performed.
The coexistence of AF with coronary artery disease is very common in patients ≥80 years of age. The use of triple antithrombotic therapy in the setting of percutaneous coronary intervention leads to a significantly increased risk of bleeding (68). Studies have shown that the combination of a P2Y12 inhibitor with either dose-adjusted warfarin or rivaroxaban 15 mg daily could be an option in patients who require stent(s) and need anticoagulation for AF, without increasing the risk of adverse events (69,70). Recently, a study of dual therapy including dabigatran showed similar results when compared with triple therapy (71). However, there was a significant age-treatment interaction for the 150-mg dose of dabigatran, with a higher bleeding risk in patients age > 80 years.
Left atrial appendage occlusion devices have been approved by the U.S. Food and Drug Administration as an alternative to oral anticoagulants in AF patients with contraindications to anticoagulation. The PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) Trial (72) included older patients (mean age 74 years) compared with prior studies. Although there was a lower rate of adverse events (2.2%), the study failed to show noninferiority of the Watchman device to warfarin in terms of efficacy. Left atrial appendage occlusion devices may be considered in carefully selected elderly AF patients with prohibitive bleeding risks.
RATE VERSUS RHYTHM CONTROL
Randomized controlled trials have failed to show the superiority of a rhythm control strategy over a rate control strategy in patient populations with a mean age of 61 to 69 years. Because very elderly patients have more advanced structural and electrical remodeling of atria, the success rate of maintaining sinus rhythm in this age group may be even worse. Also, elderly patients are more likely to have associated comorbidities, which can limit the options for treatment. Fortunately, many elderly patients tend to be less symptomatic. Therefore, it can be argued that elderly patients should be treated initially with a rate control strategy. Beta-blockers followed by calcium-channel blockers are the most effective rate-controlling medications. There are no data to suggest that the target heart rate in the elderly with AF should be any different than that for younger patients, but it is important to be aware that elderly patients have a higher prevalence of SND and/or AV block. Therefore, it is particularly important in elderly patients to obtain 24-h Holter monitors to ensure that there is not excessive bradycardia or pauses during the night when vagal tone is highest.
A rhythm control strategy is reasonable in symptomatic AF patients. The use of AAD therapy in very elderly patients is challenging because of the risk of proarrhythmia due to age-related changes in pharmacokinetics, frailty, and polypharmacy. Amiodarone is more likely to be used in the elderly for a number of reasons. Amiodarone is the most effective of the available AADs, and it is 1 of only 2 that have not been shown to adversely affect survival in patients with left ventricular dysfunction (the other being dofetilide) (73,74). The dose of amiodarone does not need to be adjusted for renal or hepatic function. The major concern about the use of amiodarone is the long-term risk of side effects, which is a less relevant concern in the very elderly.
CATHETER ABLATION
Pulmonary vein isolation is an option for rhythm control in patients with symptomatic paroxysmal or persistent AF when AADs fail to maintain sinus rhythm. In a prospective study of elderly patients (mean age 76 years) with symptomatic persistent AF, catheter ablation was more effective in maintaining sinus rhythm and improved quality of life (75). However, 19% of patients required redo ablation and 25% continued to take AADs to maintain sinus rhythm. There was also a greater risk of stroke with catheter ablation, especially if there was a prior history of transient ischemic attack. In multiple observational studies, radiofrequency catheter ablation of AF in octogenarians has been shown to be successful at rates similar to younger patients (76–78). The complication rates that ranged from 2.5% to 7.5% were also not significantly different from younger patients (Table 3). However, octogenarians are more likely to need ablation of nonpulmonary vein triggers and modification of the atrial substrate, which is at least partly related to aging-related atrial fibrosis.
TABLE 3.
Efficacy and Safety of Catheter Ablation of AF in the Very Elderly
| First Author (Ref. #) | AF Type | Age (yrs) | Mean Follow-Up Duration | Success Rate | Complication Rate | Ablation of Nonpulmonary Vein Triggers |
|---|---|---|---|---|---|---|
| Santangeli et al. (76) | Paroxysmal and persistent | ≥80, <80 | 18 months | 69% vs. 71% after first ablation 87% vs. 85% after second ablation | 4% vs. 4.9% | 84% vs. 69%, driven by difference in paroxysmal AF |
| Bunch et al. (78) | Paroxysmal and persistent | ≥80 (n = 35), <80 (n = 717) | 12 months | 78% vs. 75% | 7.5 vs. 4.1% (p = NS) | 80% vs. 78% had additional linear ablation |
| Tan et al. (77) | Paroxysmal, persistent, and long-standing persistent | ≥80, 70–79, 60–69 | 18 months | 70% vs. 72% vs. 74%* | Total complication rate 2% | Not reported |
| Zado et al. (75) | Paroxysmal and persistent | >75 (n = 34), 65–75 (n = 185), <65 (n = 948) | 27 months | 86% vs. 84% vs. 89% | 2.9% vs. 1.7% vs. 1.6% | 13% vs. 17% vs. 12% |
More patients ≥80 years of age were maintained on antiarrhythmic drugs (26% vs. 20% vs. 16%; p < 0.05 and p < 1.01, respectively).
AF = atrial fibrillation; NS = nonsignificant.
In medically refractory AF, AV junction ablation with permanent pacing may be an attractive option for rate control in selected elderly patients who are not candidates for pulmonary venous isolation due to permanent AF, frailty, or severe comorbidities. In a study of older patients with AF refractory to medical therapy, AV junction ablation with permanent pacing led to better control of AF when compared with AF ablation, but it was associated with a higher risk of heart failure (79). When AV junction ablation is performed in the setting of a left ventricular ejection fraction <50%, strong consideration should be given to implantation of a cardiac resynchronization therapy device, given the risk of adverse outcomes with chronic right ventricular pacing (80).
ATRIAL FLUTTER
Atrial flutter can be 100× more frequent in individuals ≥80 years of age compared with those younger than 50 years of age (81). The management of atrial flutter does not vary with age. Isthmus-dependent atrial flutter is often treated by radiofrequency ablation, given that it is a highly successful and safe procedure. In 1 study, there was no difference in the efficacy and safety of atrial flutter ablation between patients ≥75 years and those <75 years of age (82). In a larger study of 1,187 patients who underwent atrial flutter ablation, patients ≥80 years of age had a procedural success rate (86%) similar to younger patients (83). The complication rates were similar in patients ≥80 years of age and those 70 to 79 years of age (7.0% vs. 7.5%), but modestly worse than in patients younger than 70 years (4.5%). Age ≥75 years has been identified as one of the predictors of new-onset AF after atrial flutter ablation (HR: 1.046; 95% CI: 1.019 to 1.075) (84).
VENTRICULAR ARRHYTHMIAS AND SCD
The incidence of SCD increases with age, although the proportion of deaths that are sudden compared with total mortality declines in the elderly (85). Krahn et al. (85) demonstrated from the Amiodarone Trialists Meta-analysis database that 26% of all deaths in patients ≥80 years of age were SCD, which decreased from 51% in those age <50 years (Figure 6) (85). Kim et al. (86) showed that, in octogenarians with out-of-hospital sudden cardiac arrest, rhythm was a stronger determinant of survival to hospital discharge than age. In fact, patients ≥80 years of age with VT or ventricular fibrillation had better survival to hospital discharge than younger patients who had non-shockable rhythms. The causes of ventricular arrhythmias and SCD differ from those of younger patients and mainly include coronary artery disease and heart failure.
FIGURE 6. Annual Sudden and All-Cause Death Rate in Amiodarone Trialists Meta-Analysis Database of 6,252 Patients With Structural Heart Disease.
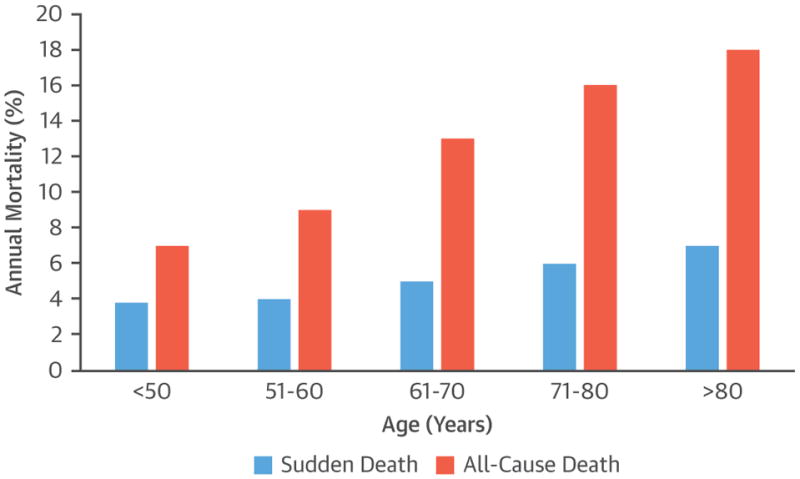
Both sudden death and nonsudden death rates increased with age, although the increase of nonsudden death with age was more dramatic. Reproduced with permission from Krahn et al. (85).
IMPLANTABLE CARDIOVERTER-DEFIBRILLATORS
ICDs are effective in the prevention of arrhythmic SCD in high-risk patients. According to the 2013 National Cardiovascular Data Registry ICD Registry Report (87), 17.9% of ICD recipients were ≥80 years of age, with 23% undergoing generator replacement. The ICD guidelines are based on multiple randomized clinical trials, but patients ≥80 years of age were not well represented in these studies. Therefore, there has been considerable debate over the efficacy and safety of ICDs at an advanced age.
A pooled meta-analysis of secondary prevention ICD trials did not show a survival benefit in patients ≥75 years of age (88). The study was underpowered (n = 252) to show a mortality difference, and most patients had nonarrhythmic deaths. In contrast, an analysis of pooled patient-level data from 5 primary prevention ICD trials showed reduced mortality in patients ≥75 years of age with a hazard ratio of 0.54 (95% posterior credible interval 0.37 to 0.78) (89). Recently, the DANISH (Defibrillator Implantation in Patients with Nonischemic Cardiomyopathy) trial (90) failed to show a survival benefit of primary prevention ICDs, especially in elderly patients. However, use of ICDs was associated with a reduction in SCD by 50%.
The reported rates of procedural complications with ICD implantation in the very elderly have been inconsistent. In 1 study, the adjusted rate of adverse events in ICD recipients ≥80 years of age was 4.5%, with an odds ratio of 1.15 (95% confidence interval: 1.01 to 1.3) compared with those age <65 years (91). In contrast, another study demonstrated a 20% lower complication rate in patients ≥80 years of age (92). The authors speculated that “a higher proportion of simpler cardiac implantable electronic device types” in the elderly may have accounted for the lower complication rate.
Clearly, there is a gap in evidence to routinely support or withhold ICD implantation in patients ≥80 years of age who otherwise meet current indications. According to the American College of Cardiology/American Heart Association/Heart Rhythm Society 2013 appropriate use criteria, it “may be appropriate” to implant defibrillators in patients ≥80 years of age with New York Heart Association functional class II or III symptoms, but it is “rarely appropriate” in patients age ≥90 years and New York Heart Association functional class I symptoms (93). Left ventricular ejection fraction <30% and renal dysfunction are predictors of poor outcomes after ICD implantation in octogenarians, with a mean survival of only 1.5 years when both risk factors are present (94). Similarly, left ventricular ejection fraction ≤20% is the strongest predictor of 1-year mortality (38.2%) in octogenarians after ICD implantation (95). Therefore, the patient’s underlying cardiovascular condition, competing non-cardiovascular comorbidities that would attenuate the benefit of ICD therapy or affect life expectancy, functional quality of life, patient preference, and advanced directives should be carefully considered when contemplating ICD implantation in an elderly patient. The same strategy of shared decision making applies when considering generator change due to end-of-battery-life, device malfunction, or infection.
Although subcutaneous ICDs have become available in the past few years, there are no studies specifically addressing their use in the elderly. The important limitation of such devices in the elderly could be the potential need for transvenous pacing, either because of underlying SND or AV block; the need for cardiac resynchronization therapy (CRT); or the desirability of antitachycardia pacing.
PREVENTION OF RECURRENT VENTRICULAR ARRHYTHMIAS
Although ICDs are effective in preventing SCD, appropriate ICD therapy (shocks or antitachycardia pacing) that occurs in about one-quarter of patients is associated with a 3- to 5-fold increase in mortality as well as impaired quality of life (96). In a meta-analysis of randomized trials, both AADs and catheter ablation significantly reduced recurrent VT but without improving mortality (97). The benefit of AADs in reducing recurrent VT was mainly driven by amiodarone. All the evidence for catheter ablation of VT in very elderly patients comes from retrospective studies. In the largest retrospective study (n = 285) of catheter ablation for recurrent post-infarction VT refractory to AADs, patients ≥70 and <70 years of age had similar rates of procedural success (79.2% vs. 87.8%), periprocedural complications (5.6% vs. 2.3%), and recurrent VT (63.9% vs. 60.1%) (98). A more recent study of catheter ablation of VT refractory to AADs comparing octogenarians (mean age 82 years) to a younger cohort (mean age 67 years) found a lower mean left ventricular ejection fraction (29% vs. 34%), more frequent complications (18% vs. 2%), more ICD shocks (28% vs. 15%), but a similar survival rate (99). Catheter ablation of refractory VT may be considered in selected patients of this age group who have reasonable functional capacity.
CARDIAC RESYNCHRONIZATION THERAPY
CRT is primarily a treatment for heart failure in patients with systolic heart failure and evidence of ventricular dyssynchrony manifested as a prolonged QRS duration and typically left bundle branch block. There is an antiarrhythmic effect of CRT that is seen in patients who have significant improvement of left ventricular end-systolic volume, reflective of reverse ventricular remodeling. A substudy of the MADIT CRT (Multicenter Automated Defibrillator Therapy Cardiac Resynchronization therapy) trial showed a 20% reduction in ventricular tachyarrhythmias for every 10% improvement in left ventricular end-systolic volume (100). Octogenarians do not differ from younger patients in terms of the antiarrhythmic response to CRT (101,102), and the procedural complication rates were not significantly different either. However, CRT is underutilized in the very elderly, as shown in the Swedish Heart Failure Study. Although 37% of octogenarians met an indication, only 4% received a CRT device (103). CRT has also been shown to improve quality of life, gait speed, and frailty score in the elderly. In patients with frailty and multiple comorbidities including renal dysfunction, a CRT pacemaker may be preferable to a CRT defibrillator to achieve the benefits of CRT on symptoms and quality of life without adding defibrillator capability, if the latter is not desired by the patient. Thus, shared decision making with the patient and family is essential.
SPECIAL CONSIDERATIONS
Because of age-related changes in pharmacodynamics and pharmacokinetics, elderly patients often require dose adjustment of antiarrhythmic drugs and anticoagulants. Decreased hepatic metabolism and decreased renal elimination of antiarrhythmic drugs may necessitate the use of lower doses compared with younger patients. In addition, elderly patients usually require lower doses of warfarin and the direct oral anticoagulants for thromboprophylaxis in AF.
Although the indications for implantable devices and ablation do not differ based on age, the possibility of higher complication rates must be taken into account. The coexistence of multiple comorbidities as well as frailty may affect the outcomes, including survival, of therapeutic procedures.
FRAILTY
Frailty is more prevalent with advancing age, occurring in about one-fourth of patients ≥80 years of age. Although AF patients with frailty are at a higher risk of stroke and stroke-related mortality, 1 study found severe frailty as a predictor for withholding anticoagulation in octogenarians (104). In addition, frailty has been associated with less frequent use of a rhythm control strategy for AF (105). Frailty could also have implications regarding when to recommend ICD therapy in octogenarians. An analysis of Medicare data of ICD patients showed that frailty was associated with a higher annual mortality rate (22%) that could attenuate the benefit of ICDs. In addition, CRT-defibrillator implantation in frail elderly patients with nonischemic cardiomyopathy is associated with a higher annual rate of decompensated heart failure compared with nonfrail patients (55.6% vs. 16.4%) (106).
END-OF-LIFE ISSUES
About one-fourth of patients may receive ICD shocks during the last 24 h of life, leading to unnecessary suffering (107). Although it is reasonable to consider deactivating ICDs in terminally ill patients, many patients are not aware of this option, as most physicians may not bring up the subject because they are uncomfortable with it or question the legality (108). It is important that a shared decision about ICD therapy be made with the patient and the family at the time of device implantation or generator change, and as a part of advanced care planning for terminally ill patients.
KNOWLEDGE GAPS AND FUTURE DIRECTIONS
There are critical knowledge gaps in the management of arrhythmias in the very elderly population, highlighted in a 2016 American College of Cardiology/American Heart Association/American Geriatrics Society scientific statement (109). We have summarized some important gaps in Table 4. Current evidence is mostly based on observational studies, registries, or post hoc analyses of randomized trials and are prone to inherent selection bias. Therefore, future randomized studies should include the elderly population and look at outcomes, such as quality of life, functional status, and health care costs, in addition to clinical endpoints and mortality.
TABLE 4.
Key Areas With Knowledge Gaps in the Management of Arrhythmias in the Elderly
| Atrial Fibrillation |
|
|
| The difference between rate and rhythm control strategies on quality of life and functional status. |
| The risks and benefits of AF ablation with respect to short- and long-term outcomes, including quality of life. |
| The impact of AV node ablation with pacemaker implantation outcomes, including quality of life. |
|
|
| Ventricular Arrhythmias |
|
|
| Better noninvasive risk stratification to predict sudden cardiac death. |
| Risks and benefits of catheter ablation of ventricular arrhythmias. |
|
|
| Device-Based Therapy |
|
|
| The impact of ICD implantation for primary and secondary prevention of sudden cardiac death on outcomes, including procedural complications, quality-adjusted life-years gained, and health care costs. |
| Decision making at the time of generator replacement for battery end-of-life. |
Adapted from Rich et al. (109).
AF = atrial fibrillation; AV = atrioventricular; ICD = implantable cardioverter-defibrillator.
CONCLUSIONS
The prevalence of arrhythmias in very elderly patients is rising. The management of arrhythmias in the very elderly is complex due to altered pharmacology, limited life expectancy, competing comorbidities, frailty, and varied treatment goals (Central Illustration). Key points related to management of arrhythmias in the elderly are summarized in Table 5.
TABLE 5.
Key Perspectives on Management of Arrhythmias in the Elderly
| Because of age-related changes in pharmacodynamics and pharmacokinetics, elderly patients often require dose-adjustment of antiarrhythmic drugs and anticoagulants. |
| Very old patients with AF have an increased risk of stroke and bleeding, but there is a net benefit favoring the use of oral anticoagulants. |
| The procedural success and complication rates of catheter ablation of AF, SVT, AV junctional arrhythmia, and ventricular tachycardia in very elderly patients are similar to those in younger patients. However, the results of observational studies may be due, at least in part, to selection bias. Therefore, these procedures may be performed in the elderly with careful consideration of comorbidities, life expectancy, frailty, and patient preference. |
| The indications for implantable device therapy (ICDs, pacemakers, or CRT) are the same as in younger patients, and complication rates are comparable. The use of primary prevention ICDs in the very elderly population should take into account the goals of therapy, life expectancy, and competing comorbidities that would attenuate the survival benefit of ICDs. |
| There should be emphasis on shared decision-making and advanced care planning based on a candid discussion about the relative efficacy and possible harm from various treatments, competing comorbidities, frailty, patient preference, and advanced directives. |
CRT = cardiac resynchronization therapy; SVT = supraventricular tachycardia; other abbreviations as in Table 4.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. Dr. Curtis has received honoraria for speaking and research study from Medtronic and Abbott; and has served on the advisory boards of Abbott, Novartis, Sanofi-Aventis, and Daiichi-Sankyo. Dr. Sharma has received support from the National Institutes of Health/National Heart, Lung, and Blood Institute grant 1K08HL131987. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. David Cannom, MD, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- AV
atrioventricular
- CRT
cardiac resynchronization therapy
- ICD
implantable cardioverter-defibrillator
- SCD
sudden cardiac death
- SND
sinus node dysfunction
- SVT
supraventricular tachycardia
- VT
ventricular tachycardia
References
- 1.United Nations Department of Economic and Social Affairs, Population Division. World population prospects: the 2017 revision, key findings and advance tables. [Accessed March 28, 2018];Working Paper No. ESA/P/WP/248. 2017 Available at: https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_KeyFindings.pdf.
- 2.Bell FC, Mille ML. The 2014 Life Tables for the United States Social Security Area 1900–2100. [Accessed March 28, 2018];Acturial Study No. 120. Available at: https://www.ssa.gov/OACT/NOTES/as120/LifeTables_Body.html.
- 3.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karagueuzian HS, Nguyen TP, Qu Z, Weiss JN. Oxidative stress, fibrosis, and early after depolarization-mediated cardiac arrhythmias. Front Physiol. 2013;4:19. doi: 10.3389/fphys.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kistler PM, Sanders P, Fynn SP, et al. Electro-physiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–16. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28:539–53. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper LL, Odening KE, Hwang MS, et al. Electromechanical and structural alterations in the aging rabbit heart and aorta. Am J Physiol Heart Circ Physiol. 2012;302:H1625–35. doi: 10.1152/ajpheart.00960.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo CT, Wu JM, Lin KH, Young ML. The effects of aging on AV nodal recovery properties. Pacing Clin Electrophysiol. 2001;24:194–8. doi: 10.1046/j.1460-9592.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- 10.Sangaralingham SJ, Huntley BK, Martin FL, et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–7. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Fortuno MA, Querejeta R, et al. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–62. doi: 10.1016/s0008-6363(03)00498-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DR, Jalife J. Synergistic research between the Center of Arrhythmia Research and the Michigan Biology of Cardiovascular Aging at the University of Michigan. Circ Res. 2017;121:1221–3. doi: 10.1161/CIRCRESAHA.117.311374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein RM, Vester EG, Brehm MU, et al. Inflammation of the myocardium as an arrhythmia trigger. Z Kardiol. 2000;89(Suppl 3):24–35. [PubMed] [Google Scholar]
- 14.Kantelip JP, Sage E, Duchene-Marullaz P. Findings on ambulatory electrocardiographic monitoring in subjects older than 80 years. Am J Cardiol. 1986;57:398–401. doi: 10.1016/0002-9149(86)90760-5. [DOI] [PubMed] [Google Scholar]
- 15.Manolio TA, Furberg CD, Rautaharju PM, et al. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: the Cardiovascular Health Study. J Am Coll Cardiol. 1994;23:916–25. doi: 10.1016/0735-1097(94)90638-6. [DOI] [PubMed] [Google Scholar]
- 16.Jensen PN, Gronroos NN, Chen LY, et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–8. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mymin D, Mathewson FA, Tate RB, Manfreda J. The natural history of primary first-degree atrio-ventricular heart block. N Engl J Med. 1986;315:1183–7. doi: 10.1056/NEJM198611063151902. [DOI] [PubMed] [Google Scholar]
- 18.Upshaw CB., Jr Comparison of the prevalence of first-degree atrioventricular block in African-American and in Caucasian patients: an electrocardiographic study III. J Natl Med Assoc. 2004;96:756–60. [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok CS, Rashid M, Beynon R, et al. Prolonged PR interval, first-degree heart block and adverse cardiovascular outcomes: a systematic review and meta-analysis. Heart. 2016;102:672–80. doi: 10.1136/heartjnl-2015-308956. [DOI] [PubMed] [Google Scholar]
- 20.Rajala S, Haavisto M, Kaltiala K, Mattila K. ECG findings and survival in very old people. Eur Heart J. 1985;6:247–52. doi: 10.1093/oxfordjournals.eurheartj.a061848. [DOI] [PubMed] [Google Scholar]
- 21.Sutton R. Mobitz type 1 second degree atrio-ventricular block: the value of permanent pacing in the older patient. Heart. 2013;99:291–2. doi: 10.1136/heartjnl-2012-303062. [DOI] [PubMed] [Google Scholar]
- 22.Coumbe AG, Naksuk N, Newell MC, Somasundaram PE, Benditt DG, Adabag S. Long-term follow-up of older patients with Mobitz type I second degree atrioventricular block. Heart. 2013;99:334–8. doi: 10.1136/heartjnl-2012-302770. [DOI] [PubMed] [Google Scholar]
- 23.Grover FL, Vemulapalli S, Carroll JD, et al. 2016 Annual report of the Society of Thoracic Surgeons/American College of Cardiology Trans-catheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69:1215–30. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98:2494–500. doi: 10.1161/01.cir.98.22.2494. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JF, Thomas HE, Kreger BE, McNamara PM, Sorlie P, Kannel WB. Newly acquired right bundle-branch block: The Framingham Study. Ann Intern Med. 1980;92:37–44. doi: 10.7326/0003-4819-92-1-37. [DOI] [PubMed] [Google Scholar]
- 26.Fisch GR, Zipes DP, Fisch C. Bundle branch block and sudden death. Prog Cardiovasc Dis. 1980;23:187–224. doi: 10.1016/0033-0620(80)90021-3. [DOI] [PubMed] [Google Scholar]
- 27.Kalscheur MM, Donateo P, Wenzke KE, et al. Long-term outcome of patients with bifascicular block and unexplained syncope following cardiac pacing. Pacing Clin Electrophysiol. 2016;39:1126–31. doi: 10.1111/pace.12946. [DOI] [PubMed] [Google Scholar]
- 28.de Vries LM, Dijk WA, Hooijschuur CA, Leening MJ, Stricker BH, van Hemel NM. Utilisation of cardiac pacemakers over a 20-year period: Results from a nationwide pacemaker registry. Neth Heart J. 2017;25:47–55. doi: 10.1007/s12471-016-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med. 2008;23(Suppl 1):13–9. doi: 10.1007/s11606-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998;338:1097–104. doi: 10.1056/NEJM199804163381602. [DOI] [PubMed] [Google Scholar]
- 31.Rosenqvist M, Obel IW. Atrial pacing and the risk for AV block: is there a time for change in attitude? Pacing Clin Electrophysiol. 1989;12:97–101. doi: 10.1111/pace.1989.12.p1.97. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen JC, Thomsen PE, Hojberg S, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686–96. doi: 10.1093/eurheartj/ehr022. [DOI] [PubMed] [Google Scholar]
- 33.Udo EO, van Hemel NM, Zuithoff NP, et al. Long-term outcome of cardiac pacing in octogenarians and nonagenarians. Europace. 2012;14:502–8. doi: 10.1093/europace/eur329. [DOI] [PubMed] [Google Scholar]
- 34.Armaganijan LV, Toff WD, Nielsen JC, et al. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol. 2012;35:131–4. doi: 10.1111/j.1540-8159.2011.03240.x. [DOI] [PubMed] [Google Scholar]
- 35.Mandawat A, Curtis JP, Mandawat A, Njike VY, Lampert R. Safety of pacemaker implantation in nonagenarians: an analysis of the healthcare cost and utilization project-nationwide inpatient sample. Circulation. 2013;127:1453–65. 1465.e1–2. doi: 10.1161/CIRCULATIONAHA.113.001434. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–41. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 37.Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intra-cardiac leadless pacemaker. N Engl J Med. 2015;373:1125–35. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 38.Dukes JW, Dewland TA, Vittinghoff E, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–9. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luber S, Brady WJ, Joyce T, Perron AD. Paroxysmal supraventricular tachycardia: outcome after ED care. Am J Emerg Med. 2001;19:40–2. doi: 10.1053/ajem.2001.20030. [DOI] [PubMed] [Google Scholar]
- 40.Sosunov EA, Anyukhovsky EP, Rosen MR. Adrenergic-cholinergic interaction that modulates repolarization in the atrium is altered with aging. J Cardiovasc Electrophysiol. 2002;13:374–9. doi: 10.1046/j.1540-8167.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 41.Brembilla-Perrot B, Houriez P, Beurrier D, et al. Influence of age on the electrophysiological mechanism of paroxysmal supraventricular tachycardias. Int J Cardiol. 2001;78:293–8. doi: 10.1016/s0167-5273(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen SA, Lee SH, Wu TJ, et al. Initial onset of accessory pathway-mediated and atrioventricular node reentrant tachycardia after age 65: clinical features, electrophysiologic characteristics, and possible facilitating factors. J Am Geriatr Soc. 1995;43:1370–7. doi: 10.1111/j.1532-5415.1995.tb06616.x. [DOI] [PubMed] [Google Scholar]
- 43.Brembilla-Perrot B, Zinsch AM, Sellal JM, et al. Age-related prognosis of syncope associated with a preexcitation syndrome. Pacing Clin Electro-physiol. 2013;36:803–10. doi: 10.1111/pace.12110. [DOI] [PubMed] [Google Scholar]
- 44.Orejarena LA, Vidaillet H, Jr, DeStefano F, et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–7. doi: 10.1016/s0735-1097(97)00422-1. [DOI] [PubMed] [Google Scholar]
- 45.Boulos M, Hoch D, Schecter S, Greenberg S, Levine J. Age dependence of complete heart block complicating radiofrequency ablation of the atrioventricular nodal slow pathway. Am J Cardiol. 1998;82:390–1. doi: 10.1016/s0002-9149(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 46.Rostock T, Risius T, Ventura R, et al. Efficacy and safety of radiofrequency catheter ablation of atrioventricular nodal reentrant tachycardia in the elderly. J Cardiovasc Electrophysiol. 2005;16:608–10. doi: 10.1111/j.1540-8167.2005.40717.x. [DOI] [PubMed] [Google Scholar]
- 47.Brembilla-Perrot B, Olivier A, Sellal JM, et al. Influence of advancing age on clinical presentation, treatment efficacy and safety, and long-term outcome of pre-excitation syndromes: a retrospective cohort study of 961 patients included over a 25-year period. BMJ Open. 2016;6:e010520. doi: 10.1136/bmjopen-2015-010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SA, Tai CT, Chiang CE, Ding YA, Chang MS. Focal atrial tachycardia: reanalysis of the clinical and electrophysiologic characteristics and prediction of successful radiofrequency ablation. J Cardiovasc Electrophysiol. 1998;9:355–65. doi: 10.1111/j.1540-8167.1998.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 49.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 50.Magnani JW, Wang N, Benjamin EJ, et al. Atrial fibrillation and declining physical performance in older adults: the Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2016;9:e003525. doi: 10.1161/CIRCEP.115.003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santangeli P, Di Biase L, Bai R, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9:1761–8. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Wallace ER, Siscovick DS, Sitlani CM, et al. Incident atrial fibrillation and disability-free survival in the cardiovascular health study. J Am Geriatr Soc. 2016;64:838–43. doi: 10.1111/jgs.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 54.Brunetti ND, De Gennaro L, Pellegrino PL, Dellegrottaglie G, Antonelli G, Di Biase M. Atrial fibrillation with symptoms other than palpitations: incremental diagnostic sensitivity with at-home tele-cardiology assessment for emergency medical service. Eur J Prev Cardiol. 2012;19:306–13. doi: 10.1177/1741826711406060. [DOI] [PubMed] [Google Scholar]
- 55.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–84. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 56.Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739–49. doi: 10.1160/TH11-05-0364. [DOI] [PubMed] [Google Scholar]
- 57.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–72. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) Circulation. 2014;130:138–46. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 59.Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc. 2016;5:e003432. doi: 10.1161/JAHA.116.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauw MN, Eikelboom JW, Coppens M, et al. Effects of dabigatran according to age in atrial fibrillation. Heart. 2017;103:1015–23. doi: 10.1136/heartjnl-2016-310358. [DOI] [PubMed] [Google Scholar]
- 61.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 62.Scowcroft AC, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: an analysis using the General Practice Research Database (GPRD) 2000–2009. Heart. 2013;99:127–32. doi: 10.1136/heartjnl-2012-302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 64.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–85. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 66.Orkaby AR, Ozonoff A, Reisman JI, Miller DR, Zhao S, Rose AJ. Continued use of warfarin in veterans with atrial fibrillation after dementia diagnosis. J Am Geriatr Soc. 2017;65:249–56. doi: 10.1111/jgs.14573. [DOI] [PubMed] [Google Scholar]
- 67.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–8. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 68.Sambola A, Mutuberria M, Garcia Del Blanco B, et al. Impact of triple therapy in elderly patients with atrial fibrillation undergoing percutaneous coronary intervention. PLoS One. 2016;11:e0147245. doi: 10.1371/journal.pone.0147245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–15. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 70.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–34. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 71.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after pci in atrial fibrillation. N Engl J Med. 2017;377:1513–24. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 72.Holmes DR, Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 73.Julian DG, Camm AJ, Frangin G, et al. for the European Myocardial Infarct Amiodarone Trial Investigators. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet. 1997;349:667–74. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 74.Kober L, Bloch Thomsen PE, Moller M, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–8. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 75.Zado E, Callans DJ, Riley M, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in the elderly. J Cardiovasc Electrophysiol. 2008;19:621–6. doi: 10.1111/j.1540-8167.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 76.Santangeli P, Di Biase L, Mohanty P, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol. 2012;23:687–93. doi: 10.1111/j.1540-8167.2012.02293.x. [DOI] [PubMed] [Google Scholar]
- 77.Tan HW, Wang XH, Shi HF, et al. Efficacy, safety and outcome of catheter ablation for atrial fibrillation in octogenarians. Int J Cardiol. 2010;145:147–8. doi: 10.1016/j.ijcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 78.Bunch TJ, Weiss JP, Crandall BG, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol. 2010;33:146–52. doi: 10.1111/j.1540-8159.2009.02604.x. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh MH, Tai CT, Lee SH, et al. Catheter ablation of atrial fibrillation versus atrioventricular junction ablation plus pacing therapy for elderly patients with medically refractory paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:457–61. doi: 10.1111/j.1540-8167.2005.40632.x. [DOI] [PubMed] [Google Scholar]
- 80.Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–93. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 81.Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–6. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 82.Da Costa A, Zarqane-Sliman N, Romeyer-Bouchard C, et al. Safety and efficacy of radio-frequency ablation of common atrial flutter in elderly patients: a single center prospective study. Pacing Clin Electrophysiol. 2003;26:1729–34. doi: 10.1046/j.1460-9592.2003.t01-1-00259.x. [DOI] [PubMed] [Google Scholar]
- 83.Brembilla-Perrot B, Sellal JM, Olivier A, et al. Risk and outcome after ablation of isthmus-dependent atrial flutter in elderly patients. PLoS One. 2015;10:e0127672. doi: 10.1371/journal.pone.0127672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen K, Bai R, Deng W, et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm. 2015;12:1483–9. doi: 10.1016/j.hrthm.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 85.Krahn AD, Connolly SJ, Roberts RS, Gent M for the ATMA Investigators. Diminishing proportional risk of sudden death with advancing age: implications for prevention of sudden death. Am Heart J. 2004;147:837–40. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 86.Kim C, Becker L, Eisenberg MS. Out-of-hospital cardiac arrest in octogenarians and nonagenarians. Arch Intern Med. 2000;160:3439–43. doi: 10.1001/archinte.160.22.3439. [DOI] [PubMed] [Google Scholar]
- 87.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: version 2. 1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 88.Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J. 2007;28:1746–9. doi: 10.1093/eurheartj/ehl438. [DOI] [PubMed] [Google Scholar]
- 89.Hess PL, Al-Khatib SM, Han JY, et al. Survival benefit of the primary prevention implantable cardioverter-defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–86. doi: 10.1161/CIRCOUTCOMES.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Køber L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 91.Tsai V, Goldstein MK, Hsia HH, et al. Influence of age on perioperative complications among patients undergoing implantable cardioverter-defibrillators for primary prevention in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:549–56. doi: 10.1161/CIRCOUTCOMES.110.959205. [DOI] [PubMed] [Google Scholar]
- 92.Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–94. doi: 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318–68. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 94.Koplan BA, Epstein LM, Albert CM, Stevenson WG. Survival in octogenarians receiving implantable defibrillators. Am Heart J. 2006;152:714–9. doi: 10.1016/j.ahj.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Ertel D, Phatak K, Makati K, et al. Predictors of early mortality in patients age 80 and older receiving implantable defibrillators. Pacing Clin Electrophysiol. 2010;33:981–7. doi: 10.1111/j.1540-8159.2010.02729.x. [DOI] [PubMed] [Google Scholar]
- 96.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 97.Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–9. doi: 10.1016/j.hrthm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Inada K, Roberts-Thomson KC, Seiler J, et al. Mortality and safety of catheter ablation for antiarrhythmic drug-refractory ventricular tachycardia in elderly patients with coronary artery disease. Heart Rhythm. 2010;7:740–4. doi: 10.1016/j.hrthm.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 99.Barra S, Begley D, Heck P, Turner I, Agarwal S. Ablation of ventricular tachycardia in the very elderly patient with cardiomyopathy: how old is too old? Can J Cardiol. 2015;31:717–22. doi: 10.1016/j.cjca.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 100.Barsheshet A, Wang PJ, Moss AJ, et al. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2011;57:2416–23. doi: 10.1016/j.jacc.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 101.Killu AM, Wu JH, Friedman PA, et al. Outcomes of cardiac resynchronization therapy in the elderly. Pacing Clin Electrophysiol. 2013;36:664–72. doi: 10.1111/pace.12048. [DOI] [PubMed] [Google Scholar]
- 102.Achilli A, Turreni F, Gasparini M, et al. Efficacy of cardiac resynchronization therapy in very old patients: the Insync/Insync ICD Italian Registry. Europace. 2007;9:732–8. doi: 10.1093/europace/eum143. [DOI] [PubMed] [Google Scholar]
- 103.Lund LH, Benson L, Stahlberg M, et al. Age, prognostic impact of QRS prolongation and left bundle branch block, and utilization of cardiac resynchronization therapy: findings from 14,713 patients in the Swedish Heart Failure Registry. Eur J Heart Fail. 2014;16:1073–81. doi: 10.1002/ejhf.162. [DOI] [PubMed] [Google Scholar]
- 104.Lefebvre MC, St-Onge M, Glazer-Cavanagh M, et al. The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF Study. Can J Cardiol. 2016;32:169–76. doi: 10.1016/j.cjca.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 105.Steinberg BA, Holmes DN, Ezekowitz MD, et al. Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2013;165:622–9. doi: 10.1016/j.ahj.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dominguez-Rodriguez A, Abreu-Gonzalez P, Jimenez-Sosa A, et al. The impact of frailty in older patients with non-ischaemic cardiomyopathy after implantation of cardiac resynchronization therapy defibrillator. Europace. 2015;17:598–602. doi: 10.1093/europace/euu333. [DOI] [PubMed] [Google Scholar]
- 107.Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation. 2014;129:422–9. doi: 10.1161/CIRCULATIONAHA.113.002648. [DOI] [PubMed] [Google Scholar]
- 108.Kramer DB, Kesselheim AS, Brock DW, Maisel WH. Ethical and legal views of physicians regarding deactivation of cardiac implantable electrical devices: a quantitative assessment. Heart Rhythm. 2010;7:1537–42. doi: 10.1016/j.hrthm.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rich MW, Chyun DA, Skolnick AH, et al. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67:2419–40. doi: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]