The onset of symptomatic heart failure (HF) remains associated with a poor prognosis despite many advances in therapies and enormous resources spent on both treatment and research for advanced symptomatic HF; therefore, prevention of HF is critical. Reviewing and mirroring successful strategies that have helped in prevention of atherosclerotic cardiovascular disease (ASCVD), in which significant advances have been made in recent decades, may help guide our efforts in preventing HF.
Quantitative assessment of an individual’s ASCVD risk using tools such as the Framingham risk score have been used in guidelines throughout the world as the initial step to guide intensity of risk factor modification using lifestyle and drug therapy. After initial efforts in classifying individuals at risk for HF as Stage A/B resulted in the majority of individuals being classified as “at risk,” HF-specific clinical risk scores were recently developed; however, most primary care providers and even cardiologists are unaware of their existence. The 2013 ACC/AHA HF guidelines do not endorse any specific risk assessment tool, but the 2017 update and the recent AHA scientific statement review the potential value of biomarkers in HF risk assessment.
These advances in HF risk estimation using algorithms that include biomarkers allow quantitative identification of higher risk individuals. Multiple studies, including the Atherosclerosis Risk in Communities study, have shown the value of candidate biomarkers such as troponin T (measured with a highly sensitive assay) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) in predicting HF risk.1 In fact, of the outcomes studied (ASCVD, death, and HF) the best prediction performance was for HF, not ASCVD. A “lab model” (including age, gender, race, troponin T, and NT-proBNP) developed subsequently was as powerful as clinical scores in predicting HF risk and can be reported with lab test results,2 similar to estimated glomerular filtration rate. Other biomarkers (e.g., galectin-3, ST2) and genes are under evaluation. Advances in imaging now allow better study of the relaxation and stiffness of the heart and blood vessels and the interaction between the heart and blood vessels. Hence, routine HF risk assessment in clinical practice needs to be endorsed, encouraged, and emphasized, as it is now possible, with the guidance of biomarkers and imaging, to identify individuals at higher risk of HF with greater precision.
Considerable controversy arose when the ACC/AHA risk calculator was introduced in 2013 as to whether it overestimated risk for ASCVD events (expanded to include stroke in addition to myocardial infarction). Unfortunately, this reflects the myopic view that preventive cardiology is limited to ASCVD. How can a patient–physician discussion not include discussion of risk for developing HF, when incident HF hospitalization is more frequent than incident stroke and myocardial infarction combined? Atrial fibrillation may also need to be considered in an expanded definition of cardiovascular diseases.
Global risk scores that include outcomes of HF along with ASCVD have been recently described.3 Routine provision of global CVD risk in addition to coronary heart disease–, stroke-, and HF-specific risk may help direct global and disease-specific preventive strategies as they emerge (Figure). A global risk estimate could spur an individual to improve his/her lifestyle, while disease-specific risk could direct therapy targeting risk factors common to cardiovascular diseases. For example, treatment of an individual at higher risk for HF may include a Dietary Approaches to Stop Hypertension (DASH)–style diet, a diuretic for hypertension, and perhaps a medication such as empagliflozin for diabetes and avoidance of a pure alpha-blocker.
Figure. A framework to approaching comprehensive primary prevention of global cardiovascular disease risk with a focus on component cardiovascular disease.
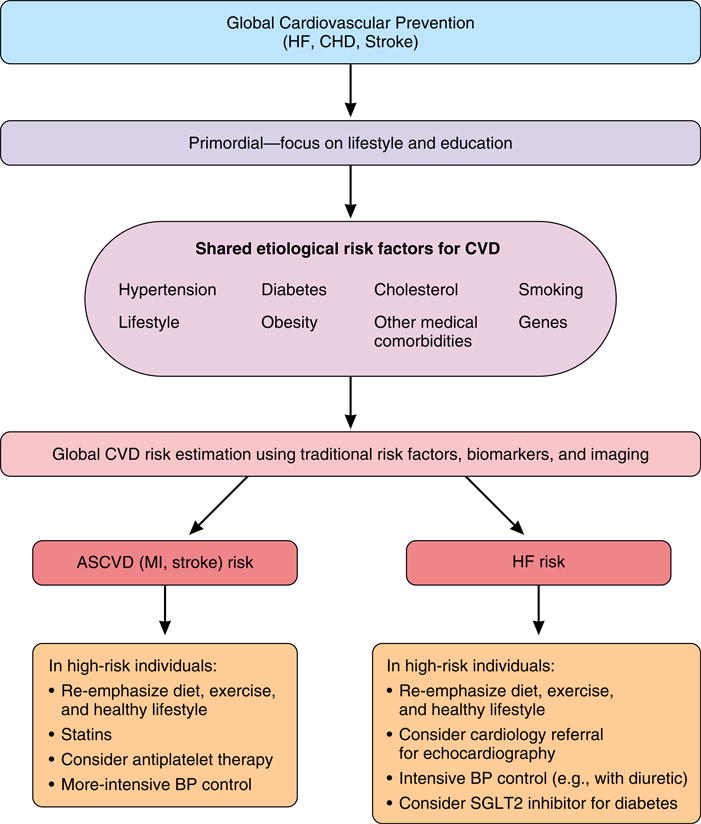
Abbreviations: Afib: atrial fibrillation; BP: blood pressure; CHD: coronary heart disease; CVD: cardiovascular disease; HF: heart failure; RAAS: renin–angiotensin–aldosterone system.
Part of the clinical inertia in risk assessment may be the lack of an answer to a clinician’s question, “what would I do with this information?,” as thus far no unique therapies prevent HF. However, recent studies testing biomarker-based approaches may provide guidance. For example, the St Vincent’s Screening to Prevent Heart Failure (STOP-HF) study reported improved outcomes in individuals with BNP >50 pg/ml randomized to receive an echocardiogram with cardiology evaluation versus usual care.4 BNP-based screening and collaborative care reduced combined rates of left ventricular dysfunction with or without heart failure as well as the incidence of emergency hospitalization for major cardiovascular events. Similarly, the NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients without a History of Cardiac Disease (PONTIAC) trial randomized 300 subjects with type 2 diabetes and NT-proBNP >125 pg/ml to regular care in diabetes care units or intensified care in which renin–angiotensin antagonists and beta-blockers were up-titrated in a cardiology setting. Intensive treatment resulted in a significant reduction in the primary endpoint of hospitalization for any cardiac cause.5 We are now studying if randomizing individuals with systolic blood pressure of 120–150 mmHg and an estimated 10-year HF risk >5% to spironolactone or carvedilol will result in improved strain measurements on echocardiogram and improved vascular stiffness compared with usual care (Clinicaltrials.gov NCT02230891). A similar approach using echocardiogram-based strain measurements and acting on changes in this parameter is advocated in cancer chemotherapy.
Primary prevention of HF currently has no clear home. In general, preventive cardiologists have focused on prevention of ASCVD, while HF experts have focused on secondary prevention and management of HF. As a community, we need to pay greater attention and dedicate more resources to what is becoming the most costly cardiovascular disease that cardiologists need to manage. Although HF has various etiologies and pathways, the greatest contributor to HF remains the traditional risk factors. We propose that the following strategies be considered and debated: 1) include HF routinely as a major adverse cardiovascular event and an endpoint in any study evaluating therapy for CVD; 2) include HF risk in estimating global CVD risk and report risk estimates for the component events (e.g., stroke, HF, coronary heart disease); 3) implement dietary and antihypertensive strategies already shown to work; and 4) conduct clinical trials enriched with individuals at higher risk for HF (identified by risk scores) to test a strategy of more aggressive therapy compared with routine care (as was done in the Systolic Blood Pressure Intervention Trial [SPRINT]) for HF prevention.
Acknowledgments
The authors would like to thank Kerrie Jara for her editorial assistance.
Nambi: Research Grant; Merck. Consultant/Advisory Board; Sanofi Regeneron. Provisional patent (# 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine, Roche. Honorarium; Siemens for event adjudication. Dr. Nambi is supported by a VA MERIT grant (1I01CX001112-01: Nambi V (PI) 04/01/2014-03/31/2019 Biomarker guided therapies in Stage A/B Heart Failure).
Ballantyne: Grant/Research Support- All significant. (All paid to institution, not individual): Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, Takeda, NIH, AHA, ADA. Consultant- Abbott Diagnostics, Amarin, Amgen*, Astra Zeneca*, Eli Lilly, Esperion, Genzyme, Isis, Matinas BioPharma Inc, Merck*, Novartis, Pfizer*, Regeneron, Roche, Sanofi-Synthelabo (*Significant where noted [>$10,000]; remainder modest [<$10,000]). Provisional patent (# 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine, Roche.
Footnotes
The views expressed here are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs.
Disclosures:
Deswal: None
References
- 1.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, Boerwinkle E, Hoogeveen RC, Aguilar D, Astor BC, Srinivas PR, Deswal A, Mosley TH, Coresh J, Folsom AR, Heiss G, Ballantyne CM. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk—the Atherosclerosis Risk in Communities study. Clin Chem. 2013;59:1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lemos JA, Ayers CR, Levine B, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, Drazner MH, Budoff M, Greenland P, Ballantyne CM, Khera A. Multimodality strategy for cardiovascular risk assessment: performance in 2 population-based cohorts. Circulation. 2017;135:2119–2132. doi: 10.1161/CIRCULATIONAHA.117.027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 5.Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, Adlbrecht C, Prager R, Luger A, Pacher R, Clodi M. PONTIAC (NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62:1365–1372. doi: 10.1016/j.jacc.2013.05.069. [DOI] [PubMed] [Google Scholar]


