LETTER TO THE EDITOR
The efficacy of early bispecific antibodies redirecting T-cells to eradicate cancer cells was partly limited because of suboptimal effector cell engagement.1 More efficient T-cell activation has been obtained with single-chain variable fragment (scFv) antibodies, notably Bispecific T-cell Engagers (BiTEs).2 Activity of the CD19/CD3 BiTE blinatumomab in adults and children with chemotherapy-resistant CD19+ B-cell acute lymphoblastic leukemia (B-ALL) led to regulatory drug approval in Europe and the United States. Many other BiTEs, all relying on CD3 signaling without providing co-stimulation, are in clinical testing in several solid tumors and hematologic malignancies.2,3
The experience with blinatumomab demonstrates many patients fail BiTE therapy for poorly-understood reasons despite target antigen expression on their cancer cells.4,5 Recent data from our group and others have shown that expression of CD80 or CD86, which signal through CD28, on cancer cells increases BiTE-induced cytotoxicity in vitro,6,7 as does co-treatment with a monoclonal CD28 antibody.6 These data suggest the importance of co-receptor activation for maximal anti-tumor efficacy of BiTEs, a finding reminiscent of data obtained with T-cells expressing chimeric antigen receptors (CARs), when significant improvements in potency were achieved after inclusion of co-stimulatory signaling moieties in the CAR constructs. Since non-specific, cancer cell-independent boosting of CD28-mediated co-stimulation can lead to overwhelming cytokine production, as shown with the superagonist antibody TGN1412,8 we envisioned a novel immunotherapeutic approach built on two BiTEs (or “Simultaneous Multiple Interaction T-cell Engaging [SMITE] bispecifics”; Fig. 1a), each of which binds cancer cells and either CD3 or CD28 to provide T-cell costimulation when employed together in the presence of the target antigen-expressing cancer.
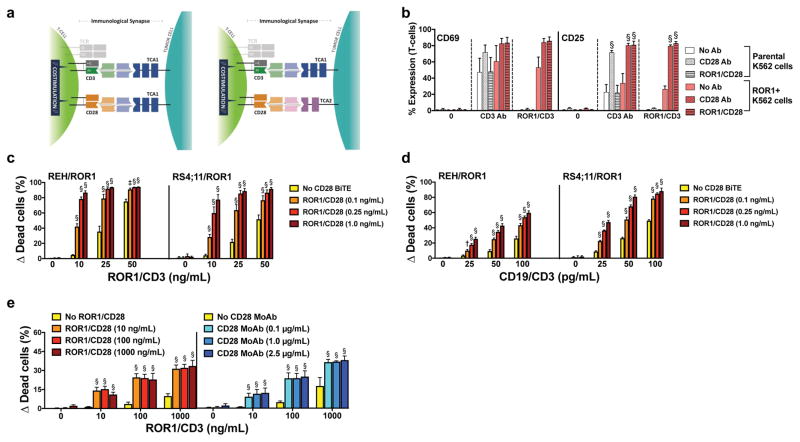
Figure 1. T-cell co-activation with CD28 BiTE antibody enhances anti-tumor efficacy of CD3 BiTE antibodies strictly dependent on the presence of target antigen-positive cancer cells.
(a) Schemes of co-activation of T-cells conferred by pairs of CD3- and CD28-directed BiTEs recognizing the same cancer cell antigen (left-hand scheme) or two separate cancer cell antigens (right-hand scheme). (b) CellVue dye-labeled parental (ROR1-negative) K562 cells and ROR1-transduced K562 cells were left untreated or incubated with CD3 antibody or a ROR1/CD3 BiTE together with unlabeled T-cells at an E:T ratio of 1:1 with or without a CD28 antibody or a ROR1/CD28 BiTE as indicated. After 24 hours, T-cell activation was quantified by flow cytometry via determination of cell surface expression of CD69 and CD25 on CellVue dye-negative cells. Results (mean ± SEM) are shown from 3 independent experiments. (c) CD19+ lymphoid REH and RS4;11 cells transduced to express ROR1 were incubated with CellVue dye-labeled T-cells at an E:T ratio of 1:1 either alone or in the presence of various concentrations of a ROR1/CD3 BiTE and/or a ROR1/CD28 BiTE as indicated. After 48 hours, drug-induced cytotoxicity of CellVue dye-negative cells was determined by flow cytometry. Increases in the percentage of DAPI-positive cells in BiTE-treated cells are compared with corresponding cells that were incubated without BiTEs, and results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells. (d) Similar experiments as described in panel c except that cells were treated with a CD19/CD3 rather than a ROR1/CD3 BiTE. (e) ROR1-transduced K562 cells were incubated with T-cells at an E:T ratio of 1:1 either alone or in the presence of various concentrations of a ROR1/CD3 BiTE and/or either a ROR1/CD28 BiTE or a monoclonal CD28 antibody as indicated. After 48 hours, drug-induced cytotoxicity was determined by flow cytometry. Increases in the percentage of DAPI-positive cells in BiTE-treated cells are compared with corresponding cells that were incubated without treatment, and results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells. Throughout, two-sided P values were calculated using repeated measure one-way or two-way ANOVA with Tukey’s multiple comparison test as appropriate. For all panels, *P<0.05, †P<0.01, ‡P<0.001, and §P<0.0001 vs. corresponding control.
To develop our platform, we generated a series of CD3- and CD28-directed bispecific antibodies targeting the cancer cell-associated antigens CD19 and receptor tyrosine kinase-like orphan receptor 1 (ROR1)9 as well as a CD28-directed bispecific antibody targeting PD-L1. Briefly (see Online Supplement for detailed methods), we used variable domain sequences available from the literature (Supplementary Table 1) for CD19 (blinatumomab), ROR1 (clone R12), CD3 (blinatumomab), CD28 (TGN1412), and PD-L1 (atezolizumab) to build molecules in the canonical BiTE format (Supplementary Fig. 1a). Protein sequences were reverse-translated using human codons and cloned into a modified pCVL lentiviral vector which was then used to transduce Freestyle™ 293-F cells. Secreted protein was extracted from the conditioned media via immobilized metal-affinity chromatography and subsequently polished via size exclusion chromatography (Supplementary Fig. 1b–d). Fractions corresponding to the monomeric proteins were pooled, quantitated, and further analyzed by SDS-PAGE (Supplementary Fig. 1b–d, insets). As source for T-cells, unstimulated peripheral blood mononuclear cells were collected from healthy adult volunteers via leukapheresis under research protocols approved by the Fred Hutch Institutional Review Board after written informed consent was obtained. T-cells were enriched via negative selection through magnetic cell sorting (Pan T-Cell Isolation Kit; Miltenyi Biotec, Auburn, CA, USA), and stored in aliquots in liquid nitrogen.10 Thawed cell aliquots were used, unlabeled or labeled with CellVue dye (eBioscience, San Diego, CA, USA), in assays without prior pre-stimulation.6,10–12 To obtain well-controlled experimental models, sublines of human myeloid K562 and human lymphoid CD19+ RCH-ACV and REH cells overexpressing ROR1 were generated through transduction with a pMP71 retrovirus (kindly provided by Dr. Stanley R. Riddell, Fred Hutchinson Cancer Research Center, Seattle, WA). Sublines of cells overexpressing PD-L1 were generated through transduction with a pRRLsin.cPPT.MSCV lentivirus11,13,14 containing a human PD-L1-IRES-Enhanced Green Fluorescent Protein cassette.6 To quantify T-cell activation, target antigen-negative and target antigen-positive cancer cells were labeled with CellVue dye and incubated in 96-well plates together with unlabeled healthy-donor T-cells at an effector:target (E:T) ratio of 1:1. Parallel cultures were treated with a CD3 antibody (clone OKT3, low endotoxin/azide-free; BioLegend, San Diego, CA, USA) or a CD3 BiTE in combination with either a CD28 antibody (clone CD28.2, low endotoxin/azide-free; BioLegend) or a CD28 BiTE. After 12–24 hours, T-cell activation was assessed by flow cytometry after staining of cells with fluorescently labeled antibodies recognizing CD3 (clone UCHT1, FITC-labeled; BD Biosciences, San Jose, CA, USA), CD4 (clone SK3, BV786-labeled; BD Biosciences), CD8 (clone RPA-T8, PE-Cy7-labeled; BD Biosciences), CD25 (clone M-A2451, APC-labeled; BD Biosciences), and CD69 (clone FN50, PE-labeled; BD Biosciences). Using 4′,6-diamidino-2-phenylindole (DAPI) to separate non-viable cells, induction of CD25 and CD69 was analyzed on CellVue dye-negative cells with FlowJo Software (Tree Star, Ashland, OR). To quantify antibody-induced cytotoxicity, cancer cells were incubated in 96-well plates with various concentrations of monoclonal or bispecific antibodies as well as CellVue dye-labeled T-cells at different E:T cell ratios.6,10–12 After 48 hours, cell numbers and drug-induced cytotoxicity, using DAPI to detect non-viable cells, were determined by flow cytometry. AML cells were identified by forward/side scatter properties and negativity for the CellVue dye. Repeated measures one-way or two-way ANOVA method with Tukey’s multiple comparison testing was used for statistical analysis with provision of two-sided P-values (Prism 7.0c; GraphPad [La Jolla, CA, USA]).
Unlike antibodies to CD3 and CD28, which can dimerize targets, BiTEs like those we generated will depend upon cancer cell binding to engage and co-activate T-cells because the CD3 and CD28 binding is monovalent and occurs via two separate molecules, avoiding unwanted immune stimulation. This feature is exemplified with human K562 cells engineered to express ROR1 and parental ROR1- cells (Fig. 1b), in which healthy-donor T-cell activation by a ROR1/CD3 BiTE, as estimated by cell surface expression of CD69 and CD25, and co-activation by a ROR1/CD28 BiTE (i.e. left-hand scheme depicted in Fig. 1a) is dependent on ROR1 display on cancer cells. In contrast, monoclonal CD3 antibody (clone OKT3) activates T-cells independent of ROR1 expression. Likewise, as long as T-cells are stimulated via CD3, monoclonal CD28 antibody (clone CD28.2) provides T-cell co-activation to both ROR+ and ROR1- K562 cells.
Consistent with the data on T-cell activation, CD28 BiTEs were ineffective alone but significantly augmented the cytotoxic effects of CD3 BiTEs in a dose-dependent fashion (Fig. 1c [for ROR1] and Supplementary Fig. 2 [for CD19]), resulting in maximal cell killing at much lower antibody doses in combination than what could be accomplished with CD3 BiTE alone. Significant enhancement of CD3 BiTE-induced cancer cell killing was also found when the CD28 BiTE was directed at a second cancer cell-associated antigen (i.e. right-hand scheme depicted in Fig. 1a), as shown in Fig. 1d and Supplementary Fig. 3, allowing for the selective targeting of cancers expressing two independent antigens. The magnitude of this effect was similar to that achieved with a CD28 antibody (Fig. 1e), although direct comparisons are limited by the fact that the antibody sequence used in the CD28 BiTE differed from the monoclonal CD28 antibody we had available for our studies.
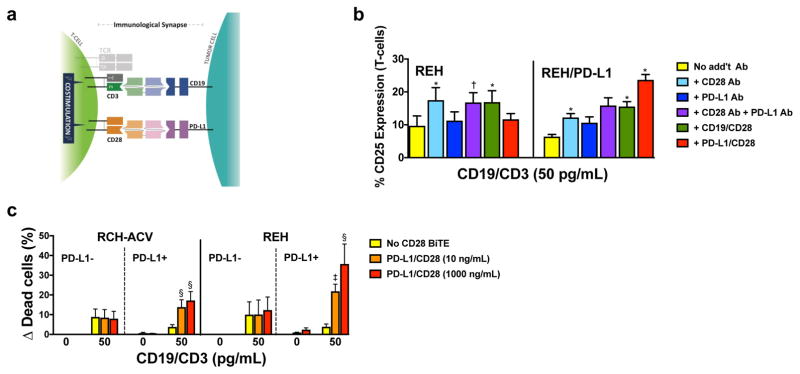
Because two different cancer cell antigens can be targeted with SMITE bispecific pairs, we tested the ability of this immunotherapy approach in overcoming inhibitory T-cell signaling. Of particular interest is PD-L1 (Fig. 2a), which we and others identified as a ligand conferring resistance to CD3 BiTEs in mechanistic studies in vitro.6,7,15 Consistent with this notion, PD-L1 was found to be increased on B-ALL cells of patients refractory to blinatumomab.7 Given this data, we investigated the potential of a PD-L1/CD28 BiTE to overcome PD-L1-mediated drug resistance. Although PD-L1 may be too widely expressed to target with a direct toxin like an antibody-drug conjugate, the requirement for CD3 costimulation with a SMITE bispecific approach allows PD-L1 to be considered as one of the two targeted cancer antigens. Similar to the other CD28-directed bispecific molecules we studied, T-cell co-activation with the PD-L1/CD28 BiTE was strictly dependent on the expression of PD-L1 target antigen and the engagement of CD3 with CD19/CD3 (Fig. 2b). Consistent with these T-cell activation data, co-treatment with a PD-L1/CD28 BiTE did not affect CD19/CD3 BiTE-induced cytotoxicity in parental, PD-L1-negative CD19+ B-ALL cells. However, this BiTE not only reversed the relative PD-L1-mediated resistance in paired cells expressing PD-L1 but led to increased cytotoxic effects relative to parental cells treated with CD19/CD3 BiTE antibody alone (Fig. 2c). Thus, the PD-L1/CD28 BiTE undermined PD-L1, not only blocking its immunosuppressive signal but converting it into a potent costimulatory one.
Figure 2. PD-L1/CD28 BiTE can reverse checkpoint inhibition into T-cell activation to overcome BiTE resistance.
(a) Scheme of co-activation of T-cells conferred by a PD-L1/CD28 BiTE and a paired CD3-directed BiTE recognizing another antigen on a PD-L1-expressing cancer cell. (b) Parental (PD-L1-negative) REH cells and PD-L1-transduced REH cells incubated with a CD19/CD3 BiTE (blinatumomab; 50 pg/mL) without additional antibody or with a CD28 antibody, a PD-L1 antibody, a CD19/CD28 BiTE, or a PD-L1/CD28 BiTE together with T-cells at an E:T ratio of 1:1 as indicated. After 24 hours, T-cell activation was quantified by flow cytometry via determination of cell surface expression of CD25. Results (mean ± SEM) are shown from 3 independent experiments. (c) Parental CD19+ lymphoid RCH-ACV and REH and sublines transduced to express PD-L1 were incubated with T-cells at an E:T ratio of 1:1 either alone or in the presence of a CD19/CD3 BiTE (blinatumomab; 50 pg/mL) with or without various concentrations of a PD-L1/CD28 BiTE as indicated. After 48 hours, drug-induced cytotoxicity was determined by flow cytometry. Increases in the percentage of DAPI-positive cells in BiTE-treated cells are compared with corresponding cells that were incubated without antibody, and results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells. Throughout, two-sided P values were calculated using repeated measure one-way or two-way ANOVA with Tukey’s multiple comparison test as appropriate. For all panels, *P<0.05, †P<0.01, ‡P<0.001, and §P<0.0001 vs. corresponding control.
In summary, our data indicate CD28 BiTEs can potently co-activate T cells stimulated with a CD3 BiTE at concentrations where either agent is inactive (or minimally active) alone, and the strict requirement for the presence of target antigen(s) suggests the possibility for both high selectivity and potency. Such pairs of bispecific antibodies can conceptually target any cancer cell surface antigen(s) and will thus provide a versatile platform exploitable for a wide variety of cancers. As our studies demonstrate, pairs of BiTEs can be utilized to neutralize a cellular resistance factor such as checkpoint inhibition and reverse it into T-cell co-activation for optimized therapeutic efficacy. While our studies focused on CD28, demonstrating that signaling via CD28 is a key modulator of the therapeutic efficacy of BiTE antibodies, future efforts will develop SMITE bispecific pairs targeting other co-receptor signaling pathways.
Supplementary Material
Acknowledgments
This work was supported by grants from the Leukemia & Lymphoma Society (Translational Research Program grant no. 6489-16), Alex’s Lemonade Stand Foundation/Cure4Cam Childhood Cancer Foundation (Innovation Grant), Hyundai Hope on Wheels (Scholar Grant), Bezos Family Immunotherapy Initiative (Pilot Award), and the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (NIDDK/NIH: P30-DK56465, Co-operative Center of Excellence in Hematology). C.D.G. is supported by a fellowship training grant from the National Heart, Lung, and Blood Institute/NIH (NHLBI/NIH: T32-HL007093). R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Footnotes
Supplementary information is available at Leukemia’s website.
Conflict of Interest: R.B.W. has received laboratory research grants and/or clinical trial support from Amgen Inc., Amphivena Therapeutics, Inc., Covagen AG, Aptevo Therapeutics, Inc., and Seattle Genetics, Inc.; has ownership interests with Amphivena Therapeutics, Inc.; and is (or has been) a consultant to Amphivena Therapeutics, Inc., Covagen AG, Emergent Biosolutions, Inc. (now Aptevo Therapeutics, Inc.), Pfizer, Inc., and Seattle Genetics, Inc. The other authors declare no competing financial interests.
References
- 1.Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun. 2012;12:12. [PMC free article] [PubMed] [Google Scholar]
- 2.Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin Pharmacol Ther. 2017;101(5):634–645. doi: 10.1002/cpt.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 5.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 6.Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 2015;5:e340. doi: 10.1038/bcj.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feucht J, Kayser S, Gorodezki D, Hamieh M, Döring M, Blaeschke F, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 9.Shabani M, Naseri J, Shokri F. Receptor tyrosine kinase-like orphan receptor 1: a novel target for cancer immunotherapy. Expert Opin Ther Targets. 2015;19(7):941–955. doi: 10.1517/14728222.2015.1025753. [DOI] [PubMed] [Google Scholar]
- 10.Reusch U, Harrington KH, Gudgeon CJ, Fucek I, Ellwanger K, Weichel M, et al. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Cancer Res. 2016;22(23):5829–5838. doi: 10.1158/1078-0432.CCR-16-0350. [DOI] [PubMed] [Google Scholar]
- 11.Laszlo GS, Gudgeon CJ, Harrington KH, Dell’Aringa J, Newhall KJ, Means GD, et al. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood. 2014;123(4):554–561. doi: 10.1182/blood-2013-09-527044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington KH, Gudgeon CJ, Laszlo GS, Newhall KJ, Sinclair AM, Frankel SR, et al. The broad anti-AML activity of the CD33/CD3 BiTE antibody construct, AMG 330, is impacted by disease stage and risk. PLoS One. 2015;10(8):e0135945. doi: 10.1371/journal.pone.0135945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105(3):1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 14.Laszlo GS, Harrington KH, Gudgeon CJ, Beddoe ME, Fitzgibbon MP, Ries RE, et al. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget. 2016;7(28):43281–43294. doi: 10.18632/oncotarget.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Köhnke T, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30(2):484–491. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.