Abstract
Uniform and strong expression of CD19, a cell surface antigen, on cells of B-cell lineage is unique to hematologic malignancies. Tumor-associated antigen (TAA) targets in solid tumors exhibit heterogeneity with regards to intensity and distribution, posing a challenge for chimeric antigen receptor (CAR) T-cell therapy. Novel CAR designs, such as dual TAA-targeted CARs, tandem CARs, and switchable CARs, in conjunction with inhibitory CARs, are being investigated as means to overcome antigen heterogeneity. In addition to heterogeneity in cancer-cell antigen expression, the key determinants for antitumor responses are CAR expression levels and affinity in T cells. Herein, we review CAR T-cell therapy clinical trials for patients with lung or pancreatic cancers, and provide detailed translational strategies to overcome antigen heterogeneity.
INTRODUCTION
Chimeric antigen receptors (CARs) are genetically engineered synthetic receptors that are transduced into patient T cells to recognize and bind to cancer cell surface antigens, thus resulting in T-cell activation and cancer cell lysis. CAR-transduced T cells are expanded ex vivo and adoptively transferred back to the patient with the goal of eliminating tumor cells and creating immunologic memory against the targeted antigen. CD19-targeted CAR T cells have demonstrated dramatic clinical responses in hematologic malignancies, such as B-cell acute lymphoblastic leukemia (B-ALL), and were approved for use by the U.S. Food and Drug Administration. Translating successful CAR T-cell therapies to solid tumors requires overcoming several barriers such as finding an ideal tumor-associated antigen (TAA) to target and overcoming antigen expression heterogeneity. In our review, we discuss potential strategies to overcome the barrier of antigen heterogeneity to achieving effective CAR T-cell therapies for solid tumors using lung and pancreatic cancers as examples.
THE STRUCTURE AND EVOLUTION OF CAR DESIGNS
CARs consist of an antigen-binding domain that is derived from a single-chain variable fragment (scFv) of a monoclonal antibody, a flexible spacer/hinge region, a trans-membrane domain, and a CD3-ζ or Fc-γ intracellular signaling domain [1]. CARs can recognize TAAs on the surface of cancer cells without the need for antigen presentation through peptide-major histocompatibility complexes. First generation CARs contain a target-specific receptor fused to an activation signaling domain and they have produced limited therapeutic responses [2]. Second and third generation CARs incorporate one or two co-stimulatory molecules such as CD28, 4-1BB, and OX40. Both second and third generation CAR T cells exhibit greater antitumor potency due to increased signaling strength and enhanced cell proliferation [3]. To improve efficacy, CARs that produce cytokines or are resistant to checkpoint inhibition and immunosuppressive signals in the tumor microenvironment have also been developed [4,5]. The inhibitory CAR (iCAR) fuses an antigen recognition domain (usually an antigen expressed on normal tissue) with an inhibitory intracellular domain (programmed cell death protein 1 [PD-1] or cytotoxic T-lymphocyte-associated protein 4 [CTLA-4]). When co-transduced with a regular CAR, activation of the iCAR can inhibit the activity of the co-expressed CAR, which limits undesired CAR activation [6]. Novel designs, such as tandem CARs (TanCAR) [7] and switchable CARs [8,9], broaden the spectrum of TAAs that can be targeted simultaneously. Suicide genes, such as inducible caspase-9 or truncated EGFR, have also been incorporated into CAR design to improve safety [10,11]
CAR T-CELL THERAPY FOR LUNG AND PANCREATIC CANCERS
Our group has reported on the prognostic significance of a higher ratio of effector to suppressive cellular immune responses in non-small cell lung cancer (NSCLC) patients [12,13]. Promoting effector cellular immune responses by developing CAR T-cell therapy for solid tumors, such as lung and pancreatic cancers, poses challenges that include suitable tumor antigen target selection, promotion of efficient T-cell infiltration to the tumor, and generation of a potent and sustained cellular immune response in an immunologically suppressive tumor microenvironment. In finding a candidate target antigen for CAR T-cell therapy for NSCLC, our group and others have investigated mesothelin (MSLN), EGFR, HER2, mucin 1 (MUC1), and carcinoembryonic antigen (CEA) (Table 1) [14]. CAR T-cell therapies that target MSLN, prostate stem cell antigen (PSCA), MUC1, HER2, and EGFR are currently being evaluated in clinical trials for pancreatic cancer [15].
Table 1.
Current Clinical Trials for Lung and Pancreatic Cancers on ClinicalTrials.gov
| Malignancy type | Target antigen | Phase | Location | Co-stimulatory Signal (★CD28, ❖4-1BB) | Pre-condition | Additional info. | NCT# |
|---|---|---|---|---|---|---|---|
| Lung Cancer | Mesothelin | I | MSK | ★ | Cy | Contains iCasp9 suicide gene | NCT02414269 |
| ROR1 | I | Fred Hutchinson | Cy/Flu | NCT02706392 | |||
| GPC3 | I | Shanghai, China | Cy/Flu | NCT02876978 | |||
| PSCA | I | Guangdong China | ★ | NCT03198052 | |||
| EGFR family | I/II | Shanghai, China | Anti-PD-1Ab expressing CAR | NCT02862028 | |||
| EGFR | I/II | Beijing, China | ❖ | NCT01869166 | |||
| Pancreatic Cancer | CD133 | I | Beijing, China | ❖ | NCT02541370 | ||
| Mesothelin, CD19 | I | UPenn/UCSF | ❖ | Cy | CART19 is included to attack B cells and impede the antibody response against CAR T-cell meso T cells | NCT02465983 | |
| Mesothelin | I | Shanghai, China | Cy | Transcatheter arterial infusion | NCT02706782 | ||
| Mesothelin | I | UPenn | ❖ | NCT01897415 | |||
| Mesothelin | I | Beijing, China | NCT02580747 | ||||
| PSCA | I | Baylor Sammons Cancer Center | Inducible MyD88/CD40 | Rimiducid as dimerization agent to stimulate CAR T cells | NCT02744287 | ||
| CD70 | I/II | NCI | Cy/Flu | Co-administration of aldesleukin | NCT02830724 | ||
| EpCAM | I/II | Chengdu, China | ★ | Yes, no details | NCT03013712 | ||
| Mesothelin | I/II | Shanghai, China | NCT02959151 | ||||
| Lung and Pancreatic Cancers | CEA | I | Chongqing, China | NCT02349724 | |||
| HER2 | I/II | Chongqing, China | NCT 02713984 | ||||
| Mesothelin | I/II | NCI | ★ | Cy/Flu | Co-administration of aldesleukin | NCT01583686 | |
| MUC1 | I/II | Jiangsu, China | NCT02587689 |
Abbreviations: CEA, carcinoembryonic antigen; Cy, cyclophosphamide; Flu, fludarabine; iCasp9, inducible caspase 9; MSK, Memorial Sloan Kettering Cancer Center; MUC1, mucin 1; NCI, National Cancer Institute; PD-1, programmed cell death protein 1; PSCA, prostate stem cell antigen; UCSF, University of California, San Francisco; UPenn, University of Pennsylvania
The desmoplastic matrix in pancreatic adenocarcinoma (PDA) can serve as a physical barrier to potentially impede CAR T-cell infiltration. Smith et al. described localized delivery of CAR T-cells to the surface of solid tumors via biopolymer implants [16]. CAR T cells that target stromal cells [17] and degrade the extracellular matrix component [18] can also promote T-cell infiltration and antitumor activity. Combining TAA-specific and stroma-targeting CARs may synergize antitumor efficacy in stroma-rich solid tumors. In the presence of high antigen burden, tumor-infiltrating CAR T cells may be exhausted by upregulation of PD-1. In order to rescue exhausted T cells and improve their functional persistence, CAR T cells are engineered to co-express a PD-1 dominant negative receptor (DNR) [5] or secret anti-PD-1 antibody [19]. Recent studies have shown that serial infusions of engineered T cells [20] or co-expression of cytokine receptors that reverse inhibitory signals to stimulating signals [21] can enhance T-cell functionality in the immunosuppressive tumor microenvironment of PDA.
Currently, there are more than 30 clinical trials evaluating CAR T-cell therapy in lung and pancreatic cancers (Table 1). In a Phase I clinical trial evaluating EGFR-targeted CAR T cells for refractory NSCLC (NCT01869166), 2 out of 11 patients obtained a partial response and 5 had stable disease for a period of 2 to 8 months [22]. In another study (NCT01355965), anti-MSLN CAR T cells were able to traffic to tumor tissue, elicit a cellular immune response, and induce humoral epitope spreading in a metastatic PDA patient [23]. A recent Phase I study using anti-HER2 CAR T cells to treat HER2-positive advanced biliary tract cancers and pancreatic cancer (NCT01935843) showed that 1 out of 11 patients obtained a partial response and 5 achieved stable disease [24]. Pre-conditioning chemotherapy used in many of these trials (cyclophosphamide alone or in combination with fludarabine) can facilitate the engraftment of adoptively transferred T cells and help decrease suppressive immune cells, such as Tregs and MDSCs, in the tumor microenvironment.
HETEROGENEOUS EXPRESSION OF TARGETED TUMOR-ASSOCIATED ANTIGENS IN LUNG AND PANCREATIC TUMORS
Unlike B-ALL and other hematologic malignancies, the antigen heterogeneity (varying levels of expression intensity and distribution of antigen-positive cells) of solid tumors is a challenge to efficacious CAR T-cell therapy. We have published that, although MSLN is overexpressed in NSCLC compared with normal tissue, tumor cells exhibit varying levels of MSLN expression [10,25]. Compared with NSCLC, pleural mesothelioma and PDA tumor cells express relatively higher percentages and intensity of MSLN expression [10].
HER2 is another commonly targeted TAA in solid tumors. In a study of patients with advanced NSCLC, 40% of tumor samples showed HER2 overexpression with varying staining intensity, as demonstrated by immunohistochemical analysis [26]. The intratumoral heterogeneity of other CAR T-cell therapy targets, such as MUC1, PSCA, and epithelial cell adhesion molecule (EpCAM), has also been reported [27-31]. In vitro experiments have demonstrated that tumor cells expressing high levels of a specific antigen were preferentially eliminated, whereas those with the lowest expression survived [32,33]. Conversely, the presence of multiple TAAs within the same tumor, such as co-expression of MSLN and EpCAM [16], MSLN and MUC16 [34], and PSCA and MUC1 in pancreatic cancer [32], creates an opportunity for using dual-antigen CAR T cells to simultaneously target multiple TAAs.
CAR DENSITY AND BINDING AFFINITY, AND T-CELL ACTIVATION STRENGTH
In addition to the heterogeneous distribution and density of TAAs on tumor cells, CAR T-cell variables, such as CAR density and scFv affinity, can also influence their efficacy. Due to central and peripheral tolerance mechanisms, naturally occurring T-cell receptors (TCRs) usually have a lower affinity to tumor-associated self-antigens than foreign antigens. However, TCRs can recognize very low levels of antigens via the serial triggering mechanism [35]. By contrast, CAR T-cell activation requires TAA density to be above a certain threshold [36]. A higher density is required to induce cytokine production and cell proliferation (activating threshold) compared with triggering cytolytic activity (lytic threshold) [37,38]. Above the lytic threshold, CAR T-cell cytotoxicity correlates with antigen density until a plateau is reached.
CAR T-cell activation is also regulated by the expression level of CARs on the T-cell surface [39]. Lower CAR density results in sub-activation of the CAR T cells, whereas CAR overexpression can result in antigen independent activation, accelerated cell differentiation and exhaustion, or apoptosis [40]. Additionally, CAR density is also affected by antigen-mediated downregulation of CARs from the cell surface [41-43]. The level of downregulation is independent of CAR affinity and associated with tumor-cell antigen density and T-cell CAR density [44]. Depending on the CAR design, CAR downregulation rates range from minutes to hours after antigen encounter and downregulation levels range from 50% to a near complete loss of CAR surface expression. Downregulation of CAR surface expression below critical levels may increase antigen threshold and limit sequential killing of targets, thus preventing CAR T cells from eliminating tumor cells with lower antigen expression. Potentially, the outcome may depend on CAR density pre-antigen encounter; CAR T cells that do not initially express a sufficient number of CARs may experience impaired effector function following post-antigen exposure-mediated CAR downregulation.
The scFv in CARs usually has a higher affinity than TCRs. Since most TAAs are overexpressed self-antigens that are also expressed on normal tissue, CAR T cells containing high-affinity scFvs can initiate an undesired attack on normal tissue [45]. This raises safety concerns over the on-target, off-tumor toxicities. Recent studies have focused on tuning scFvs to an optimal affinity to enable CAR T cells to preferentially target tumor cells with overexpressed TAAs [46,47]. Although the optimal scFv affinity reported varied depending on targets and CAR design, it seems that the Kd in a range of 10-6 to 10-7M, which is close to TCR “natural affinity,” best distinguishes overexpressed TAAs on tumor cells and antigens expressed on normal cells. Notably, using the light-chain exchange technology, a large panel of new antibodies that target the same epitope with a wide range of affinity can be generated, thus making it a feasible approach to screen many different scFvs simultaneously to determine the optimal scFv affinity for CAR T-cell activation [48].
STRATEGIES TO OVERCOME TUMOR-ASSOCIATED ANTIGEN HETEROGENEITY AND TUMOR IMMUNE ESCAPE
Given the heterogeneous nature of solid tumors, single-antigen CAR T-cell therapy can lead to tumor resistance due to outgrowth of target TAA-negative cancer cells or tumor relapse due to antigen escape (Figure 2). Recent studies suggest that simultaneously targeting two TAAs may serve as an effective therapeutic strategy (Table 2). In a B-ALL relapse model, combining anti-CD19 and anti-CD123 CAR T-cells effectively eliminated CD19+ tumor cells and CD19-CD123+ B-ALL precursors, prevented CD19 antigen loss, and suppressed tumor progression [49]. Natural killer (NK) cells that express a CAR that recognizes a common epitope in EGFR and EGFRvIII showed superior antitumor activity compared with single-specific CAR-NK cells that target EGFR or EGFRvIII alone in a glioblastoma (GBM) model [50]. Similarly, simultaneously targeting PSCA and MUC1 in pancreatic cancer and NSCLC [32,51], and sequential infusion of anti-EGFR and anti-CD133 CAR T cells in a patient with advanced cholangiocarcinoma [52], has resulted in enhanced antitumor efficacy. Clinical trials have been designed to test combined or sequential infusion of two CAR T cells that treat B-cell malignancies (NCT02903810, NCT02737085, and NCT03207178).
Figure 2.

(A) Multiplex immunofluorescent staining of a human lung adenocarcinoma that demonstrates heterogeneous antigen expression of MSLN and MUC16 on tumor cells. (B) Addressing TAA heterogeneity in solid tumors: (1) Single TAA-targeted CAR T-cell therapy may result in antigen escape or the outgrowth of tumor cells that either express very low levels of TAA (below CAR T-cell activation threshold) or do not express the targeted TAA. Targeting two TAAs simultaneously, either by co-administration of CAR T cells targeting different antigens (2) or using a TanCAR (3), can mitigate tumor escape. A broad spectrum of TAAs can be targeted simultaneously with switchable CAR-transduced T cells (4).
CAR, chimeric antigen receptor; MSLN, mesothelin; MUC16, mucin 16; TAA, tumor-associated antigen; TanCAR, tandem CAR
Table 2.
Dual-Antigen Targeting Tumors with CAR T cells
| Target antigens | Malignancy | Clinical Setting | Co-stimulatory Signal (★CD28❖4-1BB) | Reference (PMID) |
|---|---|---|---|---|
| CD19 / CD20 | B-cell Leukemia | - | ❖ | 26759369 |
| CD19 / CD20 | B-cell Lymphoma | - | ❖ | 27059623 |
| CD19 / CD22 | Acute Lymphoblastic Leukemia | - | ❖ | 26759368 |
| CD19 / CD123 | Relapsed B-cell Acute Leukemia | - | ❖ | 27571406 |
| HER2 / IL13Rα2 | Glioblastoma | - | ★ | 27427982 |
| EGFR / EGFRvIII | Glioblastoma | - | ★ | 27141401 |
| MUC1 / PSCA | Non-small cell Lung Cancer | - | ★ | 28405515 |
| MUC1 / PSCA | Pancreatic Cancer | - | ★ | 24213558 |
| EGFR / CD133 | Cholangiocarcinoma | + | ❖ | 28057014 |
| CD19 / CD20 | Diffuse Large B-cell Lymphoma | + | NCT02737085 | |
| CD19 / CD20 | Recurrent/Refractory B-cell malignancy | + | NCT03207178 | |
| CD19 / CD22 | B-cell Hematologic Malignancy | + | ❖ | NCT02903810 |
Non-clinical (in vivo animal models); + Clinical trial
Abbreviations: MUC1, mucin 1; PSCA, prostate stem cell antigen
The novel design of tandem CARs (TanCARs) fuses two TAA-specific scFvs with one intracellular signaling moiety. The “proof of concept” TanCAR that targets CD19 and HER2 recognizes each antigen individually and enables synergistic activation when both scFvs are simultaneously engaged with the antigens [53]. Importantly, TanCAR T cells exhibit functional persistence upon losing one antigen expression on tumor cells, which suggests that TanCAR is a valid approach to address tumor immune escape due to antigen loss. In an orthotopic GBM model, TanCARs that target HER2 and IL13Rα2 exhibited superior antitumor activity compared with other combinations (TanCAR > co-expressed CAR > pooled CAR > individual CAR). This effect is, at least partially, attributed to the ability of TanCARs to induce high-density clustering of HER2 and IL13Rα2 in the bivalent immune synapse [7]. A study conducted by Zah et al. revealed that the length of linker and spacer has a great impact on the activity of TanCARs [54], thus highlighting the importance of designing the optimal configuration for TanCARs.
The development of antibody-based switchable CARs connects TAA-specific antibodies with CAR T cells. This expands the possibility of targeting multiple TAAs with one CAR construct. By incorporating a “tag” (peptide neo-epitope or fluorescein isothiocyanate) into a TAA-specific antibody, tag-specific CAR T cells were redirected to and eliminated TAA-expressing tumor cells in vitro and in vivo [8,9]. The activation of CAR T cells has been shown to be antigen-specific and dose-titratable. However, unlike T cells that can actively enter extravascular spaces and migrate into the tumor nest, the distribution of an antibody is mainly through diffusion. Therefore, the antitumor efficacy of the switchable CAR T cells may be largely dependent on the capacity of the “tagged” antibodies penetrating the tumor nest.
CONCLUSIONS
Expanding knowledge of cancer cell antigen expression heterogeneity matched with understanding the dynamic interplay between tumor-cell antigen density, T-cell CAR density, and affinity helps develop strategies to improve CAR T-cell therapy for solid tumors.
Figure 1.
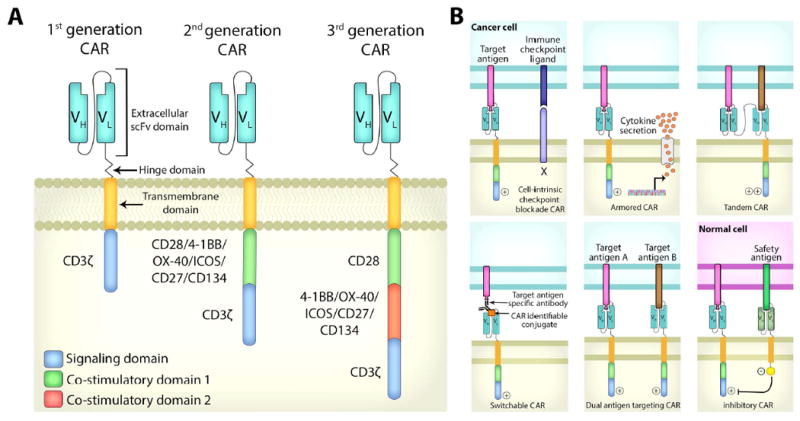
(A) CAR structure: First generation CARs contain an antigen recognition domain fused with an intracellular activation domain. Second and third generation CARs integrate one or two co-stimulatory signals such as CD28, 4-1BB, or OX40. (B) Novel strategies to augment the antitumor efficacy of CARs: CAR T cells rendered resistant to immune checkpoint blockade by co-expression of a dominant negative receptor, armored CARs that secret antitumor-potentiating cytokines, tandem CARs that express two linked scFvs to recognize different antigens, and switchable CARs that recognize a tagged epitope on therapeutic antibodies binding to the cell surface antigen on cancer cells. Co-expression of two CARs enables T cells to simultaneously recognize two TAAs. iCARs (inhibitory CARs that inhibit T-cell activation) express an intracellular inhibitory domain that is fused with an extracellular scFv that recognizes a “safety antigen” expressed on normal cells.
CAR, chimeric antigen receptor; scFv, single chain variable fragment; TAA, tumor-associated antigen
HIGHLIGHTS.
Solid tumor-specific antigen expression is a limitation for CAR T-cell therapy.
Affinity of CARs can significantly influence T-cell effector function.
Novel CAR design and targeting strategies can overcome above obstacles.
Acknowledgments
We thank Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
Funding: The authors’ laboratory research is supported by grants from the National Institutes of Health (P30 CA008748); the U.S. Department of Defense (BC132124 and LC160212); the Derfner Foundation; the Joanne and John DallePezze Foundation; Mr. William H. Goodwin and Mrs. Alice Goodwin; the Commonwealth Foundation for Cancer Research; and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE AND RECOMMENDED READING
- 1.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Yu Z, Muranski P, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 2013;20:575–580. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. This study describes a CAR T-cell intrinsic strategy to augment CAR T-cell antitumor efficacy by use of a PD-1 DNR that acts as a decoy receptor to bind to tumor-cell expressed inhibitory ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. This study describes a new design of tandem CAR (TanCAR) that simultaneously targets HER2 and IL13Rα2. TanCAR cells mitigate tumor antigen escape and exhibit superior antitumor activity compared with pooled CAR T cells or co-transduced T cells in a mouse glioblastoma model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A. 2016;113:E459–468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A. 2016;113:E450–458. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiesgen S, Chicaybam L, Chintala NK, Adusumilli PS. Chimeric antigen receptor (CAR) T-cell therapy for thoracic malignancies. Journal of Thoracic Oncology. 2017 doi: 10.1016/j.jtho.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, Sadelain M, Adusumilli PS. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadota K, Nitadori J, Ujiie H, Buitrago DH, Woo KM, Sima CS, Travis WD, Jones DR, Adusumilli PS. Prognostic Impact of Immune Microenvironment in Lung Squamous Cell Carcinoma: Tumor-Infiltrating CD10+ Neutrophil/CD20+ Lymphocyte Ratio as an Independent Prognostic Factor. J Thorac Oncol. 2015;10:1301–1310. doi: 10.1097/JTO.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeltsman M, Dozier J, McGee E, Ngai D, Adusumilli PS. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res. 2017;187:1–10. doi: 10.1016/j.trsl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSelm CJ, Tano ZE, Varghese AM, Adusumilli PS. CAR T-cell therapy for pancreatic cancer. J Surg Oncol. 2017;116:63–74. doi: 10.1002/jso.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD, Stephan MT. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 2017;127:2176–2191. doi: 10.1172/JCI87624. This study demonstrates a method to deliver high concentration of CAR T cells directly to the surface of solid tumors with an implantable biopolymer. Co-delivery of stimulator of interferon genes agonists stimulates host immune responses to eliminate tumor cells that are not CAR T-cell targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21:524–529. doi: 10.1038/nm.3833. This study discovers that ex vivo expanded human T cells lack expression of heparanase (HPSE), which degrades heparan sulfate proteoglycans, the main componnets of the extracelluar matrix. Co-expression of HSPE enhances CAR T-cell tumor infiltration and antitumor activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, et al. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 20.Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, et al. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2015;28:638–652. doi: 10.1016/j.ccell.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, Brenner MK, Fisher WE, Leen AM, Vera JF. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, Han W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 23.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Antitumor Activity in Solid Malignancies. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2017 doi: 10.1007/s13238-017-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch VW, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20:1020–1028. doi: 10.1158/1078-0432.CCR-13-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinmoller P, Gross C, Beyser K, Schmidtgen C, Maass G, Pedrocchi M, Ruschoff J. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res. 2003;9:5238–5243. [PubMed] [Google Scholar]
- 27.Situ D, Wang J, Ma Y, Zhu Z, Hu Y, Long H, Rong T. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol. 2011;28(Suppl 1):S596–604. doi: 10.1007/s12032-010-9752-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi T, Sho M, Tojo T, Yamato I, Nomi T, Hotta K, Hamada K, Suzaki Y, Sugiura S, Kushibe K, et al. Clinical significance of prostate stem cell antigen expression in non-small cell lung cancer. Jpn J Clin Oncol. 2010;40:319–326. doi: 10.1093/jjco/hyp181. [DOI] [PubMed] [Google Scholar]
- 29.Ali A, Brown V, Denley S, Jamieson NB, Morton JP, Nixon C, Graham JS, Sansom OJ, Carter CR, McKay CJ, et al. Expression of KOC, S100P, mesothelin and MUC1 in pancreatico-biliary adenocarcinomas: development and utility of a potential diagnostic immunohistochemistry panel. BMC Clin Pathol. 2014;14:35. doi: 10.1186/1472-6890-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 31.Pak MG, Shin DH, Lee CH, Lee MK. Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J Surg Oncol. 2012;10:53. doi: 10.1186/1477-7819-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anurathapan U, Chan RC, Hindi HF, Mucharla R, Bajgain P, Hayes BC, Fisher WE, Heslop HE, Rooney CM, Brenner MK, et al. Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol Ther. 2014;22:623–633. doi: 10.1038/mt.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song DG, Ye Q, Poussin M, Chacon JA, Figini M, Powell DJ., Jr Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level. J Hematol Oncol. 2016;9:56. doi: 10.1186/s13045-016-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Vallina L, Russell SJ. Efficient discrimination between different densities of target antigen by tetracycline-regulatable T bodies. Hum Gene Ther. 1999;10:559–563. doi: 10.1089/10430349950018634. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, Sakemura R, Goto T, Hanajiri R, Imahashi N, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 38.Chmielewski M, Hombach AA, Abken H. CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene Ther. 2011;18:62–72. doi: 10.1038/gt.2010.127. [DOI] [PubMed] [Google Scholar]
- 39**.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. This study shows that knocking in CD19 CARs to the endogenous T-cell receptor α constant (TRAC) locus in human peripheral blood T cells reduces tonic signaling, delays effector T-cell differentialtion and exhaustion, and outperforms conventionally generated CAR T cells in a mouse model of acute lymphoblastic leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE, Posey AD, Jr, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, Lopomo P, Vigny M, Fry TJ, Orentas RJ, et al. Tumor Antigen and Receptor Densities Regulate Efficacy of a Chimeric Antigen Receptor Targeting Anaplastic Lymphoma Kinase. Mol Ther. 2017;25:2189–2201. doi: 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Davenport AJ, Jenkins MR, Cross RS, Yong CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK, Neeson PJ. CAR-T Cells Inflict Sequential Killing of Multiple Tumor Target Cells. Cancer Immunol Res. 2015;3:483–494. doi: 10.1158/2326-6066.CIR-15-0048. This study reports that CAR T cells can kill multiple tumor target cells in a sequential manner. The efficiency of killing decreases over time due to downregulation of CARs from the cell surface. [DOI] [PubMed] [Google Scholar]
- 43.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, Biagi E, Tettamanti S, Varani L. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol Ther. 2017;25:1933–1945. doi: 10.1016/j.ymthe.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, Martens ACM, Zweegman S, van de Donk N, Groen RWJ, et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther. 2017;25:1946–1958. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genssler S, Burger MC, Zhang C, Oelsner S, Mildenberger I, Wagner M, Steinbach JP, Wels WS. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5:e1119354. doi: 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X, Lai Y, Li J, Qin L, Xu Y, Zhao R, Li B, Lin S, Wang S, Wu Q, et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology. 2017;6:e1284722. doi: 10.1080/2162402X.2017.1284722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, Huang JH, Yang QM, Han WD. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. doi: 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schonfeld K, Koch J, Dotti G, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]


