Abstract
Evodiamine, a major component of Evodia rutaecarpa, has been reported to possess various pharmacological activities, including anti-inflammatory, antioxidative stress, and neuroprotective effects. Our previous study has shown that the potential effects of evodiamine on the learning and memory impairments in the transgenic mouse model of Alzheimer’s disease (AD). The present study was designed to investigate neuroprotective mechanism and therapeutic potential of evodiamine against intracerebroventricular streptozotocin (ICV-STZ)-induced experimental sporadic Alzheimer’s disease in mice. STZ was injected twice intracerebroventrically (3 mg/kg ICV) on alternate days (day 1 and day 3) in mice. Daily oral administration with evodiamine (50 or 100 mg/kg per day) starting from the first dose of STZ for 21 days showed an improvement in STZ induced cognitive deficits as assessed by novel object recognition and Morris water maze test. Evodiamine significantly decreased STZ induced elevation in acetylcholinesterase activity and malondialdehyde level, and significantly increased STZ induced reduction in glutathione activities and superoxide dismutase activities in the hippocampus compared to control. Furthermore, evodiamine inhibited significantly glial cell activation and neuroinflammation (TNF-α, IL-1β, and IL-6 levels) in the hippocampus. Moreover, evodiamine increased the activity of AKT/GSK-3β signalling pathway and inhibited the activity of nuclear factor κB. In summary, our study suggests that evodiamine can be a novel therapeutic agent for the management of sporadic AD.
Keywords: Evodiamine, Alzheimer’s disease, Streptozotocin, Neuroinflammation, AKT/GSK-3β signalling, NF-κB
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterized by a progressive decline in cognitive functions (Querfurth and LaFerla 2010). Despite the clinic symptoms are similar; AD can be either sporadic or familial form. There have been significant advances in our understanding about AD pathology; however, the exact etiology of AD remains unknown. Collecting evidence indicates that cerebral hypometabolism, synaptic failure, oxidative stress, insulin resistance, and neuroinflammation are involved in the pathogenesis and progression of AD.
Intracerebroventricular (ICV) injection of streptozotocin (STZ), in a sub diabetogenic dose in rat or mouse has been likened to sporadic dementia of AD (Gutierres et al. 2014; Javed et al. 2011). Accumulating studies also demonstrate that ICV-STZ injection can induce remarkable behavioral and pathological alterations mimicking AD characters in rodents, like apparent spatial memory impairments (Lannert and Hoyer 1998; Shoham et al. 2003), cholinergic deficits (Blokland and Jolles 1993), glial activation (Rai et al. 2014), oxidative stress-related neurodegeneration (Javed et al. 2011; Pathan et al. 2006), hyperphosphorylation of tau protein, and the expression of amyloid-β (Grunblatt et al. 2007; Salkovic-Petrisic et al. 2011). The huge similarities in pathological symptoms with those of AD patients qualify ICV-STZ injection a suitable experimental method to explore the underlying molecular and pathophysiological mechanism of AD and their therapeutic intervention for drug development against AD pathology (Deshmukh et al. 2009; Grieb 2016; Santos et al. 2015).
Evodiamine, a quinozole alkaloidmainly, is the major component isolated from the fruits of Evodia rutaecarpa, which has long been utilized in the traditional Chinese medical treatments. With respect to the pharmacological actions of evodiamine, more attention has been paid to its beneficial effects involving anti-inflammmatory (Liu et al. 2009; Yuan et al. 2011), immune modulation (Chang et al. 1995), anti-tumor action (Rasul et al. 2012; Shi et al. 2016) and retarding development of atherosclerosis (Wei et al. 2013). Furthermore, evodiamine has been found to be effective in treating neurodegenerative disorders including cerebral ischemic injury (Zhao et al. 2014), chronic unpredictable mild stress induced depression (Jiang et al. 2015), and (Wu et al. 2016). Our previous studies have shown that evodiamine can ameliorate Aβ-induced cognitive impairments in a genetic AD model (fAD model) (Yuan et al. 2011). Since the mechanisms of fAD and sAD are different, and there is no obvious evidence to show evodiamine would ameliorate the cognitive deficits in sAD, in the present study, we use a sAD model induced by ICV-STZ to investigate the protective effects of evodiamine and explore the underlying mechanisms.
Materials and methods
Animals
C57BL/6 mice were housed individually in plastic rodent cages and maintained on a 12 h light/dark cycle with ad libitum access to conventional standard rodent chow and water, with the constant temperature (23 ± 1 °C) and relative humidity (65%). Protocols were conducted according to the University Policies on the Use and Care of Animals and were approved by the Institutional Animal Experiment Committee of Henan University of Science and Technology, China.
Group and treatment
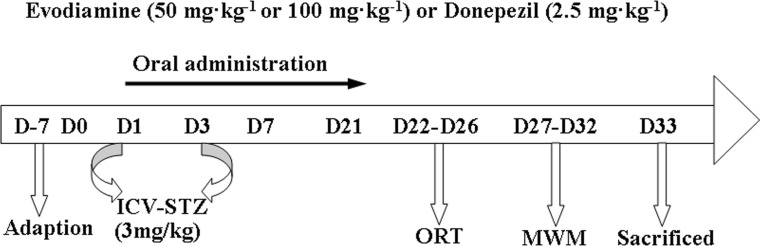
Four-month-old male mice were randomly divided into five groups (n = 15): control group, STZ group, Donepezil group, STZ/Evo 50 group, STZ/Evo 100 group. Mice were administrated ICV injection of either STZ (3 mg/kg, on day 1 and 3) or its solvent (ACSF). Additionally, mice received either evodiamine (50 or 100 mg kg−1 day−1, oral administration) or donepezil (2.5 mg kg−1 day−1, oral administration) for consecutive 21 days. STZ (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in freshly made artificial cerebral spinal fluid (ACSF) (25 mg/ml) solution. Evodiamine was of high purity (> 98%, molecular weight of 450.40) as determined by HPLC analysis and purchased from Bellancom Chemistry (U.S.A.). The dose of evodiamine was selected based on our previous study (Yuan et al. 2011). The administered dose of donepezil, an inhibitor of acetyl-cholinesterase, was calculated from the weight of the mice to be equivalent to the human dose. Upon conversion of animal dose to the equivalent human dose [human dose (mg/kg) = mouse dose (mg/kg) × (3/37)] (Reagan-Shaw et al. 2008), a dose of 0.2 mg kg−1 day−1 donepezil in humans corresponded to 2.5 mg kg−1 day−1 in mice. The experimental procedures were shown in Fig. 1. After the behavioural testing, mice were sacrificed and brain tissue was collected immediately for experiments or stored at − 70 °C.
Fig. 1.
A schematic diagram of drug treatment and protocol design
Surgery and i.c.v. microinjections
Mice anesthetized with 10% chloral hydrate (300 mg/kg, i.p.), were shaved on the dorsal skull surface and cleaned with 70% isopropyl alcohol followed by 10% betadine iodine solution, then were transferred to a stereotactic apparatus (Stoelting Company, Wood Dale, IL, USA). A 26-gauge stainless-steel guide cannula (Plastics One, Roanoke, VA, USA) was directed toward the midhypothalamus in the third ventricle using flat-skull coordinates from bregma (AP 0 mm, ML 0 mm, DV − 5.1 mm). The guide cannula was secured with cyanoacrylate gel (Plastics One) and acrylic dental cement (Jet Denture Repair, Lang Dental Manufacturing Co., Wheeling, IL, USA); the incision was closed with 4-0 silk suture (Syneture; Tyco Healthcare Group, Mansfield, MA, USA). Mice were received corresponding treatment or its solvent alone, slowly infused through a 30-gauge internal cannula (Plastics One) with a 2-μl Hamilton syringe (Fisher Scientific; Nepean, ON, Canada). Correct placement of the cannula was confirmed by injection of angiotensin II (50 ng). Animals not displaying a prompt and sustained drinking response were excluded from the study.
Behavioural tests
Novel object recognition test
The test procedure consisted of three sessions: habituation, training, and retention. Each mouse was habituated to the box (30 × 30 × 35 cm), with 10 min of exploration in the absence of objects for 3 days (habituation session). During the training session, mice were placed in the experimental apparatus and allowed to freely explore the arena in the presence of two identical objects (blue wooden cubes of side 3 cm) for 5 min. The test phase was performed 24 h later. Each mouse was placed in the arena with an object they explored during the training phase (familiar object) and a new (novel) object (a yellow wooden cylinder of diameter 3 cm and height 3 cm). The training and test sessions were video recorded by a tracking system (Ethovison XT, version 4;Noldus Information Technology, Wageningen, Netherlands) and an observer who was blinded to drug treatment scored the time spent exploring the objects. A mouse was scored as exploring an object when its head was oriented toward the object within a distance of 1 cm or when the nose was touching the object. Sitting on or going around the objects was not considered exploratory behavior. A recognition index, a ratio of the amount of time spent exploring any one of the two objects (training session) or the novel object (retention session) over the total time spent exploring both objects, was used to measure cognitive function. To control for odor cues, the OF arena and the objects were thoroughly cleaned with 10% odorless soap, dried, and ventilated for a few minutes between mice (Bevins and Besheer 2006; Takamura et al. 2011).
Morris water maze
Spatial learning and memory was tested using the Morris water maze, performed 1 day after the end of the novel object recognition test. The protocol for the Morris water maze test was modified from previously reported methods (Laczo et al. 2009; Liang et al. 1994). Briefly, the apparatus included a pool with a diameter of 100 cm that was filled with opaque water at approximately 22 ± 1 °C. An escape platform (15 cm in diameter) was placed 0.5 cm below the water surface. Geometric objects with contrasting colours were set at the remote ends of the water tank as references. Room temperature was constant, and the lighting was even throughout the room. Spatial memory is assessed by recording the latency time for the animal to escape from the water onto a submerged escape platform during the learning phase. The mice were subjected to four trials per day for 5 consecutive days. Twenty-four hours after the learning phase, the mice swam freely in the water tank without the platform for 60 s, and the time spent in the region, and number of passes through the region and the quadrant of the original platform were recorded. Performance was monitored with a video tracking system (Noldus Ltd., Ethovision XT, Wageningen, The Netherland). The behavioral studies were performed by an investigator blinded to the experimental group.
Biochemical parameters
Animals were sacrificed by decapitation and brains were removed and cut along the longitudinal fissure of the cerebrum using a surgical knife, and the regions posterior to lambda (midbrain, hindbrain, and cerebellum) were cut off. Place the cerebral hemisphere medial side up and, using forceps, carefully remove the diencephalon (thalamus and hypothalamus) under a dissection microscope. The medial side of the hippocampus would be exposed. Sever the fornix and then gently push the hippocampus out of the cortex (roll away) by inserting a spatula in the ventricle. Then the hippocampus was isolated and homogenized with ice-cold 0.1 M phosphate buffer (pH 7.4) in a volume 10 times the weight of the tissue. The homogenate was centrifuged at 10,000g for 15 min (4 °C), and aliquots of supernatant were separated and used for biochemical estimations.
Ache activity
The Ache activity was measured as previously described (Yang et al. 2013; Yang et al. 2014a). Absorbance was recorded at 412 nm in a spectrophotometer. One unit of Ache activity was defined as the number of micromolesofacetylth iocholineiodide hydrolyzed per minute per milligram of protein. The specific activity of Ache was expressed as U/mg protein.
Malondialdehyde
The quantitative measurement of malondialdehyde (MDA)—an end product of lipid peroxidation—in the brain homogenate was performed according to the method of Wills (1996). The amount of MDA was measured by reaction with thiobarbituric acid at 532 nm using a Perkin Elmer Lambda 20 spectrophotometer. The values were calculated using the molar extinction coefficient of chromophore [1.56 × 105 M−1 cm−1].
Reduced glutathione
Reduced glutathione (GSH) in hippocampus and cortex was estimated according to the method described by Ellman (1959). Results were calculated using molar extinction coefficientof chromophore (1.36 × 104 M−1 cm−1) and expressed as percentage of control.
Superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was assayed by the method of Kono (1978). The assay system consisted of EDTA 0.1 mM, sodium carbonate 50 mM, and 96 mM of nitro blue tetrazolium (NBT). In the cuvette, 2 ml of the above mixture, 0.05 ml of hydroxylamine, and 0.05 ml of the supernatant were added and the autooxidation of hydroxylamine was measured for 2 min at 30-s interval by measuring the absorbance at 560 nm using a Perkin Elmer Lambda 20 spectrophotometer.
Immunohistochemistry
Brains were fixed in formalin and embedded in paraffin. For each specimen, 15 serial sections of 5 μm thickness were coronally sliced for two to three such series, spaced 50 μm apart. Sections were deparaffinized in xylenes and rehydrated via an ethanol gradient. Antigen retrieval was performed using 88% formic acid treatment for 5 min and incubated for 30 min with 0.3% H2O2 thereafter. Sections were blocked with 10% goat serum for 1 h and incubated at 4 °C overnight with anti-Iba1 and anti-GFAP in PBS containing 3% goat serum. The immunoreactivity was visualized by DAB. The light microscopy was used to observe sections, and the intensity of the stained area of each group was analyzed using Aperio’s ImageScope Viewer software (Aperio. Technologies). All evaluations were performed by a researcher blind to the experimental design.
Estimation of proinflammtory cytokines (TNF-α, IL-1β, and IL-6) levels
Mouse hippocampus was sampled and 100 mg of tissue per animal was homogenized in 1.0 ml of 0.9% NaCl solution containing 0.1% PMSF (Sigma, MO, USA). After centrifugation at 14,000g for 15 min at 4 °C, the resulting supernatants were sampled in triplicate to detect the levels of TNF-α, IL-1β, and IL-6 by an ELISA kit (R&D Systems and Invitrogen) according to the provided instructions.
Western blot analysis
Following behavioral assessment, the hippocampus was isolated and directly homogenised in RIPA buffer containing 0.1% PMSF and 0.1% protease inhibitor cocktail (Sigma, MO, USA). The lysates were centrifuged at 14,000g for 30 min at 4 °C and the supernatant was used for protein analyses. The protein concentration in supernatants was determined using the BCA method. Equal amounts of soluble protein were separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Immobilon NC; Millipore, Molsheim, France). Immunoblotting was performed with antibodies specific for GFAP (1:1000), Iba-1 (1:1000), p-AKT (Ser473, 1:1000), AKT (1:1000), p-GSK3β (Ser9, 1:1000), GSK3β (1:1000), p-p65-NF-κB (1:1000), p65-NF-κB (1:1000), p-IκB-α (1:1000), and IκB-α (1:1000) (Cell Signaling Technology). Primary antibodies were visualized using anti-rabbit HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) and a chemiluminescent detection system (Western blotting Luminal Reagent; Santa Cruz Biotechnology, Inc.). Images were visualized and collected on Bio-Rad Chemidoc Imaging System. The bands were scanned; intensities of the bands were measured using Image-Pro Plus 6.0 analysis software.
Statistical analysis
All data were expressed as the mean ± SEM. For the Morris water maze tests, escape latency in the hidden platform trial were analysed with two-way ANOVA of repeated measures. Firstly, Mauchly’s test of sphericity should be used to judge whether there were relations among the repeatedly measured data. If any (p < 0.05), and the Greenhouse–Geisser corrected results should be taken. With multivariate ANOVA, data in different treated group of each measurement time could be compared pairwise. For the Morris water maze tests, one-way ANOVA was conducted on the data obtained from the probe trial. The recognition index in the novel object recognition test and the other data were analysed by one-way ANOVA followed by Bonferroni post hoc test for multiple comparisons. All analyses were performed with SPSS statistical package (version 13.0 for Windows, SPSS Inc., USA). Differences were considered significant at a p value < 0.05. Any animal that was 2.5 standard deviations from the mean was considered an outlier and excluded from data analysis. This criterion resulted in the removal of one animal.
Results
Behavioural test
Evodiamine enhances recognition memory of ICV-STZ treated mice in novel object recognition
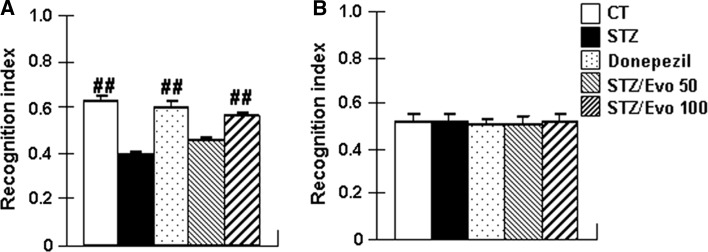
As shown in Fig. 2, a novel object recognition test was carried out in all the mice. In the test section, there was a significant overall group difference in the recognition index (F (4, 70) = 27.21, p < 0.01) among the five groups. Compared with control mice, the recognition index (p < 0.01) was significantly reduced in STZ mice. Mice treated with donepezil and 100 mg/kg evodiamine markedly increased recognition index by 53.0 and 43.6%, respectively. The 50 mg/kg evodiamine-treated group showed increase of the recognition index, however, this increase was not statistically significant. In addition, there was no significant difference in the recognition index (Fig. 1b) between the five groups of mice during training session (p > 0.05).
Fig. 2.
The effect of evodiamine on the recognition memory in ICV-STZ treated mice detected by a novel object recognition test. Control mice (CT), STZ mice, Donepezil mice, STZ/Evo 50 mice, STZ/Evo 100 mice were included. The recognition index in the test section (a) and training section (b) were measured. All data are presented as the mean ± SEM. The analysis was performed using one-way ANOVA with a LSD post hoc test between groups (n = 15, ##p < 0.01 vs. STZ mice)
Evodiamine improves spatial memory deficits of ICV-STZ treated mice in the Morris water maze
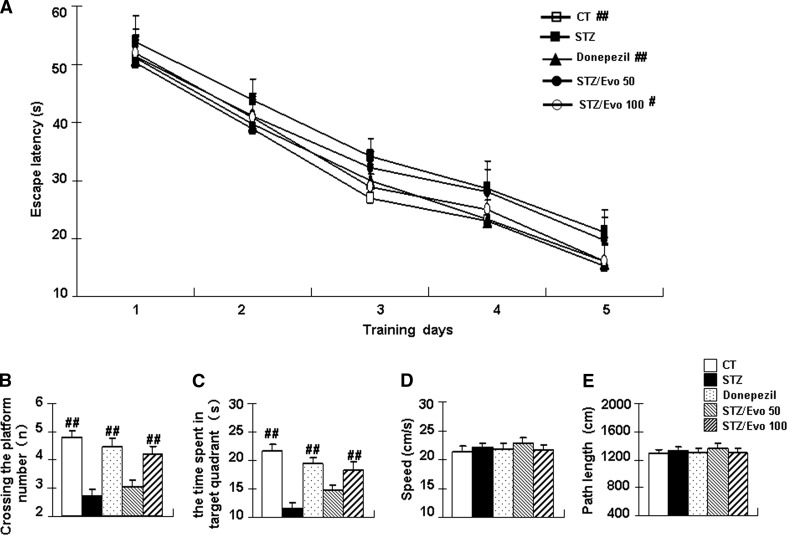
To assess spatial reference learning and memory function, all mice underwent testing in the Morris water maze after administration. In the hidden platform test (Fig. 3a), all animals, regardless of treatment, demonstrated learning over the 5-day test period as indicated by a decrease in latency (training day effect: F (4, 280) = 364.05, p < 0.01). The STZ mice showed significantly increased escape latencies from day 2 compared to the control group (p < 0.05 for day 2–4; p < 0.01 for day 5). Evodiamine-treated mice showed decreased escape latency compared with the STZ mice, especially in the 100 mg/kg evodiamine group (p < 0.01 for day 4–5). The 50 mg/kg evodiamine group showed a shortening of the escape latency. However, this decrease was not statistically significant. Twenty-four hours after the final trial, the probe test was performed. As shown in Fig. 3b, c, there was a significant overall group difference in the crossing-target number (F (4, 70) = 16.99, p < 0.01) and in the time spent in target quadrant (F (4, 70) = 14.18, p < 0.01) amongst the five groups. The STZ mice showed an obvious decrease in crossing-target number (p < 0.01) and the time spent in target quadrant (p < 0.01) compared to the controls. Compared to the STZ mice, the crossing-target numbers and the time spent in target quadrant were significantly increased by 55.6 and 55.7%, respectively, in the 100 mg/kg evodiamine treatment mice. These effects were analogous to the donepezil-treated mice. In addition, there was no significant difference in swimming speed (Fig. 3d) and path length (Fig. 3e) in the probe test between the five groups of mice (p > 0.05).
Fig. 3.
The effect of evodiamine on the spatial memory in ICV-STZ treated mice examined by the Morris water maze. Escape latency during 5 days of hidden platform tests (a), the crossing-target number (b), the time spent in target quadrant (c), the speed (d), and the path length (e) in the probe test were tabulated. All data are presented as mean ± SEM (n = 15, #p < 0.05, ##p < 0.01 vs. STZ mice)
Evodiamine reduces acetylcholinesterase activities in the hippocampus of ICV-STZ treated mice
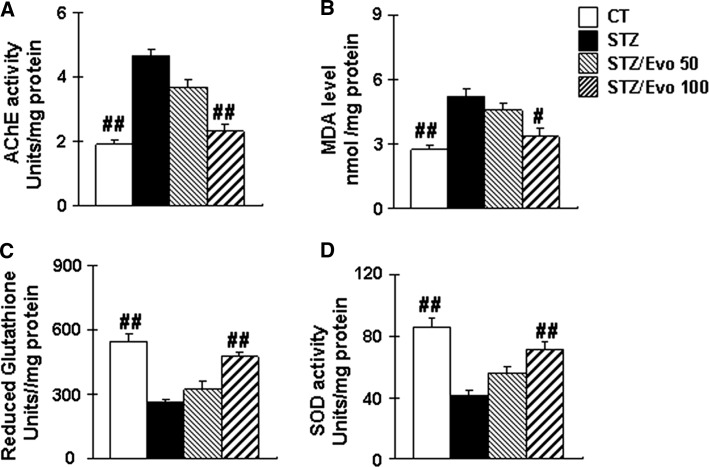
Ache is a marker of loss of cholinergic neurons in the brain. To observe beneficial effects of evodiamine on learning and memory may be due to its ability to restore cholinergic function, we measured the Ache activity in the hippocampus of each group. As shown in Fig. 4, the Ache activity significantly increased in the hippocampus of the STZ group when compared with the control group. Compared to the STZ mice, evodiamine (100 mg kg−1 day−1) treatment significantly reduced Ache activity by 50.3% (p < 0.01) in the hippocampus.
Fig. 4.
Evodiamine decreases Ache activity and ameliorates oxidative stress in ICV-STZ treated mice. Hippocampus Ache activity (a), MDA level (b), GSH activities (c), and SOD activity (d) in the hippocampus of ICV-STZ treated mice. All data are presented as mean ± SEM (n = 5, #p < 0.05, ##p < 0.01 vs. STZ mice)
Evodiamine attenuates oxidative stress in the hippocampus of ICV-STZ treated mice
When comparing the level of MDA in STZ mice to that of control mice, level was increased by 89.8%, and GSH and SOD activity were reduced by 52.9% and 52.3%, respectively (p < 0.01). Treatment with evodiamine (100 mg kg−1 day−1) significantly alleviates oxidative stress as exhibited by the reduction of MDA level by 35.3% (p < 0.05), and the increase of GSH and SOD activity by 82.2% (p < 0.01), and 73.2% (p < 0.01), respectively in the hippocampus (Fig. 4).
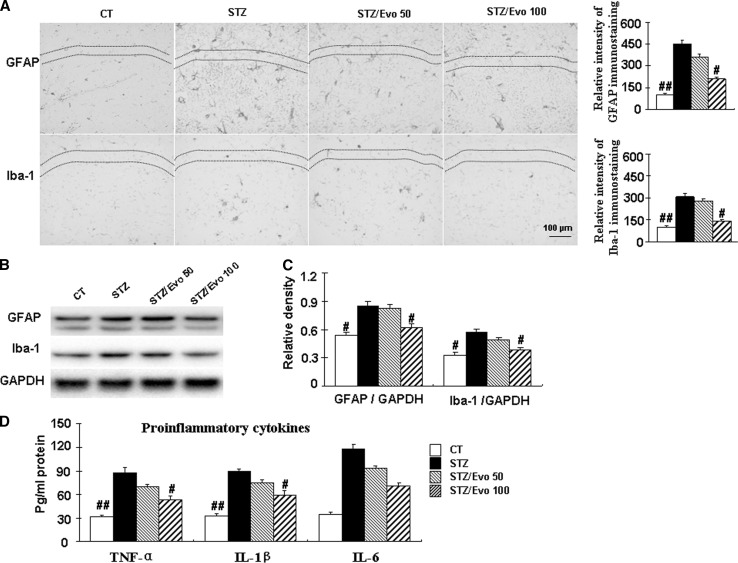
Evodiamine inhibits glial cell activation and decreases proinflammatory cytokines levels in the hippocampus of ICV-STZ treated mice
Neuroinflammation plays a crucial role in the pathogenesis and development of cognitive impairments. Thereafter, the effects of evodiamine on the glial cell activation and the levels of neuroinflammatory molecules, such as TNF-α, IL-1β, and IL-6 in the brain were examined. As shown in Fig. 5, the inflammatory response was manifested by elevation in the levels of TNF-α, IL-1β, and IL-6 in the brain of STZ mice. Moreover, glia activation was observed with increased Iba-1 and GFAP immunoreactivity, as well as the expression of proteins in the hippocampus of STZ mice. Evodiamine (100 mg kg−1 day−1) administration significantly inhibited neuroinflammatory response, as indicated by the decreased levels of TNF-α by 39.8% (p < 0.05), IL-1β by 44.9% (p < 0.05), and IL-6 by 39.8% (p < 0.05), reduced number of Iba-1 and GFAP positive cells, and the expression compared with those in the STZ mice.
Fig. 5.
Evodiamine inhibits glial activation and reduces proinflammatory cytokines levels in ICV-STZ treated mice. Brain tissues from Control mice (CT), STZ mice, STZ/Evo 50 mice, STZ/Evo 100 mice were utilized in standard pathological procedures. Immunohistochemistry of GFAP and Iba-1 in the CA1 area of hippocampus (scale bars, 100 µm) and the representative sections of CA1 area of hippocampus from four mice were shown (a). The relative levels of GFAP and Iba-1 expression were detected by Western blotting from hippocampus tissues, and a representative experiment was shown (b). The quantitative analysis of GFAP and Iba-1 expression were used GAPDH as normalization (c). Hippocampus TNF-α level, IL-1β level, and IL-6 level were analyzed (d). All data are presented as mean ± SEM (n = 5, #p < 0.05, ##p < 0.01 vs. STZ mice)
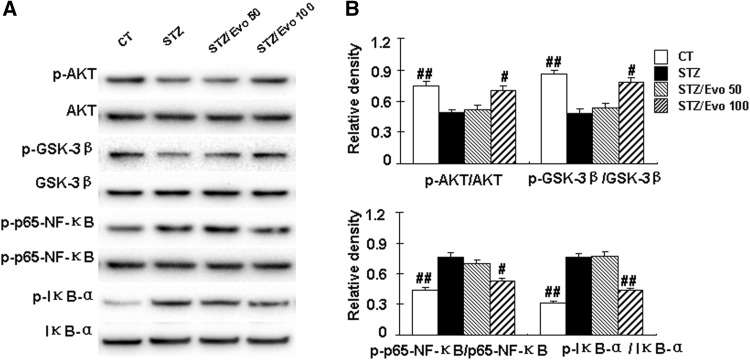
Evodiamine increases the activity of AKT/GSK-3β and inhibits the activation of NF-κB in the hippocampus of ICV-STZ treated mice
To further elucidate the potential mechanism of the effects of evodiamine, we investigated the activity of AKT, GSK-3β, and NF-κB, which play a pivotal role in the neuroinflammatory response and cognition. The STZ mice showed an obvious decrease in the expression levels of p-AKT and p-GSK-3β and an increase in the expression levels of p-p65-NF-κB and p-IκB-α in the hippocampus compared to the control mice (p < 0.01). Treatment with evodiamine significantly increases the activity of AKT/GSK-3β as evidenced by increasing the expression levels of p-AKT and p-GSK-3β by 31.0% and 39.7%, respectively, and inhibits the activation of NF-κB as indicated by decreasing the expression levels of p-NF-κB and p-IκB-α by 31.6% and 43.4%, respectively, in the hippocampus compared to the STZ mice (Fig. 6).
Fig. 6.
Evodiamine increases the activity of AKT/GSK-3β and inhibits the activation of NF-κB in the hippocampus of ICV-STZ treated mice. The relative levels of p-AKT, p-GSK-3β, p-p65-NF-κB, and p-IκB-α were detected by Western blotting from hippocampus tissues, and a representative experiment was shown (a). The quantitative analysis of p-AKT, p-GSK-3β, p-NF-κB, and p-IκB-α using AKT, GSK-3β, p65-NF-κB, and IκB-α as normalization, respectively (b). All data are presented as mean ± SEM (n = 5, ## p < 0.01 vs. STZ mice)
Discussion
The present study demonstrates the neuroprotective potential of evodiamine against STZ-induced neurotoxicity in mice. In line with earlier studies, this present studies indicated that the ICV infusion of STZ produced behavioral, biochemical, and neuopatholgial changes as seen in sAD. Evodiamine could ameliorate cognitive impairments, decrease Ache activity, and attenuate oxidative stress and neuroinflammation in ICV-STZ mice. Furthermore, NF-κB and AKT/GSK-3β signal alternations were restored by evodiamine in the hippocampus of ICV-STZ mice.
The etiology of sporadic Alzheimer’s disease (sAD) remains unclear, but numerous risk factors including insulin desensitization/resistance state, oxidative stress, neuroinflammation, synapse dysfunction, tau hyperphosphorylation, and deposition of Aβ in the brain have been identified that increase the chance of developing AD. Previous studies have shown that ICV-STZ produces similar characteristic pathology of sAD such as oxidative stress, neuroinflammation, insulin signaling dysfunction, synaptic injury, tau hyperphosphorylation, Aβ deposition, and neuronal apoptosis. These alterations mediated by ICV-STZ can be used to explore the underlying molecular and pathophysiological mechanism of AD (especially sAD).
Brain cholinergic system is well known to be closely related to neurodegenerative disease such as AD and plays a crucial role in the pathogenesis of AD (Francis et al. 1999). Acetylcholine (ACh) is a neurotransmitter necessary for memory functions and retrieval and is regulated by Ache. The activity of Ache, a hydrolyzing enzyme for ACh, has been considered as one of the markers of cholinergic function. Furthermore, Ache regulates the cholinergic neurons and neuromuscular transmission. Increased activity of Ache leads to rapid break down of ACh which correlates cholinergic system abnormalities with intellectual impairment. ICV-STZ administration has been shown an increased Ache activity and resultant cholinergic dysfunction in the brain (Javed et al. 2015; Liu et al. 2016a, b; Sonkusare et al. 2005). Consistent with this, we significantly observed an increased activity of Ache in ICV-STZ mice. In this present study, Evodiamine decreased Ache activity and improved cognitive deficits. The amelioration of cholinergic system dysfunction in CNS is an effective way of preventing or reversing the cognitive deficits (Ferreira-Vieira et al. 2016; Schliebs and Arendt 2006). Thus, we speculated that the protective effects of evodiamine on cholinergic system are beneficial to learning and memory.
Neuroinflammation is known to develop the pathology of neurodegenerative diseases including AD (Kamat et al. 2012; Zilka et al. 2006). Glial cells act as neuronal supportive cells and maintain the health of the neurons, however, overactivation of the glial cells can produce proinflammatory mediators and neuotoxic factors resulting in severe and chronic neuroinflammatory cycle that actually promotes neurodegenerative diseases (Ifuku et al. 2012; Mrak and Griffin 2001). During AD progression, it has been found that there is a steady-state increase in the number of reactive astrocyte (Simpson et al. 2010) and microglia (Edison et al. 2008). In the present study, STZ significantly activates glial cells as evidenced by a significant increase in GFAP and Iba-1 immunoreactivity, as well as the expression of proteins. The proinflammatory cytokine (TNF-α, IL-1β and IL-6), which are released by activated astrocytes and microglia (Heneka and O’Banion 2007; Liu et al. 2016a, b; Tan et al. 1999), are elevated in the AD patients (Perry et al. 2001). In neuroinflammatory process, NF-κB may be initiated and regulate the transcription of several proinflammatory cytokines (Kim et al. 2017). Activation of NF-κB and increase in proinflammatory cytokines levels have been demonstrated to occur following STZ administration in experimental animal models (Ghosh et al. 2017; Yin et al. 2016). Consistent with this, our results showed an augment neuroinflammation as evidenced by increased NF-κB phophorylation and TNF-α as well as IL-1β, IL-6 in ICV-STZ treated mice. Evodiamine treatment significantly attenuated STZ-induced microgliosis along with astrogliosis. In addition to this, evodiamine administration significantly inhibited the activation of NF-κB and thus decreased production of downstream cytokins.
The Akt/PKB signaling pathway is known as one of the most relevant pathways in regulating neuronal survival and cognition. GSK-3β, an important substrate of Akt, plays an important role in the inflammatory response in AD (Yuskaitis and Jope 2009). Several proinflammatory cytokines including the interleukins and TNF activate GSK-3β signaling activity, and the inhibition of GSK-3β causes anti-inflammatory effects (Ly et al. 2013; Yuskaitis et al. 2009). Numerous studies have suggested that GSK-3β regulates gene transcription in an NF-κB-dependent manner, and GSK-3β and NF-κB signaling pathways are involved in regulating inflammatory responses (Steinbrecher et al. 2005; Takada et al. 2004). In the present study, ICV-STZ treated mice were characterized by decreased Akt/GSK-3β activities which were associated with augmented neuroinflammation. These changes were normalized with evodiamine administration. The results suggest that interfering with the GSK-3β and NF-κB pathways can result in evodiamine-mediated neuroprotective effects in ICV-STZ treated mice. However, this hypothesis needs to be investigated further.
In conclusion, evodiamine exerts profound neuroprotective and cognitive preserving effects in an ICV-STZ induced animal model of sporadic AD. Althrough the neuroprotective mechanism of evodiamine requires further study, the results of the current study suggest that the beneficial effects of evodiamine are multifactorial effects, and that evodiamine is recommended as a possible candidate for the prevention and therapy of cognitive deficits in Alzheimer disease.
Acknowledgements
The present work was supported by National Natural Science Foundation of China (81601225), the Young Backbone Teachers Assistance Scheme of Henan Province Colleges and Universities.
Abbreviations
- Ache
Acetylcholinesterase
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- ACSF
Artificial cerebral spinal fluid
- fAD
Familial Alzheimer’s disease
- GSH
Glutathione
- ICV
Intracerebroventricular
- MDA
Malondialdehyde
- NF-κB
Nuclear factor κB
- Evo
Evodiamine
- sAD
Sporadic Alzheimer’s disease
- STZ
Streptozotocin
- SOD
Superoxide dismutase
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Dongmei Wang, Email: wdmzgadyx@hotmail.com.
Sanqiang Li, Email: sanqiangli2001@163.com.
References
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Blokland A, Jolles J. Spatial learning deficit and reduced hippocampal ChAT activity in rats after an ICV injection of streptozotocin. Pharmacol Biochem Behav. 1993;44:491–494. doi: 10.1016/0091-3057(93)90497-H. [DOI] [PubMed] [Google Scholar]
- Chang CP, Chang JY, Wang FY, Tseng J, Chang JG. The effect of Evodia rutaecarpa extract on cytokine secretion by human mononuclear cells in vitro. Am J Chin Med. 1995;23:173–180. doi: 10.1142/S0192415X95000237. [DOI] [PubMed] [Google Scholar]
- Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—a PDE1 inhibitor. Eur J Pharmacol. 2009;620:49–56. doi: 10.1016/j.ejphar.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Chen F, Wu F, Tang SS, Hu M, Long Y, Sun HB, Kong LY, Hong H. CysLT1R downregulation reverses intracerebroventricular streptozotocin-induced memory impairment via modulation of neuroinflammation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;73:19–30. doi: 10.1016/j.pnpbp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53:1741–1752. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- Gutierres JM, Carvalho FB, Schetinger MR, Marisco P, Agostinho P, Rodrigues M, et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014;96:7–17. doi: 10.1016/j.lfs.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Ifuku M, Katafuchi T, Mawatari S, Noda M, Miake K, Sugiyama M, Fujino T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J Neuroinflammation. 2012;9:197. doi: 10.1186/1742-2094-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed H, Khan MM, Khan A, Vaibhav K, Ahmad A, Khuwaja G, Ahmed ME, Raza SS, Ashafaq M, Tabassum R, Siddiqui MS, El-Agnaf OM, Safhi MM, Islam F. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011;1389:133–142. doi: 10.1016/j.brainres.2011.02.072. [DOI] [PubMed] [Google Scholar]
- Javed H, Vaibhav K, Ahmed ME, Khan A, Tabassum R, Islam F, Safhi MM. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J Neurol Sci. 2015;348:51–59. doi: 10.1016/j.jns.2014.10.044. [DOI] [PubMed] [Google Scholar]
- Jiang ML, Zhang ZX, Li YZ, Wang XH, Yan W, Gong GQ. Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci Lett. 2015;588:154–158. doi: 10.1016/j.neulet.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Tota S, Rai S, Swarnkar S, Shukla R, Nath C. A study on neuroinflammatory marker in brain areas of okadaic acid (ICV) induced memory impaired rats. Life Sci. 2012;90:713–720. doi: 10.1016/j.lfs.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ, Ham YW, Hellstrom M, Han SB, Kim HS, Park EK, Hong JT. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Laczo J, Vlcek K, Vyhnalek M, Vajnerova O, Ort M, Holmerova I, Tolar M, Andel R, Bojar M, Hort J. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202:252–259. doi: 10.1016/j.bbr.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037/0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Liang KC, Hon W, Tyan YM, Liao WL. Involvement of hippocampal NMDA and AMPA receptors in acquisition, formation and retrieval of spatial memory in the Morris water maze. Chin J Physiol. 1994;37:201–212. [PubMed] [Google Scholar]
- Liu Y, Zhang XJ, Yang CH, Fan HG. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression. Brain Res. 2009;1268:174–180. doi: 10.1016/j.brainres.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu Y, Zha S, Liu M, Wang Y, Yang G, Ma K, Fei Y, Zhang Y, Hu X, Yang W, Qian Y. Treatment effects of tanshinone IIA against intracerebroventricular streptozotocin induced memory deficits in mice. Brain Res. 2016;1631:137–146. doi: 10.1016/j.brainres.2015.11.040. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen HQ, Huang Y, Qiu YH, Peng YP. Transforming growth factor-beta1 acts via TbetaR-I on microglia to protect against MPP(+)-induced dopaminergic neuronal loss. Brain Behav Immun. 2016;51:131–143. doi: 10.1016/j.bbi.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Investig. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol Aging. 2001;22:903–908. doi: 10.1016/S0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Pathan AR, Viswanad B, Sonkusare SK, Ramarao P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79:2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/S0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rai S, Kamat PK, Nath C, Shukla R. Glial activation and post-synaptic neurotoxicity: the key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacol Biochem Behav. 2014;117:104–117. doi: 10.1016/j.pbb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Rasul A, Yu B, Zhong L, Khan M, Yang H, Ma T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol Rep. 2012;27:1481–1487. doi: 10.3892/or.2012.1694. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Osmanovic-Barilar J, Bruckner MK, Hoyer S, Arendt T, Riederer P. Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm (Vienna) 2011;118:765–772. doi: 10.1007/s00702-011-0651-4. [DOI] [PubMed] [Google Scholar]
- Santos DB, Colle D, Moreira EL, Peres KC, Ribeiro RP, dos Santos AA, de Oliveira J, Hort MA, de Bem AF, Farina M. Probucol mitigates streptozotocin-induced cognitive and biochemical changes in mice. Neuroscience. 2015;284:590–600. doi: 10.1016/j.neuroscience.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm (Vienna) 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Shi L, Yang F, Luo F, Liu Y, Zhang F, Zou M, Liu Q. Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting beta-catenin-mediated angiogenesis. Tumour Biol. 2016;37:12791–12803. doi: 10.1007/s13277-016-5251-3. [DOI] [PubMed] [Google Scholar]
- Shoham S, Bejar C, Kovalev E, Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp Neurol. 2003;184:1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, Savva G, Brayne C, Wharton SB. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging. 2010;31:578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Sonkusare S, Srinivasan K, Kaul C, Ramarao P. Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci. 2005;77:1–14. doi: 10.1016/j.lfs.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, 3rd, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IκBα kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Takamura A, Okamoto Y, Kawarabayashi T, Yokoseki T, Shibata M, Mouri A, Nabeshima T, Sun H, Abe K, Urisu T, Yamamoto N, Shoji M, Yanagisawa K, Michikawa M, Matsubara E. Extracellular and intraneuronal HMW-AbetaOs represent a molecular basis of memory loss in Alzheimer’s disease model mouse. Mol Neurodegener. 2011;6:20. doi: 10.1186/1750-1326-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after β-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Wei J, Ching LC, Zhao JF, Shyue SK, Lee HF, Kou YR, Lee TS. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol (Oxf) 2013;207:299–307. doi: 10.1111/apha.12005. [DOI] [PubMed] [Google Scholar]
- Wu Y, Pan X, Xu Y, Lu X, He S, He R, Gong M. Optimization of combinations of ginsenoside-Rg1, ginsenoside-Rb1, evodiamine and rutaecarpine for effective therapy of mouse migraine. J Nat Med. 2016;70:207–216. doi: 10.1007/s11418-015-0960-2. [DOI] [PubMed] [Google Scholar]
- Yin C, Deng Y, Gao J, Li X, Liu Y, Gong Q. Icariside II, a novel phosphodiesterase-5 inhibitor, attenuates streptozotocin-induced cognitive deficits in rats. Neuroscience. 2016;328:69–79. doi: 10.1016/j.neuroscience.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Yuan SM, Gao K, Wang DM, Quan XZ, Liu JN, Ma CM, Qin C, Zhang LF. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(DeltaE9) transgenic mouse models of Alzheimer’s disease. Acta Pharmacol Sin. 2011;32:295–302. doi: 10.1038/aps.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang X, Zhao Y, Zhang L, Bai X, Zhang J, Zhao X, Chen L, Wang L, Cui L. Pretreatment by evodiamine is neuroprotective in cerebral ischemia: up-regulated pAkt, pGSK3beta, down-regulated NF-kappaB expression, and ameliorated BBB permeability. Neurochem Res. 2014;39:1612–1620. doi: 10.1007/s11064-014-1356-5. [DOI] [PubMed] [Google Scholar]
- Zilka N, Ferencik M, Hulin I. Neuroinflammation in Alzheimer’s disease: protector or promoter? Bratisl Lek Listy. 2006;107:374–383. [PubMed] [Google Scholar]