Abstract
Background:
AIS is the most common spinal deformity disease, yet its etiology remains uncertain. Significant associations have been found between AIS risk and vitamin D receptor (VDR) gene polymorphisms; however, some of these results are controversial. The aim of this study was to determine whether VDR BsmI rs1544410 and ApaI rs7975232 polymorphisms are correlated with AIS.
Methods:
Databases, including PubMed, EMBASE, Web of Science, the Cochrane Library, the Chinese Biomedical Literature Database, and the Wanfang Database, were systematically searched, and eligible case–control studies that explored the association of VDR (BsmI and ApaI) and the susceptibility to AIS were selected. The pooled odds ratio (OR) with 95% confidence interval (95% CI) was calculated to assess the associations, and subgroup meta-analyses were performed according to the ethnicity of the study population.
Results:
A total of 5 studies with 717 cases and 554 controls fulfilled the inclusion criteria after assessment by 2 reviewers. Generally, significant correlations were found between the BsmI polymorphism and AIS risk in overall populations and in Asian populations (overall population: B vs b: OR = 2.12, 95% CI = 1.21–3.75, P = .009; BB vs bb: OR = 3.38, 95% CI = 1.08–10.57, P = .036; Bb vs bb: OR = 2.50, 95% CI = 1.29–4.82, P = .006; BB/Bb vs bb: OR = 2.71, 95% CI = 1.31–5.63, P = .007; Asian population: B vs b: OR = 2.42, 95% CI = 1.27–4.61, P = .007; BB vs bb: OR = 4.09, 95% CI = 1.03–16.22, P = .045; Bb vs bb: OR = 2.94, 95% CI = 1.42–6.10, P = .004; BB/Bb vs bb: OR = 3.23, 95% CI = 1.42–7.35, P = .005). There was no significant association observed in Caucasian populations (all P > .05). With regard to the ApaI polymorphism, we found that it significantly decreased the risk of AIS (Aa vs AA: OR = 0.43, 95% CI = 0.24–0.77, P = .004; Aa/aa vs AA: OR = 0.52, 95% CI = 0.30–0.91, P = .023); however, we could not draw a definitive conclusion for Caucasian populations, as no studies have been conducted in this group to determine the role of the VDR ApaI polymorphism in AIS etiology and development.
Conclusion:
VDR BsmI was significantly associated with AIS susceptibility in the overall and Asian populations, while the VDR ApaI polymorphism only played a key role in AIS etiology and development in Asian populations.
Keywords: AIS, meta-analysis, systematic review, vitamin D receptor, polymorphism
1. Introduction
Scoliosis is the bony structural deformity characterized by coronal, lateral, and rotational curvature of spine that may be associated with dysfunction of the neuromuscular system (neuromuscular scoliosis), transformation of soft tissue (systemic syndromes), disturbance of neural metabolism (neurofibromatosis), abnormal formation of vertebrae and thorax (congenital deformity), and other issues.[1] The prevalence of idiopathic scoliosis is approximately 2% to 3% worldwide[2] and is thought to be the most common type of scoliosis. Adolescent idiopathic scoliosis (AIS) is a common form that affects approximately 80% to 90% of the spinal deformity population, is prevalent in 1% to 4% of individuals aged 10 to 18 years, and has a great impact on children's physical and psychological health.[3,4] There is an established correlation between curve progression and the age of the child.[5,6] AIS is found in early puberty with rapid growth until sexual maturity, and females have a higher incidence rate and more aggravated spinal curves. It is well known that there is rapid curve progression in AIS children following diagnosis with AIS in adolescence. Thus, some researchers believe that the regulation of hormones during the adolescent period might be correlated with spinal curve initiation and progression.[7,8] As AIS has a strong influence on patients’ physical and psychological condition and social burdens, early diagnosis and suitable therapy are essential to prevent curvature deterioration.
The etiology and pathogenesis of AIS remain poorly understood, despite numerous studies. However, no studies have exactly illustrated the etiology of scoliotic formation. In recent years, many pathogenetic factors have been found to be potential contributors to susceptibility to AIS, including heritage and genetic factors,[9,10] environment,[11] and low bone mineral density (BMD).[12,13] In addition, different mechanisms have been revealed, such as bone growth, aberrant musculoskeletal system, neurological system defects, and metabolism abnormalities.[7,14,15] However, the exact etiology and mechanisms of scoliotic progression remain unclear.
In recent years, many studies have focused on the roles of genes and genetic polymorphisms in the etiology and development of AIS. Several reports with multiple twin series have suggested a higher concordance in monozygotic twins than in dizygotic twins.[16] One study reported that 15% of families presented with a locus on the X chromosome that could be connected to X-linked dominant inheritance among familial idiopathic scoliosis patients.[17] Other genetics-related studies demonstrated that some chromosomes and gene polymorphisms had strong correlations with the susceptibility to AIS, including chromosomes 6, 9, 16, and 17.[18–20] In addition, more gene polymorphisms have been found to be significantly associated with the etiology and development of AIS. Nikolova et al[21] performed a case–control study in Bulgarian patients and found significant relationships between an interleukin-6 (IL-6, rs1800795) polymorphism and the susceptibility to IS and the severity of curve deformation, suggesting that the IL-6 gene polymorphism could be a susceptibility and modifying factor for IS. Another study in a Chinese population revealed that the CC genotype of the boron neutron capture synovectomy (BNCS) rs10738445 polymorphism was found with higher frequency in AIS cases than in controls, and there was a significant correlation with the magnitude of curves.[22] In addition, a meta-analysis showed that the rs11109087, r678741, and rs625039 polymorphisms of the ladybird homeobox 1 (LBX1) gene were important contributors to AIS susceptibility in some populations.[23] Candidate gene approaches and genome-wide association studies (GWAS) have also suggested that there is a network relationship between different genes such as IL-6, estrogen receptor 1 (ESR1), estrogen receptor 2 (ESR2), vitamin D receptor (VDR), transforming growth factor beta 1 (TGFB1), and insulin-like growth factor 1 (IGF1) in the etiology and development of AIS.[24]
As is known, the VDR gene encodes for a nuclear receptor for vitamin D metabolites and is located on chromosome 12q12-q14.[24,25] It has been well established that VDR is an important contributor to the biological function of vitamin D, and it plays an important role in the regulation of BMD and skeletal metabolism and development. In addition, previous studies have shown strong relationships between VDR polymorphisms and some kinds of bone disorders, such as osteoarthritis and osteoporosis.[26,27] Due to the key roles of VDR in the etiology and development of many diseases and the potential impact of gene polymorphisms and hormones in AIS etiology, more studies have been performed to explore the relationships between VDR and AIS etiology.[24,28–30] The VDR gene polymorphisms, FokI, BsmI, and Cdx2, are considered key contributors to AIS etiology, and BsmI might play an important role in BMD.[24,25,31] Several studies demonstrated that the frequencies of the BsmI B allele and Bb genotype in AIS were higher than those in control cases in Asian populations.[29,31–33] However, other studies have shown no association between the ApaI A or a allele and the clinical characteristics of AIS patients.[29,32,33] Due to these conflicting results, we performed this review and systemic meta-analysis to determine whether there were significant relationships between the VDR BsmI rs1544410 and ApaI rs7975232 polymorphisms and AIS risk.
2. Methods
2.1. Literature search
This study was approved by the ethics committee of our hospital (First Affiliated Hospital of PLA General Hospital, Beijing). Databases, including PubMed, EMBASE, Web of Science and the Cochrane Library, the Chinese Biomedical Literature database, and the Wanfang database, were searched for case–control studies that focused on the relationships between the BsmI (rs1544410) and ApaI (rs7975232) polymorphisms and susceptibility to AIS. The following search terms were used: (“adolescent idiopathic scoliosis” OR “AIS”) AND (“vitamin D receptor” OR “VDR”) AND (“polymorphism” OR “single nucleotide polymorphism” OR “SNP” OR “variation”). AIS is defined by lateral curvature of the spine in children aged between 10 and 16 years with a Cobb angle of at least 10°. In all included studies, the definition of AIS and individuals recruited in these studies were identified with these parameters. Then, studies were selected that involved the association between the BsmI rs1544410 or ApaI rs7975232 polymorphisms and AIS.
No language restrictions were applied during the literature search. Secondary screens of unpublished literature were conducted by searching the reference lists of the selected studies and reviews. Reviews and comments were also examined to identify eligible studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria of our meta-analysis were as follows: case–control study; evaluation of AIS risk and at least 1 of the 2 identified gene polymorphisms (BsmI rs1544410 or ApaI rs7975232); and sufficient data, including number or frequency of alleles and genotypes. The exclusion criteria were as follows: reviews or case reports that were not case–control studies; no available data reported; and duplicated reports.
3. Data extraction
Data from the eligible studies were extracted according to the inclusion and exclusion criteria by 2 authors, and a consensus was reached. For each study, the following data were collected: author list, year of publication, ethnicity, sample size, alleles, and genotypes of BsmI rs1544410 or ApaI rs7975232 polymorphisms.
The odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the association between the BsmI rs1544410 polymorphism and AIS. An allele contrast model (B vs b), heterozygote model (Bb vs bb), homozygote model (BB vs bb), dominant model (BB/Bb vs bb), and recessive model (BB vs BB/Bb) were examined for the BsmI rs1544410 polymorphism. The strength of association between the ApaI rs7975232 polymorphism and AIS susceptibility was evaluated by OR and 95% CI according to the allele contrast model (A vs a), heterozygote model (Aa vs AA), homozygote model (aa vs AA), dominant model (Aa/aa vs AA), and recessive model (AA vs Aa/aa). The assumption that there was heterogeneity was verified by a Chi-squared based Q statistical test and quantified by the I2 metric value. If the I2 value was >50% or P < .10, suggesting obvious heterogeneity, ORs were pooled by the random effect model. Otherwise, the fixed effect model was used. Sensitivity analysis was performed to assess the impact of each study on the combined effect of the present meta-analysis and subgroup analysis according to the ethnicity of the study populations. All meta-analyses were performed using Stata 12.0 software (StataCorp, College Station, TX), and a P value below .05 indicated statistical significance. Power analysis was performed using the Power and Precision V4 software (Biostat Inc, Englewood, NJ).
4. Results
4.1. Study characteristics
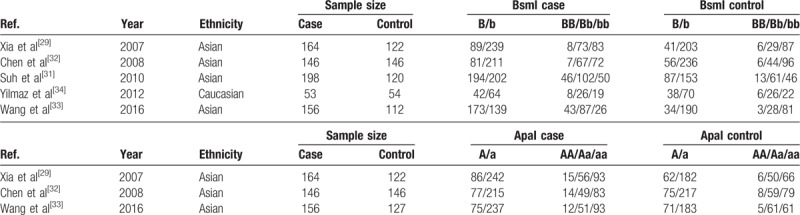
We searched all the common databases, including PubMed, EMBASE, Web of Science, the Cochrane Library, the Chinese Biomedical Literature database, and the Wanfang database, to find all eligible studies that investigated the relationships between the BsmI (rs1544410) and ApaI (rs7975232) polymorphisms and susceptibility to AIS. We found 6 studies that could be included in our meta-analysis. The data in 1 study were not available, and the authors only reported their conclusions. We attempted to obtain the data by contacting the corresponding author but received no response. Therefore, we excluded this study. In the end, a total of 5 studies[29,31–34] with 717 cases and 544 controls met the selection criteria and were included in our study. Four studies[29,31–33] were performed in Asian populations, and 1 study[34] focused on a Caucasian population. Each study[29,31–34] included the genotype distribution detail for the BsmI B/b polymorphism, and 3 studies[29,32,33] included genotype distribution for ApaI A/a. The study selection and inclusion process are presented in Fig. 1. The general demographic characteristics of the subjects in this meta-analysis are summarized in Table 1. Before this meta-analysis was performed, a power analysis was conducted by using Power and Precision V4 software to verify whether the included studies could offer adequate power (>80%). The statistical power in our study was sufficient to detect the associations between VDR gene polymorphisms and AIS risk.
Figure 1.
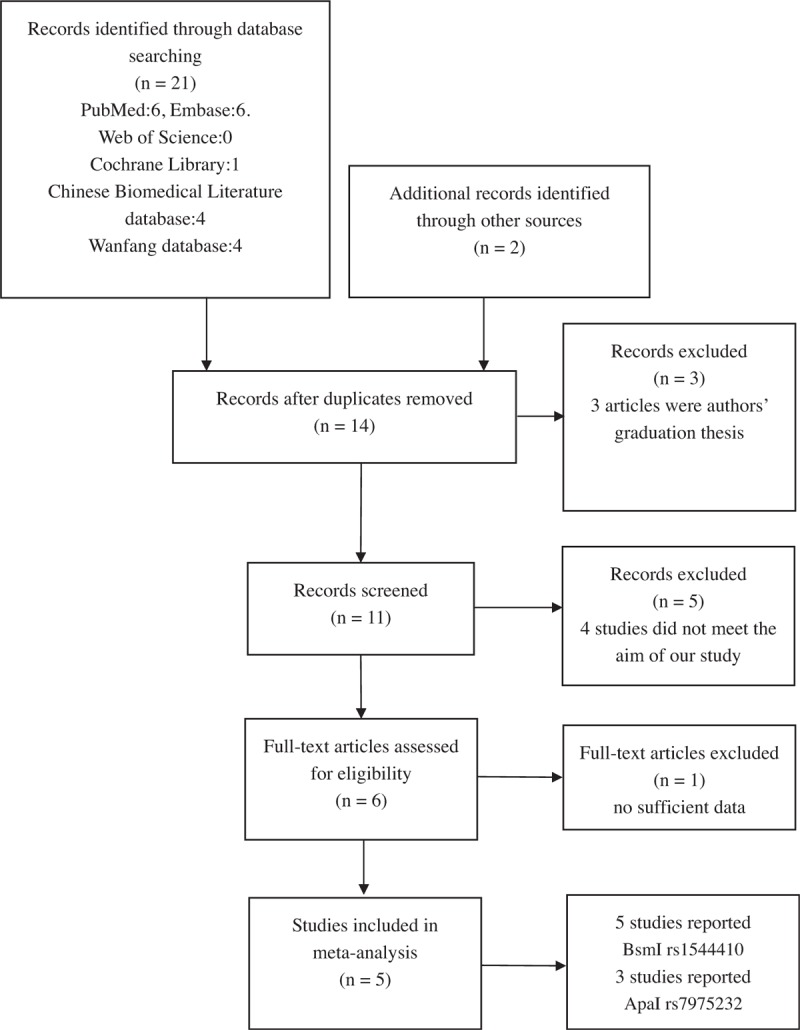
The study selection and inclusion process.
Table 1.
General characteristics of studies included in the meta-analysis.
4.2. Meta-analysis results
This meta-analysis showed a significant correlation between the BsmI rs1544410 polymorphism and the risk of AIS in several genetic models in the overall population (B vs b: OR = 2.12, 95% CI = 1.21–3.75, P = .009; BB vs bb: OR = 3.38, 95% CI = 1.08–10.57, P = .036; Bb vs bb: OR = 2.50, 95% CI = 1.29–4.82, P = .006; BB/Bb vs bb: OR = 2.71, 95% CI = 1.31–5.63, P = .007), as indicated in Table 2 and Fig. 2. In addition, the results of subgroup analysis indicated a significant association between the BsmI rs1544410 polymorphism and AIS susceptibility in the Asian population (B vs b: OR = 2.42, 95% CI = 1.27–4.61, P = .007; BB vs bb: OR = 4.09, 95% CI = 1.03–16.22, P = .045; Bb vs bb: OR = 2.94, 95% CI = 1.32–6.10, P = .004; BB/Bb vs bb: OR = 3.23, 95% CI = 1.42–7.35, P = .005), but no significant association was observed in the Caucasian population (Table 2, Fig. 3).
Table 2.
Results of genetic models for BsmI polymorphisms and adolescent idiopathic scoliosis.
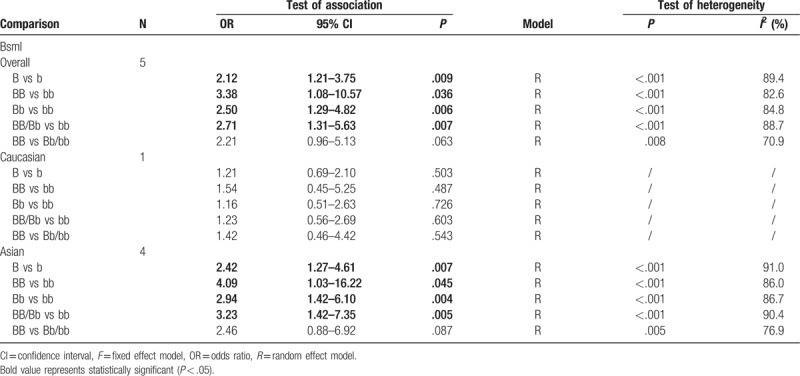
Figure 2.
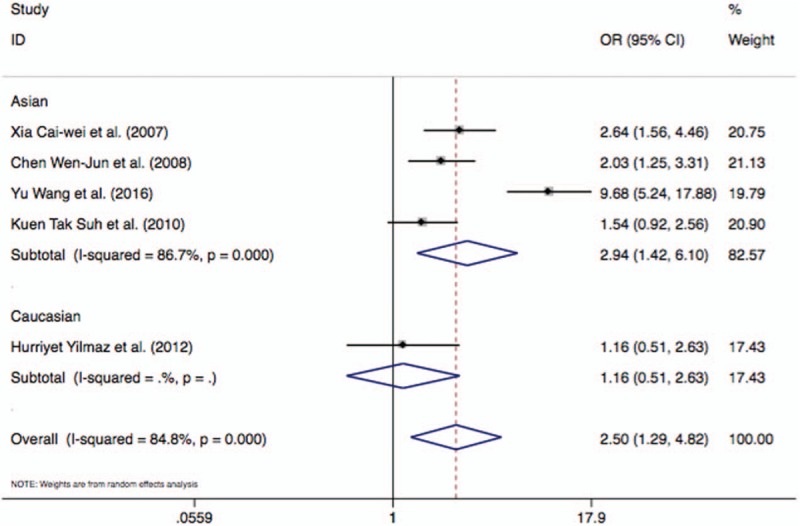
Forest plot describing the meta-analysis under the heterozygote model for the association between BsmI rs1544410 B/b polymorphism and AIS risk (Bb vs bb).
Figure 3.

Forest plot describing the meta-analysis under the dominant model for the association between BsmI rs1544410 B/b polymorphism and AIS risk (BB/Bb vs bb).
Regarding the ApaI rs7975232 polymorphism, the results indicated a stronger association with AIS risk in two genetic models in the Asian populations (Aa vs AA: OR = 0.43, 95% CI = 0.24–0.77, P = .004; Aa/aa vs AA: OR = 0.52, 95% CI = 0.30–0.91, P = .023), as indicated in Table 3, Figs. 4 and 5. However, whether the ApaI rs7975232 polymorphism plays a key role in AIS etiology in either Caucasian populations or the overall population remains unclear, as no articles studying the relationship between the ApaI rs7975232 polymorphism and AIS risk were found in our database search.
Table 3.
Results of genetic models for ApaI polymorphisms and adolescent idiopathic scoliosis.
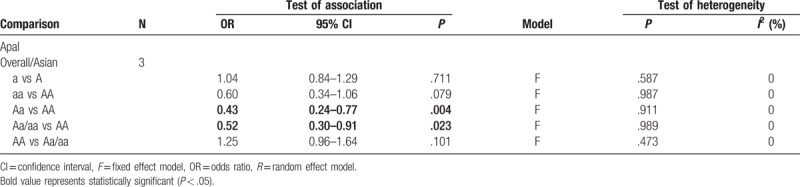
Figure 4.

Forest plot describing the meta-analysis under the heterozygote model for the association between ApaI rs7975232 A/a polymorphism and AIS risk (Aa vs AA).
Figure 5.

Forest plot describing the meta-analysis under the dominant model for the association between ApaI rs7975232 A/a polymorphism and AIS risk (Aa/aa vs AA).
4.3. Sensitivity analysis and publication bias
The corresponding pooled results did not change dramatically when each study was removed. Therefore, all these results were of high stability, and we can conclude that our meta-analysis data are relatively stable and credible. We did not evaluate publication bias because it might be difficult to assess this when fewer than 10 studies were included.
5. Discussion
Multiple factors contribute to the etiology and pathogenesis of AIS. Several studies have shown low bone mass and osteopenia in AIS patients.[31,35,36] The etiology and pathogenesis of AIS remain poorly understood. Numerous studies have indicated significant linkages through genetic analysis, suggesting that AIS might be a complex genetic disorder influenced by multiple genes.[28] The roles of the VDR gene have been verified by a genome-wide study, and this gene encodes a protein that affects the vitamin D endocrine system, for example, by regulating calcium and phosphate absorption from food.[25] In addition, VDR is considered to play important roles in various systems, such as the immune, neural, epithelial, and skeletal muscular systems.[37] Moreover, importance of VDR gene polymorphisms is increasing in susceptibility to many diseases, such as systemic lupus erythematosus,[38,39] osteoporosis,[40,41] and rheumatoid arthritis (RA).[42,43]
Thus far, several single nucleotide polymorphisms (SNPs) of the VDR gene at or near the 3’UTR region (TaqI, BsmI, and ApaI) and exon 2 (FokI) have been identified.[44] The TaqI, BsmI, and ApaI polymorphisms are named T-t, B-b, A-a, and F-f, respectively.[31] These polymorphisms can regulate the stability and vitality of VDR mRNA.[25] Therefore, it is well established that the VDR gene plays important roles in the etiology of many metabolic diseases, and more diseases have been found to be significantly associated with VDR gene polymorphisms. Bermúdez-Morales et al[45] reported a correlation between the VDR TaqI and BsmI polymorphisms and multiple sclerosis in Mexican adults. One study demonstrated the association between VDR polymorphisms and the risk of systemic lupus erythematosus in a Chinese population.[46] Another study suggested that VDR polymorphisms were related to aggressive bone loss in women with RA and that VDR genotypes might be a predictor for screening for bone loss in women with early-stage RA.[47] A systematic review and meta-analysis showed that VDR polymorphisms played a protective role in intervertebral disc degeneration.[48]
As associations have been observed between VDR gene polymorphisms and many diseases, more attention has been focused on whether VDR gene polymorphisms also play important roles in the risk of AIS.[29,31–34] However, the results have been conflicting. To the best of our knowledge, no systematic assessment of the relationship between VDR gene polymorphisms (BsmI rs1544410 and ApaI rs7975232 polymorphism) and AIS risk has been conducted. Thus, we executed a meta-analysis to estimate the associations of the BsmI rs1544410 or ApaI rs7975232 gene polymorphisms with AIS risk.
BsmI consists of 2 nucleotide variants A-G in intron 8 of the VDR gene. Morriso et al[49] first reported that these VDR alleles might predict BMD, suggesting that BsmI was a key factor for BMD and that the B allele of VDR was associated with low BMD. Other studies showed similar results, suggesting a tendency for lower bone mass with the B allele in various ethnicities.[37,50,51] Regarding the BsmI polymorphism, studies concluded that the Bb genotype and B variant appeared to lead to low bone density and delayed menstruation in comparison to the b allele,[52,53] and these characteristics of low bone density and delayed menstruation are significantly associated with progressive spinal curvature.[54] Xia et al[29] found that the frequencies of the B allele and the Bb genotype were higher in the AIS group than in controls in the Chinese population and that young Chinese AIS patients with the Bb genotype had a risk of low bone density and delayed menarche, which was also found to be associated with the progressive process of AIS. Chen et al[32] reported a higher frequency of the Bb genotype in an AIS group than in the control group among Asian young women, but there was no significant difference between ApaI and BsmI and BMD in the lumbar spine or femoral neck in AIS.[32] The study by Wang et al[33] showed that there were higher frequencies of the BsmI Bb genotype and B allele in AIS in a Chinese population. However, Yilmaz et al[34] did not find any differences in the distribution of BsmI genotypes between AIS cases and controls in Caucasian populations. In our meta-analysis study, a significant relationship between the allele and genotype frequencies of the BsmI polymorphism and AIS patients was observed in the overall population. In the subgroup analysis of ethnicity, our study showed that the VDR BsmI polymorphisms were significantly associated with susceptibility to AIS in the Asian population, which was consistent with 4 studies[29,31–33] that we included. However, no significant associations were observed in Caucasian populations. In our opinion, the small sample size in the study by Yilmaz et al [34] might contribute to these conflicting results. Another important contributor to significant differences in Asian and Caucasian populations was ethnicity. Each ethnicity has its specific gene background that might have a different role in the risk and pathogenesis of AIS. Therefore, the relationship between VDR BsmI and susceptibility to AIS in Caucasian populations should be further studied.
Similar to BsmI, the ApaI polymorphism is located in the 3’ end of the VDR gene.[55] One study demonstrated that the recessive “aa” allele of ApaI might be correlated with a higher level of vitamin D in serum.[56] Wu et al[41] reported that there were significant differences in Ward triangle BMD among each genotype of VDR, in which the aa allele was found in the lower BMD group among a total of 378 patients (normal BMD, 234 cases; decreased BMD, 65 cases; osteoporosis with osteoporotic fracture, 79 cases). This finding suggests that ApaI plays a core role in osteoporosis risk in a Chinese population. However, another study indicated no significant difference between the frequency of ApaI in the control and case groups in a Caucasian population, suggesting no association between the ApaI allele and the osteoporotic population.[40] The study by Xia et al[29] indicated that the main distribution of the ApaI allele was “aa” with rare distribution of “AA,” but no differences were observed between controls and AIS patients. Another 2 studies[32,33] concluded the same results. Our data showed a significant association between ApaI polymorphism and susceptibility to AIS in an Asian population, and the “aa” allele was a protective factor. This finding is inconsistent with that of the studies[29,32,33] included in our meta-analysis. In our opinion, sample size, genotyping techniques, and selection biases might be important contributors to this difference. Compared with the results of these studies,[29,32,33] our results provide a more exact conclusion, as the sample size and statistical power were expanded in our meta-analysis, which could give us a better understanding of the role of the VDR ApaI polymorphism in AIS etiology. In addition, whether the VDR ApaI polymorphism plays a key role in AIS etiology in either Caucasian or overall populations remains unclear, as no articles studying the relationship between ApaI rs7975232 polymorphism and AIS risk were found in our database search. This aspect needs to be further studied.
Although this was a comprehensive analysis of the relationship between BsmI rs1544410 or ApaI rs7975232 and AIS risk, there are still some limitations that should be addressed. First, only 5 studies were included in our study. One study was excluded in our meta-analysis due to unavailable data, despite numerous attempts to contact authors requesting the original data. Second, the sample sizes were relatively small in all the studies, which might lead to contrasting results and influence the conclusions. Third, only 1 study was conducted to explore the relationship between VDR BsmI and AIS risk in a Caucasian population, and this may not be sufficient to draw a convincing conclusion on overall populations. Lastly, we did not perform an adjusted analysis in our study because of insufficient data, and this is a limitation that should not be ignored.
In recent years, genetic analyses of human diseases with quantitative traits have primarily focused on identifying common variants through GWAS, and many novel susceptibility genes implicating specific biological pathways have been identified. The successes of GWAS for common diseases have provided critical insights into the contribution of genetic variants to common diseases and stimulated interest in follow-up studies of disease etiologies. However, there are still some limitations in GWAS that we should not neglect. Despite the discovery of multiple validated SNPs for diseases, GWAS have only revealed a small proportion of the genetic components of complex diseases. In addition, although GWAS have identified numerous SNPs that are associated with several complex diseases or traits, most GWAS-identified SNPs are located in noncoding regions and serve as markers for all SNPs in the same haplotype block. A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. However, there are still many problems with a meta-analysis, such as publication bias, heterogeneity, and the omission of gene–gene and gene–environment interactions. Although there are many shortcomings of a meta-analysis, our study was the first such study that was conducted to explore the associations between the VDR BsmI rs1544410 and ApaI rs7975232 polymorphisms and AIS risk. This study has provided us with information that has furthered our understanding of AIS etiology.
6. Conclusion
The VDR BsmI polymorphism was significantly associated with AIS susceptibility in the overall and Asian populations, while the VDR ApaI polymorphism only played a key role in AIS etiology and development in Asian populations.
Acknowledgments
We would like to express our sincere thanks to Dr Yang Mingyuan for his invaluable advice, constant encouragement, and precise modification of the manuscript. We would also like to express our appreciation to all the members who participated in the study, because they provided help in this study. In addition, we would also like to thank Dr Jeffrey C. Wang, who provided the opportunity for a collaboration and taught with patience.
Footnotes
Abbreviations: AIS = adolescent idiopathic scoliosis, BMD = bone mineral density, BNCS = boron neutron capture synovectomy, ESR1 = estrogen receptor 1, ESR2 = estrogen receptor 2, GWASs = genome-wide association studies, IGF1 = insulin-like growth factor 1, IL-6 = interleukin-6, LBX1 = Ladybird Homeobox 1, RA = rheumatoid arthritis, SNPs = single nucleotide polymorphisms, TGFB1 = transforming growth factor beta 1, VDR = vitamin D receptor.
XY, HW, JG, and LZ contributed equally to this work.
There was no funding support for this study, and the authors report no conflicts of interest.
References
- [1].Weiss HR, Karavidas N, Moramarco M, et al. Long-term effects of untreated adolescent idiopathic scoliosis: a review of the literature. Asian Spine J 2016;10:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luk KD, Lee CF, Cheung KM, et al. Clinical effectiveness of school screening for adolescent idiopathic scoliosis: a large population-based retrospective cohort study. Spine (Phila Pa 1976) 2010;35:1607–14. [DOI] [PubMed] [Google Scholar]
- [3].Weinstein SL, Dolan LA, Cheng JC, et al. Adolescent idiopathic scoliosis. Lancet 2008;371:1527–37. [DOI] [PubMed] [Google Scholar]
- [4].Boswell CW, Ciruna B. Understanding idiopathic scoliosis: a new zebrafish school of thought. Trends Genet 2017;33:183–96. [DOI] [PubMed] [Google Scholar]
- [5].Hresko MT, Talwalkar V, Schwend R; AAOS, SRS, Posna. Early detection of idiopathic scoliosis in adolescents. J Bone Joint Surg Am 2016; 98:e67. [DOI] [PubMed] [Google Scholar]
- [6].Grauers A, Einarsdottir E, Gerdhem P. Genetics and pathogenesis of idiopathic scoliosis. Scoliosis Spinal Disord 2016;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Seze M, Cugy E. Pathogenesis of idiopathic scoliosis: a review. Ann Phys Rehab Med 2012;55:128–38. [DOI] [PubMed] [Google Scholar]
- [8].Kulis A, Gozdzialska A, Drag J, et al. Participation of sex hormones in multifactorial pathogenesis of adolescent idiopathic scoliosis. Int Orthop 2015;39:1227–36. [DOI] [PubMed] [Google Scholar]
- [9].Chan V, Fong GC, Luk KD, et al. A genetic locus for adolescent idiopathic scoliosis linked to chromosome 19p13.3. Am J Human Genet 2002;71:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen S, Zhao L, Roffey DM, et al. Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis in East Asians: a systematic review and meta-analysis. Spine J 2014;14:2968–75. [DOI] [PubMed] [Google Scholar]
- [11].Ji XR, Yang ZD, Yang XH, et al. Change of selenium in environment and risk of adolescent idiopathic scoliosis: a retrospective cohort study. Eur Rev Med Pharmacol Sci 2013;17:2499–503. [PubMed] [Google Scholar]
- [12].Dayer R, Haumont T, Belaieff W, et al. Idiopathic scoliosis: etiological concepts and hypotheses. J Child Orthop 2013;7:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang M, Wei X, Yang W, et al. The polymorphisms of melatonin receptor 1B gene (MTNR1B) (rs4753426 and rs10830963) and susceptibility to adolescent idiopathic scoliosis: a meta-analysis. J Orthop Sci 2015;20:593–600. [DOI] [PubMed] [Google Scholar]
- [14].Cheng JC, Castelein RM, Chu WC, et al. Adolescent idiopathic scoliosis. Nat Rev Dis Primers 2015;1:15030. [DOI] [PubMed] [Google Scholar]
- [15].Zhao J, Yang M, Li M. Association of IL-6 and MMP-3 gene polymorphisms with susceptibility to adolescent idiopathic scoliosis: a meta-analysis. J Genet 2016;95:573–9. [DOI] [PubMed] [Google Scholar]
- [16].Wise CA, Gao X, Shoemaker S, et al. Understanding genetic factors in idiopathic scoliosis, a complex disease of childhood. Curr Genomics 2008;9:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Justice CM, Miller NH, Marosy B, et al. Familial idiopathic scoliosis: evidence of an X-linked susceptibility locus. Spine (Phila Pa 1976) 2003;28:589–94. [DOI] [PubMed] [Google Scholar]
- [18].Miller NH, Justice CM, Marosy B, et al. Identification of candidate regions for familial idiopathic scoliosis. Spine (Phila Pa 1976) 2005;30:1181–7. [DOI] [PubMed] [Google Scholar]
- [19].Wise CA, Barnes R, Gillum J, et al. Localization of susceptibility to familial idiopathic scoliosis. Spine (Phila Pa 1976) 2000;25:2372–80. [DOI] [PubMed] [Google Scholar]
- [20].Yang M, Li C, Li M. The estrogen receptor alpha gene (XbaI, PvuII) polymorphisms and susceptibility to idiopathic scoliosis: a meta-analysis. J Orthop Sci 2014;19:713–21. [DOI] [PubMed] [Google Scholar]
- [21].Nikolova ST, Yablanski VT, Vlaev EN, et al. Association between IL-6 and MMP3 common genetic polymorphisms and idiopathic scoliosis in Bulgarian patients: a case-control study. Spine (Phila Pa 1976) 2016;41:785–91. [DOI] [PubMed] [Google Scholar]
- [22].Xu L, Xia C, Qin X, et al. Genetic variant of BNC2 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Mol Genet Genomics 2017;292:789–94. [DOI] [PubMed] [Google Scholar]
- [23].Cao Y, Min J, Zhang Q, et al. Associations of LBX1 gene and adolescent idiopathic scoliosis susceptibility: a meta-analysis based on 34,626 subjects. BMC Musculoskelet Disord 2016;17:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang W, Ma J, Li SY, et al. [Advance on genetic mechanism of adolescent idiopathic scoliosis and genetic relationship map]. Zhongguo Gu Shang 2015;28:854–60. [PubMed] [Google Scholar]
- [25].Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004;338:143–56. [DOI] [PubMed] [Google Scholar]
- [26].Xu G, Mei Q, Zhou D, et al. Vitamin D receptor gene and aggrecan gene polymorphisms and the risk of intervertebral disc degeneration: a meta-analysis. PLoS One 2012;7:e50243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martirosyan NL, Patel AA, Carotenuto A, et al. Genetic alterations in intervertebral disc disease. Front Surg 2016;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang P, Li Q, Qi J, et al. Association between vitamin D receptor gene polymorphism and ankylosing spondylitis in Han Chinese. Int J Rheum Dis 2017;20:1510–6. [DOI] [PubMed] [Google Scholar]
- [29].Xia CW, Qiu Y, Sun X, et al. [Vitamin D receptor gene polymorphisms in female adolescent idiopathic scoliosis patients]. Zhonghua Yi Xue Za Zhi 2007;87:1465–9. [PubMed] [Google Scholar]
- [30].Bell NH, Morrison NA, Nguyen TV, et al. ApaI polymorphisms of the vitamin D receptor predict bone density of the lumbar spine and not racial difference in bone density in young men. J Lab Clin Med 2001;137:133–40. [DOI] [PubMed] [Google Scholar]
- [31].Suh KT, Eun IS, Lee JS. Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 2010;19:1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen WJ, Qiu Y, Zhu F, et al. [Vitamin D receptor gene polymorphisms: no association with low bone mineral density in adolescent idiopathic scoliosis girls]. Zhonghua Wai Ke Za Zhi 2008;46:1183–6. [PubMed] [Google Scholar]
- [33].Wang Y, Cui ZQ, Luo TB, et al. Correlations of VDR and VDBP genetic polymorphisms with susceptibility to adolescent idiopathic scoliosis and efficacy of brace treatment. Genomics 2016;108:194–200. [DOI] [PubMed] [Google Scholar]
- [34].Yilmaz H, Zateri C, Uludag A, et al. Single-nucleotide polymorphism in Turkish patients with adolescent idiopathic scoliosis: curve progression is not related with MATN-1, LCT C/T-13910, and VDR BsmI. J Orthop Res 2012;30:1459–63. [DOI] [PubMed] [Google Scholar]
- [35].Cheng JC, Qin L, Cheung CS, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res 2000;15:1587–95. [DOI] [PubMed] [Google Scholar]
- [36].Eun IS, Park WW, Suh KT, et al. Association between osteoprotegerin gene polymorphism and bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 2009;18:1936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 1998;13:325–49. [DOI] [PubMed] [Google Scholar]
- [38].Hu W, Niu G, Lin Y, et al. Impact of the polymorphism in vitamin D receptor gene BsmI and the risk of systemic lupus erythematosus: an updated meta-analysis. Clin Rheumatol 2016;35:927–34. [DOI] [PubMed] [Google Scholar]
- [39].Azab SF, Ali YF, Farghaly MA, et al. Vitamin D receptor gene BsmI polymorphisms in Egyptian children and adolescents with systemic lupus erythematosus: a case-control study. Medicine 2016;95:e5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dabirnia R, Mahmazi S, Taromchi A, et al. The relationship between vitamin D receptor (VDR) polymorphism and the occurrence of osteoporosis in menopausal Iranian women. Clin Cases Miner Bone Metab 2016;13:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu J, Shang DP, Yang S, et al. Association between the vitamin D receptor gene polymorphism and osteoporosis. Biomed Rep 2016;5:233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Song GG, Bae SC, Lee YH. Vitamin D receptor FokI, BsmI, and TaqI polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Z Rheumatol 2016;75:322–9. [DOI] [PubMed] [Google Scholar]
- [43].Cavalcanti CA, Silva Jde A, Pita Wde B, et al. Vitamin D receptor polymorphisms and expression profile in rheumatoid arthritis Brazilian patients. Mol Biol Rep 2016;43:41–51. [DOI] [PubMed] [Google Scholar]
- [44].Carvalho C, Marinho A, Leal B, et al. Association between vitamin D receptor (VDR) gene polymorphisms and systemic lupus erythematosus in Portuguese patients. Lupus 2015;24:846–53. [DOI] [PubMed] [Google Scholar]
- [45].Bermúdez -Morales VH, Fierros G, Lopez RL, et al. Vitamin D receptor gene polymorphisms are associated with multiple sclerosis in Mexican adults. J Neuroimmunol 2017;306:20–4. [DOI] [PubMed] [Google Scholar]
- [46].Chen XE, Chen P, Chen SS, et al. A population association study of vitamin D receptor gene polymorphisms and haplotypes with the risk of systemic lupus erythematosus in a Chinese population. Immunol Res 2017;65:750–6. [DOI] [PubMed] [Google Scholar]
- [47].Di Spigna G, Del Puente A, Covelli B, et al. Vitamin D receptor polymorphisms as tool for early screening of severe bone loss in women patients with rheumatoid arthritis. Eur Rev Med Pharmacol Sci 2016;20:4664–9. [PubMed] [Google Scholar]
- [48].Pabalan N, Tabangay L, Jarjanazi H, et al. Association between the FokI and ApaI polymorphisms in the vitamin D receptor gene and intervertebral disc degeneration: a systematic review and meta-analysis. Genet Test Mol Biomarkers 2017;21:24–32. [DOI] [PubMed] [Google Scholar]
- [49].Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature 1994;367:284–7. [DOI] [PubMed] [Google Scholar]
- [50].Ferrari S, Rizzoli R, Chevalley T, et al. Vitamin-D-receptor-gene polymorphisms and change in lumbar-spine bone mineral density. Lancet 1995;345:423–4. [DOI] [PubMed] [Google Scholar]
- [51].Salamone LM, Ferrell R, Black DM, et al. The association between vitamin D receptor gene polymorphisms and bone mineral density at the spine, hip and whole-body in premenopausal women. Osteoporos Int 1996;6:63–8. [DOI] [PubMed] [Google Scholar]
- [52].Xu H. Interaction effects between estrogen receptor α and vitamin D receptor genes on age at menarche in Chinese women. Acta Pharmacol Sin 2005;26:860–4. [DOI] [PubMed] [Google Scholar]
- [53].Stavrou I, Zois C, Ioannidis JP, et al. Association of polymorphisms of the oestrogen receptor alpha gene with the age of menarche. Hum Reprod (Oxford, England) 2002;17:1101–5. [DOI] [PubMed] [Google Scholar]
- [54].Lee WT, Cheung CS, Tse YK, et al. Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int 2005;16:1924–32. [DOI] [PubMed] [Google Scholar]
- [55].Faraco JH, Morrison NA, Baker A, et al. ApaI dimorphism at the human vitamin D receptor gene locus. Nucleic Acids Res 1989;17:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Karpinski M, Galicka A, Milewski R, et al. Association between Vitamin D receptor polymorphism and serum Vitamin D levels in children with low-energy fractures. J Am Coll Nutr 2017;36:64–71. [DOI] [PubMed] [Google Scholar]


