Abstract
Epicatechin (EC) is a monomeric flavan-3-ol. We have previously demonstrated that glucose-intolerant rats fed flavan-3-ols exhibit improved pancreatic islet function corresponding with an increase in circulating EC-derived metabolites. Thus, we speculate that EC may act as a cellular signaling molecule in vivo to modulate insulin secretion. In this study we further examined the effects of different concentrations of EC on H2O2 or hyperglycemia-induced ROS production, as well as on saturated fatty acid (SFA)-impaired glucose-stimulated insulin secretion (GSIS) in INS-1 cell line in vitro. We showed that EC at a high concentration (30 μmol/L), but not a low concentration (0.3 μmol/L), significantly decreased H2O2 or hyperglycemia-induced ROS production in INS-1 cells. However, EC (0.3 μmol/L) significantly enhanced SFA-impaired GSIS in INS-1 cells. Addition of KN-93, a CaMKII inhibitor, blocked the effect of EC on insulin secretion and decreased CaMKII phosphorylation. Addition of GW1100, a GPR40 antagonist, significantly attenuated EC-enhanced GSIS, but only marginally affected CaMKII phosphorylation. These results demonstrate that EC at a physiological concentration promotes GSIS in SFA-impaired β-cells via activation of the CaMKII pathway and is consistent with its function as a GPR40 ligand. The findings support a role for EC as a cellular signaling molecule in vivo and further delineate the signaling pathways of EC in β-cells.
Keywords: epicatechin, flavonoids, hyperglycemia, oxidative stress, insulin secretion, CaMKII signaling pathway, GPR40, KN-93, GW1100
Introduction
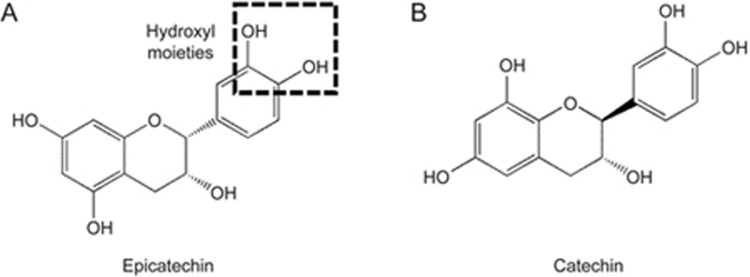
Epicatechin (EC) is a monomeric flavan-3-ol that belongs to a subgroup of flavonoids (Figure 1) and is a ubiquitous secondary metabolite in plants1. Dietary EC mainly derives from tea and cocoa products, grains such as beans and peas, and fruits such as grapes, berries, and apples2. In vitro studies demonstrate that EC can be taken up and metabolized in enterocytes and hepatocytes 3,4. Evidence from both animal and human studies shows that monomeric flavanols are extensively metabolized to glucuronides, sulfates, and O-methylated forms by phase II enzymes in the intestine and liver5,6,7. Their metabolites are found in plasma and accumulate in organs such as the brain, liver, heart, intestine, and kidney8,9. Therefore, EC likely affects some functions of these organs.
Figure 1.
Structure of epicatechin (A) and catechin (B).
EC is well known for its antioxidant activity. Its half-maximal inhibitory concentration (IC50) as a protective antioxidant is approximately 200–400 μmol/L in the intestine10. However, the concentrations of accumulated EC in vivo are much lower: in plasma, EC metabolites peak at about 40 μmol/L, whereas in other tissues, their concentration is approximately 0.1–0.3 μmol/L8,9,11. Therefore, whether EC acts as an antioxidant in vivo remains debatable.
Current evidence suggests that the interaction of EC with molecular targets could explain its bioactivities. EC may exert antioxidant effects indirectly by modulating redox enzymes. When rats are fed EC at a dose of 20 mg per kg of body weight per day for 8 weeks, oxidative stress in the heart induced by dietary fructose overload is prevented via increasing superoxide dismutase activity, decreasing superoxide anion production, and a reduced oxidized/reduced glutathione ratio12. In tert-butylhydroperoxide (t-BOOH)-treated INS-1E beta-cells, 20 μmol/L of cocoa-derived EC prevents glutathione (GSH) depletion, recovers the activity of glutathione peroxidase and glutathione reductase, and reduces reactive oxygen species (ROS)13. The Ca2+/calmodulin-dependent protein kinase (CaMK) II pathway may also be modulated by EC. In the presence of Ca2+, EC (1 μmol/L) induces the synthesis of nitric oxide (NO) through endothelial nitric oxide synthase (eNOS) activation via the CaMKII pathway in human coronary artery endothelial cells14. Improved basal synaptic transmission and long-term potentiation in hippocampus slices are revealed after 0.3 μmol/L EC metabolite treatment11. The underlying mechanism is associated with the activation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) signaling via the CaMKII pathway11. Furthermore, recent studies support the existence of an EC cell membrane receptor. Moreno-Ulloa et al15,16 observed cell responses in human coronary artery endothelial cells using a cell-membrane impermeable form of EC. In addition, Kado et al.17 found that the hydroxyl or methoxyl moieties on the aromatic ring of flavonoids can potentially act on GPR40 to activate the CaMKII pathway during stimulation of glucagon-like peptide 1 (GLP-1) secretion in a murine GLUTag cell line. These findings indicate that EC with two hydroxyl moieties on the aromatic ring (Figure 1) may act on cell membrane receptors such as GPR40 to modulate cellular signaling pathways.
EC enhances glucose-stimulated insulin secretion (GSIS) from healthy β-cell lines and isolated islets18,19. In oxidant-stressed β-cell lines, isolated islets, or animals, recovery of β-cell mass and function is observed in response to EC treatment13,20. Nonetheless, the mechanisms of these phenomena are less understood. Although not yet reported, the concentrations of EC and its metabolites accessible to pancreatic β-cells are most likely similar to those of other organs (0.1–0.3 μmol/L)8,9,11. Therefore, at these concentrations, EC and its metabolites may act as a cellular signaling modulatory compound in pancreatic β-cells. In addition, studies have suggested that CaMKII is a key Ca2+ sensor that controls Ca2+ homeostasis in pancreatic β-cells21. Elevated intracellular Ca2+ activates CaMKII, thereby enhancing GSIS, whereas inhibition of CaMKII reduces GSIS21. Considering the intracellular targets of EC found in other cell types, EC may modulate the Ca2+/CaMKII pathway in pancreatic β-cells.
Previously, we demonstrated that glucose-intolerant rats fed oligomeric flavan-3-ols had improved pancreatic islet function as suggested by enhanced GSIS from isolated islets22. Correspondingly, an increase in serum metabolites derived from flavan-3-ols was observed, including EC22. Therefore, we speculate that EC modulates the CaMKII pathway in pancreatic β-cells to improve GSIS. In addition, existing evidence suggests that the EC concentration at the organ level is approximately 0.3 μmol/L11. Therefore, we investigated the effects of EC at a physiological concentration (0.3 μmol/L) versus an antioxidant concentration (30 μmol/L) on INS-1 cells stressed by treatment with saturated fatty acid. We hypothesized that EC at the lower concentration can recover insulin secretion via the Ca2+/CaMKII pathway by activating GPR40 in SFA-treated pancreatic β-cells.
Materials and methods
Cell culture
The rat insulinoma β-cell line INS-1 (clone 832/13) (a gift from Dr Peter LIGHT, Alberta Diabetes Institute, Canada) was maintained in a humidified incubator containing 5% CO2 at 37°C and grown in Roswell Park Memorial Institute RPMI-1640 medium with 2 mmol/L L-glutamine (Gibco, Burlington, ON, Canada) supplemented with 10% FBS (Sigma, Oakville, ON, Canada), 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Fisher, NJ, USA), 1 mmol/L sodium pyruvate (Gibco, Burlington, ON, Canada), 50 μmol/L β-mercaptoethanol (Sigma, Oakville, ON, Canada), and 1× antibiotic antimycotic solution (Sigma, Oakville, ON, Canada).
Measurement of EC modulation of glucose- or H2O2-induced ROS production
Cellular ROS were quantified using the DCFDA Cellular ROS Detection Assay Kit (Abcam, Cambridge, MA, USA). INS-1 cells (5×105 cells per well) were seeded in 96-well clear-bottom black-side plates and allowed to grow until 70% confluent. The cells were then cultured for 24 h in medium containing vehicle, 0.3 μmol/L N-acetyl cysteine (NAC, Sigma, Oakville, ON, Canada), or 0.3 μmol/L epicatechin (EC; purity >98%; Sigma, Oakville, ON, Canada). For glucose-induced ROS, the medium was changed to Krebs–Ringer bicarbonate buffer (KRB: 116 mmol/L NaCl, 24 mmol/L NaHCO3, 4.4 mmol/L KCl, 1.5 mmol/L KH2PO4, 1.2 mmol/L MgSO4·7H2O, 2.5 mmol/L CaCl2) supplemented with 10 mmol/L HEPES, 0.1% BSA, and 2.8 mmol/L glucose for a quiescent period of 30 min. Then, the cells were incubated in KRB-HEPES containing 2.8 or 16.5 mmol/L glucose, the latter with vehicle and 0.3 μmol/L or 30 μmol/L EC or with 0.3 μmol/L or 30 μmol/L NAC as a positive control for 3 h. Next, the medium was removed, and 100 μL of 5 μmol/L 2′,7′–dichlorofluorescin diacetate (DCFDA) in Hanks' Balanced salt solution (HBSS) without phenol red (Sigma, Oakville, ON, Canada) was added to the wells for 30 min at 37 °C. After diffusion into the cell, DCFDA is deacetylated by cellular esterases to a non-fluorescent compound, which is later oxidized by ROS into 2′, 7′–dichlorofluorescein (DCF), which emits fluorescence. ROS generation was evaluated in a fluorescent microplate reader (Infinite 200 PRO Multimode Reader, Tecan Group Ltd, Germany) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The results were expressed in arbitrary units of fluorescence emission at 535 nm. Six or eight independent experiments were run in which each condition was tested in quadruplicates.
For hydrogen peroxide (H2O2), INS-1 cells were cultured as described above. The medium was then removed, and 100 μL of 5 μmol/L DCFDA in HBSS without phenol red (Sigma, Oakville, ON, Canada) was added to the wells for 30 min at 37°C. The antioxidant status of the cells was determined by adding 50 μmol/L H2O2 (Sigma, Oakville, ON, Canada) to each well and intracellular ROS were immediately measured in the microplate reader as described earlier. Six or seven independent experiments were run in which each condition was tested in quadruplicate.
Glucose-stimulated insulin secretion
INS-1 cells after 24-h treatments (as indicated below in Treatments 1, 2, and 3) were washed and incubated in KRB-HEPES with 0.1% BSA for a quiescent period of 90 min. Then, the cells were incubated in KRB-HEPES-BSA containing 2.8 or 16.5 mmol/L glucose for 90 min. Insulin secreted into the medium was evaluated by radioimmunoassay (RIA). Total cell insulin content was calculated by adding insulin secreted into the supernatant plus that remaining in the cells as determined by RIA. From this, the percentage of total insulin secreted was calculated for each data point to eliminate variance caused by cell number. Seven independent experiments were run in which each condition was tested in triplicate.
Treatment 1: RPMI, RPMI+0.4 mmol/L SFA, RPMI+0.4 mmol/L SFA+0.3 μmol/L EC or NAC, RPMI+0.4 mmol/L SFA+30 μmol/L EC or NAC;
Treatment 2: RPMI, RPMI+0.4 mmol/L SFA, RPMI+10 μmol/L KN-93 (a CaMKII inhibitor; Sigma, Oakville, ON, Canada), RPMI+0.4 mmol/L SFA+0.3 μmol/L EC or NAC, RPMI+0.4 mmol/L SFA+0.3 μmol/L EC or NAC+10 μmol/L KN-93;
Treatment 3: RPMI, RPMI+0.4 mmol/L SFA, RPMI+10 μmol/L GW1100 (a GPR40 antagonist; Cayman Chemical, Ann Arbor, MI, USA), RPMI+0.4 mmol/L SFA+0.3 μmol/L EC or NAC, RPMI+0.4 mmol/L SFA+0.3 μmol/L EC or NAC+10 μmol/L GW1100.
Stearic acid (SFA; Sigma, Oakville, ON, Canada) was dissolved in 75% ethanol at 50°C with 100 r/min shaking, and then added to RPMI medium with 5% BSA to prepare a 4 mmol/L stock. The stock was added to cell culture medium to yield a final concentration of 0.4 mmol/L. The vehicle control was RPMI+0.75% ethanol.
Western blot analysis
INS-1 cells collected after 24-h treatment 2 or 3 were lysed in a radioimmunoprecipitation assay buffer (150 mmol/L NaCl; 1.0% NP-40; 0.5% sodium deoxycholate; 0.1% SDS; 50 mmol/L Tris, pH 8.0) with 2 μg/mL aprotinin, 10 μL/mL protease inhibitor cocktail, and 1 mmol/L NaF. All of the chemicals were obtained from Sigma (Oakville, ON, Canada). Total protein was measured using a Lowry protein assay (Thermo Scientific, Pierce Biotechnology, Rockford, IL, USA).
Protein samples (20 μg) were subjected to SDS-PAGE followed by blotting onto nitrocellulose membranes. The membranes were blocked in 3% BSA–0.1% Tween–Tris-buffered saline for 1 h followed by overnight incubation at 4°C with primary antibodies diluted 1:1000 in the blocking solution. Primary antibodies included anti-Ca2+/calmodulin-dependent protein kinase II (CaMKII), antiphospho-CaMKII (p-CaMKII), anti-extracellular regulated kinases (ERKs), antiphospho-ERKs (p-ERKs), anti-protein kinase A (PKA), and antiphospho-PKA (p-PKA), all from Cell Signaling Technology (Danvers, MA, USA). The membranes were subsequently incubated with the appropriate HRP-conjugated secondary antibodies (1:5000; Sigma, Saint Louis, MO, USA) for 1 h at room temperature. Labeling of each phosphorylated protein was compared with its respective total protein. The membranes were developed using ECL Prime (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA) and digital images were captured using a ChemiDoc MPTM imaging system (Bio-Rad Laboratories, Inc., Mississauga, ON, Canada). Band intensity was quantified using Quantity One v4.6.2 (Bio-Rad Laboratories, Inc, Hercules, CA, USA).
Statistical analysis
The results are presented as the mean±the standard error of the mean (SEM). ANOVA followed by Bonferroni's post hoc test, with the P-value adjusted for the number of comparisons, was used to assess differences between different conditions using GraphPad Prism 6 (GraphPad Software, Inc, San Diego, CA, USA). Nonparametric Kruskal-Wallis tests were used for data with non-normal distributions. P<0.05 was considered statistically significant.
Results
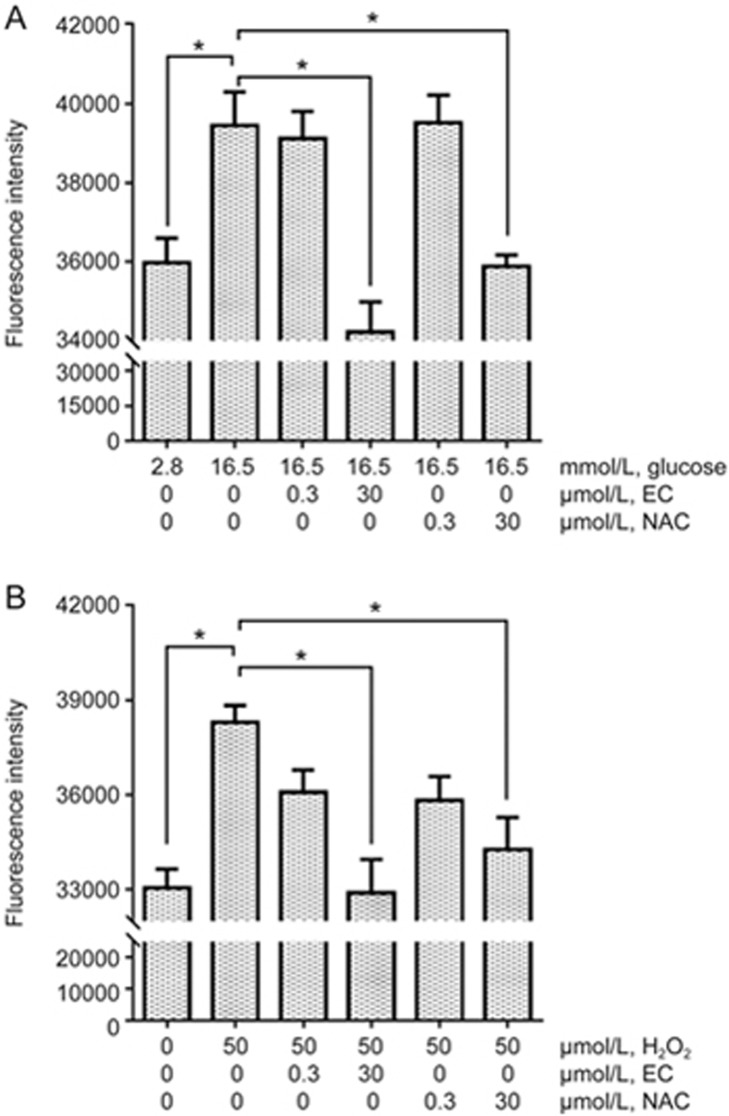
EC (30 μmol/L) protects against ROS in INS-1 cells
Incubation of INS-1 cells with high glucose for 3 h significantly increased ROS compared with cells treated in low glucose (P<0.01, Figure 2A). Treatment with 30 μmol/L EC significantly reduced high glucose-induced ROS production to a level comparable to that with low glucose treatment (P<0.001 vs high glucose, Figure 2A). In contrast, 0.3 μmol/L EC had no effect on ROS production induced by high glucose. Similar results were found when using the same concentrations of NAC to treat INS-1 cells: a concentration of 30 μmol/L significantly (P<0.01, Figure 2A) decreased ROS in high glucose-treated cells while 0.3 μmol/L showed no improvement.
Figure 2.
EC at 30 μmol/L protects against ROS in INS-1 cells. (A) INS-1 cells incubated for 24 h with or without EC or NAC were exposed to low or high glucose medium for 3 h in the continuous presence or absence of EC or NAC. The antioxidant status of the cells was then determined by measuring intracellular ROS accumulation after loading the cells with 5 μmol/L of the ROS dye DCFDA for 30 min. (B) INS-1 cells incubated for 24 h with or without EC or NAC were loaded with 5 μmol/L of the fluorescent ROS dye DCFDA for 30 min. The cells were then exposed to 50 μmol/L H2O2 to increase intracellular ROS for 2 min. The results were expressed in arbitrary units of fluorescence emission at 535 nm. Six to eight independent experiments were run in which each condition was tested in quadruplicate. The data were analyzed using one-way ANOVA with Bonferroni's multiple comparisons. *P<0.05.
We then tested whether EC treatment could reduce H2O2-induced ROS in INS-1 cells. H2O2 treatment for 10 min can lead to impaired insulin secretion in β-cells23. Therefore, we measured ROS concentrations immediately (within 2 min) after exposing cells to H2O2. ROS production was significantly increased in the cells after short-term exposure to H2O2 compared with that in untreated cells (P<0.001, Figure 2B). Cells pretreated with 30 μmol/L EC had significantly lower levels of ROS (P<0.001, Figure 2B). Again, no significant reduction in H2O2-induced ROS was found in cells treated with 0.3 μmol/L EC. When using the antioxidant NAC at the same concentrations, 30 μmol/L NAC was effective in decreasing H2O2-induced ROS, whereas 0.3 μmol/L NAC was not.
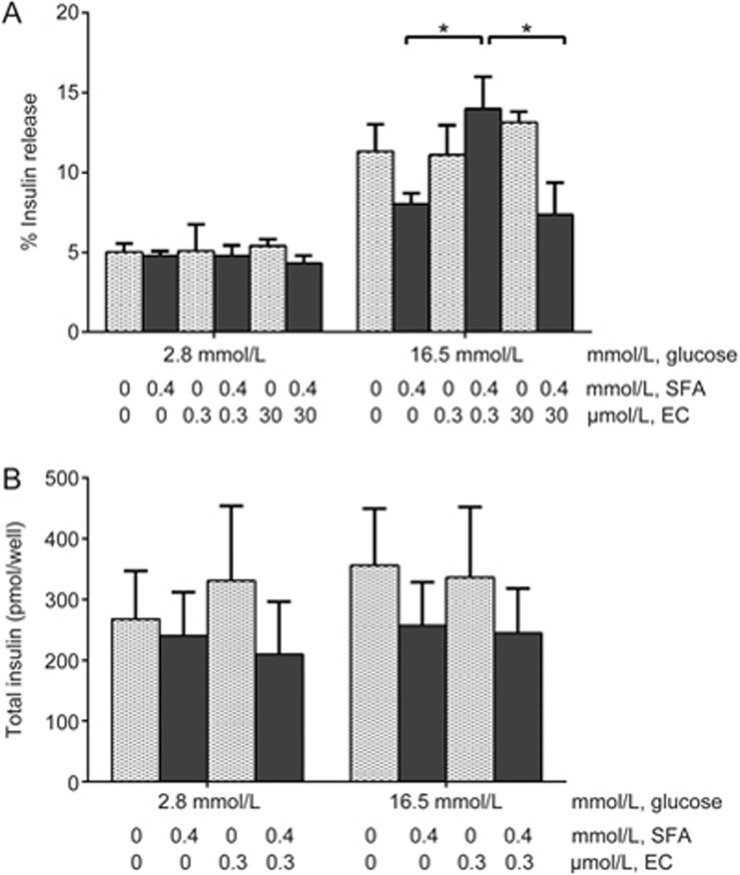
EC (0.3 μmol/L) enhances insulin secretion from INS-1 cells treated with SFA
Long-term SFA exposure impairs insulin secretion from β-cells24. In our study, 24-h incubation with 0.4 mmol/L SFA was used to impair β-cell function. After treatment, the mean insulin secretion in response to high glucose was reduced by 30% (P>0.05, Figure 3A). The addition of EC (0.3 μmol/L) in the culture medium significantly increased GSIS compared with that of SFA-treated cells (P<0.05, Figure 3A). In contrast, SFA-impaired insulin secretion was not increased by a high concentration of EC (30 μmol/L), remaining comparable to that of SFA-treated cells. EC alone did not stimulate insulin secretion as shown in cells incubated with 2.8 mmol/L glucose and treated with 0.3 μmol/L EC (Figure 3A). Thus, EC-enhanced insulin secretion is dependent on the presence of high glucose stimulation.
Figure 3.
EC (0.3 μmol/L) enhances insulin secretion from INS-1 cells treated with SFA. INS-1 cells were incubated for 24 h with or without EC in the continuous presence or absence of SFA. Then, the cells were exposed to low or high glucose medium for 90 min in the continuous presence or absence of EC after a quiescent period of 90 min. (A) Insulin secreted into the medium and remaining in the cells was measured by RIA to calculate the % release at each glucose concentration. (B) Total insulin synthesized was calculated as the sum of insulin secreted and remaining in the cells. Five independent experiments were run in which each condition was tested in triplicate. The data were analyzed using two-way ANOVA with Bonferroni's multiple comparisons. *P<0.05.
The improved insulin secretion observed in 0.3 μmol/L EC-treated cells could be caused by increases in either cell numbers or secretory capacity. However, neither SFA nor EC treatment had significant effects (Figure 3B, P>0.05) on the insulin content in these cells, suggesting that 0.3 μmol/L EC reverses insulin secretion by modulating cell secretory capacity.
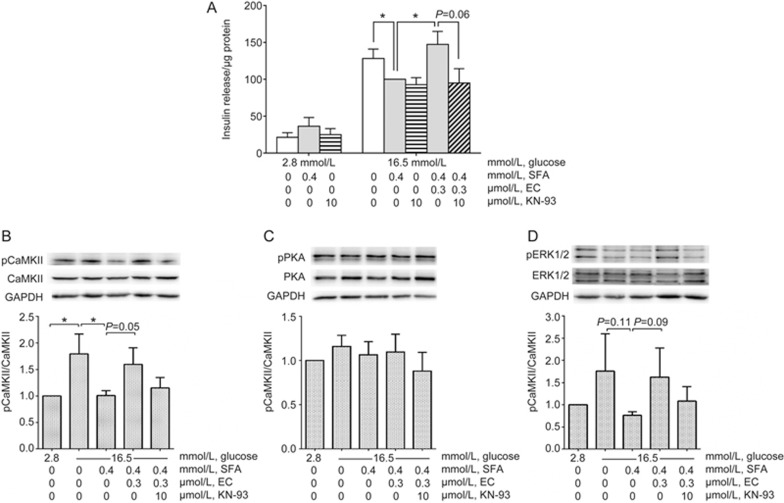
EC regulates insulin secretion via the CaMKII pathway
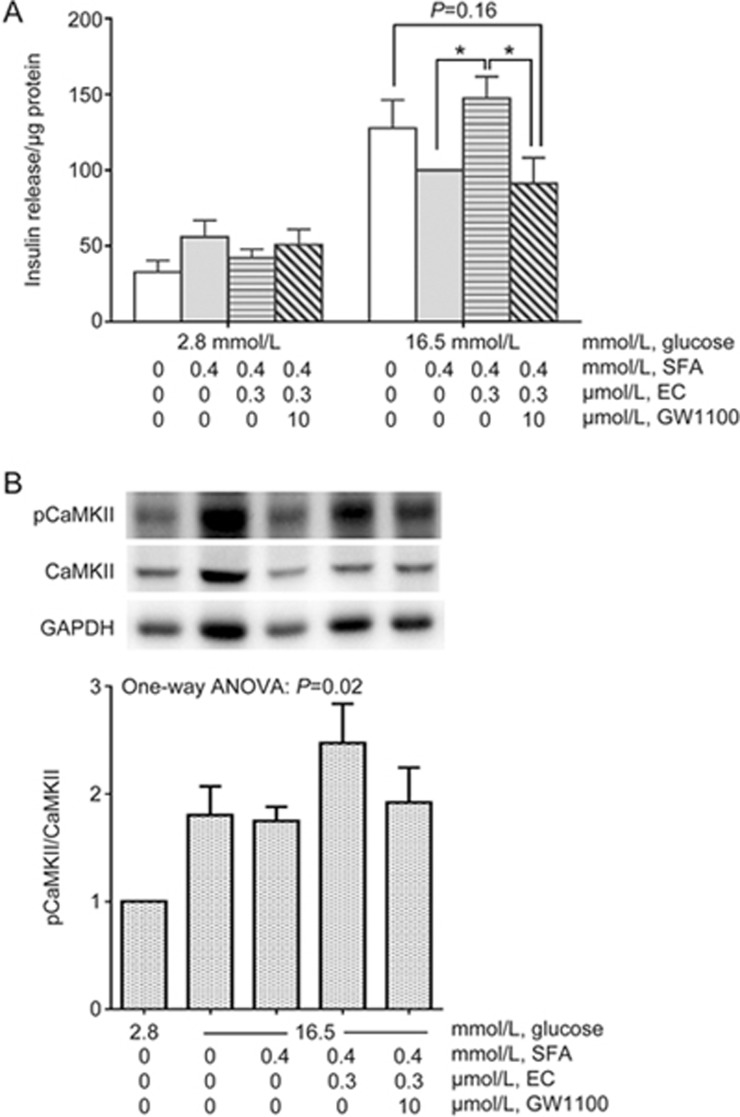
CaMKII is a Ca2+ sensor and regulator that promotes insulin secretion in pancreatic β-cells25. EC has been shown to activate the CaMKII pathway in other cell types11,14,17. Therefore, it may potentiate insulin secretion by the same mechanism. We tested this hypothesis by blocking CaMKII using KN-93 and measuring insulin secretion in the presence of 0.3 μmol/L EC in INS-1 cells. As expected, EC-enhanced GSIS was attenuated by adding 10 μmol/L KN-93, although statistical significance was not reached (P=0.06, Figure 4A). Insulin secretion from KN-93-treated normal cells occurred at a similar level to that of SFA-treated cells (Figure 4A).
Figure 4.
EC regulates insulin secretion via the CaMKII pathway. INS-1 cells were incubated for 24 h with or without EC in the continuous presence or absence of SFA and KN-93. Then, the cells were exposed to low or high glucose medium in the continuous presence or absence of EC and KN-93 for 90 min after a quiescent period of 90 min. Insulin secreted into the medium was measured by RIA. The cells were lysed and quantified for total protein. Insulin release (A) was expressed as per μg of protein. The expression of target proteins was measured by western blotting. Phosphorylated CaMKII (B), PKA (C), and ERK (D) were normalized to the total density of each respective enzyme, which was normalized to GAPDH. Four independent experiments were run in which each condition was tested in triplicate. The data were analyzed using one-way ANOVA with Bonferroni's multiple comparisons (due to missing matched groups, two-way ANOVA cannot be performed for insulin release data. Therefore, insulin secretion at 16.5 mmol/L glucose was compared using one-way ANOVA). *P<0.05.
To further characterize the EC-induced activation of intracellular signaling pathways, we examined the extent of phosphorylation/activation of CaMKII, PKA, and ERK1/2 in INS-1 cells in the absence or presence of the inhibitor KN-93, which blocks the activation of CaMKII. Glucose at 16.5 mmol/L increased CaMKII phosphorylation (Figure 4B), while the addition of SFA to 16.5 mmol/L glucose decreased CaMKII phosphorylation to basal levels (Figure 4B, P<0.05). The addition of 0.3 μmol/L EC reversed SFA-mediated inhibition of CaMKII phosphorylation (P=0.05). EC-induced phosphorylation of CaMKII was decreased to a level similar to that of non-stimulated cells by preincubating cells with 10 μmol/L KN-93. In the same experiments, PKA phosphorylation was not affected (Figure 4C), but examination of the effects of CaMKII antagonism by KN-93 on downstream ERK1/2 phosphorylation revealed a similar pattern to that for CaMKII phosphorylation; however, no significant differences were found among the treatment groups (one-way ANOVA: P=0.098, Figure 4D).
GPR40 is a potential cell membrane receptor of EC
We then examined whether GPR40 was involved in EC-mediated insulin secretion. The effect of the GPR40 antagonist GW1100 on EC-stimulated insulin secretion was tested (Figure 5A), which significantly decreased insulin secretion in EC-treated cells compared to that in cells treated without GW1100. CaMKII phosphorylation was affected by GW1100 treatment (P<0.05, Figure 5B). GW1100 partially but not significantly reduced CaMKII phosphorylation compared to that in EC-treated cells without GW1100 (Figure 5B).
Figure 5.
GPR 40 is a potential cell membrane receptor of EC. INS-1 cells were incubated for 24 h with or without EC in the continuous presence or absence of SFA and GW1100. Then, the cells were exposed to low or high glucose medium in the continuous presence or absence of EC and GW1100 for 90 min after a quiescent period of 90 min. Insulin secreted into the medium was measured by RIA. The cells were lysed and quantified for total protein. Insulin release (A) was expressed as per μg of protein. The expression of target proteins was measured by Western blotting. Phosphorylated CaMKII (B) was normalized to the total density of CaMKII. Four independent experiments were run in which each condition was tested in triplicate. Insulin release data were analyzed using two-way ANOVA, and Western blotting data were tested by one-way ANOVA, with Bonferroni's multiple comparisons. *P<0.05.
Discussion
Many studies have investigated the effects of EC-rich foods or extracts on glucose homeostasis in humans and animal models. Although controversy remains, several studies have reported the effectiveness of EC in improving glucose handling and insulin sensitivity in diabetes19,20,26,27,28,29. One theory regarding how EC exerts its beneficial effects suggests that EC interacts with intracellular molecules to modulate cell signaling pathways30. In this study, we showed that a concentration (0.3 μmol/L) of EC attainable via dietary intake of flavonoid-rich foods such as PAC-containing pea seed coats could modulate insulin secretion from pancreatic β-cells22. The results suggest that EC enhances insulin secretion by activating CaMKII in the presence of high glucose, possibly involving GPR40 activation.
Chronic elevation of free saturated fatty acids (FSFAs) in plasma plays a key role in the pathology of insulin resistance and type 2 diabetes31. FSFAs also represent a crucial link between insulin resistance and β-cell dysfunction32. In vitro studies have found that SFA at 0.25 mmol/L can blunt insulin secretion in response to glucose in isolated islets treated for 72 h33 and induce apoptosis in INS-1 cells and human islets after 24 h of exposure34. Long-term exposure (≥ 48 h) of insulin-secreting β-cell lines to palmitic acid (0.4 mmol/L), a type of FSFA, leads to impaired GSIS from the cells24. In this study, we used 0.4 mmol/L SFA to treat INS-1 cells for 24 h to impair insulin secretion in the cells. Our results showed a 30% reduction in the percentage of insulin secretion from the cells treated with SFA, whereas adding 0.3 μmol/L EC normalized insulin secretion in SFA-treated cells.
CaMKII is a multifunctional serine/threonine kinase that plays an important role in regulating nutrient metabolism, gene expression, membrane excitability, the cell cycle, and neuronal communication35. CaMKII is expressed in pancreatic β-cells and several β-cell lines, such as INS-1, MIN6, and HIT T5 cells36,37,38. Insulin secretagogues induce elevated cytosolic Ca2+, which activates CaMKII and leads to autophosphorylation of the kinase. The autophosphorylation of CaMKII is thought to be crucial in extending the activation period and maintaining activity in the absence of Ca2+/calmodulin35. Studies have found that CaMKII activation is closely correlated with insulin secretion39. Overexpression of CaMKII in β-cells potentiates insulin secretion upon stimulation38, whereas inhibition of the kinase impairs Ca2+ entry and insulin secretion during glucose stimulation21,40. Long-term palmitate exposure reportedly blunts the activation of CaMKII, which results in reduced glucose- or amino acid-induced insulin release40. In our study, we also found decreases in GSIS that correlated with reduced CaMKII phosphorylation in SFA-treated cells. EC at 0.3 μmol/L was able to reverse this reduction. CaMKII inhibitor KN-93 alone decreased insulin release and CaMKII phosphorylation to a similar level as SFA, and it totally blocked EC-mediated effects in SFA-treated cells. Therefore, our results suggest that EC may promote insulin secretion by reversing the inactivation of CaMKII by SFA.
In pancreatic β-cells, a glucose-induced increase in cytosolic Ca2+ activates CaMKII, and activation of CaMKII coincides with insulin secretion39. In addition, PKA can be activated by glucose via increasing intracellular Ca2+ and cyclic adenosine monophosphate (cAMP), and PKA activity correlates closely with Ca2+ spikes41 and potentiates both acute and sustained insulin release42. In this study, we failed to detect significant changes in PKA phosphorylation by EC treatment. It has been reported that in the hypothalamus, cAMP-activated PKA is essential in CaMKII signaling in long-term potentiation (LTP) by inhibiting protein phosphatase–1 (PP1), which specifically dephosphorylates CaMKII43,44,45. Likewise, PKA may have a similar effect on CaMKII in pancreatic β-cells. As a result, EC treatment may specifically affect only CaMKII phosphorylation, but not PKA. Alternatively, KN-93 treatment may also have impacted CaMKIV activation, which regulates β-cell Irs2 expression and GSIS46. However, to our knowledge, the benefits of CaMKIV are not known to be dependent on prior stress due to saturated fatty acids as in the case of CaMKII40. A schematic diagram illustrating the proposed central role of CaMKII in mediating EC effects is shown in Figure 6.
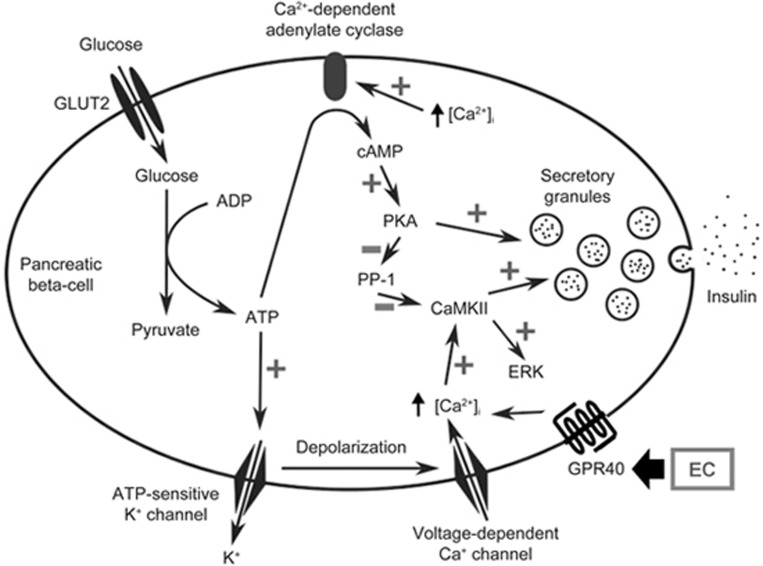
Figure 6.
Schematic diagram of the possible pathways for EC-mediated insulin secretion in pancreatic β-cells. An increased cellular adenosine triphosphate (ATP)/adenosine diphosphate (ADP) ratio primarily induced by oxidative phosphorylation closes ATP-sensitive K+ channels, which subsequently causes membrane depolarization and opening of voltage-dependent Ca2+ channels, leading to increased cytosolic [Ca2+]i. Epicatechin (EC) can activate G-protein coupled receptor (GPR) 40 to further increase cytosolic [Ca2+]i. The increased [Ca2+]i activates Ca2+/calmodulin-dependent protein kinase II (CaMKII), which serves as the triggering signal in glucose-induced insulin secretion and increases extracellular signal-regulated kinase (ERK) phosphorylation. The increased [Ca2+]i also leads to activation of Ca2+-dependent adenylate cyclase, which increases the cyclic adenosine monophosphate (cAMP) level, leading to protein kinase A (PKA) activation. Activated PKA then prevents dephosphorylation of CaMKII by inhibiting protein phosphatase–1 (PP1), which specifically dephosphorylates CaMKII.
Our results are consistent with the hypothesis that GPR40 plays a role in EC effects on insulin secretion. The GPR40 antagonist GW1100 was able to block EC-enhanced GSIS in SFA-treated cells. However, CaMKII phosphorylation was not significantly reduced by GW1100, although a trend was observed. Further confirmation of whether CaMKII phosphorylation is modulated by GPR40 activation is required. The effect of EC on GPR40 is also likely related to its conformational structure (Figure 1). In addition to the presence of hydroxyl groups on the aromatic ring, Ramirez-Sanchez et al report that catechin, a stereoisomer of EC, fails to mimic the ability of EC to induce NO synthesis in human coronary artery endothelial cells, which is mediated by endothelial nitric oxide synthase (eNOS) activation via the CaMKII pathway14. Furthermore, the substrates of CaMKII in β-cells include microtubule-associated protein 2 (MAP-2) and synapsin I25. Therefore, it is conceivable that CaMKII may also play a role in the transport of insulin granules to exocytosis sites in β-cells. Whether EC treatment could affect MAP-2 and synapsin I via the CaMKII pathway to enhance insulin secretion is worthy of exploration. In addition, genetic knockdown of GRP40 in INS-1 cells will be of interest to further confirm GRP40's role in mediating the effect of EC.
Another interesting finding is the differences in the effects of EC on ROS reduction at low and high concentrations. We found that at 30 μmol/L, EC was effective in inhibiting ROS production induced by high glucose and acute exposure to H2O2, which was similar to the action of equimolar NAC. Neither EC nor NAC at 0.3 μmol/L reversed the increase in ROS. Pancreatic β-cells are highly sensitive to ROS mainly due to their low expression and activity of antioxidant enzymes47. Glucose stimulation results in endogenous H2O2 accumulation in β-cells, which is thought to be necessary for insulin secretion48, while excessive and/or sustained ROS production causes oxidative stress and leads to deterioration of β-cell function23,49. When applying high glucose stimulation or H2O2 exposure in β-cells, intracellular ROS presumably increase only moderately above the physiological level. Therefore, it is possible that 30 μmol/L EC is capable of neutralizing increased ROS in our experimental settings, even though the suggested concentration of EC as an antioxidant is at least 10 times higher than 30 μmol/L10. Indeed, Martín et al reported that 5–20 μmol/L EC is effective in reducing ROS induced by 50 μmol/L t-BOOH in INS-1E cells13. Overall, with EC and NAC showing similar effects, we assume that the inhibitory effect on ROS production observed after high EC exposure is due to its direct antioxidant ability.
On the other hand, 30 μmol/L EC was unsuccessful in enhancing SFA-impaired insulin secretion, which contradicts our initial hypothesis that 30 μmol/L EC may reduce oxidative stress to promote insulin secretion from β-cells and suggests that EC may exert other effects in β-cells besides serving as an antioxidant. Possibly, EC inhibits protein phosphorylation at 30 μmol/L, as suggested by Schroeter et al50. Consequently, SFA-impaired CaMKII activation (phosphorylation) would not be rescued by 30 μmol/L EC to improve GSIS.
EC treatment also promotes pancreatic β-cell regeneration in diabetic animal models in vivo20,51. Martín et al found that EC from cocoa increases the viability of t-BOOH-treated INS-1E cells13. In contrast to their findings, this study found that EC at 0.3 μmol/L did not enhance the viability of INS-1 cells treated with 0.4 mmol/L SFA (data not shown). This is not surprising because compared with their cell model induced by limited 2-h 50 μmol/L t-BOOH treatment, we applied SFA incubation for 24 h to impair cells, which has multiple deleterious effects, including elevated ROS, disrupted lipid metabolism, mitochondrial dysfunction, altered β-cell gene expression and signaling, impaired insulin secretion, upregulated inflammatory responses and apoptosis32,52. In addition, we adopted a much lower but physiologically relevant concentration of EC at 0.3 μmol/L, whereas their effective concentrations of EC ranged from 5–20 μmol/L, which is 20–60-times higher. Most importantly, the improved GSIS reported by Martín et al could be due to increased cell numbers. In contrast, in this study, the insulin content was not changed by either SFA or EC treatment. Therefore, we suggest that 0.3 μmol/L EC potentiated insulin secretion by modulating cellular signaling rather than enhancing cell viability.
In summary, our findings support that EC at a physiological concentration (0.3 μmol/L) can act as a signaling molecule to stimulate insulin secretion via activation of CaMKII, possibly through the GPR40 receptor. EC at 30 μmol/L has similar effects to those of NAC on ROS reduction, but with no effect on the promotion of insulin secretion. Due to its varied actions at different concentrations, application of EC as a nutraceutical would have to be performed with careful evaluation of the dose according to specific conditions. Meanwhile, further investigation is needed to characterize the signaling pathways of EC in β-cells.
Abbreviation
EC: epicatechin; CaMKII: calmodulin-dependent protein kinase II; GPR40: G-protein coupled receptor 40; SFA: saturated fatty acid; ROS: reactive oxygen species; GSIS: glucose-stimulated insulin secretion; t-BOOH: tert-butylhydroperoxide; cAMP: cyclic adenosine monophosphate; DCFDA: 2′,7′–dichlorofluorescin diacetate; ERK: extracellular regulated kinases; PKA: protein kinase A; NO: nitric oxide.
Author contribution
Catherine B CHAN and Kaiyuan YANG conceived and designed the study; Kaiyuan YANG performed the experiments, analyzed the data, and drafted the article; Kaiyuan YANG and Catherine B CHAN interpreted the data and revised and approved the final version to be published. Catherine B CHAN is the guarantor of this work, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
This work was supported by an operating grant from Alberta Innovates BioSolutions and the Alberta Pulse Growers Commission. Neither funder placed any restrictions on publication of the present study. K YANG received stipend support from China Scholarship Council.
The authors would like to thank Ms A CLOSE, Mr K SUZUKI, and Mr K TAO for technical support.
References
- Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res 2008; 52: 79–104. [DOI] [PubMed] [Google Scholar]
- Guyot S. Flavan-3-Ols and Proanthocyanidins. In: Nollet LML, and Toldrá F, editors. Handbook of analysis of active compounds in functional foods. CRC Press; 2012. p317–48.
- Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signal 2001; 3: 957–67. [DOI] [PubMed] [Google Scholar]
- Clifford MN, van der Hooft JJ, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr 2013; 98: 1619S–1630S. [DOI] [PubMed] [Google Scholar]
- Roowi S, Stalmach A, Mullen W, Lean MEJ, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem 2010; 58: 1296–304. [DOI] [PubMed] [Google Scholar]
- Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, et al. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr 2005; 94: 170–81. [DOI] [PubMed] [Google Scholar]
- Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr 2008; 138: 1535S–1542S. [DOI] [PubMed] [Google Scholar]
- Serra A, Macià A. Romero M-P, Anglès N, Morelló JR, Motilva MJ. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct 2011; 2: 562–8. [DOI] [PubMed] [Google Scholar]
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 2002; 33: 1693–702. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res 2000; 33: 819–30. [DOI] [PubMed] [Google Scholar]
- Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, et al. Brain-targeted proanthocyanidin metabolites for Alzheimer's disease treatment. J Neurosci 2012; 32: 5144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabró V, Piotrkowski B, Fischerman L, Vazquez Prieto MA, Galleano M, Fraga CG. Modifications in nitric oxide and superoxide anion metabolism induced by fructose overload in rat heart are prevented by (−)-epicatechin. Food Funct 2016; 7: 1876–83. [DOI] [PubMed] [Google Scholar]
- Martín MÁ, Fernández-Millán E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol Nutr Food Res 2014; 58: 447–56. [DOI] [PubMed] [Google Scholar]
- Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (-)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 2010; 55: 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Ulloa A, Romero-Perez D, Villarreal F, Ceballos G, Ramirez-Sanchez I. Cell membrane mediated (-)-epicatechin effects on upstream endothelial cell signaling: Evidence for a surface receptor. Bioorg Med Chem Lett 2014; 24: 2749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Ulloa A, Mendez-Luna D, Beltran-Partida E, Castillo C, Guevara G, Ramirez-Sanchez I, et al. The effects of (−)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol Res 2015; 100: 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Tani T, Terahara N, Tsuda T. The anthocyanin delphinidin 3-rutinoside stimulates glucagon-like peptide-1 secretion in murine GLUTag cell line via the Ca2+/calmodulin-dependent kinase II pathway. PLoS One 2015; 10: e0126157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemler JA, Lock LT, Koffas MAG, Tzanakakis ES. Standardized biosynthesis of flavan-3-ols with effects on pancreatic beta-cell insulin secretion. Appl Microbiol Biotechnol 2007; 77: 797–807. [DOI] [PubMed] [Google Scholar]
- Hii CS, Howell SL. Effects of epicatechin on rat islets of Langerhans. Diabetes 1984; 33: 291–6. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Ryu GR. Chung J-S, Sim SS, Min DS, Rhie DJ, et al. Protective effects of epicatechin against the toxic effects of streptozotocin on rat pancreatic islets: in vivo and in vitro. Pancreas 2003; 26: 292–9. [DOI] [PubMed] [Google Scholar]
- Dadi PK, Vierra NC, Ustione A, Piston DW, Colbran RJ, Jacobson D A. Inhibition of pancreatic β-cell Ca2+/calmodulin-dependent protein kinase ii reduces glucose-stimulated calcium influx and insulin secretion, impairing glucose tolerance. J Biol Chem 2014; 289: 12435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hashemi Z, Han W, Jin A, Yang H, Ozga J, et al. Hydrolysis enhances bioavailability of proanthocyanidin-derived metabolites and improves β-cell function in glucose intolerant rats. J Nutr Biochem 2015; 26: 850–9. [DOI] [PubMed] [Google Scholar]
- Maechler P. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem 1999; 274: 27905–13. [DOI] [PubMed] [Google Scholar]
- Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell Metab 2009; 10: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom RA. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 1999; 48: 675–84. [DOI] [PubMed] [Google Scholar]
- Liu X, Wei J, Tan F, Zhou S, Würthwein G, Rohdewald P. Antidiabetic effect of Pycnogenol French maritime pine bark extract in patients with diabetes type II. Life Sci 2004; 75: 2505–13. [DOI] [PubMed] [Google Scholar]
- Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003; 26: 3215–8. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zhang Z, Dai X, Jiang Y, Bao L, Li Y, et al. Grape seed proanthocyanidins ameliorate pancreatic beta-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stress. Nutr Metab (Lond) 2013; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Millán E, Cordero-Herrera I, Ramos S, Escrivá F, Alvarez C, Goya L, et al. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol Nutr Food Res 2015; 59: 820–4. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 2004; 36: 838–49. [DOI] [PubMed] [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997; 46: 3–10. [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002; 32 Suppl 3: 14–23. [DOI] [PubMed] [Google Scholar]
- Ayvaz G, Balos Törüner F, Karakoç A, Yetkin I, Cakir N, Arslan M. Acute and chronic effects of different concentrations of free fatty acids on the insulin secreting function of islets. Diabetes Metab 2002; 28: 3S7–S12; discussion 3S108–S112. [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 2003; 144: 4154–63. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol 1995; 57: 417–45. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ramanadham S, Ma ZA, Bao S, Mancuso DJ, Gross RW, et al. Group VIA phospholipase A2 forms a signaling complex with the calcium/calmodulin-dependent protein kinase IIbeta expressed in pancreatic islet beta-cells. J Biol Chem 2005; 280: 6840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki I, Okazaki K, Saitoh M, Niki A, Niki H, Tamagawa T, et al. Presence and possible involvement of Ca2+/calmodulin-dependent protein kinases in insulin release from the rat pancreatic beta cell. Biochem Biophys Res Commun 1993; 191: 255–61. [DOI] [PubMed] [Google Scholar]
- Tabuchi H, Yamamoto H, Matsumoto K, Ebihara K, Takeuchi Y, Fukunaga K, et al. Regulation of insulin secretion by overexpression of Ca2+/calmodulin-dependent protein kinase II in insulinoma MIN6 cells. Endocrinology 2000; 141: 2350–60. [DOI] [PubMed] [Google Scholar]
- Easom RA, Filler NR, Ings EM, Tarpley J, Landt M. Correlation of the activation of Ca2+/calmodulin-dependent protein kinase II with the initiation of insulin secretion from perifused pancreatic islets. Endocrinology 1997; 138: 2359–64. [DOI] [PubMed] [Google Scholar]
- Watson ML, Macrae K, Marley AE, Hundal HS. Chronic effects of palmitate overload on nutrient-induced insulin secretion and autocrine signalling in pancreatic MIN6 beta cells. PLoS One 2011; 6: e25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol 2011; 7: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaihara KA, Dickson LM, Jacobson DA, Tamarina N, Roe MW, Philipson LH, et al. β-cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes 2013; 62: 1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munton RP, Vizi S, Mansuy IM. The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett 2004; 567: 121–8. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, et al. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 1998; 280: 1940–2. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 2002; 3: 175–90. [DOI] [PubMed] [Google Scholar]
- Persaud SJ, Liu B, Sampaio HB, Jones PM, Muller DS. Calcium/calmodulin-dependent kinase IV controls glucose-induced Irs2 expression in mouse beta cells via activation of cAMP response element-binding protein. Diabetologia 2011; 54: 1109–20. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996; 20: 463–6. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007; 56: 1783–91. [DOI] [PubMed] [Google Scholar]
- Li N, Brun T, Cnop M. Cunha D a, Eizirik DL, Maechler P. Transient oxidative stress damages mitochondrial machinery inducing persistent β-cell dysfunction. J Biol Chem 2009; 284: 23602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Bahia P, Spencer JPE, Sheppard O, Rattray M, Cadenas E, et al. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J Neurochem 2007; 101: 1596–606. [DOI] [PubMed] [Google Scholar]
- Cordero-Herrera I. Martín MÁ, Fernández-Millán E, Álvarez C, Goya L, Ramos S. Cocoa and cocoa flavanol epicatechin improve hepatic lipid metabolism in in vivo and in vitro models. Role of PKCζ. J Funct Foods 2015; 17: 761–73. [Google Scholar]
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002; 51: 7–18. [DOI] [PubMed] [Google Scholar]