Abstract
Reactive nitrogen species (RNS) play important roles in mediating cerebral ischemia-reperfusion injury. RNS activate multiple signaling pathways and participate in different cellular events in cerebral ischemia-reperfusion injury. Recent studies have indicated that caveolin-1 and matrix metalloproteinase (MMP) are important signaling molecules in the pathological process of ischemic brain injury. During cerebral ischemia-reperfusion, the production of nitric oxide (NO) and peroxynitrite (ONOO−), two representative RNS, down-regulates the expression of caveolin-1 (Cav-1) and, in turn, further activates nitric oxide synthase (NOS) to promote RNS generation. The increased RNS further induce MMP activation and mediate disruption of the blood-brain barrier (BBB), aggravating the brain damage in cerebral ischemia-reperfusion injury. Therefore, the feedback interaction among RNS/Cav-1/MMPs provides an amplified mechanism for aggravating ischemic brain damage during cerebral ischemia-reperfusion injury. Targeting the RNS/Cav-1/MMP pathway could be a promising therapeutic strategy for protecting against cerebral ischemia-reperfusion injury. In this mini-review article, we highlight the important role of the RNS/Cav-1/MMP signaling cascades in ischemic stroke injury and review the current progress of studies seeking therapeutic compounds targeting the RNS/Cav-1/MMP signaling cascades to attenuate cerebral ischemia-reperfusion injury. Several representative natural compounds, including calycosin-7-O-β-D-glucoside, baicalin, Momordica charantia polysaccharide (MCP), chlorogenic acid, lutein and lycopene, have shown potential for targeting the RNS/Cav-1/MMP signaling pathway to protect the brain in ischemic stroke. Therefore, the RNS/Cav-1/MMP pathway is an important therapeutic target in ischemic stroke treatment.
Keywords: Ischemic stroke, caveolin-1, reactive nitrogen species (RNS), MMPs, natural compound
Pathophysiology of acute ischemic stroke
Stroke is one of the most prevalent diseases with high mortality and disability all over the world1. Ischemic stroke and hemorrhage stroke are two major subtypes, among which ischemic stroke accounts for more than 80 percent of stroke incidences2. Currently, tissue plasminogen activator (t-PA) is the only FDA approved drug for ischemic stroke, and its efficacy is limited by the restrictive golden time window of 4.5 h3 with the potential risk of hemorrhagic transformation when given beyond this time window4,5. The development of novel therapeutic agents has become timely and important for improving the outcome of ischemic stroke treatment.
Ischemic stroke involves different pathophysiological cascades, including energy failure, oxidative stress, acidosis, disruption of ion homeostasis, calcium overload, neuronal cell excitotoxicity, inflammation, etc6,7,8,9,10,11. In ischemic stroke, the obstruction of blood flow dramatically reduces glucose and oxygen supply in ischemic brain region and triggers “ischemic cascades”12,13. Lack of ATP synthesis with low oxygen supply leads to accumulation of lactate and malfunction of ion pumps, including the Na+/K+-ATPase and Ca2+/H-ATPase14, subsequently inducing membrane depolarization and calcium ion (Ca2+) overload15. In the meantime, membrane depolarization causes the release of excitotoxic amino acids, especially leading to glutamate translocation into the extracellular compartment. Glutamate can act to induce neurotoxicity, activate glutamate receptors and promote the influx of Ca2+16. A substantial elevation in intracellular Ca2+ activates various calcium-dependent enzymes, including protein kinase C, phospholipase A2, phospholipase C, cyclooxygenase, calcium-dependent nitric oxide synthase (NOS), calpain, proteases and endonucleases, resulting in necrotic and apoptotic cell death17,18. Inflammation is another important process of cell death in ischemic stroke19. Ischemic cascades activate resident microglia and astrocytes, together with infiltrated T lymphocytes, neutrophils, and macrophages, subsequently inducing the release of multiple inflammatory factors such as cytokines, chemokines, enzymes and free radicals19,20. Therefore, ischemic stroke is a complicated pathophysiological process involving the activation of regulatory networks in response to stroke.
Free radicals are considered to be important players in ischemic stroke, particularly in cerebral ischemia-reperfusion injury. There are two species of free radicals including reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS are comprised of superoxide, hydroxyl radical, singlet oxygen, hydrogen peroxide, etc. ROS at a low concentration serve as redox signaling molecules to maintain biological functions under physiological conditions, whereas large amounts of ROS produced from ischemic brains exacerbate brain injury through different mechanisms21,22. For example, ROS enhance inflammatory responses by activating adhesion molecules and promoting leucocyte infiltration23. ROS induce the release of glutamate and calcium overload24. ROS activate inflammation factors, mediate lipid peroxidation, and induce neural cell death, disrupting the integrity of the blood-brain barrier (BBB) and enlarging the infarction volume22. As representative antioxidants, edaravone, NXY-059, and allopurinol improved outcomes in acute ischemic stroke patients25,26,27. Hence, free radicals aggravate the brain damage in ischemic stroke, and antioxidants may be beneficial in ischemic stroke treatment.
While ROS-mediated ischemic brain injury has been intensively investigated, the roles of RNS remain relatively unexplored. RNS, including NO and ONOO−, mediate the BBB disruption, infarction enlargement and apoptotic cell death in cerebral ischemia-reperfusion injury28. RNS-mediated matrix metalloproteinase (MMP) activation is one of the critical pathological processes in cerebral ischemia-reperfusion injury29,30. MMP-9 has been used as a biomarker for monitoring brain damage and predicting hemorrhagic transformation in thrombolytic treatment for ischemic stroke31. NG-nitro-L-arginine methyl ester (L-NAME), a nonselective NOS inhibitor, significantly reduced the BBB breakdown and MMP-9 activity in a middle cerebral artery occlusion (MCAO) animal model32. In the past decade, we have made great efforts in the exploration of the roles of RNS in cerebral ischemia-reperfusion injury. In this mini-review, we mainly focus on the roles of RNS/caveolin-1/MMP signaling cascades in acute ischemic brain injury. Subsequently, we review the potential natural compounds targeting RNS/caveolin-1/MMP signaling pathways for ameliorating cerebral ischemia-reperfusion injury.
Detection of NO and ONOO− in cerebral ischemia-reperfusion injury
As representative RNS, NO and ONOO− are produced at both the ischemia and reperfusion stages in cerebral ischemia-reperfusion injury. A low concentration of NO produced from endothelial nitric oxide synthase (eNOS) has physiological functions, whereas a high concentration of NO produced from inducible NOS (iNOS) and neuronal NOS (nNOS) is detrimental to the ischemic brain28. When NO and superoxide (O2−) are simultaneously produced in the ischemic brain, they rapidly react with each other to produce ONOO− at a diffusion-limited rate28. By using electron paramagnetic resonance (EPR) spin trapping technology, we monitored the production of NO in a rat MCAO model and found that cerebral ischemia-reperfusion resulted in a biphasic increase in NO production in the ischemic core and penumbra, with first-phase NO production at the ischemic phase and the second-phase increase of NO at the reperfusion stage33. Notably, a large amount of superoxide (O2−) was generated from neurons and endothelial cells by activating NADPH oxidase34, xanthine oxidase35, and cyclooxygenase (COX)36,37. The reaction of O2− and NO rapidly forms ONOO−. Peroxynitrite induces protein tyrosine nitration by the addition of a nitro group to the hydroxyl group of the tyrosine residue to form 3-nitrotyrosine (3-NT), a footprint marker for ONOO− production38. The production of NO from iNOS and nNOS appears to be important for ONOO− formation as iNOS or nNOS knockout mice did not show nitrotyrosine-positive staining39,40. Due to the concerns of the sensitivity and specialty of 3-NT for ONOO−41, we have made great efforts to develop novel specific and sensitive probes for ONOO− detection42,43,44. With ONOO− probes, we directly visualized the ONOO−-induced fluorescence in ischemic brains in vivo as well as hypoxic neurons in vitro. Our results suggest that targeting ONOO− could be an important strategy not only for attenuating cerebral ischemia-reperfusion injury45,46 but also for reducing hepatic ischemia-reperfusion injury47.
Roles of peroxynitrite in cerebral ischemia-reperfusion injury
Peroxynitrite has a much higher cytotoxicity than NO and O2−. Peroxynitrite has an approximately 400 times higher penetrating capacity across the lipid membrane than superoxide anions48,49. Peroxynitrite penetrates the lipid bilayers of the membrane and induces DNA damage50, protein nitration51,52, and lipid peroxidation53, as well as enzyme and ion channel inactivation54,55. Peroxynitrite, rather than NO, directly mediated poly(ADP-ribose) synthase (PARS) activation and suppressed cellular viability56. The peroxynitrite decomposition catalyst FeTMPyP reduced brain infarct volume, inhibited neuronal cell death in the Cornu Ammonis 1 (CA1) region of the hippocampus and improved functional outcomes57,58. Our recent study showed that FeTMPyP significantly reduced the ONOO− level in ischemic brains and attenuated neuronal apoptosis59. Uric acid, an ONOO− scavenger, rescued over 70 percent of the ischemic cortex and striatum60. In addition to neuronal injury, cerebrovascular injury was also induced by peroxynitrite. The ONOO− donor 3-morpholino sydnonimine (SIN-1) further reduced the expression of tight junction protein ZO-1 and exacerbated BBB disruption in a cerebral ischemia-reperfusion animal model61. Intravenous administration of FeTMPyP significantly reduced neurovascular injury in a prolonged brain ischemia model62. In clinical studies, a high level of uric acid in the blood was correlated with excellent outcomes in stroke patients63,64. A meta-analysis involving 10 studies with 8131 ischemic stroke patients also showed a positive correlation of serum uric acid with good neurological outcomes65. In addition, the plasma 3-NT level was positively correlated with the magnitude of the brain injury among ischemic stroke patients66. Together, these works indicate that ONOO− could be an important target for ischemic stroke.
Interaction of RNS and MMPs in ischemic brain injury
MMPs are proteolytic enzymes that are capable of disrupting the extracellular matrix (ECM) to mediate ischemic brain injuries67,68. MMPs have a common configuration that includes a zinc-dependent catalytic site, propeptide cysteine switch and other entities, such as a transmembrane domain, fibronectin-binding site and so on69. MMP-9 and MMP-2 are two well-known MMPs that contribute to cerebral ischemia-reperfusion injury. The basal level of MMPs in the adult brain is low, but ischemic insults trigger acute activation of several MMPs70,71,72. Stroke patients have a significantly higher serum level of MMP-2 and MMP-9 than healthy controls73. Tissue plasminogen activator (t-PA) treatment further enhanced the serum MMP-9 level73. Neutrophils and microvessels are major sources of MMP-9 activation and contribute to hemorrhagic transformation in the presence or absence of t-PA during ischemic stroke74,75. Inhibition of MMPs protected against the sustained loss of tight junction proteins such as claudin-5 and occludin in rodent MCAO models30,76,77. Broad-spectrum and specific MMP-9 inhibitors notably attenuated hippocampal neuronal damage in a transient global cerebral ischemia model78,79. The MMP-9-neutralizing antibody greatly decreased infarction size in ischemic brain injury80. MMP-9 KO mice showed a smaller lesion volume than wild-type mice after cerebral ischemia81. These results together indicate that MMP-9 plays an important role in mediating cerebral ischemia-reperfusion injury.
RNS activate MMPs during ischemic brain injury82. Peroxynitrite was co-localized with MMP-9 in brain microvessels of the area showing Evans blue leakage, suggesting that ONOO− may induce MMP-9 activation and contribute to BBB disruption32. The nonselective NOS inhibitor N(omega)-nitro-L-arginine (L-NA) reduced the 3-NT level and attenuated MMP-9 expression and EB extravasation during cerebral ischemia-reperfusion83. Consistently, S-nitrosoglutathione (GSNO) inhibited MMP-9 activation, up-regulated the expression of tight junction protein ZO-1, and ameliorated BBB leakage in ischemic brains61. Intravenous administration of FeTMPyP at the reperfusion stage significantly reduced MMP-9 and MMP-2 expression in ischemic brains62. Our recent study showed that ONOO−-mediated MMP-9 activation contributed to hemorrhagic transformation (HT) in a rodent ischemic stroke model with delayed tissue plasminogen activator (t-PA) treatment84. Delayed t-PA treatment beyond 4.5 h after MCAO ischemia significantly up-regulated the expression of 3-NT and MMP-9 and aggravated HT in the ischemic brain area. FeTMPyP treatment significantly down-regulated MMP-9 activity, attenuated HT and improved the neurological outcomes84. Furthermore, other studies have shown that ONOO− production mediates the activation of purified human proMMP-1, -8, and -9 in the presence of similar concentrations of GSH via S-nitrosoglutathione85. Peroxynitrite activated MMP-2 in the presence of glutathione by modifying the cysteine residue in the auto-inhibitory domain of the zymogen86. Taken together, ONOO−-mediated MMP activation plays crucial roles in BBB damage and hemorrhagic transformation during cerebral ischemia-reperfusion injury.
Role of caveolin-1 in acute ischemic brain injury
Caveolae are flask-shaped lipid rafts in the cell membrane, ranging from 50 to 100 nm in size, that regulate transport and cell signaling. Caveolins, which are 19–22-kDa integral membrane proteins located at caveolae, are abundant in adipocytes, endothelial cells, and fibroblasts and are critical for caveolae formation87,88,89,90,91,92,93. Caveolins have three subtypes including caveolin-1, -2, and -3, with an NH2-terminal membrane attachment domain (N-MAD, Residues 82–101) and COOH-terminal membrane attachment domain (C-MAD, residues 135–150) that binds to membranes with high affinity94,95,96.
Caveolin-1 (Cav-1) binds to all isoforms of NOS via the Cav-binding motif and inhibits NOS activity97,98,99,100. Caveolin-1 has two cytoplasmic domains including the scaffolding domain (amino acids 61-101) and C-terminal tail (amino acids 135-178), which are able to bind with eNOS. Peptides derived from the scaffolding domains of Cav-1 and Cav-3 inhibited eNOS, iNOS and nNOS activities101 and subsequently reduced NO production in blood vessels and endothelial cells99. Overexpression of Cav-1 significantly attenuated eNOS enzyme activity in endothelial cells102,103. Loss of Cav-1 persistently activated eNOS both in mice and human subjects104. Under a transient MCAO ischemia-reperfusion condition, Cav-1 KO mice had a larger infarction volume than wild-type mice105. Interestingly, Cav-1 expression was significantly down-regulated in ischemic brains during cerebral ischemia-reperfusion injury compared to that in control brains. NOS inhibitors including L-NAME, N6-(1-iminoethyl)-lysine (NIL) and 7-NI all prevented the loss of Cav-1 in ischemic brains33, indicating that NO down-regulates Cav-1 in ischemic brain injury. The interaction between NO and Cav-1 forms a positive feedback loop for the regulation of NO production in cerebral ischemia-reperfusion injury. Notably, the roles of NO in the modulation of Cav-1 expression appear to be different in neuroblastoma cells. An NO donor up-regulated the expression of Cav-1, while both the non-selective NOS inhibitor L-NAME and iNOS inhibitor 1400W abolished the induction of Cav-1 in neuroblastoma SK-N-MC cells. Increased Cav-1 expression may be an adaptive mechanism in neuroblastoma cells in response to hypoxic stimulation106. Consistent results have also been found in lung cancer cells107. Thus, the interaction of Cav-1 and NO could be an important cellular signaling pathway to cope with different pathological processes whose defensive or detrimental effects might be related to cell types and pathological conditions.
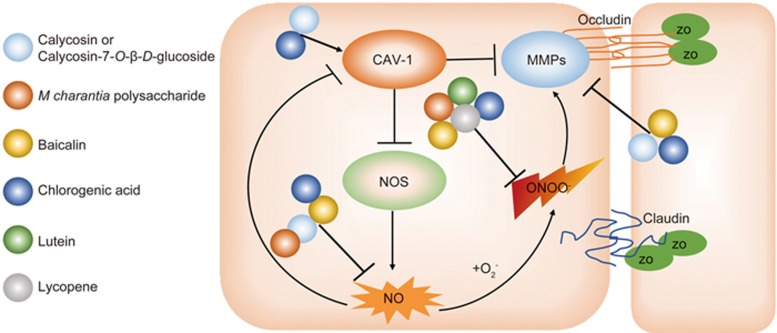
The interaction of Cav-1 and NO impacts BBB permeability through modulation of MMP activation in cerebral ischemia-reperfusion injury108. Cav-1 was co-localized with MMP-2 on the surface of endothelial cells109,110. NO modulated the expression and distribution of Cav-1 and MMP-9 at the endothelial cell/tumor cell interface111. Treatment of Cav-1 peptide protected BBB integrity from chemokine-induced damage as evidenced by the up-regulation of TJ and adherent junction proteins in BMECs in vitro112. Cav-1 KO mice had increased eNOS activity and NO production in endothelial cells along with endothelial hyper-permeability compared to wild-type mice113. To explore the roles of Cav-1 in the regulation of BBB permeability, we compared the activities and expression of MMPs and the BBB permeability in a mouse MCAO model. After wild-type mice were subjected to cerebral ischemia-reperfusion injury, Cav-1 expression was down-regulated, accompanied with increased MMP-2 and -9 activities, decreased ZO-1 expression and enhanced BBB permeability in ischemic brains. The roles of Cav-1 in the modulation of MMPs and BBB permeability were further confirmed by using Cav-1 KO mice in vivo and Cav-1 RNAi brain microvascular endothelial cells (BMECs) in vitro. Knockout or knockdown of Cav-1 aggravated the BBB permeability and cell damage. Furthermore, L-NAME treatment partly inhibited MMP activation and protected the BBB integrity in Cav-1 KO mice114]. The results suggest that NO production directly contributes to MMP activation and BBB disruption even without Cav-1 involvement. Cav-1 only partly contributes to the BBB damage. Similar results have also been reported by others115,116. Lentiviral-mediated re-expression of Cav-1 inhibited MMP activation, protected TJ protein expression and decreased brain edema in Cav-1 KO mice115. Thus, we conclude that the NO/Cav-1/MMP signaling cascades play critical roles in mediating BBB damage during cerebral ischemia-reperfusion injury114. In addition, peroxynitrite also affected Cav-1 expression in endothelial cells. The expression of 3-NT was co-localized with Cav-1 in the endothelial cells of the diabetes mellitus (DM) patients, and exogenous peroxynitrite decreased the caveolae structure and Cav-1 expression, which led to NOS uncoupling117. Interestingly, similar results were also found in hepatic ischemia/reperfusion injury, showing Cav-1 KO mice have more 3-NT expression in liver tissues than wild-type mice47. Therefore, the interaction of RNS and Cav-1 may be an important cellular signaling pathway in both cerebral and hepatic ischemia-reperfusion injury47,108,114,116,118. However, controversial results in different neurological disease models have also been reported. In a rat cortical cold-injury model, increased Cav-1 expression and phosphorylation were co-localized with decreased occludin and claudin-5 expression in the brain area with increased BBB permeability119. Expression of phosphorylated Cav-1 was increased in endothelial cells after cortical cold injury, which was associated with BBB disruption and edema in brain injury120. The exact mechanisms and explanations for those controversial results are unclear. Since those studies only presented a phenomenon in which increased Cav-1 expression co-existed with BBB disruption in the rat cortical cold-injury model, further investigations should be conducted for the proof-of-concept of the roles of Cav-1 in BBB permeability. Recently, we stepped forward to investigate the roles of Cav-1 in the modulation of the BBB permeability in neuroinflammation diseases by using a laboratory murine model of experimental autoimmune encephalomyelitis for mimicking multiple sclerosis. Increased expression of Cav-1 in the serum and spinal cord was associated with disease incidence and severity in wild-type mice with active encephalomyelitis. After immunization, Cav-1 KO mice showed a remarkably lower disease incidence and fewer clinical symptoms than wild-type littermates. The Cav-1 KO mice also had fewer encephalitogenic T cells trafficking into the CNS and decreased expression of adhesion molecules ICAM-1 and VCAM-1 within the lesions. Thus, we concluded that Cav-1 could mediate CNS-directed lymphocyte trafficking across the BBB via interacting with adhesion molecules ICAM-1 and VCAM-1, subsequently aggravating neuroinflammation and degeneration in EAE pathology121,122. The above results indicate that Cav-1 has different functions in different neurological diseases. Particularly for ischemic brain injury, we concluded that the interaction of RNS, Cav-1 and MMPs could form a positive feedback loop, amplifying the impact of RNS in BBB disruption and ischemic brain injury (Figure 1). Therefore, targeting the RNS/Cav-1/MMP pathway is a promising therapeutic strategy for protecting against cerebral ischemia-reperfusion injury28,108,123.
Figure 1.
Schematic illustrating the involvement of RNS/caveolin-1/MMPs in mediating the ischemic brain injury and the natural compounds regulating related targets. Upon cerebral ischemia, reduction of caveolin-1 (CAV-1) activates nitric oxide synthase (NOS) and overproduces NO. NO is accumulated and down-regulates CAV-1 expression in ischemic brains, which further activates NOS and forms a feedback interaction to amplify the detrimental signals. MMPs activity is negatively regulated by CAV-1, and loss of CAV-1 leads to higher activity of MMPs. In addition, NO reacts with O2− to generate ONOO−. ONOO− is highly toxic and could also activate MMPs. Active MMPs cleave tight junction proteins, including occludin, claudin, and ZO-1, leading to blood-brain barrier damage. Calycosin or Calycosin-7-O-β-D-glucoside targets on CAV-1 (Ref 125), NO (Ref 124,125,130), and MMPs (Ref 125,127,128); M charantia polysaccharide targets on NO (Ref 45) and ONOO− (Ref 45); Baicalin targets on NO (Ref 150,151), ONOO− (Ref 46,59,151), and MMPs (Ref 46,152,153); Chlorogenic acid targets on CAV-1 (Ref 181), NO (Ref 173), ONOO− (Ref 168,169,170,171), and MMPs (172, 175, 176); lutein targets on ONOO− (Ref 182); lycopene targets on ONOO− (Ref 187,188,189). Ref, reference.
Natural active compounds targeting ONOO−/Cav-1/MMP-9 signaling pathway for neuroprotection in ischemic stroke
Based on abundant experience and accumulated histological evidence, Chinese herbal medicine has been used for the treatment of stroke in China for centuries. Herbal formulas or single herbs are great sources for drug discovery. Herein, we summarize the current progress in the exploration of active compounds from Chinese medicinal herbs that modulate the RNS/Cav-1/MMP signaling pathways and their implications for neuroprotection in the treatment of ischemic stroke.
Calycosin and calycosin-7-O-β-D-glucoside
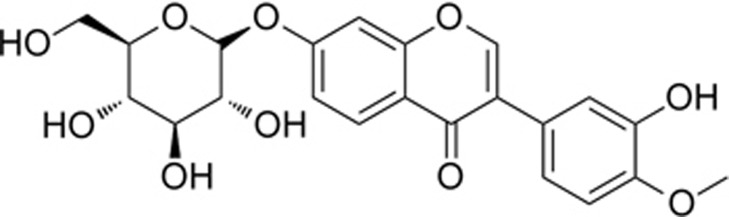
Calycosin and its glycoside form calycosin-7-O-β-D-glucoside (CG) are two representative isoflavones isolated from Astragali Radix, a medicinal herb used for ischemic stroke for hundreds of years in China124. The chemical structure of CG is shown in Figure 2. We investigated the neuroprotective effects of CG on modulating the NO/Cav-1/MMP signaling pathway and reducing infarction volume and BBB permeability in a rat MCAO cerebral ischemia-reperfusion model125. CG inhibited MMP activation, maintained the expression of Cav-1 and tight junction proteins, attenuated BBB disruption, reduced infarction volume and improved the neurological outcomes in cerebral ischemia-reperfusion injury125,126. Calycosin also demonstrated bioactivities of scavenging free radicals and inhibiting MMP-9 activity in other cellular or non-cellular systems127,128,129. Calycosin and calycosin-7-O-β-D-glucoside decreased the production of NO, O2−, and TNF-α in lipopolysaccharide (LPS)-stimulated microglial or RAW 264.7 macrophages124,130 and attenuated the neurotoxicity induced by various pathological factors including LPS, glutamate, mongholicus and xanthine (XA)/xanthine oxidase (XO)130,131,132. In addition, calycosin attenuated the permeability of human umbilical vein endothelial cells (HUVECs) under hypoxic conditions, possibly through inhibiting ROS production and preserving cytoskeleton structure133. These results suggest that the inhibition of the RNS/MMP-9 signaling pathway contributes to the neuroprotective and vascular protective effects of calycosin and CG. Nevertheless, other mechanisms could also account for the neuroprotective effects of calycosin and CG. For example, calycosin up-regulated transient receptor potential canonical 6 (TRPC6) and induced phosphorylation of CREB in ischemic brains134. Calycosin modulated the positive feedback of estrogen receptor ER-α and microRNA-375 in cerebral ischemia-reperfusion injury135. Calycosin was shown to act as a noncompetitive calcium channel blocker to prevent calcium overload136. CG activated the PI3K/Akt pathway and had neuroprotective effects in cerebral ischemia-reperfusion injury137. Therefore, calycosin and CG are able to modulate multiple signaling targets to exert their neuroprotective effects on ischemic brain injury. With better bioavailability than calycosin, CG has greater potential for further translational research125.
Figure 2.
Calycosin-7-O-β-D-glucoside.
Baicalin
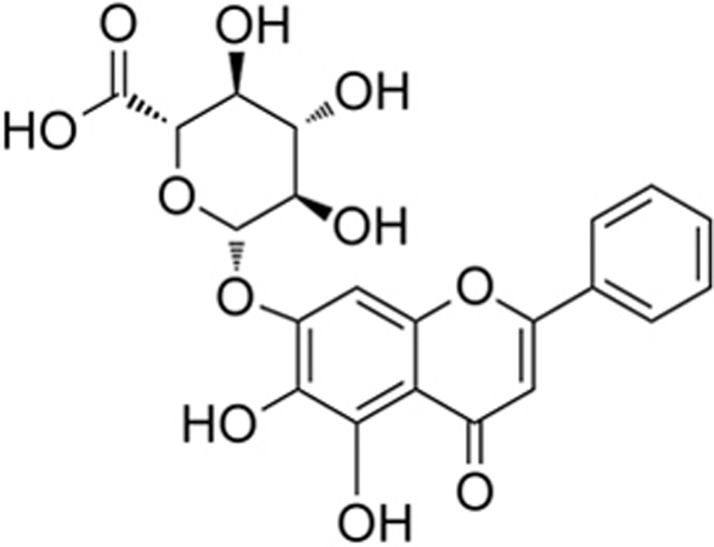
Baicalin is one of the major flavonoids isolated from the dried root of Scutellaria baicalensis, a medicinal herb used for ischemic stroke in China138. The chemical structure of baicalin is shown in Figure 3. Baicalin promoted neuronal differentiation of neural progenitor cells139,140. Baicalin reduced brain infarction volume, brain edema, BBB damage and brain inflammation in rodent ischemic stroke models138,141,142,143,144,145. Baicalin decreased BBB permeability and protected brain microvascular endothelial cells (BMVECs) in vivo and in vitro46,145,146 and reduced neurotoxicity under an OGD condition147,148,149. The neurovascular protective effects of baicalin may be attributed to its antioxidant effects138,150,151. By using mass spectrometry and EPR spin trapping experiments, we demonstrated the direct scavenging activities of baicalin on ONOO− and superoxide59. Baicalin inhibited the formation of 3-nitrotyrosine in ischemic brain tissues59. By using our newly developed peroxynitrite-specific probe, HK-Yellow AM, we directly visualized the production of ONOO− in ischemic brains. Baicalin also inhibited ONOO− production and reduced MMP-9 activity46, protected the expression of the tight junction protein occludin, and attenuated the BBB damage and brain edema in both permanent MCAO model and intracerebral hemorrhage model152,153. Notably, a proteomic study and gene microarray indicated that baicalin acted in a regulatory network to induce its neuroprotective effects against cerebral ischemic injury142,154. Further studies revealed that baicalin inhibited toll-like receptor 2/4 and NF-kB pathways143,151,155, reduced the phosphorylation of CaMKII156 and up-regulated AMPK alpha signaling157. Thus, baicalin is a good drug candidate for the treatment of stroke142,158,159.
Figure 3.
Baicalin.
M charantia polysaccharide (MCP)
M charantia polysaccharide (MCP) is one of the important bioactive components of Momordica charantia (MC), also named the bitter melon. MC has antioxidant and anti-hyperglycemic effects on cerebral ischemia-reperfusion injury in diabetic mice160. MCP showed its antioxidant effects through promoting endogenous antioxidant enzyme activities in a rat myocardial infarction model161,162. Our recent studies showed that MCP dose-dependently reduced infarction volume and attenuated neuronal apoptosis in animal models of four-vessel occlusion (4-VO) and MCAO. MCP had scavenging effects on NO and ONOO−, inhibited the release of cytochrome c from mitochondria and modulated the activation of the JNK3, c-Jun, and Fas-L signaling pathways in ischemic brains45. NO was reported to mediate the activation of JNK3 signaling via S-nitrosylation, and antioxidant N-acetylcysteine down-regulated JNK3 signaling and protected neurons from ischemic brain injury163,164. Thus, the neuroprotective effects of MCP may be attributed to inhibiting the free radical-mediated c-Jun N-terminal kinase 3 signaling pathway to protect against cerebral ischemia-reperfusion injury.
Chlorogenic acid
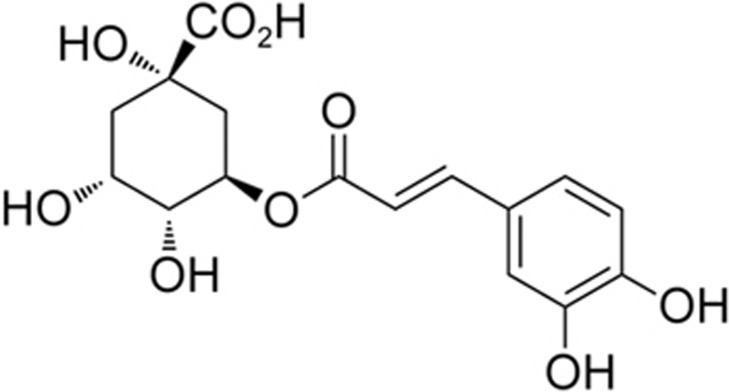
Chlorogenic acid (CGA) is a dietary phenylpropanoid molecule derived from a variety of natural products such as aubergine, blueberries and coffee165,166,167. The chemical structure of CGA is shown in Figure 4. CGA directly reacted with ONOO− with a rate constant of 1.6±0.7×105 M−1·s−1 and prevented DNA damage168,169,170,171. CGA is also a strong MMP-9 inhibitor with an IC50 of 30–50 nmol/L172. CGA inhibited the excess production of NO in LPS/gamma-interferon (IFN-gamma)-treated C6 astrocytes173. CGA improved the behavioral outcome in a rabbit small clot embolic stroke model174. CGA and its metabolite dihydrocaffeic acid (DHCA) inhibited MMP-2/9 activity, attenuated BBB damage and reduced brain infarction and brain edema175,176. CGA also exerted anti-inflammatory effects against cerebral ischemia-reperfusion injury177,178. CGA protected against glutamate-induced neurotoxicity in primary cultured cortical neurons179. CGA was shown to cross the BBB180. In an alcoholic liver injury model, CGA up-regulated the expression of Cav-1 and inhibited Stat3/iNOS signaling and hepatic lipid accumulation and peroxidation181. Thus, CGA could target the RNS/Cav-1/MMP signaling pathways to potentially protect the brain against ischemic stroke. Notably, CGA has synergistic effects with tissue plasminogen activator (t-PA), the only FDA-approved drug, in improving the neurological outcomes174. As a thrombolytic treatment, t-PA has a restrictive therapeutic time window within 4.5 h, and treatment beyond this time window increases the risk of hemorrhagic transformation (HT)67. BBB disruption is a critical process of delayed t-PA-induced HT, which involves ONOO− generation and MMP activation67,84. With the bioactivities of inhibiting ONOO− and MMPs, further study of the potential of CGA as an adjunct agent for protecting BBB integrity and preventing t-PA-mediated HT during thrombolytic treatment for ischemic stroke is valuable.
Figure 4.
Chlorogenic acid.
Other compounds
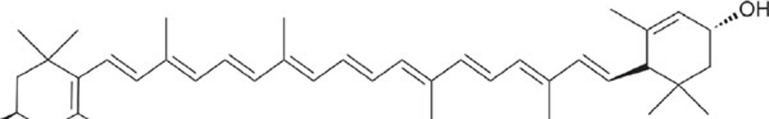
Other compounds such as lutein and lycopene may also target RNS to protect ischemic brains. For example, lutein (Figure 5), a xanthophyll rich in green leafy vegetables, directly reacted with peroxynitrite and nitrogen dioxide radicals182 and protected human neuroblastoma cells from DNA damage induced by peroxynitrite183. Lutein treatment ameliorated oxidative stress and inflammation, reduced brain infarction volume and protected against neuronal apoptosis in mouse MCAO models184,185. Interestingly, ischemic stroke patients with a poor early outcome showed significantly lower plasma lutein levels than those who remained functionally stable186. These results suggest that lutein may protect ischemic brains through its antioxidant effects. As lutein is a safe daily supplement for ocular health, its potential application for stroke treatment merits further study.
Figure 5.
Lutein.
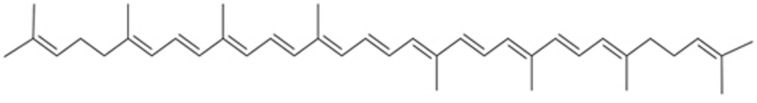
Lycopene (Figure 6) is another compound that has been shown to directly react with ONOO−. Lycopene prevented protein nitration and DNA damage in lung fibroblast cells187,188,189. Lycopene exerted antioxidative stress effects and inhibited neuronal apoptosis in rodent transient cerebral ischemia/reperfusion models190,191. A recent meta-analysis of 116 127 participants and 1989 cases showed that circulating lycopene was negatively associated with the risk of stroke192. Similarly, a prospective study also demonstrated the potential of lycopene for reducing the risk of stroke onset193. Hence, lycopene is valuable for further studies as a neuroprotective agent for ischemic stroke. In addition, other compounds, such as resveratrol, curcumin, apocynin, caffeic acid, and tanshinone IIA, have been noted as good candidates for inhibiting RNS-mediated brain damage in cerebral ischemia-reperfusion injury or ischemic brain injury. We have reviewed their values as potential therapeutic agents in our previous articles28,82,158. The details have been discussed before and should not be repeated here.
Figure 6.
Lycopene.
Discussion
Ischemic stroke is a major cause of death and long-lasting disability worldwide. Identifying new therapeutic targets is important for drug development to treat ischemic stroke. In this review, we have highlighted the important role of the RNS/Cav-1/MMP pathway in mediating cerebral ischemia-reperfusion injury (Figure 1). As shown in Figure 1, we have summarized the effects of several representative compounds targeting the RNS/Cav-1/MMP signaling pathway to demonstrate their neuroprotective mechanisms in ischemic stroke treatment.
The following points regarding studies on drug discovery for ischemic stroke should be noted. First, optimal therapeutic strategies should be considered since RNS has complex functions at different stages of stroke pathology. For example, at a low concentration, free radicals could also contribute to redox signaling. NO can be a cellular signaling molecule that promotes neuronal proliferation and migration and improves neurological outcomes at the recovery stage in post-ischemic brains194. MMP-9 has also been shown to exert its beneficial effects on neuronal plasticity and brain remodeling at the recovery phase in post-stroke brains195. Treatment with an MMP-9 inhibitor beginning at day 7 exacerbated brain injury and impaired the functional outcomes of rats at day 14 after MCAO ischemia195. Consistently, we found that caveolin-1 inhibited the neuronal differentiation of neural stem cells via the VEGF pathway, and Cav-1 KO mice showed more abundant newborn neurons in brains196. Cav-1 KO mice revealed a better proliferation capacity of adult neural stem cells than wild-type mice197. Hence, the RNS/Cav-1/MMP pathways may be beneficial for brain repair at the recovery phase of ischemic stroke. For drug treatment, the half-life of the aforementioned compounds is quite short, within several hours, which is still in the acute phase of stroke. For example, calycosin-7-O-β-D-glucoside had an elimination half-life of approximately 2.18 h after oral gavage in rats198. Baicalin had an elimination half-life of 0.12 h after intravenous injection in rats199. The CGA metabolite level was decreased to less than 50% within 1.5 h after intraperitoneal injection200. To reach the goal of the best neuroprotective outcome without interrupting the brain repair process, the selection of the optimal intervention time, dosage and frequency by integrating the knowledge of the functions of cellular signaling pathways at different stages of brain damage and repair and the pharmacological activities, pharmacokinetics and pharmacodynamics of those compounds is important69,201. Thus, understanding the dynamic changes of RNS, Cav-1 and MMPs and their impact on brain injury and brain repair after ischemic stroke is a prerequisite.
We should also consider the therapeutic time window of these compounds. In current studies, most of the compounds were applied within 2 h after ischemia onset59,125. In the past, many neuroprotective compounds have failed in clinical trials despite animal studies showing promising neuroprotective effects. One of the important reasons for this failure in clinical trials might be the limited therapeutic time windows of those compounds202. A compound that shows a neuroprotective effect when treated at 2 h after experimental stroke attack may not guarantee its therapeutic effect on stroke patients, especially when those treatments are launched several hours after stroke onset. Therefore, the compounds that show a broad therapeutic time window in an experimental stroke model are favorable. A series of experiments should be conducted to determine the therapeutic time window of those compounds in stroke treatment.
For drug development, we should consider the following key issues. First, the pharmacokinetics and pharmacodynamics of the candidate compounds should be taken into consideration. The BBB is a critical factor limiting drug distribution into the brain203,204. The BBB penetration of the drug is usually determined by two major parameters: one is the physicochemical properties of the compounds (such as molecular weight, rotatable bonds, solvent-accessible surface areas, H-bond capacity), and the other is the binding affinity of the compounds to the central nervous system (CNS) drug efflux pumps (most often the P-glycoprotein)204. Some simple rules based on the physiochemical features help to predict the BBB penetration of the compounds. For example, if the number of N+O in a compound is no more than five, the compound is likely to cross the BBB205. A CNS drug has been proposed to have an in vitro passive permeability more than 150 nm/s and should not be a good P-glycoprotein substrate206. Nevertheless, experimental data should be collected to directly show the penetration of the compounds into the CNS. Cerebral-spinal fluid (CSF) studies are usually conducted to calculate the CNS exposure of drug candidates204. An animal study showed that baicalin could cross the BBB and was detected in the CSF after single intravenous injection at a dosage of 24 mg/kg207. CGA was also detected in the CSF of rats after oral administration and reached the level of pharmacological effect208. These preclinical results suggest the potential of these compounds to enter the ischemic brains to exert their neuroprotective effects.
Another key issue is the acquisition of direct evidence of the compound-target interaction in the treatment of ischemic brain injury204. Although many papers have reported the therapeutic effects of candidate compounds with in vivo and in vitro data, most of the aforementioned studies did not provide data regarding the direct interaction of the active compounds with the observed targets, such as the RNS/Cav-1/MMP signaling pathway, in the experimental systems. Moreover, we should note that those compounds might have multiple targets, and their neuroprotection should not be simply explained by targeting a single signaling pathway. For example, calycosin-7-O-β-D-glucoside also modulated TRPC6 and ER-α signaling in ischemic brain injury134,135. Baicalin was also revealed to inhibit TLR-2/-4 signaling and attenuate brain inflammation143. Therefore, further exploration of the molecular targets of natural compounds and differentiation of the direct and indirect effects of those compounds on certain cellular signaling pathways and disease progression are desirable.
Recent advances in brain imaging technology highlight positron emission tomography as a useful tool to directly assess drug distribution and drug-target interaction204,209. The technique enables scientists to further evaluate the binding affinity and efficacy of the compounds to a target of interest in different brain regions among different species209,210. By using this method, we could guide the dosage selection by determining the target occupancy and its relationship to the blood-drug concentration210.
The third key issue is the downstream biological effects of these compounds in human subjects. Preclinical studies of those compounds seem promising. To evaluate the overall drug efficacy, clinical trials are needed to evaluate neurological scores, brain infarction, brain edema, etc. In addition, measurements of serum biomarkers will help assess the modulation of related pathways as well as the drug effectiveness. For example, 3-NT and MMP-9 are potential biomarkers of ischemic stroke and are associated with the prognosis of stroke outcomes66,73. Therefore, serum 3-NT and MMP-9 levels may help monitor the effects of the compounds on the RNS/caveolin-1/MMP-9 signaling pathways.
In summary, we propose that the RNS/Cav-1/MMP pathway plays an important role in mediating cerebral ischemia-reperfusion injury. Targeting this novel signaling pathway could provide a new clue for drug discovery for the treatment of ischemic stroke.
Acknowledgments
This work was supported by the Hong Kong General Research Fund (GRF No 17102915, GRF No 17118717), Research Grant Council, Hong Kong SAR and Health and Medical Research Fund, Hong Kong SAR (No 13142901), AoE/P-705/16 Areas of Excellence Scheme, RGC, Hong Kong SAR, China, and the SIRI/04/04/2015/06 Shenzhen Basic Research Plan Project.
References
- Esenwa C, Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag 2015; 11: 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011; 123: e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NT, Kim AS. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist 2015; 5: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrue V, von Kummer R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator. Stroke 2001; 32: 438–41. [DOI] [PubMed] [Google Scholar]
- Balami JS, Sutherland BA, Buchan AM. Complications associated with recombinant tissue plasminogen activator therapy for acute ischaemic stroke. CNS Neurol Disord Drug Targets 2013; 12: 155–69. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener 2011; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 2014; 115: 157–88. [DOI] [PubMed] [Google Scholar]
- Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, et al. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2017. pii: S0028-3908(17)30520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke 2007; 38: 674–6. [DOI] [PubMed] [Google Scholar]
- Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol 2017; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Song M. Disrupted ionic homeostasis in ischemic stroke and new therapeutic targets. J Stroke Cerebrovasc Dis 2017; 26: 2706–19. [DOI] [PubMed] [Google Scholar]
- Smith WS. Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J Vasc Interv Radiol 2004; 15: S3–12. [DOI] [PubMed] [Google Scholar]
- Kaur H, Prakash A, Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology 2013; 92: 324–34. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Adv Neurol 1996; 71: 209–33. [PubMed] [Google Scholar]
- Phan TG, Wright PM, Markus R, Howells DW, Davis SM, Donnan GA. Salvaging the ischaemic penumbra: more than just reperfusion? Clin Exp Pharmacol Physiol 2002; 29: 1–10. [DOI] [PubMed] [Google Scholar]
- Park CK, Nehls DG, Teasdale GM, McCulloch J. Effect of the NMDA antagonist MK-801 on local cerebral blood flow in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab 1989; 9: 617–22. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999; 79: 1431–568. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007; 87: 179–97. [DOI] [PubMed] [Google Scholar]
- Gu Y, Chen J, Shen J. Herbal medicines for ischemic stroke: combating inflammation as therapeutic targets. J Neuroimmune Pharmacol 2014; 9: 313–39. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001; 21: 2–14. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005; 38: 1433–44. [DOI] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol 2000; 190: 255–66. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Cherici G, Alesiani M, Carlà V, Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free-radical formation. J Neurochem 1988; 51: 1960–3. [DOI] [PubMed] [Google Scholar]
- Nakase T, Yoshioka S, Suzuki A. Free radical scavenger, edaravone, reduces the lesion size of lacunar infarction in human brain ischemic stroke. BMC Neurol 2011; 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med 2006; 354: 588–600. [DOI] [PubMed] [Google Scholar]
- Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, et al. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke. Stroke 2008; 39: 3303–7. [DOI] [PubMed] [Google Scholar]
- Chen XM, Chen HS, Xu MJ, Shen JG. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin 2013; 34: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab 1999; 19: 1020–8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 2007; 27: 697–709. [DOI] [PubMed] [Google Scholar]
- Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis 2011; 20: 47–54. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 2004; 35: 1449–53. [DOI] [PubMed] [Google Scholar]
- Shen J, Ma S, Chan P, Lee W, Fung PC, Cheung RT, et al. Nitric oxide down-regulates caveolin-1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J Neurochem 2006; 96: 1078–89. [DOI] [PubMed] [Google Scholar]
- Miller AA, Dusting GJ, Roulston CL, Sobey CG. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res 2006; 1111: 111–6. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985; 312: 159–63. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med 2006; 12: 225–9. [DOI] [PubMed] [Google Scholar]
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab 1995; 15: 242–7. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: The beginning or the end of the end of dopamine neurons? J Neurochem 2004; 89: 529–36. [DOI] [PubMed] [Google Scholar]
- Hirabayashi H, Takizawa S, Fukuyama N, Nakazawa H, Shinohara Y. Nitrotyrosine generation via inducible nitric oxide synthase in vascular wall in focal ischemia-reperfusion. Brain Res 2000; 852: 319–25. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci 1999; 19: 5910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen H, Deng R, Shen J. Pros and cons of current approaches for detecting peroxynitrite and their applications. Biomed J 2014; 37: 120. [DOI] [PubMed] [Google Scholar]
- Yang D, Sun ZN, Peng T, Wang HL, Shen JG, Chen Y, et al. Synthetic fluorescent probes for imaging of peroxynitrite and hypochlorous acid in living cells. Methods Mol Biol 2010; 591: 93–103. [DOI] [PubMed] [Google Scholar]
- Yang D, Wang HL, Sun ZN, Chung NW, Shen JG. A highly selective fluorescent probe for the detection and imaging of peroxynitrite in living cells. J Am Chem Soc 2006; 128: 6004–5. [DOI] [PubMed] [Google Scholar]
- Peng T, Chen X, Gao L, Zhang T, Wang W, Shen J, et al. A rationally designed rhodamine-based fluorescent probe for molecular imaging of peroxynitrite in live cells and tissues. Chem Sci 2016; 7: 5407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Sun F, Li Y, Zhou X, Duan Z, Duan F, et al. Momordica charantia polysaccharides could protect against cerebral ischemia/reperfusion injury through inhibiting oxidative stress mediated c-Jun N-terminal kinase 3 signaling pathway. Neuropharmacology 2015; 91: 123–34. [DOI] [PubMed] [Google Scholar]
- Chen H, Guan B, Chen X, Chen X, Li C, Qiu J, et al. Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurological outcome in ischemic stroke rats with delayed t-PA treatment: Involvement of ONOO(-)-MMP-9 pathway. Transl Stroke Res 2017. doi:10.1007/s12975-017-0598-3. [DOI] [PubMed]
- Gao L, Chen X, Peng T, Yang D, Wang Q, Lv Z, et al. Caveolin-1 protects against hepatic ischemia/reperfusion injury through ameliorating peroxynitrite-mediated cell death. Free Radic Biol Med 2016; 95: 209–15. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med 2005; 39: 1291–304. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett 2003; 140–141: 113–24. [DOI] [PubMed] [Google Scholar]
- Salgo MG, Squadrito GL, Pryor WA. Peroxynitrite causes apoptosis in rat thymocytes. Biochem Biophys Res Commun 1995; 215: 1111–8. [DOI] [PubMed] [Google Scholar]
- Tajes M, Ill-Raga G, Palomer E, Ramos-Fernandez E, Guix FX, Bosch-Morato M, et al. Nitro-oxidative stress after neuronal ischemia induces protein nitrotyrosination and cell death. Oxid Med Cell Longev 2013; 2013: 826143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991; 288: 481–7. [DOI] [PubMed] [Google Scholar]
- Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 2000; 278: H1883–90. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Ashraf QM, Delivoria-Papadopoulos M, Mishra OP. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci Lett 2000; 296: 5–8. [DOI] [PubMed] [Google Scholar]
- Endres M, Scott G, Namura S, Salzman AL, Huang PL, Moskowitz MA, et al. Role of peroxynitrite and neuronal nitric oxide synthase in the activation of poly(ADP-ribose) synthetase in a murine model of cerebral ischemia-reperfusion. Neurosci Lett 1998; 248: 41–4. [DOI] [PubMed] [Google Scholar]
- Dhar A, Kaundal RK, Sharma SS. Neuroprotective effects of FeTMPyP: a peroxynitrite decomposition catalyst in global cerebral ischemia model in gerbils. Pharmacol Res 2006; 54: 311–6. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol 2004; 142: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Chen X, Gu Y, Peng T, Yang D, Chang RCC, et al. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J Ethnopharmacol 2013; 150: 116–24. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 1998; 53: 613–25. [DOI] [PubMed] [Google Scholar]
- Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, Singh AK, et al. The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J Neurochem 2012; 123: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suofu Y, Clark J, Broderick J, Wagner KR, Tomsick T, Sa Y, et al. Peroxynitrite decomposition catalyst prevents matrix metalloproteinase activation and neurovascular injury after prolonged cerebral ischemia in rats. J Neurochem 2010; 115: 1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu M, Chen M, Ge QM, Pan SM. Serum uric acid is neuroprotective in Chinese patients with acute ischemic stroke treated with intravenous recombinant tissue plasminogen activator. J Stroke Cerebrovasc Dis 2015; 24: 1080–6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Heo SH, Kim JH, Lee D, Lee JS, Kim YS, et al. Effects of uric acid levels on outcome in severe ischemic stroke patients treated with intravenous recombinant tissue plasminogen activator. Eur Neurol 2014; 71: 132–9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lin Y, Liu Y, Chen Y, Wang B, Li C, et al. Serum uric acid levels and outcomes after acute ischemic stroke. Mol Neurobiol 2016; 53: 1753–9. [DOI] [PubMed] [Google Scholar]
- Bas DF, Topcuoglu MA, Gursoy-Ozdemir Y, Saatci I, Bodur E, Dalkara T. Plasma 3-nitrotyrosine estimates the reperfusion-induced cerebrovascular stress, whereas matrix metalloproteinases mainly reflect plasma activity: a study in patients treated with thrombolysis or endovascular recanalization. J Neurochem 2012; 123: 138–47. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab 2014; 34: 185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandooren J, Van Damme J, Opdenakker G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog Brain Res 2014; 214: 193–206. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res 2015; 1623: 30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 624–33. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost 2007; 5: 1732–9. [DOI] [PubMed] [Google Scholar]
- Sole S, Petegnief V, Gorina R, Chamorro A, Planas AM. Activation of matrix metalloproteinase-3 and agrin cleavage in cerebral ischemia/reperfusion. J Neuropathol Exp Neurol 2004; 63: 338–49. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke 2003; 34: 2165–70. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008; 39: 1121–6. [DOI] [PubMed] [Google Scholar]
- Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol 2008; 84: 207–14. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 2008; 28: 9451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol 2011; 762: 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Tsuji K, Lee SR, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci 2004; 24: 671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 2005; 25: 6401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998; 29: 1020–30. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 2001; 21: 7724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Guan B, Shen J. Targeting ONOO-/HMGB1/MMP-9 signaling cascades: potential for drug development from Chinese medicine to attenuate ischemic brain injury and hemorrhagic transformation induced by thrombolytic treatment. Integr Med Int 2016; 3: 32–52. [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke 2000; 31: 1974–80. [DOI] [PubMed] [Google Scholar]
- Chen HS, Chen XM, Feng JH, Liu KJ, Qi SH, Shen JG. Peroxynitrite decomposition catalyst reduces delayed thrombolysis-induced hemorrhagic transformation in ischemia-reperfused rat brains. CNS Neurosci Ther 2015; 21: 585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 2001; 276: 29596–602. [DOI] [PubMed] [Google Scholar]
- Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, et al. Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol 2009; 77: 826–34. [DOI] [PubMed] [Google Scholar]
- Vinten J, Johnsen AH, Roepstorff P, Harpoth J, Tranum-Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta 2005; 1717: 34–40. [DOI] [PubMed] [Google Scholar]
- Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab 2008; 8: 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow JH Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 2010; 86: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 2004; 84: 1341–79. [DOI] [PubMed] [Google Scholar]
- Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv 2003; 3: 445–64. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med 2004; 36: 584–95. [DOI] [PubMed] [Google Scholar]
- Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Front Physiol 2012; 2: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuzova A, Wang L, Wang J, Hangyas-Mihalyne G, Murray D, Honig B, et al. Membrane binding of peptides containing both basic and aromatic residues. Experimental studies with peptides corresponding to the scaffolding region of caveolin and the effector region of MARCKS. Biochemistry 2000; 39: 10330–9. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem 2000; 275: 21605–17. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Schwab RB, Scherer PE, Lisanti MP. A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1. The caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. J Biol Chem 1999; 274: 22660–7. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 1997; 272: 25437–40. [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 1997; 272: 18522–5. [DOI] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 2000; 6: 1362–7. [DOI] [PubMed] [Google Scholar]
- Sato Y, Sagami I, Shimizu T. Identification of caveolin-1-interacting sites in neuronal nitric-oxide synthase. Molecular mechanism for inhibition of NO formation. J Biol Chem 2004; 279: 8827–36. [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BSS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem 1997; 272: 25437–40. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 1997; 272: 15583–6. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 1997; 272: 25907–12. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 2009; 119: 2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin JF, Malhotra S, Singh Dhallu M, Mercier I, Rosenbaum DM, Lisanti MP. Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res 2007; 100: 721–9. [DOI] [PubMed] [Google Scholar]
- Shen J, Lee W, Li Y, Lau CF, Ng KM, Fung ML, et al. Interaction of caveolin-1, nitric oxide, and nitric oxide synthases in hypoxic human SK-N-MC neuroblastoma cells. J Neurochem 2008; 107: 478–87. [DOI] [PubMed] [Google Scholar]
- Chanvorachote P, Nimmannit U, Lu Y, Talbott S, Jiang BH, Rojanasakul Y. Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J Biol Chem 2009; 284: 28476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Dee CM, Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed) 2011; 3: 1216–31. [DOI] [PubMed] [Google Scholar]
- Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res 2001; 262: 28–36. [DOI] [PubMed] [Google Scholar]
- Chow AK, Cena J, El-Yazbi AF, Crawford BD, Holt A, Cho WJ, et al. Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J Mol Cell Cardiol 2007; 42: 896–901. [DOI] [PubMed] [Google Scholar]
- Phillips PG, Birnby LM. Nitric oxide modulates caveolin-1 and matrix metalloproteinase-9 expression and distribution at the endothelial cell/tumor cell interface. Am J Physiol Lung Cell Mol Physiol 2004; 286: L1055–65. [DOI] [PubMed] [Google Scholar]
- Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 2007; 109: 1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, et al. Caveolin-1–eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol 2011; 193: 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Zheng G, Xu M, Li Y, Chen X, Zhu W, et al. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem 2012; 120: 147–56. [DOI] [PubMed] [Google Scholar]
- Choi KH, Kim HS, Park MS, Kim JT, Kim JH, Cho KA, et al. Regulation of Caveolin-1 expression determines early brain edema after experimental focal cerebral ischemia. Stroke 2016; 47: 1336–43. [DOI] [PubMed] [Google Scholar]
- Choi KH, Kim HS, Park MS, Lee EB, Lee JK, Kim JT, et al. Overexpression of caveolin-1 attenuates brain edema by inhibiting tight junction degradation. Oncotarget 2016; 7: 67857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, et al. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 2014; 63: 1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 2012; 32: 3044–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol 2007; 114: 459–69. [DOI] [PubMed] [Google Scholar]
- Nag S, Manias JL, Stewart DJ. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol 2009; 35: 417–26. [DOI] [PubMed] [Google Scholar]
- Wu H, Deng R, Chen X, Wong WC, Chen H, Gao L, et al. Caveolin-1 is critical for lymphocyte trafficking into central nervous system during experimental autoimmune encephalomyelitis. J Neurosci 2016; 36: 5193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Shen J. Focusing on caveolin-1 in CNS autoimmune disease: multiple sclerosis. Neural Regen Res 2016; 11: 1920–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chen X, Shen J. Reactive nitrogen species as therapeutic targets for autophagy: implication for ischemic stroke. Expert Opin Ther Targets 2017; 21: 305–17. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Liu HK, Hsiao PC, Kuo LM, Lee IJ, Wu TS, et al. New isoflavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J Agric Food Chem 2011; 59: 1131–7. [DOI] [PubMed] [Google Scholar]
- Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X, et al. Calycosin-7-O-β-D-glucoside regulates nitric oxide/caveolin-1/matrix metalloproteinases pathway and protects blood–brain barrier integrity in experimental cerebral ischemia–reperfusion injury. J Ethnopharmacol 2014; 155: 692–701. [DOI] [PubMed] [Google Scholar]
- Guo C, Tong L, Xi M, Yang H, Dong H, Wen A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol 2012; 144: 768–74. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Y, Feng C, Wu G, Ye Y, Tian J. Calycosin inhibits the migration and invasion of human breast cancer cells by down-regulation of Foxp3 expression. Cell Physiol Biochem 2017; 44: 1775–84. [DOI] [PubMed] [Google Scholar]
- Quan GH, Wang H, Cao J, Zhang Y, Wu D, Peng Q, et al. Calycosin suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-kappaB. Int J Mol Sci 2015; 16: 29496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DH, Bao YM, Wei CL, An LJ. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed Environ Sci 2005; 18: 297–301. [PubMed] [Google Scholar]
- Chen HQ, Wang XJ, Jin ZY, Xu XM, Zhao JW, Xie ZJ. Protective effect of isoflavones from Trifolium pratense on dopaminergic neurons. Neurosci Res 2008; 62: 123–30. [DOI] [PubMed] [Google Scholar]
- Yu D, Duan Y, Bao Y, Wei C, An L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J Ethnopharmacol 2005; 98: 89–94. [DOI] [PubMed] [Google Scholar]
- Yu DH, Bao YM, An LJ, Yang M. Protection of PC12 cells against superoxide-induced damage by isoflavonoids from Astragalus mongholicus. Biomed Environ Sci 2009; 22: 50–4. [DOI] [PubMed] [Google Scholar]
- Fan Y, Wu DZ, Gong YQ, Zhou JY, Hu ZB. Effects of calycosin on the impairment of barrier function induced by hypoxia in human umbilical vein endothelial cells. Eur J Pharmacol 2003; 481: 33–40. [DOI] [PubMed] [Google Scholar]
- Guo C, Ma Y, Ma S, Mu F, Deng J, Duan J, et al. The role of TRPC6 in the neuroprotection of calycosin against cerebral ischemic injury. Sci Rep 2017; 7: 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dong X, Li Z, Wang W, Tian J, Chen J. Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J Neurol Sci 2014; 339: 144–8. [DOI] [PubMed] [Google Scholar]
- Wu XL, Wang YY, Cheng J, Zhao YY. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol Sin 2006; 27: 1007–12. [DOI] [PubMed] [Google Scholar]
- Ren M, Wang X, Du G, Tian J, Liu Y. Calycosin-7-O-β-D-glucoside attenuates ischemia-reperfusion injury in vivo via activation of the PI3K/Akt pathway. Mol Med Rep 2016; 13: 633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull 2011; 85: 396–402. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhuang P, Shen B, Zhang Y, Shen J. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res 2012; 1429: 36–42. [DOI] [PubMed] [Google Scholar]
- Zhuang PW, Cui GZ, Zhang YJ, Zhang MX, Guo H, Zhang JB, et al. Baicalin regulates neuronal fate decision in neural stem/progenitor cells and stimulates hippocampal neurogenesis in adult rats. CNS Neurosci Ther 2013; 19: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Li P, Wang Z, Li PT, Zhang WS, Sun ZH, et al. A comparative study on the individual and combined effects of baicalin and jasminoidin on focal cerebral ischemia-reperfusion injury. Brain Res 2006; 1123: 188–95. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wu R, Li P, Liu F, Zhang W, Zhang P, et al. Baicalin administration is effective in positive regulation of twenty-four ischemia/reperfusion-related proteins identified by a proteomic study. Neurochem Int 2009; 54: 488–96. [DOI] [PubMed] [Google Scholar]
- Li HY, Yuan ZY, Wang YG, Wan HJ, Hu J, Chai YS, et al. Role of baicalin in regulating Toll-like receptor 2/4 after ischemic neuronal injury. Chin Med J (Engl) 2012; 125: 1586–93. [PubMed] [Google Scholar]
- Zhou QB, Duan CZ, Jia Q, Liu P, Li LY. Baicalin attenuates focal cerebral ischemic reperfusion injury by inhibition of protease-activated receptor-1 and apoptosis. Chin J Integr Med 2014; 20: 116–22. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L, et al. Baicalin reduces the permeability of the blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J Ethnopharmacol 2012; 141: 714–20. [DOI] [PubMed] [Google Scholar]
- Luo S, Li S, Zhu L, Fang SH, Chen JL, Xu QQ, et al. Effect of baicalin on oxygen-glucose deprivation-induced endothelial cell damage. Neuroreport 2017; 28: 299–306. [DOI] [PubMed] [Google Scholar]
- Liu LY, Wei EQ, Zhao YM, Chen FX, Wang ML, Zhang WP, et al. Protective effects of baicalin on oxygen/glucose deprivation- and NMDA-induced injuries in rat hippocampal slices. J Pharm Pharmacol 2005; 57: 1019–26. [DOI] [PubMed] [Google Scholar]
- Ge QF, Hu X, Ma ZQ, Liu JR, Zhang WP, Chen Z, et al. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacol Res 2007; 55: 148–57. [DOI] [PubMed] [Google Scholar]
- Li H, Hu J, Ma L, Yuan Z, Wang Y, Wang X, et al. Comprehensive study of baicalin down-regulating NOD2 receptor expression of neurons with oxygen-glucose deprivation in vitro and cerebral ischemia-reperfusion in vivo. Eur J Pharmacol 2010; 649: 92–9. [DOI] [PubMed] [Google Scholar]
- Kim DH, Cho KH, Moon SK, Kim YS, Kim DH, Choi JS, et al. Cytoprotective mechanism of baicalin against endothelial cell damage by peroxynitrite. J Pharm Pharmacol 2005; 57: 1581–90. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Shi SS, Chen Y, Wang CH, Chen CM, et al. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation 2011; 34: 463–70. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Liang RS, Shi SS, Chen JP, Chen CM, et al. Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem Res 2011; 36: 2022–8. [DOI] [PubMed] [Google Scholar]
- Zhou QB, Jin YL, Jia Q, Zhang Y, Li LY, Liu P, et al. Baicalin attenuates brain edema in a rat model of intracerebral hemorrhage. Inflammation 2014; 37: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Wang Z, Zhang XY, Ying K, Liu JX, Wang YY. Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats. Acta Pharmacol Sin 2005; 26: 307–14. [DOI] [PubMed] [Google Scholar]
- Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F, Jiang EN, et al. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor kappaB p65 activation. Biochem Biophys Res Commun 2010; 403: 398–404. [DOI] [PubMed] [Google Scholar]
- Wang P, Cao Y, Yu J, Liu R, Bai B, Qi H, et al. Baicalin alleviates ischemia-induced memory impairment by inhibiting the phosphorylation of CaMKII in hippocampus. Brain Res 2016; 1642: 95–103. [DOI] [PubMed] [Google Scholar]
- Li S, Sun X, Xu L, Sun R, Ma Z, Deng X, et al. Baicalin attenuates in vivo and in vitro hyperglycemia-exacerbated ischemia/reperfusion injury by regulating mitochondrial function in a manner dependent on AMPK. Eur J Pharmacol 2017; 815: 118–26. [DOI] [PubMed] [Google Scholar]
- Chen HS, Qi SH, Shen JG. One-compound-multi-target: combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr Neuropharmacol 2017; 15: 134–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Chopra K. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol Biochem Behav 2014; 120: 33–42. [DOI] [PubMed] [Google Scholar]
- Malik ZA, Singh M, Sharma PL. Neuroprotective effect of Momordica charantia in global cerebral ischemia and reperfusion induced neuronal damage in diabetic mice. J Ethnopharmacol 2011; 133: 729–34. [DOI] [PubMed] [Google Scholar]
- Raish M. Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-kappaB signaling pathway. Int J Biol Macromol 2017; 97: 544–51. [DOI] [PubMed] [Google Scholar]
- Tan HF, Gan CY. Polysaccharide with antioxidant, alpha-amylase inhibitory and ACE inhibitory activities from Momordica charantia. Int J Biol Macromol 2016; 85: 487–96. [DOI] [PubMed] [Google Scholar]
- Yu HM, Xu J, Li C, Zhou C, Zhang F, Han D, et al. Coupling between neuronal nitric oxide synthase and glutamate receptor 6–mediated c-Jun N-terminal kinase signaling pathway via S-nitrosylation contributes to ischemia neuronal death. Neuroscience 2008; 155: 1120–32. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Tian H, Li HC, Zhang GY. Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett 2006; 408: 159–64. [DOI] [PubMed] [Google Scholar]
- Jung HA, Park JC, Chung HY, Kim J, Choi JS. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch Pharm Res 1999; 22: 213–8. [DOI] [PubMed] [Google Scholar]
- Yen WJ, Wang BS, Chang LW, Duh PD. Antioxidant properties of roasted coffee residues. J Agric Food Chem 2005; 53: 2658–63. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem 2003; 51: 502–9. [DOI] [PubMed] [Google Scholar]
- Grace SC, Salgo MG, Pryor WA. Scavenging of peroxynitrite by a phenolic/peroxidase system prevents oxidative damage to DNA. FEBS Lett 1998; 426: 24–8. [DOI] [PubMed] [Google Scholar]
- Jung HA, Chung HY, Kang SS, Hyun SK, Kang HS, Choi JS. A phenolic glucoside isolated from Prunus serrulata var spontanea and its peroxynitrite scavenging activity. Arch Pharm Res 2005; 28: 1127–30. [DOI] [PubMed] [Google Scholar]
- Ketsawatsakul U, Whiteman M, Halliwell B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem Biophys Res Commun 2000; 279: 692–9. [DOI] [PubMed] [Google Scholar]
- Kono Y, Kobayashi K, Tagawa S, Adachi K, Ueda A, Sawa Y, et al. Antioxidant activity of polyphenolics in diets: rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim Biophys Acta 1997; 1335: 335–42. [DOI] [PubMed] [Google Scholar]
- Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, et al. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci 2005; 77: 2760–9. [DOI] [PubMed] [Google Scholar]
- Soliman KF, Mazzio EA. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc Soc Exp Biol Med 1998; 218: 390–7. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. The phenylpropanoid micronutrient chlorogenic acid improves clinical rating scores in rabbits following multiple infarct ischemic strokes: synergism with tissue plasminogen activator. Exp Neurol 2007; 205: 407–13. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee JS, Jang HJ, Kim SM, Chang MS, Park SH, et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur J Pharmacol 2012; 689: 89–95. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee BJ, Bu Y. Protective effects of dihydrocaffeic acid, a coffee component metabolite, on a focal cerebral ischemia rat model. Molecules 2015; 20: 11930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Cao L, Li R, Fang X, Miao Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi Pharm J 2017; 25: 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos MD, Almeida MC, Lopes NP, de Souza GE. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull 2006; 29: 2236–40. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Yamazawa T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci 2015; 139: 69–74. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79: 727–47. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Hsu JD, Lin WL, Kao SH, Wang CJ. Upregulation of caveolin-1 by mulberry leaf extract and its major components, chlorogenic acid derivatives, attenuates alcoholic steatohepatitis via inhibition of oxidative stress. Food Funct 2017; 8: 397–405. [DOI] [PubMed] [Google Scholar]
- Tsuboi M, Etoh H, Yomoda Y, Kato K, Kato H, Kulkarni A, et al. Nitration reaction of lutein with peroxynitrite. Tetrahedron Lett 2010; 51: 676–8. [Google Scholar]
- Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G. Lutein, zeaxanthin and astaxanthin protect against DNA damage in SK-N-SH human neuroblastoma cells induced by reactive nitrogen species. J Photochem Photobiol B 2007; 88: 1–10. [DOI] [PubMed] [Google Scholar]
- Sun YX, Liu T, Dai XL, Zheng QS, Hui BD, Jiang ZF. Treatment with lutein provides neuroprotection in mice subjected to transient cerebral ischemia. J Asian Nat Prod Res 2014; 16: 1084–93. [DOI] [PubMed] [Google Scholar]
- Li SY, Yang D, Fu ZJ, Woo T, Wong D, Lo AC. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol Dis 2012; 45: 624–32. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Cherubini A, Stahl W, Senin U, Sies H, Mecocci P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radic Res 2002; 36: 265–8. [DOI] [PubMed] [Google Scholar]
- Yokota T, Ohtake T, Ishikawa H, Inakuma T, Ishiguro Y, Terao J, et al. Quenching of peroxynitrite by lycopene in vitro. Chem Lett 2003; 33: 80–1. [Google Scholar]
- Muzandu K, Ishizuka M, Sakamoto KQ, Shaban Z, El Bohi K, Kazusaka A, et al. Effect of lycopene and β-carotene on peroxynitrite-mediated cellular modifications. Toxicol Appl Pharmacol 2006; 215: 330–40. [DOI] [PubMed] [Google Scholar]
- Panasenko OM, Sharov VS, Briviba K, Sies H. Interaction of peroxynitrite with carotenoids in human low density lipoproteins. Arch Biochem Biophys 2000; 373: 302–5. [DOI] [PubMed] [Google Scholar]
- Lei X, Lei L, Zhang Z, Cheng Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia-reperfusion in rats: The role of the Nrf2/HO-1 signaling pathway. Mol Med Rep 2016; 13: 412–8. [DOI] [PubMed] [Google Scholar]
- Fujita K, Yoshimoto N, Kato T, Imada H, Matsumoto G, Inakuma T, et al. Lycopene inhibits ischemia/reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue. Neurochem Res 2013; 38: 461–9. [DOI] [PubMed] [Google Scholar]
- Xinli L, Jiuhong X. Dietary and circulating lycopene and stroke risk: a meta-analysis of prospective studies. Sci Rep 2014; 4: 5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppi J, Laukkanen JA, Sivenius J, Ronkainen K, Kurl S. Serum lycopene decreases the risk of stroke in men A population-based follow-up study. Neurology 2012; 79: 1540–7. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, LaPointe M, et al. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol 2001; 50: 602–11. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006; 12: 441–5. [DOI] [PubMed] [Google Scholar]
- Li Y, Luo J, Lau WM, Zheng G, Fu S, Wang TT, et al. Caveolin-1 plays a crucial role in inhibiting neuronal differentiation of neural stem/progenitor cells via VEGF signaling-dependent pathway. PLoS One 2011; 6: e22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin JF, Yang M, Iacovitti L, Lisanti MP. Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle 2009; 8: 3978–83. [DOI] [PubMed] [Google Scholar]
- Wen XD, Qi LW, Li P, Bao KD, Yan XW, Yi L, et al. Simultaneous determination of calycosin-7-O-β-D-glucoside, ononin, astragaloside IV, astragaloside I and ferulic acid in rat plasma after oral administration of Danggui Buxue Tang extract for their pharmacokinetic studies by liquid chromatography–mass spectrometry. J Chromatogr B 2008; 865: 99–105. [DOI] [PubMed] [Google Scholar]
- Xing J, Chen X, Zhong D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci 2005; 78: 140–6. [DOI] [PubMed] [Google Scholar]
- Azuma K, Ippoushi K, Nakayama M, Ito H, Higashio H, Terao J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J Agric Food Chem 2000; 48: 5496–500. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke 2007; 38: 748–52. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci 1999; 22: 535–40. [DOI] [PubMed] [Google Scholar]
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 2014; 13: 419–31. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Greig NH. Drug discovery and development: Role of basic biological research. Alzheimers Dement (N Y) 2017; 3: 651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norinder U, Haeberlein M. Computational approaches to the prediction of the blood-brain distribution. Adv Drug Deliv Rev 2002; 54: 291–313. [DOI] [PubMed] [Google Scholar]
- Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, et al. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther 2002; 303: 1029–37. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang Y, Yang R, Tang X. Determination of baicalin in rat cerebrospinal fluid and blood using microdialysis coupled with ultra-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B 2008; 874: 77–83. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen H, Li H, Tang Y, Yang L, Cao S, et al. Antidepressant potential of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver Bark with neuron protection and promotion of serotonin release through enhancing synapsin I expression. Molecules 2016; 21: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PM, Rabiner EA, Passchier J, Gunn RN. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol 2012; 73: 175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth S, Rabiner EA, Gunn RN, Plisson C, Wilson AA, Comley RA, et al. Evaluation of 11C-GSK189254 as a novel radioligand for the H3 receptor in humans using PET. J Nucl Med 2010; 51: 1021–9. [DOI] [PubMed] [Google Scholar]