Abstract
Background
Patients with hemiplegic migraine (HM) may sometimes develop progressive neurological deterioration of which the pathophysiology is unknown.
Patient
We report a 16-year clinical and neuroradiological follow-up of a patient carrying a de novo p.Ser218Leu CACNA1A HM mutation who had nine severe HM attacks associated with seizures and decreased consciousness between the ages of 3 and 12 years.
Results
Repeated ictal and postictal neuroimaging revealed cytotoxic oedema during severe HM attacks in the symptomatic hemisphere, which later showed atrophic changes. In addition, progressive cerebellar atrophy was observed. Brain atrophy halted after cessation of severe attacks, possibly due to prophylactic treatment with flunarizine and sodium valproate.
Conclusion
Severe HM attacks may result in brain atrophy and prophylactic treatment of these attacks might be needed in an early stage of disease to prevent permanent brain damage.
Keywords: Hemiplegic migraine, MRI, DWI, migraine prophylaxis
Introduction
Familial (FHM) and sporadic hemiplegic migraine (SHM) are rare subtypes of migraine characterised by transient motor weakness during the aura phase and caused by mutations in CACNA1A, ATP1A2 or SCN1A (1). Here, we report a unique 16-year follow-up of a SHM patient. Repeated ictal and postictal neuroimaging revealed cytotoxic oedema during attacks leading to brain atrophy which halted after cessation of severe HM attacks.
Case report
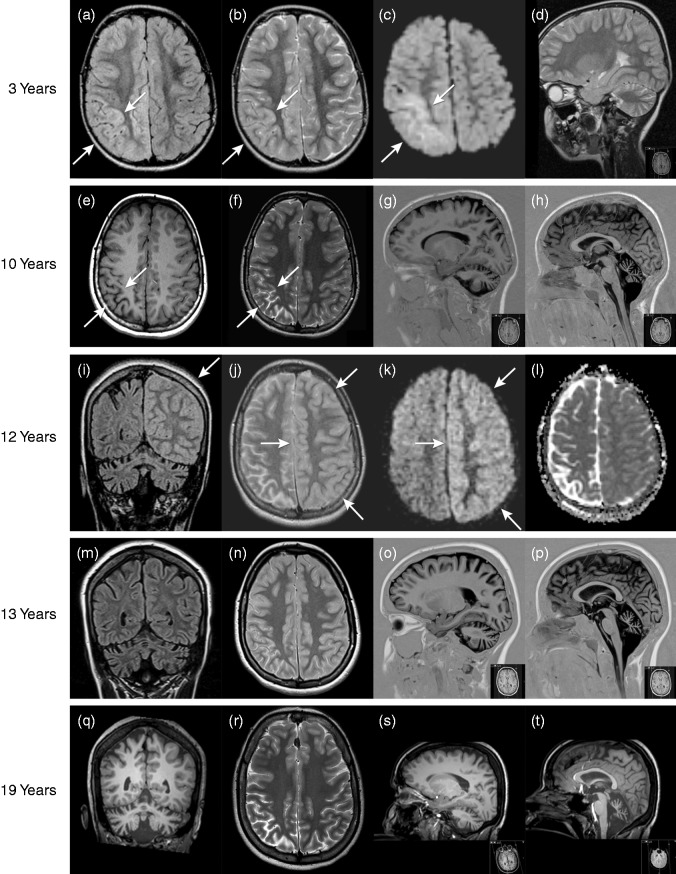
This currently 19-year-old woman was diagnosed with SHM due to a de novo p.Ser218Leu CACNA1A mutation during childhood. She was also diagnosed with Brugada syndrome, an inherited cardiac disorder. Some features of this patient were previously briefly reported by our group (2). Apart from ‘early seizures and cerebral oedema after trivial head trauma’ (ESCEATHT) the p.Ser218Leu CACNA1A mutation has been associated with hemiconvulsion–hemiplegia–epilepsy syndrome (2,3). The comprehensive phenotype thus illustrates the often-described co-occurrence of SHM/FHM and epilepsy (4). Shortly after birth, our SHM patient developed apnoea but after resuscitation and artificial ventilation she breathed spontaneously. She showcased impaired psychomotor development and cerebellar ataxia from infancy. Upon first hospital admission at the age of three years, she showed loss of consciousness and left-sided hemiconvulsions for 2 h, starting 30 min after minor head trauma. After two days, brain MRI revealed right hemispheric cortical oedema with cytotoxic oedema, most notable in the right parieto-occipital region. Mild cerebellar atrophy was also evident (Fig. 1a–d). The attack was followed by a week-long left-sided neglect and hemiparesis. At the age of ten years, interictal brain MRI displayed marked cortical atrophy corresponding to the areas of cytotoxic oedema from when she was three years old (Fig. 1e–h). At the age of 12 years, she was admitted with decreased consciousness, right hemiparesis, fever and vomiting. Brain MRI revealed left hemispheric cortical oedema with cytotoxic oedema (Fig. 1i–l). Nine months later, she suffered a similar attack with decreased consciousness, left deviation of eyes and head, and a right hemiparesis. She had a fever (38.7℃) and an EEG showed asymmetric slow high-voltage activity over the entire left hemisphere. Some abnormalities suspect for epileptic discharges were noted over the right hemisphere. During hospital admission, she had several short-lasting epileptic seizures. A follow-up EEG several days later showed an improved pattern and no signs of epilepsy. Due to difficulties swallowing and fear of respiratory failure, she was shortly admitted to the intensive care unit. She remained in a state of decreased consciousness for nine days and was discharged from hospital after 12 days. Four months later, mild left-hemispheric cortical atrophy was observed on interictal brain MRI, corresponding to the areas of cytotoxic oedema during the previous attack, as well as progressive right-hemispheric and cerebellar atrophy (Fig. 1m–p). In total, between the ages of three and 12 years, she suffered nine of these severe HM attacks. After each severe attack, her psychomotor skills deteriorated, improving only slowly and incompletely. Since the age of 12 years, she has been treated with flunarizine and sodium valproate, after which severe attacks halted. At 16 years, her IQ was scored below 50. Interictal examination, at 18 years, revealed gaze-evoked nystagmus, mild ataxia in both arms and ataxic gait but no motor weakness. She continues having mild attacks during which her arm or leg feels ‘strange’ and some motor weakness appears to be present which resolves completely within hours. She presented with further deterioration of psychomotor skills at the age of 19 years. However, interictal brain MRI revealed no progression of cerebral and cerebellar atrophy compared with the interictal MRI at 13 years (Fig. 1q–t).
Figure 1.
At the age of three years, during a hemiplegic migraine attack, FLAIR (a) and T2-weighted (T2W) images (b) showed right hemispheric diffuse cortical swelling. Diffusion-weighted imaging (DWI) (c) showed diffusion restriction, most notable in the right parieto-occipital region (arrows). Mild cerebellar atrophy was present, as illustrated on the sagittal T2W image (d). At the age of ten years, interictal axial T1-weighted (T1W) (e) and T2W images (f) showed marked cortical atrophy in the right parieto-occipital regions, corresponding with the previous area of diffusion restriction (c). Progressive cerebellar atrophy was observed on sagittal T1W images (g, h). At the age of 12 years, during a hemiplegic migraine attack, coronal FLAIR (i) and axial T2W images (j) showed diffuse cortical swelling of the whole left hemisphere (arrow) with slight diffusion restriction on DWI and apparent diffusion coefficient images (k, l). Follow-up MRI at 13 years showed progressive cerebral and cerebellar atrophy on the coronal FLAIR (m), axial T2W (n) and sagittal T1W images (o, p). After the start of prophylactic medication and subsequent ceasing of severe hemiplegic attacks, follow-up MRI at 19 years revealed no evident progression of cerebellar and cerebral atrophy as visible on coronal T1W (q), axial T2W (r) and sagittal T1W images (s, t).
Discussion
In our patient, progression of brain atrophy halted after cessation of severe HM attacks, as was reported in an HM patient with a p.Thr666Met CACNA1A mutation (5). Cytotoxic oedema during HM attacks is thought to be attributable to enhanced susceptibility to cortical spreading depression (CSD), the electrophysiological correlate of migraine aura, which may be accompanied by transient neuronal swelling (6). The p.Ser218Leu CACNA1A mutation particularly enhances susceptibility to CSD (6). As a result, even weak stimuli may trigger repetitive and prolonged CSDs associated with cerebral oedema. This is in line with the severe phenotype of patients with this specific mutation, with severe, sometimes fatal, cerebral oedema (2). Repetitive CSDs may eventually result in neuronal cell death (7), which is illustrated by the severe brain atrophy in our case. In contrast, cerebellar atrophy in HM is thought to be caused by a different mechanism in which a direct effect on Purkinje cells causes neuronal cell loss (8).
A limitation of our report is that effects of the prophylactic treatment are difficult to assess due to several reasons: (i) we describe a single case; (ii) the low attack frequency in our patient; (iii) spontaneous reduction in attack frequency may occur with age in (hemiplegic) migraine (9,10), although usually not at such a young age. However, since treatment with flunarizine and sodium valproate, no severe HM attack has occurred and the cortical atrophy seems not to have progressed. The clinical psychomotor deterioration at the age of 19 years in our patient could not be related to progression of cerebral atrophy and thus may have been due to other unknown chronic mechanisms in HM. Furthermore, cognitive complaints can occur with the use of sodium valproate (11).
This unique case illustrates development of secondary cortical atrophy in previously affected brain areas during severe HM attacks and suggests that adequate prophylaxis may be critical to prevent permanent brain damage in HM patients.
Clinical implications
Severe hemiplegic migraine attacks may result in brain atrophy.
Adequate prophylaxis may be critical to prevent permanent brain damage in HM patients.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N. Pelzer reports support for conference visits from Menarini; M.D. Ferrari reports grants and consultancy or industry support from Medtronic and independent support from the European Community, NWO, NIH and the Dutch Heart Foundation; G.M. Terwindt reports independent support from NWO, ZonMW, European Community, Dutch Heart Foundation and Dutch Brain Foundation. The other authors report no disclosures.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Netherlands Organization for Scientific Research (NWO) (VIDI 91711319 to G.M.T.) and the European Community (EC) (FP7-EUROHEADPAIN – no. 602633 to M.D.F.). The funding agencies had no role in the design or conduct of the study.
References
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 2.Stam AH, Luijckx GJ, Poll-The BT, et al. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J Neurol Neurosurg Psychiatry 2009; 80: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki S, Ikeno K, Abe T, et al. Hemiconvulsion-hemiplegia-epilepsy syndrome associated with CACNA1A S218L mutation. Pediatr Neurol 2011; 45(3): 193–196. [DOI] [PubMed] [Google Scholar]
- 4.Prontera P, Sarchielli P, Caproni S, et al. Epilepsy in hemiplegic migraine: Genetic mutations and clinical implications. Cephalalgia. Epub ahead of print 1 January 2017. DOI: 10.1177/0333102416686347. [DOI] [PubMed]
- 5.Tashiro Y, Yamazaki T, Nagamine S, et al. Repeated encephalopathy and hemicerebral atrophy in a patient with familial hemiplegic migraine type 1. Intern Med 2014; 53: 2245–2250. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015; 14: 65–80. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghian H, Jafarian M, Karimzadeh F, et al. Neuronal death by repetitive cortical spreading depression in juvenile rat brain. Exp Neurol 2012; 233: 438–446. [DOI] [PubMed] [Google Scholar]
- 8.Gao ZY, Todorov B, Barrett CF, et al. Cerebellar ataxia by enhanced Ca(V)2.1 currents is alleviated by Ca2+-dependent K+-channel activators in Cacna1a(S218L) mutant mice. J Neurosci 2012; 32: 15533–15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 2010; 30(9): 1065–1072. [DOI] [PubMed] [Google Scholar]
- 10.Haan J, Terwindt GM, Bos PL, et al. Familial hemiplegic migraine in The Netherlands. Dutch Migraine Genetics Research Group. Clin Neurol Neurosurg 1994; 96(3): 244–249. [DOI] [PubMed] [Google Scholar]
- 11.Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem 2013; 46: 1323–1338. [DOI] [PubMed] [Google Scholar]