Abstract
BACKGROUND
Psychotic-like experiences (PLEs) are associated with lower social and occupational functioning, and lower executive function. Emerging evidence also suggests that PLEs reflect neural dysfunction resembling that of psychotic disorders.
METHODS
The present study examined dynamic connectivity related to a measure of PLEs derived from the Achenbach Adult Self-Report, in an otherwise-healthy sample of adults from the Human Connectome Project. 76 PLE-endorsing and 153 control participants were included in the final sample. To characterize network dysfunction, dynamic connectivity states were examined across large-scale resting-state networks using Dynamic Conditional Correlation (DCC) and k-means clustering.
RESULTS
Three dynamic states were identified. The PLE-endorsing group spent more time than controls in State 1, a state reflecting hyper-connectivity within Visual regions and hypo-connectivity within the Default Mode Network, and less time in State 2, a state characterized by robust within-network connectivity for all networks and strong Default Mode Network anti-correlations. Within the PLE-endorsing group, worse Executive Function was associated with more time spent in and more transitions into State 1 and less time spent in and fewer transitions into State 3.
CONCLUSIONS
PLEs are associated with altered large-scale brain dynamics, which tip the system away from spending more time in states reflecting more “typical” connectivity patterns toward more time in states reflecting Visual hyper-connectivity and Default Mode hypo-connectivity.
Keywords: Psychotic Experiences, Psychosis, Dynamic Connectivity, Executive Function, Network, Default Mode Network
Background
There is a growing consensus that psychosis occurs along a continuum that spans from subsyndromal symptoms observed in healthy individuals to clinically-significant symptoms observed in psychotic disorders. Subsyndromal symptoms, often referred to as Psychotic-Like Experiences (PLEs) include hallucinations and delusions, which are quantitatively but not qualitatively different from clinically significant psychotic symptoms (1). While only about 0.72% of individuals will be diagnosed with schizophrenia in their lifetime (2), the number that will experience PLEs is substantially higher, with prevalence estimates of 7.2% in adults (1, 3) and up to 40–60% (usually transitory experiences) in children and adolescents (4, 5). Individuals with PLEs may have no history of clinical diagnosis and may never develop a disorder; however, they tend to exhibit impairments in social and occupational functioning similar to, albeit less severe than, those observed in schizophrenia (6), supporting the notion that PLEs represent a more subtle form of psychosis. While in some cases, PLEs are indicative of risk for a psychotic disorder; in the majority of cases, symptoms either remit entirely or persist sub-clinically (3).
Consistent with the notion that they represent the low end of the psychosis continuum (1, 7), PLEs are associated with neural alterations that often resemble attenuated versions of those observed in schizophrenia (8). These include functional and structural changes across the brain, particularly within cognitive and reward systems (9–12). A number of studies have found alterations to the cingulo-opercular and default mode systems (11, 13–15), including reduced within-network connectivity and reduced between-network anti-correlation. In youths with PLEs, connections across these two systems emerged as the most-profoundly impacted across the brain (11). In addition, network efficiency is reduced within both of these systems in individuals endorsing PLEs and has been shown to scale with symptom severity (13).
Patients with psychotic disorders also exhibit alterations in frontoparietal connections. Both functional and structural connections are reduced between frontal and parietal nodes of this network in first-episode psychosis and schizophrenia (8, 16, 17). These regions have an established role in working memory and executive function and pathological activation patterns have been associated with impaired working memory in these disorders (18–20) as well as in healthy populations (19, 21). Moreover, alterations of the executive network have been found in individuals at genetic (19, 22, 23) and clinical (10, 24, 25) risk for schizophrenia, indicating that such deficits are present to varying degrees along the psychosis spectrum. In individuals endorsing PLEs, the findings have been mixed (14) with some suggesting that stronger frontoparietal connections may be a protective factor for individuals with sub-clinical PLEs that prevents or delays conversion (1, 14, 16).
While much of the resting-state literature has focused on static connectivity, or the correlation between regions across the scan duration, there has been recent interest in dynamic functional connectivity. Dynamic connectivity assesses changes in connectivity throughout the scan duration. Reoccurring connectivity patterns are commonly characterized as “states” that the brain moves in and out of over time. While alterations in connectivity have been identified in individuals with PLEs, to-date no published studies have examined alterations in dynamic connectivity associated with PLEs. Compared to static measures, dynamic connectivity improves classification accuracy (26, 27); and some studies that find few or no group differences in static connections, find more extensive disrupted connectivity when examining dynamic states (28, 29). Numerous studies find altered dynamic states in schizophrenia patients (26, 27, 29–31), suggesting that altered function may be due to disrupted dynamics for particular brain states. We therefore expect that examining large-scale dynamic connectivity states will reveal novel patterns of network alteration associated with PLEs.
The current study examined re-occurring dynamic states in the Human Connectome Project (HCP). The HCP is ideal for examining dynamic connectivity due to its high temporal resolution, long scan lengths, and multiple scan sessions. PLEs were derived from this dataset using four questionnaire items on the Achenbach Adult Self-Report (ASR) for ages 18–59 (32). These items have previously been used to examine PLEs and were robustly associated with graph metrics of functional connectivity in selected brain networks (13). For the current study, a large-scale network approach was taken to determine whether altered connectivity associated with PLEs reflect altered dynamic states.
Methods and Materials
Participants
Data for 820 healthy adult participants were available from the HCP 900 Subjects Data Release (http://humanconnectome.org/documentation/S900). To identify PLEs, ratings were summed across four items reflecting psychosis-like symptoms on the ASR:
I hear sounds or voices that other people think aren’t there
I see things that other people think aren’t there
I do things that other people think are strange
I have thoughts that other people would think are strange.
Participants could rate a 0, 1, or 2 for each item, reflecting: 0 not true, 1 somewhat or sometimes true, or 2 very true or often true. Across the full HCP sample, participants’ summed ratings ranged between 0 and 6 across the four items, with the majority of participants scoring a total of 0. We defined the “high sub-clinical PLE” group (HP) as those participants that rated a 2 or more and the “low sub-clinical PLE” group (LP) as those participants that rated a 0 across the four ASR items. Fifty participants with a rating of 1 were excluded from the analysis in order to maximize group differences and to reduce noise that may have been related to participants’ fuzzy interpretation of the Achenbach items.
The full HCP dataset included sets of siblings. To ensure that group differences were not affected by relatedness, only unrelated participants were included in the analyses. If two or more participants were related, the person that met criteria for the HP group was chosen and the other siblings were excluded. If at least two participants met for the HP group or at least two met for the LP group then one was randomly chosen and the other siblings were excluded. This resulted in 76 unrelated HP and 205 unrelated LP participants. The two groups were then matched for age, sex, handedness, race, ethnicity, and two measures of scan motion (mean absolute motion and mean relative motion, i.e. root mean square frame-wise displacement) by excluding LP participants. After matching, the final groups consisted of 76 HP and 153 LP participants, between 22 and 37 years old (Table 1).
Table 1.
Participant Characteristics for the Low Psychosis (LP) and High Psychosis (HP) Groups
| LP | HP | p-value | |
|---|---|---|---|
|
| |||
| N | 153.00 | 76.00 | - |
| Age | 27.90 | 27.67 | 0.66 |
| Sex (% male) | 50.00% | 46.41% | 0.61 |
| Handedness | 66.41 | 64.74 | 0.77 |
| Race (% caucasian) | 63.16% | 71.24% | 0.23 |
| Ethnicity (% hispanic) | 11.84% | 13.73% | 0.69 |
| Absolute Motion | 0.76 | 0.72 | 0.32 |
| Relative Motion | 0.080 | 0.078 | 0.42 |
| Income | 5.14 | 4.36 | 0.010 |
| Executive Function | 113.22 | 111.24 | 0.039 |
| Thought Problems | 51.75 | 62.34 | 0.000 |
HCP Imaging Acquisition and Data Processing
As part of the HCP protocol, each participant completed four 14 minute, 33 second resting-state fMRI (rs-fMRI) runs on two separate days. The resting-state runs were eyes-open and participants were instructed to keep their eyes on the fixation cross. Scans were acquired on a Siemens connectome-Skyra 3T scanner with 32-channel head coil (multiband sequence, acceleration factor of 8, TR = 0.72 sec, 2 mm isotropic spatial resolution) (33).
Dynamic connectivity measures were computed on the time-courses from the “Parcellation-Timeseries-Netmats (PTN) extensively processed resting-state fMRI dataset” that were included in the HCP900 Data Release. This publically-released dataset had undergone the following preprocessing by the HCP (34, 35): artifact removal using ICA+FIX (36, 37), temporal demeaning and variance normalization (38), and data reduction using MELODIC for Incremental Group-PCA (39) and then spatial Group-ICA at several dimensionalities (34, 38). For the current study, the time-courses from the 300-dimensional ICA, that were extracted using the dual-regression approach, were used to examine dynamic connectivity.
Network Identification
The volumetric MNI152 3D-space version of the 300-dimensional Group-ICA component spatial maps were automatically labeled based on spatial overlap with the Yeo 7-network parcellation (40). This consisted of first thresholding each of the 300 component ICA spatial maps so that only 5% of voxels with the highest intensity values were included. Each thresholded component map was then labeled as one of the seven networks: Visual (VN), Somatomotor (SMN), Dorsal Attention (DAN), Ventral Attention (VAN), Limbic (LN), Frontoparietal (FPN), or Default Mode (DMN), if: (1.) at least 500 suprathreshold voxels overlapped with one network; and (2.) over 55% of suprathreshold voxels fell within one network.
If the component failed these classification criteria for all seven networks, then it was labeled as “noise”. This resulted in 65 “signal” components (29 VN, 8 SMN, 3 DAN, 2 VAN, 8 FPN, and 15 DMN). Dynamic connectivity measures were computed for the time-courses corresponding to these 65 “signal” components. The remaining 235 “noise” components were excluded from further processing and analysis.
Dynamic Connectivity
Dynamic connectivity was computed using the Dynamic Conditional Correlation (DCC) approach (https://github.com/canlab/Lindquist_Dynamic_Correlation), a multivariate volatility method (41). Unlike sliding-window approaches that estimate connectivity over a fixed window length, this is a model-based method that estimates the contribution of surrounding time-points to the covariance matrix. Pairwise-dynamic connectivity values were obtained for every time-point of each participant’s four resting state runs. This resulted in a matrix of connectivity values that was 1200 (time-points) × 2080 (connections) for each participant and each run.
K-means clustering was performed to identify the commonly occurring ‘brain states’ across the set of 2080 connections. This was done for each of the four runs by concatenating the matrix of connectivity values across all participants, resulting in a matrix that was 274,000 by 2080 (i.e. 229 subjects × 1200 time-points by 2080 connections). This matrix served as the input to the k-means clustering algorithm. Clustering was performed using the squared Euclidean distance to minimize distance to the centroid and was replicated five times for each run using different initial centroid values. The clustering was performed using a varying number of states (k = 2, …, 9) and the optimal cluster solution was identified by computing the Within-Cluster Sums of each time-point’s Euclidean Distance to Centroid (sumd) for each cluster solution. This is done by plotting the sumd for each cluster solution choosing the optimal solution as the point at which reductions in sumd taper off. In addition, the reliability of the State Centroids across the four runs was assessed using the Intra-Class Correlation (ICC) coefficient to validate the optimal cluster solution.
The following dynamic connectivity summary measures were computed and used to investigate group differences: 1. Dwell Time - the percent of time-points in each state. 2. Transitions - the sum of time-points in which the state changed from time t-1 to time t. 3. Distance to Centroid - the average of the squared Euclidean distance between connectivity at each time-point and the cluster centroid. Previous examination has shown low reliability of dynamic connectivity state summary measures across runs, which may be due to the low occurrence of some states for some participants (42). For this reason, each measure was first computed within each run and then averaged across the four runs to get a single value per participant.
Primary models tested for group by state interactions and included root mean square Frame-wise Displacement (FD) as a covariate to account for the effects of scan-to-scan motion on the dynamic connectivity measures. For Dwell Time and Transitions, multinomial logistic regression models included the proportion of time-points in each of the k-states compared to the total of any state as a response variable and assumed a multinomial distribution with a logit link function. Psychosis group (LP or HP) and FD were included as explanatory variables. For Distance to Centroid, a repeated-measures ANOVA model tested for a group by state interaction. Group membership was the between-subjects factor, while state was the within-subjects factor. Before ANOVA testing, the Distance to Centroid measures were natural log-transformed to ensure that they were normally distributed for each state.
Primary analyses were multiple-comparisons corrected by dividing p = 0.05 by 3 to get a critical p-value of p = 0.017 (i.e. by performing Bonferroni correction). For those dynamic connectivity measures in which significant group by state interactions were found at this corrected threshold, post-hoc tests examining group differences, while accounting for FD, were performed. In the case of Dwell Time and Transitions, binomial logistic regression was used for post-hoc tests of group effects in each state; while in the case of Distance to Centroid, this was done using general linear models testing for group effects in each state.
Phenotype associations were examined for the Thought Problems summary score of the ASR. In addition, a measure of Executive Function was created by averaging the unadjusted scores from the NIH toolbox (www.nihtoolbox.org) Executive Function tests: Card Sort, Flanker, and Working Memory/List Sort. Multinomial logistic regression (for Dwell Time and Transitions) and ANOVA (for Distance to Centroid) models were similar to the primary models testing for group effects, except that, in addition to testing the group effect, the models also included terms for phenotype and for the group by phenotype interaction. These models tested for whether the two groups differed in their phenotype by state interactions and included FD as a covariate. Multiple-comparisons were again accounted for by Bonferroni correction. In this case, six analyses were performed (i.e. testing the effects of 3 dynamic connectivity measures * 2 phenotypes), which resulted in a corrected p-value of 0.0083. If the group by phenotype interaction was determined to be significant, follow-up analyses were performed that tested for group, phenotype and group by phenotype interactions within each state. Analyses examining the phenotype association, which also included FD as a covariate, were also performed within the HP group alone to ensure that associations existed within that group. Finally, if there were no group by phenotype associations (at a subthreshold p-value of p<0.05), then associations across the two groups were performed by examining models that tested for group and phenotype effects (while excluding the group by phenotype interaction terms).
Results
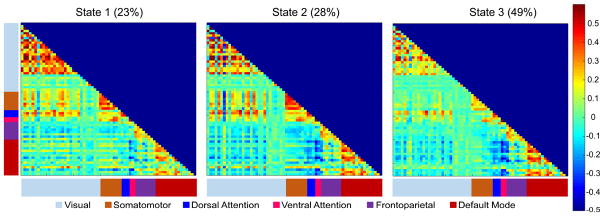
Using the elbow criterion to select the minimum-distance solution, k=3 was identified as optimal (Figure S1). Dynamic state summary measures were computed for the three-state solution. The centroid connectivity matrices for each of the three states were matched across the four runs by minimizing the sum of the Euclidean Distance. This resulted in high reliability of the State Centroids across the four runs (ICC = 0.994, 0.996, and 0.997 for States 1, 2, and 3, respectively). Due to the high similarity of the State Centroids between runs (Figure S2), each of the State Centroids were averaged across the four runs (Figure 1).
Figure 1.
Dynamic Connectivity States for k = 3. Each State Represents the Mean Cluster Centroid Across the Four Resting-State Runs. The Colorbar on the Right Represents the Strength of Each Connection and Ranges Between a Correlation of −0.5 to +0.6. Each Row and Column is One of the 65 Independent Components Classified as a “Signal Component” and the Color Labels on the X- and Y-Axes Indicate Their Network Affiliation. The Mean Dwell Time for All Participants is Displayed Next to Each State Title
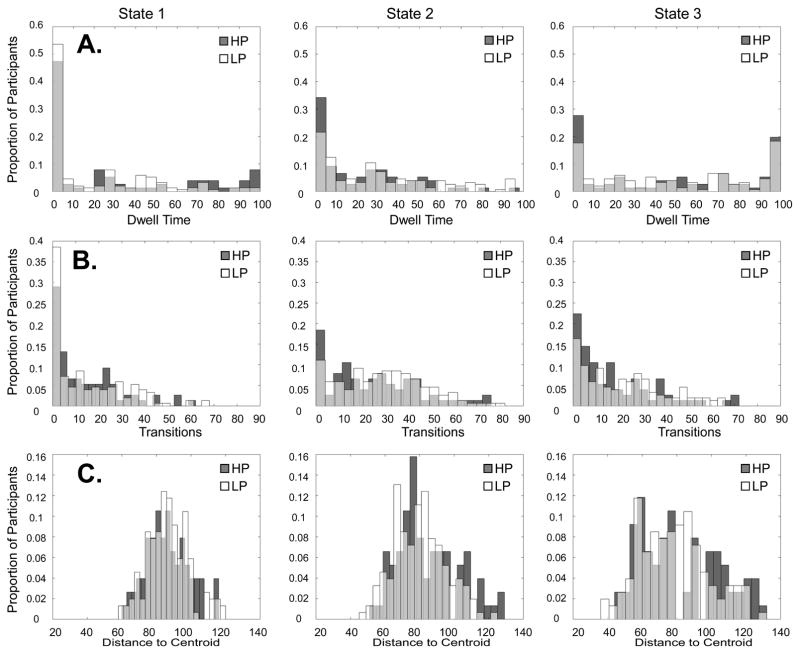
All three states generally showed the strongest connectivity for within-network connections in the seven labeled networks and weak or negative connectivity between the default mode and other networks. The most common state was State 3 with an average Dwell Time, or percent of time spent in state, of 49%, followed by State 2, with an average Dwell Time of 28%, and then State 1, with an average Dwell Time of 23%. Figure 2 displays the distributions for each state’s Dwell Time, Transitions, and Distance to Centroid for both groups.
Figure 2.
Distributions for (A.) Dwell Time - Percent of Time in State, (B.) Total Transitions – Number of Timepoints Transitioning Into State, and (C.) Distance to Centroid – Average Distance to the Cluster Centroid, for the Low Psychosis (LP) and High Psychosis (HP) Groups
Table S1 displays the connectivity values for each state averaged over each network. State 3, the most frequent state, was characterized by weak within-network VN and SMN connectivity, and weak between-network connectivity for these two networks. This state also showed strong within-network DMN connectivity and strong anti-correlations between the DMN and other task-positive networks, particularly with the DAN and VAN. State 2, showed strong within-network connectivity for all networks and strong connectivity within and between the DAN and VAN components. In addition, like State 3, connectivity in State 2 tended to be more anti-correlated between the DMN and the DAN, VAN, and VN. State 1, the least frequent state, was characterized by stronger connectivity within Visual regions, but weaker connectivity within the DMN. In this state, DMN anti-correlations with the DAN and VAN were also weaker and connectivity within and between the DAN and VAN were weaker.
Table 2 displays the results for the psychosis by state effects for Dwell Time, Transitions, and Distance to Centroid. There was a significant group by state interaction in Dwell Time for States 1 and 2 (p= 0.74, p = 0.0062) and a marginal group by state interaction in Dwell Time for States 1 and 3 (B = 0.46, p = 0.049). However, after applying the multiple-comparisons threshold (α = 0.017), only the Dwell Time effect for States 1 and 2 was significant. Follow-up tests of the group effect in States 1 and 2 revealed that the probability of spending time in State 1 was higher for the HP group (B = 0.56, p = 0.014), while the probability of spending time in State 2 was lower for the HP group (B = −0.44, p = 0.023). Examination of group by state interactions for Transitions revealed a marginal interaction for States 1 and 2 (B = 0.25, p = 0.047). However, this effect was not significant after multiple-comparisons correction. The group by state interaction for Distance to Centroid was also marginal (F(1.48,333.61) = 3.60, p = 0.042), but was not significant after multiple comparisons correction.
Table 2.
Primary Results Examining Group by State Effects for Dwell Time, Transitions, and Distance to Centroid. The Findings Reported in Dark Blue are Significant at the Multiple-Comparisons Corrected Threshold of p < 0.017, While Findings in Light Blue are No Longer Significant After Correction. Significant Follow-up Tests for the Individual States are Highlighted in Orange. The Mean and Standard Deviation (SD) for the Low Psychosis (LP) and High Psychosis (HP) Groups are Displayed Below the Statistical Results for Each Measure
| p-value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | |
| intercept | −0.730 | −1.330 | −0.601 | 0.125 | 0.002 | 0.136 |
| psychosis | 0.742 | 0.460 | −0.282 | 0.006 | 0.049 | 0.235 |
| motion | 3.829 | 5.271 | 1.442 | 0.486 | 0.282 | 0.762 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | |
|
| ||||||
| intercept | −1.769 | −0.801 | 0.000 | 0.014 | ||
| psychosis | 0.559 | −0.437 | 0.014 | 0.023 | ||
| motion | 4.754 | −0.307 | 0.316 | 0.936 | ||
|
| ||||||
| mean | SD | |||||
|
| ||||||
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | |
|
| ||||||
| LP | 20.06 | 30.46 | 49.48 | 26.44 | 27.43 | 36.12 |
| HP | 30.20 | 22.07 | 47.73 | 35.89 | 22.53 | 38.92 |
| Transitions | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | |
| intercept | −0.920 | −0.646 | 0.274 | 0.000 | 0.005 | 0.151 |
| psychosis | 0.248 | 0.214 | −0.034 | 0.047 | 0.100 | 0.753 |
| motion | 1.319 | 0.961 | −0.358 | 0.610 | 0.721 | 0.873 |
|
| ||||||
| mean | SD | |||||
|
| ||||||
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | |
|
| ||||||
| LP | 14.96 | 29.05 | 21.90 | 16.04 | 18.19 | 17.62 |
| HP | 14.20 | 25.13 | 18.11 | 14.69 | 19.69 | 17.94 |
| Distance to Centroid | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| F | df effect | df error | p-value | |||
|
|
||||||
| psychosis | 0.846 | 1.000 | 226.000 | 0.359 | ||
| motion | 5.826 | 1.000 | 226.000 | 0.017 | ||
| state | 5.359 | 1.476 | 333.607 | 0.011 | ||
| state × psychosis | 3.600 | 1.476 | 333.607 | 0.042 | ||
|
| ||||||
| mean | SD | |||||
|
| ||||||
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | |
|
| ||||||
| LP | 87.02 | 79.94 | 78.46 | 12.10 | 14.94 | 20.06 |
| HP | 86.34 | 84.42 | 82.09 | 12.22 | 17.78 | 23.46 |
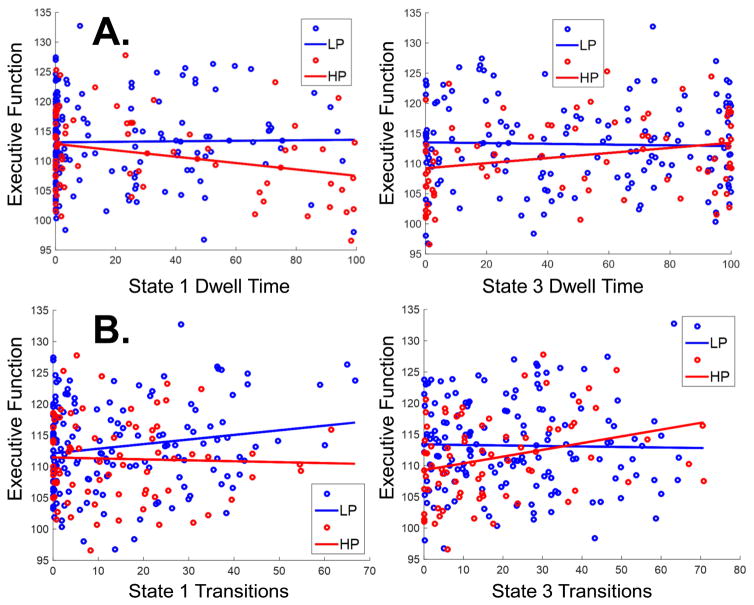
Results from the tests for phenotypic associations are displayed in Tables 3 and 4. Table 3 shows that Thought Problems were not associated with Dwell Time. Although there was a large group difference in Thought Problems (p = 4.35 × 10−26), this was not associated with Dwell Time when group effects were included in the model. Executive Function was differentially associated with dynamic connectivity measures for States 1 and 3 (Table 4). For Dwell Time, there was a subthreshold group by phenotype interaction (B = −0.093, p = 0.010). In the HP group, there was a differential association between Dwell Time and Executive Function in States 1 and 3 (B = −0.092, p = 0.005). More Dwell Time in State 1 was associated with worse Executive Function (B = −0.082, p = 0.016), while more Dwell Time in State 3 was associated with better Executive Function (B = 0.065, p = 0.028) (Figure 3A). For Transitions, there was a significant group by phenotype interaction (B = −0.068, p = 0.001) for States 1 and 3. Transitions were also significantly associated with Executive Function for the HP group only. For the HP group, more Transitions into State 1 were associated with worse Executive Function (B = −0.048, p = 0.009) and more Transitions into State 3 were associated with better Executive Function (B = 0.036, p = 0.009) (Figure 3B).
Table 3.
Phenotypic Results Examining Group by State Effects for Thought Problems (TP). The Findings Reported in Dark Blue are Significant at the Multiple-Comparisons Corrected Threshold of p <0.0083, While Findings in Light Blue are No Longer Significant After Correction. Significant Follow-up Tests for the Individual States are Highlighted in Orange.
| p-value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
| intercept | 3.568 | 0.591 | 2.976 | 0.174 | 0.780 | 0.177 |
| psychosis | −2.767 | 1.344 | −4.110 | 0.431 | 0.640 | 0.183 |
| TP | −0.014 | −0.017 | 0.003 | 0.709 | 0.588 | 0.934 |
| psychosis × TP | −0.043 | 0.031 | −0.074 | 0.501 | 0.540 | 0.180 |
| motion | −3.226 | −5.636 | 2.409 | 0.561 | 0.254 | 0.618 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | 2.350 | 1.519 | 0.832 | 0.139 | 0.254 | 0.552 |
| psychosis | −0.399 | −0.417 | 0.018 | 0.325 | 0.233 | 0.960 |
| TP | −0.032 | −0.004 | −0.028 | 0.277 | 0.878 | 0.281 |
| motion | −3.632 | −5.266 | 1.634 | 0.510 | 0.283 | 0.733 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | −1.201 | −1.303 | −0.103 | 0.653 | 0.555 | 0.967 |
| Thought Problems | 0.015 | 0.014 | −0.002 | 0.695 | 0.671 | 0.966 |
| motion | 7.233 | −0.208 | −7.441 | 0.485 | 0.980 | 0.438 |
| Transitions | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
| intercept | 0.740 | −0.335 | 1.075 | 0.522 | 0.776 | 0.246 |
| psychosis | 0.071 | 0.467 | −0.396 | 0.964 | 0.773 | 0.766 |
| TP | −0.002 | 0.005 | −0.007 | 0.896 | 0.778 | 0.637 |
| psychosis × TP | 0.006 | 0.014 | −0.008 | 0.841 | 0.625 | 0.720 |
| motion | −1.331 | −1.078 | −0.253 | 0.611 | 0.693 | 0.911 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | 0.590 | −0.413 | 1.003 | 0.585 | 0.714 | 0.278 |
| psychosis | −0.242 | −0.195 | −0.048 | 0.055 | 0.138 | 0.667 |
| TP | 0.003 | 0.009 | −0.006 | 0.755 | 0.336 | 0.420 |
| motion | −1.128 | −0.338 | −0.791 | 0.672 | 0.903 | 0.732 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | −0.586 | 0.307 | 0.893 | 0.627 | 0.806 | 0.409 |
| Thought Problems | 0.001 | −0.007 | −0.008 | 0.944 | 0.709 | 0.610 |
| motion | −0.778 | −3.022 | −2.244 | 0.864 | 0.518 | 0.575 |
| Distance to Centroid | |||||
|---|---|---|---|---|---|
|
| |||||
| both groups | F | df effect | df error | p-value | |
|
|
|||||
| psychosis | 1.456 | 1.000 | 223.000 | 0.229 | |
| TP | 0.039 | 1.000 | 223.000 | 0.844 | |
| psychosis × TP | 5.567 | 1.000 | 223.000 | 0.009 | |
| motion | 6.702 | 1.000 | 223.000 | 0.010 | |
| state | 0.930 | 1.470 | 327.917 | 0.370 | |
| state × psychosis | 0.190 | 1.470 | 327.917 | 0.758 | |
| state × TP | 0.495 | 1.470 | 327.917 | 0.553 | |
| state × psychosis × TP | 0.712 | 1.470 | 327.917 | 0.246 | |
|
| |||||
| HP only | F | df effect | df error | p-value | |
|
| |||||
| Thought Problems | 1.609 | 1.000 | 73.000 | 0.209 | |
| motion | 0.668 | 1.000 | 73.000 | 0.416 | |
| state | 0.185 | 1.417 | 103.411 | 0.864 | |
| state × Thought Problems | 0.147 | 1.417 | 103.411 | 0.789 | |
Table 4.
Phenotypic Results Examining Group by State Effects for Executive Function (EF). The Findings Reported in Dark Blue are Significant at the Multiple-Comparisons Corrected Threshold of p <0.0083, While Findings in Light Blue are No Longer Significant After Correction. Significant Follow-up Tests for the Individual States are Highlighted in Orange.
| p-value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
| intercept | 0.767 | 2.267 | −1.501 | 0.779 | 0.367 | 0.498 |
| psychosis | −7.421 | −10.825 | 3.404 | 0.108 | 0.008 | 0.397 |
| EF | 0.059 | 0.084 | −0.025 | 0.092 | 0.005 | 0.422 |
| psychosis × EF | −0.061 | −0.093 | 0.033 | 0.144 | 0.010 | 0.359 |
| motion | −2.289 | −3.739 | 1.449 | 0.686 | 0.462 | 0.768 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | 6.742 | 10.228 | 3.486 | 0.127 | 0.008 | 0.373 |
| Executve Function | −0.060 | −0.092 | −0.032 | 0.110 | 0.005 | 0.338 |
| motion | 2.970 | −6.143 | −9.113 | 0.773 | 0.459 | 0.340 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 | State 2 | State 3 | State 1 | State 2 | State 3 |
|
| ||||||
| intercept | 8.480 | −7.884 | 0.032 | 0.024 | ||
| Executve Function | −0.082 | 0.065 | 0.016 | 0.028 | ||
| motion | −3.295 | 7.129 | 0.700 | 0.370 | ||
| Transitions | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| both groups | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
| intercept | 2.352 | 2.108 | 0.244 | 0.064 | 0.112 | 0.819 |
| psychosis | −5.428 | −7.837 | 2.409 | 0.009 | 0.000 | 0.183 |
| EF | 0.034 | 0.055 | −0.021 | 0.030 | 0.001 | 0.116 |
| psychosis × EF | −0.047 | −0.068 | 0.022 | 0.013 | 0.001 | 0.174 |
| motion | −1.114 | −0.392 | −0.722 | 0.673 | 0.887 | 0.753 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| both groups | State 1 | State 2 | State 3 | State 1 | State 2 | State 3 |
|
| ||||||
| intercept | −2.361 | 1.172 | 0.334 | 0.577 | ||
| psychosis | 9.679 | −7.479 | 0.014 | 0.038 | ||
| EF | −0.076 | 0.058 | 0.010 | 0.035 | ||
| psychosis × EF | 0.082 | −0.066 | 0.020 | 0.039 | ||
| motion | 3.199 | −2.586 | 0.513 | 0.566 | ||
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 | State 1 vs State 2 | State 1 vs State 3 | State 2 vs State 3 |
|
| ||||||
| intercept | 3.795 | 6.845 | 3.050 | 0.047 | 0.001 | 0.067 |
| Executve Function | −0.037 | −0.061 | −0.023 | 0.022 | 0.001 | 0.100 |
| motion | −3.014 | −6.325 | −3.310 | 0.497 | 0.171 | 0.401 |
|
| ||||||
| beta | p-value | |||||
|
| ||||||
| HP only | State 1 | State 2 | State 3 | State 1 | State 2 | State 3 |
|
| ||||||
| intercept | 4.464 | −5.011 | 0.036 | 0.002 | ||
| Executve Function | −0.048 | 0.036 | 0.009 | 0.009 | ||
| motion | −4.491 | 4.339 | 0.357 | 0.252 | ||
| Distance to Centroid | |||||
|---|---|---|---|---|---|
|
| |||||
| both groups | F | df effect | df error | p-value | |
|
|
|||||
| psychosis | 2.344 | 1.000 | 226.000 | 0.127 | |
| EF | 1.525 | 1.000 | 226.000 | 0.218 | |
| psychosis × EF | 2.172 | 1.000 | 226.000 | 0.142 | |
| motion | 4.889 | 1.000 | 226.000 | 0.028 | |
| state | 0.756 | 1.483 | 332.223 | 0.434 | |
| state × psychosis | 6.381 | 1.485 | 332.223 | 0.012 | |
| state × EF | 5.077 | 1.485 | 332.223 | 0.013 | |
| state × psychosis × EF | 4.704 | 1.485 | 332.223 | 0.018 | |
|
| |||||
| HP only | F | df effect | df error | p-value | |
|
| |||||
| EF | 0.135 | 1.000 | 226.000 | 0.713 | |
| motion | 5.381 | 1.000 | 226.000 | 0.021 | |
| state | 0.421 | 1.485 | 335.707 | 0.596 | |
| state × EF | 0.875 | 1.485 | 335.707 | 0.390 | |
Figure 3.
Phenotypic Associations with Dynamic Connectivity Measures for (A.) Executive Function with Dwell Time in States 1 and 3, and (B.) Executive Function with Transitions into States 1 and 3. The Low Psychosis (LP) group is displayed in blue and the High Psychosis (HP) group is displayed in red.
Discussion
The current study examined functional dynamics underlying psychotic experiences in otherwise healthy individuals. Our sample was drawn from the HCP dataset, which is ideal for examining dynamic connectivity due to its high temporal resolution, large number of datapoints (i.e. four ~14.5 minute sessions), and high-dimensional spatial decomposition, which are well-suited for detecting recurring large-scale brain states. Dynamic Conditional Correlation (DCC) was used to compute dynamic connectivity at each timepoint. Unlike sliding-window approaches, this method does not require the a priori selection of a sliding-window length. Further, DCC provides more reliable connectivity estimates, which are less susceptible to noise (41). We found robust alterations in large-scale functional dynamics associated with PLEs. The results corroborate previous findings of profound functional alterations associated with psychosis, even for relatively mild symptoms in otherwise healthy individuals (13, 14). Control participants, those who did not endorse any PLEs, spent significantly more time in State 2. In addition, there were marginal effects suggesting that control participants made slightly more Transitions into State 2, and had lower Distance to the Centroid for State 2. This state generally reflected strong within-network connectivity for all networks, strong connectivity within and between DAN and VAN regions, and strong anti-correlation between the DMN and the DAN, VAN, and most Visual components; which is a relatively typical connectivity pattern as evidenced by static connectivity (43). Therefore, this state may reflect coordination between visual and higher-order networks for goal-directed behavior. Both our observed group differences and phenotypic associations suggest that State 2 reflects a healthier network configuration associated with lower symptomatology; while State 1 reflects pathology associated with PLEs. Within the HP group, spending more time in State 1 was also associated with worse Executive Function, suggesting that spending less time in State 1 is associated with healthier cognition.
The results are consistent with previous dynamic connectivity findings in the schizophrenia literature, in which there was altered connectivity in particular states (28, 44, 45). For example, one study focused on dynamic connectivity within the DMN and found that healthy controls spent more time in a state which reflected stronger connectivity between anterior and posterior sub-networks of the DMN; while patients with schizophrenia spent more time in a dis-connected DMN state (44). This is in line with the current finding that relative to those with PLEs, control participants spent more time in a state associated with robust DMN connectivity (State 2) and less time in a state with reduced DMN connectivity (State 1). This is particularly interesting, as it demonstrates how dynamic investigations can expand our understanding of the previously-observed static connectivity findings of disrupted executive-DMN network interactions in schizophrenia (46–49). It may be that not only overall static connectivity differences between the DMN and other networks is important, but also that the amount of time spent in states reflecting connectivity or dysconnectivity within the DMN may be important for psychosis.
Most studies examining PLEs have specifically focused on cognitive networks, such as the cingulo-opercular network, FPN, and DMN. However, the current study finds that dysfunction within those networks is only part of the story. Individuals experiencing PLEs spend less time in a state characterized by more typical connectivity within and between both cognitive and sensory networks (State 2); however, perhaps more interestingly, they also spent more time in a state characterized by not just DMN hypo-connectivity but also visual hyper-connectivity (State 1). While a few studies have reported atypical connectivity in sensory regions associated with psychosis (14, 50), visual hyper-connectivity may be integral to PLEs and should be explored further. For example, visual hyper-activity observed in this state could potentially be the basis for certain psychotic symptoms, such as visual hallucinations. Future investigations could focus on the relationship between these states and severity of specific symptom domains.
Future Considerations
PLEs were currently assessed in a large publically-available dataset using self-report items that were not specifically designed to examine psychosis. For this reason, assessment accuracy may be lower than that of other studies using assessments designed to examine subclinical psychosis. In addition, symptom variability was limited. Future studies, which recruit individuals with psychotic-like symptoms, could provide more accurate assessment and greater variability in psychosis symptoms.
The current study used k-means clustering across all timepoints and participants to identify re-occurring dynamic connectivity states. While this method is commonly used, other clustering algorithms may improve detection of aberrant connectivity patterns by combining group-level as well as subject-level information (28), or using non-linear boundaries to more accurately identify sub-states (51). Future studies may find that other algorithms are even more sensitive to atypical dynamic connectivity associated with PLEs.
Finally, the current study examined neural differences between psychosis-spectrum and control individuals. Future studies should examine how these differences relate to dynamic connectivity in individuals with a psychotic disorder. Some previous findings suggest that neural alterations underlying subclinical psychosis are similar to that of psychotic disorders; while others suggest that some neural alterations show compensatory patterns, which may confer resilience to conversion (7).
Conclusions
Here we have demonstrated that psychotic-like experiences are associated with alterations in large-scale network dynamics. Specifically, individuals experiencing PLEs differed in their time spent in two dynamic connectivity states. They spent less time in a state exhibiting a more “typical” connectivity pattern across networks (State 2); and they spent more time in a state reflecting hyper-connectivity within Visual regions and hypo-connectivity in the DMN (State 1). Consistent with findings of executive alterations across the psychosis spectrum, spending more time in State 1 and less time in State 3 was associated with worse Executive Function in individuals experiencing PLEs. Overall, results suggest that the pattern observed in State 1 reflects pathology related to psychosis. This finding highlights the need to investigate not only static connectivity, but dynamic fluctuations in functional connectivity, and indicates that such dynamic fluctuations are sensitive to even subtle symptomatic change, potentially representing a novel endophenotype for psychosis.
Supplementary Material
Figure S1. Summary Measures of Centroid Distance and Centroid Reliability for k = 2…9 Cluster Solutions. (A.) Within-Cluster Sums of Each Timepoint’s Euclidean Distance to Centroid (sumd) for each of the k-Cluster Solutions. (B.) Mean Intraclass Correlation (ICC) Averaged Over the k-States for Each of the k-Cluster Solutions. The Red Arrow Points to the Value at k = 3, the Optimal Solution.
Figure S2. State Centroids for Each of the Four Resting-State Runs.
Figure S3. Network Maps. Each Network Map Represents the Average of All Thresholded Component Maps Classified as that Network.
Table S1. Mean Network Connectivity Values for Each State and Each Network Pair. All Timepoints Classified as Each State were First Averaged Across All Connections Within Each Network Pair and Then Averaged Across Participants.
Acknowledgments
This research was supported by the following NIH grants: R01 MH101506, R01 MH108654, R01 EB016061, and P41 EB015909.
Footnotes
Financial Disclosures
The authors have no biomedical financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeRosse P, Karlsgodt KH. Examining the Psychosis Continuum. Current behavioral neuroscience reports. 2015;2:80–89. doi: 10.1007/s40473-015-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic reviews. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 3.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychological medicine. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- 4.Wigman JT, Vollebergh WA, Raaijmakers QA, Iedema J, van Dorsselaer S, Ormel J, et al. The structure of the extended psychosis phenotype in early adolescence--a cross-sample replication. Schizophrenia bulletin. 2011;37:850–860. doi: 10.1093/schbul/sbp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurens KR, Hobbs MJ, Sunderland M, Green MJ, Mould GL. Psychotic-like experiences in a community sample of 8000 children aged 9 to 11 years: an item response theory analysis. Psychological medicine. 2012;42:1495–1506. doi: 10.1017/S0033291711002108. [DOI] [PubMed] [Google Scholar]
- 6.Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophrenia bulletin. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt A, Diwadkar VA, Smieskova R, Harrisberger F, Lang UE, McGuire P, et al. Approaching a network connectivity-driven classification of the psychosis continuum: a selective review and suggestions for future research. Frontiers in human neuroscience. 2014;8:1047. doi: 10.3389/fnhum.2014.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsgodt KH, Sun D, Cannon TD. Structural and Functional Brain Abnormalities in Schizophrenia. Current directions in psychological science. 2010;19:226–231. doi: 10.1177/0963721410377601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smieskova R, Roiser JP, Chaddock CA, Schmidt A, Harrisberger F, Bendfeldt K, et al. Modulation of motivational salience processing during the early stages of psychosis. Schizophrenia research. 2015;166:17–23. doi: 10.1016/j.schres.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Fusar-Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. European psychiatry: the journal of the Association of European Psychiatrists. 2012;27:181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Satterthwaite TD, Vandekar SN, Wolf DH, Bassett DS, Ruparel K, Shehzad Z, et al. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Molecular psychiatry. 2015;20:1508–1515. doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, et al. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA psychiatry. 2015;72:456–465. doi: 10.1001/jamapsychiatry.2014.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheffield JM, Kandala S, Burgess GC, Harms MP, Barch DM. Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biological psychiatry: cognitive neuroscience and neuroimaging. 2016;1:498–506. doi: 10.1016/j.bpsc.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr JM, Turner JA, Mittal VA. Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. NeuroImage Clinical. 2014;4:343–351. doi: 10.1016/j.nicl.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fryer SL, Woods SW, Kiehl KA, Calhoun VD, Pearlson GD, Roach BJ, et al. Deficient Suppression of Default Mode Regions during Working Memory in Individuals with Early Psychosis and at Clinical High-Risk for Psychosis. Frontiers in psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, Malhotra AK. Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry research. 2014;224:296–302. doi: 10.1016/j.pscychresns.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of general psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 19.Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophrenia research. 2009;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Effects of working memory demand on neural mechanisms of motor response selection and control. Journal of cognitive neuroscience. 2013;25:1235–1248. doi: 10.1162/jocn_a_00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon TD, van Erp TG, Glahn DC. Elucidating continuities and discontinuities between schizotypy and schizophrenia in the nervous system. Schizophrenia research. 2002;54:151–156. doi: 10.1016/s0920-9964(01)00362-0. [DOI] [PubMed] [Google Scholar]
- 23.Glahn DC, Williams JT, McKay DR, Knowles EE, Sprooten E, Mathias SR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biological psychiatry. 2015;77:75–83. doi: 10.1016/j.biopsych.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsgodt KH, van Erp TG, Bearden CE, Cannon TD. Altered relationships between age and functional brain activation in adolescents at clinical high risk for psychosis. Psychiatry research. 2014;221:21–29. doi: 10.1016/j.pscychresns.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cetin MS, Houck JM, Rashid B, Agacoglu O, Stephen JM, Sui J, et al. Multimodal Classification of Schizophrenia Patients with MEG and fMRI Data Using Static and Dynamic Connectivity Measures. Frontiers in neuroscience. 2016;10:466. doi: 10.3389/fnins.2016.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, et al. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clinical. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y, Pearlson GD, Lin D, Sui J, Chen J, Salman M, et al. Identifying dynamic functional connectivity biomarkers using GIG-ICA: Application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Human brain mapping. 2017 doi: 10.1002/hbm.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Q, Erhardt EB, Sui J, Du Y, He H, Hjelm D, et al. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. NeuroImage. 2015;107:345–355. doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2009. [Google Scholar]
- 33.Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, et al. The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE transactions on medical imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Hyvarinen A, Varoquaux G, Miller KL, Beckmann CF. Group-PCA for very large fMRI datasets. NeuroImage. 2014;101:738–749. doi: 10.1016/j.neuroimage.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindquist MA, Xu Y, Nebel MB, Caffo BS. Evaluating dynamic bivariate correlations in resting-state fMRI: a comparison study and a new approach. NeuroImage. 2014;101:531–546. doi: 10.1016/j.neuroimage.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choe AS, Nebel MB, Barber AD, Cohen JR, Xu Y, Pekar JJ, et al. Comparing test-retest reliability of dynamic functional connectivity methods. NeuroImage. 2017;158:155–175. doi: 10.1016/j.neuroimage.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Y, Pearlson GD, Yu Q, He H, Lin D, Sui J, et al. Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophrenia research. 2016;170:55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in human neuroscience. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological medicine. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 47.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Human brain mapping. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophrenia bulletin. 2013;39:168–178. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophrenia bulletin. 2012;38:803–813. doi: 10.1093/schbul/sbq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry. 2015;72:882–891. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreopoulos B, An A, Wang X, Schroeder M. A roadmap of clustering algorithms: finding a match for a biomedical application. Briefings in bioinformatics. 2009;10:297–314. doi: 10.1093/bib/bbn058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary Measures of Centroid Distance and Centroid Reliability for k = 2…9 Cluster Solutions. (A.) Within-Cluster Sums of Each Timepoint’s Euclidean Distance to Centroid (sumd) for each of the k-Cluster Solutions. (B.) Mean Intraclass Correlation (ICC) Averaged Over the k-States for Each of the k-Cluster Solutions. The Red Arrow Points to the Value at k = 3, the Optimal Solution.
Figure S2. State Centroids for Each of the Four Resting-State Runs.
Figure S3. Network Maps. Each Network Map Represents the Average of All Thresholded Component Maps Classified as that Network.
Table S1. Mean Network Connectivity Values for Each State and Each Network Pair. All Timepoints Classified as Each State were First Averaged Across All Connections Within Each Network Pair and Then Averaged Across Participants.