Abstract
The nematode C. elegans exhibits complex thermal experience-dependent navigation behaviors in response to environmental temperature changes of as little as 0.01°C over a >10°C temperature range. The remarkable thermosensory abilities of this animal are mediated primarily via the single pair of AFD sensory neurons in its head. In this review, we describe the contributions of AFD to thermosensory behaviors and temperature-dependent regulation of organismal physiology. We also discuss the mechanisms that enable this neuron type to adapt to recent temperature experience and to exhibit extraordinary thermosensitivity over a wide dynamic range.
Keywords: Thermosensation, C. elegans, AFD, receptor guanylyl cyclases, phosphodiesterases, adaptation
The terrestrial nematode C. elegans is commonly found associated with decaying plant material and in compost heaps [30,24,22], environments with large localized temperature gradients. Although the laboratory reference strain is derived from animals first isolated in Bristol, England [30], this nematode species is a global citizen; C. elegans has been found in diverse locales although largely concentrated within temperate zones [36,1]. Thus, the habitat of C. elegans is not only subject to temperatures that fluctuate locally, but that also change daily and seasonally. C. elegans is, therefore, a temperature ‘generalist’, and is able to survive and reproduce in a relatively wide temperature range of about 12°C −26°C [2,73,3,80]. Moreover, these tiny 1 mm long worms are exquisitely thermosensitive, and detect temperature changes of 0.01°C or less across this thermal range [51,67]. Here we review the current knowledge of the molecular and neuronal mechanisms by which a single thermosensory neuron pair, the AFD neurons, regulates this organism’s extraordinary thermosensory abilities. The AFD neurons detect additional and ubiquitous physical stimuli including CO2 gas [10], and possibly magnetic fields [86]. Since little is known about whether or how these functions influence temperature-sensing, these functions will not be discussed here. In addition, while sensory neurons other than AFD have also been shown to respond to temperature, these neurons play more minor roles in temperature-regulated behavioral and physiological responses [42,8,6,60], and are also not further considered here.
Thermosensory navigation behaviors
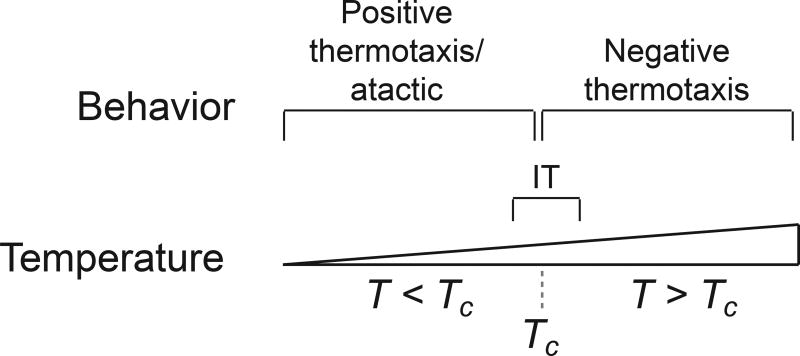
When placed on a spatial thermal gradient within the animals’ physiological temperature range, worms exhibit one of four distinct behaviors. They move up the gradient towards warmer temperatures (PT; positive thermotaxis), down the gradient towards cooler temperatures (NT; negative thermotaxis), track isotherms perpendicular to the gradient (IT: isothermal tracking), or are insensitive to thermal gradients (atactic) [29]. The specific behavior that will be exhibited is dictated by: a) the worms’ temperature experience (Tc), i.e. the temperature to which they were exposed for 3–5h prior to the assay, b) the temperature they experience on the gradient (T), and c) their satiety state [29]. At T>Tc, worms exhibit NT; at T<Tc, they exhibit PT under a circumscribed set of conditions close to Tc [68,34] or are atactic; and at T=Tc ±∼2°C, they perform IT (Figure 1). If Tc is altered, the temperature ranges at which these behaviors are exhibited also shift correspondingly. If animals are starved for 3–5h at a constant temperature, animals are largely atactic [29,68,15]. Thus, in these behavioral assays, C. elegans does not exhibit a rigid preferred temperature. Instead, the temperature to which these animals navigate is flexible, and is determined primarily via comparison of their temperature experience with the ambient temperature under well-fed conditions. Simulation of thermoregulatory behaviors in soil-like environments suggests that this Tc-dependent behavioral plasticity may permit C. elegans to be robust to temperature changes these animals are likely to experience in their natural environments [68].
Figure 1.
C. elegans exhibits distinct navigation behaviors at temperatures relative to their recent temperature experience. Positive thermotaxis - movement towards warmer temperatures; Atactic - movement without regard to temperature; Negative thermotaxis - movement towards cooler temperatures; IT - isothermal tracking behavior. T - ambient temperature on spatial thermal gradient; Tc - temperature experienced 3–5h prior to assay. Adapted with permission from [95].
Detailed tracking of worm locomotion has described the behavioral strategies that worms employ to navigate spatial thermal gradients. NT behavior is primarily achieved by klinokinesis. In this strategy, the duration of forward movement (or runs) is extended as temperatures fall, and conversely, the frequency of reorientation movements is increased as temperatures rise [70,19,52], resulting in net displacement towards cooler temperatures. In addition, following reorientation maneuvers, animals preferentially bias their runs towards cooler temperatures [52]. In contrast, PT behavior does not employ klinokinesis, but is driven by biasing runs towards warmer temperatures following a reorientation [52].
IT behavior is particularly intriguing. Worms do not actively seek isotherms on a spatial thermal gradient [51,29,70]. However, if they are oriented on an isotherm within the permissive range of T=Tc ±∼2°C, the temperature variations detected by the side-to-side movement of their heads is harnessed via unknown mechanisms to maintain forward movement on the isotherm and suppress reorientations [51]. Characterization of the shallowest gradient steepness that permits IT behavior suggests that worms may be able to detect temperature differences of as little as 0.005°C behaviorally in order to track isotherms [70,51].
These behaviors raise several interesting questions. How and where is Tc experience encoded? How do worms sense and compare T with their Tc ‘memory’ to drive specific behaviors? How do worms detect tiny temperature changes over a broad temperature range to modulate their navigation behaviors?
Temperature responses in the AFD thermosensory neurons
The bilateral pair of bipolar AFD sensory neurons resides in the head of C. elegans, with dendrites extending to the tip of the nose, and axons entering into the nerve ring, the major neuropil in the head [89,91]. Physical or genetic ablation of these neurons abolishes IT and severely affects NT/PT behaviors [53,51,88], establishing AFD as the dominant regulator of worm thermosensory behaviors. Temperature-sensing by AFD neurons may also enable worms to navigate toward appropriately humid environments [69].
The AFD neurons are unique in their ability to detect and respond to tiny thermal fluctuations. A recent study monitoring calcium dynamics in dozens of neurons simultaneously showed that activity in only the AFD neurons is strongly correlated with thermal fluctuations in freely moving worms [85]. Such responses are evident not only in freely moving animals traversing spatial thermal gradients [18,83], but also in immobilized animals exposed to time-varying thermal stimuli [35,17,90]. Remarkably, these responses are largely preserved in AFD neurons dissociated from embryos and maintained in culture [38], suggesting that these responses are generated cellautonomously.
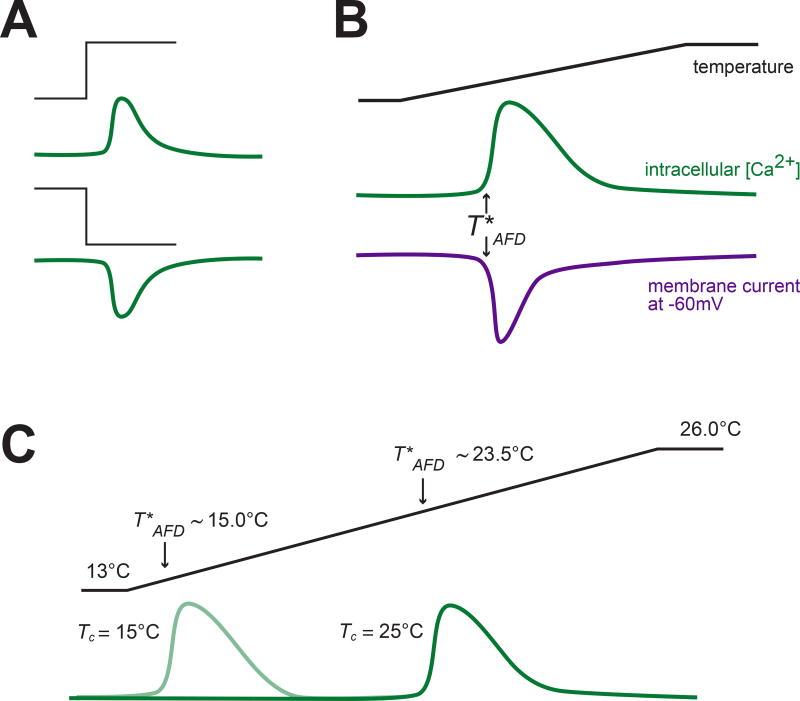
The AFD neurons are activated by thermal fluctuations and do not appear to signal during prolonged sojourns at a constant temperature [35,17,19,83]. Such response dynamics are evident in the response to cooling and warming steps that elicit transient decreases and increases in intracellular calcium, respectively [35,17,19] (Figure 2A). Similarly, cooling hyperpolarizes and warming depolarizes AFD by inhibiting and activating a non-selective cation current, respectively [67]. Consistent with activation of this current by a soluble second messenger, tiny and rapid temperature shifts modulate the current with a latency of ∼100 ms [67]. How sensitive is AFD to temperature changes? Though imperfect [87], Q10 is a common metric used to distinguish the ordinary temperature-dependence expected of biochemical reactions from the extraordinary temperature sensitivity underpinning the function of thermoreceptor molecules and cells like the AFD neurons. The temperature dependence of the thermoreceptor current in AFD is extraordinary, having an aggregate Q10 of >1020 [67]. Such sensitivity is specific to the thermoreceptor currents, since voltage-activated currents in AFD have a Q10 of less than 3 [67]. This extraordinary thermosensitivity reflects the action of a nonlinear amplification cascade (see below) akin to the one that mediates phototransduction and enables vertebrates, including humans, to detect single photons of visible light [28,82,31].
Figure 2.
The AFD sensory neurons are activated by warming and inhibited by cooling. A) Idealized calcium transients (green) evoked by thermal up-steps (top) and down-steps (bottom) [35,17]. B) Idealized calcium transients (green) and thermoreceptor currents (violet) evoked by a rising thermal ramp [67]. Arrows indicate T*AFD C) Acclimation to a new Tc shifts T*AFD. Calcium transients are shown in green. Black traces in A–C show thermal stimuli delivered to immobilized animals.
Interestingly, activity in response to thermal fluctuations is observed in AFD only at and above a response threshold (T*AFD) that is closely correlated with Tc, as determined by imaging AFD calcium dynamics in vivo [35,17,8] and in vitro [38], and by recording thermoreceptor currents in ex vivo preparations [67] (Figure 2B). Shifting animals to a new Tc results in a concomitant shift in T*AFD [35,17,8,67,38] (Figure 2C), suggesting that behavioral acclimation to Tc is reflected in part via adaptation of T*AFD [95,67,88,8] as well as AFD synaptic output [8]. A recent study has shown that the T*AFD acclimation process occurs in two phases [95]. Under the specific conditions used, the first phase is brief and has a time constant of minutes and the second is much longer with a time constant of nearly 5 hours that matches the time required for behavioral adjustments to Tc [95]. In these experiments, acclimation to temperature downshifts was found to be slower than acclimation to temperature upshifts and could be fit by a single exponential [95,8,35,17]. In electrophysiological experiments, the threshold for warming-evoked currents, was shown to adapt to higher and lower holding temperatures with time constants of ∼4 minutes and ∼8 minutes, respectively [67]. This pattern and timing is similar to short-term adaptation in calcium signaling, suggesting that a common molecular mechanism is responsible for adaptation on this time scale. Regardless of the mechanism, such adaptation ensures that AFD retains its extraordinary sensitivity to small deviations from T*AFD, across a wide range of temperatures.
Can temperature responses in AFD alone account for the experience-dependent thermosensory behaviors exhibited by C. elegans? This is unlikely to be the case. Responses of AFD to thermal fluctuations overlap with the temperature range at which animals exhibit NT or PT behavior [52,67,35,17]. In addition, the ability of AFD to respond to small sinusoidal thermal fluctuations around T*AFD, but not at warmer temperatures, enables IT behavior [90]. However, although a few studies have addressed this issue [17,7,59,55], how temperature responses in AFD are translated through the circuit into distinct behavioral strategies at temperatures relative to Tc is not yet fully described. Moreover, T*AFD adaptation and temperature-modulated responses are unaffected in AFD in starved animals [83,67] even though thermosensory behaviors are abolished, suggesting that circuit mechanisms downstream or in in parallel with AFD modulate thermosensory behavioral output in response to internal satiety state. Together, these results indicate that while the extraordinary thermoresponsive properties of AFD are critical for thermosensory behaviors in C. elegans, integration of AFD-dependent signals with additional context-and experience-dependent cues in the circuit likely drive specific behaviors in a Tc experience-dependent manner.
Molecular mechanisms of thermotransduction in AFD
Any thermotransduction mechanisms and pathways in AFD must account for the extraordinary thermosensitivity and broad dynamic range of this neuron type. Diverse sensory systems such as those that detect chemicals and light achieve such feats via multiple molecular mechanisms. These include the expression of a receptor(s) with high affinity for the ligand, amplification of the initial signal via soluble second messengers, and rapid adaptation to maintain sensitivity to stimulus changes even at saturating stimulus levels. It has long been hypothesized that cGMP is likely the major transducer of the thermosensory signal in AFD [20,41,29], such that warming increases intracellular cGMP concentrations and activates a cGMP-gated ion channel. But how do temperature changes regulate cGMP flux in AFD?
The C. elegans genome encodes 27 receptor-type transmembrane guanylyl cyclases (rGCs) that catalyze cGMP from GTP [94,62]. Of these, the GCY-8, GCY-18, and GCY-23 rGCs cluster together in the genome and in a phylogenetic tree, are expressed exclusively in AFD, and are localized to their sensory endings [94,33]. Animals mutant for one or two of these AFD-specific rGCs (henceforth referred to as AFD-rGCs) exhibit lower T*AFD, alter the ability of AFD to follow small amplitude temperature oscillations around T*AFD, and disrupt IT behavior or the temperature range in which IT behavior is exhibited [90]. However, NT behavior is only partially disrupted in these animals [33,88], and their AFD neurons retain the ability to respond to a rising oscillatory temperature ramp [90,78]. In contrast, animals triply mutant for all three rGCs are atactic [33,90,88], and temperature changes fail to elicit thermoreceptor current or calcium flux in the AFD neurons of these triple mutants [78,67]. Together, these observations suggest that while the functions of all three AFD-rGCs contribute to precisely shaping AFD thermosensory properties, these rGCs act partly redundantly to mediate thermotransduction in AFD.
Besides being necessary for thermotransduction, might these AFD-rGCs be sufficient to confer thermosensory responses with high Q10 onto non-thermosensory cells? GCY-18 and GCY-23, although not GCY-8, was found to confer robust temperature responses upon misexpression in chemosensory neurons in C. elegans as measured via imaging of intracellular calcium dynamics [78]. GCY-23 was also sufficient to confer temperature responses onto vulval muscles [78]. Moreover, within the limitations of calcium imaging, quantification of the aggregate Q10 value of rGC-conferred temperature responses in chemosensory neurons showed that these molecules are sufficient to confer robust and sensitive temperature responses upon misexpression in non-thermosensory cell types [78]. However, these AFD-rGCs do not exhibit a defined temperature response threshold. Instead, the threshold of temperature response is determined by the individual rGC protein and the cellular context, suggesting that the temperatures at which these proteins become activated are flexible [78]. These experiments suggest that temperature modulates cGMP flux in AFD in part via regulation of rGC activity. Interestingly, the GC-G rGC was recently also shown to be both necessary and sufficient for sensing cool temperatures in the rodent Grueneberg ganglion [12], raising the possibility that rGCs may represent a new and conserved family of thermoreceptor molecules.
Temperature-dependent changes in intracellular cGMP levels in AFD are unlikely to be mediated solely via modulation of rGC activity. As in all signaling pathways, mechanisms must be in place that terminate signaling and allow adaptation. Moreover, since the Q10 value of the AFD-rGC-conferred temperature responses on chemosensory neurons is lower than that calculated for AFD via similar methods [78], additional mechanisms may also contribute to AFD thermosensitivity. Temperature-dependent regulation of cGMP hydrolysis is one plausible additional way to fine-tune sensitivity to thermal fluctuations and govern T*AFD. Indeed, the PDE-2 cGMP-selective phosphodiesterase was shown to be expressed in AFD; animals with a deletion in the pde-2 gene exhibit thermotaxis behavioral defects under specific growth and assay conditions [88]. Importantly, while the Q10 of temperature-evoked currents is not altered, T*AFD is higher, and thermoreceptor currents are prolonged, in the AFD neurons of pde-2 mutants [88]. The AFD neurons also express additional PDE genes which may also modulate AFD properties [88,76]. These results suggest that together with the rGCs, PDEs play a critical role in regulating temperature-dependent cGMP levels in AFD, and in shaping the threshold and temporal dynamics of the response. Whether one or more PDEs themselves are responsive to temperature changes remains to be determined.
How are cGMP levels translated into neuronal depolarization? The AFD neurons express multiple cyclic nucleotide-gated channel proteins, a subset of which is essential for thermotransduction [41,20,29]. While the TAX-4 alpha and TAX-2 beta subunits can form heteromeric channels in heterologous cells, TAX-4 also forms homomeric channels with a remarkably high affinity for cGMP (K1/2 - 0.4 µM) [40,63,48]. Loss of function of either protein results abolishes temperature responses in AFD as measured by either calcium imaging or electrophysiology [67,35], and both tax-2 and tax-4 mutants are atactic [29,53]. The AFD neurons also express the CNG-3 alpha subunit which is implicated in thermotolerance [16], but a role for this subunit in AFD thermotransduction has not been described. Similar to other sensory neurons in C. elegans, the primary TAX-2/TAX-4-dependent calcium influx is expected to be amplified by depolarization-activated calcium channels [25,46,96,11,79]. Whether such channels, calcium release from intracellular stores, or both factors play a role in AFD thermotransduction has not yet been established.
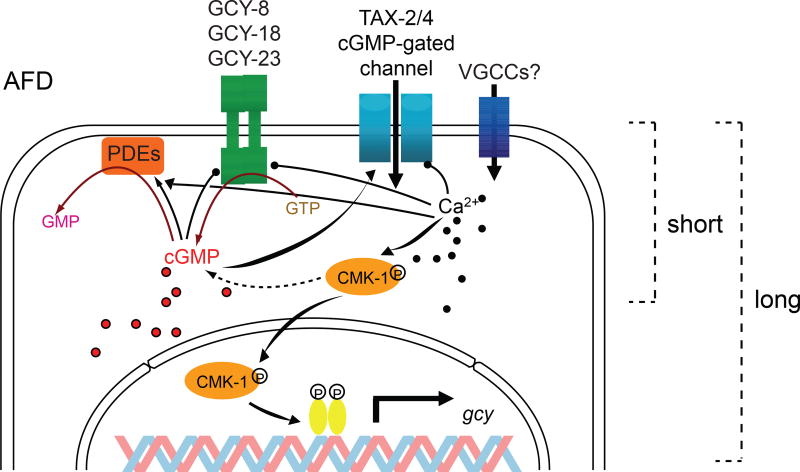
Taken together, the current working model for thermotransduction (summarized in Figure 3) in AFD posits that at T>T*AFD, the rGCs are activated to generate cGMP. Rising cGMP concentrations activate TAX-2/TAX-4 channels to permit cation influx and depolarization. Rising temperatures and cGMP and or calcium concentrations may also activate PDEs such as PDE-2 which subsequently hydrolyzes cGMP to terminate the response. Amplification of the T response by a cGMP-dependent signaling pathway together with the high cGMP affinity of the cyclic nucleotide-gated channels underlies in part the extraordinary thermosensitivity of AFD.
Figure 3.
Model of thermosensory transduction and adaptation pathways in AFD. Warming activates the AFD-rGCs GCY-8, GCY-18 and GCY-23 to increase intracellular cGMP concentrations. cGMP gates TAX-2/TAX-4-encoded cation channels. cGMP hydrolysis is mediated by PDEs such as PDE-2 whose activity may also be temperature-regulated. cGMP and/or Ca2+ feeds back to terminate signaling and promote rapid adaptation via inhibition of rGCs, activation of PDEs, and/or decreasing the sensitivity of the TAX-2/TAX-4 channels. Long-term adaptation of T*AFD also requires CMK-1-mediated changes in AFD-rGC and other gene expression. VGCCs - voltage-gated calcium channels (hypothesized). Adapted with permission from [95].
Temperature adaptation mechanisms in AFD
As noted above, adaptation of AFD thermosensory response occurs on both a fast (mins) and slow (hrs) timescale [67,95,7,29,88]. The mechanisms underlying fast adaptation of T*AFD are not yet fully understood. However, given that this adaptation occurs on a timescale of minutes [88,95,67], this mechanism is likely to be transcription-independent. Calcium buffering was shown to slow adaptation [67], suggesting that a calcium-dependent mechanism regulates this short-term neuronal plasticity. In addition, modulation of intracellular cGMP levels affects T*AFD adaptation; genetic or pharmacological manipulations predicted to lower or raise intracellular cGMP levels decrease or increase T*AFD[90,88], respectively. However, whether these perturbations specifically alter short-term T*AFD adaptation or also affect long-term plasticity has not yet been explored. Nevertheless, these observations suggest that intracellular cGMP and/or calcium feedback terminates signaling and enables adaptation.
The cellular targets of this feedback in regulating rapid adaptation are currently unclear. Animals mutant for the calcium-regulated NCS-1 frequenin-like protein exhibit defects in T*AFD adaptation [88]. Frequenin promotes phosphodiesterase activity [72] suggesting that PDE-2 may be a potential substrate for regulation [88] (Figure 3). In vertebrate phototransduction, neuronal calcium sensor proteins play a critical role in restoring cellular cGMP levels following a light pulse via interaction with the intracellular domains of retinal rGCs [49,75,74,39]. Calcium-dependent inhibition of AFD-rGC activity via calcium sensor proteins could similarly play a role in rapid T*AFD adaptation (Figure 3). A subset of these adaptation mechanisms is likely to be AFD-specific. Thus, while AFD-rGCs exhibit Tc-correlated adaptation in AFD, their response thresholds are largely Tc-independent in misexpressing cells [78]. Moreover, analyses of the response thresholds of chimeric AFD-rGC proteins suggest that the response threshold is partly determined by the rGC intracellular domains [78]. A possible unifying explanation for these observations is that cell-specific levels of intracellular cGMP or calcium concentrations set the response threshold to different values via interaction with AFD-rGC intracellular domains, and that in misexpressing cells, the absence of cGMP/calcium-dependent feedback mechanisms such as those present in AFD fail to shift the response threshold in a Tc-dependent manner. Finally, gating of cyclic nucleotide-gated channels via interaction with calcium/calmodulin or cGMP-dependent protein kinases has been implicated in olfactory adaptation in both C. elegans and vertebrates [44,54,13,9,58]; these channels may also represent a target of short-term adaptation mechanisms in AFD (Figure 3).
In addition to the rapid feedback mechanisms to terminate signaling, long-term adaptation of T*AFD to Tc-correlated values also requires temperature-dependent changes in gene expression. Expression of gcy-8, gcy-18 and gcy-23 is 3 to 5-fold higher when worms are grown at 25°C as compared to at 15°C [95]. Animals mutant for the cmk-1 calcium/calmodulin-dependent protein kinase I (CaMKI) gene exhibit decreased expression of all three AFD-rGC genes that is not further altered upon growth at warmer temperatures [95,71], indicating that CMK-1 plays a key role in upregulating AFD-rGC gene expression upon long-term exposure to a warmer temperature (Figure 3). In cmk-1 mutants, while T*AFD shifts at the same rate upon temperature upshift, the magnitude of the shift is significantly decreased [95]. Correspondingly, cmk-1 mutants exhibit lower T*AFD regardless of Tc, with the defect being significantly stronger upon cultivation at warmer temperatures [95,38]. These results suggest that upon temperature upshift, both CMK-1-independent adaptation processes such as the feedback mechanisms described above, as well as a CMK-1-mediated increase in the number of AFD-rGC molecules are required to reset T*AFD to the correct Tc-correlated value (Figure 3). It is likely that the expression of additional genes is also affected in a temperature -dependent manner in cmk-1 mutants, and that this altered expression further contributes to correct long-term adaptation. The molecular mechanisms by which CMK-1 regulates gene expression in AFD is currently unclear but may in part require the Raf kinase pathway [38], and possibly CREB ([57] but also see [95,38]. In addition to the described mechanisms, temperature-regulated systemic signals from tissues such as the intestine may also fine-tune T*AFD[77].
Although T*AFD exhibits both fast and slow adaptation upon temperature shift, behavioral adaptation to Tc experience only occurs on the timescale of hours [29,7]. In other words, animals must be exposed to a new temperature for hours in order to shift the temperature range at which NT, PT and IT behaviors will be exhibited. These observations suggest that adaptation of synaptic output threshold in AFD is linked to the long- but not short-term adaptation of T*AFD. How is AFD sensory activity coupled to adaptation of its presynaptic output? Loss-of-function mutations in the dgk-3 diacylglycerol kinase gene were found to decrease the rate of behavioral adaptation as well as adaptation of AFD synaptic output threshold (measured as the response threshold in the AIY interneurons, the major postsynaptic partners of AFD) without altering the rate of T*AFD adaptation [7]. Similarly, mutations in the pkc-1 (also called ttx-4) protein kinase C gene were shown to alter the operating range of thermotaxis behaviors without affecting temperature response dynamics in AFD [52,61]. In recent studies, PKC-2 has also been implicated in regulating temperature-modulated synaptic output from AFD [45]. These results imply that adaptation of T*AFD is translated into changes in synaptic diacylglycerol levels and protein kinase C activity to alter the threshold of AFD synaptic output, and that this presynaptic adaptation occurs on a timescale that resembles the long timescale of T*AFD adaptation. Thus, the single AFD neuron pair exhibits both short-term transcription-independent, and long-term transcription- and experience-dependent, plasticity mechanisms, features that are generally characteristic of circuits comprised of multiple neurons in more complex organisms [27,32,26].
The specialized sensory endings of AFD shape their thermoresponsive properties
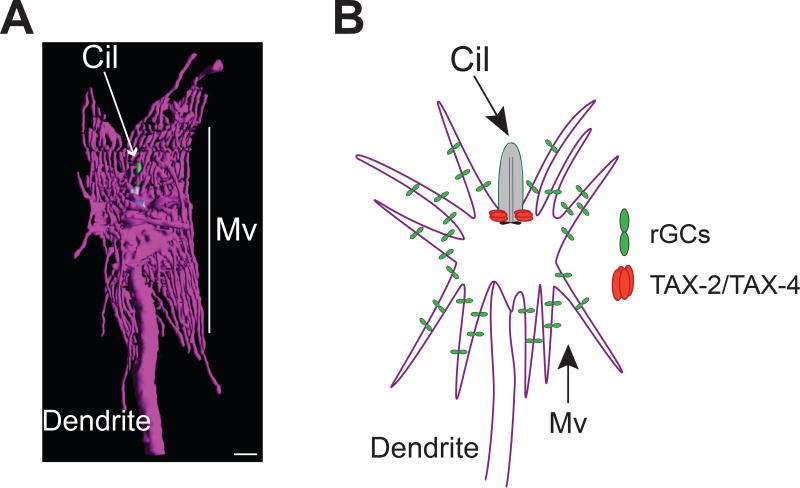
While signal amplification and adaptation are major contributors to the experience-dependent and extraordinary thermosensitive properties of AFD, additional mechanisms are also likely to shape the thermoresponsive features of this neuron type. A major factor is the unique architecture of the AFD sensory endings. Similar to other sensory neurons in C. elegans, a microtubule-based short rod-like cilium is present at the dendritic ends of AFD at the nose of the animal [64,21] (Figure 4A). However, unique to AFD, these endings also contain numerous actin-based microvilli [64,21,56] (Figure 4A) whose structures rely on the integrity of surrounding amphid sheath glial cells [5]. Subcellular localization studies have indicated that while TAX-4 cGMP-gated channels are localized to proximal region of the cilium, other thermotransduction molecules including the AFD-rGCs are present in these ‘finger’-like microvilli [56] (Figure 4B). The dramatically increased membrane surface area to volume ratio potentially allows for the localization of more rGCs and other signaling molecules than would be possible in endings with a relatively simple structure. Analogous to vertebrate photoreceptors, the concentration and organization of thermotransduction molecules in the specialized sensory endings is likely a major contributor to the extraordinary thermosensitivity of AFD.
Figure 4.
Thermotransduction molecules are localized to the specialized sensory endings of AFD. A) 3D reconstruction model of the sensory endings of an AFD neuron. Cil: cilium (green); Mv: microvilli. Scale bar: 500 nm. Adapted from [21]. B) Schematic showing localization of the AFD-rGCs and cGMP-gated channels at the base of the cilium and in the microvilli, respectively. Adapted from [56].
Interestingly, AFD microvilli architecture appears to be homeostatically regulated by intracellular cGMP levels such that prolonged high levels of cGMP (for 24h or more) result in dramatic shortening of microvilli via modulation of the actin cytoskeleton [76]. Similar to a subset of rGCs in other systems [43], GCY-8 exhibits basal levels of catalytic activity [76] which must be inhibited upon growth at low temperatures to ensure the correct setting of T*AFD to low values [90,88]. Animals in which GCY-8 is inappropriately activated, or additional manipulations that increase intracellular cGMP levels, lead to shortened AFD microvilli, particularly at low temperatures [76]. How is basal GCY-8 activity regulated at low temperatures to maintain AFD sensory ending integrity? It has now been shown that GCY-8 catalytic activity is inhibited via binding of Cl- ions to its extracellular domain [76]. Neither GCY-23 nor GCY-18 appear to contain a similar Cl− binding site in their extracellular domains, suggesting that GCY-8 activity alone may be modulated by Cl− [76]. In turn, extracellular Cl- ion concentrations are regulated via the KCC-3 K+/Cl− cotransporter expressed in the ensheathing amphid sheath glial cells [76,93]. Reduced Cl- concentrations as expected in kcc-3 mutants also shorten microvilli [76], and affect thermotaxis behaviors and AFD temperature response properties [76,93]. These experiments raise the possibility that temperature-dependent modulation of the ionic environment by glial cells regulates AFD sensory ending architecture, and may thereby also influence AFD thermosensitivity.
Regulation of systemic temperature responses by AFD
In principle, since all physiological and biochemical processes are temperature-sensitive, and the body temperature of a small ectotherm such as C. elegans is not different than the ambient temperature, a dedicated thermosensory system may not be essential to regulate whole body thermal homeostasis. However, work from a number of labs has now suggested that AFD not only directs navigation on thermal gradients in response to temperature changes, but is also important for regulating long-term animal physiology.
In one set of studies, AFD has been suggested to play a critical role in coordinating heat shock responses throughout the body via serotonin-mediated signaling [65,81]. Animals mutant for thermotransduction in AFD fail to mount appropriate heat shock responses in multiple somatic tissues, thereby reducing organismal thermotolerance [65]. Conversely, optogenetic activation of AFD is sufficient to induce HSF1 expression in somatic tissues even in the absence of a heat shock [81].
In a second series of experiments, AFD has been implicated in regulating temperature-dependent longevity. Ectotherms are known to exhibit longer lifespan at lower temperatures [50,23]. Consistent with this notion, C. elegans lives longer at cooler temperatures than it does at warmer ones [37,47,84]. Interestingly, AFD was shown to antagonize the heat-mediated reduction of lifespan, such that AFD-ablated animals exhibit an even shorter lifespan than wild-type animals at 25°C [47]. AFD promotes longevity at warmer temperatures by CMK-1-dependent upregulation of the FLP-6 neuropeptide in AFD; FLP-6 in turn regulates insulin and sterol hormone signaling to increase lifespan [14]. In contrast, the cold-dependent promotion of longevity is AFD-independent, and has been suggested to be mediated via TRPA1 channel function in the intestine and elsewhere [92]. Together, these observations suggest that AFD not only signals to synaptically connected neurons to drive behavior, but can also govern systemic temperature responses on different timescales via multiple signaling pathways.
Open questions and future directions
The AFD neurons of C. elegans provide a fascinating single neuron system in which to explore the molecular basis of thermosensation and plasticity of behaviors linked to thermosensation. Although there has been much progress on both fronts in recent years, several questions remain to be fully addressed.
Are the AFD-rGCs direct thermosensors? Although these molecules are both necessary and sufficient to mediate thermosensation in C. elegans, in the absence of successful expression in heterologous systems, is not yet firmly established that these molecules themselves respond to temperature.
Why does the AFD neuron require the function of three rGCs? Although all three AFD-rGCs act partly redundantly to mediate thermosensation in AFD, each of these proteins also has unique properties in AFD. For instance, GCY-8, but not GCY-18 and GCY-23, is regulated by Cl− [76], and single and double AFD-rGC mutants exhibit subtle but distinct phenotypes [90,33].
What are the mechanisms by which rapid adaptation to temperature experience is achieved in AFD? How do intracellular cGMP and/or Ca2+ homeostatically reset the response threshold of AFD?
What is the complete complement of genes whose expression is regulated by Tc experience via CMK-1 to set T*AFD and the threshold of synaptic output?
How does AFD direct the exhibition of distinct behavioral strategies in different temperature regimes relative to the animal’s Tc experience? While a role for distinct AFD synaptic outputs in shaping NT and PT behaviors is being described [59,55], little is known about the AFD-driven circuit that regulates IT behavior.
How does temperature experience shape the sensory architecture of AFD, and how does this architecture in turn influence AFD thermosensory properties?
Thermosensation in AFD exhibits features remarkably similar to those described previously in mammalian phototransduction. In both systems, signal amplification via cGMP production and hydrolysis, concentration and organization of signaling molecules in elaborate specialized sensory structures, and feedback-mediated adaptation via intracellular calcium levels contributes to their extraordinary sensitivity and response range [66,4,39]. It will be interesting to establish the extent to which principles similar to vertebrate phototransduction, as well as organism-specific mechanisms, shape the unique properties of this sensory neuron and dictate the amazing thermoresponsive behaviors of this animal.
Acknowledgments
Related work in the authors’ labs is supported in part by the NIH (R35 GM22463 and P01 GM103770 - P.S., and R01 NS047715 - M.B.G.) and funding from the Mathers Foundation (M.B.G.).
References
- 1.Andersen EC, Gerke JP, Shapiro JA, Crissman JR, Ghosh R, Bloom JS, Felix MA, Kruglyak L. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet. 2012;44:285–290. doi: 10.1038/ng.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JL, Albergotti L, Ellebracht B, Huey RB, Phillips PC. Does thermoregulatory behavior maximize reproductive fitness of natural isolates of Caenorhabditis elegans? BMC Evol Biol. 2011;11:157. doi: 10.1186/1471-2148-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angilletta MJ. Thermal Adaptation: A theoretical and empirical synthesis. Oxford University Press; Oxford, UK: 2009. [Google Scholar]
- 4.Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beverly M, Anbil S, Sengupta P. Degeneracy and signaling within a sensory circuit contributes to robustness in thermosensory behaviors in C. elegans. J Neurosci. 2011;31:11718–11727. doi: 10.1523/JNEUROSCI.1098-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, Sengupta P, Samuel AD. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci. 2006;9:1499–1505. doi: 10.1038/nn1796. [DOI] [PubMed] [Google Scholar]
- 8.Biron D, Wasserman SM, Thomas JH, Samuel AD, Sengupta P. An olfactory neuron responds stochastically to temperature and modulates C. elegans thermotactic behavior. Proc Natl Acad Sci USA. 2008;105:11002–11007. doi: 10.1073/pnas.0805004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, de Bono M. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69:1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch KE, Laurent P, Soltesz Z, Murphy RJ, Faivre O, Hedwig B, Thomas M, Smith HL, de Bono M. Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat Neurosci. 2012;15:581–591. doi: 10.1038/nn.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao YC, Chen CC, Lin YC, Breer H, Fleischer J, Yang RB. Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 2015;34:294–306. doi: 10.15252/embj.201489652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen TY, Yau KW. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Chen HJ, Tseng WC, Hsu JM, Huang TT, Chen CH, Pan CL. A C elegans thermosensory circuit regulates longevity through crh-1/CREB-dependent flp-6 neuropeptide signaling. Dev Cell. 2016;39:209–223. doi: 10.1016/j.devcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Chi CA, Clark DA, Lee S, Biron D, Luo L, Gabel CV, Brown J, Sengupta P, Samuel AD. Temperature and food mediate long-term thermotactic behavioral plasticity by association-independent mechanisms in C. elegans. J Exp Biol. 2007;210:4043–4052. doi: 10.1242/jeb.006551. [DOI] [PubMed] [Google Scholar]
- 16.Cho SW, Choi KY, Park CS. A new putative cyclic nucleotide-gated channel gene, cng-3, is critical for thermotolerance in Caenorhabditis elegans. Biochem Biophys Res Commun. 2004;325:525–531. doi: 10.1016/j.bbrc.2004.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Clark DA, Biron D, Sengupta P, Samuel ADT. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in C. elegans. J Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark DA, Gabel CV, Gabel H, Samuel AD. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J Neurosci. 2007;27:6083–6090. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark DA, Gabel CV, Lee TM, Samuel AD. Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J Neurophysiol. 2007;97:1903–1910. doi: 10.1152/jn.00892.2006. [DOI] [PubMed] [Google Scholar]
- 20.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 21.Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in C. elegans. eLife. 2014;3:e01948. doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Flouris AD, Piantoni C. Links between thermoregulation and aging in endotherms and ectotherms. Temperature. 2015;2:73–85. doi: 10.4161/23328940.2014.989793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frezal L, Felix MA. C. elegans outside the Petri dish. eLife. 2015;4:05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frokjaer-Jensen C, Kindt KS, Kerr RA, Suzuki H, Melnik-Martinez K, Gerstbreih B, Driscol M, Schafer WR. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J Neurobiol. 2006;66:1125–1139. doi: 10.1002/neu.20261. [DOI] [PubMed] [Google Scholar]
- 26.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21:519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bullet. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 28.Hecht S, Shlaer S, Pirenne MH. Energy, Quanta, and Vision. J Gen Physiol. 1942;25:819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes R, Victora M, Wang RF, Kwiat PG. Measuring temporal summation in visual detection with a single-photon source. Vision Res. 2017;140:33–43. doi: 10.1016/j.visres.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurado P, Kodama E, Tanizawa Y, Mori I. Distinct thermal migration behaviors in response to different thermal gradients in Caenorhabditis elegans. Genes Brain Behav. 2010;9:120–127. doi: 10.1111/j.1601-183X.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- 35.Kimura KD, Miyawaki A, Matsumoto K, Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14:1291–1295. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Kiontke KC, Felix MA, Ailion M, Rockman MV, Braendle C, Penigault JB, Fitch DH. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol Biol. 2011;11:339. doi: 10.1186/1471-2148-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, Nakano S, Amano M, Tsuboi D, Nishioka T, Ikeda S, Yokoyama G, Kaibuchi K, Mori I. Single-cell memory regulates a neural circuit for sensory behavior. Cell Rep. 2016;14:11–21. doi: 10.1016/j.celrep.2015.11.064. [DOI] [PubMed] [Google Scholar]
- 39.Koch KW, Dell’Orco D. Protein and signaling networks in vertebrate photoreceptor cells. Front Molec Neurosci. 2015;8:67. doi: 10.3389/fnmol.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsu H, Jin YH, L’Etoile N, Mori I, Bargmann CI, Akaike N, Ohshima Y. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 1999;821:160–168. doi: 10.1016/s0006-8993(99)01111-7. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu H, Mori I, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 42.Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura KD, Inada H, Matsumoto K, Mori I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science. 2008;320:803–807. doi: 10.1126/science.1148922. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev. 2016;96:751–804. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 44.L’Etoile ND, Coburn CM, Eastham J, Kistler A, Gallegos G, Bargmann CI. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–1089. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- 45.Land M, Rubin CS. A Calcium- and diacylglycerol-stimulated protein kinase C (PKC), Caenorhabditis elegans PKC-2, links thermal signals to learned behavior by acting in sensory neurons and intestinal cells. Mol Cell Biol. 2017;37:e00192–17. doi: 10.1128/MCB.00192-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsch J, Flavell SW, Liu Q, Gordus A, Albrecht DR, Bargmann CI. A circuit for gradient climbing in C. elegans chemotaxis. Cell Rep. 2015;12:1748–1760. doi: 10.1016/j.celrep.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19:715–722. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Zhou X, Wang S, Michailidis I, Gong Y, Su D, Li H, Li X, Yang J. Structure of a eukaryotic cyclic-nucleotide-gated channel. Nature. 2017;542:60–65. doi: 10.1038/nature20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim S, Dizhoor AM, Ames JB. Structural diversity of neuronal calcium sensor proteins and insights for activation of retinal guanylyl cyclase by GCAP1. Front Molec Neurosci. 2014;7:19. doi: 10.3389/fnmol.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loeb J, Northrop JH. Is there a temperature coefficient for the duration of life? Proc Natl Acad Sci USA. 1916;2:456–457. doi: 10.1073/pnas.2.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo L, Clark DA, Biron D, Mahadevan L, Samuel AD. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J Exp Biol. 2006;209:4652–4662. doi: 10.1242/jeb.02590. [DOI] [PubMed] [Google Scholar]
- 52.Luo L, Cook N, Venkatachalam V, Martinez-Velazquez LA, Zhang X, Calvo AC, Hawk J, Macinnis BL, Frank M, Ng JH, Klein M, Gershow M, Hammarlund M, Goodman MB, Colon-Ramos DA, Zhang Y, Samuel AD. Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. Proc Natl Acad Sci USA. 2014;111:2776–2781. doi: 10.1073/pnas.1315205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 54.Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau KW, Zufall F, Reed RR. Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayan A, Laurent G, Sternberg PW. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108:9667–9672. doi: 10.1073/pnas.1106617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen PA, Liou W, Hall DH, Leroux MR. Ciliopathy proteins establish a bipartite signaling compartment in a C. elegans thermosensory neuron. J Cell Sci. 2014;127:5317–5330. doi: 10.1242/jcs.157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishida Y, Sugi T, Nonomura M, Mori I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 2011;12:855–862. doi: 10.1038/embor.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Halloran DM, Altshuler-Keylin S, Zhang XD, He C, Morales-Phan C, Yu Y, Kaye JA, Brueggemann C, Chen TY, L’Etoile ND. Contribution of the cyclic nucleotide gated channel subunit, CNG-3, to olfactory plasticity in Caenorhabditis elegans. Sci Rep. 2017;7:169. doi: 10.1038/s41598-017-00126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohnishi N, Kuhara A, Nakamura F, Okochi Y, Mori I. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011;30:1376–1388. doi: 10.1038/emboj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohta A, Ujisawa T, Sonoda S, Kuhara A. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat Commun. 2014;5:4412. doi: 10.1038/ncomms5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–2137. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortiz CO, Etchberger JF, Posy SL, Frokjaer-Jensen C, Lockery S, Honig B, Hobert O. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paoletti P, Young EC, Siegelbaum SA. C-Linker of cyclic nucleotide-gated channels controls coupling of ligand binding to channel gating. J Gen Physiol. 1999;113:17–34. doi: 10.1085/jgp.113.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 65.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opin Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 67.Ramot D, MacInnis BL, Goodman MB. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci. 2008;11:908–915. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramot D, MacInnis BL, Lee HC, Goodman MB. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J Neurosci. 2008;28:12546–12557. doi: 10.1523/JNEUROSCI.2857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell J, Vidal-Gadea AG, Makay A, Lanam C, Pierce-Shimomura JT. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2014;111:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryu WS, Samuel AD. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined thermal stimuli. J Neurosci. 2002;22:5727–5733. doi: 10.1523/JNEUROSCI.22-13-05727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satterlee JS, Ryu WS, Sengupta P. The CMK-1 CaMKI and the TAX-4 Cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr Biol. 2004;14:62–68. doi: 10.1016/j.cub.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 72.Schaad NC, De Castro E, Nef S, Hegi S, Hinrichsen R, Martone ME, Ellisman MH, Sikkink R, Rusnak F, Sygush J, Nef P. Direct modulation of calmodulin targets by the neuronal calcium sensor NCS-1. Proc Natl Acad Sci USA. 1996;93:9253–9258. doi: 10.1073/pnas.93.17.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schulenburg H, Felix MA. The natural biotic environment of Caenorhabditis elegans. Genetics. 2017;206:55–86. doi: 10.1534/genetics.116.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma RK, Duda T. Ca(2+)-sensors and ROS-GC: interlocked sensory transduction elements: a review. Front Molec Neurosci. 2012;5:42. doi: 10.3389/fnmol.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma RK, Duda T, Makino CL. Integrative signaling networks of membrane guanylate cyclases: Biochemistry and physiology. Front Molec Neurosci. 2016;9:83. doi: 10.3389/fnmol.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singhvi A, Liu B, Friedman CJ, Fong J, Lu Y, Huang XY, Shaham S. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell. 2016;165:936–948. doi: 10.1016/j.cell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011;14:984–992. doi: 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- 78.Takeishi A, Yu YV, Hapiak V, Bell HW, O’Leary T, Sengupta P. Receptor guanylyl cyclases confer thermosensory responses in C. elegans. Neuron. 2016;90:235–244. doi: 10.1016/j.neuron.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanimoto Y, Yamazoe-Umemoto A, Fujita K, Kawazoe Y, Miyanishi Y, Yamazaki SJ, Fei X, Busch KE, Gengyo-Ando K, Nakai J, Iino Y, Iwasaki Y, Hashimoto K, Kimura KD. Calcium dynamics regulating the timing of decision-making in C. elegans. eLife. 2017;6:e21629. doi: 10.7554/eLife.21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK. Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol. 2012;2:2151–2202. doi: 10.1002/cphy.c110055. [DOI] [PubMed] [Google Scholar]
- 81.Tatum MC, Ooi FK, Chikka MR, Chauve L, Martinez-Velazquez LA, Steinbusch HW, Morimoto RI, Prahlad V. Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr Biol. 2015;25:163–174. doi: 10.1016/j.cub.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tinsley JN, Molodtsov MI, Prevedel R, Wartmann D, Espigule-Pons J, Lauwers M, Vaziri A. Direct detection of a single photon by humans. Nat Commun. 2016;7:12172. doi: 10.1038/ncomms12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsukada Y, Yamao M, Naoki H, Shimowada T, Ohnishi N, Kuhara A, Ishii S, Mori I. Reconstruction of spatial thermal gradient encoded in thermosensory neuron AFD in Caenorhabditis elegans. J Neurosci. 2016;36:2571–2581. doi: 10.1523/JNEUROSCI.2837-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Voorhies WA, Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc Natl Acad Sci USA. 1999;96:11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venkatachalam V, Ji N, Wang X, Clark C, Mitchell JK, Klein M, Tabone CJ, Florman J, Ji H, Greenwood J, Chisholm AD, Srinivasan J, Alkema M, Zhen M, Samuel AD. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2016;113:E1082–1088. doi: 10.1073/pnas.1507109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vidal-Gadea A, Ward K, Beron C, Ghorashian N, Gokce S, Russell J, Truong N, Parikh A, Gadea O, Ben-Yakar A, Pierce-Shimomura J. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. Elife. 2015;4 doi: 10.7554/eLife.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voets T. Quantifying and modeling the temperature-dependent gating of TRP channels. Reviews of physiology, biochemistry and pharmacology. 2012;162:91–119. doi: 10.1007/112_2011_5. [DOI] [PubMed] [Google Scholar]
- 88.Wang D, O’Halloran D, Goodman MB. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J Gen Physiol. 2013;142:437–449. doi: 10.1085/jgp.201310959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 90.Wasserman SM, Beverly M, Bell HW, Sengupta P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr Biol. 2011;21:353–362. doi: 10.1016/j.cub.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Transact R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 92.Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZ. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoshida A, Nakano S, Suzuki T, Ihara K, Higashiyama T, Mori I. A glial K /Cl cotransporter modifies temperature-evoked dynamics in C. elegans sensory neurons. Genes Brain Behav. 2015;15:429–440. doi: 10.1111/gbb.12260. [DOI] [PubMed] [Google Scholar]
- 94.Yu S, Avery L, Baude E, Garbers DA. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu YV, Bell HW, Glauser DA, Goodman MB, Van Hooser SD, Sengupta P. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron. 2014;84:919–926. doi: 10.1016/j.neuron.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zahratka JA, Williams PD, Summers PJ, Komuniecki RW, Bamber BA. Serotonin differentially modulates Ca2+ transients and depolarization in a C. elegans nociceptor. J Neurophysiol. 2015;113:1041–1050. doi: 10.1152/jn.00665.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]