Abstract
Children’s early language exposure impacts their later linguistic skills, cognitive abilities, and academic achievement, and large disparities in language exposure are associated with family socioeconomic status (SES). However, there is little evidence about the neural mechanisms underlying the relation between language experience and linguistic and cognitive development. Here, language experience was measured from home audio recordings of 36 SES-diverse 4- to 6-year-old children. During a story-listening functional MRI task, children who had experienced more conversational turns with adults—independently of SES, IQ, and adult-child utterances alone—exhibited greater left inferior frontal (Broca’s area) activation, which significantly explained the relation between children’s language exposure and verbal skill. This is the first evidence directly relating children’s language environments with neural language processing, specifying both an environmental and a neural mechanism underlying SES disparities in children’s language skills. Furthermore, results suggest that conversational experience impacts neural language processing over and above SES or the sheer quantity of words heard.
Keywords: language, socioeconomic status, fMRI, LENA, turn taking, open data, open materials
Children’s early life experiences during sensitive periods of neural plasticity shape the brain structures and functions underlying their cognitive aptitudes. One critical experience is language exposure. Specifically, the language quantity (e.g., number of words) and quality (e.g., sentence complexity, lexical diversity) that young children hear are the foundation of later language and literacy skills (Hirsh-Pasek et al., 2015; Rodriguez & Tamis-LeMonda, 2011; Rowe, 2012) and nonverbal capacities, including executive functioning (Sarsour et al., 2011), math ability (Levine, Suriyakham, Rowe, Huttenlocher, & Gunderson, 2010), and social skills (Connell & Prinz, 2002).
Children’s language exposure varies substantially in relation to their socioeconomic status (SES). SES represents the social and economic resources of an individual or group, and children from lower-SES backgrounds hear fewer and less complex utterances, on average, than their more advantaged peers (Hart & Risley, 1995; Rowe, 2008). In a landmark study, Hart and Risley (1995) estimated that by age 3, children from higher-SES backgrounds had heard 30 million more words than children from lower-SES backgrounds, and other studies report similar trends (Hoff, 2006). Until recently, such studies required time-consuming transcription of parent-child exchanges that limited the amount of data that could be collected. Technological advances now allow for longer, more comprehensive, and less intrusive recordings of naturalistic language exposure. One such device, the Language Environment Analysis (LENA) system, records 16-hr days from the child’s perspective and automatically characterizes children’s language environments. Studies using LENA have confirmed substantial variation in the amount of language children experience in association with SES (Gilkerson et al., 2017).
This broad or distal association between SES and children’s language development must be distinguished from the direct or proximal association between language exposure and language development (Bronfenbrenner & Morris, 2007). SES is a broad characterization of many correlated factors, including income, educational access, other environmental resources, stress, health, and nutrition. Development, however, depends on specific, proximal factors that directly affect the child, such as immediate language exposure. Indeed, the separability of distal SES from proximal language experience is evident in the considerable variation in early language exposure within each SES band (Gilkerson et al., 2017; Hirsh-Pasek et al., 2015; Rowe, Pan, & Ayoub, 2005; Weisleder & Fernald, 2013). When SES is controlled, children’s language exposure remains strongly associated with variation in their language abilities (Rowe, 2012; Weisleder & Fernald, 2013), and differences in exposure partially or fully explain the SES-related gap in language skills (Hoff, 2006).
Despite considerable behavioral research linking children’s language exposure to their language abilities, there is currently no evidence about the neural mechanisms underlying this relationship. There is, however, a growing body of evidence that SES disproportionately affects language ability and language neural systems compared with other neurocognitive domains (Farah, 2017). Structurally, lower SES is associated with reduced gray matter in left perisylvian regions underlying phonological, semantic, and syntactic components of language comprehension and production (Noble et al., 2015; Noble, Houston, Kan, & Sowell, 2012), as well as with bilateral occipitotemporal regions involved in reading (Jednoróg et al., 2012; Mackey et al., 2015). Additionally, functional neuroimaging with language tasks has revealed SES-related differences in left inferior frontal (Raizada, Richards, Meltzoff, & Kuhl, 2008), superior temporal, and fusiform regions (Noble, Wolmetz, Ochs, Farah, & McCandliss, 2006).
Although these studies provide valuable insight on the relation between brain development and SES, they have not aimed to relate brain measures directly to children’s language environments—the proximal factor assumed to directly influence children’s linguistic abilities (Noble et al., 2012; Perkins, Finegood, & Swain, 2013). Relating specific and objectively measureable language experiences to brain development is of particular interest because such experiences can become practical and efficacious targets for intervention (Roberts & Kaiser, 2011). Only two neuroimaging studies have related home language experiences to brain functions. One study using functional MRI (fMRI) with children ages 8 to 12 reported a relation between videotaped home language and right prefrontal activation on a complex nonverbal task (Sheridan, Sarsour, Jutte, D’Esposito, & Boyce, 2012). Another study with infants reported a relation between LENA-measured adult word counts and event-related potentials (ERPs) to phonetic contrasts in left frontal electrodes (Garcia-Sierra, Ramírez-Esparza, & Kuhl, 2016). However, neither study examined the joint roles of SES and language input in relation to linguistic brain functions.
In the present study, we aimed to elucidate how variation in children’s natural language experience relates to brain function underlying language processing and, in turn, to linguistic abilities. Specifically, we hypothesized that LENA measures of language exposure—over and above SES—would be associated with children’s language skills and language-related brain activation, especially in left perisylvian neocortices known to support language.
Method
Participants
Thirty-six children (22 male) between the ages of 4 years, 6 months and 6 years, 10 months (M = 5.8 years, SD = 0.63) and their parents completed this study (see the Supplemental Material available online for justification of sample size). Boys and girls did not significantly differ on any behavioral (all ps > .15), demographic (all ps > .33), language exposure (all ps > .76), or neural measure (maximum z = 1.2). Children were native-English speakers and typically developing, with no history of premature birth, neurological disorders, developmental delay, speech-language therapy, or grade repetition, and all bilaterally passed a four-pure-tone hearing screening (0.5 KHz, 1 KHz, 2 KHz, 4KHz) on the day of assessment. Nineteen children were initially assessed and excluded for not meeting these inclusion criteria.
Twenty of the 36 participants additionally participated in a larger randomized controlled intervention study on parenting practices; only their baseline data (before learning of group assignment) were used here. Twenty-seven other children participated but did not have complete data sets, either because they did not complete the home recordings (n = 6), did not participate in the fMRI scan (n = 11), fell asleep during the fMRI scan (n = 3, details below), or exhibited excessive movement during the fMRI scan (n = 7, details below). These participants did not differ from those in the included sample on SES or on any behavioral scores or language exposure measures. All procedures were approved by the Massachusetts Institute of Technology Institutional Review Board, and written informed consent was obtained from parents.
Behavioral and demographic assessments
Children completed standardized behavioral assessments to characterize verbal and nonverbal cognitive skills (see the Supplemental Material for additional information on executive function assessments). These included the Matrix Reasoning, Picture Memory, and Bug Search subtests of the Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition (WPPSI-IV; Wechsler, 2012); the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4; Dunn & Dunn, 2007); and the Sentence Comprehension, Word Structure, Formulated Sentences, and Recalling Sentences subtests of the Clinical Evaluation of Language Fundamentals, Fifth Edition (CELF-5; Wiig, Semel, & Secord, 2013), which together form the CELF-5 Core Language Score (CLS). Age-normed scaled scores from the three WPPSI-IV subtests were averaged to create a nonverbal composite score. Inclusion criteria required all participants to have a nonverbal composite score, PPVT-4 standard score, and CELF-5 CLS greater than or equal to 1 standard deviation below the mean (16th percentile). Because the CELF-5 only provides age-based norms for children 5 years old or more, 4-year-olds were required to score greater than or equal to the age equivalent for their raw scores on each of the four subtests. Composite verbal scores were created by averaging the PPVT-4 standard score and the CELF-5 CLS.
Additionally, parents filled out questionnaires about the child’s developmental history and family demographics, including highest level of education obtained by both parents and annual household income. When a father was present in the home, maternal and paternal years of education were averaged to create a parental education metric (1 = high school or less, 2 = some college/associate’s degree, 3 = bachelor’s degree, 4 = master’s/professional degree, 5 = doctoral-level degree).
Neuroimaging data acquisition
Neuroimaging took place at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at the Massachusetts Institute of Technology. First, children were acclimated to the MRI environment and practiced lying still in a mock MRI scanner. Data were then acquired on a 3 Tesla Siemens MAGNETOM Trio Tim scanner (Siemens, Erlangen, Germany) that was equipped for echo-planar imaging and had a 32-channel phased-array head coil. An automated scout image was acquired, and shimming procedures were performed to optimize field homogeneity. A whole-head, high-resolution T1-weighted multiecho magnetization-prepared rapid-acquisition gradient echo structural image was acquired using a protocol optimized for movement-prone pediatric populations—repetition time (TR) = 2,530 ms; echo times (TEs) = 1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms; inversion time (TI) = 1,400 ms; flip angle = 7°; resolution = 1-mm isotropic. Whole-brain functional images were acquired with a continuous gradient echoplanar T2*-weighted sequence—T2*-weighted images, TR = 2,500 ms, TE = 30 ms, flip angle = 90°, bandwidth = 2,298 Hz/Px, echo spacing = 0.5 ms, 41 transverse slices with field of view = 192 × 192, in-plane resolution = 3 mm × 3 mm. Before each scan, six dummy volumes were acquired and discarded to reach equilibrium, and online prospective acquisition correction was applied to the echo-planar image sequence throughout the scan.
Functional MRI task
Children passively listened to short, simple stories derived from the Narrative Language Measures (Petersen & Spencer, 2012), the content of which includes events that young children are likely to be familiar with (e.g., playing games, getting hurt). All stories had consistent narrative structure, word count, and language complexity and were recorded by a female native-English speaker. A block design paradigm presented 15-s-long trials consisting of a single story either played normally or in reverse (backward speech), followed by 5 s of silent rest. A third condition (not analyzed here) involved dichotic speech with a different story played in each ear. One run consisted of 6 trials of each condition (18 trials total), such that the run lasted 6 min, with condition order pseudorandomized so the same condition never repeated twice in a row. Each participant was randomly assigned to hear one of two stimulus lists containing all different stories with equal story interest ratings. A female stick figure appeared on a gray screen throughout auditory stimulation to remind children to listen. During the scan, an experimenter stood at the foot of the bore and monitored participants’ attentiveness. If participants closed their eyes for more than 5 s, they were considered asleep, and their data was discarded (n = 2, mentioned above). Before entering the scanner, children completed a short practice with stories not heard in the scanner and were required to correctly answer two of four free-response comprehension questions to ensure familiarity with the task. In the scanner, participants were reminded to listen carefully to the stories to earn prizes on task completion. Participants were not instructed to memorize the passages because the goal was to record brain responses during natural language comprehension. Pilot data from children and adults indicated that participants had very low levels of incidental memory for the passages; thus, no post-MRI comprehension or retention test was administered to avoid burdensome additional testing that would be uninformative. All stimuli and scripts are available for download at http://dx.doi.org/10.7910/DVN/DIDBMQ.
Neuroimaging analysis
Functional MRI data preprocessing and analysis was executed with Nipype (Gorgolewski et al., 2011), utilizing FSL Version 5.0.9 (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012) and FreeSurfer Version 5.3.0 (Fischl, 2012). Functional images were realigned to the first volume of the run, coregistered to the corresponding anatomical image (which had been processed and manually edited as necessary in FreeSurfer to ensure correct gray and white matter boundaries), and then to a standard Montreal Neurological Institute 152 (MNI152) template. Functional time-series outliers (global mean intensity > 3 SDs or volume-to-volume motion > 2 mm) were identified by Artifact Detection Tools (ART; https://www.nitrc.org/projects/artifact_detect/) and removed from the analysis by adding one regressor per outlier to subject-level general linear models (GLMs). Participants with outliers in more than 20% of volumes were excluded from the study (n = 7, mentioned above). Time-series data were high-pass-filtered at 120 s, spatially smoothed using a 6-mm full width half maximum Gaussian kernel, and convolved with the canonical double-gamma hemodynamic response function in FSL, and GLMs were used to create contrast maps for each subject. Subject-level results were combined in mixed effects models using FSL’s FEAT with FMRIB’s Local Analysis of Mixed Effects (FLAME) Stage 1. Results were corrected for multiple comparisons using a conservative cluster-forming threshold of p < .001, connectivity of 26 (voxels must be connected by at least a point), and a family-wise error rate of p < .05 and fractionally projected orthogonally to the surface for visualization purposes. Average activations were extracted from subject-level cortical parcellations (according to the Desikan-Killiany gyral-based atlas) for mediation analysis.
Home audio recordings
Parents were given two LENA Pro digital language processors (DLPs), which are 2-ounce digital recorders that fit in a child’s shirt pocket and store up to 16 hr of digitally recorded audio. Parents were instructed to collect full-day recordings from a consecutive Saturday and Sunday, beginning when the child woke up. The average number of days between assessment or MRI and LENA recording was 8.97 (SD = 5.81), with a maximum of 21 intervening days. After return of the DLPs, the LENA Pro processing system automatically analyzed the audio and provided estimates of the total number of adult words spoken in the recording (i.e., word tokens), the total number of child utterances, and the total number of adult-child conversational turns, defined as a discrete pair of an adult utterance followed by a child utterance, or vice versa, with no more than a 5-s pause between the two. Whereas adult words and child utterances are simple linguistic measures, conversational turns incorporate both linguistic information and nonverbal communicative aspects such as temporal contiguity, adult responsiveness, joint social attention, and exchange of communicative information. As such, conversational turns may represent a more holistic measure of interpersonal conversational engagement.
LENA speech-identification algorithms have been determined to be highly reliable, yielding measures approximately 82% accurate for adult speech and 76% accurate for the speech of infants and young children up to 3 years old (Gilkerson et al., 2017; Zimmerman et al., 2009). Although primarily designed to analyze speech of children younger than 4 years old, the same algorithms were applied to recordings from all participants, such that any potential inaccuracies would be consistent. Running totals for each speech category were calculated for each consecutive 60 min across the 2 days in 5-min increments (e.g., 7:00 a.m.–8:00 a.m., 7:05 a.m.–8:05 a.m, etc.), and the per-participant highest hourly totals of adult words, child utterances, and conversational turns were separately extracted for further analysis. This metric helped minimize differences in daily totals solely due to different recording lengths or loud activities that may have masked speech and misrepresented language input. It also attempted to reduce the amount of overheard speech that was not child directed, since peak language periods are shown to be more similar to engaged structured play situations (Tamis-LeMonda, Kuchirko, Luo, Escobar, & Bornstein, 2017). Such measures of peak naturalistic observations are consistent with those used in other studies utilizing LENA (Garcia-Sierra et al., 2016; Ramírez-Esparza, García-Sierra, & Kuhl, 2014).
Results
Behavioral results
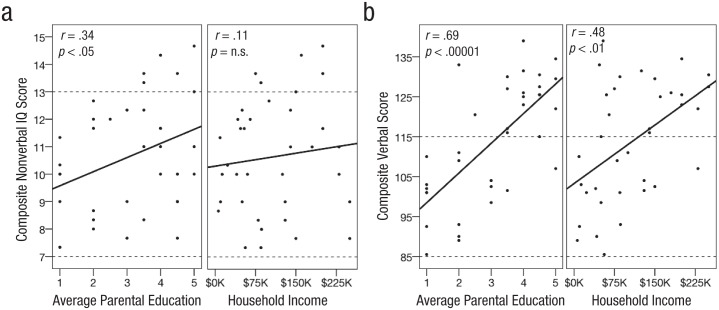
All data (to the extent that they are available to share) are freely available for download at http://dx.doi.org/10.7910/DVN/DIDBMQ. Children’s verbal and nonverbal ability, according to standardized assessments, ranged from low average to above average (verbal composite standard score: range = 86–139, M = 114, SD = 15; nonverbal composite scaled score: range = 7.3–14.7, M = 10.6, SD = 2.1). Parental education ranged from partial high school to doctorate-level degree (M = some college), and familial income ranged from $6,000 to $250,000 per year, with a median of $85,500 per year, consistent with the median familial income in Massachusetts of $90,590. Parental education, but not income, was positively correlated with children’s nonverbal ability (education: r = .34, 95% confidence interval, or CI = [.02, .67], p < .05; income: r = .11, n.s.; Fig. 1a). Although both education and income were correlated with children’s verbal ability (education: r = .69, 95% CI = [.44, .94], p < .00001; income: r = .48, 95% CI = [.17, .79], p < .01; Fig. 1b), linear regression revealed that income predicted no unique variance in child verbal ability after accounting for parental education (education: β = 8.25, 95% CI = [4.07, 12.44], p < .001, income: β < 0.01, n.s.).
Fig. 1.
Scatterplots showing composite (a) nonverbal and (b) verbal score as a function of parental education level (mother and father averaged) and household income. Standardized nonverbal assessments evaluated fluid reasoning, nonverbal working memory, and processing speed. Standardized verbal assessments evaluated vocabulary, receptive and expressive morphosyntax, and verbal working memory skill. Dotted lines indicate the average range of scores (within 1 standard deviation of the population mean). Solid lines indicate best-fitting regressions.
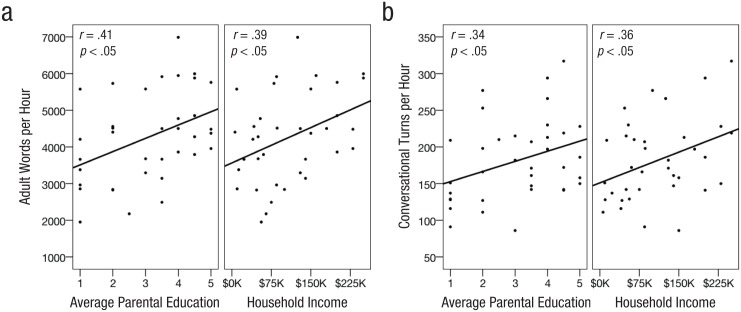
There was great individual variability in language exposure measures, including the number of adult words per peak hour (M = 4,260, SD = 1,225, range = 1,953–6,991), the number of child utterances per hour (M = 743, SD = 261, range = 300–1,275), and the number of conversational turns per hour (M = 181, SD = 56, range = 86–330). Higher parental education and income correlated significantly with more adult words (education: r = .41, 95% CI = [.09, .73], p < .05; income: r = .39, 95% CI = [.06, .71], p < .05) and more conversational turns (education: r = .34, 95% CI = [.02, .67], p < .05; income: r = .37, 95% CI = [.04, .69], p < .05; Fig. 2), but neither SES measure was significantly correlated with child utterances (education: r = .25, n.s.; income: r = .24, n.s.). If these peak-hour measures were extrapolated, children in the top and bottom SES quartiles would experience an annual adult word gap of 5 million words, which could accumulate to approximately 30 million words by age of enrollment in this study, similar to the gap originally reported by Hart and Risley (1995). However, SES explained only a moderate share of the variability in language exposure (11%–17%), indicating that there was wide variability of language exposure within families of similar SES.
Fig. 2.
Scatterplots showing peak hourly (a) adult words and (b) conversational turns as a function of parental education level (mother and father averaged) and household income. Solid lines indicate best-fitting regressions.
All three measures of language experience correlated with children’s scores on behavioral language assessments, although conversational turns most strongly predicted the verbal composite score (conversational turns: r = .51, 95% CI = [.21, .81], p < .001; adult words: r = .36, 95% CI = [.04, .69], p < .05; child utterances: r = .34, 95% CI = [.01, .66], p < .05). Multiple regression models were constructed to predict verbal composite scores as a function of parental education, family income, and each of the three language experience measures. In all three models, parental education significantly predicted verbal scores (all βs > 7.70, p < .001, partial r > .55), whereas income did not (all βs < 0.1). Only conversational turns significantly predicted additional variance in verbal scores after education and income were partialled out (β = 0.09, 95% CI = [0.02, 0.16], p = .01, partial r = .43, R2 change = .10; Fig. 3). Thus, children’s composite verbal score increased by 1 point for every additional 11 conversational turns experienced per hour, independently of SES. The relation between conversational turns and verbal scores remained significant (all βs > 0.08, p < .05) when adult words or child utterances were added to the model, suggesting that the number of conversational turns was not just a proxy for adult speech or child talkativeness. Furthermore, a bootstrap mediation analysis revealed that the number of conversational turns significantly mediated the relationship between parental education and verbal composite scores (indirect effect = 1.16, 95% CI = [0.22, 2.92], indirect/total effect = 0.16); specifically, variation in conversational turns could account for 16% of the total relationship between parental education and children’s verbal scores.
Fig. 3.
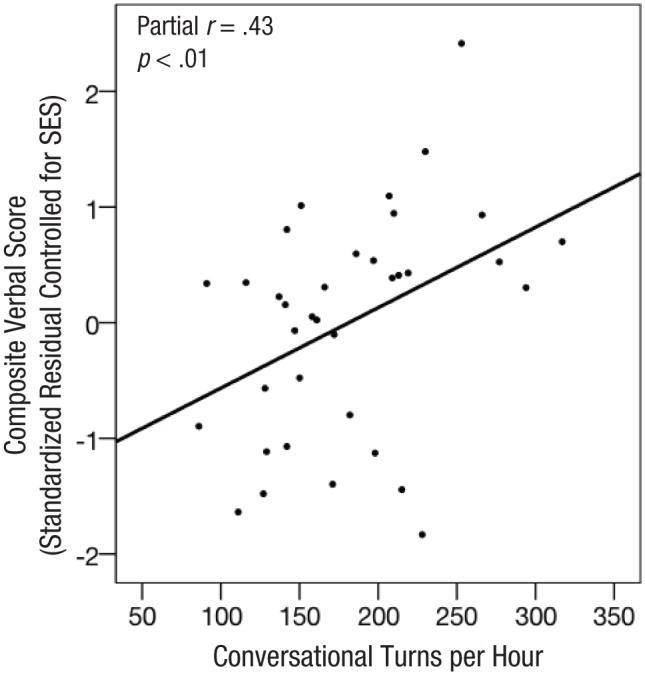
Scatterplot showing the relationship between children’s composite verbal score (controlled for parental education level and income) and the number of hourly conversational turns. The solid line indicates the best-fitting regression.
Neuroimaging results
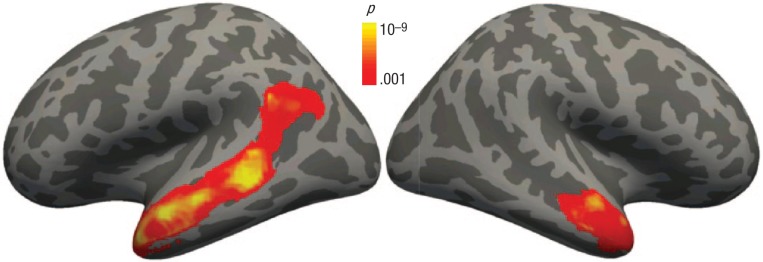
The contrast of interest was activation during the comprehensible forward-speech condition versus the incomprehensible backward-speech condition, which yielded activation specific to higher-level language processing involved in comprehending heard stories, roughly controlling for auditory characteristics. As a group, this task yielded significant activation along bilateral superior temporal sulci (STS), with a leftward lateralization (Fig. 4; see also Table S1 in the Supplemental Material); in the left hemisphere, a cluster extended from the temporal pole to supramarginal/angular gyri, while in the right hemisphere, a cluster was restricted to the anterior portion of the STS.
Fig. 4.
Regions where activation was significantly greater while listening to forward speech than backward speech, averaged across all participants. Clusters include the whole of the left superior temporal sulcus and the anterior portion of the right superior temporal sulcus.
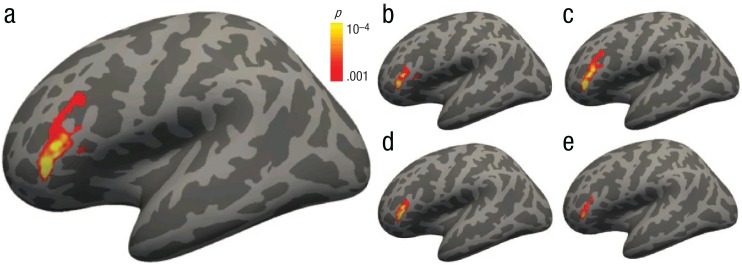
Whole-brain correlations with the three LENA measures were conducted to detect individual differences in activation related to language exposure. While there were no significant correlations with the number of adult words or child utterances, the number of conversational turns correlated positively with activation in a single cluster (Fig. 5a; see also Table S2 in the Supplemental Material; 766 total voxels) spanning left pars triangularis (Brodmann’s area 45) extending into pars opercularis (Brodmann’s area 44), which together comprise Broca’s area. This cluster remained significantly correlated with conversational turns after controlling for parental education and income (Fig. 5b), verbal and nonverbal composite scores (Fig. 5c), adult words and child utterances counts (Fig. 5d), and all of these covariates together (Fig. 5e), indicating that this relationship was not driven simply by any of these factors. In other words, the more conversational turns children experienced, the greater their activation in Broca’s area during language processing, independent of the child’s SES, cognitive ability, or sheer numbers of adult words and child utterances. There were no clusters exhibiting significant correlations with any demographic variables (age, gender, parental education, income) or cognitive variables (verbal, nonverbal scores).
Fig. 5.
Correlations between activation during language processing and the number of hourly conversational turns children experienced. The brain image in (a) shows the zero-order correlation between the number of conversational turns and activation in the forward > backward speech contrast. Correlations remained significant when controlling for (b) parental education and income, (c) verbal and nonverbal assessment scores, (d) individual numbers of adult words and child utterances, and (e) all of these covariates.
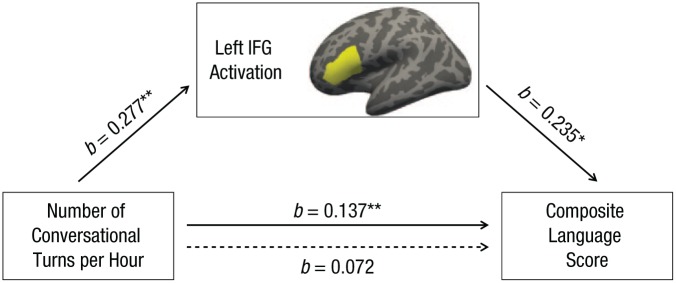
We then asked whether Broca’s area activation helped explain the relation between children’s language exposure and verbal scores. The magnitudes of children’s Broca’s area activations (averaged over anatomically defined opercular and triangular regions, as shown in Fig. 6), significantly mediated the relation between the number of conversational turns and verbal composite scores (indirect effect = 0.065, 95% CI = [0.02, 0.11], indirect/total effect = 0.48), rendering the relation between conversational turns and verbal scores insignificant. This suggests that conversational turns may support children’s verbal skills in part by influencing Broca’s area activation during language processing. Further, this neural pattern explained 48% of the relation between children’s conversational turns and their verbal scores.
Fig. 6.
Mediation model showing the effect of conversational turns on language assessment scores as mediated by activation in the left inferior frontal gyrus (IFG), shaded in yellow. Activation significantly mediated the relation between the number of conversational turns children experience and their language scores. Solid arrows represent direct paths, whereas the dotted arrow represents the indirect (mediated) path. Asterisks indicate significant paths (*p < .01, **p < .001).
Finally, conversational turns and Broca’s area activation jointly mediated the relationship between parental education and children’s language scores (indirect effect = 1.69, 95% CI = [0.24, 3.75], indirect/total effect = 0.23), indicating that conversational turns and Broca’s area activation during language processing could account for 23% of the total relationship between SES and children’s language skills.
Discussion
This study provides the first evidence of the neural activation patterns underlying the relation between children’s early language exposure and verbal skills. Using at-home, real-world audio recorders, we replicated behavioral findings that higher SES is correlated with both greater language experience and verbal abilities in children between the ages of 4 and 6 years. Specifically, it was the number of conversational turns between children and adults (and not the sheer number of adult words) that significantly mediated the SES/verbal-ability relationship. Further, neuroimaging revealed a neural mechanism by which language experience may influence brain development; namely, children who experienced more conversational turns exhibited greater activation in left inferior frontal regions (Broca’s area) during language processing, which explained nearly half the relationship between children’s language exposure and verbal abilities. Finally, conversational turns and Broca’s area activation jointly mediated the relationship between SES and children’s language abilities, demonstrating both environmental and neural mechanisms underlying SES disparities in early language skills.
These findings are consistent with evidence that qualitative aspects of children’s language experience (such as turn taking) may have a greater impact on language development than sheer quantitative measures (Hirsh-Pasek et al., 2015; Zimmerman et al., 2009). While the conversational turn count likely included more child-directed speech than the adult word count (which also included any overheard speech), it is unlikely that the quantity of child-directed speech alone explains the significance of the conversational turn measure. Studies of child-directed speech suggest that contiguity (temporal connectedness) and contingency (contextual relevancy) with children’s utterances are critical for word learning (Roseberry, Hirsh-Pasek, & Golinkoff, 2014) and that the fluency, connectedness, and joint engagement of communication predict later language skills over and above the number of adult words (Hirsh-Pasek et al., 2015). In fact, conversational turns fully explain the effect of adult words on 2- to 48-month-old children’s language skills (Zimmerman et al., 2009). The present results extend the importance of conversational turns to language skills at age 6, suggesting a continued role for this essentially social aspect of language development.
Conversational turns may be particularly important for language development because they provide increased opportunities for children to practice language and receive feedback from adults. Furthermore, this creates a feedback loop to help adults hone their own speech to the optimal complexity to best support children’s language development (Zimmerman et al., 2009). While it is possible that children with better language abilities may better engage in these conversations, child utterances had the weakest relation to language scores and brain functions, suggesting that the strong effect of conversational turns is not simply a reflection of more talkative children. More broadly, the importance of conversational turns supports theories that language development crucially relies on social interaction and social neural circuitry (Kuhl, 2007) and that prelinguistic communicative turn taking was essential for the evolution of language (Levinson, 2016).
The present study is the first to provide evidence of a localized (left inferior frontal) neural mechanism that underlies the relation between children’s direct language exposure and language processing. This is consistent with findings that language input is related to infants’ ERP responses in left frontal regions during a phonological task (Garcia-Sierra et al., 2016). Thus, linguistic experience appears to have a particular influence on language processes in left prefrontal cortex, beginning in infancy and continuing through early childhood.
The finding that participants as a group yielded left-lateralized superior temporal activation is likely indicative of a relative invariance in activation related to the acoustic/subdiscourse aspects of language. However, variation in participants’ language experience correlated exclusively with activation in Broca’s area. The localization of this brain-behavior relationship may be related to the nature of conversational turns as a higher-level, supralexical language process. Although Broca’s area is classically associated with speech production, research suggests it plays a much broader role in both receptive and expressive language processing, as well as a variety of nonlinguistic functions. The specific role of Broca’s area in passive language comprehension is still a matter of debate, although it may function as a convergence zone, in which small, independent elements of language (e.g., phonemes, words) are unified into a coherent overall representation (Hagoort, 2014). The present functional task—listening to meaningful, connected stories—requires integration across phonological, semantic, and syntactic units; thus, greater activation in Broca’s area may represent a deeper engagement with the linguistic structure of the stories. Alternatively, regions of Broca’s area also support several domain-general functions, including action perception, working memory, and executive functioning and cognitive control (Fedorenko, Duncan, & Kanwisher, 2012); in this view, greater activation could indicate a neural representation of the speaker’s or characters’ movements or relating of current verbal information to recently heard sentences or stories. Conversational experience could plausibly contribute to either or both neural systems, and future studies are needed to delineate the precise cognitive processes associated with language exposure.
Several limitations of the present study are noted. To study typical development, we excluded children with language disorders and delays or language scores below the 16th percentile. Given the strong relation between SES and language scores, this may have disproportionately excluded lower-SES children, which some argue may itself be considered a learning disability (Ryan, 2013). Therefore, future studies should delineate the generalization of these findings to children with a greater variety of language abilities. Additionally, participants’ young age required minimization of in-scanner tasks; thus, the functional task was passive in nature. Although children were required to demonstrate listening comprehension before entering the scanner, monitored for alertness during scanning, and incentivized to listen closely, children could have varied in their level of task engagement. However, it is unlikely that this wholly accounted for activation differences, because there were no temporal-lobe differences in relation to language experience and because this task has revealed robust perisylvian activation even in young, sleeping children (Redcay, Haist, & Courchesne, 2008). Nevertheless, any functional activation is constrained by the nature of the task and material used in an experiment, and further studies will be needed to characterize the scope and limits of the present findings. Finally, while LENA provides immense, naturalistic data on the quantity of speech experienced, it does not parse what is said, and thus provides little information about other qualitative aspects of language, such as lexical diversity and grammatical complexity. Future studies should determine the precise relation between conversational quantity and quality on brain and language development.
Although it has been theorized that the home language environment underlies the link between SES and the structure and function of canonical language-related brain regions (Noble et al., 2012; Perkins et al., 2013), this is the first study to reveal a direct relation between a specific aspect of language exposure, namely conversational turns, and brain function during language processing. While causation cannot be implied, results suggest that early language exposure, a proximal aspect of children’s environment, may alter the way in which their brains process language. These findings also have clear practical implications. While many early intervention programs aim to increase the amount of language parents address to their children, these findings suggest programs should also encourage parents to talk with their children by engaging in more interactive, back-and-forth conversation (Leech, Wei, Harring, & Rowe, 2018; McGillion, Pine, Herbert, & Matthews, 2017). Future longitudinal studies may determine whether increasing the number of conversational turns affects the neural patterns supporting language processing and whether there is a critical or sensitive period for such neural changes. Nevertheless, the present study provides initial information on the neural mechanisms underlying the link between children’s linguistic exposure and their language development.
Supplemental Material
Supplemental material, Open_Practices_Disclosure for Beyond the 30-Million-Word Gap: Children’s Conversational Exposure Is Associated With Language-Related Brain Function by Rachel R. Romeo, Julia A. Leonard, Sydney T. Robinson, Martin R. West, Allyson P. Mackey, Meredith L. Rowe, and John D. E. Gabrieli in Psychological Science
Supplemental Material
Supplemental material, Supplemental_Material for Beyond the 30-Million-Word Gap: Children’s Conversational Exposure Is Associated With Language-Related Brain Function by Rachel R. Romeo, Julia A. Leonard, Sydney T. Robinson, Martin R. West, Allyson P. Mackey, Meredith L. Rowe, and John D. E. Gabrieli in Psychological Science
Acknowledgments
We thank the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research (Massachusetts Institute of Technology); Atshusi Takahashi, Steve Shannon, and Sheeba Arnold for data collection support; Kelly Halverson, Emilia Motroni, Lauren Pesta, Veronica Wheaton, and Christina Yu for assistance in administering behavioral assessments; Megumi Takada for help with data collection and organization; Anne Fernald for insight on Language Environment Analysis (LENA) data analysis; Joshua Segaran and Hannah Grotzinger for MRI quality assurance; Tyler Perrachione for thoughtful conversations; Andrea Imhof for comments on the manuscript; and Transforming Education, John Connolly and Glennys Sanchez from 1647 Families, and Ethan Scherer from the Boston Charter Research Collaborative for extensive recruitment support.
Footnotes
Action Editor: Ralph Adolphs served as action editor for this article.
Author Contributions: R. R. Romeo and J. D. E. Gabrieli developed the study concept. R. R. Romeo, J. A. Leonard, A. P. Mackey, M. L. Rowe, and J. D. E. Gabrieli designed the study. R. R. Romeo, J. A. Leonard, and S. T. Robinson collected the data. R. R. Romeo analyzed and interpreted the data under the supervision of J. D. E. Gabrieli and M. L. Rowe. R. R. Romeo drafted the manuscript, and J. A. Leonard, M. R. West, A. P. Mackey, M. L. Rowe, and J. D. E. Gabrieli provided critical revisions. All authors approved the final version of the manuscript for submission.
ORCID iD: Rachel R. Romeo  https://orcid.org/0000-0002-0315-4385
https://orcid.org/0000-0002-0315-4385
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This research was funded by a grant from the Walton Family Foundation (to M. R. West), the National Institute of Child Health and Human Development (Grant No. F31HD086957 to R. R. Romeo), a Harvard Mind Brain Behavior Grant (to R. R. Romeo), and a gift from David Pun Chan.
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617742725
Open Practices:


Home audio recordings and automated Language Environment Analysis (LENA) output measures cannot be shared publicly because this would violate confidentiality. However, we have made all summary measures available, which are all that is needed to replicate the reported results. This data, along with the study materials, have been made publicly available via Harvard Dataverse and can be accessed at www.dx.doi.org/10.7910/DVN/DIDBMQ. The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617742725. This article has received badges for Open Data and Open Materials. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.
References
- Bronfenbrenner U., Morris P. A. (2007). The bioecological model of human development. In Damon W., Lerner R. M. (Eds.), Handbook of child psychology, Vol. 1: Theoretical models of human development (6th ed., pp. 793–828). Hoboken, NJ: Wiley. [Google Scholar]
- Connell C. M., Prinz R. J. (2002). The impact of childcare and parent–child interactions on school readiness and social skills development for low-income African American children. Journal of School Psychology, 40, 177–193. [Google Scholar]
- Dunn L. M., Dunn D. M. (2007). Peabody Picture Vocabulary Test (4th ed.). Bloomington, MN: Pearson. [Google Scholar]
- Farah M. J. (2017). The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron, 96, 56–71. [DOI] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., Kanwisher N. (2012). Language-selective and domain-general regions lie side by side within Broca’s area. Current Biology, 22, 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sierra A., Ramírez-Esparza N., Kuhl P. K. (2016). Relationships between quantity of language input and brain responses in bilingual and monolingual infants. International Journal of Psychophysiology, 110, 1–17. [DOI] [PubMed] [Google Scholar]
- Gilkerson J., Richards J. A., Warren S. F., Montgomery J. K., Greenwood C. R., Kimbrough Oller D., . . . Paul T. D. (2017). Mapping the early language environment using all-day recordings and automated analysis. American Journal of Speech-Language Pathology, 26, 248–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C. D., Madison C., Clark D., Halchenko Y. O., Waskom M. L., Ghosh S. S. (2011). Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics, 5, Article 13. doi: 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. (2014). Nodes and networks in the neural architecture for language: Broca’s region and beyond. Current Opinion Neurobiology, 28, 136–141. [DOI] [PubMed] [Google Scholar]
- Hart B., Risley T. (1995). Meaningful differences in the everyday experience of young American children. Baltimore, MD: Paul H. Brookes. [Google Scholar]
- Hirsh-Pasek K., Adamson L. B., Bakeman R., Owen M. T., Golinkoff R. M., Pace A., . . . Suma K. (2015). The contribution of early communication quality to low-income children’s language success. Psychological Science, 26, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Hoff E. (2006). How social contexts support and shape language development. Developmental Review, 26, 55–88. [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C., . . . Ramus F. (2012). The influence of socioeconomic status on children’s brain structure. PLOS ONE, 7(8), e42486. doi: 10.1371/journal.pone.0042486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C. F., Behrens T. E. J., Woolrich M. W., Smith S. M. (2012). FSL. NeuroImage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kuhl P. K. (2007). Is speech learning ‘gated’ by the social brain? Developmental Science, 10, 110–120. [DOI] [PubMed] [Google Scholar]
- Leech K. A., Wei R., Harring J., Rowe M. L. (2018). A brief parent-focused intervention to improve preschoolers’ conversational skills and school readiness. Developmental Science, 54, 15–28. [DOI] [PubMed] [Google Scholar]
- Levine S. C., Suriyakham L. W., Rowe M. L., Huttenlocher J., Gunderson E. A. (2010). What counts in the development of young children’s number knowledge? Developmental Science, 46, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S. C. (2016). Turn-taking in human communication – Origins and implications for language processing. Trends in Cognitive Sciences, 20, 6–14. [DOI] [PubMed] [Google Scholar]
- Mackey A. P., Finn A. S., Leonard J. A., Jacoby-Senghor D. S., West M. R., Gabrieli C. F. O., Gabrieli J. D. E. (2015). Neuroanatomical correlates of the income-achievement gap. Psychological Science, 26, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillion M., Pine J. M., Herbert J. S., Matthews D. (2017). A randomised controlled trial to test the effect of promoting caregiver contingent talk on language development in infants from diverse socioeconomic status backgrounds. Journal of Child Psychology and Psychiatry, 58, 1122–1131. [DOI] [PubMed] [Google Scholar]
- Noble K. G., Engelhardt L. E., Brito N. H., Mack L. J., Nail E. J., Angal J., . . . Elliott A. J. (2015). Socioeconomic disparities in neurocognitive development in the first two years of life. Developmental Psychobiology, 57, 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K. G., Houston S. M., Kan E., Sowell E. R. (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K. G., Wolmetz M. E., Ochs L. G., Farah M. J., McCandliss B. D. (2006). Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9, 642–654. [DOI] [PubMed] [Google Scholar]
- Perkins S. C., Finegood E. D., Swain J. E. (2013). Poverty and language development: Roles of parenting and stress. Innovations in Clinical Neuroscience, 10(4), 10–19. [PMC free article] [PubMed] [Google Scholar]
- Petersen D. B., Spencer T. D. (2012). The narrative language measures: Tools for language screening, progress monitoring, and intervention planning. Perspectives on Language and Learning Education, 19, 119–129. [Google Scholar]
- Raizada R. D., Richards T. L., Meltzoff A., Kuhl P. K. (2008). Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage, 40, 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Esparza N., García-Sierra A., Kuhl P. K. (2014). Look who’s talking: Speech style and social context in language input to infants are linked to concurrent and future speech development. Developmental Science, 17, 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Haist F., Courchesne E. (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science, 11, 237–252. [DOI] [PubMed] [Google Scholar]
- Roberts M. Y., Kaiser A. P. (2011). The effectiveness of parent-implemented language interventions: A meta-analysis. American Journal of Speech-Language Pathology, 20, 180–199. [DOI] [PubMed] [Google Scholar]
- Rodriguez E. T., Tamis-LeMonda C. S. (2011). Trajectories of the home learning environment across the first 5 years: Associations with children’s vocabulary and literacy skills at prekindergarten. Child Development, 82, 1058–1075. [DOI] [PubMed] [Google Scholar]
- Roseberry S., Hirsh-Pasek K., Golinkoff R. M. (2014). Skype me! Socially contingent interactions help toddlers learn language. Child Development, 85, 956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. L. (2008). Child-directed speech: Relation to socioeconomic status, knowledge of child development and child vocabulary skill. Journal of Child Language, 35, 185–205. [DOI] [PubMed] [Google Scholar]
- Rowe M. L. (2012). A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Development, 83, 1762–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. L., Pan B. A., Ayoub C. (2005). Predictors of variation in maternal talk to children: A longitudinal study of low-income families. Parenting Science and Practice, 5, 285–310. [Google Scholar]
- Ryan J. E. (2013). Poverty as disability and the future of special education law. Georgetown Law Journal, 101, 1455–1503. [Google Scholar]
- Sarsour K., Sheridan M., Jutte D., Nuru-Jeter A., Hinshaw S., Boyce W. T. (2011). Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society, 17, 120–132. [DOI] [PubMed] [Google Scholar]
- Sheridan M. A., Sarsour K., Jutte D., D’Esposito M., Boyce W. T. (2012). The impact of social disparity on prefrontal function in childhood. PLOS ONE, 7(4), Article e35744. doi: 10.1371/journal.pone.0035744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda C. S., Kuchirko Y., Luo R., Escobar K., Bornstein M. H. (2017). Power in methods: Language to infants in structured and naturalistic contexts. Developmental Science, 20, Article e12456. doi: 10.1111/desc.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2012). Wechsler Preschool and Primary Scale of Intelligence (4th ed.). Bloomington, MN: Pearson. [Google Scholar]
- Weisleder A., Fernald A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24, 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig E. H., Semel E. M., Secord W. (2013). Clinical Evaluation of Language Fundamentals (5th ed.). Bloomington, MN: Pearson. [Google Scholar]
- Zimmerman F. J., Gilkerson J., Richards J. A., Christakis D. A., Xu D., Gray S., Yapanel U. (2009). Teaching by listening: The importance of adult-child conversations to language development. Pediatrics, 124, 342–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Open_Practices_Disclosure for Beyond the 30-Million-Word Gap: Children’s Conversational Exposure Is Associated With Language-Related Brain Function by Rachel R. Romeo, Julia A. Leonard, Sydney T. Robinson, Martin R. West, Allyson P. Mackey, Meredith L. Rowe, and John D. E. Gabrieli in Psychological Science
Supplemental material, Supplemental_Material for Beyond the 30-Million-Word Gap: Children’s Conversational Exposure Is Associated With Language-Related Brain Function by Rachel R. Romeo, Julia A. Leonard, Sydney T. Robinson, Martin R. West, Allyson P. Mackey, Meredith L. Rowe, and John D. E. Gabrieli in Psychological Science