Abstract
Background
We previously showed that children undergoing cardiac surgery are at high risk for long-term chronic kidney disease (CKD) and hypertension, although postoperative acute kidney injury (AKI) is not a risk factor for worse long-term kidney outcomes. We evaluated if renal injury biomarkers 5 years after cardiac surgery are associated with postoperative AKI or long-term CKD and hypertension.
Methods
This prospective cohort study recruited children 1 month to 18 years old undergoing cardiopulmonary bypass. At 5 years post-cardiac surgery, we measured urine interleukin-18, kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein-1 (MCP-1), YKL-40, and neutrophil gelatinase–associated lipocalin (NGAL). Biomarker levels were compared between patients with AKI VS. without. We also performed a cross-sectional analysis of the association between these biomarkers with CKD and hypertension.
Results
305 subjects survived hospitalization: 4 (1.3%) died after discharge; 110 (42%) participated in the 5-year follow-up. 49/110 (45%) had AKI. Patients with VS. without post-operative AKI did not have significantly different biomarker concentrations at 5 years. None of the biomarker concentrations were associated with CKD or hypertension at 5-years, although CKD and hypertension were associated with a higher proportion of participants with 5-year abnormal NGAL.
Conclusions
Post-operative pediatric AKI is not associated with urinary kidney injury biomarkers 5 years after surgery. This may represent a lack of chronic renal injury after AKI, imprecise GFR estimation, that longer follow-up is needed to detect chronic renal damage, or that our studied biomarkers are inadequate for evaluating subclinical chronic renal injury.
Keywords: biomarker, acute kidney injury, long-term outcomes, CKD, cardiopulmonary bypass, children
Introduction
Pediatric acute kidney injury (AKI) is common after cardiac surgery and is associated with increased inpatient mortality, length of ventilation, and length of stay [1, 2]. However, much less is known about the long-term outcomes after AKI in children [3, 4]. We previously reported that hypertension and chronic kidney disease (CKD) were common five years after cardiac surgery, although postoperative AKI was not associated with an increased risk of long-term kidney outcomes [5]. Likewise, Cooper et al. found that seven years after cardiac surgery there was no association of AKI with CKD, but that patients with AKI had an elevation of urine kidney injury biomarkers at follow-up [6]. Most recently, a retrospective study evaluating administrative data from Denmark found that children with AKI after cardiac surgery are 3.8 times more likely to develop CKD [7]. Research findings in adult cohorts have clearly demonstrated the association between AKI and risk of long-term CKD, in multiple settings including cardiac surgery [8, 9].
One of the explanations for the conflicting findings on AKI outcomes in the pediatric literature may be the limitations of our current CKD biomarkers, serum creatinine and proteinuria. Proteinuria and serum creatinine may increase late in the course of CKD [10]. Up to 50% of renal mass may be lost without any change in serum creatinine levels, due to compensatory glomerular hypertrophy and hyperfiltration [11]. Therefore, serum creatinine may go unchanged for years in children with CKD [12]. Additionally, serum creatinine is a marker of glomerular filtration and is not specific for identifying tubular or interstitial damage. Proteinuria has up to a 40% intraday variability and is often absent in patients with CKD [10]. Thus, there is clearly a need for more sensitive and predictive biomarkers to study patients at risk for CKD.
A number of novel urinary biomarkers have been studied as candidate proteins of chronic kidney injury, primarily in adults [6, 13, 14]. These urinary biomarkers may rise earlier than serum creatinine and therefore may be used for the early prediction of incident CKD or CKD progression. Additionally, novel biomarkers may capture the variability in CKD progression left unexplained by serum creatinine and proteinuria. Since CKD is a multifactorial disease process we measured a biomarker panel in children five years after cardiac surgery that assessed tubular injury (interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), neutrophil gelatinase–associated lipocalin (NGAL)), inflammation/repair (monocyte chemoattractant protein-1 (MCP-1)), and fibrosis/repair (YKL-40). There is pre-clinical or human research to suggest that all five candidate biomarkers may detect late kidney injury and CKD [15–21]. We measured two biomarkers, MCP-1 and YKL-40, for the first time in this cohort at five years because they are candidate biomarkers of renal repair after injury [21–23]. The primary goal of the present study is to determine if AKI after cardiac surgery is associated with long-term evidence of subclinical kidney injury, detected using novel biomarkers. We hypothesized that children with at least stage I AKI would have higher five-year urinary biomarker levels when compared to children without AKI. We also evaluated the extent to which biomarkers (indexed and not indexed to urine creatinine or when expressed as abnormal using gender and age-specific threshold values), and the change in biomarker concentrations from preoperative to five-year follow-up are associated with 5-year CKD or hypertension.
Materials and Methods
Study Cohort
This is a prospective cohort study of children with congenital heart disease who were enrolled between July 2007 and December 2009. Children one month to 18 years old undergoing cardiac surgery with cardiopulmonary bypass at Cincinnati Children’s Hospital, Montreal Children’s Hospital, and Yale New-Haven Children’s Hospital, were enrolled in the Translational Research Investigating Biomarker End Points in AKI (TRIBE-AKI) study [24–26]. Children were excluded if they had a history of renal transplantation or dialysis. Details around preoperative patient recruitment are published in previous reports [27, 28]. The children who consented to future contact during the index hospitalization were invited to participate in the current study. Informed consent was obtained from parents or legal guardians, along with assent, when appropriate, from children. This study was approved by the institutional review board of each participating institution.
Study visits
Five-year in-person visits were performed by a research nurse at the hospital clinic or at subject’s home as previously described [5]. Height, weight, and blood pressure were measured and blood and urine were collected at the time of the visits. Percentiles and z-scores of height, weight, and BMI were calculated using CDC growth charts [29]. Blood pressure percentiles were calculated as per The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents [29]. During the visit other details on the medical history, interim hospital admissions, medication history, and laboratory testing since cardiac surgery, were also collected. Discharge summaries from hospital admissions and other medical records were obtained for each hospitalization from the respective medical institutions. Full details of the serum creatinine, urine creatinine, and albuminuria measurements, including sample collection and processing have been previously described [5].
Data from the index cardiac surgery admission
The following detailed index cardiac surgery admission information was available in all participants from our prior in-hospital study: preoperative characteristics, operative details, and postoperative complications using the definitions of the Society of Thoracic Surgeons [30]. We utilized the risk adjustment for congenital heart surgery-1 (RACHS-1) consensus-based scoring system to categorize the complexity of surgery [30]. AKI was defined as the development of at least stage one AKI, defined by at least a ≥ 50% increase or a ≥0.3 mg/dL increase from baseline serum creatinine during hospitalization after cardiac surgery [31]. We also analyzed the outcome of stage two AKI, defined as a doubling in serum creatinine from baseline or receiving acute dialysis during the hospital stay. Details of the in-hospital study have been previously described [24–26, 32].
Biomarker Measurements
We measured urinary NGAL, IL-18, and KIM-1 pre-operatively, on the first post-operative day, and at the five-year study visit. YKL-40 and MCP-1 were only measured at the five-year visit due to limited urine volumes. Perioperative NGAL and IL-18 were measured using the ARCHITECT® assay (Abbott Diagnostics, Abbott Park, IL) while KIM-1 was measured using a Sekisui Diagnostic assay (Sekisui Diagnostics LLC, Stamford, CT). Urine NGAL, IL-18, KIM-1, YKL-40, and MCP-1 at five years after surgery were measured using a Mesoscale Discovery (MSD) multiplex assay with intra-assay coefficients of variation of 2.1%, 7.1%, 7.6%, 1.7%, and 1.4%, respectively [25, 26]. MSD uses patterned arrays and an electrochemiluminescence detection method which is quantified using the MSD Quickplex SQ 120 instrument. To evaluate differences between the perioperative and five-year assays, 75 perioperative samples were re-measured on the MSD platform. The correlation between platforms was excellent (0.90 for IL-18 and 0.89 for KIM-1), but KIM-1 measurements from MSD were systematically lower than from Sekisui. Therefore, we did not include preoperative KIM-1 measurements in this study. Five-year biomarkers were primarily expressed as concentrations. For IL-18 and NGAL we also evaluated absolute change in biomarkers (delta) from preoperative to five-year follow-up concentrations. Biomarker age- and gender-specific abnormal thresholds (cutoffs) were available for urine KIM-1, IL-18, and NGAL. Based on prior published biomarker reference ranges in children, we determined whether five-year biomarker levels of IL-18, NGAL, and KIM-1 were greater than published age- and gender-specific cutoffs [33]. The 95th percentile biomarker cutoffs by age and gender were used as abnormal biomarker thresholds for NGAL [3–5 years old (yo) (Male(M) 26.1 ng/ml, Female(F) 52.2 ng/ml), 5–10 yo (M 10.9 ng/ml, F 139.5 ng/ml), 10–15 yo (M 25.5 ng/ml, 72.3 ng/ml), 15–18 yo (M 50.0 ng/ml, 138.6 ng/ml)], IL-18 [3–5 yo (M 78.3 pg/ml, F 100.5 pg/ml), 5–10 yo (M 41.7 pg/ml, F 79.0 pg/ml), 10–15 yo (M 58.5 pg/ml, 111.1 pg/ml), 15–18 yo (M 71.2 pg/ml, 273.1 pg/ml)], and KIM-1 [3–5 yo (M 983.7 pg/ml, F 1291.7 pg/ml), 5–10 yo (M 1276.6 pg/ml, F 1212.9 pg/ml), 10–15 yo (M 1156.6 pg/ml, 1103.5 pg/ml), 15–18 yo (M 1877.3 pg/ml, 1934.5 pg/ml)] [33].
Five-year outcome definitions: hypertension and CKD
For participants up to 18 years old, hypertension was defined as systolic or diastolic blood pressure ≥95th percentile for age, gender, and height [29]. For participants >18 years old, hypertension was defined as systolic or diastolic blood pressure >140 mmHg or >90 mmHg, respectively [34]. Additionally, subjects were classified as having hypertension if they reported a history of hypertension. We calculated estimated glomerular filtration rate (eGFR) using the serum creatinine-based eGFR equation and the cystatin C-based eGFR equation of the Chronic Kidney Disease in Children study (CKiD) [35]. Albuminuria was defined as urine albumin to creatinine ratio >30 mg/g. CKD was defined as a serum creatinine-based eGFR <90 ml/min/1.73m2 or albuminuria [36].
Statistical analysis
Baseline characteristics of participants who completed and did not complete follow-up visits were compared. For each outcome, only those participants with non-missing values were included in the analysis. Continuous variables were compared with two-sample t-test or Wilcoxon rank sum test, and dichotomous variables with the chi-squared test or Fisher’s exact test. Five-year urine biomarker concentrations were compared between patients with and without post-operative AKI and multiple linear regression was performed to evaluate this association, adjusting for age, race, gender, RACHS-1, cardiopulmonary bypass time, and study site. Patients with VS. without CKD and patients with VS. without hypertension were compared for five-year biomarker concentrations, preoperative to five-year biomarker concentration change and presence/absence of elevated biomarkers at five years. SAS version 9.4 was used for analyses.
Results
Study population
Of the 311 children enrolled in the TRIBE-AKI cohort, 305 survived the index hospitalization (Figure 1). 105 children declined future contact and therefore 200 children were eligible for consent. 131 children participated in the five-year follow-up study of which urine samples were available in 110 at a median of 5.4 years of follow-up. Patient baseline and perioperative characteristics by AKI status are shown in Table 1. Children with postoperative AKI had a higher pre-operative eGFR, longer cardiopulmonary bypass time, and a higher RACHS-1 category when compared to children without postoperative AKI. Out of 110 children, 49 (44%) children developed AKI after their index cardiac surgery.
Figure 1.
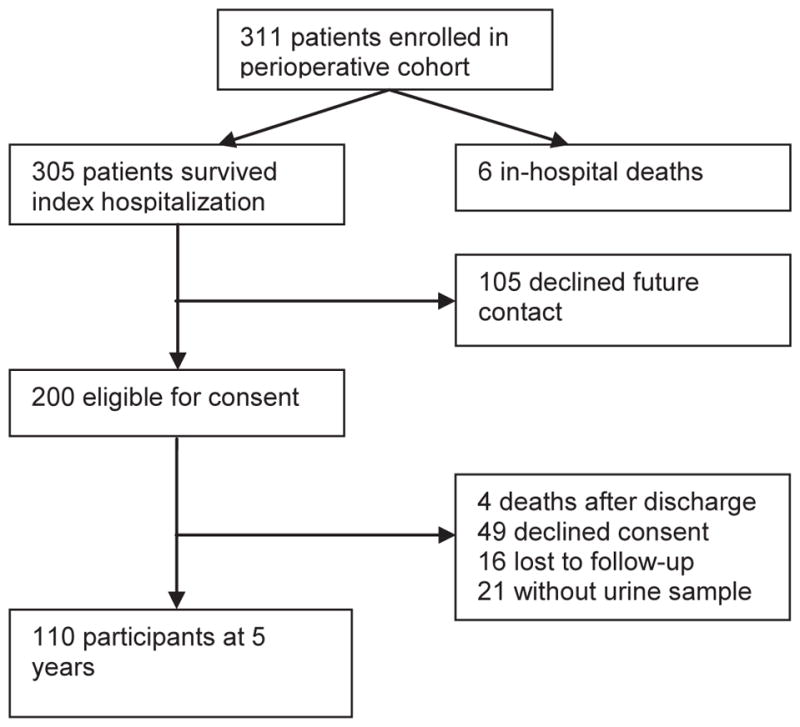
Study Population. TRIBE-AKI Long Term Follow-Up
Table 1.
Baseline and post-operative characteristics of study cohort by AKI status
| Characteristic | Overall n=110 | AKI (n=49) | No AKI (n=61) | P value |
|---|---|---|---|---|
| Pre-operative/baseline | ||||
| Age (years), (mean (SD) | 3.73 (3.9) | 3.54 (4.17) | 3.89 (3.7) | 0.33 |
| Male gender | 57 (52%) | 28 (57%) | 29 (48%) | 0.32 |
| Non-white | 14 (13%) | 7 (14%) | 7 (11%) | 0.66 |
| Pre-operative estimated SCr-GFR (mean (SD)) | 97.59 (25.82) | 103.33 (30.04) | 92.9 (20.89) | 0.05 |
| CPB time (minutes) | 104.88 (66.29) | 124.59 (81.2) | 89.05 (46.23) | 0.03 |
| RACHS-1 surgical category 1 | 7 (6%) | 0 | 7 (11%) | 0.01 |
| RACHS-1 surgical category 2 | 48 (44%) | 21 (43%) | 27 (44%) | |
| RACHS-1 surgical category 3 | 50 (45%) | 23 (47%) | 27 (44%) | |
| RACHS-1 surgical category 4 | 5 (5%) | 5 (10%) | 0 | |
| Elective vs. Urgent Surgery | ||||
| Elective | 105 (95%) | 46 (94%) | 59 (97%) | 0.48 |
| Urgent | 5 (5%) | 3 (6%) | 2 (3%) | |
| Type of Surgery | ||||
| Septal defect repair | 36 (35%) | 14 (30%) | 22 (38%) | 0.47 |
| Inflow/outflow tract or valve procedure | 23 (22%) | 9 (20%) | 14 (24%) | |
| Combined procedure | 45 (43%) | 23 (50%) | 22 (38%) | |
| Index Hospitalization | ||||
| Peak post-op SCr rise from baseline, mg/dL (median (IQR)) | 0.2 (0.3, 0.7) | 0.7 (0.5, 1) | 0.2 (0, 0.3) | <0.001 |
| Length of hospital stay (days) | 8.03 (9.25) | 10.76 (12.38) | 5.84 (4.67) | <0.001 |
| Length of ICU stay (days) | 3.62 (5.53) | 5.53 (7.77) | 2.08 (1.37) | <0.001 |
| Renal replacement | 3 (2%) | 3 (5%) | 0 | 0.04 |
Abbreviations: AKI, Acute Kidney Injury, IQR, interquartile range, SCr, serum creatinine (in mg/dl. 1mg/dl = 88.4 μmole/L), GFR, glomerular filtration rate (ml/min/1.73m2), CPB, cardiopulmonary bypass, RACHS-1, risk adjustment for congenital heart surgery, mg, milligram, dL, deciliter, CRRT, continuous renal replacement therapy.
Postoperative AKI and five-year biomarkers
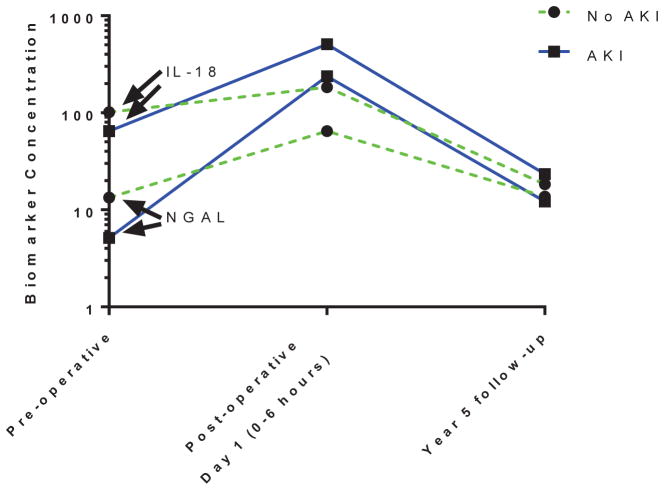
Figure 2 displays AKI VS. non-AKI biomarker concentrations from preoperative, postoperative and five-year time points for KIM-1, NGAL, and IL-18. As shown, KIM-1 changed very little throughout all time points, whereas NGAL and IL-18 rose acutely postoperatively but then decreased at the five-year follow-up. At the five-year visit, median urine IL-18, KIM-1, MCP-1, YKL-40, and NGAL were not significantly different in patients with VS. without stage 1 or worse AKI (Table 2). Similarly, stage 2 or worse AKI was not associated with five-year biomarker levels (data not shown). In multivariable analyses (adjusted for age, race, gender, RACHS-1, cardiopulmonary bypass time, and study site), there was still no association between postoperative AKI and five-year biomarker levels (Supplementary Table 1).
Figure 2.
Urine IL-18 and urine NGAL at pre-operative, post-operative, and year five follow-up. The biomarkers are presented in the following units: IL-18 (pg/mL), NGAL (ng/ml).
Table 2.
Five-year biomarkers by AKI status
| Overall n=110 | AKI (n=49) | No AKI (n=61) | P value | |
|---|---|---|---|---|
| Urine albumin/Cr (mg/g) | 5.1 [2.8, 12.6] | 5.8 [2.8, 10.3] | 5.0 [2.8, 14.3] | 0.79 |
| Estimated GFRa | 113 [103, 126] | 118 [105, 132] | 112 [98, 123] | 0.09 |
| Urine IL-18 (pg/mL) | 14.7 [8.6, 23.4] | 16.6 [9.5, 24.3] | 13.9 [7.9, 21.8] | 0.26 |
| Urine KIM-1 (pg/mL) | 250.6 [157.6, 568.5] | 366.1 [157.6, 656.8] | 336.3 [175.8, 525.4] | 0.40 |
| Urine MCP-1 (pg/mL) | 109.9 [46.4, 169.4] | 103.4 [45.3,169.4] | 116.4 [47.4,167.3] | 0.83 |
| Urine YKL-40 (pg/mL) | 338.6 [157.1, 615.1] | 344.5 [184.2, 645.9] | 330.1 [149.6, 614.8] | 0.58 |
| Urine NGAL (ng/ml) | 4.4 [2.1, 10.2] | 4.7 [2.2, 7.5] | 4.29 [2.0, 14.3] | 0.86 |
Values are expressed as median [interquartile range]. GFR, glomerular filtration rate; Cr, creatinine; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1: NGAL, neutrophil gelatinase–associated lipocalin; AKI, acute kidney injury,
Estimated GFR is displayed as ml/min/1.73m2 and calculated with the serum creatinine-based Chronic Kidney Disease in Children Study
Five-year biomarkers and kidney outcomes
At the five-year follow-up visit, the median (IQR) urine albumin to creatinine ratio and serum creatinine-based eGFR in children was 5.1 (2.8–12.6) mg/g and 113 (103–126) mL/min/1.73m2, respectively (Table 2). Hypertension and CKD was present in 20 (18%) and 18 (17%) children, respectively. Five-year median urine IL-18, KIM-1, MCP-1, YKL-40, and NGAL were not significantly higher in children with VS. without hypertension or CKD at five-years (Table 3 for hypertension, Table 4 for CKD). Median five-year urine KIM-1 was lower in patients with CKD (0.33 [IQR: 0.19–0.55) than in patients without CKD (0.63 [IQR: 0.3–1.0) (p=0.04). Similarly, there was no significant difference in absolute change in urine biomarker concentrations (KIM-1, NGAL, IL-18) from preoperative to five-years in patients with VS. without hypertension (Table 3) or CKD (Table 4). Both hypertension (VS. no hypertension) and CKD (VS. no CKD) were associated with a higher proportion of patients with five-year NGAL concentrations above normal for age (Tables, 3 and 4, respectively); they were not associated with abnormal five-year KIM-1 or IL-18 concentrations (Tables 3 and 4). There was no change in our five-year biomarker results in patients with or without CKD when we used a cystatin C-based equation to estimate GFR (data not shown). Additionally, the lack of association with AKI, CKD, and hypertension remained unaffected when we adjusted five-year biomarker concentrations for urinary creatinine (Supplementary Tables 2, 3, and 4).
Table 3.
Urinary Biomarkers and Hypertension at the Five-Year Follow-up Visit
| Hypertension (N=20) | No Hypertension (N=89) | P value | |
|---|---|---|---|
| IL-18 Year 5 (pg/mL) | 17.1 (10.9, 40.7) | 14.4 (8, 21.8) | 0.24 |
| IL-18 Elevated at Year 5 | 1 (5%) | 3 (3%) | 0.73 |
| IL18 (Change from pre-op) (pg/mL) | 2.7 (-25.5, 16.1) | -0.8 (-22.0, 9.1) | 0.32 |
| KIM-1 Year 5 (ng/mL) | 0.48 (0.4, 0.8) | 0.6 (0.3, 1.0) | 0.78 |
| KIM-1 Elevated at Year 5 | 1 (5%) | 15 (17%) | 0.18 |
| NGAL Year 5 (ng/mL) | 7.07 (2.1, 32.2) | 4.3 (2.2, 8.4) | 0.25 |
| NGAL Elevated at Year 5 | 2 (10%) | 1 (1%) | 0.03 |
| NGAL (Change from pre-op) (ng/mL) | 2.7 (-2.5, 39.4) | 0.8 (-3.3, 4.2) | 0.14 |
| MCP1 MSD Year 5 (pg/mL) | 94.3 (38.8, 164.8) | 112.3 (47.1, 169.4) | 0.67 |
| YKL-40 MSD Year 5 (pg/mL) | 423.5 (187.5, 693.0) | 317.7 (157.1, 614.8) | 0.53 |
Values are expressed as median [interquartile range] or Number (%). IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1: NGAL, neutrophil gelatinase–associated lipocalin. Change from pre-op signifies the difference between the five-year biomarker level and the preoperative biomarker level
Table 4.
Urinary Biomarkers and CKD at Five-Year Follow-up Visit
| CKD (N=18) | No CKD (N=89) | P value | |
|---|---|---|---|
| IL-18 Year 5 (pg/mL) | 14.4 (7.3, 19.8) | 15.1 (8.7, 23.9) | 0.35 |
| IL-18 Elevated at Year 5 | 0 (0%) | 4 (4%) | 0.36 |
| IL18 (Change from pre-op) (pg/mL) | -4.6 (-8.8, 2.4) | -0.6 (-22.1, 12.9) | 0.50 |
| KIM-1 Year 5 (ng/mL) | 0.3 (0.2, 0.6) | 0.6 (0.3, 1.0) | 0.04 |
| KIM-1 Elevated at Year 5 | 2 (11%) | 14 (16%) | 0.62 |
| NGAL Year 5 (ng/mL) | 3.5 (1.6, 12.8) | 4.7 (2.3, 9.3) | 0.67 |
| NGAL Elevated at Year 5 | 2 (11%) | 1 (1%) | 0.02 |
| NGAL (Change from pre-op) (ng/mL) | -0.08 (-3.4, 2.1) | 1.5 (-2.5, 5.0) | 0.43 |
| MCP1 MSD Year 5 (pg/mL) | 85.9 (29.8, 176.2) | 103.4 (47.4, 163.2) | 0.56 |
| YKL-40 MSD Year 5 (pg/mL) | 223.5 (157.1, 442.0) | 379.9 (151.4, 639.4) | 0.31 |
Values are expressed as median [interquartile range] or Number (%). IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1: NGAL, neutrophil gelatinase–associated lipocalin; CKD, chronic kidney disease. Change from pre-op signifies the difference between the five-year biomarker level and the preoperative biomarker level
Discussion
This is the largest prospective study to examine the association between postoperative AKI and long-term kidney injury biomarkers in children who underwent cardiopulmonary bypass. We found that postoperative AKI was not associated with later elevations of urine biomarkers of kidney injury and that children with five-year CKD and hypertension did not generally have higher kidney biomarkers at follow-up.
A previous study by Cooper et al. found that children with AKI after cardiac surgery had significantly higher IL-18, KIM-1, and L-FABP at approximately 7 years post-operatively, when compared to children without AKI [6]. These findings suggested that despite a lack of association of AKI with later CKD, there may have been ongoing subclinical kidney injury in the long-term, only detectable by more sensitive urine kidney injury biomarkers. Several differences between our study and the Cooper study may explain our differing findings. The Cooper study had a smaller sample size (n=51) from a single center, but was enriched with patients who developed severe AKI (postoperative doubling of serum creatinine). However, when we evaluated stage two AKI or worse, we did not find an association between more severe AKI and 5-year biomarker concentrations. The follow-up in the Cooper study was longer, with a median of 7 years; it is thus conceivable that patients in their cohort had more time to develop differential biomarker concentrations between AKI groups over time. Finally, patient characteristics between the two studies also differed, with the Cooper study including neonates at the time of surgery, longer cardiopulmonary bypass time, and higher RACHS-1 scores which may have placed them at overall increased risk for long-term subclinical renal damage [37]. However, when we performed a multivariable regression analysis and controlled for potential confounders, there was still no association with AKI and five-year biomarkers. Importantly, biomarker concentrations were quite different between the two cohorts and were measured with different assays, which lends to difficulties in comparing the two studies. Our study does not confirm the findings of the Cooper study that AKI leads to long-term subclinical renal injury, as evidenced by elevated kidney injury biomarkers, but also highlights the importance of validation of biomarker studies in larger cohorts from multiple centers.
We evaluated the cross-sectional association between five-year urine biomarkers and the presence of functional kidney abnormalities or hypertension, with the rationale that if urine injury biomarkers were in fact indicative of chronic injury in this clinical context, they should be higher in patients with functional impairment. Overall, the utility of renal injury biomarkers in evaluating subclinical chronic kidney injury is still an active area of investigation; thus negative findings are as important as positive findings [16, 20, 38] [15] [39] [18, 40]. Biomarker concentrations were not significantly different in our cohort of children with (VS. without) CKD or hypertension. Although it is intriguing that children with hypertension and those with CKD had a higher prevalence of abnormally high urine NGAL, these results must be interpreted with caution, given the multiple testing performed in our study and the risk of spurious results. That said, several previous studies in patients with CKD associated with other diseases have also found that urine NGAL is associated with worse renal function [39, 41]. It is thus worth evaluating in future research the utility of abnormal NGAL levels to screen for and predict progression of renal disease after cardiac surgery. We found at the five-year visit, urine KIM-1 was lower in children with CKD as compared to those without CKD. Since KIM-1 is a tubular protein detected with kidney injury, this association is not supported by previous KIM-1 research and may be influenced by confounding factors [17, 42, 43].
Our overall lack of association of urine injury biomarkers with CKD or with hypertension may also be explained by other issues. It is possible that our studied biomarkers are not sensitive to detect renal damage in the non-acute setting in patients after cardiac surgery. Secondly, it is possible that our methods of evaluating renal disease (CKD and hypertension) are non-specific to chronic tubular injury-associated renal disease in the cardiac surgery population; other factors (e.g., hormonal, humoral, vascular, cardiac) which are not related to these kidney injury biomarkers may play a more important role in the renal disease pathophysiology. Thus, while our negative findings are relevant in directing future studies in the pediatric cardiac surgery long-term outcomes research, they should be evaluated separately in distinct populations (e.g., nephrotoxic AKI or non-cardiac surgery critical illness-associated AKI).
Our study has important limitations. We did not enroll infants less than one-month old and thereby we excluded many of the infants with the most complex cardiac disease. Due to the substantial limitations of serum creatinine as well as the pediatric AKI and CKD definitions available, there may have been misclassification of exposure status and outcomes. Importantly, we used the CKiD equation to estimate GFR even though it has not been validated in healthy children without CKD. Additionally, this pediatric cohort may have been sub-optimal to study CKD after AKI as many of our patients had non-progressive mild AKI, which may not lead to long-term kidney damage in children. Given this uncertainty, future research should consider focusing on severe AKI as a risk factor for CKD. There were a large number of children who declined participation in this long-term follow-up study, although baseline characteristics were not different between those who did and did not participate [5]. When we determined the absolute change in urine biomarker concentrations we used different biomarker assays at the perioperative and five-year time points, although we measured inter-assay variability. Future prospective studies measuring the change of biomarkers in serial biospecimens should make an effort to use identical biomarker assays. There was also a wide age range at study enrollment and the temporal change of urinary biomarkers over five years may be influenced by substantial age-related changes in biomarker concentrations over time [33, 44].
We have previously shown that children undergoing cardiac surgery are at very high risk for long-term CKD and hypertension; however, we have not found an association of AKI with CKD or hypertension development, and the current study does not support the existence of a relationship between urine injury biomarkers and long-term post-cardiac surgery CKD or hypertension. Future research should determine which renal injury biomarkers detect the presence of renal disease or of hypertension in the unique and high-risk cardiac surgery population, while accounting for effects of age and other patient and disease characteristics on determining biomarker concentrations. Future research should also determine the extent to which our studied biomarkers are (or are not) predictive of post-AKI development of CKD or hypertension in other, non-cardiac surgery, populations, in order to guide future AKI outcome translational research. Such research may identify markers of early subclinical renal injury to allow intervention prior to development of renal functional decline or cardiovascular effects of hypertension.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (NIH) (R01HL085757 to C.R.P.) to fund the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. J.H.G. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH under award number K08DK110536. C.R.P. is supported by the NIH (K24DK090203) and P30 DK079310-07 O'Brien Center Grant. C.R.P. and P.D. are members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01DK082185). P.D. is also supported by P50DK096418.
Footnotes
Compliance with ethical standards
Informed consent was obtained from parents or legal guardians, along with assent, when appropriate, from children. This study was approved by the institutional review board of each participating institution.
Disclosures
Dr. Devarajan reported being a coinventor on the neutrophil gelatinase–associated lipocalin patent. No other disclosures were reported.
References
- 1.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8:1661–1669. doi: 10.2215/CJN.00270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR TRIBE-AKI Consortium. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016;170:1071–1078. doi: 10.1001/jamapediatrics.2016.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P, Krawczeski CD. Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI) Clin J Am Soc Nephrol. 2016;11:21–29. doi: 10.2215/CJN.04240415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen NL, Goldstein SL, Froslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92:751–756. doi: 10.1016/j.kint.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, Slinin Y, Ensrud KE. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 9.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Woods LL. Intrarenal mechanisms of renal reserve. Semin Nephrol. 1995;15:386–395. [PubMed] [Google Scholar]
- 12.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 13.Mansour SG, Puthumana J, Coca SG, Gentry M, Parikh CR. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: a systematic review. BMC Nephrol. 2017;18:72. doi: 10.1186/s12882-017-0490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chron Kidney Dis. 2010;17:469–479. doi: 10.1053/j.ackd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubowska M, Wyka K, Fendler W, Mlynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers. 2013;35:811–818. doi: 10.1155/2013/369784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienias B, Zajaczkowska M, Borzecka H, Sikora P, Wieczorkiewicz-Plaza A, Wilczynska B. Early Markers of Tubulointerstitial Fibrosis in Children With Idiopathic Nephrotic Syndrome: Preliminary Report. Medicine. 2015;94:e1746. doi: 10.1097/MD.0000000000001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 18.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 19.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 20.Vianna HR, Soares CM, Silveira KD, Elmiro GS, Mendes PM, de Sousa Tavares M, Teixeira MM, Miranda DM, Simões E, Silva AC. Cytokines in chronic kidney disease: potential link of MCP-1 and dyslipidemia in glomerular diseases. Pediatr Nephrol. 2013;28:463–469. doi: 10.1007/s00467-012-2363-x. [DOI] [PubMed] [Google Scholar]
- 21.Puthumana J, Hall IE, Reese PP, Schroppel B, Weng FL, Thiessen-Philbrook H, Doshi MD, Rao V, Lee CG, Elias JA, Cantley LG, Parikh CR. YKL-40 Associates with Renal Recovery in Deceased Donor Kidney Transplantation. J Am Soc Nephrol. 2017;28:661–670. doi: 10.1681/ASN.2016010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, Parikh CR, Cantley LG. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24:309–319. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour SG, Puthumana J, Reese PP, Hall IE, Doshi MD, Weng FL, Schroppel B, Thiessen-Philbrook H, Bimali M, Parikh CR. Associations between Deceased-Donor Urine MCP-1 and Kidney Transplant Outcomes. Kidney Int Rep. 2017;2:749–758. doi: 10.1016/j.ekir.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR TRIBE-AKI Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery – a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG TRIBE-AKI Consortium. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 30.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR TRIBE-AKI Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol. 2015;30:677–685. doi: 10.1007/s00467-014-2989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–163. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 37.Morgan C, Al-Aklabi M, Garcia Guerra G. Chronic kidney disease in congenital heart disease patients: a narrative review of evidence. Can J Kidney Health Dis. 2015;2:27. doi: 10.1186/s40697-015-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghobrial EE, El Hamshary AA, Mohamed AG, Abd El Raheim YA, Talaat AA. Urinary monocyte chemoattractant protein-1 as a biomarker of lupus nephritis activity in children. Saudi J Kidney Dis Transpl. 2015;26:507–515. doi: 10.4103/1319-2442.157350. [DOI] [PubMed] [Google Scholar]
- 39.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY Chronic Renal Insufficiency Cohort study investigators. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83:909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P Investigators of the HUS-SYNSORB Pk Multicenter Clinical Trial. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 41.Malyszko J, Malyszko JS, Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S, Mysliwiec M. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41:158–161. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 42.Ucakturk A, Avci B, Genc G, Ozkaya O, Aydin M. Kidney injury molecule-1 and neutrophil gelatinase associated lipocalin in normoalbuminuric diabetic children. J Pediatr Endocrinol Metab. 2016;29:145–151. doi: 10.1515/jpem-2015-0138. [DOI] [PubMed] [Google Scholar]
- 43.van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007;84:1625–1630. doi: 10.1097/01.tp.0000295982.78039.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg JH, Parikh CR. Biomarkers for Diagnosis and Prognosis of AKI in Children: One Size Does Not Fit All. Clin J Am Soc Nephrol. 2017;12:1551–1557. doi: 10.2215/CJN.12851216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.